Significance
A relative fall in tissue oxygen levels (hypoxia) is a common feature of many human diseases, including heart failure, lung diseases, anemia, and many cancers, and can compromise normal cellular function. Hypoxia also occurs in healthy humans at high altitude due to low barometric pressures. Human populations resident at high altitude in the Himalayas have evolved mechanisms that allow them to survive and perform, including adaptations that preserve oxygen delivery to the tissues. Here, we studied one such population, the Sherpas, and found metabolic adaptations, underpinned by genetic differences, that allow their tissues to use oxygen more efficiently, thereby conserving muscle energy levels at high altitude, and possibly contributing to the superior performance of elite climbing Sherpas at extreme altitudes.
Keywords: metabolism, altitude, skeletal muscle, hypoxia, mitochondria
Abstract
The Himalayan Sherpas, a human population of Tibetan descent, are highly adapted to life in the hypobaric hypoxia of high altitude. Mechanisms involving enhanced tissue oxygen delivery in comparison to Lowlander populations have been postulated to play a role in such adaptation. Whether differences in tissue oxygen utilization (i.e., metabolic adaptation) underpin this adaptation is not known, however. We sought to address this issue, applying parallel molecular, biochemical, physiological, and genetic approaches to the study of Sherpas and native Lowlanders, studied before and during exposure to hypobaric hypoxia on a gradual ascent to Mount Everest Base Camp (5,300 m). Compared with Lowlanders, Sherpas demonstrated a lower capacity for fatty acid oxidation in skeletal muscle biopsies, along with enhanced efficiency of oxygen utilization, improved muscle energetics, and protection against oxidative stress. This adaptation appeared to be related, in part, to a putatively advantageous allele for the peroxisome proliferator-activated receptor A (PPARA) gene, which was enriched in the Sherpas compared with the Lowlanders. Our findings suggest that metabolic adaptations underpin human evolution to life at high altitude, and could have an impact upon our understanding of human diseases in which hypoxia is a feature.
At high altitude, low barometric pressure is accompanied by a fall in the partial pressure of inspired O2, resulting in hypobaric hypoxia. The cellular response to hypoxia is orchestrated by the hypoxia-inducible factor (HIF) transcription factors, with HIF-1α and HIF-2α, respectively, mediating responses to short-term and more sustained hypoxia (1). In normoxia, prolyl-hydroxylases target HIFα subunits for destruction (2). Under low O2 partial pressures, however, HIF-1α and HIF-2α are stabilized and dimerize with the nuclear HIF-1β subunit. This dimer interacts with hypoxia-response elements in promoter regions to increase expression of specific genes, for example, encoding erythropoietin (EPO) and vascular endothelial growth factor A (VEGFA) (3).
The Tibetan Plateau has an average altitude of some 4,500 m. Humans were first present on the plateau ∼30,000 y ago, with the earliest permanent settlements appearing 6,000–9,000 y ago (4), a period sufficient to drive the natural selection of genetic variants (and associated features) favoring survival and performance in sustained hypoxia (5, 6). Evidence supports the selection of genetic variants encoding components of the HIF pathway, such as EPAS1 (encoding HIF-2α) (7) and EGLN1 [prolyl-hydroxylase-2 (PHD2)] (8) in Tibetan populations. One population, the Sherpas, migrated from Tibet to eastern Nepal ∼500 y ago and exhibits remarkable physical performance at extreme altitude (9).
Although the human adaptive response to hypoxia is incompletely understood, mitigation against the fall in convective O2 delivery plays an important role. In Lowlanders, increased ventilation and cardiac output, as well as the production of more O2-carrying red blood cells, help to sustain O2 delivery and content (10, 11). Likewise, exhaled concentrations of nitric oxide (NO), a key regulator of blood flow, are higher in Tibetans than Lowlanders (12), as are circulating NO metabolites and limb blood flow (13). The rise in red cell mass in response to hypobaric hypoxia is not as great in Tibetans as in Lowlanders, however (14, 15), suggesting that adaptation involves more than just increased O2 delivery. In fact, acclimatization also involves alterations in O2 use. In Lowlander muscle, mitochondrial density declines with sustained exposure to extreme altitude (16–18), whereas exposure to more moderate high altitude is associated with a reprogramming of muscle metabolism (19) even without altered mitochondrial density (20), including down-regulation of electron transfer complexes (19) and tricarboxylic acid (TCA) cycle enzymes (21), loss of fatty acid oxidation (FAO) capacity (19, 20), and improved oxidative phosphorylation (OXPHOS) coupling efficiency (20). Sherpas have lower muscle mitochondrial densities than unacclimatized Lowlanders (22), but little is known of their metabolic adaptation to hypoxia, or any genetic selection that might underpin it. A role has been suggested for peroxisome proliferator-activated receptor α (PPARα), a transcriptional regulator of FAO in liver, heart, and muscle. HIF down-regulates PPARα in some tissues (23), although there is evidence for selection of variants in its encoding gene (PPARA) in some Tibetan subgroups (8, 24). We hypothesized that metabolic adaptation and PPARα, in particular, play a central role in the Sherpa adaptation to hypobaric hypoxia.
Results and Discussion
Selection of PPARA Variants in Sherpas.
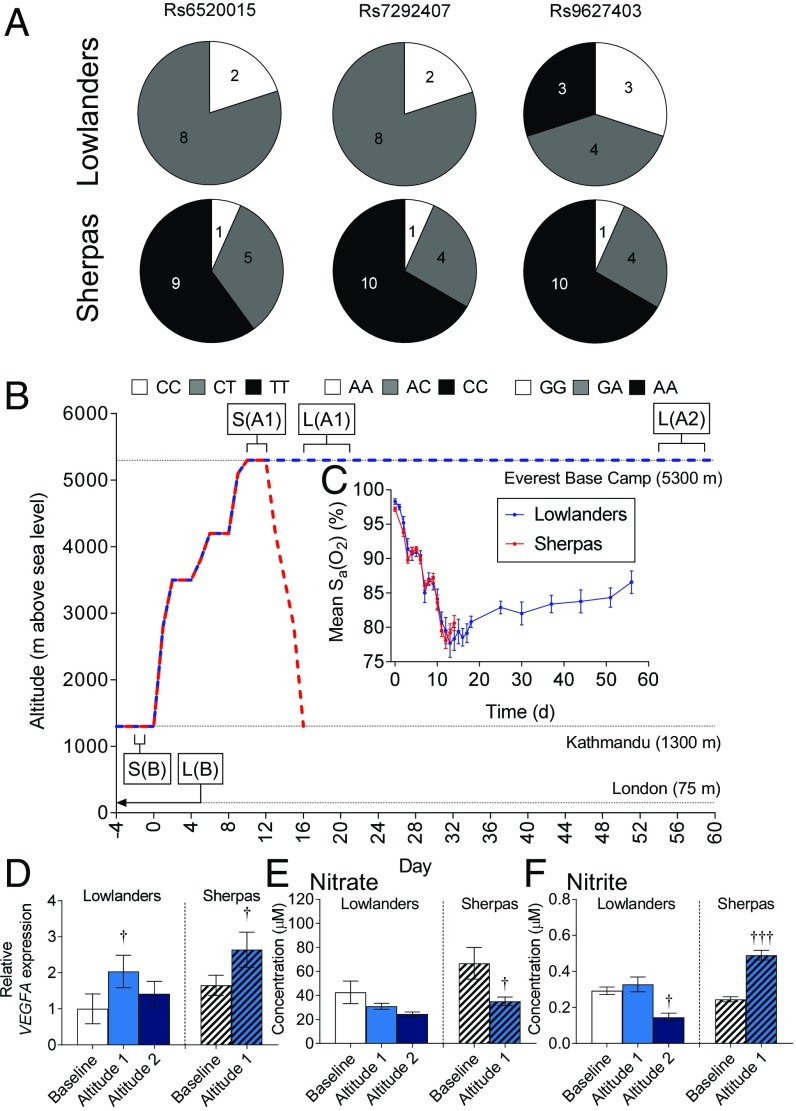
Lowlander and Sherpa subjects were participants of the research expedition, Xtreme Everest 2 (25). The Lowlanders comprised 10 investigators selected to operate the Mount Everest Base Camp (EBC) laboratory. Sherpas (n = 15) were a gender-matched (73% male, compared with 70% in Lowlanders) and age-matched (26.8 ± 1.2 y, compared with 28.0 ± 1.6 y in Lowlanders) group living in Kathmandu and the Solukhumbu and Rolwaling valleys. No subject ascended higher than 4,200 m in the 3 mo preceding the trek, or above 2,500 m in the preceding 3 wk. In addition, Sherpas presented evidence of sole Sherpa ancestry for two generations (i.e., four Sherpa grandparents). The frequency of putatively advantageous PPARA alleles (8) was higher in Sherpas than Lowlanders (Fig. 1A and Table S1), with genotype frequencies of the cohorts being significantly different at two single-nucleotide polymorphisms (SNPs), rs6520015 and rs7292407 (P = 0.0091), although not at rs9627403. This finding reflected patterns reported in some other Tibetan groups (26).
Fig. 1.
Subject genetics, ascent profile, arterial blood O2 saturation, muscle hypoxia, and circulating NO metabolites. (A) Genotypes of Lowlanders and Sherpas at three PPARA SNPs. Subjects homozygous for the putatively advantageous allele are shown in black, heterozygous subjects are shown in gray, and subjects homozygous for the nonadvantageous allele are shown in white (digits in segments refer to the number of subjects with a specific genotype). (B) Ascent profile, including timing of biopsies. A1, early-altitude exposure; A2, late-altitude exposure; B, baseline; L, Lowlanders; S, Sherpas. Arterial hemoglobin-O2 saturations (C), muscle VEGFA expression (D), and plasma nitrogen oxides (E and F) in Lowlanders and Sherpas at baseline and at early and late altitudes are shown. Mean ± SEM (n = 4–15). Sa, arterial blood O2 saturation. †P ≤ 0.05, †††P ≤ 0.001 at B vs. A1 within cohort.
Table S1.
PPARA SNP positions and putatively advantageous alleles
| PPARA SNP | HG 18 position* | Selected allele | Alternate allele | TaqMan SNP Genotyping Assay ID |
| rs9627403 | Chr22: 44827140 | A | G | C_30661738_10 |
| rs7292407 | Chr22: 44832376 | C | A | C_189279291_10 |
| rs6520015 | Chr22: 44842095 | T | C | C_26019862_10 |
SNP positions and alleles are shown as identified by Simonson et al. (8) and by TaqMan SNP Genotyping Assay ID information.
Based on University of California Santa Cruz (UCSC) Genome Browser Human Reference Build 18 (HG 18). Chr, chromosome.
Muscle Hypoxia and Circulating NO Metabolites.
