Significance
Cellular growth in plants is constrained by cell walls; hence, loosening these structures is required for growth. The long-standing acid growth theory links auxin signaling, apoplastic pH homeostasis, and cellular expansion, providing a conceptual framework for cell expansion in plant shoots. Intriguingly, this model remains heavily debated for roots. Here, we present a fluorescent dye that allows for the correlation of cell size and apoplastic pH at a cellular resolution in Arabidopsis thaliana. This enabled us to elucidate a complex involvement of auxin in root apoplastic pH homeostasis, which is important for root cell expansion and gravitropic response. These findings shed light on the poorly understood acid growth mechanism in roots.
Keywords: apoplastic pH, auxin, cellular expansion, root growth, root gravitropism
Abstract
Plant cells are embedded within cell walls, which provide structural integrity, but also spatially constrain cells, and must therefore be modified to allow cellular expansion. The long-standing acid growth theory postulates that auxin triggers apoplast acidification, thereby activating cell wall-loosening enzymes that enable cell expansion in shoots. Interestingly, this model remains heavily debated in roots, because of both the complex role of auxin in plant development as well as technical limitations in investigating apoplastic pH at cellular resolution. Here, we introduce 8-hydroxypyrene-1,3,6-trisulfonic acid trisodium salt (HPTS) as a suitable fluorescent pH indicator for assessing apoplastic pH, and thus acid growth, at a cellular resolution in Arabidopsis thaliana roots. Using HPTS, we demonstrate that cell wall acidification triggers cellular expansion, which is correlated with a preceding increase of auxin signaling. Reduction in auxin levels, perception, or signaling abolishes both the extracellular acidification and cellular expansion. These findings jointly suggest that endogenous auxin controls apoplastic acidification and the onset of cellular elongation in roots. In contrast, an endogenous or exogenous increase in auxin levels induces a transient alkalinization of the extracellular matrix, reducing cellular elongation. The receptor-like kinase FERONIA is required for this physiological process, which affects cellular root expansion during the gravitropic response. These findings pinpoint a complex, presumably concentration-dependent role for auxin in apoplastic pH regulation, steering the rate of root cell expansion and gravitropic response.
Plant cells are surrounded by a rigid cell wall, which provides form and stability, enabling plants to grow to extreme heights despite the absence of a skeleton. However, these advantages come with the price that plant cells are encased within the stiff cell wall matrix, which must be remodeled to allow for cellular elongation. How cell walls are modified to enable cellular expansion has been of scientific interest since the 1930s, as insight into this physiological process would provide a wealth of knowledge on how plants grow (1). In the early 1970s, a physiological mechanism explaining cell expansion, the acid growth theory, was proposed (2–4). This theory postulates that the plant hormone auxin triggers the activation of plasma membrane (PM)-localized H+-ATPases (proton pumps), resulting in acidification of the intercellular space (apoplast). The reduction in apoplastic pH activates cell wall-loosening enzymes, which, in concert with turgor pressure, enables cellular expansion (1). Auxin was the first plant hormone shown to be involved in processes important for plant growth and development, including tissue growth, apical dominance, wound response, flowering, and tropisms, such as the gravitropic response (5). Auxin is known to play a complex role in plant growth regulation, as it can both stimulate and inhibit tissue expansion, depending on the tissue and its concentration (6–8). A positive effect of auxin on growth was hypothesized by the acid growth theory (1). Subsequent literature provided significant insight into the molecular mechanisms of auxin-triggered acid growth in shoots (9–13). However, in roots, the acid growth theory remains the subject of debate. On one hand, several studies report the stimulating effect of apoplast acidification on cell expansion in roots, as well as the requirement of functional PM H+-ATPases for root growth (14–16). On the other hand, high auxin concentrations are known to inhibit root cell expansion and overall root growth (8, 17). Moreover, exogenous auxin application has been described to trigger apoplast alkalization in roots, which is the opposite effect as in shoots (18–20). Notably, a recent study provides substantial transcriptomic insight into auxin-triggered cell wall modification and cell expansion in Brachipodium distachion roots (21). However, the authors also observed that medium acidification does not correlate with root cell elongation (21). Notably, most of the aforementioned studies indirectly investigated apoplast acidification by measuring pH alterations in the medium, thereby failing to directly assess the apoplastic pH at cellular resolution. The discrepancies in the current literature point to a complex role for auxin in apoplastic pH homeostasis and highlight the need to reassess the acid growth theory at the cellular level.
Here, we introduce 8-hydroxypyrene-1,3,6-trisulfonic acid trisodium salt (HPTS) as a suitable fluorescent pH indicator for assessing apoplastic pH at a cellular resolution. Using HPTS, we dissected the apoplastic pH dynamics in A. thaliana roots and show that root cell expansion correlates with its acidification and increased nuclear auxin signaling. In agreement, interference with endogenous auxin levels or signaling abolishes acidification and elongation. However, we also find that exogenous and endogenous increases in cellular auxin accumulation lead to a transient alkalization of the apoplast, correlating with the inhibition of root cell expansion. A significant proportion of this transient alkalization is dependent on the receptor-like kinase FERONIA. Taken together, our data suggest a complex role of auxin in apoplastic pH regulation, which is important for root organ growth and gravitropic response.
Results
HPTS Enables the Assessment of Apoplastic pH at a Cellular Resolution.
To efficiently dissect acid growth in A. thaliana roots, we aimed to identify a fluorescent dye that would enable the assessment of apoplastic pH with a cellular resolution. We screened the literature for nontoxic, fluorescent, pH-sensitive dyes that are also water soluble so they would easily penetrate the root apoplast, but not enter the root cells (22). Our search identified HPTS as a suitable candidate to assess apoplastic pH in A. thaliana roots. HPTS is a water-soluble fluorescent dye displaying pH-dependent spectral characteristics (23). This fluorescent pH indicator has been previously described to be suitable for the pH assessment of neuronal organelles, as well as liposomes designed for drug delivery (24–26). In plants, HPTS has been used to define the pH of extracted apoplastic fluid from plant tissues (27, 28).
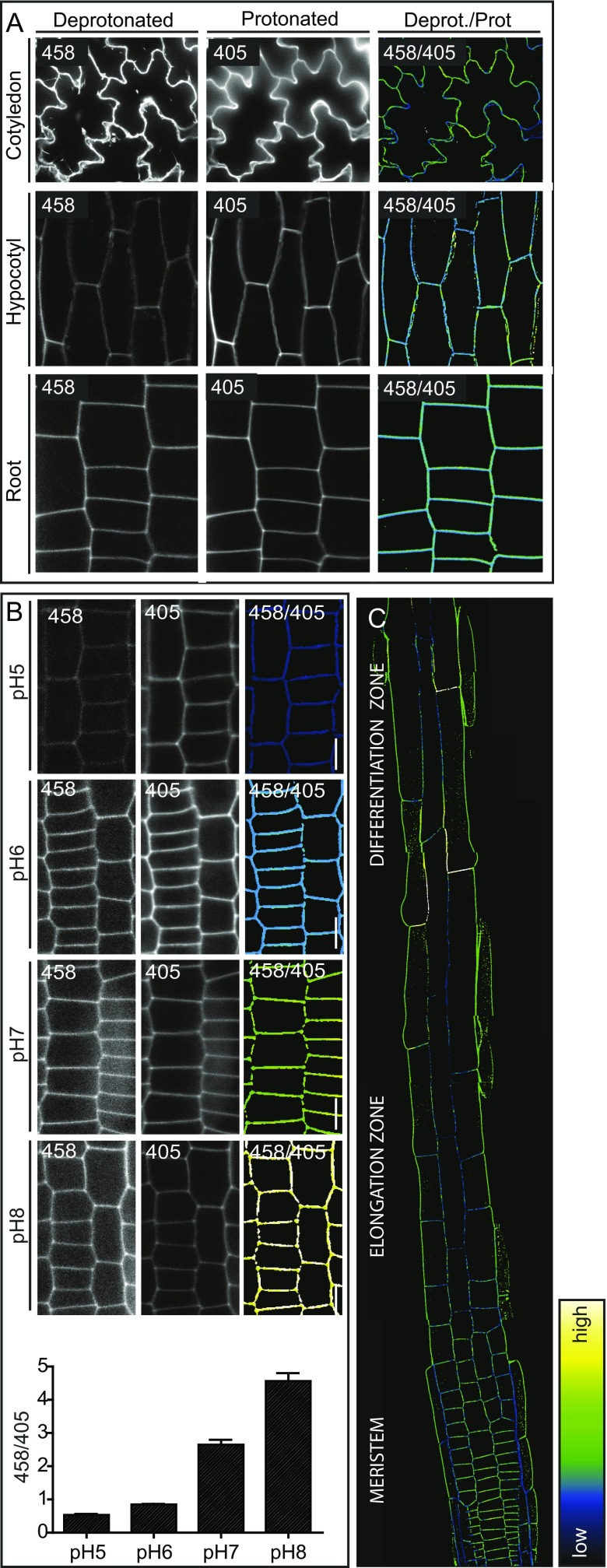
To test whether HPTS can be used to directly assess apoplastic pH with a cellular resolution in planta, we immersed 4-d-old seedlings in HPTS containing liquid growth medium for 30 min and imaged roots, hypocotyls, and cotyledons, using confocal microscopy (Fig. S1A). The protonated and deprotonated forms of HPTS were visualized in 2 different channels with excitation wavelengths of 405 and 458 nm, respectively. The signal was restricted to the apoplastic area, confirming that the cells do not take up HPTS (Fig. S1A). This is crucial for the specific assessment of pH in the cell wall.
Fig. S1.
(A and B) HPTS stained Col-0-WT seedlings under standard conditions (A) and incubated for 30 min in liquid growth medium of different pH (B). (Left) Deprotonated (basic) version of HPTS (λex, 458 nm; λem, 514 nm). (Middle) Protonated (acidic) version of HPTS (λex, 405 nm; λem, 514 nm). (Right) Ratiometric image where, for each pixel, the 458 intensity has been divided by the 405 intensity. The 458/405 ratio values correlate with the apoplastic pH. (C) HPTS stained 4-d-old seedling root. Color code (black to white) depicts (low to high) 458/405 intensity, and thus pH values. Error bars represent SEM.
