Abstract
Identifying accurate biomarkers of cognitive decline is essential for advancing early diagnosis and prevention therapies in Alzheimer’s Disease. The Alzheimer’s Disease DREAM Challenge was designed as a computational crowdsourced project to benchmark the current state-of-the-art in predicting cognitive outcomes in Alzheimer’s Disease based on high-dimensional, publicly available genetic and structural imaging data. This meta-analysis failed to identify a meaningful predictor developed from either data modality, suggesting that alternate approaches should be considered for to prediction of cognitive performance.
2. Background
The Alzheimer’s Disease DREAM Challenge (http://dx.doi.org/10.7303/syn2290704) was designed to provide an unbiased assessment of current capabilities for estimation of cognition and prediction of cognitive decline using genetic and imaging data from public data resources using a crowd-sourced approach. The ability to predict rate of cognitive decline – both prior to and following diagnosis – is essential to effective trial design for the development of therapies for Alzheimer’s Disease (AD) prevention and treatment. Major collaborative efforts in the field are assessing the association of genetic loci with AD diagnosis and the application of structural imaging for development of early biomarkers of diagnosis, but the utility of these approaches to estimate cognition or predict cognitive decline is not well established. This project was designed under the advisement of a panel of experts in the field to evaluate whether these questions could be meaningfully addressed with current methodologies given existing public data sources. To ensure that these questions were tested across a broad spectrum of the latest analytical approaches, the study was designed as a crowdsourced, community-based challenge in which participants were invited to address one or more of the following three problems: (1) The prediction of cognitive decline over time based on genetic data. (2) The prediction of resilience to cognitive decline in individuals with elevated amyloid burden based on genetic data. (3) The estimation of cognitive state based on structural magnetic resonance (MR) imaging data.
3. Results
3.1 Study design and data harmonization
To ensure that predictors were detecting true biological variation rather than study-specific technical variation, this project required inclusion of data from multiple study sources. While genetic and imaging data have been generated within many rich longitudinal cohorts across the field, the procurement and harmonization of these data sets was a non-trivial problem that required solutions to overcome political, ethical, and technical barriers. For example, the generation of whole genome sequencing data across multiple AD cohorts within the NIH-funded AD sequencing project has resulted in a powerful resource for genetic analysis in the field but longitudinal information on cognitive traits is not readily available in those datasets. Despite limitations on data accessibility, multiple relevant data sources were identified and used in this project including: the Alzheimer’s Disease Neuroimaging Initiative (ADNI)(1), the Rush Alzheimer’s Disease Center Religious Orders Study(2) and Memory and Aging Project (MAP)(3) and the European AddNeuroMed(4) study, which is part of InnoMed, a precursor to the Innovative Medicines Initiative. Data selection and processing was performed based on data availability across these three datasets. As such, cognition was defined using Mini Mental State Examination (MMSE) scores(5), genetic data was provided based on imputation across array-based genotype data, and structural MR imaging data was reprocessed in each cohort using a common processing pipeline. Genetic and imaging data was supplemented with a limited set of covariates including diagnosis, initial MMSE score, age at the initial examination, years of education, gender, and APOE haplotype. Participants were provided with data from ADNI to train algorithms over a four-month period and, to ensure that participation was not limited by access to compute resources, they were offered use of the IBM z-Enterprise cloud to perform analyses. The challenge generated significant interest with 527 individuals from around the world registered to participate. A leaderboard displayed accuracy of submissions throughout the duration of the challenge: 1,157 submissions were made for problem 1,478 submissions for problem 2, and 434 submissions for problem 3. Thirty-two teams submitted final results that were scored based on prediction/estimation of blinded outcomes within ROS/MAP for genetic predictions and AddNeuroMed for imaging-based estimations (Figure 1).