Baseline testing, including blood sampling, muscle biopsy sampling, high-resolution respirometry of permeabilized muscle fibers, and oral glucose tolerance tests (OGTTs), took place in London (35 m) for Lowlanders and in Kathmandu (1,300 m) for Sherpas (25). All subjects then followed an identical ascent (Fig. 1B) from Kathmandu to EBC (5,300 m), whereupon further testing took place at an early time point (A1: 15–20 d postdeparture for Lowlanders, 11–12 d for Sherpas) and a late time point (A2: 54–59 d postdeparture) for Lowlanders only. At the time of sampling, both groups had passed through the acute phase of hypoxic exposure (<24 h) (1) and had been sufficiently exposed to chronic hypoxia for acclimatization to have occurred. Indeed, arterial hemoglobin-O2 saturations were similarly low in both groups (Fig. 1C), whereas muscle expression of the HIF-target VEGFA increased in all subjects (Fig. 1D), indicating a molecular response to hypoxia. Following measurements at the early time point, the Lowlanders remained at EBC for 2 mo to carry out research, presenting an opportunity to collect data pertaining to longer term metabolic acclimatization. Interestingly, VEGFA expression was no longer elevated by this time point, suggesting further acclimatization had occurred.
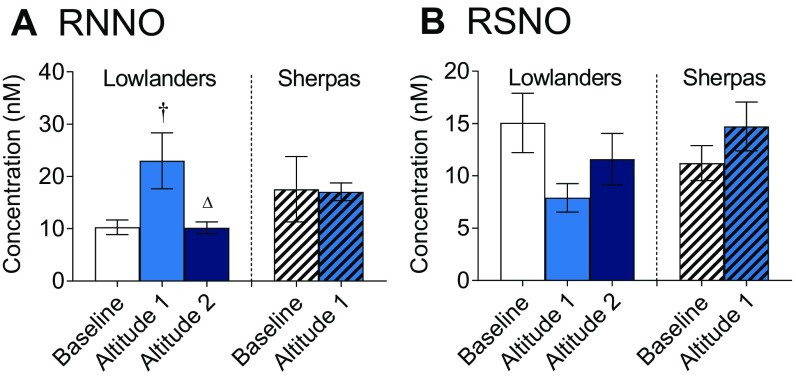
To our surprise, there were no differences in circulating N-nitrosamine (RNNO), S-nitrosothiol, nitrate, or nitrite concentrations between Lowlanders and Sherpas at baseline (Fig. 1 E and F and Fig. S1). In Lowlanders, a transient increase in plasma RNNO levels occurred upon arrival at EBC (P < 0.05) but disappeared by the later time point (Fig. S1A). In Sherpas, plasma nitrate levels fell at altitude (P < 0.05; Fig. 1E) and nitrite levels increased (P < 0.05; Fig. 1F), whereas nitrite levels fell by the later time point (P < 0.05) in Lowlanders. The absence of large differences in NO metabolites between the groups at baseline or at altitude suggested an adaptive phenotype in Sherpas that is distinct from other Tibetan highlanders (13).
Fig. S1.
Circulating nitrogen oxide levels. (A) N-nitrosamine (RNNO) and (B) S-nitrosothiol (RSNO) concentrations in blood plasma of Lowlanders and Sherpas at baseline (B) and at early (A1) and late (A2) altitudes are shown. Mean ± SEM (n = 10–12). †P ≤ 0.05 at B vs. A1 within cohort. △P < 0.001 at A1 vs. A2 within cohort.
Lower FAO Capacity in Sherpas.
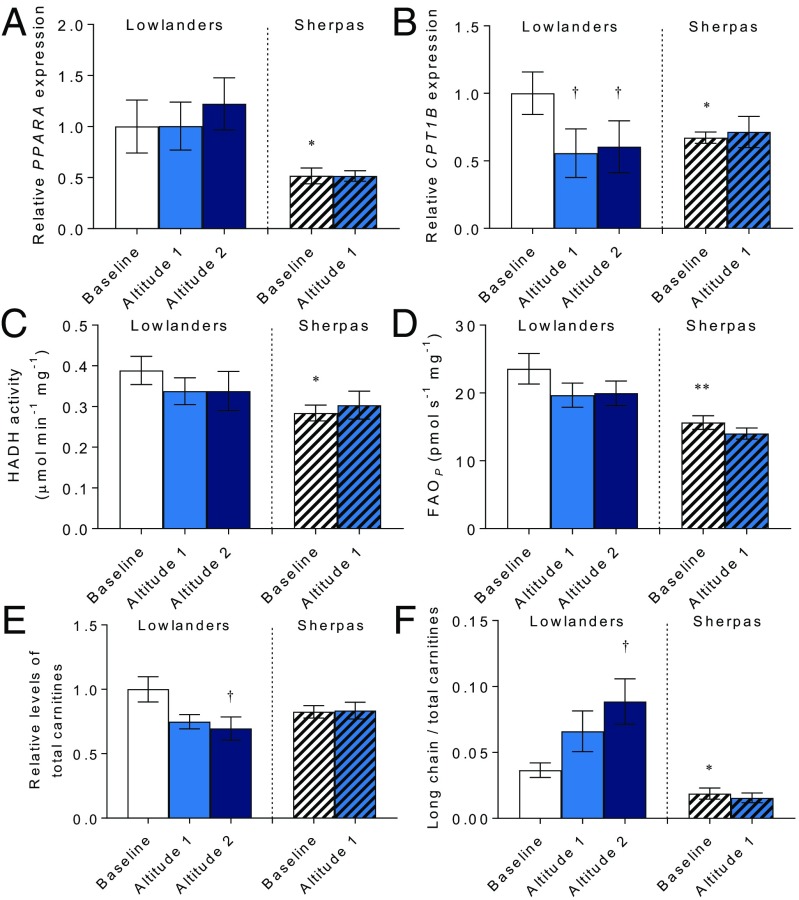
Skeletal muscle biopsies revealed marked differences in gene expression and FAO capacity between Sherpas and Lowlanders. Expression of PPARA mRNA was 48% lower in Sherpas than Lowlanders (P < 0.05; Fig. 2A); thus, the putatively advantageous PPARA allele is associated with diminished expression. Correspondingly, expression of the PPARα target CPT1B was 32% lower in Sherpas at baseline compared with Lowlanders (P < 0.05; Fig. 2B). The PPARA gene contains 139 SNPs. One of the tagging SNPs reported by Simonson et al. (8) is rs6520015; however, it appears to be a noncoding variant. It is thus uncertain whether the SNP itself affects transcriptional regulation or whether it tags a functional variant elsewhere, modifying expression or mRNA stability. Ascent to EBC did not alter PPARA expression in either group; however, despite this finding, CPT1B expression decreased by 44% in Lowlanders (P < 0.05) but did not decrease further in Sherpas. This result suggests that the Lowlander response to hypoxia involves decreased PPARα transcriptional activity without changes in PPARA expression, similar to hypoxic rat skeletal muscle (27).
Fig. 2.
FAO and regulation in muscle. PPARA expression (A), CPT1B expression (B), 3-hydroxyacyl-CoA dehydrogenase (HADH) activity (C), oxidative phosphorylation with octanoyl carnitine and malate (FAOP) (D), total carnitine (E), and long chain/total carnitine ratio (F) in Lowlanders and Sherpas are shown. Gene expression and carnitine levels are expressed relative to Lowlanders at baseline. Mean ± SEM (n = 6–13). *P ≤ 0.05, **P ≤ 0.01 in Lowlanders vs. Sherpas at baseline. †P ≤ 0.05 at baseline vs. altitude within cohort.
Gene expression changes do not necessarily reflect protein levels or activity; therefore, we measured activity of the β-oxidation enzyme 3-hydroxyacyl–CoA dehydrogenase, finding it to be 27% lower in Sherpas than Lowlanders at baseline (P < 0.05), and not changing in either group following ascent (Fig. 2C). Moreover, fatty acid oxidative phosphorylation capacity (FAOP) was measured as the oxygen flux in saponin-permeabilized muscle fibers with octanoyl carnitine, malate, and ADP, using high-resolution respirometry (28). FAOP was 34% lower in Sherpas than Lowlanders at baseline (P < 0.01), and did not change in either group following ascent (Fig. 2D and Fig. S2). Ex vivo measurements may be particular to assay conditions used; therefore, we also measured muscle metabolite levels to indicate changes in metabolism in vivo. Total carnitine concentrations decreased in Lowlanders with time spent at EBC (P < 0.05), although they were not significantly different from total carnitine concentrations in Sherpas at baseline (Fig. 2E). The ratio of long-chain acylcarnitines to total carnitines, however, increased in Lowlanders with time at altitude (P < 0.05; Fig. 2F), suggesting incomplete FAO results in accumulation of potentially harmful lipid intermediates (29). In Sherpa muscle, however, the ratio of long-chain acylcarnitines to total carnitines was lower than in Lowlanders at baseline (P < 0.05), perhaps resulting from lower expression of CPT-1. In further contrast to Lowlanders, the ratio of long-chain acylcarnitines to total carnitines remained low in Sherpa muscle at altitude.
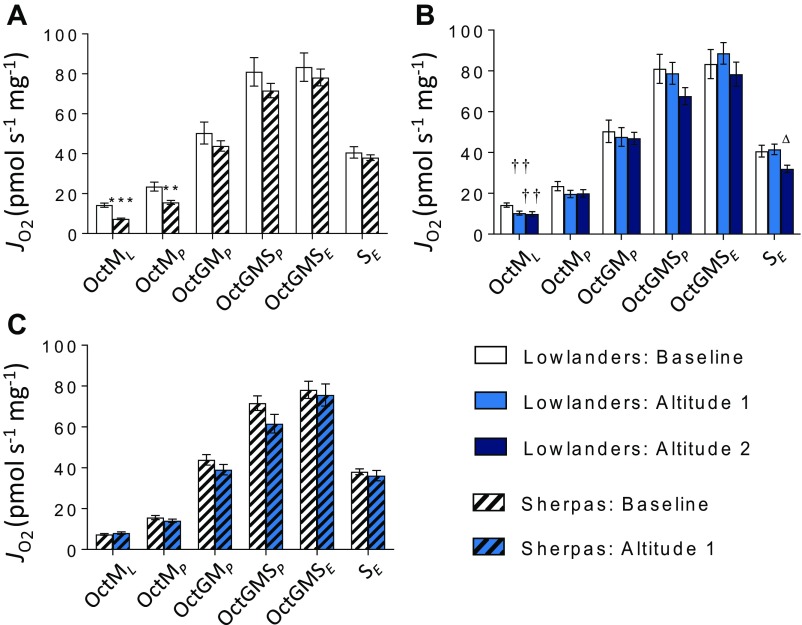
Fig. S2.
