Significance
A decline in organic brain health paralleled by degraded perceptual and cognitive abilities is a well-established aspect of human and animal aging. Here, in a rodent model, an elementary form of attention-demanding auditory training completed in young adulthood resulted in positive changes in modulation-rate discrimination abilities that carried forward into older age. These sustained behavioral impacts for processing speed were paralleled by enduring changes in physical and functional aspects of perceptual processing, documented at the level of the primary auditory cortex. Additionally, an important generalization of training impacts was recorded in the hippocampus, a brain structure for which physical and functional declines have been long associated with the progression to infirmity and dementia in aging.
Keywords: aging, auditory cortex, behavioral training, cortical processing, inhibition
Abstract
Progressive negative behavioral changes in normal aging are paralleled by a complex series of physical and functional declines expressed in the cerebral cortex. In studies conducted in the auditory domain, these degrading physical and functional cortical changes have been shown to be broadly reversed by intensive progressive training that improves the spectral and temporal resolution of acoustic inputs and suppresses behavioral distractors. Here we found older rats that were intensively trained on an attentionally demanding modulation-rate recognition task in young adulthood substantially retained training-driven improvements in temporal rate discrimination abilities over a subsequent 18-mo epoch—that is, forward into their older age. In parallel, this young-adult auditory training enduringly enhanced temporal and spectral information processing in their primary auditory cortices (A1). Substantially greater numbers of parvalbumin- and somatostatin-labeled inhibitory neurons (closer to the numbers recorded in young vigorous adults) were recorded in the A1 and hippocampus in old trained versus untrained age-matched rats. These results show that a simple form of training in young adulthood in this rat model enduringly delays the otherwise expected deterioration of the physical status and functional operations of the auditory nervous system, with evident training impacts generalized to the hippocampus.
A degradation of the status of physical brain machinery, expressed by a decline in its temporal processing abilities, has been repeatedly associated with its deteriorating functional status in normal aging (1–4). Recent studies have shown that the machinery that supports processing accuracy and speed, as well as the processes supporting attention control and distractor suppression, can be substantially rejuvenated via simple forms of intensive training in the aged-rat model (4, 5). With auditory perceptual training, in parallel with recovery in behavioral abilities to that matching young-animal performance levels, serials of key physical, chemical, and functional aspects of cortical processing machinery in the trained rats were shown to be restored to a physical or functional status that approached that normally recorded in vigorous young-adult animals.
In human studies, substantial changes in speed of processing (SOP) and in other spectro-temporal (or spatiotemporal) signal resolution of performance abilities have been shown to result from attention-demanding, speed-challenged auditory (3, 5–7) or visual training (8–11). For example, the accurate behavioral identification and stimulus-order reconstruction of rapidly successive auditory stimuli was restored in human individuals trained in their eighth decade of life to a performance level normally typifying human performance abilities recorded in their third or fourth decade (3, 6).
Our goal here was to define the magnitude and endurance of an intense dose of attention-demanding modulation discrimination training on the forward progression of physical and functional aging. Specifically, we asked if a simple form of modulation-rate recognition training, in which stimuli were presented in a continuous performance format with target stimuli interleaved with nontarget distractors, can confer resilience against expected age-related deterioration, measured behaviorally, and/or in the physical and functional cerebral cortex. This task form was chosen because it embodied aspects of the two forms of training applied earlier in studies in older aged rats (i.e., focusing on improving receptive accuracy at speed and exercising selective memory and distractor suppression processes), which has been shown to be necessary to broadly physically and functionally rejuvenate cortical systems (4, 5).
We begin reporting on this series of studies here, by documenting (i) sustained modulation-rate signal recognition abilities attributable to training, 18 mo after training was completed in young, sexually mature animals; (ii) the endurance of functional changes in the cortex that plausibly support requisite, rapid-successive signal discrimination and recognition; and (iii) the status of specific cell populations in the cortex known to contribute to accurate and speeded operations in cortical networks (12–15). We also explored training impacts on a higher level (i.e., hippocampal) process, as an initial index of possible generalized brain health impacts.
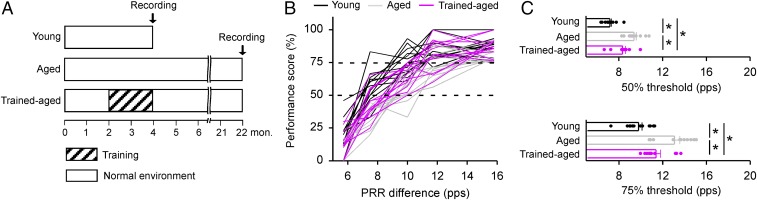
In our studies, ∼2-mo-old (young adult) rats were trained over a period of ∼2 mo on a modified go/no-go behavioral task (SI Materials and Methods). Temporal (modulatory) rate recognition abilities for pulsed stimuli were determined, and cortical responses of neurons representing spectral and temporal dimensions of acoustic signals were documented in the primary auditory cortex (A1) in trained animals ∼18 mo after training completion (i.e., at ∼22 mo of age). These data from this young adult-trained, now older-aged cohort were compared with two control groups: (i) age-matched but untrained rats, and (ii) untrained young-adult (∼4 mo old) rats (Fig. 1A for experimental timelines of different experimental groups). By comparing behavioral and physical measures with these two control populations, our goal was to determine impacts (if any) on the endurance of high-level modulation response recognition that normally distinguished young from older adult rats and on cortical neuronal processing plausibly accounting for any better sustained temporal processing performance abilities. In addition, changes in cortical and hippocampal expression of markers for parvalbumin (PV) and somatostatin (SOM) inhibitory neurons were measured in all three cohorts, to elucidate the possible cellular and molecular mechanisms for sustained behavioral and cortical plasticity induced by early training.
Fig. 1.
Performance on the temporal rate discrimination task. (A) Experimental timelines for young, aged-untrained, and trained-aged rats. Note that aged-untrained and trained-aged rats were reared under identical housing conditions before behavioral testing, to assure that any sustained impacts could be attributed to early training. (B) Individual psychometric curves obtained from young (n = 13), aged (n = 12), and trained-aged (n = 11) rats. Dashed lines show 50% and 75% of the maximal score, respectively. pps, pulses per second; PRR, pulse repetition rate. (C) Individual discrimination thresholds determined at a 50% (Top) or 75% (Bottom) performance score on the psychometric curve, for different groups of rats. Bars display average thresholds (±SEM). *P < 0.01–0.0001.
SI Materials and Methods
Female SD rats were used in the study. All procedures were approved by the Institutional Animal Care and Use Committee and complied with NIH standards.
Behavioral Training.
Rats were trained to identify and respond to a target auditory stimulus that was randomly set by the training system at the beginning of each training day, presented with a set of distractor stimuli to receive a food reward. Auditory stimuli involved pulse trains of the same duration (520 ms) containing different numbers (5, 6, 7, 9, or 11) of noise bursts, corresponding to repetition rates of 8.3, 10, 12.5, 16.7, or 20 pps, respectively. Only one pulse train was presented in each trial. Rats were rewarded for making a “go” response within a limited time window after the target pulse train with a specific repetition rate was presented.
