Abstract
Giant cell tumor of bone (GCT) is a primary bone neoplasm with unique cytogenetic findings including telomeric associations. Elevated expression of message RNA for transforming growth factor beta (TGFβ), but not transforming growth factor alpha (TGFα), has been reported in this tumor. Further investigation of GCT was undertaken to determine whether genetic loci for TGFβ in GCT patients with and without chromosome abnormalities are altered.
Due to the reported TGFβ overexpression in GCT, qualitative and quantitative Southern blot analyses with TGFβ1 and TGFβ2 and an internal control probe (p3–21) were performed with tumor DNA and DNA from normal tissue on ten patients with GCT and control individuals. No obvious TGFβ1 or TGFβ2 gene alterations were detected. Normal copy numbers were calculated when comparing tumor and normal DNA Rom GCT patients as well as DNA from control individuals.
Abnormal chromosome findings, including telomeric associations, marker chromosomes, double minutes, chromosome fragments, ring chromosomes (possibly representing intra-chromosome telomeric associations), and polyploid cells were observed in seven of the ten patients with GCT. Chromosomes 11, 16, 19, 20, and 21 were most commonly observed in telomeric associations, with the terminus of the long arm of chromosome 19 being the most frequent.
We conclude that there are no TGFβ1 or TGFβ2 gene alterations detected in GCT with the methodologies described, and that telomeric associations are a reproducible cytogenetic characteristic of this neoplasm.
INTRODUCTION
Giant cell tumor of bone (GCT) is a solid, primary bone neoplasia composed of stromal mononuclear cells and large multinucleated cells which resemble osteoclasts. These musculoskeletal tumors occur most frequently in the ends of long tubular bones of young adults and constitute 5% of all primary bone tumors. GCT is usually considered benign but has an unpredictable pattern of biological aggressiveness and is treated by surgical resection. The local recurrence rate is 20–60%, with a 2% rate for benign pulmonary metastasis.
Chromosome abnormalities including telomeric associations (defined as the end-to-end fusion of chromosomes without apparent loss of DNA) have been reported previously in GCT [1–5] and, recently, consistent transforming growth factor overexpression was reported in GCT for TGFβ1 and TGFβ2 but not for TGFβ3 or TGFα [2]. Because the 19q terminus is frequently involved in telomeric associations of GCT [1–5] and the gene locus for TGFβ1 is on 19q, we undertook cytogenetic studies and determined the DNA status of TGFβ1 as well as TGFβ2 genes in patients with this neoplasm.
MATERIALS AND METHODS
Cytogenetic Studies
Cytogenetic and DNA studies were undertaken on GCTs excised and histologically confirmed from ten patients (six men and four women ranging in age from 12–53 years; see Table 1). These patients had not received any form of chemotherapy or radiation treatment prior to tumor removal.
Table 1.
Clinical, cytogenetic, and DNA densitometric data on patients with giant cell tumor of bone
| Patient | Age (years) | Race | Sex | Tumor location | TASa | Copy number for TGFβ2 tumor/blood |
|---|---|---|---|---|---|---|
| BC | 40 | B | F | Proximal tibia | + | 1.9/2.5 |
| DB | 20 | W | M | Distal femur | + | 2.3/2.1 |
| BP | 12 | W | F | Sacrum | − | 1.8/10 |
| BH | 47 | W | M | Distal femur | + | −/− |
| SS | 26 | W | F | Proximal tibia | − | 1.9/2.0 |
| GL | 53 | B | M | Distal femur | + | 2.1/1.8 |
| FH | 32 | W | M | Distal radius | + | 2.2/1.7 |
| KW | 39 | O | M | Proximal tibia | + | 2.4/− |
| MG | 39 | W | M | Proximal tibia | − | 1.7/2.5 |
| MS | 25 | W | F | Scapula | + | 1.9/2.1 |
Telomeric associations.
GCT cells from the ten patients were sterilely harvested, cultured, and cytogenetically analyzed. The solid tumor (1 cc) was minced and digested in an enzyme solution containing collagenase. The tumor cell suspension was centrifuged and the enzyme solution removed. The cells were placed in sterile T-25 flasks containing 4 mL RPMI 1640 cell culture medium supplemented with 20% fetal calf serum, penicillin, streptomycin, and L-glutamine. Short-term cultures (less than 4 weeks) were harvested when the cells reached confluency. Colcemid (2 μg final concentration) was added to each flask for the final 3 hours. Microscope slides were made and the chromosomes banded with Giemsa stain after trypsinization.