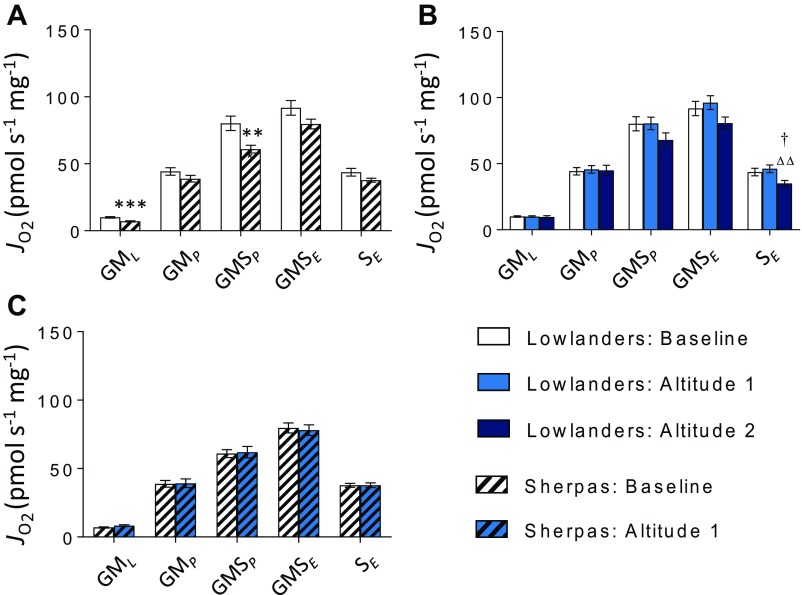
Mitochondrial respiratory function by SUIT protocol 1, in the presence of Oct. FAO-LEAK (OctML), FAO-OXPHOS (OctMP), N-OXPHOS (OctGMP), NS-OXPHOS (OctGMSP), NS-ETS capacity (OctGMSE), and S-ETS capacity (SE) are illustrated. Lowlanders vs. Sherpas at baseline (B) (A), Lowlanders at B and at early-altitude (A1) and late-altitude (A2) time points (B), and Sherpas at B and A1 time points (C). Mean ± SEM (n = 10–11). **P ≤ 0.01, ***P ≤ 0.001 for Lowlanders vs. Sherpas at B. ††P ≤ 0.01 at B vs. A1 within cohort. △P ≤ 0.05 at A1 vs. A2 within cohort. JO2, oxygen flux.
TCA Cycle Regulation at High Altitude.
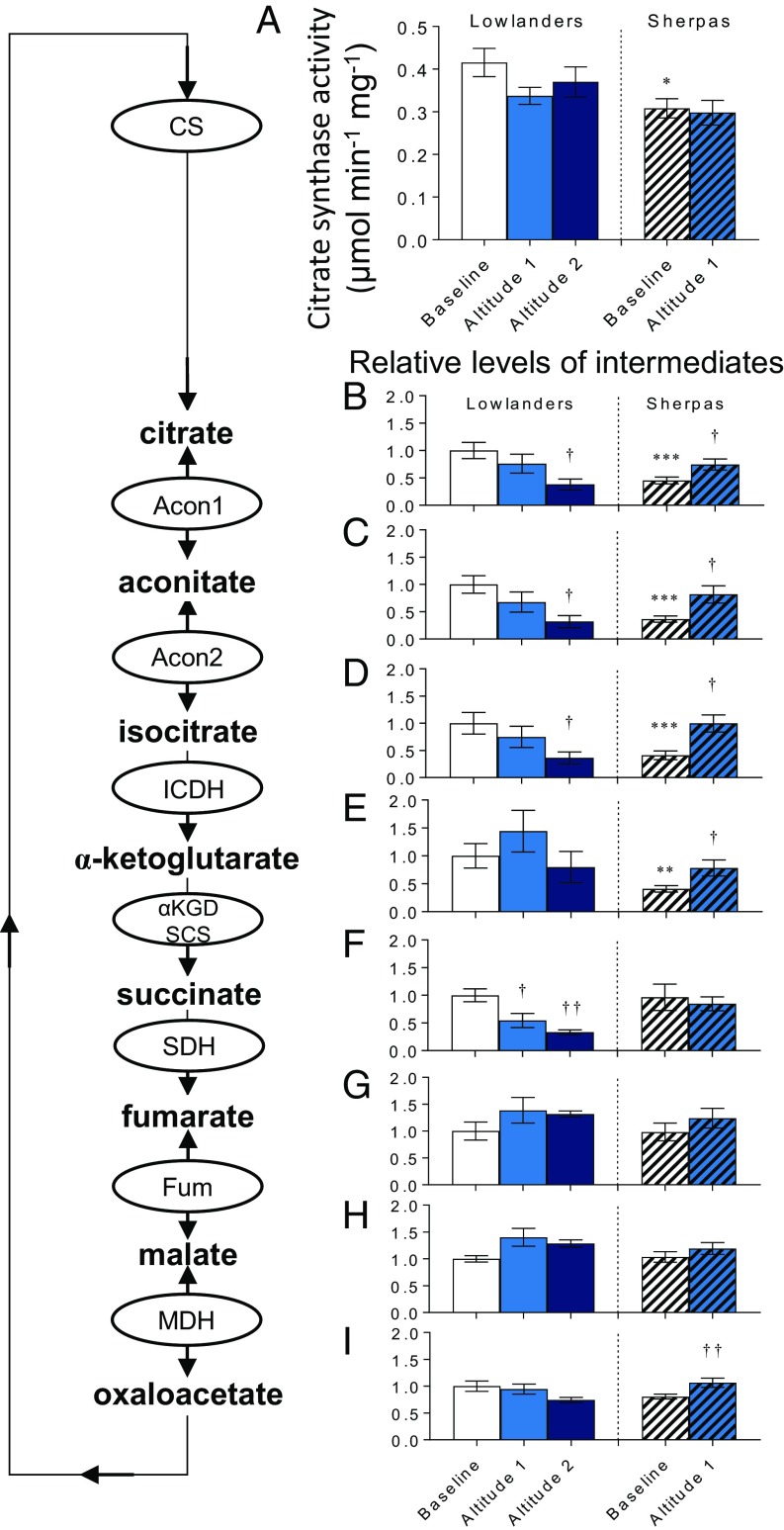
We therefore sought to understand whether there were differences between the populations in other aspects of mitochondrial metabolism. The TCA cycle enzyme citrate synthase (CS) is a candidate marker of mitochondrial content in human muscle (30). At baseline, Sherpas had 26% lower muscle CS activity than Lowlanders (P < 0.05; Fig. 3A), in agreement with findings of 17–33% lower mitochondrial volume density in Sherpa vastus lateralis compared with Lowlanders (22). In accordance with lower CS activity, concentrations of 6- and 5-carbon intermediates downstream of CS (citrate, aconitate, isocitrate, and α-ketoglutarate) were lower in Sherpas than Lowlanders (P < 0.001). However, concentrations of 4-carbon intermediates (succinate, fumarate, malate, and oxaloacetate) were not different (Fig. 3 B–I). This finding suggests an alternative strategy to supply the TCA cycle with succinate. Intriguingly, recent analysis of a large SNP dataset from low- and high-altitude–adapted populations in the Americas and Asia (31) aimed to identify pathways of convergent evolution, and highlighted fatty acid ω-oxidation as the most significant cluster of overlapping gene sets between high-altitude groups (32). ω-Oxidation is normally a minor pathway in vertebrates, becoming more important when β-oxidation is defective (33); through successive cycles, it oxidizes fatty acids to adipate and succinate in the endoplasmic reticulum, after which succinate enters the mitochondria with anaplerotic regulation of the TCA cycle (34).
Fig. 3.
TCA intermediates and activity in muscle. CS activity (A) and TCA cycle intermediates (B–I) in Lowlanders and Sherpas are shown. Metabolite levels are expressed relative to Lowlanders at baseline. Mean ± SEM (n = 7–14). *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 in Lowlanders vs. Sherpas at baseline. †P ≤ 0.05, ††P ≤ 0.01 at baseline vs. altitude within cohort.
Upon ascent to altitude, 6- and 5-carbon TCA cycle intermediates increased in Sherpa muscle (P < 0.05; Fig. 3 B–E), suggesting improved coupling of intermediary metabolism, TCA cycle, and oxidative phosphorylation. In Lowlanders, however, citrate, aconitate, and isocitrate decreased at altitude (P < 0.05; Fig. 3 B–D), despite no significant change in CS activity, perhaps reflecting impairments upstream. Interestingly, α-ketoglutarate concentrations were maintained in Lowlanders at altitude (Fig. 3E), despite decreased succinate downstream, which could be explained by the fall in both α-ketoglutarate dehydrogenase and isocitrate dehydrogenase, as reported previously in Lowlanders following an identical ascent to EBC (21). α-Ketoglutarate plays regulatory roles in hypoxia, including suppression of HIF stabilization (35), but also supporting glutathione synthesis (36). Taken together, these results indicate different TCA cycle regulation in Sherpas and Lowlanders. The replete TCA cycle of Sherpas at altitude contrasts sharply with the depletion of TCA cycle intermediates in Lowlanders, and suggests a coupling of the TCA cycle in Sherpa muscle to its distinct intermediary substrate metabolism.
Greater Mitochondrial Coupling Efficiency in Sherpas.