Next, we tested whether HPTS can reliably record pH changes. We therefore adjusted the apoplastic pH of 4-d-old seedlings by incubating them for 30 min in HPTS containing liquid growth medium with different pH levels, and imaged them as described earlier. Dividing the signal intensity of the 458-nm channel by the 405-nm channel for each pixel resulted in a ratiometric image from which the intensity values correlate with the apoplastic pH (Fig. S1B). We designed a custom-made Fiji (29) script to enable fast and consistent ratiometric image conversion with an easy color code-based read out (Dataset S1). Indeed, increases in the pH of the media led to an increase in the apoplastic pH, as indicated by the 458/405 ratio (Fig. S1B). This indicates that HPTS is suitable to detect exogenously imposed apoplastic pH changes.
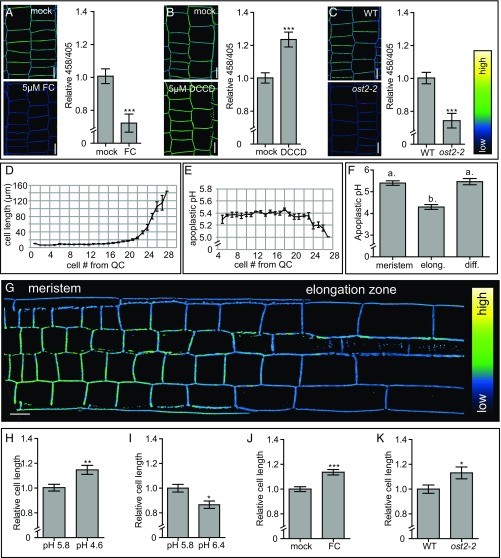
Next, we tested whether HPTS allows for the detection of physiological changes in apoplastic pH. To accomplish this, we pharmacologically modulated the activity of the PM-localized H+-ATPases and analyzed the 458/405 ratio after HPTS staining and imaging. Fusicoccin treatment, which activates the PM H+-ATPases (30), resulted in decreased 458/405 ratio values, suggesting a decrease in apoplastic pH (Fig. 1A). To test whether we can also measure an increase in apoplastic pH, we used N,N′-dicyclohexylcarbodiimide (DCCD), which is a chemical known to inhibit the proton-conducting activity of H+-ATPases, including the PM H+-ATPases (31). DCCD treatment gave rise to higher 458/405 values, indicating a higher apoplastic pH (Fig. 1B). Finally, we used HPTS to measure apoplastic pH on genetic perturbation by analyzing OPEN STOMATA 2 (ost2-2) mutant seedlings. The ost2-2 mutant expresses the constitutively active PM H+-ATPase AHA1 (32). Compared with WT seedlings, ost2-2 mutants displayed lower 458/405 values (Fig. 1C), confirming the apoplastic acidification in ost2-2 mutants (29). These data show that HPTS is able to report on physiological changes in apoplastic pH.
Fig. 1.
Apoplastic pH and epidermal cell length correlation in A. thaliana roots. (A and B) The effect of 5 μM fusicoccin (FC) treatment for 60 min and 5 μM DCCD treatment for 17 h on the apoplastic pH in A. thaliana roots, visualized using HPTS staining. y-axis: mean 458/405 values of epidermal cells in root meristems of the pharmacologically treated seedlings relative to mock-treated seedlings. (C) Comparison of the apoplastic pH in WT and ost2-2 mutant seedlings, visualized by HPTS staining. y-axis: the mean 458/405 values of the ost2-2 mutant roots relative to the WT. (A–C) Error bars represent SEM (n ≥ 13 roots per line/condition). Student t test P values: *P < 0.05, **P < 0.01, ***P < 0.001. (D, E, and G) Epidermal cell length with the corresponding absolute apoplastic pH, as visualized using HPTS staining. Error bars represent SEM (n = 11 roots). (F) Apoplastic pH of the epidermal apoplast in the root meristem, elongation, and differentiation zones (Fig. S1C). Error bars represent SEM. Statistical significance (0.05%) was tested using a one-way ANOVA test with a Tukey-Kramer post hoc test (n ≥ 14 roots). (H and I) Epidermal cell length in the distal meristem of seedlings grown on pH 5.8 growth medium and transferred for 2.5 h on pH 4.6 or 6.4 growth medium. (J) The effect of 19 h of 10 μM FC treatment on the root epidermal cell length. (K) Root epidermal cell length of WT and ost2-2 seedlings. (H–K) Error bars represent SEM. (n ≥ 13 roots). Student t test P values: *P < 0.05, **P < 0.01, ***P < 0.001. (A–C and G) Color code (black to white) depicts (low to high) 458/405 intensity, and thus, pH values. (Scale bars: 10 μm.)
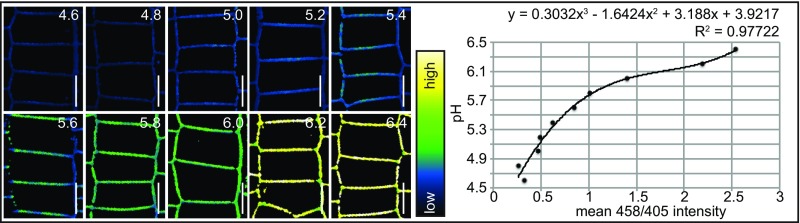
To obtain a reliable measure of the apoplastic pH in Arabidopsis roots, we performed an in vivo calibration of the HPTS dye. For this, we stained 4-d-old seedlings for 30 min in HPTS and rinsed the roots with medium adjusted to pH values between 4.6 and 6.4. The 458/405 ratios were used to plot a calibration curve from which a best-fitting regression curve was calculated (Fig. S2). Although we cannot exclude the possibility that a potential buffering capacity of the root (33) affects our measurements, these short-term treatments allowed us to approximate an absolute pH value of 5.4 in the cell wall of meristematic cells (Fig. 1 E and F). Accordingly, we conclude that HPTS is a suitable dye for assessing apoplastic pH in Arabidopsis roots at a cellular resolution.
Fig. S2.
In vivo HPTS calibration. Apoplastic epidermal root meristem 458/405 values of seedlings incubated for 30 min in liquid growth medium with pH 4.6–6.4. x-axis: mean 458/405 value. y-axis: pH. (Top) Regression analysis derived-equation enabling apoplastic pH calculation from the obtained 458/405 values.
Apoplastic pH Determines Cellular Expansion in A. thaliana Roots.
Having established an accurate method for assessing apoplastic pH, our next step in dissecting acid growth in A. thaliana roots was to determine the relationship between apoplastic pH and root cell expansion. Several studies have reported a stimulating effect of apoplast acidification on root cell expansion in maize, pea, and bean (14, 15). To assess apoplastic pH dynamics in A. thaliana roots, we measured the epidermal cell length of root cells, beginning at the height of the quiescent center, and recorded their corresponding apoplastic pH (Fig. 1 D–G). Under our growth and experimental conditions, cells in the meristematic zone harbor an approximate apoplastic pH of 5.4. During cellular elongation, we observed that the apoplastic pH decreases below pH 5 (Fig. 1 D–G). When root cells reach their final length in the differentiation zone, the apoplastic pH increases again, suggesting apoplast acidification only occurs in elongating cells (Fig. 1F and Fig. S1C).
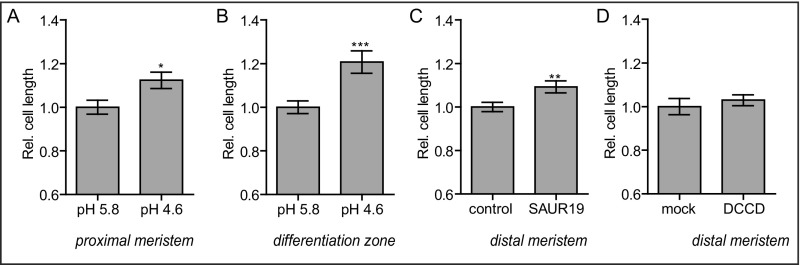
This correlation led us to test whether apoplast acidification induces root cell elongation. Therefore, we transferred 4-d-old seedlings (grown on standard medium with pH 5.8) to growth medium with a pH of 4.6 for 2.5 h. These seedlings displayed longer epidermal cells in the distal (late) meristem (the last 4 cells of the meristem) compared with seedlings transferred to control medium with a pH of 5.8 (Fig. 1I). These data indicate that exogenously imposed apoplast acidification is sufficient to trigger root cell expansion. Seedlings transferred to pH 4.6 medium also display longer epidermal cells in the proximal (early) meristem and differentiation zone compared with control seedlings, indicating that these cells are also responsive to exogenously imposed apoplast acidification (Fig. S3 A and B). We next tested the opposite response: whether apoplast alkalization can inhibit cell elongation. Indeed, seedlings transferred to pH 6.4 medium for 2.5 h displayed shorter epidermal cells in the distal meristem compared with seedlings transferred to control medium (Fig. 1I). These data indicate that apoplast acidification correlates with and affects root cell expansion in A. thaliana. We note that, in this study, we focus on epidermal root cells in the stage just before elongation. From here on, the term root epidermal cells comprises the root epidermal cells in the distal meristem (including the last four cells of the meristem).
Fig. S3.
(A and B) Epidermal cell length (y-axis) in the proximal (early) meristem (A) and differentiation zone (B) of seedlings grown on pH 5.8 medium and transferred for 2.5 h to pH 4.6 growth medium relative to seedlings transferred on pH 5.8 growth medium. (C) Root epidermal cell length of estradiol-induced GFP-SAUR19 seedlings relative to control (empty vector) seedlings, as visualized by PI staining. (D) The effect of 17 h of 5 μM DCCD treatment on the root epidermal cell length relative to mock-treated seedlings. Error bars represent SEM. (n ≥ 24 roots per line/condition). t test P values: *P < 0.05, **P < 0.01, ***P < 0.001.