Training was conducted in an acoustically transparent training chamber (20 × 20 × 18 cm, length × width × height) enclosed within a sound-attenuated chamber. The rats’ behavioral responses were classified as go or “no-go.” The interruption of the photobeam on the nose-poke device signaled a go. All other conditions were considered no-go. Rats were rewarded for making a go response (i.e., a hit) within 3 s after target presentation; failure to respond within this time window was scored as a “miss.” A go response within 3 s of a nontarget stimulus was scored as a “false positive”; the absence of a response was scored as a “withhold.” The fifth state, “false alarm,” was defined as a go response that occurred 3 s or more after stimulus presentation. A hit triggered the delivery of a 45-mg food pellet (BioServe). A miss, false positive, or false alarm initiated a 9-s “time-out” period, during which time no stimuli were presented and the house lights were turned off. A withhold did not produce a reward or a time out. An input and output system (photobeam detector, food dispenser, sound card, and house light; Med Associates) was used to control behavioral training.
Behavioral performance was shaped in three phases. Rats were initially pretrained to make a nose-poke response to obtain a food reward. They were then trained to make a nose-poke after the presentation of a pulsed-noise train of 12.5 pps. During the training stage, rats were conditioned to make a discriminative response to the target stimulus when a set of pulse trains of various repetition rates were randomly delivered; one of these pulse trains with a specific repetition rate was randomly chosen as the target, at the beginning of each training day. Note that a trained rat identified that target on the basis of rewards and time outs in the first or second training block of each training day.
Trials were grouped into blocks of 50. At the conclusion of each block, a hit ratio (H; hits per number of target trials, expressed as a percentage) and a false-positive ratio (F; false-positives per number of nontarget trials) were calculated. Discrimination ability was quantified by a performance score, calculated as H – F × H (18).
An animal was considered well-trained if it achieved a performance score of ≥80% for at least two of the last four daily training blocks. Upon reaching this performance level, rats were continuously trained for an additional ∼2 wk using trains of tone pips, again tasked with recognizing repetition rates of these tone-pip sequences. The carrying frequency (either 4, 9, 13, 19, or 24 kHz) of stimulus trains (including target and nontarget) was randomly chosen at the beginning of each training day and hold identical for the whole training sessions of that day. Pseudorandomization assured that every rat was trained to distinguish different modulated-rate stimuli at each of these carrier frequencies on at least 2 training days.
Passive Exposure.
For passive exposure, rats were put in the training apparatus and passively exposed to training sounds that were identical to those delivered to trained rats each day but were given free access to food across the same epoch (i.e., they were not engaged in the above-described tasks).
Sound Discrimination Assessment.
Rats were first trained to respond to a 520-ms pulse train of four noise bursts (i.e., nontarget; repetition rate, 6.3 pps), distinguishing it from a nontarget 11-burst train of the same duration (target; repetition rate, 20 pps). When animals reached steady performance scores after ∼1 wk of training, their temporal rate discrimination abilities were tested by randomly delivering 520-ms nontarget pulse trains with pulse rates of 6.3, 8.3, 10, 12.5, or 14.3 pps. The target was always 20 pps during testing. The input and output system used to control the behavioral performance was as described in Behavioral Training.
Trials were again grouped into blocks of 50. The performance score was calculated at the conclusion of each block (18, 22).
ABR Measurement and Cortical Mapping.
The recording was conducted in a shielded, double-walled sound chamber. Under pentobarbital anesthesia (50 mg per kg body weight), ABR was measured by placing three electrodes subdermally at the scalp midline, posterior to the stimulated ear, and on the midline of the back 1–2 cm posterior to the neck of the animal. Tone pips (4, 10, or 24 kHz) at different intensities were generated using TDT System III (Tucker-Davis Technologies) and delivered to the left ear through a calibrated earphone with a sound tube positioned inside the external auditory meatus. ABR thresholds were defined as the lowest sound intensity capable of eliciting a response pattern characteristic of that observed at higher intensities.
For cortical recording, the skull was secured in a head holder, leaving the ears unobstructed. After reflecting the right temporalis muscle, the auditory cortex was exposed and the dura resected. The cortex was maintained under a thin layer of viscous silicone oil to prevent desiccation. A state of areflexia was maintained with supplemental doses of 8 mg/mL dilute pentobarbital injected intraperitoneally (i.p.) throughout the surgical procedures and during recording.
Cortical responses were recorded with parylene-coated tungsten microelectrodes (1–2 megohms at 1 kHz; FHC). At each recording site, the microelectrode was lowered orthogonally into the cortex to a depth of ∼550 µm (layers 4–5), where evoked spikes of a neuron or a small cluster of neurons were recorded. Acoustic stimuli were generated and delivered to the contralateral ear relative to the recording site through a calibrated earphone with a sound tube. Acoustic calibration was performed with a 1/2-inch Brüel & Kjær microphone sealed to the sound delivery tube so that the speaker output could be corrected to ensure a flat frequency response over a range of frequencies spanning 1–30 kHz. A software package (SigCal, SigGen, and Brainware; Tucker-Davis Technology) was used to calibrate the earphone, generate acoustic stimuli, monitor cortical response properties on-line, and store data for off-line analysis.
Frequency tuning curves of A1 neurons were reconstructed by presenting pure tones (25 ms duration) of 50 frequencies (1–30 kHz) at eight sound intensities (0–70 dB SPL in 10 dB increments) in a random, interleaved sequence at a rate of 2 pps. The overall boundaries of the A1 were functionally determined using nonresponsive sites and responsive sites that did not have a well-defined pure tone-evoked response area (i.e., non-A1 sites). As previously described (18, 45), A1 has a unique rostral-to-caudal tonotopy and reliable neuronal responses to tone pips of selective frequencies.
To document cortical RRTFs, trains of six tonal pulses (25 ms duration with 5 ms ramps at 60 dB SPL) were delivered four times at each of eight repetition rates (2, 4, 7, 10, 12.5, 15, 17.5, and 20 pps) in a randomly interleaved sequence. The tone frequency was set at the CF of each site. To reduce the variability resulting from different numbers of neurons included in the different multiunit responses recorded, the normalized cortical response for each repetition rate was calculated as the average response to the last five pulses divided by the response to the first pulse. The RRTF, therefore, represents the normalized cortical response as a function of the temporal rate.
Vector strength was calculated using the following equation:
where n is the total number of spikes, ti (i = 1, 2 … n) is the time between the onset of the first pulses and the ith spike, and T is the interstimulus interval. Spikes that occurred during a 6T period after the onset of the first tonal pulse were included to compute vector strength.
MRs were calculated using the van Rossum spike train distance metric (18, 20). Each spike train was convolved with an exponential function, N(t) = N0e−t/t, to obtain a filtered function. The distance between two spike trains was defined as the integral of the squared difference of the two functions. Distances were computed for all spike trains at a t of 10 ms for every combination of spike trains in response to stimulus trains of different repetition rates. Confusion matrices were then constructed by calculating the average distance and standard deviation between spike trains, with a misclassification recorded when the distance was less (for spike trains obtained with dissimilar stimuli) or more than (for spike trains obtained with identical stimuli) 1 standard deviation away from the average distance.
Immunohistochemistry.
As described in our earlier studies (18, 19, 22), rats received a lethal dose of pentobarbital (85 mg/kg body weight) and were perfused intracardially with saline solution followed by 4% paraformaldehyde in 0.1 M potassium PBS (pH 7.2). Brains were removed and placed in the same fixative containing 20% sucrose for 12–24 h. Fixed material was sliced in the coronal plane on a freezing microtome at a thickness of 40 μm. Free-floating sections were preincubated in blocking solution to suppress nonspecific binding. The sections were then incubated at 4 °C for 48–72 h with anti-PV (Sigma) or anti-SOM (Sigma). After exposure to biotinylated IgG (Vector) at room temperature for 1 h, samples were treated further with streptavidin-conjugated Cy3 (Jackson ImmunoResearch) again at room temperature for 1 h.