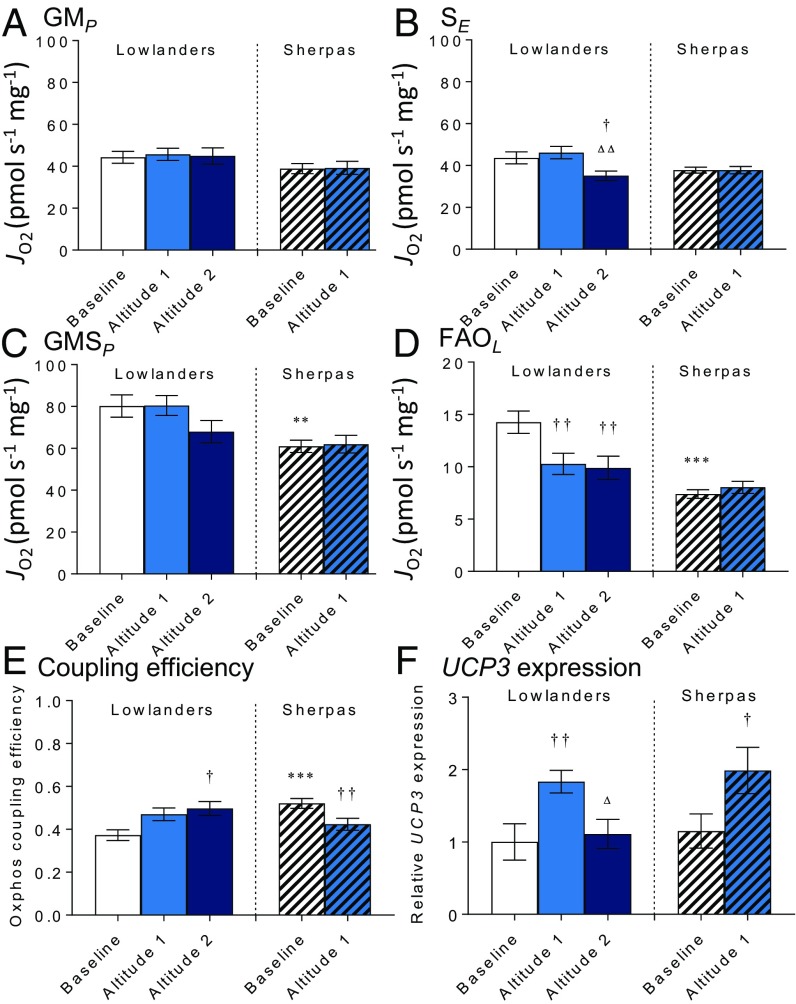
To understand further whether mitochondrial function differs between Sherpas and Lowlanders, we used high-resolution respirometry to probe electron transfer system (ETS) capacity and coupling efficiency in permeabilized muscle fibers. At baseline, there was no significant difference between the two groups in OXPHOS or ETS capacities with either malate and glutamate (N-pathway through complex I) or succinate (S-pathway through complex II; Fig. 4 A and B and Fig. S3) as a substrate, but Sherpas had a lower OXPHOS capacity with malate, glutamate, and succinate combined to reconstitute TCA cycle function (NS pathway; P < 0.01; Fig. 4C). There were no early changes in either group upon ascent. By the later time point, however, succinate-linked respiration had fallen in Lowlanders (P < 0.05), consistent with previous findings of decreased succinate dehydrogenase (complex II) levels in subjects with sustained exposure at >5,300 m (21).
Fig. 4.
Mitochondrial oxygen consumption, efficiency, and uncoupling protein expression. N-OXPHOS (GMP) (A), S-ETS capacity (SE) (B), and NS-OXPHOS capacity (GMSP) (C) in permeabilized muscle fibers from Lowlanders and Sherpas are shown. Octanoyl carnitine and malate-supported LEAK (FAOL) (D) and OXPHOS coupling efficiency (E) are shown. (F) Muscle UCP3 expression relative to Lowlanders at baseline. Mean ± SEM (n = 7–11). **P ≤ 0.01, ***P ≤ 0.001 in Lowlander vs. Sherpas at baseline. †P ≤ 0.05, ††P ≤ 0.01 at baseline vs. altitude within cohort. △P ≤ 0.05, △△P ≤ 0.01 at altitude 1 vs. altitude 2 within cohort. G, glutamate; JO2, oxygen flux; M, malate, S, succinate.
Fig. S3.
Mitochondrial respiratory function by SUIT protocol 2, in the absence of Oct. N-LEAK (GML), N-OXPHOS (GMP), NS-OXPHOS (GMSP), NS-ETS capacity (GMSE), and SE are illustrated in Lowlanders vs. Sherpas at B (A), Lowlanders at B at A1 and A2 time points (B), and Sherpas at B and A1 time points (C). Mean ± SEM (n = 10–11). **P ≤ 0.01, ***P ≤ 0.001 for Lowlanders vs. Sherpas at B. †P ≤ 0.05 at B vs. altitude within cohort. △△P ≤ 0.01 at A1 vs. A2 within cohort.
In addition, we measured muscle fiber respiration in the absence of ADP (LEAK) (i.e., O2 consumption without ADP phosphorylation). Expressing LEAK relative to OXPHOS capacity, it is possible to calculate OXPHOS coupling efficiency (37, 38). At baseline, Sherpa muscle mitochondria had lower LEAK respiration and greater coupling efficiency than Lowlander mitochondria (P < 0.001; Fig. 4 D and E), indicating more efficient use of O2. Upon ascent to EBC and with sustained time at altitude, LEAK decreased in Lowlanders (P < 0.01), although it remained higher than in Sherpas (Fig. 4D), and coupling efficiency improved (P < 0.05; Fig. 4E). In Sherpas at altitude, LEAK did not change, although coupling efficiency decreased (P < 0.01). One possible explanation for these differences in coupling efficiency might be the altered expression of uncoupling protein 3 (UCP3). UCP3 is a transcriptional target of PPARα, and lower UCP3 levels at altitude might improve the efficiency of O2 utilization. In previous studies, however, muscle UCP3 expression increased with acute hypoxia (17, 39), which may offer some protective benefit considering its possible role as an antioxidant (39). Notably, however, UCP3 levels decreased with more sustained exposure to extreme altitude (17). Here, UCP3 was up-regulated in Sherpas at altitude in association with decreased coupling efficiency (P < 0.05; Fig. 4F). However, UCP3 expression also increased in Lowlanders in the short term (P < 0.01), in whom there was decreased LEAK respiration. Moreover, UCP3 expression returned to baseline in Lowlanders with longer term exposure with no further change in LEAK respiration. Overall, our results indicate that Sherpa muscle mitochondria are characterized by a lower OXPHOS capacity and greater, albeit declining, efficiency, whereas the OXPHOS efficiency of Lowlanders improved with acclimatization.
Glycolysis and Glucose Metabolism.
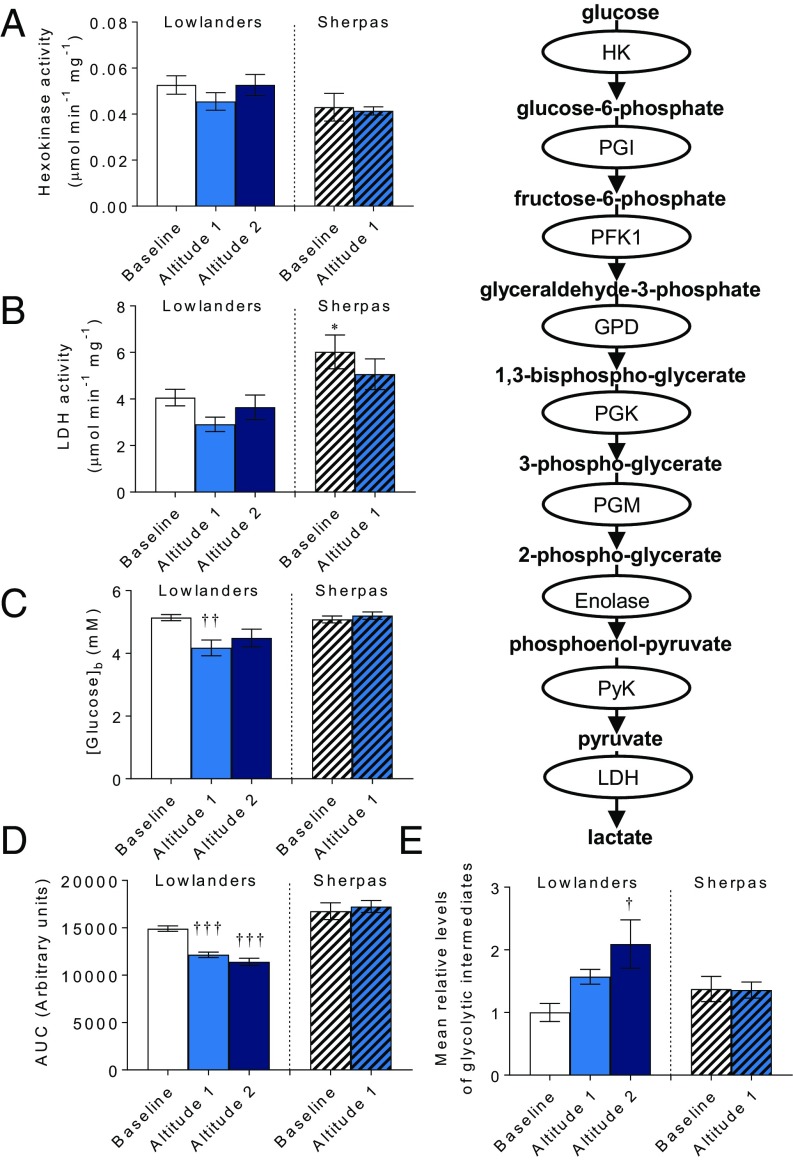
Next, we investigated the capacity to derive cellular energy via glycolysis, which is increased in hypoxic cells (40), because glycolysis may allow ATP levels to be maintained when O2 is limited. Hexokinase activity was the same in both groups at baseline, and did not change at altitude (Fig. 5A); however, lactate dehydrogenase activity was 48% higher in Sherpa muscle than in Lowlanders (P < 0.05), indicating greater capacity for anaerobic lactate production (Fig. 5B). Fasting blood glucose was the same in Sherpas and Lowlanders at baseline, and decreased upon ascent in Lowlanders (P < 0.01; Fig. 5C), who also showed faster clearance of glucose during an OGTT (P < 0.001; Fig. 5D) in agreement with previous reports (41). In Sherpas, however, there was no indication of altered glucose homeostasis. Meanwhile, over time at altitude, glycolytic intermediates increased in Lowlander muscle (Fig. 5E), with increased glucose-6-phosphate/fructose-6-phosphate and 2-phosphoglycerate/3-phosphoglycerate (Table S2). In contrast, total glycolytic intermediates did not change in Sherpa muscle, although 2-phosphoglycerate/3-phosphoglycerate decreased. These findings might be explained to some extent by altered HIF activities. Many genes encoding glycolytic enzymes are up-regulated by HIF-1 (42), whereas hypoglycemia is seen in Chuvash polycythemia (CP), an autosomal recessive disorder in which HIF degradation is impaired (43). Taken together, our findings suggest an increased reliance on glucose by Lowlanders under resting conditions at altitude compared with Sherpas but a greater capacity for lactate production in Sherpas, which may prove effective upon exertion.
Fig. 5.
Muscle glycolysis and blood glucose homeostasis. Hexokinase (A) and lactate dehydrogenase (LDH) (B) activity are shown. Fasting blood glucose (C) and glucose clearance during OGTT (D) are shown. (E) Total muscle glycolytic intermediates relative to Lowlanders at baseline. Mean ± SEM (n = 5–14). AUC, area under the curve. *P ≤ 0.05 in Lowlanders vs. Sherpas at baseline. †P ≤ 0.05, ††P ≤ 0.01, †††P ≤ 0.001 at baseline vs. altitude within cohort.
Table S2.