After having established that our dye faithfully reports pH changes, we studied the relationship between cell elongation and apoplastic pH. To test whether root cell elongation is affected by a physiological change in apoplastic pH, we treated seedlings with fusicoccin for 19 h and observed longer epidermal root cells compared with in mock-treated seedlings (Fig. 1J). In agreement with this, ost2-2 mutant seedlings, which had a lower apoplastic pH compared with WT seedlings (Fig. 1C), also displayed longer root epidermal cells compared with WT seedlings (Fig. 1K). Even though DCCD treatment significantly increased the root apoplastic pH (Fig. 1B), we did not observe differences in cell length under our growth and experimental conditions (Fig. S3D). However, previous literature reports an inhibiting effect of DCCD on root cell elongation in A. thaliana roots (34). Altogether, our data show that apoplast acidification and alkalization promotes and inhibits epidermal root cell expansion, respectively.
Auxin Signaling Affects Apoplastic pH Regulation.
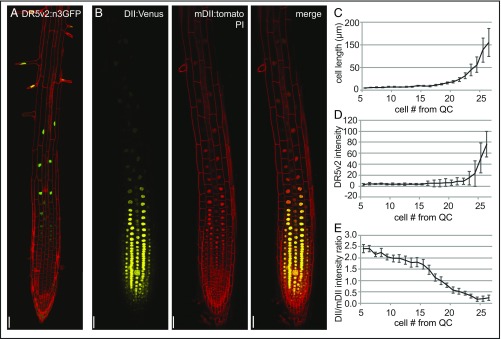
The phytohormone auxin has been shown to be indispensable for plant growth and development because of its involvement in a broad spectrum of plant developmental processes (35). In plant aerial tissues, auxin is known to trigger the activation of PM-localized proton pumps (PM H+-ATPases), resulting in acidification of the apoplast (1). Our data suggest apoplast acidification is also important for root cell elongation in A. thaliana. To investigate the role of auxin in controlling apoplast acidification, and thereby root cell expansion, we used the auxin response reporter lines DR5v2:n3GFP and R2D2. DR5v2:n3GFP expresses a GFP reporter with a nuclear localization signal under a synthetic auxin inducible promoter (36). R2D2 seedlings express a Venus-tagged auxin degradable reporter protein (DII:n3xVenus) under control of an RPS5A promoter along with an RFP-tagged undegradable protein (mDII:ntdTomato), allowing for the quantitative assessment of auxin responses (36). In root epidermal cells, DR5v2:n3GFP expression is very low in the meristem, but gradually increases toward the elongation zone (Fig. 2 A, C, and D). DII-Venus signal intensity, normalized by the mDII signal, is strong in epidermal, meristematic root cells and gradually decreases toward the elongation zone (Fig. 2 B, C, and E). The increased DR5v2:n3GFP expression along with the decreased DII/mDII signal intensity ratio, both indicating increased auxin signaling, suggest an increase in auxin signaling in epidermal root cells at the onset of elongation (Fig. 2 A–E). This raises the question of whether auxin could be involved in the apoplast acidification required for root cell expansion.
Fig. 2.
Epidermal cell-length correlation with auxin response reporter activity. (A and B) DR5v2:GFP, DII-Venus and mDII-tomato reporter activity in roots of 4-d-old Col-0-WT seedlings (n ≥ 9 roots per line). (C–E) Average epidermal cell size with the corresponding DR5v2:GFP signal intensity and DII-Venus/mDII-tomato intensity ratio. Error bars represent SEM (n = 6–9 roots per data point). (Scale bars: 50 μm.)
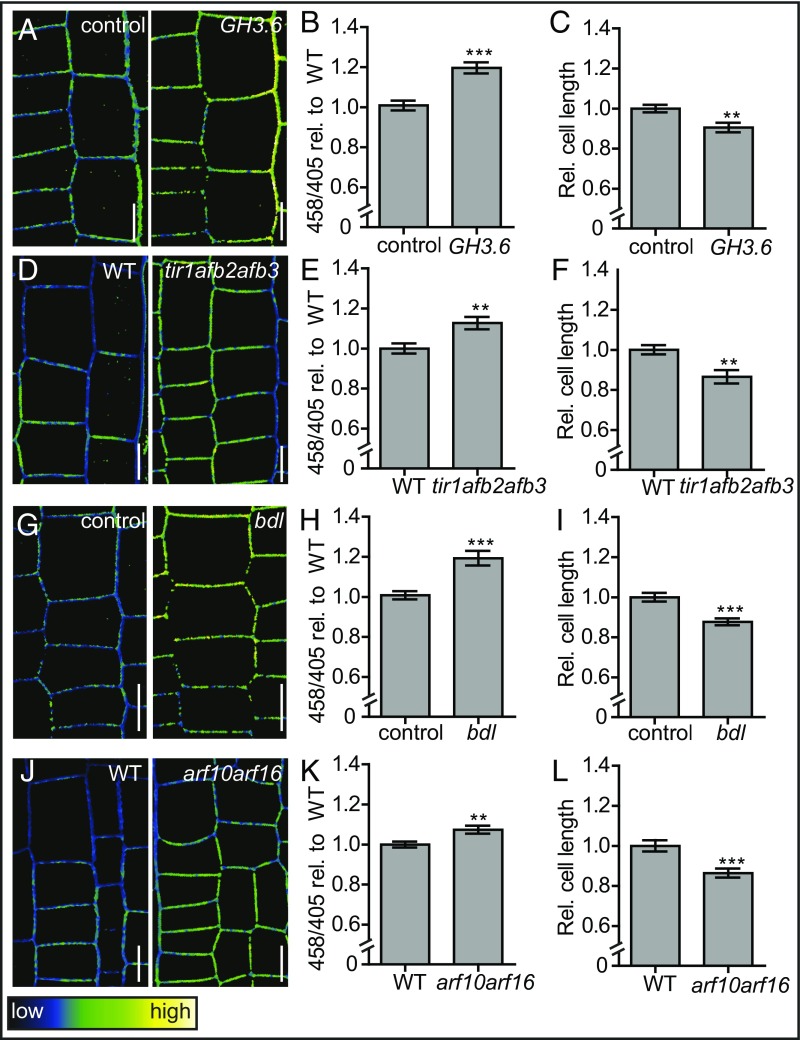
To test this hypothesis, we analyzed apoplastic pH as well as root epidermal cell length in mutants that are compromised in auxin content and signaling. Proteins from the GRETCHEN HAGEN 3 family are conjugating enzymes, of which some, including GH3.5 and GH3.6, conjugate auxin to amino acids (37), and thus lower the levels of cellular free auxin. To transiently decrease free auxin levels, we induced GH3.6 expression in an estradiol-inducible GH3.6 line and analyzed the effects on apoplastic pH and cell size. On 6 h of induction, we observed higher apoplastic pH in GH3.6-induced seedlings compared with control (empty vector expressing) seedlings (Fig. 3 A and B). At that point, we did not observe a significant effect of GH3.6 expression on cell length. However, 19 h of GH3.6 expression significantly decreased the epidermal root cell size compared with control seedlings (Fig. 3C). These data suggest decreased endogenous auxin levels give rise to a higher apoplastic pH and subsequent inhibition of cell elongation.
Fig. 3.
Apoplastic pH homeostasis in auxin signaling mutants. (A–C) Apoplastic pH and corresponding epidermal cell length in estradiol-induced GH3.6 and control (empty vector) seedlings, as visualized by HPTS staining. (D–F) Apoplastic pH and corresponding root epidermal cell length of WT and tir1afb2afb3 mutant seedlings, as visualized by HPTS staining. (G–I) Apoplastic pH and corresponding root epidermal cell length in estradiol-induced bdl and control (empty vector) seedlings, as visualized by HPTS staining. (J–L) Apoplastic pH and corresponding root epidermal cell length of WT and arf10arf16 mutant seedlings, as visualized by HPTS staining. (A, D, G, J) Color code (black to white) depicts (low to high) 458/405 intensity, and thus, pH values. Error bars represent SEM (n ≥ 19). Student t test P values: *P < 0.05, **P < 0.01, ***P < 0.001. (Scale bars: 10 μm.)
Next, we genetically interfered with the auxin signaling pathway. Transcriptional auxin responses are known to be mediated by the nuclear TIR/AFB auxin signaling pathway. Auxin binding to members of the TIR1/AFB F-box family receptors triggers the proteasomal degradation of AUX/IAA repressors. This degradation causes the subsequent release of AUXIN RESPONSE FACTOR (ARF) transcription factors, which then execute auxin responsive gene expression (38). Auxin receptor triple mutant seedlings, tir1afb2afb3, display a higher apoplastic pH along with smaller epidermal cells compared with WT seedlings (Fig. 3 D–F). This suggests TIR/AFB-dependent auxin signaling is required for normal apoplast acidification with a concomitant effect on root cell elongation.
We also aimed to test the effect of transiently compromised auxin signaling. bodenlos (bdl) is a mutant allele of IAA12 that produces a nondegradable form of the AUX/IAA repressor IAA12 (39). Hence, induction of bdl expression inhibits auxin signaling (40). We analyzed the apoplastic pH and root cell size of seedlings in which bdl expression was induced. On 6 h induction, bdl-expressing seedlings displayed a higher apoplastic pH compared with control seedlings containing an empty vector (Fig. 3 G and H). Similar to GH3.6 expression, we only observed an inhibitory effect on root cell elongation on 19 h of bdl induction (Fig. 3I), again suggesting pH regulation precedes growth.
Next, we assessed the apoplastic pH and cell length of seedlings defective in ARF10 and ARF16. ARFs are transcription factors that act in the nuclear auxin signaling pathway that activate the expression of auxin responsive genes in the presence of auxin (41). ARF10 and ARF16 are readily expressed in root epidermal cells, and arf10 arf16 mutant roots are impaired in auxin responses (42). Similar to the auxin receptor triple mutant, we observed a higher apoplastic pH and smaller cells in arf10arf16 mutant seedlings compared with WT (Fig. 3 J–L). Finally, we induced the expression of the auxin responsive gene SMALL AUXIN UP RNA 19 (SAUR19) fused to a GREEN FLUORESCENT PROTEIN (GFP) in an estradiol-inducible GFP-SAUR19 line. SAUR19 was shown to acidify the apoplast through PM H+-ATPase activation (12). Moreover, GFP-SAUR19 overexpression increases the size of hypocotyls in A. thaliana, as well as in tomato (43, 44). Because of the emission of the GFP fluorophore, which overlaps with the HPTS spectrum, we were not able to directly confirm the GFP-SAUR19 effect on the root apoplastic pH. However, we observed longer epidermal root cells in the GFP-SAUR19-induced line compared with estradiol-treated control seedlings (expressing an empty vector) (Fig. S3C). These data show that the ectopic expression of the auxin response gene GFP-SAUR19 is sufficient to trigger epidermal cell elongation in A. thaliana roots. Notably, it has been shown that the stabilizing effect of the GFP tag is required for the GFP-SAUR19-dependent phenotypes (43). It has to be seen whether the ectopic overexpression of an untagged SAUR19 is able to trigger cell expansion in A. thaliana roots.