Samples from different groups of rats were always processed together during the immunostaining procedures to limit variations related to antibody penetration, incubation time, and the postsectioning condition of the tissue. Fluorescence in the immunostained material was assessed and images acquired using a Leica DM4000B (Leica Microsystems) epifluorescent microscope equipped with a Leica DFC 450C digital camera (Leica Microsystems). From these photographs, the number of immunostained neurons was evaluated for an individual 400 μm-wide A1 zone that was centered in the cortical layers 2/3, 4, or 5/6. A neuron was counted only if the staining revealed a complete soma perimeter and the neuron was clearly distinguished from the background. The data for each rat group were then averaged and compared.
Results
Performance in an Acoustic Temporal Rate Discrimination Task.
As described in SI Materials and Methods, young rats at an age of 2 mo were progressively trained in a modified go/no-go behavioral procedure over an intensive 2-mo training period. Sound temporal rate discrimination assessment was conducted in these trained animals ∼18 mo after training completion (i.e., at ∼22 mo of age). These data from this young adult-trained, now older-aged cohort were then compared with two control groups—that is, age-matched but untrained rats and untrained young rats. Our first goal was to define the magnitudes of possible, enduring impacts of this early training on acoustic temporal rate identification performance in trained-aged versus aged (i.e., age-matched untrained) or young control rats (Fig. 1A for experimental timelines of different groups of rats).
As shown in Fig. 1B, a psychometric curve summarizing data from each animal in these three cohorts was obtained by plotting their performance scores as a function of pulse repetition rate (PRR) differences between target and nontarget stimuli. The discrimination threshold, defined as the PRR difference corresponding to a 50% performance score on the psychometric curve, provided a simple index of the temporal rate recognition abilities of each animal. Average thresholds obtained in trained-aged rats were modestly higher than in young controls but significantly lower than in aged rats (Fig. 1C, Top; one-way ANOVA with post hoc student Newman–Keuls test, both P < 0.005). This demonstration of sustained temporal processing advantages in trained-aged versus aged rats was further confirmed by summary statistics for the discrimination threshold determined at a 75% performance score on the psychometric curve (Fig. 1C, Bottom; one-way ANOVA with post hoc student Newman–Keuls test, P < 0.01–0.001). These results show that early training results in a stronger modulation-rate discrimination performance, sustained on a surprisingly high level for ∼18 mo after training.
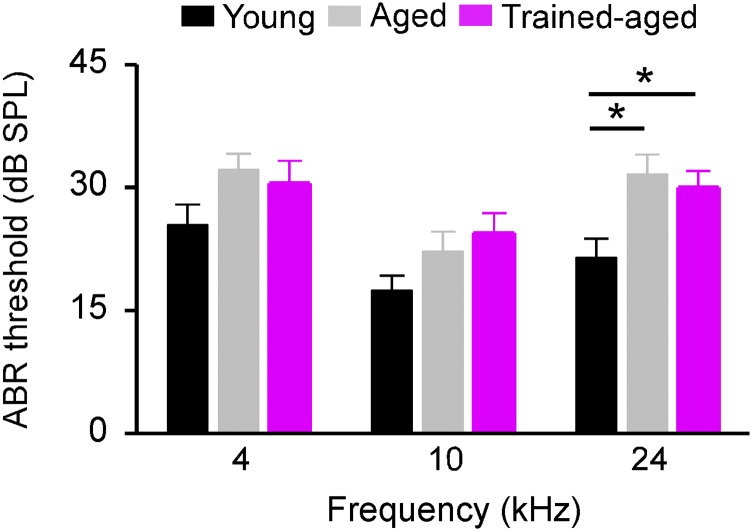
To assure that these enhanced behavioral abilities for trained-aged rats could not be simply due to training-induced changes in peripheral hearing thresholds, we compared auditory brainstem response (ABR) thresholds recorded from different rat cohorts (Fig. S1). Consistent with earlier studies (16, 17), ABR thresholds recorded in aged rats were all higher than those in young controls, particularly at higher frequencies (one-way ANOVA with post hoc student Newman–Keuls test, P < 0.01 at high frequency but >0.05 at both low and middle frequencies). Importantly, ABR thresholds for trained-aged rats did not differ from those of aged rats (one-way ANOVA with post hoc student Newman–Keuls test, all P > 0.05).
Fig. S1.
ABR thresholds at three frequency ranges for young (n = 10), aged (n = 9), and trained-aged (n = 9) rats. Note that the ABR thresholds of trained-aged rats did not differ from those of aged rats at all three frequencies tested (one-way ANOVA with post hoc student Newman–Keuls test, all P > 0.05). Values shown are mean ± SEM. *P < 0.05.
Temporal and Spectral Responses of Cortical Neurons.
Changes in cortical response selectivity and reliability in the dimensions of time and frequency have been argued to underlie the neurological encoding of the details of acoustic inputs and to account for the limits of auditory perceptual abilities (18, 19). We therefore primarily focused on documenting temporal and spectral processing by neurons in the cortical field A1 in our investigation of the neural basis for these sustained, training-induced behavioral changes, while recognizing that any cortical changes that we might record were likely also contributed to by subcortical differences. Cortical temporal responses to modulated stimuli documented in A1 for trained-aged rats (recording sites, 262 from 6 rats) were again compared with those recorded from age-matched untrained (recording sites, 338 from 8 rats) and young control rats (recording sites, 330 from 8 rats; see Fig. 1A for experimental timelines of different groups of rats). Unless otherwise specified, all subsequent quantitative analyses are based on these samples.
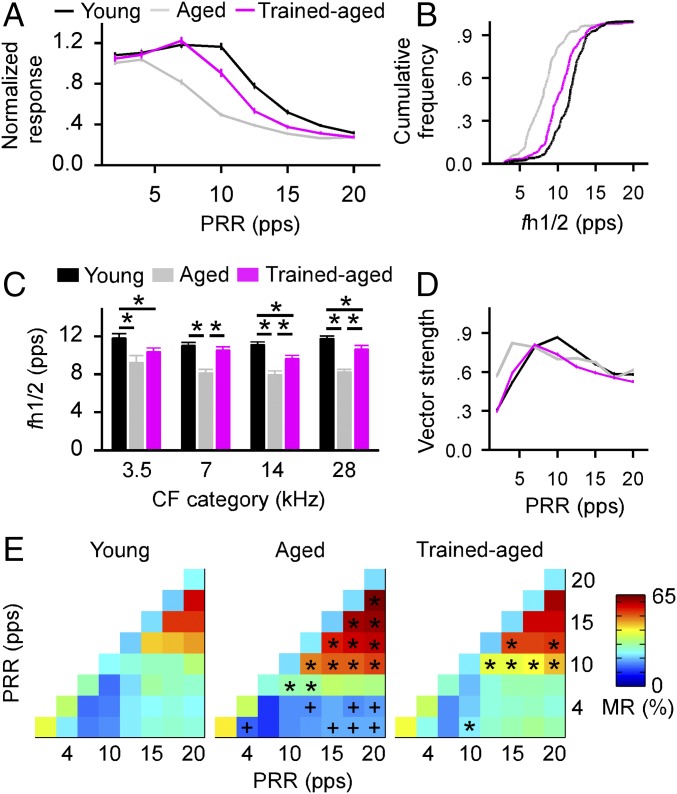
Repetition rate transfer functions (RRTFs) were derived to characterize cortical temporal responses for different rat groups (SI Materials and Methods). As shown in Fig. 2A, normalized responses in aged rats were in general weaker than those recorded in young control rats at all repetition rates tested (one-way ANOVA with student Newman–Keuls post hoc test, all P < 0.001 for repetition rates of 7–17.5 pulses per second, pps). Responses in trained-aged rats were stronger than in aged rats at repetition rates of 7–15 pps (one-way ANOVA with student Newman–Keuls post hoc test, P < 0.05–0.001) but were weaker than in young rats between 10 and 17.5 pps (one-way ANOVA with student Newman–Keuls post hoc test, all P < 0.001).