Relative levels of glycolytic intermediates
| Intermediate | L(B) | L(A1) | L(A2) | S(B) | S(A1) |
| Glucose-6-phosphate and fructose-6-phosphate | 1.00 ± 0.17 | 1.44 ± 0.25 | 3.29 ± 0.95† | 1.33 ± 0.43 | 1.76 ± 0.35 |
| Dihydroxyacetone phosphate | 1.00 ± 0.18 | 1.20 ± 0.25 | 0.86 ± 0.13 | 1.23 ± 0.26 | 1.40 ± 0.12 |
| 2-Phosphoglycerate and 3-phosphoglycerate | 1.00 ± 0.16 | 2.07 ± 0.28† | 2.13 ± 0.22† | 1.56 ± 0.22 | 0.92 ± 0.08†† |
Two pairs of metabolites, glucose- and fructose-6-phosphate and 2- and 3-phosphoglycerate, could not be distinguished from each other, so combined levels are shown. Levels of all intermediates are shown relative to Lowlanders at baseline as mean ± SEM (n = 7–14 per group). A1, early-altitude exposure; A2, late-altitude exposure; B, baseline; L, Lowlanders; S, Sherpas.
P < 0.05, †† P < 0.01 B vs. A1 within cohort.
Energetics and Oxidative Stress.
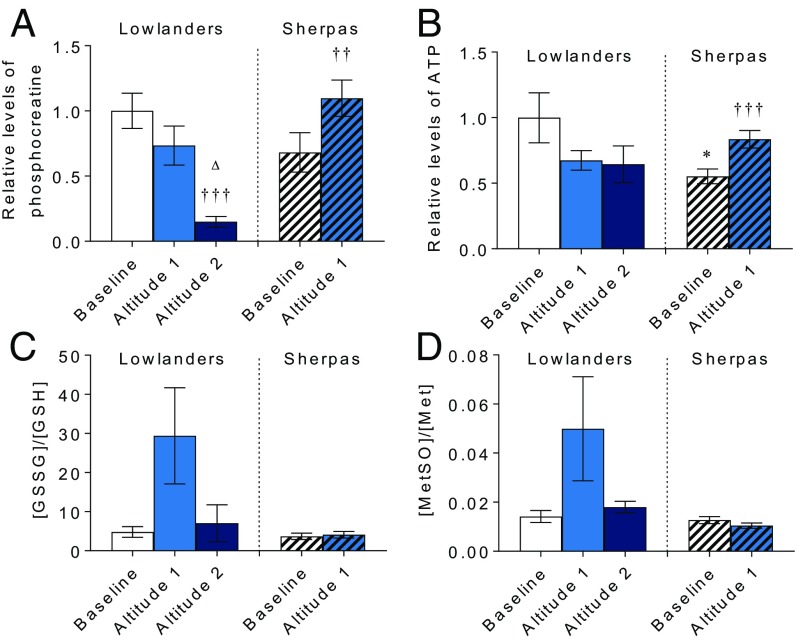
Finally, to understand the implications of Sherpa metabolic adaptation, we investigated muscle energetics and redox homeostasis. At altitude, Lowlanders showed progressive loss of muscle phosphocreatine (PCr; P < 0.001; Fig. 6A), indicating a loss of energetic reserve, which may relate to down-regulation of muscle creatine kinase, as reported previously (21). By contrast, in Sherpa muscle, PCr increased at altitude (P < 0.01). Similarly, Sherpa muscle ATP levels, which were lower than in Lowlanders at baseline (P < 0.05), increased at altitude (P < 0.001; Fig. 6B), illustrating that Sherpa metabolism is better suited to maintaining muscle energetics at altitude than Lowlander metabolism either in the short term or following acclimatization. Moreover, with short-term exposure, markers of oxidative stress (reduced/oxidized glutathione and methionine sulfoxide) increased in Lowlander muscle but not Sherpa muscle (Fig. 6 C and D), indicating superior redox homeostasis in the Sherpas. Antioxidant protection may represent another outcome of convergent evolution, having been reported in Andean subjects in association with protection of fetal growth (44), whereas glutathione levels are raised in CP, suggesting a possible role for HIF activation (45).
Fig. 6.
Muscle energetics and oxidative stress. PCr (A), ATP (B), oxidized/reduced glutathione (GSSG/GSH) (C), and sulfoxide/total methionine (MetSO/Met) (D), all expressed relative to Lowlanders at baseline, are shown. Mean ± SEM (n = 8–14). ††P ≤ 0.01, †††P ≤ 0.001 at baseline vs. altitude within cohort. △P ≤ 0.05 at altitude 1 vs. altitude 2 within cohort.
Conclusions
It has long been suspected that Sherpa people are better adapted to life at high altitude than Lowlanders (46). Recent findings have suggested a genetic basis to adaptation in populations around the world (6), and we show here that Sherpas have a metabolic adaptation associated with improved muscle energetics and protection against oxidative stress. Genetic selection on the PPARA gene is associated with decreased expression, and thus lower fatty acid β-oxidation and improved mitochondrial coupling compared with Lowlanders, with a possible compensatory increase in fatty acid ω-oxidation. Sherpas also have a greater capacity for lactate production. With acclimatization to altitude, Lowlanders accumulate potentially harmful lipid intermediates in muscle as a result of incomplete β-oxidation, alongside depletion of TCA cycle intermediates, accumulation of glycolytic intermediates, a loss of PCr despite improved mitochondrial coupling, and a transient increase in oxidative stress markers. In Sherpas, however, there are remarkably few changes in intermediary metabolism at altitude but increased TCA cycle intermediates and PCr and ATP levels, with no sign of oxidative stress.
Genetic selection, by definition, requires an increased likelihood of advantageous gene variants being passed on to offspring. Hence selection might occur if the disadvantageous variant is associated with poorer survival to reproductive age and beyond, including greater fetal/neonatal mortality. Evidence supports precisely such effects, with fetal growth at altitude being poorer in Lowlander populations than in many native highlanders (47), including Tibetans (48) and Sherpas (49). Likewise, gene variants may affect survival through childhood or fecundity/fertility in the hypoxic environment. We cannot speculate on the mechanism by which PPARA variants prove advantageous; however, PPAR isoforms are expressed in the placenta (50) and influence female reproductive function (51). It would be of interest to seek association of the PPARA variants with birth weight and measures of placentation in high-altitude natives and Lowlanders exposed to hypoxia.
Our findings suggest a metabolic basis to Sherpa adaptation that may permit the population to survive and perform at high altitude. Such adaptations may also underpin the superior performance of elite climbing Sherpas at extreme high altitude.
Materials and Methods
Subjects were selected from the participants of Xtreme Everest 2 (25). All Lowlanders were born and lived below 1,000 m, were not descended from a high-altitude–dwelling population, and were of European (Caucasian) origin. Subjects gave written consent, and underwent medical screening. All protocols were approved by the University College London Research Ethics Committee and Nepal Health Research Council. Vastus lateralis biopsies were taken from the midthigh, muscle fibers were prepared for respirometry (28), and respiration was measured using substrate-uncoupler-inhibitor titrations (Tables S3 and S4). Enzyme activities were assayed as described elsewhere (27). RNA was extracted, and Taqman assays were used to analyze gene expression (Table S5). For metabolite analysis, a methanol/chloroform extraction (52) was followed by liquid chromatography mass spectrometry. OGTTs were carried out on fasted subjects on the day after biopsies. Blood plasma NO metabolites were quantified as described (53). Genomic DNA was isolated from whole blood, and PPARA SNPs were genotyped using TaqMan assays for allelic discrimination (Applied Biosystems; Table S1). To compare cohorts at baseline, an unpaired, two-tailed Student’s t test was used (significance at P ≤ 0.05). Genotype frequencies were compared using a χ2 test. To assess the effects of altitude, a one-way ANOVA with repeated measures was used. Post hoc pairwise comparisons were carried out with a Tukey correction.
Table S3.
SUIT protocol 1
| No. | SUIT | State | Figs. |
| 1 | Malate, 5 mM | Fig. 4D and Fig. S2 | |
| Octanoyl carnitine, 0.2 mM | OctML | ||
| 2 | ADP, 10 mM* | OctMP | Fig. 2D and Fig. S2 |
| 3 | Glutamate, 10 mM | OctGMP | Fig. S2 |
| 4 | Succinate, 10 mM | OctGMSP | Fig. S2 |
| 5 | Cytochrome c, 10 μM | OctGMScP | |
| 6 | FCCP, 0.25–1.5 μM† | OctGMSE | Fig. S2 |
| 7 | Rotenone, 0.5 μM | SE | Fig. S2 |
Reagents were added in the order 1–7 (stub column) to give the final concentrations shown. c, cytochrome c; L, LEAK state respiration; P, OXPHOS state respiration; SE, S-ETS capacity.
The minimum amount of ADP added was 10 mM. Higher concentrations were required to reach saturation in some cases.
FCCP was titrated in 0.25 μM steps until an inhibitory effect was observed.
Table S4.
SUIT protocol 2
| No. | SUIT | State | Figs. |
| 1 | Malate, 5 mM | ||
| Glutamate, 10 mM | GML | Fig. S3 | |
| 2 | ADP, 10 mM* | GMP | Fig. 4A and Fig. S3 |
| 3 | Cytochrome c, 10 μM | GMcP | |
| 4 | Succinate, 10 mM | GMSP | Fig. 4C and Fig. S3 |
| 5 | FCCP, 0.25–1.5 μM† | GMSE | Fig. S3 |
| 6 | Rotenone, 0.5 μM | SE | Fig. 4B and Fig. S3 |
Reagents were added in the order 1–6 (stub column) to give the final concentrations shown.
The minimum amount of ADP added was 10 mM. Higher concentrations were required to reach saturation in some cases.
FCCP was titrated in 0.25 μM steps until an inhibitory effect was observed.
Table S5.
Details of Taqman assays selected to assess gene expression
| Gene | Amplicon size | Assay no. |
| ACTB* | 63 | Hs01060665_g1 |
| HPRT1* | 82 | Hs02800695_m1 |
| PPIA* | 97 | Hs04194521_s1 |
| RNA18S* | 90 | Hs03928985_g1 |
| VEGFA | 59 | Hs00900055_m1 |
| PPARA | 62 | Hs00947536_m1 |
| UCP3 | 74 | Hs01106052_m1 |
| CPT1B | 133 | Hs03046298_s1 |
Housekeeping genes used as controls for normalization of target genes.
SI Materials and Methods
Study Design.
The design and conduct of Xtreme Everest 2 have been described previously (25). Healthy Lowlanders (n = 10, seven male) and healthy age- and gender-matched Sherpas (n = 15, 11 male) were selected from recruited participants. All Lowlander subjects were born and lived below 1,000 m, were not descended from a high-altitude–dwelling population (e.g., Tibetan, Andean, Ethiopian), and were of European (Caucasian) origin. These subjects were Xtreme Everest 2 investigators selected to be resident at the EBC laboratory throughout the expedition for the purpose of conducting research. The researchers were selected on the basis of their availability and ability to contribute to the scientific program of the expedition, but not due to any proven ability to perform at high altitude; indeed, a number of these subjects were altitude-naive at the time of departure. Sherpa subjects were drawn from communities in the Solokhumbu and Rolwaling valleys and were required to provide evidence that all parents and grandparents were Nepali Sherpas. Two of the Sherpa subjects were first cousins, but no other subjects (Sherpa or Lowlander) were related.