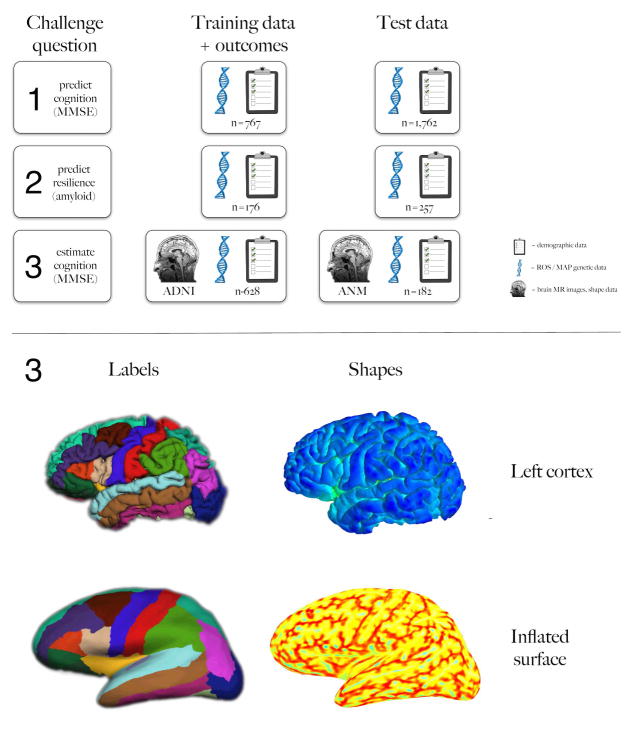
Fig. 1. Challenge overview.
The top schematic summarizes the three challenge questions on the left column, the training data in the middle, and the test data on the right, including numbers of subjects. The symbols represent sources of data (demographic, ROS/MAP genetic, and ADNI or ANM brain images and shape information). The bottom panel provides example brain image labels and shape information provided to the participants for question 3. Anatomical labels for left cortical regions are shown on the left and just a couple of the cortical surface shape measures are shown on the right (travel depth on top and mean curvature below), for both uninflated and inflated surfaces (top and bottom rows, respectively).
3.2 Genetic prediction of cognitive decline
The first challenge question assessed the ability of current methods to predict change in cognitive examination performance based on genetic data. High prediction accuracy would signal the potential for noninvasive biomarkers of cognition to have a major clinical impact on early AD diagnosis and prevention. Previous efforts to develop predictors of change in cognitive function have not succeeded in providing robust and replicable models(6–8). Genetic variation has been demonstrated to influence AD status: rare genetic mutations at several loci are implicated in familial forms of early-onset disease(9) while common variation contributes 33% to variance in sporadic AD and 22 loci have been implicated by large-scale genetic association analyses(10, 11). However, with the exception of the APOE4 haplotype, there has been little success in transforming these genetic associations into meaningful clinical predictions of cognitive decline. For this purpose, participants were challenged to predict 2-year changes in MMSE scores based on genotypes imputed from SNP array data. Participants trained their algorithms with 767 ADNI samples and the algorithms’ predictions were evaluated on a test set of 1,175 ROS/MAP samples with blinded outcome measures. The algorithm with the best predictive performance at the midpoint of the challenge did not contain any genetic features beyond APOE haplotype. Since the goal of this subchallenge was to assess genetic contribution to prediction of cognitive decline, this top-ranked algorithm was openly shared across teams as an interim baseline upon which to incorporate additional genetic predictors (http://dx.doi.org/10.7303/syn2838779). Eighteen teams submitted final predictions. The majority of methods performed significantly better than a permutation-based random model prediction (Figure 2a). A cluster of six methods performed significantly better than the others (including the interim baseline model) but were statistically indistinguishable amongst themselves (Figure 2d). Of these, the prediction with the best overall score (team GuanLab_umich from the University of Michigan) achieved a Pearson correlation of 0.382 and a Spearman correlation of 0.433 (the overall score was a rank-based combination of these two measures of performance; see online Supplement and Supplementary Methods: http://dx.doi.org/10.7303/syn3383106). However, no significant contribution of genetics beyond APOE haplotype to predictive performance was observed across any of the submissions. Given the small sample size, no conclusions can be inferred from this analysis regarding the existence of genetic loci associated with cognitive decline. Rather, these observations suggest that predictors of cognitive decline developed based on genetic data will not be useful within the clinical setting.