Fig. 2.
Cortical temporal responses. (A) Normalized responses to stimuli presented at different PRRs recorded from young, aged, and trained-aged rats. Values shown are mean ± SEM. (B) Cumulative frequency histograms showing fh1/2 distributions for different groups of rats. Leftward shifts of fh1/2 distributions for aged and trained-aged rats compared with young rats indicate reduced cortical rate-following abilities. (C) Average fh1/2s for all recording sites in different groups of rats. *P < 0.05–0.001. (D) Average vector strengths for different groups of rats. (E) Average MRs for different groups of rats. * or +, MRs that were significantly larger or smaller than in young control rats.
Comparisons of repetition rates at which the normalized responses were at half maximum (i.e., fh1/2) for each RRTF quantified the cortical capacity for processing repetitive stimuli in a second way. A comparison of distributions for fh1/2s, shown in Fig. 2B, documented a significant leftward shift (i.e., a reduced cortical capacity for processing repetitive stimuli) for aged versus young control rats (Kolmogorov–Smirnov test, P < 0.0001). Early training increased fh1/2s, with trained-aged rats clearly superior to age-matched untrained controls in cortical stimulus rate-following abilities. Significant but more modest differences in fh1/2 distributions distinguished young rats from trained-aged rats (Kolmogorov–Smirnov test, P < 0.001). These cohort differences for fh1/2s held for neurons across all characteristic frequency (CF) ranges (Fig. 2C; one-way ANOVA with student Newman–Keuls post hoc test, P < 0.05–0.001).
We next calculated vector strengths to evaluate the phase locking of cortical responses to repetitive stimuli as another index of temporal processing fidelity. As shown in Fig. 2D, average vector strengths as a function of stimulus temporal rates shifted leftward and peaked at lower rates in aged versus young rats (peak at 4 pps in aged rats vs. 10 pps in young rats). The vector strengths of trained-aged rats again matched the values of young rats at lower repetition rates (i.e., 2–7 pps; one-way ANOVA with student Newman–Keuls post hoc test, all P > 0.05) but were smaller relative to young rats at most higher repetition rates (one-way ANOVA with student Newman–Keuls post hoc test, all P < 0.001 except for 17.5 pps, where P > 0.05). These results indicate stronger effects of early training on phase-locking properties of cortical responses to lower rate than to higher rate stimuli.
To further determine the reliability and precision of cortical responses to repetitive stimuli, we also calculated the misclassification rate (MR) for every possible combination of stimulus pulse trains by using the Van Rossum spike train distance metric (20) (see also SI Materials and Methods). MR quantifies the similarity between spike trains recorded using different or identical stimulus trains, while taking into account spike number and timing. Smaller MR values indicate more reliable and precise spiking, representing the temporal structures of stimulus trains. As shown in Fig. 2E, MRs of aged rats for combinations of dissimilar middle and high pulse rates (i.e., 10–20 pps) were significantly larger compared with young control rats (denoted with asterisks in Fig. 2E, Middle; all P < 0.01). Early training had reduced the MRs such that values for some combinations of pulse rates for trained-aged rats were now comparable with young control rats (Fig. 2E, Right; all P > 0.05 except for those denoted with asterisks, where P < 0.01).
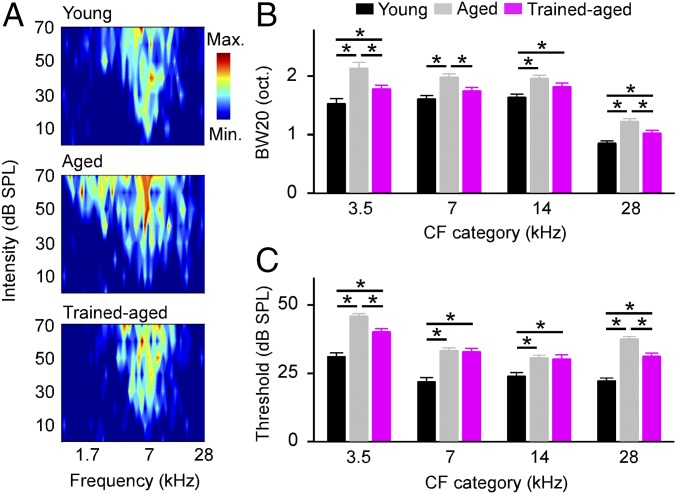
Finally, we assessed cortical frequency selectivity by constructing frequency-versus-intensity response areas (i.e., tuning curves; Fig. 3A) and then measuring tuning bandwidths 20 dB above the threshold (i.e., BW20s). The average BW20s of cortical neurons were significantly wider in aged compared with young control rats (Fig. 3B; one-way ANOVA with student Newman–Keuls post hoc test, all P < 0.001). Modest but significant decreases in BW20s were observed for trained-aged versus aged rats (one-way ANOVA with student Newman–Keuls post hoc test, all P < 0.005, except for the CF category of 14 kHz, where P = 0.077). Bandwidths from trained-aged rats differed from those recorded in young controls (one-way ANOVA with student Newman–Keuls post hoc test, all P < 0.05, except for the CF category of 7 kHz, where P = 0.63) but to a lesser extent than for comparisons with aged rats. Consistent with earlier studies (4), cortical response thresholds were higher for aged versus young control rats (Fig. 3C; one-way ANOVA with student Newman–Keuls post hoc test, all P < 0.001). At the same time, there were significantly lower response thresholds recorded in trained-aged rats for neurons with low and high CFs than in aged rats (one-way ANOVA with student Newman–Keuls post hoc test, both P < 0.001).
Fig. 3.
Cortical frequency selectivity. (A) Representative examples of tonal receptive fields recorded from young, aged, and trained-aged rats. (B) Average receptive field bandwidths at 20 dB above threshold (BW20s) for all recordings in different groups of rats. Values shown are mean ± SEM. *P < 0.05–0.001. (C) Average response thresholds for all recordings in different groups of rats. *P < 0.001.
All of the above analyses indicate that early training slowed a normal age-related regression in cortical processing, with sustained advantages in response feature representation likely accounting for greater, behaviorally documented modulation-rate recognition abilities.
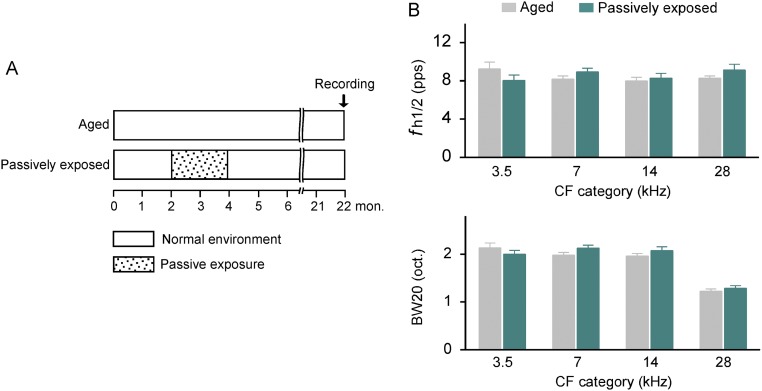
As a final check to assure that sustained impacts on cortical processing were attributed to training but not to mere sound exposure, we examined the neurological status of a subsequent group of aged rats that were passively exposed to training stimuli as young adults (these rats thereafter were referred to as passively exposed rats). Data then were compared with those recorded in age-matched untrained controls (Fig. S2A). As shown in Fig. S2B, no differences in fh1/2s and BW20s of cortical neurons were recorded between passively exposed rats and aged controls (unpaired t test, all P > 0.09). This finding is consistent with earlier claims that ambient auditory inputs are probably not sufficient for generating persisting cortical plasticity in the mature brain (21, 22).