Subjects gave written consent for participation, and were subjected to medical screening before the expedition, which involved completion of a health questionnaire and a check-up with the chief medical officer. Potential participants with serious cardiac or respiratory disease were excluded. A local, medically qualified translator was present at all times to ensure effective communication between scientific investigators and Sherpa subjects. All protocols were approved by the University College London Research Ethics Committee and the Nepal Health Research Council. All subjects from both cohorts were free from altitude exposure for at least 3 mo before the expedition and were physically active, but neither particularly sedentary nor highly trained. Subjects were flown from Kathmandu, Nepal (1,300 m) to Lukla in the Solokhumbu region (2,800 m), before ascending on foot to EBC (5,300 m) by a matched 10-d ascent profile. Diet was not strictly controlled; however, all subjects were presented with similar communal fare at tea houses, lodges, and camps throughout the expedition, and this diet did not include large quantities of foods known to be rich in nitrogen oxides, such as green leafy vegetables or cured meats.
Muscle Sample Collection and Preparation.
Biopsies of the vastus lateralis muscle were taken from the midthigh using Tilley–Henckel forceps under local anesthesia (2% lignocaine, 1:80,000 adrenaline) of the skin and superficial muscle fascia. A 5-mm incision was made, and 150 mg of wet-weight tissue was collected, with repeat biopsies taken adjacent to previous biopsies. Sherpa biopsies were taken in Kathmandu and again 11–12 d after departure (1–2 d at 5,300 m). Lowlander biopsies were taken in London (35 m) before the expedition, 15–20 d after departure (5–10 d at 5,300 m), and again 54–59 d after departure (44–49 d at 5,300 m). Atmospheric parameters from the three laboratories have been reported elsewhere (25). The London and Kathmandu biopsies were taken to assess baseline metabolic profile. Thereafter, biopsies were taken at EBC within 21 d of the start of the ascent to assess the effects of shorter term high-altitude exposure on metabolism, whereas biopsies taken after 55 d indicated the effects of more sustained high-altitude exposure.
Ideally, biopsies would have been carried out on the subjects at the same times following the onset of exposure; however, Sherpas and Lowlanders were studied on different days after arrival at EBC for logistical reasons. The Lowlander subjects, being Xtreme Everest 2 investigators, needed to establish camp; construct the laboratory; and unpack, calibrate, and validate equipment for high-resolution respirometry upon arrival. Because these measurements cannot be made on frozen samples, biopsy sampling only occurred once respirometry could be carried out. The Sherpa subjects, however, arrived and departed within guided trekking groups after the laboratory had been established. These treks followed preordained ascent/descent schedules to and from Kathmandu, with the subjects spending three nights at EBC. There was therefore a narrow window of opportunity during which Sherpas could be studied. Following measurements at the high-altitude exposure time point, the Lowlander subjects were scheduled to remain at EBC for a further 2 mo for the purpose of carrying out research, presenting us with the additional opportunity of collecting further valuable data pertaining to longer term metabolic acclimatization to hypobaric hypoxia in a group resident at 5,300 m. It was not, however, possible for us to collect comparable longer term data for the Sherpa subjects on this expedition, although we acknowledge that such data would have been of interest.
No food or caffeine was allowed within the 12 h preceding each biopsy. The muscle sample was immediately placed in ice-cold biopsy preservation medium (BIOPS) [CaK2EGTA (2.77 mM), K2EGTA (7.23 mM), MgCl2⋅6H2O (6.56 mM), taurine (20 mM), PCr (15 mM), imidazole (20 mM), DTT (0.5 mM), MES (50 mM), and Na2ATP (5.77 mM) at pH 7.10], which was filtered and stored at −40 °C or lower until use to prevent bacterial growth. Following this procedure, the muscle sample was cleared of any fat or connective tissue and divided into sections as follows: 15 mg was snap-frozen in liquid nitrogen for metabolomics, 20 mg was snap-frozen in liquid nitrogen for gene expression and enzyme activity assays, and 50 mg was retained in ice-cold biopsy preservation medium for high-resolution respirometry. Frozen samples were flown back to the United Kingdom on liquid nitrogen and stored at −80 °C until use.
Measurement of NO Metabolites.
Venesection was performed for the measurement of circulating biomarkers. Plasma was separated from blood cells by centrifugation of whole blood at 800 × g for 15 min and immediately frozen in 1-mL aliquots in liquid nitrogen. Samples were stored under liquid nitrogen for the duration of the expedition, transported back to the United Kingdom on dry ice, and kept at −80 °C until analysis.
NO metabolite concentrations were quantified immediately after thawing of frozen plasma aliquots in the presence of an excess of N-ethylmaleimide (NEM; in PBS, 10 mM final concentration). For the analysis of circulating total nitroso species, aliquots of NEM-treated EDTA plasma were directly injected into a triiodide-containing reaction chamber, and the NO produced from the reduction of protein nitroso species was quantified by gas phase chemiluminescence (CLD 77sp; EcoMedics), as described elsewhere (52). The concentration of nitroso species in these samples was estimated from the difference in quantification of the NO signal after sample pretreatment with mercuric chloride with sulfanilamide vs. sulfanilamide alone. For nitrite/nitrate analysis, NEM-treated samples were deproteinized with ice-cold methanol (1:1 vol/vol), separated by centrifugation, and subjected to analysis by high-pressure liquid chromatography using a dedicated nitrite/nitrate analyzer (ENO20; Eicom). Sample processing was performed in a staggered fashion to ensure reproducible processing times, and reported values are corrected for background contaminant levels.
Genetics.
Total genomic DNA was isolated from whole-blood samples using LGC Genomics’ DNA extraction service (www.lgcgroup.com/services/extraction/dna-extraction/). In brief, samples were extracted using detergent-driven cell lysis, followed by guanidinium isothiocyanate-mediated DNA binding to silica. Contaminants were removed by washing, and DNA was subsequently eluted into a low-salt buffer (10 mM Tris, 1 mM EDTA). Three SNPs on the PPARA gene (rs9627403, rs7292407, and rs6520015) were genotyped using the TaqMan platform for allelic discrimination (Applied Biosystems). PCR amplification was performed on 384-well plates using TaqMan Predesigned SNP Genotyping Assays (Applied Biosystems) and conditions recommended by the manufacturer. Reactions were analyzed by individuals blinded to subject/racial status and phenotypic data using the Applied Biosystems TaqMan 7900HT system and the sequence detection system software v2.4. All samples were genotyped twice, with 100% concordance. The TaqMan SNP Genotyping Assay ID numbers for each PPARA SNP are shown in Table S2.
OGTT.
OGTTs were carried out to assess whole-body insulin sensitivity. After an overnight fast, subjects were challenged with an oral dose of 75 g of glucose dissolved in water. Blood was collected at 0, 15, 30, 60, 90, and 120 min after ingestion, and the blood-glucose concentration was measured using a standard AccuChek Glucometer (Roche Applied Science). The area under the curve was then calculated using the trapezoidal rule. An OGTT was performed on Lowlanders in London before altitude exposure, 16–21 d after departure (6–11 d at 5,300 m), and 55–60 d after departure (45–50 d at 5,300 m). An OGTT was performed on Sherpa subjects in Kathmandu before altitude exposure, and 12–13 d after departure (2–3 d at 5,300 m). For all subjects, an OGTT was performed the day following biopsy collection to avoid confounding experiments on muscle metabolism.
High-Resolution Respirometry.
Skeletal muscle fiber bundles were prepared from the respirometry-designated sample according to previously described methods (28). After permeabilization, fiber bundles were blotted on filter paper and weighed using a microbalance (Mettler–Toledo). Respiration of fiber bundles was then measured in a mitochondrial respiration medium (MiR05) containing EGTA (0.5 mM), MgCl2⋅6H2O (3 mM), K-lactobionate (60 mM), taurine (20 mM), KH2PO4 (10 mM), Hepes (20 mM), sucrose (110 mM), and defatted BSA (1 g⋅L−1) at pH 7.4, using two substrate-uncoupler-inhibitor titration (SUIT) protocols, detailed in Tables S3 and S4. Respirometry was performed such that there was crossover of personnel between the three laboratories.
Malate (M; 5 mM) and octanoyl carnitine (Oct; 0.2 mM) were added initially to stimulate LEAK respiration (FAOL; Fig. 4D, Fig. S2, and Table S3). ADP (saturating concentration ≥ 10 mM) activated phosphorylation of ADP to ATP, resulting in OXPHOS limited by the capacity of β-oxidation (FAOP, F-OXPHOS; Fig. 2D). Addition of glutamate (G; 10 mM), followed by succinate (S; 10 mM), saturated convergent electron entry to the Q-junction in the FN-pathway (OctGMP) and the FNS-pathway (OctGMSP), respectively. Cytochrome c (10 μM) addition was used as a quality control to confirm outer mitochondrial membrane integrity; all assays with an increase in O2 consumption of >15% following cytochrome c addition were excluded from further analysis. Carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP) was used (stepwise titration of 0.25 μM) to uncouple oxidative phosphorylation and investigate ETS capacity (OctGMSE). Finally, rotenone was added (0.5 μM) to inhibit complex I [and thus FAO (38)] and isolate succinate-linked ETS capacity (SE). The OXPHOS coupling efficiency (Fig. 4E) was calculated as follows to give an indication of mitochondrial coupling (37):
where j ≈ P = OXPHOS coupling efficiency, P = OXPHOS capacity following ADP addition, and L = LEAK respiration before ADP addition.
A second SUIT protocol was used to interrogate ETS function in the absence of fatty acid substrates (Fig. S3 and Table S4). Malate (5 mM) was added initially, followed by glutamate (10 mM), to measure LEAK respiration. ADP (saturating concentration ≥ 10 mM) activated phosphorylation of ADP to ATP, resulting in N-pathway OXPHOS capacity (Fig. 4A). Addition of succinate (10 mM) stimulated convergent electron entry to the Q-junction through complexes I and II (NS-pathway; Fig 4C). Cytochrome c (10 μM) addition was used as a quality control to confirm outer mitochondrial membrane integrity; all assays with an increase in O2 consumption of >15% following cytochrome c addition were excluded from further analysis. FCCP was used (stepwise titration of 0.25 μM) to uncouple oxidative phosphorylation and investigate ETS capacity. Finally, rotenone was added (0.5 μM) to inhibit complex I and isolate succinate-linked ETS capacity (Fig. 4B).
Enzyme Activity Assays.