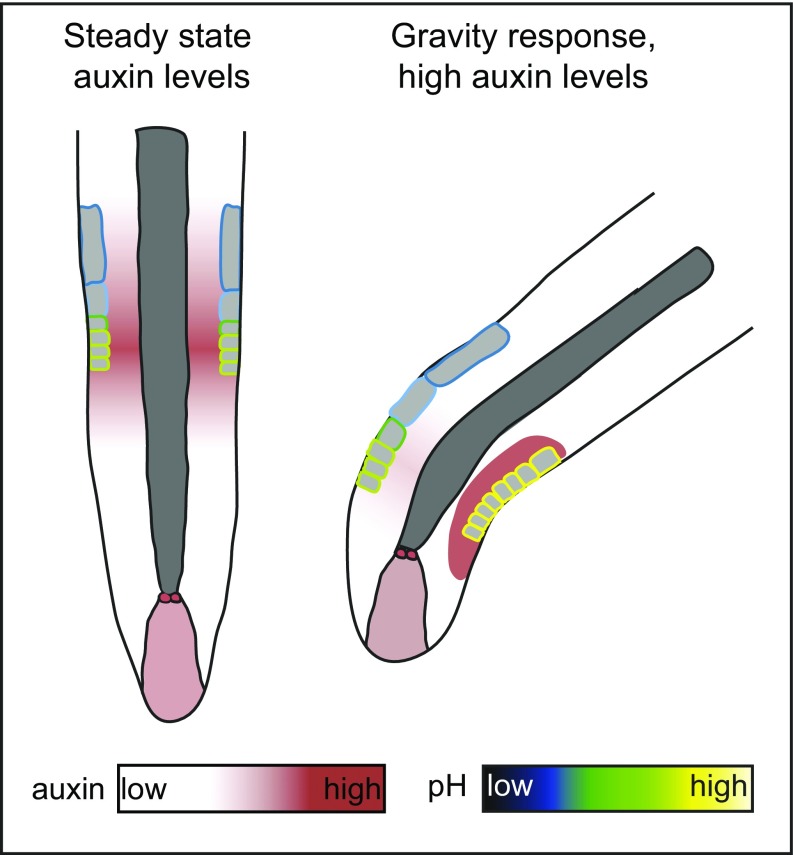
Our data suggest auxin signaling is important for apoplast acidification and cell elongation in A. thaliana roots. This implies endogenous auxin levels could trigger apoplast acidification, and thus stimulate cell expansion in the distal meristematic root epidermal cells (Fig. S4).
Fig. S4.
Schematic overview of a model linking auxin levels, pH, and gravity response. (Left) Apoplastic pH homeostasis in the root epidermis on steady state auxin levels. Our data show that, under steady-state auxin conditions, nuclear auxin signaling is required for apoplast acidification in the distal meristem and subsequent epidermal root cell elongation. (Right) Apoplastic pH regulation on highly increased auxin levels during a gravitropic stimulus. On gravistimulation, an auxin signaling maximum is formed at the lower side of the root (48). Our data suggest this auxin maximum results in a FERONIA-dependent transient apoplast alkalization that inhibits epidermal root cell elongation. This local inhibition of epidermal root cell elongation enables root bending toward the gravity vector.
Exogenous Auxin Causes a Biphasic pH Response in the Apoplast.
Our data suggest low endogenous auxin levels in the root meristem and elongation zone are required for apoplast acidification and subsequent root cell elongation. This is quite surprising, however, as increased auxin levels are assumed to inhibit root cell elongation, rather than stimulate it (8). A possible explanation could come from the fact that auxin is also known to affect growth in a concentration-dependent manner, stimulating or repressing tissue expansion in low or high concentrations, respectively, depending on the tissue in question (6, 7).
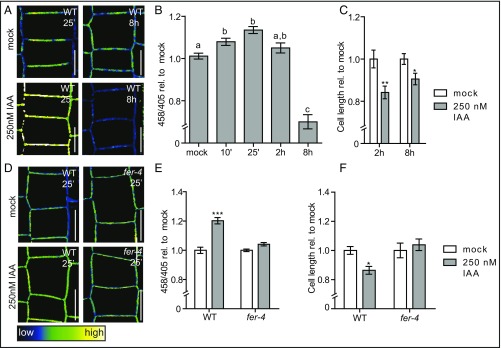
Because high auxin concentrations are known to inhibit root tissue expansion (8), we aimed to address how inhibitory levels of auxin affect root apoplastic pH and cell expansion. For this, we transferred seedlings to growth medium supplemented with 250 nM IAA. In contrast to our observations in untreated roots, and in agreement with previous reports, we observed a rapid auxin-induced alkalization of the apoplast (Fig. 4 A and B (20, 45). Notably, this alkalizing effect of auxin was transient. After 2 h of auxin treatment, the apoplastic pH returned to levels comparable to mock-treated seedlings (Fig. 4 A and B). Moreover, prolonged auxin treatments of up to 8 h decreased the apoplastic pH relative to mock-treated seedlings (Fig. 4 A and B). We conclude that the application of high auxin concentrations affects apoplastic pH in a biphasic manner. Accordingly, the contradictions in the current literature may be explained by the varying experimental conditions of previous studies, such as times. Importantly, the auxin-induced inhibition of cell expansion occurs after the alkalization phase (2 h) and precedes the later apoplast acidification (Fig. 4C).
Fig. 4.
Biphasic effect of exogenous auxin on apoplastic Ph. (A–C) Effect of 250 nM exogenous IAA on apoplastic pH and root epidermal cell length, as visualized by HPTS staining. (B) The mean 458/405 intensities of seedlings treated with 250 nM IAA over time relative to mock-treated seedlings. (C) Epidermal cell length in the root of seedlings treated with 250 nM IAA for 2 h and 8 h compared with mock-treated seedlings. (D–F) Effect of 250 nM IAA treatment on apoplastic pH and epidermal cell length in the root of WT and mutant seedlings, as visualized by HPTS staining. (E) Mean 458/405 intensities of WT and fer-4 seedlings treated with 250 nM IAA for 25 min relative to mock-treated seedlings. (F) Root epidermal cell length of WT and fer-4 seedlings treated with 250 nM IAA for 8 h. Color code (black to white) depicts (low to high) 458/405 intensity, and thus pH values. (B, C, E, and F) Error bars represent SEM (n ≥ 13 roots). Statistical significance was tested using a one-way ANOVA test with a Tukey-Kramer post hoc test (different letters depict statistically significant differences; P < 0.05) (B) or a two-way ANOVA test with Bonferroni posttests (treatment factor P values: *P < 0.05, **P < 0.01, ***P < 0.001) (C, E, and F). (Scale bars: 10 μm.)
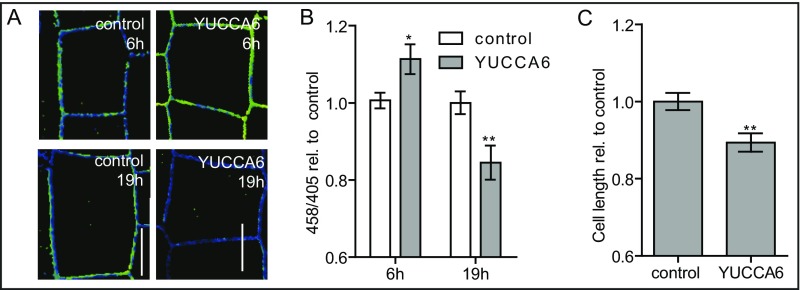
To test whether this behavior is also observable using endogenous elevation of auxin levels, we induced the expression of the auxin biosynthesis gene YUCCA6. Similar to exogenous auxin treatment, YUCCA6 induction initially resulted in increased apoplastic pH, whereas long-term YUCCA6 induction of 19 h gave rise to a lower apoplastic pH compared with control seedlings expressing an empty vector (Fig. S5 A and B). Accordingly, induction of auxin biosynthesis also inhibited epidermal root cell elongation (Fig. S5C). Overall, these data show that external auxin application in an inhibitory concentration range, as well as increased endogenous auxin levels, gives rise to a biphasic apoplastic pH response resulting in the inhibition of root cell expansion. Accordingly, we conclude that the initial apoplast alkalization could be the causal factor for repressing cellular elongation.
Fig. S5.
(A–C) The effect of YUCCA6 induction on apoplastic pH and epidermal cell length as visualized by HPTS staining (A). The mean 458/405 intensities (y-axis) of seedlings induced with YUCCA6 for 6 h relative to control seedlings expressing an empty vector (B). Epidermal cell length (y-axis) in the root of seedlings in which YUCCA6 was induced for 19 h relative to control seedlings expressing an empty vector (C). Student t test (YUCCA6-induced compared with empty vector-induced) P values: *P < 0.05, **P < 0.01, ***P < 0.001. (Scale bars: 10 μm.) n ≥ 28 roots per line.
FERONIA Impairs Auxin-Dependent Apoplast Alkalization and Cellular Elongation.
To further assess the importance of auxin-mediated cell wall alkalization for cellular expansion, we wanted to study the effects of suppressed cell wall alkalization. Apoplast alkalization has been previously reported to be mediated by the PM-localized receptor-like kinase FERONIA on the binding of its ligand rapid alkalization factor 1 (RALF1) (46). RALF-FERONIA binding triggers the phosphorylation of the PM-localized proton pumps, thereby inhibiting their proton transport capacity and resulting in apoplast alkalization (46). To test whether auxin-triggered apoplast alkalization is affected by FERONIA, we tested the effect of auxin on apoplastic pH and cell size in the fer-4 loss-of-function mutant. Indeed, fer-4 mutant seedlings displayed a substantial resistance to the apoplast alkalization, as well as an eventual inhibition of cell expansion triggered by 250 nM IAA (Fig. 4 D–F). This suggests that a significant portion of the auxin-triggered apoplast alkalization requires FERONIA. Moreover, this experiment demonstrated that auxin-induced apoplast alkalization is important for the inhibition of root cell expansion.