Fig. S2.
Effects of early passive sound exposure on cortical responses. (A) Experimental timelines for aged and passively exposed rats. Note that passively exposed rats were presented with acoustic stimuli that were every way identical in frequency, loudness, modulation, and presentation schedule to those presented to trained rats each day but were given free access to food across the same time epoch. (B) Average fh1/2s (Top) and BW20s (Bottom) for all recordings of passively exposed rats (recording sites, 119 from 3 rats) compared with aged rats (recording sites, 338 from 8 rats). Note that the values recorded in passively exposed rats were comparable with those in aged rats (unpaired t test, all P > 0.09). Values shown are mean ± SEM.
Cortical Expression of PV and SOM.
Cortical inhibitory interneurons, particularly those expressing PV or SOM, undergo significant changes in populations, morphologies, and functional powers in aging and have therefore been associated with age-related functional declines (4, 23, 24). Importantly, they have also been argued to contribute to acetylcholine-modulated plasticity processes that support local and brain system coordination and the accurate representation of spatial (in the auditory cortex, spectral) and temporal details of perceptual stimuli (12–15, 25).
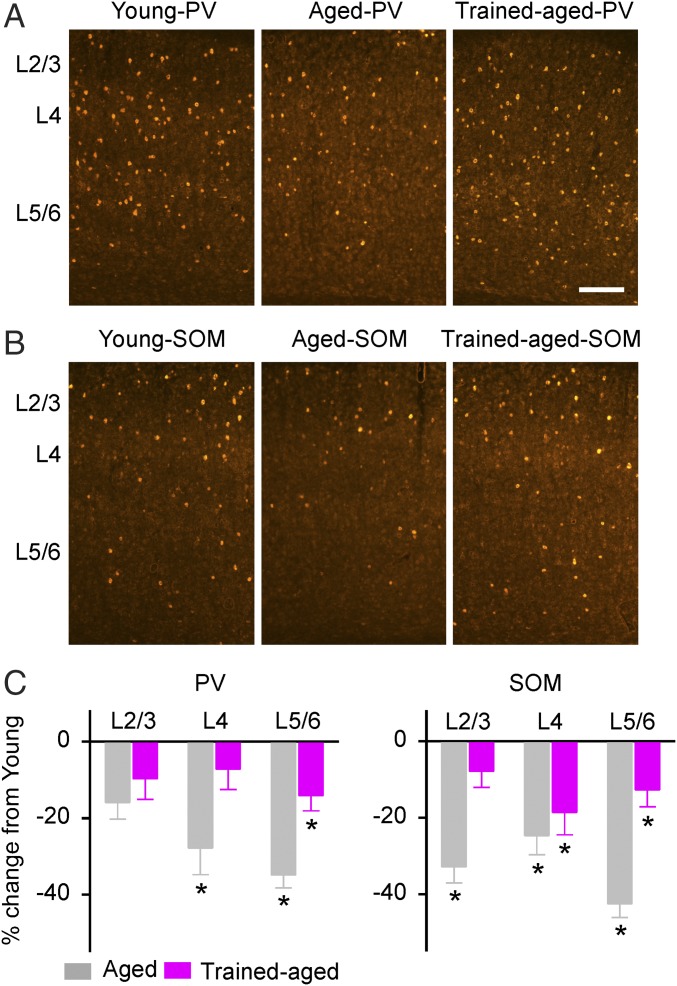
As an initial step for documenting the cellular and molecular changes at the cortical level impacted by young-adult epoch training, numbers of PV- and SOM-expressing neurons in the cortical field A1 were determined in all rats. As shown in Fig. 4, the numbers of both PV-positive (PV+) and SOM-positive (SOM+) neurons in aged rats were lower than in young rats across different cortical layers (one-way ANOVA with post hoc student Newman–Keuls test, P < 0.005–0.001, except for PV at layers 2/3, where P > 0.05). Early training delayed that age-related reduction in expression of PV and SOM such that the numbers of PV+ neurons in layers 2/3 and 4 and the number of SOM+ neurons in layers 2/3 for trained-aged rats did not differ from those seen in young rats (one-way ANOVA with post hoc student Newman–Keuls test, all P > 0.05). There were still fewer PV+ neurons in layers 5/6 and fewer SOM+ neurons in layers 4 and 5/6 in trained-aged rats than in young rats (one-way ANOVA with post hoc student Newman–Keuls test, all P < 0.05).
Fig. 4.
PV- and SOM-expressing neurons in the auditory cortex. (A) Representative photomicrographs of PV-immunostained (PV+) cortical sections for young, aged, and trained-aged rats. (Scale bar, 200 µm.) (B) Photomicrographs of SOM-immunostained (SOM+) cortical sections. (C) Quantitative analysis of PV (Left) and SOM (Right) expression within the A1 for young (10 hemispheres), aged (8 hemispheres), and trained-aged (8 hemispheres) rats. The values (mean ± SEM) shown are normalized to those of young rats. *P < 0.05–0.001.
PV and SOM Expression in the Hippocampus.
The behavioral task applied in the current study required animals to identify a remembered target stimulus presented against a background of nontarget (distracting) stimuli. Inhibitory interneurons expressing PV and SOM in the hippocampus are among the most affected cell populations impacted by aging (26, 27) and have long been argued to play critical roles in learning and memory (28–31). Here we asked if our memory-based go/no-go training task applied in young adulthood detectably impacted the status of these key neuronal populations in the dentate gyrus (DG) and the CA1 subfield of the hippocampus.
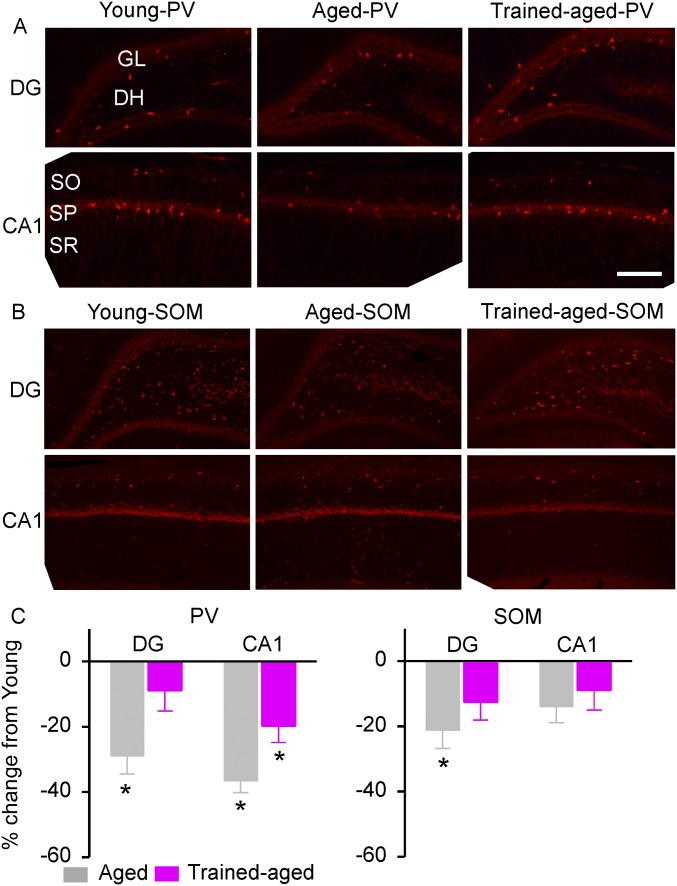
Consistent with earlier studies (32, 33), PV+ neurons were predominantly located in the hilus and the granule cell layer of the DG (Fig. S3A, Top) and in the strata oriens and pyramidale of the CA1 (Fig. S3A, Bottom). The numbers of PV+ neurons recorded in aged rats were significantly lower than in young rats, in both hippocampal areas (Fig. S3C, Left; one-way ANOVA with post hoc student Newman–Keuls test, both P < 0.002). In contradistinction, the numbers of PV+ neurons in trained-aged rats were higher in both hippocampal zones than in aged rats (one-way ANOVA with post hoc student Newman–Keuls test, both P < 0.03), although, again, labeled interneuron numbers still differed from those recorded in young rats (one-way ANOVA with post hoc student Newman–Keuls test, P < 0.001). These data indicate that early training delays an age-related decline in PV expression of inhibitory interneurons in these hippocampal sectors.