Enzyme activity assays were performed as described previously (19). Briefly, ∼10 mg of vastus lateralis from each individual was homogenized with an Eppendorf pestle in an Eppendorf tube containing 300 μL of homogenization buffer containing Hepes (20 mM), EDTA (1 mM), and Triton X-100 (0.1% vol/vol). The samples were then centrifuged (380 × g, 30 s, 4 °C), and the supernatant was collected. This supernatant was centrifuged again (380 × g, 30 s, 4 °C), and the resulting supernatant was collected to obtain a homogeneous suspension. The protein concentration of chamber and tissue homogenates was measured using the Quick Start Bradford protein assay (Bio-Rad). All assays were performed using a spectrophotometer (Evolution 220; Thermo Scientific) at 37 °C in a reaction volume of 1 mL. CS activity was quantified with homogenate diluted to 10 μg of protein per milliliter in an assay buffer containing Tris (20 mM), 5,5′-dithiobis-2-nitrobenzoic acid (0.1 mM), and acetyl-CoA (0.3 mM) at pH 8.0. The reaction was initiated by the addition of oxaloacetate (0.5 mM), and the absorbance change at 412 nm was measured. The 3-hydroxy acyl dehydrogenase activity was assayed with homogenate diluted to 20 μg of protein per milliliter in an assay buffer containing imidazole (50 mM), NADH (0.15 mM), and Triton X-100 (0.1% vol/vol) at pH 7.4. The reaction was initiated by the addition of 0.1 mM acetoacetyl-CoA (0.1 mM), and the absorbance change at 340 nm was measured. Hexokinase activity was quantified with homogenate diluted to 60 μg of protein per milliliter in an assay buffer containing imidazole (20 mM), ATP (1 mM), 7H2O⋅MgCl2 (5 mM), DTT (5 mM), NAD+ (2 mM), and glucose-6-phosphate-dehydrogenase (3.125 U) at pH 7.4. Glucose (5 mM) was added to trigger the reaction, and the absorbance change at 340 nm was measured. Activity of lactate dehydrogenase was quantified with homogenate diluted to 2 μg of protein per milliliter with an assay buffer containing Hepes (50 mM) and NADH (0.3 mM) at pH 7.0, and the reaction was triggered by the addition of pyruvate (0.5 mM). The reaction was monitored by measuring absorbance at a wavelength of 340 nm.
Reverse Transcription Quantitative PCR.
RNA was extracted from frozen skeletal muscle samples using a Qiagen RNeasy Fibrous Tissue Mini kit as per the manufacturer’s instructions, except that the incubation step with DNase I was excluded because this step was found to lower RNA yields. The Taqman assays used are detailed in Table S5.
Mass Spectrometry.
A methanol/chloroform extraction protocol was used, as described previously (51). First, 600 μL of chloroform/methanol (2:1 mixture) was added to cryovials containing ∼20 mg of frozen skeletal muscle and a metallic bead. Samples were lysed in a tissue lyser (Qiagen, 3 × 2 min, 22 s−1) and sonicated for 15 min. Metallic beads were then removed before 200 μL of chloroform and 200 μL of distilled water were added. Samples were thoroughly vortexed before centrifugation (∼20,000 × g, 15 min), which resulted in clear separation of an aqueous phase (upper), protein pellet (middle), and organic phase (lower). The aqueous and organic fractions were carefully extracted using a positive displacement pipette and transferred to separate Eppendorf tubes. A further 600 μL of chloroform/methanol (2:1 mixture) was added to the protein pellet, and the lysis, mixing, and centrifugation steps were repeated to maximize metabolite recovery. Both the aqueous and organic fractions were dried under nitrogen and stored at −80 °C until further analysis.
Due to their high polarity, compounds that contain phosphate were measured using hydrophilic interaction liquid chromatography, where an aqueous layer is formed on the surface of the stationary phase and this layer allows retention of the analytes. Samples were reconstituted in 200 μL of acetonitrile/water (7:3 mixture), vortexed, and analyzed. The instrumentation comprised an Acquity Ultra Performance Liquid Chromatography unit (Waters Ltd.) interfaced with an AB Sciex 5500 triple quadrupole (AB Sciex). Mobile phases were run at 0.6 mL·min−1, where mobile phase A consisted of 10 mM ammonium acetate adjusted to pH 9.5 with ammonia and mobile phase B was acetonitrile. Mobile phase B was held for 1 min at 70%, decreased to 40% over 2.5 min, returned to 70% by 3.6 min, and maintained for 2.4 min. The total run time was 6 min. Data were acquired in both positive and negative ionization modes using capillary spray voltages of 3.5 kV and 2.5 kV, respectively. The ion transfer tube was set to operate at 356 °C, and the vaporizer temperature was set to 420 °C. Sheath, auxiliary, and sweep gases were set to 52, 16, and 2 arbitrary units, respectively.
Other aqueous metabolites were measured using the same instrumentation, but the chromatographic separation was performed using an ACE C18-PFP 3-μm column (2.1 × 150 mm; Advanced Chromatography Technologies Ltd.). The mobile phase gradient was run at 0.5 mL⋅min−1 using water (mobile phase A) and acetonitrile (mobile phase B). The gradient started at 0% B and increased to 60% B from 1.6 to 4.5 min, followed by reequilibration for 2 min. The total run time was 6.5 min. Data were acquired in both positive and negative ionization modes using capillary spray voltages of 3.5 kV and 2.5 kV, respectively. The ion transfer tube was set to operate at 350 °C, whereas the vaporizer temperature was set to 400 °C. Sheath, auxiliary, and sweep gases were set to 50, 15, and 2 arbitrary units, respectively.
Half of the organic fraction and half of the aqueous fraction were combined with 200 μL of acetonitrile containing an internal standard mix of eight deuterated carnitines [1.63 μM (d9) free carnitine, 0.3 μM (d3) acetyl carnitine, 0.06 μM (d3) propionyl carnitine, 0.06 μM (d3) butyryl carnitine, 0.06 μM (d9) isovaleryl carnitine, 0.06 μM (d3) Oct, 0.06 μM (d9) myristoyl carnitine, and 0.12 μM (d3) palmitoyl carnitine; Cambridge Isotope Laboratories, Inc.] and dried under nitrogen. Samples were derivatized with 100 μL of 3 M HCl in butanol for 15 min at 65 °C. The resulting mixture was dried under nitrogen and finally reconstituted in 4:1 acetonitrile/0.1% formic acid in water, vortexed, and placed into autosampler vials. The strong mobile phase used for analysis was acetonitrile with 0.1% formic acid (B), and the weak mobile phase was 0.1% formic acid in water (A). The analytical ultra performance liquid chromatography (UPLC) gradient used a Synergi Polar RP phenyl ether column (50 × 2.1 mm, 2.5 μm; Phenomenex) starting with 30% B in 0.1% formic acid, followed by a linear gradient to 100% B for 3 min, and held at 100% B for the next 5 min with a further 2 min reequilibration. The total run time was 10 min, and the flow rate was 0.5 mL⋅min−1 with an injection volume of 2 μL. Analytes were measured using a multiple reaction monitoring (MRM) method, with the daughter ion being set to 85.0 Da for each compound.
Protein pellets were dissolved in 1 mL of 1 M NaOH solution and heated for 10 min at 80 °C. Samples were then centrifuged (16,000 × g, 10 min). Sample protein concentration was quantified using a bicinchoninic acid (BCA) assay kit (BCA1-1KT; Sigma), and absorbance at a wavelength of 562 nm was then quantified using a spectrophotometer (Evolution 220; Thermo Scientific).
Data were processed using the vendor software and normalized to total protein content and to the intensity of the internal standards.
It was not feasible in a field study such as this one to avoid autooxidation during sample processing, which is known to affect redox; thus, the redox ratios reported do not correspond to true physiological levels, which would have been obtainable with direct addition of thiol-alkylating agents. Nevertheless, we demonstrate that ratios change substantially, reflecting redox differences in relation to oxidative load. Addition of a thiol-alkylating agent would have compromised the analysis of other metabolites.
Statistics.
To compare Sherpa and Lowlander cohorts at baseline, an unpaired, two-tailed Student’s t test was performed (considering significance at P ≤ 0.05). Genotype frequencies were compared between Sherpas and Lowlanders using a χ2 test. To assess the effects of an ascent to high altitude on both cohorts, a one-way ANOVA with repeated measures was performed. If a significant difference was reported, post hoc pairwise comparisons were carried out with a Tukey correction.
Data Sharing.
All data are available from the University of Cambridge data repository, which is available at https://www.repository.cam.ac.uk/handle/1810/263797.
ACKNOWLEDGMENTS. Xtreme Everest 2 is a research project coordinated by the Xtreme Everest Oxygen Research Consortium, a collaboration between the UCL Centre for Altitude, Space, and Extreme Environment Medicine; the Centre for Human Integrative Physiology at the University of Southampton; and the Duke University Medical Center. Members of the Xtreme Everest 2 Research Group are as follows: S. Abraham, T. Adams,W. Anseeuw, R. Astin, B. Basnyat, O. Burdall, J. Carroll, A. Cobb, J. Coppel, O. Couppis, J. Court, A. Cumptsey, T. Davies, S. Dhillon, N. Diamond, C. Dougall, T. Geliot, E. Gilbert-Kawai, G. Gilbert-Kawai, E. Gnaiger, M. Grocott, C. Haldane, P. Hennis, J. Horscroft, D. Howard, S. Jack, B. Jarvis, W. Jenner, G. Jones, J. van der Kaaij, J. Kenth, A. Kotwica, R. Kumar, J. Lacey, V. Laner, D. Levett, D. Martin, P.Meale,K.Mitchell, Z. Mahomed, J. Moonie, A. Murray,M.Mythen, P. Mythen, K. O’Brien, I. Ruggles-Brice, K. Salmon, A. Sheperdigian, T. Smedley, B. Symons, C. Tomlinson, A. Vercueil, L. Wandrag, S. Ward, A. Wight, C. Wilkinson, and S. Wythe. Members of the Scientific Advisory Board are as follows: M. Feelisch, E. Gilbert-Kawai, M. Grocott (chair),M. Hanson, D. Levett, D.Martin, K. Mitchell, H. Montgomery, R. Moon, A. Murray, M. Mythen, and M. Peters. Some of this work was undertaken at UCL Hospital–UCL Biomedical Research Centre, which received a proportion of funding from the UK Department of Health’s National Institute for Health Research Biomedical Research Centre’s funding scheme. Some of this work was undertaken at University Hospital Southampton–University of Southampton Respiratory Biomedical Research Unit, which received a proportion of funding from the UK Department of Health’s National Institute for Health Research Biomedical Research Units funding scheme. J.L.G. thanks the Medical Research Council (MRC) (MC UP A90 1006) and AB Sciex. M.F. thanks the MRC and Faculty of Medicine, Southampton University. This work was supported by PhD Studentships from the Biotechnology and Biological Sciences Research Council (BB/F016581/1) to (J.A.H.) and British Heart Foundation (FS/09/050) (to A.O.K.), an Academic Fellowship to A.J.M. from the Research Councils UK (EP/E500552/1), a grant from the Physiological Society, and support from Oroboros Instruments. Xtreme Everest 2 was financially supported by the Royal Free Hospital National Health Service (NHS) Trust Charity, the Special Trustees of University College London (UCL) Hospital NHS Foundation Trust, the Southampton University Hospital Charity, the UCL Institute of Sports Exercise and Health, The London Clinic, UCL, University of Southampton, Duke University Medical School, the United Kingdom Intensive Care Society, the National Institute of Academic Anaesthesia, the Rhinology and Laryngology Research Fund, The Physiological Society, Smiths Medical, Oroboros Instruments (Austria), Deltex Medical, Atlantic Customer Solutions, and the Xtreme Everest 2 volunteer participants who trekked to EBC.