DNA Analysis
Telomeric associations frequently involve the long arm of chromosome 19 [2]. The TGFβ1 gene has been localized to this chromosome region and elevated TGFβ message RNA was reported in GCT [2]. Therefore, DNA analysis of TGFβ genes (e.g., TGFβ1 localized to 19q13.1–q13.2 and TGFβ2 localized to 1q41) were undertaken. Genomic DNA was extracted, following established protocols, from both normal tissue (e.g., blood) and GCT tissue from each of the ten patients with GCT studied cytogenetically, and two normal control individuals. Genomic DNA (5 μg) digested with TaqI enzyme was fractionated by electrophoresis on agarose gel and transferred to Gene Screen Plus (New England Nuclear, Boston, MA) membranes. The membrane was pre-hybridized and probed with radioactive DNA, washed, and exposed to x-ray film as previously described [6]. A 974-bp fragment of cDNA for murine TGFβ1 and a 442-bp fragment of cDNA for murine TGFβ2 (both kindly provided by Dr. Harold Moses, Vanderbilt University) were used to probe for loci [7]. A 2.2-kb fragment (p3–21 probe) of single copy DNA localized to 15q11–q12 and assigned to locus D15510 obtained from American Tissue Culture Collection, Rockville, MD, was used as an internal control probe for quantitative hybridization analysis of both TGFβ1 and TGFβ2 probes. The DNA fragments were uniformly labeled with 32P using the multi-priming method of Feinberg and Vogelstein [8]. Two probes (e.g., TGFβ1 and p3–21 or TGFΒ2 and p3–21) were concomitantly hybridized to Southern blot membranes in order to determine the presence or absence of the TGFβ1 or TGFβ2 gene copies.
The autoradiographic bands were scanned using an LKB Ultrascan soft laser densitometer. The 32P hybridization ratio figures for a given DNA lane were converted to an estimate of the copies per diploid genome assuming two copies per diploid genome for the DNA detected by the internal control probe (p3–21). To obtain a linear range of band intensities, an autoradiogram of varying amounts of radioactive DNA spotted onto a nylon membrane was subjected to a similar densitometric analysis. The relative intensities of bands on genomic Southern blots were computed from at least two lanes as described by Tantravahi et al. [9].
RESULTS
Cytogenetic Studies
Cytogenetic findings from short-term cultures are reported from tumor cells of ten patients. Figure 1 shows representative examples of chromosome abnormalities seen in our GCT patients. Detailed chromosome analysis of four of the patients are published elsewhere [2]. Tetraploid cells, chromosome aberrations (fragments, double minutes, translocations, and markers), telomeric associations, and ring chromosomes (which could represent intra-chromosome telomeric associations) were observed in the tumor cells from seven of the ten patients, whereas no chromosome abnormalities were observed in the tumor cells from three patients (Table 1). The most frequently observed chromosome abnormality was telomeric associations. Chromosomes 11, 16, 19, 20, and 21 were most commonly observed in telomeric associations with the terminus of the long arm of chromosome 19 as the most frequent in our patients. All GCT patients with telomeric associations were found to have involvement of chromosome 19 and no patient had monosomy or trisomy 19 in their tumor cells. Chromosomes 11 and 19 have been among the most frequently involved in GCT, as reported in other investigations [1–5].
Figure 1.

Representative examples of chromosomes showing telomeric associations (top row), ring chromosomes, and an acrocentric marker chromosome (bottom row) from patients with GCT. The chromosome number and the telomere involved are listed to the left of each telomeric association.
DNA Studies
Southern blotting with the TGFβ1 probe and DNA isolated from tumor cells of four patients with GCT and two control individuals did not detect obvious gene alterations (e.g., deletions) (Figure 2A). Quantitative Southern hybridization with TGFβ1 and an internal control probe (p3–21) was also undertaken with tumor derived DNA from two GCT patients (B.H. with telomeric associations and S.S. without telomeric associations, and DNA from a control individual) (Figure 2B). Again, no obvious DNA alterations of TGFβ1 gene were observed and a normal allele copy number (e.g., 1.8 for tumor DNA from B.H. and 2.2 for tumor DNA from S.S.) was calculated by densitometric analysis by comparing the TGFβ1/p3–21 ratios for the GCT patients.
Figure 2.