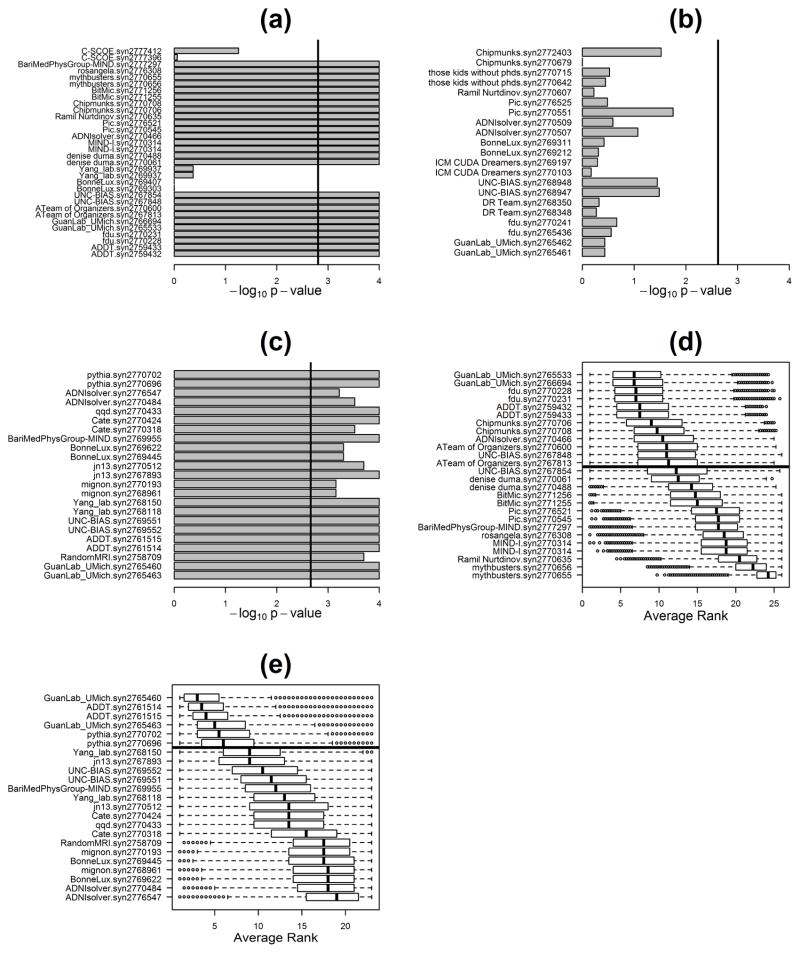
Figure 2. Performance evaluation results.
Panels a, b, and c report the p-values (in negative log10 scale) for intersection union tests investigating which teams performed better than random for questions 1, 2, and 3, respectively. Explicitly, for question 1 (panel a) we tested the null hypothesis that at least one of the four correlation coefficients (namely, Pearson/clinical, Pearson/clinical + genetics, Spearman/clinical, Spearman/clinical + genetics) is equal to zero, against the alternative that all four correlation coefficients are larger than zero. Adopting a 0.05 significance level, 26 out of the 32 submissions were statistically better than random, after Bonferroni multiple testing correction for 32 tests (submissions crossing the black vertical line). For question 2 (panel b), we tested the null hypothesis that balanced accuracy = 0.5 or AUC = 0.5, against the alternative that balanced accuracy > 0.5 and AUC > 0.5. In this case, no model performed significantly better than random and, therefore, no best performer was declared. For question 3 (panel c), we tested the null hypothesis that Pearson’s correlation (COR) or Lin’s concordance correlation coefficient (CCC) are equal to zero, against the alternative that both COR and CCC are larger than zero. Adopting a 0.05 significance level, all 23 submissions were statistically better than random, after Bonferroni correction. For all three questions, the p-values were computed from an empirical null distribution based on 10,000 permutations. Panels d and e report the bootstrapped assessment of ranks for questions 1 and 3, respectively. Samples were resampled with replacement from the original data (true outcome and team’s predictions), and the ranks of the different teams were re-assessed in each of 100,000 re-samplings. Submissions were sorted according to the median of their bootstrapped average ranking distributions. The black horizontal line represents the posterior odds cutoff from the Bayesian analysis. Teams above the black line are statistically tied to the top ranked model, according to a posterior odds threshold of 3.