Fig. S3.
PV and SOM expression in the hippocampus. (A) Photomicrographs of PV+ neurons in young, aged, and trained-aged rats in the DG and the CA1 subfield. DH, dentate hilus; GL, granule cell layer; SO, stratum oriens; SP, stratum pyramidal; SR, stratum radiatum. (Scale bar, 200 µm.) (B) Photomicrographs of SOM+ neurons in the DG and the CA1 subfield. (C) Quantitative analysis of PV (Left) and SOM (Right) expression in young (10 hemispheres), aged (8 hemispheres), and trained-aged (8 hemispheres) rats. The values (mean ± SEM) shown are normalized to those of young rats. *P < 0.05–0.001.
SOM+ neurons were predominantly located in the hilus of the DG (Fig. S3B, Top) and the strata oriens of the CA1 (Fig. S3B, Bottom). Early training again offset the age-associated reduction in SOM expression in the DG; the numbers of SOM+ neurons recorded from trained-aged rats were comparable with those from young rats (Fig. S3C, Right; one-way ANOVA with post hoc student Newman–Keuls test, P > 0.05). Interestingly, no significant differences between these three rat cohorts were observed for SOM expression in the CA1 (one-way ANOVA, P = 0.26).
Discussion
A decline in behaviorally measured SOP has long been described as a signal change indexing the progression of functional aging in humans (1, 2). Beyond a peak performance epoch in the second to third decades of life, the average modern human declines, in speeded successive-signal recognition abilities, by about 1/3 of a standard deviation/decade. By age 75, this elementary “sampling rate” index of SOP is, in different modalities, 1/3–1/2 as fast as in young adults. A deterioration of both sustained attention and distractor suppression abilities also grows in aging, with those progressive losses marking what come to be large-scale negative changes in connectivity and attention control in functional brain systems in older humans (34, 35).
The lifespan for the rat model engaged in our studies is about 36 mo. Here, we applied a modulated-stimulus recognition assessment to behaviorally index the status of this elementary aspect of SOP. Our task also required sustained attention and “top-down” working memory processes to achieve target recognition in the presence of confusable distractors. We showed that the accurate reception of rapidly successively pulsed stimuli was degraded in older adults. Fastest accurately recognized modulation rates were, again, about half the rates recorded in vigorous young adults (4, 5). In parallel, cortical temporal and spectral representational processes had all deteriorated in ways that appeared to at least substantially account for these age-related behavioral deficits.
By contrast, 22-mo-old rats trained on an attention-demanding modulation-rate recognition task as young adults had better sustained abilities for distinguishing between and recognizing these rapidly modulated stimuli. Their retained behavioral performance was much closer to that recorded in young, untrained animals than was seen in never-trained older adults. These sustained behavioral abilities recorded ∼18 mo after early-adult training were paralleled by equivalently better sustained cortical signal processing in the temporal and spectral domains and by a large, positive difference in the numbers of PV- and SOM-labeled inhibitory interneurons, all here documented in the cortical area A1.
Earlier studies in different animal species have shown that functional development of the auditory system is substantially influenced by acoustic environments and/or auditory training in early life. For example, exposing rat pups to noise during the critical period can induce a disrupted tonotopicity and degraded response tuning in the inferior colliculus and in cortical field A1, which in turn results in deteriorated behavioral function in adulthood (36). On the other hand, a period of training in rodents on a specific auditory task (i.e., sound amplitude modulation detection) during development produces a positive effect on adult perception in an identical task (37, 38). Studies in humans have also demonstrated a link between music training early in life and better auditory processing skills later in life, especially in challenging listening environments (39–41). Although early training in younger life has been shown to confer enduring neurobehavioral advantages and to contribute to positive brain health benefits sustained into older ages, scientists still incompletely understand the basis of this “cognitive reserve” (42, 43). However, higher SOP, the accurate reception of target stimuli in the presence of confusable distractors, and the retained connectional integrity of forebrain systems, all of which were intensely engaged by our simple training task, provide key indices of the measured level of protection that is commonly attributed to this “reserve” (2, 43, 44). Our results, together with those of others (37–41), therefore indicate that cognitive reserve may be amplified at any age, that the functional and physical impacts of this amplification can be long enduring, and that with appropriate training dosing, brain health and function is likely sustainable, at a high level, across the lifespan.
We documented in this study the physical and functional impacts of early training at the level of cortical field A1, because it has been compellingly argued that spectro-temporal changes in response selectivity and reliability at this system level most directly account for auditory training-driven changes in perceptual abilities (18, 19, 45–47). It should be noted that both animal and human studies have shown that subcortical plasticity, here undocumented, contributes to cortically expressed changes in temporal and spectral processing driven by this and related forms of intensive auditory training (7, 48–50). Furthermore, cortical plasticity induced by auditory training has been demonstrated in multiparametric sound domains (4, 21, 22, 45). It has been proposed that training-based cortical plasticity is restricted only to stimulus parameters that are relevant to behavioral demands (21, 22, 45). In this study, however, we found that attention-demanding modulation-rate training altered cortical processing in both temporal and spectral domains. These results indicate generalized effects of training on cortical remodeling, which amplified the benefits of training derived from this simple perceptual learning task.
Declines in the function of inhibitory circuits in the auditory cortex, which is mostly mediated by GABAergic interneurons through feed-forward or feedback connections, have been documented in detail in aging animal models (4, 23, 24, 51–53). These major types of GABAergic interneurons expressing PV and SOM are argued to contribute to cortical signal representation, local and system coordination, and experience-dependent plasticity (14, 18, 22, 54–57). For example, recent studies have shown that PV+ neurons in the auditory cortex have markedly faster response latencies than PV– neurons, indicating their critical roles in regulating the temporal precision of cortical responses (55). Cortical SOM+ neurons, on the other hand, provide delayed and more selective inhibition to modulate the processing of complex stimuli, as in human speech (58). In addition to behavioral and neurological changes, here we also observed alterations in cellular aspects of the cortical machinery (i.e., decreases in both PV+ and SOM+ cortical neurons with aging). By organizing network coordination in fast time, early training up-regulated PV+ and SOM+ neuron processes that arguably support more reliable signal representation, increase feed-forward power, enable stronger feed-forward coincident input-dependent plasticity, and increase system coordination. We therefore propose that PV+ and SOM+ interneuron status, here sustained at a high level over half the adult life of a rat by a limited, early epoch of training, provides an especially important index of the better sustained functional integrity of cortical networks and systems. It is interesting to note that early training strongly offset the age-associated reduction in PV expression at cortical layers 4 and 5/6, whereas impacts on SOM expression were stronger in layers 2/3 and 5/6. The basis of these different laminar patterns of changes in PV and SOM expression is an important subject for further study.