Qualitative and quantitative Southern blot analysis with TGFβ1 and p3–21 of tumor DNA digested with TaqI from five patients with GCT as well as DNA from two normal control individuals. A normal allele copy number for TGFβ1 was calculated in DNA isolated from GCT. (A) Lane 1 = DNA from GCT from patient S.S. with normal chromosomes. Lane 2 = DNA from GCT from patient G.L. with abnormal chromosome findings. Lane 3 = DNA from GCT from patient B.P. with normal chromosome findings. Lane 4 = DNA from GCT from patient B.C. with abnormal chromosome findings. Lane 5 = Normal DNA from a control adult woman. Lane 6 = Normal DNA from a control adult man. (B) Lanes 1 and 2 = Normal DNA from a control adult female. Lanes 3 and 4 = Tumor DNA from patient B.H. with abnormal chromosomes. Lanes 5 and 6 = Tumor DNA from patient S.S. with normal chromosomes.
Quantitative hybridization with TGFβ2 and p3–21 was undertaken with tumor-derived DNA and normal tissue DNA from the ten GCT patients and a control individual. Representative DNA lanes are shown in Figure 3. No obvious TGFβ2 gene alterations or abnormal allele copy numbers were detected for the GCT patients using DNA from healthy controls for determination of the copy number (Table 1), which is also supported by the lack of chromosome 1 abnormalities observed in our GCT patients. The copy numbers for TGFβ2 with GCT DNA ranged from 1.7 to 2.4 with an average copy number of 2.0. The copy numbers for TGFβ2 with normal DNA from the GCT patients ranged from 1.7 to 2.5 with an average copy number of 2.1.
Figure 3.
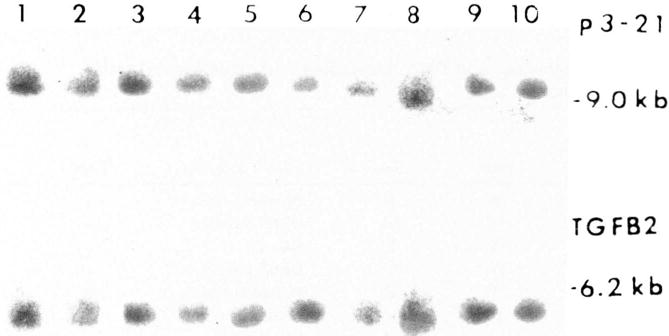
Qualitative and quantitative Southern blot analysis with TGFβ2 and p3–21 of tumor and normal DNA digested with TaqI. No obvious DNA alterations were observed after comparing tumor and normal DNA from the same GCT patients or in comparing DNA from a control individual. Lane 1 = DNA from normal tissue from S.S. Lane 2 = Tumor DNA from patient S.S. Lane 3 = DNA from normal tissue from patient EH. Lane 4 = Tumor DNA from patient F.H. Lane 5 = DNA from normal tissue from patient M.S. Lane 6 = Tumor DNA from patient M.S. Lane 7 = DNA from normal tissue from patient B.H. Lane 8 = Tumor DNA from patient B.H. Lane 9 = Tumor DNA from patient K.W. Lane 10 = DNA from normal tissue from a control adult woman.
DISCUSSION
In our cytogenetic study of GCT, we found that telomeric associations were present in short-term cultures from seven of the ten patients and the terminus of the long arm of chromosome 19 was the most frequently involved. Telomeric associations are not unique to GCT, and have been reported in at least five malignancies, of which three are solid tumors (malignant fibrous histiocytoma [10] and papillary renal cell carcinoma [11]) and three are of hematopoietic origin (B-cell lymphoid leukemia [12], T-cell lymphoid leukemia [13], and hairy cell leukemia [14]). Telomeric associations have also been reported in four benign neoplasms including GCT [1–5, 15], desmoid tumor [16], atrial myxoma [17], and renal oncocytoma [11]. Telomeric associations have also been observed in senescent fibroblasts [18] and in SV40-transformed cells [19].
Interestingly, genes that may be important in tumorigenesis are mapped to the vicinity of the end of the long arm of chromosomes 19 and 20 and the short arm of chromosome 11 (these chromosomes are predominantly seen in telomeric associations in GCT). For example, src oncogene has been localized to band 20q12–q13 [20], but no activation of the oncogene has been found in solid tumors [21]. TGFβ1 has been localized to 19q13.1–q13.2 [20] and is the focus of our molecular genetic analysis. H-ras oncogene is localized to 11p15.5; its role in the formation of GCT has recently been investigated and an overrepresentation of rare H-ras DNA polymorphisms was observed, but with no loss of heterozygosity or H-ras gene alterations detected [22].