3.3 Genetic prediction of cognitive resilience
The second question challenged participants to identify genetic predictors that could distinguish individuals who exhibit resilience to AD pathology as defined by minimal change in cognitive function despite evidence of amyloid deposition(12, 13). Identification of genetic signatures predictive of cognitive resilience would aid in the elucidation of mechanisms that may confer resilience, providing a powerful tool to help advance AD prevention strategies and treatment development. Eleven teams submitted predictions of resilience based on a training set derived from 176 ADNI subjects. Evaluations were made using data derived from 257 individuals from the ROS/MAP data. Despite using the largest such public dataset assembled to date, participants were unable to develop algorithms with predictive performances significantly better than random (see Figure 2b, online Supplement and Supplementary Methods in Synapse: http://dx.doi.org/10.7303/syn3383106). While it is likely that the study was underpowered due to small sample size and trait heterogeneity, this result suggest that information about cognitive resilience is not easily discoverable from SNP analysis.
3.4 Structural imaging-based estimation of cognition
The third question challenged participants to estimate cognitive state using structural brain image data (Figure 1, lower panel). Brain imaging has emerged as a powerful method for monitoring neurodegeneration and there is great enthusiasm in the field to make use of images for diagnosis and prediction. There have been many attempts in the past to correlate changes in brain shape with disease progression and/or diagnosis, conventionally using measures of volume for a given brain region(14, 15). More detailed shape measures of image features including cortical thickness, curvature, and depth have also been found to be relevant to a variety of neurological conditions(16). Participants were challenged to estimate MMSE scores based on structural brain images, or shape information derived from these images. Participants trained algorithms using ADNI data (N=628) and were evaluated using AddNeuromed data (N=182) for which they were blind to outcome measures. To engage as many participants as possible from both within and beyond the neuroimaging community, the data were provided both as raw MR images and as tables containing shape measures (volume, thickness, area, curvature, depth, etc.) for every labeled brain region. Thirteen teams submitted estimates for final evaluation and all teams performed better than a random model (see online Supplement and Supplementary Methods in Synapse: http://dx.doi.org/10.7303/syn3383106). Three teams performed significantly better than the others (teams GuanLab_umich from the University of Michigan, ADDT from the Karolinska Institute and Pythia from the University of Pennsylvania) (Figure 2c) but were statistically indistinguishable from one another and tied for top average rank (Figure 2e). The algorithm that generated the best absolute mean combined rank (Team GuanLab_umich) achieved a concordance correlation coefficient of 0.569 and Pearson’s correlation of 0.573 (the overall score was a rank-based combination of these two measures of performance). The most common features that contributed heavily to the MMSE estimates across the algorithms were hippocampal volume and entorhinal thickness, corroborating prior work(17–19). The top three teams also found that inclusion of shape measures of the entorhinal cortex (volume, curvature, surface area, travel and geodesic depth) improved overall estimation. Other features that contributed to predictions within the top three teams’ results included volume of inferior lateral ventricle and amygdala (see online Supplement and Supplementary Methods in Synapse: http://dx.doi.org/10.7303/syn3383106). These results validate an established relationship between structural imaging data and cognition. However, the correlative performance of these estimators was low suggesting that their application in the clinical setting may not be sufficient to inform patient care.
4. Discussion
The AD DREAM Challenge provided a formalized assessment of the ability to develop meaningful predictions of cognitive performance from public genetic or imaging data using contemporary state-of-the-art predictive algorithms. Predictive performance across all three of the subchallenges was modest and most methods performed roughly equivalently. Given this uniform performance, we do not expect that the presented results are a failure of current modeling methodologies. A more likely explanation is that the data used to address these questions were inadequate to support these tasks. We also note that the majority of research teams that participated in this challenge did not have expertise in the field of AD. Although the few teams that did posses this knowledge did not do better than the others, there remains the possibility that performance would have been improved by the inclusion of more domain experts.