Auxin Induces Transient Apoplastic Alkalization for Gravitropic Root Growth.
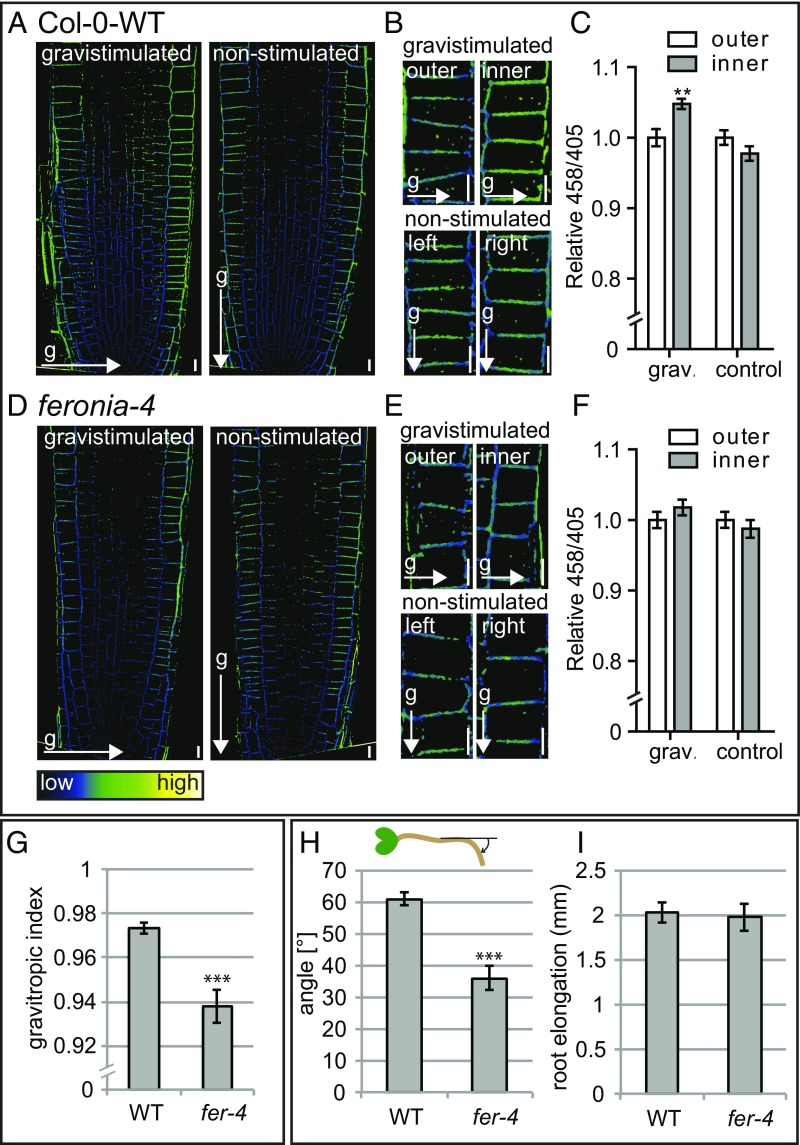
Root gravitropism, the process in which root growth is aligned with the field of gravity, is one of the most prominent processes in which auxin-dependent growth responses immediately affect cellular elongation in the root. On gravistimulation, an auxin-signaling maximum is formed at the lower side of the root, resulting in local inhibition of tissue expansion and, as a consequence, root bending (47). To assess the biological relevance of our findings, we tested the effect of gravistimulation on apoplastic pH and its requirement for gravitropic root response. In agreement with previous reports (19, 48), 45 min root gravistimulation resulted in an increased apoplastic pH at the lower side of the root (Fig. 5 A–C), which corresponds to the site of auxin accumulation (49) (Fig. S4).
Fig. 5.
Effect of gravistimulation on apoplastic pH in WT and fer4 seedlings. (A–F) Mean 458/405 signal intensities in the epidermal cell layer at the outer and inner side of the root of WT and fer-4 seedlings after 45 min gravistimulation (90°) compared with nonstimulated seedlings, visualized using HPTS staining. Error bars represent SEM (n = 17 roots per line). Statistical significance was tested using a two-way ANOVA test with Bonferroni posttests. Outer/inner factor P values: *P < 0.05, **P < 0.01, ***P < 0.001. (Scale bars: 10 μm.) (G) Gravitropic index (50) of 7-d-old WT and fer-4 seedlings. (H) Gravitropic response of 4-d-old seedlings gravistimulated for 6 h (90°). y-axis shows the mean angle of the root tip with respect to the horizontal (base of the root), as depicted in the schematic. (I) Root elongation of 4-d-old seedlings gravistimulated for 6 h (90°). (G–I) Error bars represent SEM (n ≥ 23 roots per line). Student t test P values: *P < 0.05, **P < 0.01, ***P < 0.001.
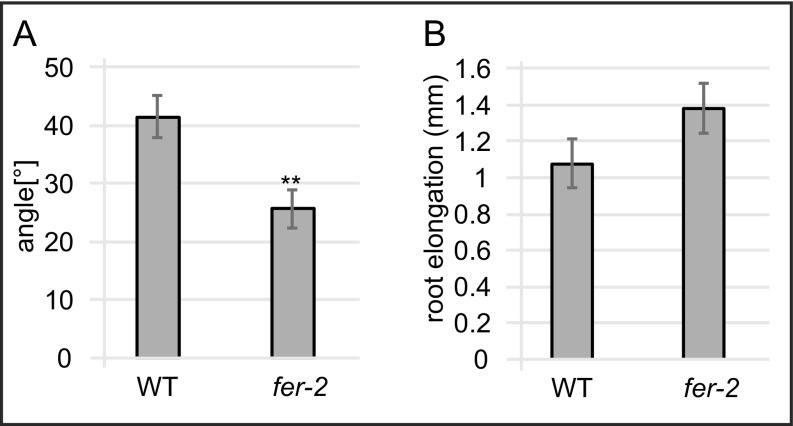
To address whether auxin-induced apoplast alkalization plays a role in this physiological process, we tested the effect of gravistimulation on apoplastic pH in the fer-4 mutant. Compared with WT seedlings, fer-4 mutants display a reduced increase in apoplastic pH at the lower side of the root on gravistimulation (Fig. 5 D–F). In agreement with this compromised cellular response, fer-4 seedling’s root growth was less aligned with the gravity vector, displaying a lower gravitropic index (50) compared with WT seedlings (Fig. 5G). Moreover, 6 h of gravistimulation revealed a reduced gravitropic response in fer-4 mutants compared with WT seedlings (Fig. 5H). In addition, we tested another feronia loss-of-function allele, fer-2, for its responsiveness to a gravitropic stimulus. Similar to fer-4 seedlings, fer-2 seedlings displayed a reduced gravitropic response compared with WT seedlings on 6 h of gravistimulation (Fig. S6A). Importantly, the overall root growth rate is not impaired in fer-4 or fer-2 (Fig. 5I and Fig. S6B), suggesting a specific delay in asymmetric growth responses.
Fig. S6.
(A) Gravitropic response of 4-d-old fer-2 and WT seedlings gravistimulated for 6 h (90°). y-axis shows the mean angle of the root tip with respect to the horizontal (base of the root) as depicted in the schematic. (B) Root elongation of 4-d-old seedlings gravistimulated for 6 h (90°). (A and B) Error bars represent SEM. (n ≥ 26 roots per line). Student t test P values: *P < 0.05, **P < 0.01, ***P < 0.001.
These data strongly suggest that auxin-triggered transient apoplast alkalization and the consequent repression of cellular expansion are required during the initial stages of the gravitropic response (Fig. S4).
Discussion
Plant cells are surrounded by a rigid cell wall, which needs to undergo loosening to allow cellular expansion. The long-standing acid growth theory postulates that the plant hormone auxin activates PM-localized proton pumps, decreasing the pH of the intercellular space. This apoplast acidification activates cell wall-loosening enzymes, enabling cell expansion (2–4). Subsequent studies confirmed the acid growth theory in shoot tissues of numerous species including Avena coleoptiles, pea stem, and maize coleoptiles, as well as Arabidopsis thaliana hypocotyls (2, 9–13). In roots, however, the acid growth theory has been intensely debated during the last decades. On one hand, previous studies demonstrated the stimulating effect of apoplast acidification on root elongation, as well as the requirement for functional PM proton pumps for root growth (14–16). In addition, low auxin concentrations are described to have a positive effect on root growth (6, 7). However, the root growth-promoting effect of low auxin concentrations remains controversial, as only a few laboratories were able to observe this presumably condition-dependent phenomenon. On the other hand, opponents raise the argument that auxin in higher concentrations inhibits, rather than stimulates, root cell expansion, as well as overall root growth (8, 17, 51). Moreover, although exogenous auxin application triggers apoplast acidification in shoots, it was proposed to trigger the opposite in roots (18–20). Interestingly, a recent study on B. distachion roots provides arguments both for and against the acid growth theory (21). Altogether, the contradictory conclusions obtained from previous studies point to a highly complex regulation of apoplastic pH homeostasis in roots. Moreover, the overall role of auxin in this physiological process is currently only poorly understood. A possible reason for this partially contradictory information may be the limited number of molecular tools available to tackle this biological question. Apoplastic pH has been previously investigated using pH-sensitive fluorescent dye combinations (48, 52–55), as well as by the transgenic expression of pH-sensitive fluorescent proteins facing the apoplast (20). Even though these approaches substantially increased our physiological insight into apoplastic pH homeostasis, they still face technical limitations in easily assessing apoplastic pH at a cellular resolution and/or assessing a large number of genotypes.