An intriguing finding of the current study was that early training also offset the age-associated reduction in expressions of PV and SOM for GABAergic inhibitory interneurons in the hippocampus. These inhibitory neurons expressing PV and SOM, among the most affected populations impacted by aging in the hippocampus (26, 27, 31, 59, 60), have been shown to play important roles in learning and memory operations (28–31, 61). As our memory-guided learning task plausibly engages hippocampal processes, the positive changes recorded in inhibitory neuronal populations presumably reflect the fact that our simple form of training has induced physical and functional changes that have had positive, generalized impacts expressed very broadly across forebrain networks.
Materials and Methods
Female Sprague Dawley (SD) rats were used in the study. All details for behavioral training, passive exposure, sound discrimination assessment, ABR measurement and cortical mapping, and immunohistochemistry are described in SI Materials and Methods. These procedures were approved by the Institutional Animal Care and Use Committee and complied with NIH standards.
Acknowledgments
We thank Dr. Dan H. Sanes for helpful comments on an earlier version of this paper. This work was supported by National Natural Science Foundation of China Grants 81570925, 91632108, 81271085, and 81530030; Key Project of Shanghai Science and Technology Innovation Plan Grant 15JC1400102; Program of Introducing Talents of Discipline to Universities Grant B16018; JRI Seed Grants for Collaborative Research Grant 20170317 JFXZ; and the Foundation of Taishan Scholar.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1707086114/-/DCSupplemental.
References
- 1.Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- 2.Salthouse TA. Aging and measures of processing speed. Biol Psychol. 2000;54:35–54. doi: 10.1016/s0301-0511(00)00052-1. [DOI] [PubMed] [Google Scholar]
- 3.Mahncke HW, Bronstone A, Merzenich MM. Brain plasticity and functional losses in the aged: Scientific bases for a novel intervention. Prog Brain Res. 2006;157:81–109. doi: 10.1016/S0079-6123(06)57006-2. [DOI] [PubMed] [Google Scholar]
- 4.de Villers-Sidani E, et al. Recovery of functional and structural age-related changes in the rat primary auditory cortex with operant training. Proc Natl Acad Sci USA. 2010;107:13900–13905. doi: 10.1073/pnas.1007885107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mishra J, de Villers-Sidani E, Merzenich M, Gazzaley A. Adaptive training diminishes distractibility in aging across species. Neuron. 2014;84:1091–1103. doi: 10.1016/j.neuron.2014.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith GE, et al. A cognitive training program based on principles of brain plasticity: Results from the improvement in memory with plasticity-based adaptive cognitive training (IMPACT) study. J Am Geriatr Soc. 2009;57:594–603. doi: 10.1111/j.1532-5415.2008.02167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson S, White-Schwoch T, Parbery-Clark A, Kraus N. Reversal of age-related neural timing delays with training. Proc Natl Acad Sci USA. 2013;110:4357–4362. doi: 10.1073/pnas.1213555110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ball K, et al. Effects of cognitive training interventions with older adults: A randomized controlled trial. JAMA. 2002;288:2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ball K, Edwards JD, Ross LA. The impact of speed of processing training on cognitive and everyday functions. J Gerontol B Psychol Sci Soc Sci. 2007;62:19–31. doi: 10.1093/geronb/62.special_issue_1.19. [DOI] [PubMed] [Google Scholar]
- 10.Wolinsky FD, Vander Weg MW, Howren MB, Jones MP, Dotson MM. A randomized controlled trial of cognitive training using a visual speed of processing intervention in middle aged and older adults. PLoS One. 2013;8:e61624. doi: 10.1371/journal.pone.0061624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elliott AF, O’Connor ML, Edwards JD. Cognitive speed of processing training in older adults with visual impairments. Ophthalmic Physiol Opt. 2014;34:509–518. doi: 10.1111/opo.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pouille F, Scanziani M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science. 2001;193:1159–1163. doi: 10.1126/science.1060342. [DOI] [PubMed] [Google Scholar]
- 13.Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- 14.Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Urban-Ciecko J, Barth AL. Somatostatin-expressing neurons in cortical networks. Nat Rev Neurosci. 2016;17:401–409. doi: 10.1038/nrn.2016.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parthasarathy A, Datta J, Torres JA, Hopkins C, Bartlett EL. Age-related changes in the relationship between auditory brainstem responses and envelope-following responses. J Assoc Res Otolaryngol. 2014;15:649–661. doi: 10.1007/s10162-014-0460-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ng CW, Navarro X, Engle JR, Recanzone GH. Age-related changes of auditory brainstem responses in nonhuman primates. J Neurophysiol. 2015;114:455–467. doi: 10.1152/jn.00663.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou X, Merzenich MM. Environmental noise exposure degrades normal listening processes. Nat Commun. 2012;3:843. doi: 10.1038/ncomms1849. [DOI] [PubMed] [Google Scholar]
- 19.Zhu X, et al. Environmental acoustic enrichment promotes recovery from developmentally degraded auditory cortical processing. J Neurosci. 2014;34:5406–5415. doi: 10.1523/JNEUROSCI.5310-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Rossum MC. A novel spike distance. Neural Comput. 2001;13:751–763. doi: 10.1162/089976601300014321. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Zhao Y, Zhu X, Sun X, Zhou X. Refining cortical representation of sound azimuths by auditory discrimination training. J Neurosci. 2013;33:9693–9698. doi: 10.1523/JNEUROSCI.0158-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu X, et al. Perceptual training restores impaired cortical temporal processing due to lead exposure. Cereb Cortex. 2016;26:334–345. doi: 10.1093/cercor/bhu258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ouda L, Burianova J, Syka J. Age-related changes in calbindin and calretinin immunoreactivity in the central auditory system of the rat. Exp Gerontol. 2012;47:497–506. doi: 10.1016/j.exger.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Ouellet L, de Villers-Sidani E. Trajectory of the main GABAergic interneuron populations from early development to old age in the rat primary auditory cortex. Front Neuroanat. 2014;8:40. doi: 10.3389/fnana.2014.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Froemke RC. Plasticity of cortical excitatory-inhibitory balance. Annu Rev Neurosci. 2015;38:195–219. doi: 10.1146/annurev-neuro-071714-034002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuruba R, Hattiangady B, Parihar VK, Shuai B, Shetty AK. Differential susceptibility of interneurons expressing neuropeptide Y or parvalbumin in the aged hippocampus to acute seizure activity. PLoS One. 2011;6:e24493. doi: 10.1371/journal.pone.0024493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cardoso A, Silva D, Magano S, Pereira PA, Andrade JP. Old-onset caloric restriction effects on neuropeptide Y- and somatostatin-containing neurons and on cholinergic varicosities in the rat hippocampal formation. Age (Dordr) 2014;36:9737. doi: 10.1007/s11357-014-9737-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawaguchi Y, Katsumaru H, Kosaka T, Heizmann CW, Hama K. Fast spiking cells in rat hippocampus (CA1 region) contain the calcium-binding protein parvalbumin. Brain Res. 1987;416:369–374. doi: 10.1016/0006-8993(87)90921-8. [DOI] [PubMed] [Google Scholar]