4.1 Use of genetic information for cognitive prediction
The modest performance observed in the subchallenges focused on genetic analysis demonstrated that contemporary algorithms were not able to leverage genetic signal to make useful predictions for cognition. These results support the prevailing expectation that genetic variants of moderate to high frequency will not support viable biomarker development in AD (9–11). Although heritability estimates and linkage studies have demonstrated that there is a considerable estimated genetic contribution to AD onset and progression (11, 20, 21), evidence both within the AD field and across other complex disease (22) traits has indicated that this overall genetic contribution is the aggregated compilation of a large number of loci with small – independent or epistatic – effects. Historically, this type of signal is difficult to capture in predictive models and unlikely to be useful in a diagnostic setting (23). Furthermore, cognition is highly influenced by a host of non-genetic factors relating to lifestyle choices and accumulated exposures that were not represented across all of these datasets and, in fact, are not fully captured in most cohorts (24–27). Non-genetic contributions to cognitive performance may themselves provide an important base for successful predictions. Within the context of genetic analysis, the absence of these factors from models confounds the ability to detect real genetic signal and impacts the ability to accurately model state-specific genetic contributions. As such, future inquiry into the use of genetic testing for prediction of cognitive performance and AD risk assessment may be better served by focusing on the contribution of rare genetic variation. Recently discovered disease-associated rare variants have larger effect sizes than common variants and confer 2 to 5 fold greater risk or protection in carriers relative to the general population (28–30). Ongoing large-scale sequencing analyses will identify additional associated rare risk variants. In sufficient numbers, the aggregate prevalence would support the development of a genetic diagnostic containing a library of rare variants.
4.2 Use of structural imaging data for cognitive estimation
While the inexpensive and noninvasive nature of genetic testing makes this approach amenable to population-level disease screening, the resource-intensive nature of image-based testing is better positioned for careful evaluation of high-risk individuals. As such, these approaches are needed to provide a higher confidence estimate of cognitive performance. Although a variety of methods developed within the context of this challenge were able to successfully estimate cognition, none of these methods were sufficiently accurate to merit clinical consideration. These observations support previous work in the field (17, 19) and highlight the imperfect relationship between brain structure and function. Newer imaging modalities that focus on brain function and/or pathology – such as FDG-PET (31) or tau imaging (32)– may prove more successful for assessing cognitive dysfunction.
4.3 Effective performance of meta-analysis across diverse cohorts
A major consideration for any meta-analysis is the issue of appropriate harmonization of data across disparate sources. Despite leveraging several of the most deeply phenotyped cohorts in the field, this challenge limited analysis to those traits that were in common across cohorts. Although this approach to data harmonization is standard practice for meta-analyses (10), it greatly reduced the depth of the information available for modeling and influenced the selection of cognitive measures for use as prediction outcomes. Because each cohort had performed a battery of study-specific tests, this greatly limited the ability for finer grained assessment across cognitive processes. A more sensible approach for future analyses may be to focus effort on more sophisticated methods to calibrate disparate cognitive phenotypes across cohorts (33). Another undesirable consequence of the focus on traits measured in common was the inability to incorporate into model development the full spectrum of non-genetic and non-imaging factors that are known to influence cognitive performance (24–27). This suggests the need for development of alternate approaches for integrating heterogeneous data and/or assessing replication across cohorts. Alternatively, smaller scale analyses that prioritize phenotypic depth over sample size may afford a more refined view of disease.
In summary, this challenge demonstrated that predictions of cognitive performance developed from genetic or structural imaging data were modest across a diverse set of contemporary modeling methods. Future efforts to identify clinically relevant predictors of cognition will benefit from a focus on alternate data sources as well as methods that work to incorporate greater phenotypic complexity.
Systematic Review
Extensive literature searches using PubMed establish this as the largest study to date using demographic, clinical, imaging and genetic data to predict cognitive decline and the first major instance of crowdsourcing analysis in AD.
Interpretation
Over 500 scientists worldwide in the analytical portion of the Challenge, demonstrating the viability of crowdsourced approaches in AD research. Unfortunately, we were unable to detect meaningful predictors of either cognitive decline or resilience through this effort.
Future Directions
This experiment in crowdsourcing AD analyses is an invaluable first-of-its-kind contribution that provides a snapshot of both the strengths and limitations in big data analytics in AD research. The relative inaccessibility and heterogeneity across data sources severely limits formalized integration. Mandates on data sharing, considerations of standardized data collection, and mechanisms to integrate heterogeneous data are necessary to address these issues. We anticipate that this work will initiate those discussions across the community.