TGFβ promotes the differentiation of mesenchymal cells [23–26] and has been implicated in bone resorption and in the activation of osteoclasts [27–30]. Because of the promotion of mesenchymal cell differentiation by TGFβ1, activation of osteoclasts, and its location on 19q, TGFβ1 could be important in the biology of GCT. In addition, elevated levels of TGFβ message RNA has been reported in GCT [2]. Hence, TGFβ1 as well as TGFβ2 were investigated in a total of ten GCT patients. Qualitative and quantitative Southern blot analyses of both TGFβ1 and TGFβ2 did not identify gene alterations. There were no differences in the DNA pattern or the allele copy number determined from normal or tumor-derived DNA in the GCT patients with or without telomeric associations, compared with healthy controls. Further detailed molecular analyses may be required to delineate the complete role, if any, of TGFβs in this tumor system.
Acknowledgments
This research was supported in part by Vanderbilt University Research Council Grant #3-62033-531 (H.S.S.) and Biomedical Research Support Grant #RR-05424 (M.G.B.). We thank Judy Haynes, G. Andy Allen, William Wright, and Lora Miller for technical assistance.
References
- 1.Schwartz HS, Jenkins RB, Dahl RJ, Dewald GW. Cytogenetic analyses on giant cell tumors of bone. Clin Orthop Rel Res. 1989;240:250–260. [PubMed] [Google Scholar]
- 2.Schwartz HS, Butler MG, Jenkins RB, Miller DA, Moses HL. Telomeric associations and consistent growth factor overexpression detected in giant cell tumor of bone. Cancer Genet Cytogenet. 1991;56:263–276. doi: 10.1016/0165-4608(91)90179-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bridge JA, Neff JR, Bhatia PS, Sanger WG, Murphey MR. Cytogenetic findings and biologic behavior of giant cell tumors of bone. Cancer. 1990;65:2697–2703. doi: 10.1002/1097-0142(19900615)65:12<2697::aid-cncr2820651217>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 4.Bridge JA, Neff JR, Mouron BJ. Giant cell tumor of bone: Chromosomal analysis of 48 specimens and review of the literature. Cancer Genet Cytogenet. 1992;58:2–15. doi: 10.1016/0165-4608(92)90125-r. [DOI] [PubMed] [Google Scholar]
- 5.Bardi G, Pandis N, Mandahl N, Helm S, Sfikas K, Willen H, Panagiotopoulos G, Rydhold A, Mitelman F. Chromosomal abnormalities in giant cell tumors of bone. Cancer Genet Cytogenet. 1991;57:161–167. doi: 10.1016/0165-4608(91)90147-m. [DOI] [PubMed] [Google Scholar]
- 6.Mbikay M, Linard CG, Sirois F, Lazure C, Seidah NG, Chretien M. Tissue-specific expression of the prostatic secretory protein PSP94 in cyanomolgus monkey (Macaca fascicularis) Cell Mol Biol. 1988;34:387–398. [PubMed] [Google Scholar]
- 7.Derynck R, Lindquist PB, Lee A, Wen D, Tamm J, Graycar JL, Rhee L, Mason AJ, Miller DA, Coffey RJ, Moses HL, Chen EY. A new type of transforming growth factor β, TgF-β3. EMBO J. 1988;7:3737–3743. doi: 10.1002/j.1460-2075.1988.tb03257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feinberg AP, Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1984;137:266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- 9.Tantravahi U, Kirschner DA, Beauregard L, Page L, Kunkel L, Latt SA. Cytologic and molecular analysis of 46,XXq – cells to identify a DNA segment that might serve as probe for a putative human X chromosome inactivation center. Hum Genet. 1983;64:33–38. doi: 10.1007/BF00289475. [DOI] [PubMed] [Google Scholar]
- 10.Mandahl N, Helm S, Arheden K, Rydholm A, Willen H, Mitelman F. Rings, dicentrics, and telomeric association in histiocytomas. Cancer Genet Cytogenet. 1988;30:23–33. doi: 10.1016/0165-4608(88)90089-1. [DOI] [PubMed] [Google Scholar]
- 11.Kovacs G, Muller-Brechlin R, Szucs S. Telomeric association in two human renal tumors. Cancer Genet Cytogenet. 1987;28:363–366. doi: 10.1016/0165-4608(87)90225-1. [DOI] [PubMed] [Google Scholar]
- 12.Fitzgerald PH, Morris CM. Telomeric association of chromosomes in B-cell lymphoid leukemia. Hum Genet. 1984;67:385–390. doi: 10.1007/BF00291396. [DOI] [PubMed] [Google Scholar]