- 29.Pawelzik H, Hughes DI, Thomson AM. Physiological and morphological diversity of immunocytochemically defined parvalbumin- and cholecystokinin-positive interneurones in CA1 of the adult rat hippocampus. J Comp Neurol. 2002;443:346–367. doi: 10.1002/cne.10118. [DOI] [PubMed] [Google Scholar]
- 30.Royer S, et al. Control of timing, rate and bursts of hippocampal place cells by dendritic and somatic inhibition. Nat Neurosci. 2012;15:769–775. doi: 10.1038/nn.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spiegel AM, Koh MT, Vogt NM, Rapp PR, Gallagher M. Hilar interneuron vulnerability distinguishes aged rats with memory impairment. J Comp Neurol. 2013;521:3508–3523. doi: 10.1002/cne.23367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kosaka T, Katsumaru H, Hama K, Wu JY, Heizmann CW. GABAergic neurons containing the Ca2+-binding protein parvalbumin in the rat hippocampus and dentate gyrus. Brain Res. 1987;419:119–130. doi: 10.1016/0006-8993(87)90575-0. [DOI] [PubMed] [Google Scholar]
- 33.Stanley EM, Fadel JR, Mott DD. Interneuron loss reduces dendritic inhibition and GABA release in hippocampus of aged rats. Neurobiol Aging. 2012;33:431.e1–431.e13. doi: 10.1016/j.neurobiolaging.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mishra J, Anguera JA, Ziegler DA, Gazzaley A. A cognitive framework for understanding and improving interference resolution in the brain. Prog Brain Res. 2013;207:351–377. doi: 10.1016/B978-0-444-63327-9.00013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wais PE, Gazzaley A. Distractibility during retrieval of long-term memory: Domain-general interference, neural networks and increased susceptibility in normal aging. Front Psychol. 2014;5:280. doi: 10.3389/fpsyg.2014.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bureš Z, Popelář J, Syka J. The effect of noise exposure during the developmental period on the function of the auditory system. Hear Res. 2016 doi: 10.1016/j.heares.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 37.Sarro EC, Rosen MJ, Sanes DH. Taking advantage of behavioral changes during development and training to assess sensory coding mechanisms. Ann N Y Acad Sci. 2011;1225:142–154. doi: 10.1111/j.1749-6632.2011.06023.x. [DOI] [PubMed] [Google Scholar]
- 38.Sarro EC, Sanes DH. The cost and benefit of juvenile training on adult perceptual skill. J Neurosci. 2011;31:5383–5391. doi: 10.1523/JNEUROSCI.6137-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parbery-Clark A, Anderson S, Hittner E, Kraus N. Musical experience offsets age-related delays in neural timing. Neurobiol Aging. 2012;33:1483.e1–1483.e4. doi: 10.1016/j.neurobiolaging.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 40.White-Schwoch T, Woodruff Carr K, Anderson S, Strait DL, Kraus N. Older adults benefit from music training early in life: Biological evidence for long-term training-driven plasticity. J Neurosci. 2013;33:17667–17674. doi: 10.1523/JNEUROSCI.2560-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skoe E, Kraus N. A little goes a long way: How the adult brain is shaped by musical training in childhood. J Neurosci. 2012;32:11507–11510. doi: 10.1523/JNEUROSCI.1949-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harrison SL, et al. Exploring strategies to operationalize cognitive reserve: A systematic review of reviews. J Clin Exp Neuropsychol. 2015;37:253–264. doi: 10.1080/13803395.2014.1002759. [DOI] [PubMed] [Google Scholar]
- 43.Yates LA, Ziser S, Spector A, Orrell M. Cognitive leisure activities and future risk of cognitive impairment and dementia: Systematic review and meta-analysis. Int Psychogeriatr. 2016;28:1791–1806. doi: 10.1017/S1041610216001137. [DOI] [PubMed] [Google Scholar]
- 44.Finkel D, Reynolds CA, McArdle JJ, Pedersen NL. Age changes in processing speed as a leading indicator of cognitive aging. Psychol Aging. 2007;22:558–568. doi: 10.1037/0882-7974.22.3.558. [DOI] [PubMed] [Google Scholar]
- 45.Zhou X, Merzenich MM. Developmentally degraded cortical temporal processing restored by training. Nat Neurosci. 2009;12:26–28. doi: 10.1038/nn.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Engle JR, Recanzone GH. Characterizing spatial tuning functions of neurons in the auditory cortex of young and aged monkeys: A new perspective on old data. Front Aging Neurosci. 2013;4:36. doi: 10.3389/fnagi.2012.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Overton JA, Recanzone GH. Effects of aging on the response of single neurons to amplitude-modulated noise in primary auditory cortex of rhesus macaque. J Neurophysiol. 2016;115:2911–2923. doi: 10.1152/jn.01098.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hornickel J, Zecker SG, Bradlow AR, Kraus N. Assistive listening devices drive neuroplasticity in children with dyslexia. Proc Natl Acad Sci USA. 2012;109:16731–16736. doi: 10.1073/pnas.1206628109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.King J, et al. Rodent auditory perception: Critical band limitations and plasticity. Neuroscience. 2015;296:55–65. doi: 10.1016/j.neuroscience.2015.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vollmer M, Beitel RE, Schreiner CE, Leake PA. Passive stimulation and behavioral training differentially transform temporal processing in the inferior colliculus and primary auditory cortex. J Neurophysiol. 2017;117:47–64. doi: 10.1152/jn.00392.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caspary DM, Ling L, Turner JG, Hughes LF. Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. J Exp Biol. 2008;211:1781–1791. doi: 10.1242/jeb.013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Caspary DM, Hughes LF, Ling LL. Age-related GABAA receptor changes in rat auditory cortex. Neurobiol Aging. 2013;34:1486–1496. doi: 10.1016/j.neurobiolaging.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stebbings KA, et al. Ageing-related changes in GABAergic inhibition in mouse auditory cortex, measured using in vitro flavoprotein autofluorescence imaging. J Physiol. 2016;594:207–221. doi: 10.1113/JP271221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Villers-Sidani E, Simpson KL, Lu YF, Lin RC, Merzenich MM. Manipulating critical period closure across different sectors of the primary auditory cortex. Nat Neurosci. 2008;11:957–965. doi: 10.1038/nn.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moore AK, Wehr M. Parvalbumin-expressing inhibitory interneurons in auditory cortex are well-tuned for frequency. J Neurosci. 2013;33:13713–13723. doi: 10.1523/JNEUROSCI.0663-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen N, Sugihara H, Sur M. An acetylcholine-activated microcircuit drives temporal dynamics of cortical activity. Nat Neurosci. 2015;18:892–902. doi: 10.1038/nn.4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sugahara H, Chen N, Sur M. Cell-specific modulation of plasticity and cortical state by cholinergic inputs to the visual cortex. J Physiol Paris. 2016;110:37–43. doi: 10.1016/j.jphysparis.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li LY, et al. Differential receptive field properties of parvalbumin and somatostatin inhibitory neurons in mouse auditory cortex. Cereb Cortex. 2015;25:1782–1791. doi: 10.1093/cercor/bht417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brady DR, Mufson EJ. Parvalbumin-immunoreactive neurons in the hippocampal formation of Alzheimer’s diseased brains. Neuroscience. 1997;80:1113–1125. doi: 10.1016/s0306-4522(97)00068-7. [DOI] [PubMed] [Google Scholar]
- 60.Thome A, Gray DT, Erickson CA, Lipa P, Barnes CA. Memory impairment in aged primates is associated with region-specific network dysfunction. Mol Psychiatry. 2016;21:1257–1262. doi: 10.1038/mp.2015.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Amilhon B, et al. Parvalbumin interneurons of hippocampus tune population activity at theta frequency. Neuron. 2015;86:1277–1289. doi: 10.1016/j.neuron.2015.05.027. [DOI] [PubMed] [Google Scholar]