Acknowledgments
The following people provided final submissions, but did not participate in the community phase: Lorna Barron1, Oliver Barron1, Riccardo Bellazzi2, Jungwoo Chang1, Marianne H Cowherd1, Grace Ganzel1, Łukasz Grad3, Inhan Lee1, Ivan Limongelli2, Simone Marini2, Szymon Migacz3, Ettore Rizzo2, Witold R Rudnicki3,4, Andrzej Sułecki3, Leo Tunkle1, Francesca Vitali2
This study was supported by the following individuals and organizations: Alan Evans (McGill University), Gaurav Pandey (MSSM), Gil Rabinovici (UCSF), Kaj Blennow (Göteborg University), Kristine Yaffe (UCSF), Maria Isaac (EMA), Nolan Nichols (University of Washington), Paul Thompson (UCLA), Reisa Sperling (Harvard), Scott Small (Columbia), Guy Eakin (BrightFocus Foundation), Maria Carillo (Alzheimer’s Association), Neil Buckholz (NIA), Alzheimer’s Research UK, European Medicines Agency, Global CEO Initiative on Alzheimer’s Disease, Pfizer, Inc, Ray and Dagmar Dolby Family Fund, Rosenberg Alzheimer’s Project, Sanofi S.A, and Takeda Pharmaceutical Company Ltd, USAgainstAlzheimer’s.
Study data were provided by the following groups:
The Alzheimer’s Disease Neuroimaging Initiative (ADNI)
ADNI is funded by the National Institutes of Health (U01 AG024904), the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; BioClinica, Inc.; Biogen Idec Inc.; Bristol-Myers Squibb Company; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; GE Healthcare; Innogenetics, N.V.; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Medpace, Inc.; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Synarc Inc.; and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California. This research was also supported by NIH grants P30 AG010129 and K01 AG030514.
The Rush Alzheimer’s Disease Center, Rush University Medical Center, Chicago
Data collection was supported through funding by NIA grants P30AG10161, R01AG15819, R01AG17917, R01AG30146, R01AG36836, U01AG32984, and U01AG46152, the Illinois Department of Public Health, and the Translational Genomics Research Institute.
European AddNeuroMed study
The AddNeuroMed data are from a public-private partnership supported by EFPIA companies, SMEs and the EU under the FP6 programme. Clinical leads responsible for data collection are Iwona Kłoszewska (Lodz), Simon Lovestone (London), Patrizia Mecocci (Perugia), Hilkka Soininen (Kuopio), Magda Tsolaki (Thessaloniki), and Bruno Vellas (Toulouse).
Footnotes
GIDAS, miRcore, 2929 Plymouth Rd. Suite 207, Ann Arbor, MI, USA
Electrical, Computer and Biomedical Engineering Department, Via Ferrata, 1, Pavia, Italy
Interdisciplinary Centre for Mathematical and Computational Modelling, Pawińskiego 5A, Warsaw, Poland
Department of Bioinformatics, University of Białystok, Ciołkowskiego 1M, Białystok, Poland
AUTHOR CONTRIBUTIONS: CJB, ECN, DWF, SHF, SSG, AK, JSKK, YK, BAL, LMM, TJM, TCN, MAP, GS, GV and NJT contributed to the challenge organization
VB, DD, PSH, RCG contributed with compute resources and scientific advice
GIA, NA, CA, DB, RB, KB, PCB, LC, CC, FC, YCC, BC, CYC, TYC, TC, SD, CD, JD, QD, JD, DD, RE, GE, EE, HF, JG, EG, YG, MYH, CH, JH, JI, PI, QJ, DK, RK, EL, ML, EL, XL, ZL, JL, AL, MM, AM, EM, JN, AN, MN, MN, RNN, YJO, YP, SP, SRP, PP, CP, VYS, PS, XS, AS, ST, AT, YAT, EV, GX, LX, YX, JX, HY, XZ, YZ, FZ, HZ and SZ participated in the challenge community phase
DAB, RJBD, SL, SJN, AS and MWW contributed with data used in the challenge