- 13.Morgan R, Jarzabek V, Jaffe JP, Hecht BK, Hecht F, Sandberg AA. Telomeric fusion in pre-T-cell acute lymphoblastic leukemia. Hum Genet. 1986;73:260–263. doi: 10.1007/BF00401240. [DOI] [PubMed] [Google Scholar]
- 14.Heerema NA, Palmer CG, Harvey W, Jansen J, Srour E, Paeratakul U, Fisher D, Taylor MW. Telomeric association in a hairy cell leukemia line (GASH) Am J Hum Genet. 1987;41(3):A122. [Google Scholar]
- 15.Schwartz HS, Allen GA, Butler MG. Telomeric associations. Applied Cytogenet. 1990;16:133–137. [PMC free article] [PubMed] [Google Scholar]
- 16.Bridge JA, Sreekantaiah C, Mouron B, Neff JR, Sandberg AA, Wolman SR. Clonal chromosomal abnormalities in desmoid tumors. Cancer. 1992;69:430–436. doi: 10.1002/1097-0142(19920115)69:2<430::aid-cncr2820690226>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 17.Dewald GW, Dahl RJ, Spurbeck JL, Carney JA, Gordon H. Chromosomally abnormal clones and nonrandom telomeric translocations in cardiac myxomas. Mayo Clin Proc. 1987;62:558–567. doi: 10.1016/s0025-6196(12)62293-9. [DOI] [PubMed] [Google Scholar]
- 18.Benn PA. Specific chromosome aberrations in senescent fibroblast cell lines derived from human embryos. Am J Hum Genet. 1976;28:465–473. [PMC free article] [PubMed] [Google Scholar]
- 19.Wolman SR, Steinberg ML, Defendi V. Simian virus 40-induced chromosome changes in human epidermal cultures. Cancer Genet Cytogenet. 1980;2:39–46. [Google Scholar]
- 20.McAlpine PJ, Boucheix C, Pakstis AJ, Stranc LC, Berent TG, Shows TB. The 1988 catalog of mapped genes and report of the nomenclature committee. Cytogenet Cell Genet. 1988;49:4–45. doi: 10.1159/000132645. [DOI] [PubMed] [Google Scholar]
- 21.Hunter TV. A tail of two src’s: Mutatis mutandis. Cell. 1987;49:1–4. doi: 10.1016/0092-8674(87)90745-8. [DOI] [PubMed] [Google Scholar]
- 22.Dahir GA, Schwartz HS, Butler MG. Dosage and allelic restriction fragment studies of the H-ras locus in giant cell tumor of bone. 1992 doi: 10.1016/0165-4608(94)90004-3. Submitted to Cancer Genet Cytogenet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centrella M, McCarthy TL, Canalis E. Transforming growth factor β is a bifunctional regulator of replication and collagen synthesis in osteoblast-enriched cell cultures from fetal rat bone. J Biol Chem. 1987;262:2869–2874. [PubMed] [Google Scholar]
- 24.Ellingsworth LR, Brennan JE, Fok K, Rosen DM, Bentz H, Piez KA, Seyedin SM. Antibodies to the N-terminal portion of cartilage-inducing factor A and transforming growth factor β. Immunohistochemical localization and association with differentiating cells. J Biol Chem. 1986;261:12362–12367. [PubMed] [Google Scholar]
- 25.Massague J. The TGFβ family of growth and differentiation factors. Cell. 1987;49:437–438. doi: 10.1016/0092-8674(87)90443-0. [DOI] [PubMed] [Google Scholar]
- 26.Seyedin SM, Thompson AY, Bentz H, Rosen DM, McPherson JM, Conti A, Siegel NR, Galluppi GR, Piez KA. Cartilage-inducing factor-A. Apparent identity to transforming growth factor-β. J Biol Chem. 1986;261:5693–5695. [PubMed] [Google Scholar]
- 27.Raisz LG, Kream BE. Regulation of bone formation (parts 1 and 2) N Engl J Med. 1983;309:29–35. doi: 10.1056/NEJM198307073090107. [DOI] [PubMed] [Google Scholar]
- 28.Rodan GA, Martin TJ. Role of osteoblasts in hormonal control of bone resorption—a hypothesis. Calcif Tissue Int. 1981;33:349–351. doi: 10.1007/BF02409454. [DOI] [PubMed] [Google Scholar]
- 29.Tashjian AH, Voelkel EF, Lazzaro M, Singer FR, Roberts AB, Derynck R, Winkler ME, Levine L. α and β human transforming growth factors stimulate prostaglandin production and bone resorption in cultured mouse calvaria. Proc Natl Acad Sci USA. 1985;82:4535–4538. doi: 10.1073/pnas.82.13.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong GL. Paracrine interactions in bone-secreted products of osteoblasts permit osteoclasts to respond to parathyroid hormone. J Biol Chem. 1984;259:4019–4022. [PubMed] [Google Scholar]