Here we present HPTS as a versatile pH indicator enabling apoplastic pH assessment at a cellular resolution. We show, via pharmacologic and genetic modulation of PM-localized proton pump activity, that HPTS reliably reports on subtle changes in apoplastic pH. In A. thaliana roots, we observed a decrease in apoplastic pH around expanding cells. Moreover, we show that media-driven acidification of the apoplast triggered root cell expansion, whereas alkalization prevented it. These data suggest that root cell expansion in A. thaliana is driven by apoplast acidification. Interestingly, recent literature reports a negative effect of apoplast acidification on cell expansion in Brachipodium roots (21). The reason for these opposing observations may be a result of the different duration of pharmacological treatments. In our study, we decided to assess the short-term effects of apoplast acidification on cell expansion to avoid secondary effects, which may complicate the interpretation of the experimental outcome. Previous literature demonstrates that the stimulating effect of apoplast acidification on cell expansion is transient (56, 57). In addition, long-term acidity is known to affect nutrient availability and is correlated with a large energy cost to maintain the cellular influx of necessary cations (58–60). On root gravistimulation, we demonstrate the biological relevance of a short/transient asymmetrical apoplast alkalization, which enables differential tissue growth, and ultimately, root bending.
Using HTPS, we show that apoplast acidification is important for root cell expansion and requires nuclear auxin signaling. Consistent with our data, a recent study on A. thaliana hypocotyls reported that auxin-induced hypocotyl growth correlates with local apoplast acidification and requires nuclear auxin signaling (13). Moreover, another study reported a correlation between reduced auxin influx and altered PM H+-ATPase phosphorylation status, resulting in reduced proton protrusion capacity and impaired root growth (61). These recently published findings support our data in suggesting that endogenous auxin levels may be required for apoplastic pH homeostasis, which is important for sustainable cell elongation during root growth (Fig. S4). In addition, we demonstrate that growth inhibitory concentrations of auxin trigger fast transient root apoplast alkalization and subsequent acidification. Our data suggest that the initial auxin-triggered apoplast alkalization is causal for the inhibition of root cell expansion (Fig. S4). It remains to be seen whether the effect of inhibitory auxin concentrations on the root apoplastic pH is indeed the result of altered PM H+-ATPase activity or whether other molecular components also contribute to the observed dynamics.
To address the biological relevance of high auxin concentrations, we assessed the apoplastic pH on root gravitropism. During root gravistimulation, auxin is known to accumulate at the inner (lower) side of the root (62). We show an asymmetric apoplast alkalization at the inner side of gravistimulated roots, which presumably inhibits cell elongation and enables root bending (Fig. S4).
It has been previously shown that auxin-triggered apoplast acidification in hypocotyls is mediated by the rapid up-regulation of SAUR19 expression, resulting in the activation of PM H+-ATPases (12). On GFP-SAUR19 induction, we also observed increased epidermal root cell elongation. Notably, no molecular players have been attributed to auxin-triggered apoplast alkalization so far. Here we show that auxin-triggered apoplast alkalization requires the PM-localized receptor-like kinase FERONIA. FERONIA was identified as an important mediator during pollen tube reception, as well as sperm cell release (63–65). Later studies reported that FERONIA, on binding to its ligand RALF, triggers phosphorylation of Ser899 of the PM H+-ATPase AHA2 (46). This phosphorylation inhibits the proton secretion capacity of the proton pump, resulting in alkalization of the apoplast (46). Intriguingly, auxin-induced root hair elongation is abolished in the absence of FERONIA (66). We demonstrate the requirement of FERONIA for auxin-triggered apoplast alkalization on exogenous auxin treatment, as well as during root gravistimulation. Moreover, fer-4 loss-of-function mutants displayed a delayed response to gravitropic stimulus compared with WT seedlings. Altogether, our data suggest a role for auxin-triggered apoplast alkalization in root gravitropic response. Intriguingly, it has been previously described that the RALFs, which are ligands of FERONIA, are involved in cell wall integrity maintenance. In particular, RALF4 seems coregulated with pectin-modifying enzymes (67), such as pectin methyl esterases (PMEs). PMEs play a role in cell wall modifications that are associated with growth transitions (68, 69) and display an alkaline pH optimum. A notable adverse effect of their enzymatic activity is cell wall acidification, which in turn inhibits PME activity. It needs to be seen whether auxin-triggered apoplast alkalization, for example during gravitropism, activates PMEs. The PME activity would subsequently lead to cell wall acidification, resulting in a PME inhibitory effect that keeps the cell wall modification process transient. Such a process would result in the local inhibition of root cell elongation, enabling efficient gravitropic response without overbending of the root.
Conclusion
In this work, we have introduced HPTS as a suitable dye for assessing apoplastic pH at a cellular resolution. Our data, obtained by HPTS-based analysis, suggest nuclear auxin signaling is required for apoplast acidification important for root cell expansion. In addition, we show that high auxin levels transiently trigger root apoplastic alkalization. Finally, we show that auxin-triggered apoplast alkalization requires FERONIA, which is important for the root gravitropic response.
Altogether, our data suggest a model in which auxin plays a complex, concentration-dependent role in apoplastic pH homeostasis during A. thaliana growth and development. This hypothesis is in line with previous literature reporting that low auxin concentrations stimulate, whereas high auxin concentrations inhibit, root growth (6–8). This view may clarify previously published contradictory results, which either supported or opposed the acid growth theory in the root. In summary, our work provides a versatile tool as well as mechanistic insight into apoplastic pH regulated root growth.
Materials and Methods
We used A. thaliana of the ecotype Colombia 0. Seeds were sterilized with 70% ethanol and stratified at 4 °C for 2 d in the dark on in vitro growth plates. Seedlings were grown vertically on half Murashige and Skoog medium [per liter: 2.16 g MS salts (Duchefa), 4.7 mM Mes, 0.8% plant agar]. Plants were grown under long-day (16 h light/8 h dark) conditions at 21 °C/18 °C. The following lines have been previously described: ost2-2 (32), DII-Venus (70), tir1-1/afb2-1/afb3-1 (49), arf10-2/arf16-2 (42), feronia-4 (66), feronia-2 (71). pER8 and pER8:YUC6 (72), pER10:bdl (40), pER8:GFP:SAUR19 (12), and R2D2 and DR5v2::n3GFP (36). The raw data files of the presented experiments have been uploaded to the public database datadryad.org (doi: 10.5061/dryad.sq7s3).
More detail can be found in SI Materials and Methods.
SI Materials and Methods
Plant Material and Growth Conditions.
We used A. thaliana of the ecotype Colombia 0. Seeds were sterilized with 70% ethanol and stratified at 4 °C for 2 d in the dark on in vitro growth plates. Seedlings were grown vertically on half Murashige and Skoog medium [per liter: 2.16 g MS salts (Duchefa), 4.7 mM Mes, 0.8% plant agar]. Plants were grown under long-day (16 h of light/8 h of dark) conditions at 21 °C/18 °C. For experiments with estradiol-inducible lines, 4-d-old estradiol-inducible lines were induced by transfer on agar plates supplemented with 5 μM estradiol (dissolved in DMSO) along with a control line harboring an empty vector. The following lines have been previously described: ost2-2 (32), DII-Venus (70), tir1-1/afb2-1/afb3-1 (49), arf10-2/arf16-2 (42), feronia-4 (66), feronia-2 (71). pER8 and pER8:YUC6 (72), pER10:bdl (40), pER8:GFP:SAUR19 (12), and R2D2 and DR5v2::n3GFP (36).
Chemicals.
Indole-3-actic acid was supplied by Duchefa, and fusicoccin, DCCD, propidium iodide, estradiol, and HPTS by Sigma Aldrich.
Chemical Treatments.
Seedlings were treated with fusicoccin by incubating them in 1 mL liquid growth medium supplemented with 5 μM fusicoccin (from 5 mM DMSO stock). Treatments with IAA and DCCD were performed by transferring 4-d-old seedlings to one-half Murashige and Skoog (1/2 MS) in vitro growth plates supplemented with 5 μM DCCD (from 5 mM DMSO stock) or 250 nM IAA (from 250 μM DMSO stock). Mock treatments were performed identically to the chemical treatments with the same amount of solvent.
Propidium Iodide Staining and Imaging.
Four-day-old seedlings were mounted on microscopy slides in liquid growth medium supplemented with 2 μM propidium iodide (from 2 mM water stock). The seedling roots were covered with a coverslip and subsequently imaged using an inverted Zeiss 700 confocal microscope. Fluorescence signals for propidium iodide (PI) staining (excitation, 536 nm; emission peak, 617 nm) were detected with a 20× (air) objective. For imaging the DII-Venus auxin reporter line, PI staining was performed as described earlier. Seedlings were, however, imaged using an inverted Zeiss 710 confocal microscope. Fluorescence signals for PI and GFP (excitation, 488 nm; emission peak, 509 nm) were detected with a 63× (water immersion) objective. Signal intensity and cell size measurements were performed using the Fiji software (fiji.sc), and data were statistically evaluated with Microsoft Excel 2011. The experiments described were performed in at least 3 biological repetitions.
HPTS Staining and Imaging.
HPTS staining in different pH media was performed by incubating 4-d-old seedlings for 30 min in liquid growth medium of the given pH supplemented with 1 mM HPTS (from 100 mM water stock). The seedlings were subsequently mounted in the same growth medium supplemented with HPTS on a microcopy slide and covered with a coverslip. For other experiments, seedlings were mounted on a slice of 1/2 MS growth medium supplemented with 1 mM HPTS and a potential chemical (IAA, DCCD, or fusicoccin) in the stated concentration and subsequently flipped into a plastic Nunc imaging chamber, as described previously (49). Seedling imaging was performed using either an inverted Zeiss 710 confocal microscope equipped with a highly sensitive GaAsP detector (Figs. 1, 3 D and G, 4, and 5 and Fig. S3) or a LEICA SP8 confocal microscope equipped with a white laser and hybrid laser detectors (Figs. 2 and 3 A and G and Figs. S2 and S5). Fluorescent signals for the protonated HPTS form (Excitation 405 nm, emission peak 514 nm), as well as the deprotonated HPTS form [excitation, 458 nm (Zeiss) or 470 (LEICA); emission peak, 514 nm] were detected with a 63× (water immersion) objective. The image analysis was performed using the Fiji software (fiji.sc), and data were statistically evaluated with Microsoft Excel 2011. The experiments described were performed in at least 3 biological repetitions. Notably, to avoid potential technical variability between different experiments, we state absolute apoplastic pH values only when a standard curve was generated during the same experiment. For other experiments, the apoplastic pH values of treated samples or mutants will be given relative to mock-treated or WT seedlings.