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mueller SG, Weiner MW, Thal LJ, Petersen RC, Jack C, Jagust W, et al. The Alzheimer’s disease neuroimaging initiative. Neuroimaging clinics of North America. 2005 Nov;15(4):869–77. xi–xii. doi: 10.1016/j.nic.2005.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett DA, Schneider JA, Arvanitakis Z, Wilson RS. Overview and findings from the religious orders study. Current Alzheimer research. 2012 Jul;9(6):628–45. doi: 10.2174/156720512801322573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and findings from the rush Memory and Aging Project. Current Alzheimer research. 2012 Jul;9(6):646–63. doi: 10.2174/156720512801322663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lovestone S, Francis P, Kloszewska I, Mecocci P, Simmons A, Soininen H, et al. AddNeuroMed--the European collaboration for the discovery of novel biomarkers for Alzheimer’s disease. Annals of the New York Academy of Sciences. 2009 Oct;1180:36–46. doi: 10.1111/j.1749-6632.2009.05064.x. [DOI] [PubMed] [Google Scholar]
- 5.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research. 1975 Nov;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 6.Ercoli LM, Siddarth P, Dunkin JJ, Bramen J, Small GW. MMSE items predict cognitive decline in persons with genetic risk for Alzheimer’s disease. Journal of geriatric psychiatry and neurology. 2003 Jun;16(2):67–73. doi: 10.1177/0891988703016002001. [DOI] [PubMed] [Google Scholar]
- 7.Hsiung GY, Alipour S, Jacova C, Grand J, Gauthier S, Black SE, et al. Transition from cognitively impaired not demented to Alzheimer’s disease: an analysis of changes in functional abilities in a dementia clinic cohort. Dementia and geriatric cognitive disorders. 2008;25(6):483–90. doi: 10.1159/000126499. [DOI] [PubMed] [Google Scholar]
- 8.Vemuri P, Wiste HJ, Weigand SD, Shaw LM, Trojanowski JQ, Weiner MW, et al. MRI and CSF biomarkers in normal, MCI, and AD subjects: predicting future clinical change. Neurology. 2009 Jul 28;73(4):294–301. doi: 10.1212/WNL.0b013e3181af79fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ridge PG, Ebbert MT, Kauwe JS. Genetics of Alzheimer’s disease. BioMed research international. 2013;2013:254954. doi: 10.1155/2013/254954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nature genetics. 2013 Dec;45(12):1452–8. doi: 10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ridge PG, Mukherjee S, Crane PK, Kauwe JS Alzheimer’s Disease Genetics C. Alzheimer’s disease: analyzing the missing heritability. PloS one. 2013;8(11):e79771. doi: 10.1371/journal.pone.0079771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006 Jun 27;66(12):1837–44. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- 13.Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Annals of neurology. 1999 Mar;45(3):358–68. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 14.Davatzikos C, Xu F, An Y, Fan Y, Resnick SM. Longitudinal progression of Alzheimer’s-like patterns of atrophy in normal older adults: the SPARE-AD index. Brain: a journal of neurology. 2009 Aug;132(Pt 8):2026–35. doi: 10.1093/brain/awp091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Misra C, Fan Y, Davatzikos C. Baseline and longitudinal patterns of brain atrophy in MCI patients, and their use in prediction of short-term conversion to AD: results from ADNI. NeuroImage. 2009 Feb 15;44(4):1415–22. doi: 10.1016/j.neuroimage.2008.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Im K, Lee JM, Seo SW, Hyung Kim S, Kim SI, Na DL. Sulcal morphology changes and their relationship with cortical thickness and gyral white matter volume in mild cognitive impairment and Alzheimer’s disease. NeuroImage. 2008 Oct 15;43(1):103–13. doi: 10.1016/j.neuroimage.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 17.Haight TJ, Jagust WJ Alzheimer’s Disease Neuroimaging I. Relative contributions of biomarkers in Alzheimer’s disease. Annals of epidemiology. 2012 Dec;22(12):868–75. doi: 10.1016/j.annepidem.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nho K, Risacher SL, Crane PK, DeCarli C, Glymour MM, Habeck C, et al. Voxel and surface-based topography of memory and executive deficits in mild cognitive impairment and Alzheimer’s disease. Brain imaging and behavior. 