Ratiometric Image Conversion.
The analysis was performed in Fiji (28), using the macro language. To shape out the cell walls, the image was processed using Background subtraction and a Laplacian of Gaussian filter. The resulting image was manually thresholded. For noise removal, the original channels were Gauss filtered, and a ratiometric image was created by dividing the pixel values of channel 1 by channel 2. A color LUT was applied to represent the ratios values, and the display was set to the same minimum and maximum values for comparison. The Fiji macro is available under Dataset S1.
Cell Size Measurements.
Epidermal cell sizes have been measured using the Fiji software. Distal (late) meristematic root epidermal cells (meristematic cells that are shortly before elongating) were defined as the 4 cells below the first cell that is twice as long as it is wide. Proximal (early) meristematic cells are the cells in the meristematic zone below these distal meristematic cells. Differentiated epidermal cells were defined as the first 3 cells in the differentiation zone identified as described (71). For statistical analysis, the average values per root have been calculated, and each root was treated as a biological replicate.
Quantitative Analysis of Root Gravitropism.
For the gravitropic response analysis, 4-d-old seedlings were transferred and stretched on 1/2 MS in vitro plates. The plates were turned 90° for 6 h of gravistimulation and subsequently scanned on a flatbed scanner. The angles formed by the root tips relative to the axis perpendicular to the gravity vector were measured. For the gravitropic index analysis, seedlings were grown for 7 d on vertical 1/2 MS plates, as described earlier, and subsequently scanned using a flatbed scanner. The image analysis was performed using the Fiji software (28) (fiji.sc), and data were statistically evaluated with Microsoft Excel 2011. To obtain the gravitropic index, the distance between the root base and the root tip was divided through the total root length (50). The experiments described were performed in at least 3 biological repetitions.
Statistics and Data Analysis.
The sample size (n) is the total number of seedlings analyzed (root growth analysis) per line/conditions. For the pairwise comparison of two experimental groups, statistical analysis has been performed using a Student t test. For comparing apoplastic pH in in the different root zones, a one-way ANOVA test with a Tukey-Kramer post hoc test has been performed. To address the statistical difference between two or more experimental groups, two-way ANOVA tests with Bonferroni post tests have been performed. For all cell biological experiments, the average values per root were considered as a single data point. For the statistical analysis of these experiments, the relative data points of the biological repetitions have been merged. For the gravitropic index, root gravitropism, and root growth experiments, statistics have been performed on the data of single experiments (n ≥ 23 roots per experiment), and a representative experiment is shown in the figures. All statistical analysis was performed in the GraphPad Prism5 software (www.graphpad.com). We included the data of all performed experiments in our analysis and emphasize that we did not exclude potential outliers.
Supplementary Material
Acknowledgments
We thank Herman Höfte, Clara Sanchez Rodriguez, Paul Larsen, Dolf Weijers, Hong-Quan Yang, William Gray, and Mark Estelle for sharing published material; J. Matthew Watson for English editing; Juergen Kleine-Vehn for expertise, access to scientific equipment, and the kind donation of the pMDC7B(pUBQ10)GH3.6 and the pMDC7B(pUBQ10)empty lines; Youssef Belkhadir for scientific discussion; the W.B. group for critical reading of the manuscript; Pawel Paserbiek, Tobias Müller, Karin Aumayr, and Gabriele Stengl from the IMP BioOptics Facility; and the VIBT imaging center for their access and expertise. This work was supported by funds from the Austrian Academy of Science through the Gregor Mendel Institute (W.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The raw data files of the presented experiments have been uploaded to the Dryad database, datadryad.org (doi: 10.5061/dryad.sq7s3).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1613499114/-/DCSupplemental.
References
- 1.Hager A. Role of the plasma membrane H+-ATPase in auxin-induced elongation growth: Historical and new aspects. J Plant Res. 2003;116:483–505. doi: 10.1007/s10265-003-0110-x. [DOI] [PubMed] [Google Scholar]
- 2.Rayle DL, Cleland R. Enhancement of wall loosening and elongation by Acid solutions. Plant Physiol. 1970;46:250–253. doi: 10.1104/pp.46.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hager A, Menzel H, Krauss A. Versuche und Hypothese zur Primärwirkung des Auxins beim Streckungswachstum. Planta. 1971;100:47–75. doi: 10.1007/BF00386886. [DOI] [PubMed] [Google Scholar]
- 4.Cleland R, Haughton PM. The effect of auxin on stress relaxation in isolated Avena coleoptiles. Plant Physiol. 1971;47:812–815. doi: 10.1104/pp.47.6.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Enders TA, Strader LC. Auxin activity: Past, present, and future. Am J Bot. 2015;102:180–196. doi: 10.3732/ajb.1400285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evans MLIH, Estelle MA. Responses of Arabidopsis roots to auxin studied with high temporal resolution: Comparison of wild type and auxin-response mutants. Planta. 1994;194:215–222. [Google Scholar]
- 7.Fu X, Harberd NP. Auxin promotes Arabidopsis root growth by modulating gibberellin response. Nature. 2003;421:740–743. doi: 10.1038/nature01387. [DOI] [PubMed] [Google Scholar]
- 8.Chadwick AV, Burg SP. An explanation of the inhibition of root growth caused by indole-3-acetic Acid. Plant Physiol. 1967;42:415–420. doi: 10.1104/pp.42.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rayle DL, Cleland R. Control of plant cell enlargement by hydrogen ions. Curr Top Dev Biol. 1977;11:187–214. doi: 10.1016/s0070-2153(08)60746-2. [DOI] [PubMed] [Google Scholar]
- 10.Lüthen H, Bigdon M, Böttger M. Reexamination of the Acid growth theory of auxin action. Plant Physiol. 1990;93:931–939. doi: 10.1104/pp.93.3.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takahashi K, Hayashi K, Kinoshita T. Auxin activates the plasma membrane H+-ATPase by phosphorylation during hypocotyl elongation in Arabidopsis. Plant Physiol. 2012;159:632–641. doi: 10.1104/pp.112.196428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spartz AK, et al. SAUR Inhibition of PP2C-D phosphatases activates plasma membrane H+-ATPases to promote cell expansion in arabidopsis. plant cell. 2014;26:2129–2142. doi: 10.1105/tpc.114.126037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fendrych M, Leung J, Friml J. TIR1/AFB-Aux/IAA auxin perception mediates rapid cell wall acidification and growth of Arabidopsis hypocotyls. eLife. 2016;5:5. doi: 10.7554/eLife.19048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moloney MM, Elliott MC, Cleland RE. Acid growth effects in maize roots: Evidence for a link between auxin-economy and proton extrusion in the control of root growth. Planta. 1981;152:285–291. doi: 10.1007/BF00388251. [DOI] [PubMed] [Google Scholar]
- 15.Lado F, DeMichaelis MI, Cerana R, Marre E. Fusicoccin-induced, K+-stimulated proton secretion and acid-induced growth of apical root segments. Plant Sci Lett. 1976;6:5–20. [Google Scholar]
- 16.Haruta M, et al. Molecular characterization of mutant Arabidopsis plants with reduced plasma membrane proton pump activity. J Biol Chem. 2010;285:17918–17929. doi: 10.1074/jbc.M110.101733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sauer M, Robert S, Kleine-Vehn J. Auxin: Simply complicated. J Exp Bot. 2013;64:2565–2577. doi: 10.1093/jxb/ert139. [DOI] [PubMed] [Google Scholar]
- 18.Mulkey TJ, Evans ML. Geotropism in corn roots: Evidence for its mediation by differential Acid efflux. Science. 1981;212:70–71. doi: 10.1126/science.212.4490.70. [DOI] [PubMed] [Google Scholar]
- 19.Monshausen GB, Miller ND, Murphy AS, Gilroy S. Dynamics of auxin-dependent Ca2+ and pH signaling in root growth revealed by integrating high-resolution imaging with automated computer vision-based analysis. Plant J. 2011;65:309–318. doi: 10.1111/j.1365-313X.2010.04423.x. [DOI] [PubMed] [Google Scholar]
- 20.Gjetting KS, Ytting CK, Schulz A, Fuglsang AT. Live imaging of intra- and extracellular pH in plants using pHusion, a novel genetically encoded biosensor. J Exp Bot. 2012;63:3207–3218. doi: 10.1093/jxb/ers040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pacheco-Villalobos D, et al. The effects of high steady state auxin levels on root cell elongation in brachypodium. Plant Cell. 2016;28:1009–1024. doi: 10.1105/tpc.15.01057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han J, Burgess K. Fluorescent indicators for intracellular pH. Chem Rev. 2010;110:2709–2728. doi: 10.1021/cr900249z. [DOI] [PubMed] [Google Scholar]
- 23.Amali AJ, Awwad NH, Rana RK, Patra D. Nanoparticle assembled microcapsules for application as pH and ammonia sensor. Anal Chim Acta. 2011;708:75–83. doi: 10.1016/j.aca.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Kano K, Fendler JH. Pyranine as a sensitive pH probe for liposome interiors and surfaces. pH gradients across phospholipid vesicles. Biochim Biophys Acta. 1978;509:289–299. doi: 10.1016/0005-2736(78)90048-2. [DOI] [PubMed] [Google Scholar]
- 25.Clement NR, Gould JM. Pyranine (8-hydroxy-1,3,6-pyrenetrisulfonate) as a probe of internal aqueous hydrogen ion concentration in phospholipid vesicles. Biochemistry. 1981;20:1534–1538. doi: 10.1021/bi00509a019. [DOI] [PubMed] [Google Scholar]
- 26.Overly CC, Lee KD, Berthiaume E, Hollenbeck PJ. Quantitative measurement of intraorganelle pH in the endosomal-lysosomal pathway in neurons by using ratiometric imaging with pyranine. Proc Natl Acad Sci USA. 1995;92:3156–3160. doi: 10.1073/pnas.92.8.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kašík I, et al. In vivo optical detection of pH in microscopic tissue samples of Arabidopsis thaliana. Mater Sci Eng C. 2013;33:4809–4815. doi: 10.1016/j.msec.2013.07.045. [DOI] [PubMed] [Google Scholar]
- 28.Villiers F, Kwak JM. Rapid apoplastic pH measurement in Arabidopsis leaves using a fluorescent dye. Plant Signal Behav. 2013;8:e22587. doi: 10.4161/psb.22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schindelin J, et al. Fiji: An open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marre E. Fusicoccin: A tool in plant physiology. Annu Rev Plant Physiol. 1979;30:273–288. [Google Scholar]
- 31.Nelson N, Harvey WR. Vacuolar and plasma membrane proton-adenosinetriphosphatases. Physiol Rev. 1999;79:361–385. doi: 10.1152/physrev.1999.79.2.361. [DOI] [PubMed] [Google Scholar]
- 32.Merlot S, et al. Constitutive activation of a plasma membrane H(+)-ATPase prevents abscisic acid-mediated stomatal closure. EMBO J. 2007;26:3216–3226. doi: 10.1038/sj.emboj.7601750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luthen HBM. Induction of elongation in maize coleoptiles by hexachloroiridate and its interrelation with auxin and fusicoccin action. Physiol Plant. 1993;89:77–86. [Google Scholar]
- 34.Staal M, et al. Apoplastic alkalinization is instrumental for the inhibition of cell elongation in the Arabidopsis root by the ethylene precursor 1-aminocyclopropane-1-carboxylic acid. Plant Physiol. 2011;155:2049–2055. doi: 10.1104/pp.110.168476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vanneste S, Friml J. Auxin: A trigger for change in plant development. Cell. 2009;136:1005–1016. doi: 10.1016/j.cell.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 36.Liao CY, et al. Reporters for sensitive and quantitative measurement of auxin response. Nat Methods. 2015;12:207–210. doi: 10.1038/nmeth.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woodward AW, Bartel B. Auxin: Regulation, action, and interaction. Ann Bot (Lond) 2005;95:707–735. doi: 10.1093/aob/mci083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peer WA. From perception to attenuation: Auxin signalling and responses. Curr Opin Plant Biol. 2013;16:561–568. doi: 10.1016/j.pbi.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 39.Hamann T, Benkova E, Bäurle I, Kientz M, Jürgens G. The Arabidopsis BODENLOS gene encodes an auxin response protein inhibiting MONOPTEROS-mediated embryo patterning. Genes Dev. 2002;16:1610–1615. doi: 10.1101/gad.229402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang JY, He SB, Li L, Yang HQ. Auxin inhibits stomatal development through MONOPTEROS repression of a mobile peptide gene STOMAGEN in mesophyll. Proc Natl Acad Sci USA. 2014;111:E3015–E3023. doi: 10.1073/pnas.1400542111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chandler JW. Auxin response factors. Plant Cell Environ. 2016;39:1014–1028. doi: 10.1111/pce.12662. [DOI] [PubMed] [Google Scholar]
- 42.Wang JW, et al. Control of root cap formation by MicroRNA-targeted auxin response factors in Arabidopsis. Plant Cell. 2005;17:2204–2216. doi: 10.1105/tpc.105.033076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spartz AK, et al. The SAUR19 subfamily of SMALL AUXIN UP RNA genes promote cell expansion. Plant J. 2012;70:978–990. doi: 10.1111/j.1365-313X.2012.04946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spartz AK, et al. Constitutive expression of Arabidopsis SMALL AUXIN UP RNA19 (SAUR19) in tomato confers auxin-independent hypocotyl elongation. Plant Physiol. 2017;173:1453–1462. doi: 10.1104/pp.16.01514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ikeda Y, et al. Local auxin biosynthesis modulates gradient-directed planar polarity in Arabidopsis. Nat Cell Biol. 2009;11:731–738. doi: 10.1038/ncb1879. [DOI] [PubMed] [Google Scholar]
- 46.Haruta M, Sabat G, Stecker K, Minkoff BB, Sussman MR. A peptide hormone and its receptor protein kinase regulate plant cell expansion. Science. 2014;343:408–411. doi: 10.1126/science.1244454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muday GK. Auxins and tropisms. J Plant Growth Regul. 2001;20:226–243. doi: 10.1007/s003440010027. [DOI] [PubMed] [Google Scholar]
- 48.Fasano JM, et al. Changes in root cap pH are required for the gravity response of the Arabidopsis root. Plant Cell. 2001;13:907–921. doi: 10.1105/tpc.13.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dharmasiri N, Dharmasiri S, Estelle M. The F-box protein TIR1 is an auxin receptor. Nature. 2005;435:441–445. doi: 10.1038/nature03543. [DOI] [PubMed] [Google Scholar]
- 50.Grabov A, et al. Morphometric analysis of root shape. New Phytol. 2005;165:641–651. doi: 10.1111/j.1469-8137.2004.01258.x. [DOI] [PubMed] [Google Scholar]
- 51.Zhao Y, et al. A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science. 2001;291:306–309. doi: 10.1126/science.291.5502.306. [DOI] [PubMed] [Google Scholar]
- 52.Bibikova TN, Jacob T, Dahse I, Gilroy S. Localized changes in apoplastic and cytoplasmic pH are associated with root hair development in Arabidopsis thaliana. Development. 1998;125:2925–2934. doi: 10.1242/dev.125.15.2925. [DOI] [PubMed] [Google Scholar]
- 53.Fuglsang AT, et al. Arabidopsis protein kinase PKS5 inhibits the plasma membrane H+ -ATPase by preventing interaction with 14-3-3 protein. Plant Cell. 2007;19:1617–1634. doi: 10.1105/tpc.105.035626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Monshausen GB, Bibikova TN, Messerli MA, Shi C, Gilroy S. Oscillations in extracellular pH and reactive oxygen species modulate tip growth of Arabidopsis root hairs. Proc Natl Acad Sci USA. 2007;104:20996–21001. doi: 10.1073/pnas.0708586104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shih HW, DePew CL, Miller ND, Monshausen GB. The cyclic nucleotide-gated channel CNGC14 regulates root gravitropism in Arabidopsis thaliana. Curr Biol. 2015;25:3119–3125. doi: 10.1016/j.cub.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 56.Kazama H, Katsumi M. Biphasic response of cucumber hypocotyl sections to auxin. Plant Cell Physiol. 1976;17:467–473. [Google Scholar]
- 57.Vanderhoef LN, Dute RR. Auxin-regulated wall loosening and sustained growth in elongation. Plant Physiol. 1981;67:146–149. doi: 10.1104/pp.67.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Poschenrieder C, Llugany M, Barceló J. Short-term effects of pH and aluminium on mineral nutrition in maize varieties differing in proton and aluminium tolerance. J Plant Nutr. 2008;18:1495–1507. [Google Scholar]
- 59.Hinsinger P, et al. P for two, sharing a scarce resource: Soil phosphorus acquisition in the rhizosphere of intercropped species. Plant Physiol. 2011;156:1078–1086. doi: 10.1104/pp.111.175331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shavrukov Y, Hirai Y. Good and bad protons: Genetic aspects of acidity stress responses in plants. J Exp Bot. 2016;67:15–30. doi: 10.1093/jxb/erv437. [DOI] [PubMed] [Google Scholar]
- 61.Inoue SI, Takahashi K, Okumura-Noda H, Kinoshita T. Auxin influx carrier AUX1 confers acid resistance for Arabidopsis root elongation through the regulation of plasma membrane H+-ATPase. Plant Cell Physiol. 2016;57:2194–2201. doi: 10.1093/pcp/pcw136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen R, Rosen E, Masson PH. Gravitropism in higher plants. Plant Physiol. 1999;120:343–350. doi: 10.1104/pp.120.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huck N, Moore JM, Federer M, Grossniklaus U. The Arabidopsis mutant feronia disrupts the female gametophytic control of pollen tube reception. Development. 2003;130:2149–2159. doi: 10.1242/dev.00458. [DOI] [PubMed] [Google Scholar]
- 64.Rotman N, Gourgues M, Guitton AE, Faure JE, Berger F. A dialogue between the SIRENE pathway in synergids and the fertilization independent seed pathway in the central cell controls male gamete release during double fertilization in Arabidopsis. Mol Plant. 2008;1:659–666. doi: 10.1093/mp/ssn023. [DOI] [PubMed] [Google Scholar]
- 65.Escobar-Restrepo JM, et al. The FERONIA receptor-like kinase mediates male-female interactions during pollen tube reception. Science. 2007;317:656–660. doi: 10.1126/science.1143562. [DOI] [PubMed] [Google Scholar]
- 66.Duan Q, Kita D, Li C, Cheung AY, Wu HM. FERONIA receptor-like kinase regulates RHO GTPase signaling of root hair development. Proc Natl Acad Sci USA. 2010;107:17821–17826. doi: 10.1073/pnas.1005366107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wolf S, Höfte H. Growth control: A saga of cell walls, ROS, and peptide receptors. Plant Cell. 2014;26:1848–1856. doi: 10.1105/tpc.114.125518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peaucelle A, et al. Arabidopsis phyllotaxis is controlled by the methyl-esterification status of cell-wall pectins. Curr Biol. 2008;18:1943–1948. doi: 10.1016/j.cub.2008.10.065. [DOI] [PubMed] [Google Scholar]
- 69.Pelletier S, et al. A role for pectin de-methylesterification in a developmentally regulated growth acceleration in dark-grown Arabidopsis hypocotyls. New Phytol. 2010;188:726–739. doi: 10.1111/j.1469-8137.2010.03409.x. [DOI] [PubMed] [Google Scholar]
- 70.Brunoud G, et al. A novel sensor to map auxin response and distribution at high spatio-temporal resolution. Nature. 2012;482:103–106. doi: 10.1038/nature10791. [DOI] [PubMed] [Google Scholar]
- 71.Deslauriers SD, Larsen PB. FERONIA is a key modulator of brassinosteroid and ethylene responsiveness in Arabidopsis hypocotyls. Mol Plant. 2010;3:626–640. doi: 10.1093/mp/ssq015. [DOI] [PubMed] [Google Scholar]
- 72.Mashiguchi K, et al. The main auxin biosynthesis pathway in Arabidopsis. Proc Natl Acad Sci USA. 2011;108:18512–18517. doi: 10.1073/pnas.1108434108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.