2012 Dec;6(4):551–67. doi: 10.1007/s11682-012-9203-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thung KH, Wee CY, Yap PT, Shen D Alzheimer’s Disease Neuroimaging I. Neurodegenerative disease diagnosis using incomplete multi-modality data via matrix shrinkage and completion. NeuroImage. 2014 May 1;91:386–400. doi: 10.1016/j.neuroimage.2014.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Escott-Price V, Sims R, Bannister C, Harold D, Vronskaya M, Majounie E, et al. Common polygenic variation enhances risk prediction for Alzheimer’s disease. Brain: a journal of neurology. 2015 Dec;138(Pt 12):3673–84. doi: 10.1093/brain/awv268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SH, Harold D, Nyholt DR Consortium AN, International Endogene C, Genetic, et al. Estimation and partitioning of polygenic variation captured by common SNPs for Alzheimer’s disease, multiple sclerosis and endometriosis. Hum Mol Genet. 2013 Feb 15;22(4):832–41. doi: 10.1093/hmg/dds491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chatterjee N, Wheeler B, Sampson J, Hartge P, Chanock SJ, Park JH. Projecting the performance of risk prediction based on polygenic analyses of genome-wide association studies. Nature genetics. 2013 Apr;45(4):400–5. 5e1–3. doi: 10.1038/ng.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manolio TA. Bringing genome-wide association findings into clinical use. Nat Rev Genet. 2013 Aug;14(8):549–58. doi: 10.1038/nrg3523. [DOI] [PubMed] [Google Scholar]
- 24.Scarmeas N, Stern Y, Mayeux R, Luchsinger JA. Mediterranean diet, Alzheimer disease, and vascular mediation. Arch Neurol. 2006 Dec;63(12):1709–17. doi: 10.1001/archneur.63.12.noc60109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Podewils LJ, Guallar E, Kuller LH, Fried LP, Lopez OL, Carlson M, et al. Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study. Am J Epidemiol. 2005 Apr 1;161(7):639–51. doi: 10.1093/aje/kwi092. [DOI] [PubMed] [Google Scholar]
- 26.Lindsay J, Laurin D, Verreault R, Hebert R, Helliwell B, Hill GB, et al. Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian Study of Health and Aging. Am J Epidemiol. 2002 Sep 1;156(5):445–53. doi: 10.1093/aje/kwf074. [DOI] [PubMed] [Google Scholar]
- 27.Wang HX, Karp A, Winblad B, Fratiglioni L. Late-life engagement in social and leisure activities is associated with a decreased risk of dementia: a longitudinal study from the Kungsholmen project. Am J Epidemiol. 2002 Jun 15;155(12):1081–7. doi: 10.1093/aje/155.12.1081. [DOI] [PubMed] [Google Scholar]
- 28.Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, et al. TREM2 variants in Alzheimer’s disease. N Engl J Med. 2013 Jan 10;368(2):117–27. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, et al. Variant of TREM2 associated with the risk of Alzheimer’s disease. N Engl J Med. 2013 Jan 10;368(2):107–16. doi: 10.1056/NEJMoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jonsson T, Atwal JK, Steinberg S, Snaedal J, Jonsson PV, Bjornsson S, et al. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature. 2012 Aug 2;488(7409):96–9. doi: 10.1038/nature11283. [DOI] [PubMed] [Google Scholar]
- 31.Gray KR, Wolz R, Heckemann RA, Aljabar P, Hammers A, Rueckert D, et al. Multi-region analysis of longitudinal FDG-PET for the classification of Alzheimer’s disease. NeuroImage. 2012 Mar;60(1):221–9. doi: 10.1016/j.neuroimage.2011.12.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.James OG, Doraiswamy PM, Borges-Neto S. PET Imaging of Tau Pathology in Alzheimer’s Disease and Tauopathies. Front Neurol. 2015;6:38. doi: 10.3389/fneur.2015.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gross AL, Sherva R, Mukherjee S, Newhouse S, Kauwe JS, Munsie LM, et al. Calibrating longitudinal cognition in Alzheimer’s disease across diverse test batteries and datasets. Neuroepidemiology. 2014;43(3–4):194–205. doi: 10.1159/000367970. [DOI] [PMC free article] [PubMed] [Google Scholar]