Acknowledgments
Full acknowledgments are provided in Supporting Information. This work was supported by PhD Studentships from the Biotechnology and Biological Sciences Research Council (BB/F016581/1) (to J.A.H.) and British Heart Foundation to (FS/09/050) (to A.O.K.), an Academic Fellowship from the Research Councils UK (EP/E500552/1) (to A.J.M.), a Physiological Society grant, and support from Oroboros Instruments.
Footnotes
Conflict of interest statement: E.G. is Chief Executive Officer and V.L. is Chief Operating Officer of Oroboros Instruments.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1700527114/-/DCSupplemental.
References
- 1.Koh MY, Powis G. Passing the baton: The HIF switch. Trends Biochem Sci. 2012;37:364–372. doi: 10.1016/j.tibs.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Willam C, Nicholls LG, Ratcliffe PJ, Pugh CW, Maxwell PH. The prolyl hydroxylase enzymes that act as oxygen sensors regulating destruction of hypoxia-inducible factor alpha. Adv Enzyme Regul. 2004;44:75–92. doi: 10.1016/j.advenzreg.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 3.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aldenderfer M. Peopling the Tibetan plateau: Insights from archaeology. High Alt Med Biol. 2011;12:141–147. doi: 10.1089/ham.2010.1094. [DOI] [PubMed] [Google Scholar]
- 5.Beall CM. Two routes to functional adaptation: Tibetan and Andean high-altitude natives. Proc Natl Acad Sci USA. 2007;104:8655–8660. doi: 10.1073/pnas.0701985104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bigham AW, Lee FS. Human high-altitude adaptation: Forward genetics meets the HIF pathway. Genes Dev. 2014;28:2189–2204. doi: 10.1101/gad.250167.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beall CM, et al. Natural selection on EPAS1 (HIF2alpha) associated with low hemoglobin concentration in Tibetan highlanders. Proc Natl Acad Sci USA. 2010;107:11459–11464. doi: 10.1073/pnas.1002443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simonson TS, et al. Genetic evidence for high-altitude adaptation in Tibet. Science. 2010;329:72–75. doi: 10.1126/science.1189406. [DOI] [PubMed] [Google Scholar]
- 9.Gilbert-Kawai ET, Milledge JS, Grocott MP, Martin DS. King of the mountains: Tibetan and Sherpa physiological adaptations for life at high altitude. Physiology (Bethesda) 2014;29:388–402. doi: 10.1152/physiol.00018.2014. [DOI] [PubMed] [Google Scholar]
- 10.Peacock AJ. ABC of oxygen: Oxygen at high altitude. BMJ. 1998;317:1063–1066. doi: 10.1136/bmj.317.7165.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grocott MP, et al. Caudwell Xtreme Everest Research Group Arterial blood gases and oxygen content in climbers on Mount Everest. N Engl J Med. 2009;360:140–149. doi: 10.1056/NEJMoa0801581. [DOI] [PubMed] [Google Scholar]
- 12.Beall CM, et al. Pulmonary nitric oxide in mountain dwellers. Nature. 2001;414:411–412. doi: 10.1038/35106641. [DOI] [PubMed] [Google Scholar]
- 13.Erzurum SC, et al. Higher blood flow and circulating NO products offset high-altitude hypoxia among Tibetans. Proc Natl Acad Sci USA. 2007;104:17593–17598. doi: 10.1073/pnas.0707462104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winslow RM, et al. Different hematologic responses to hypoxia in Sherpas and Quechua Indians. J Appl Physiol (1985) 1989;66:1561–1569. doi: 10.1152/jappl.1989.66.4.1561. [DOI] [PubMed] [Google Scholar]
- 15.Beall CM, et al. Hemoglobin concentration of high-altitude Tibetans and Bolivian Aymara. Am J Phys Anthropol. 1998;106:385–400. doi: 10.1002/(SICI)1096-8644(199807)106:3<385::AID-AJPA10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 16.Hoppeler H, Howald H, Cerretelli P. Human muscle structure after exposure to extreme altitude. Experientia. 1990;46:1185–1187. doi: 10.1007/BF01936933. [DOI] [PubMed] [Google Scholar]
- 17.Levett DZ, et al. Caudwell Xtreme Everest Research Group Acclimatization of skeletal muscle mitochondria to high-altitude hypoxia during an ascent of Everest. FASEB J. 2012;26:1431–1441. doi: 10.1096/fj.11-197772. [DOI] [PubMed] [Google Scholar]
- 18.Murray AJ, Horscroft JA. Mitochondrial function at extreme high altitude. J Physiol. 2016;594:1137–1149. doi: 10.1113/JP270079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horscroft JA, Murray AJ. Skeletal muscle energy metabolism in environmental hypoxia: climbing towards consensus. Extrem Physiol Med. 2014;3:19. doi: 10.1186/2046-7648-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobs RA, et al. Twenty-eight days at 3454-m altitude diminishes respiratory capacity but enhances efficiency in human skeletal muscle mitochondria. FASEB J. 2012;26:5192–5200. doi: 10.1096/fj.12-218206. [DOI] [PubMed] [Google Scholar]
- 21.Levett DZ, et al. Changes in muscle proteomics in the course of the Caudwell Research Expedition to Mt. Everest. Proteomics. 2015;15:160–171. doi: 10.1002/pmic.201400306. [DOI] [PubMed] [Google Scholar]
- 22.Kayser B, Hoppeler H, Claassen H, Cerretelli P. Muscle structure and performance capacity of Himalayan Sherpas. J Appl Physiol (1985) 1991;70:1938–1942. doi: 10.1152/jappl.1991.70.5.1938. [DOI] [PubMed] [Google Scholar]
- 23.Narravula S, Colgan SP. Hypoxia-inducible factor 1-mediated inhibition of peroxisome proliferator-activated receptor alpha expression during hypoxia. J Immunol. 2001;166:7543–7548. doi: 10.4049/jimmunol.166.12.7543. [DOI] [PubMed] [Google Scholar]
- 24.Peng Y, et al. Genetic variations in Tibetan populations and high-altitude adaptation at the Himalayas. Mol Biol Evol. 2011;28:1075–1081. doi: 10.1093/molbev/msq290. [DOI] [PubMed] [Google Scholar]
- 25.Gilbert-Kawai E, et al. Design and conduct of Xtreme Everest 2: An observational cohort study of Sherpa and lowlander responses to graduated hypobaric hypoxia. F1000 Res. 2015;4:90. doi: 10.12688/f1000research.6297.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ge RL, et al. Metabolic insight into mechanisms of high-altitude adaptation in Tibetans. Mol Genet Metab. 2012;106:244–247. doi: 10.1016/j.ymgme.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horscroft JA, Burgess SL, Hu Y, Murray AJ. Altered oxygen utilisation in rat left ventricle and soleus after 14 days, but not 2 days, of environmental hypoxia. PLoS One. 2015;10:e0138564. doi: 10.1371/journal.pone.0138564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pesta D, Gnaiger E. High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol Biol. 2012;810:25–58. doi: 10.1007/978-1-61779-382-0_3. [DOI] [PubMed] [Google Scholar]
- 29.Koves TR, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7:45–56. doi: 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 30.Kayser B, et al. Muscle ultrastructure and biochemistry of lowland Tibetans. J Appl Physiol (1985) 1996;81:419–425. doi: 10.1152/jappl.1996.81.1.419. [DOI] [PubMed] [Google Scholar]
- 31.Bigham A, et al. Identifying signatures of natural selection in Tibetan and Andean populations using dense genome scan data. PLoS Genet. 2010;6:e1001116. doi: 10.1371/journal.pgen.1001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foll M, Gaggiotti OE, Daub JT, Vatsiou A, Excoffier L. Widespread signals of convergent adaptation to high altitude in Asia and America. Am J Hum Genet. 2014;95:394–407. doi: 10.1016/j.ajhg.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wanders RJ, Komen J, Kemp S. Fatty acid omega-oxidation as a rescue pathway for fatty acid oxidation disorders in humans. FEBS J. 2011;278:182–194. doi: 10.1111/j.1742-4658.2010.07947.x. [DOI] [PubMed] [Google Scholar]
- 34.Nelson DL, Cox MM, Lehninger AL. Principles of Biochemistry. Freeman; New York: 2008. [Google Scholar]
- 35.MacKenzie ED, et al. Cell-permeating alpha-ketoglutarate derivatives alleviate pseudohypoxia in succinate dehydrogenase-deficient cells. Mol Cell Biol. 2007;27:3282–3289. doi: 10.1128/MCB.01927-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chinopoulos C. Which way does the citric acid cycle turn during hypoxia? The critical role of α-ketoglutarate dehydrogenase complex. J Neurosci Res. 2013;91:1030–1043. doi: 10.1002/jnr.23196. [DOI] [PubMed] [Google Scholar]
- 37.Gnaiger E, et al. Mitochondrial coupling and capacity of oxidative phosphorylation in skeletal muscle of Inuit and Caucasians in the arctic winter. Scand J Med Sci Sports. 2015;25:126–134. doi: 10.1111/sms.12612. [DOI] [PubMed] [Google Scholar]
- 38.Gnaiger E. Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. Mitochondr Physiol Network. 2014;19:1–80. [Google Scholar]
- 39.Anedda A, et al. The transcription factor Nrf2 promotes survival by enhancing the expression of uncoupling protein 3 under conditions of oxidative stress. Free Radic Biol Med. 2013;61:395–407. doi: 10.1016/j.freeradbiomed.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 40.Murray AJ. Metabolic adaptation of skeletal muscle to high altitude hypoxia: How new technologies could resolve the controversies. Genome Med. 2009;1:117. doi: 10.1186/gm117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woolcott OO, Ader M, Bergman RN. Glucose homeostasis during short-term and prolonged exposure to high altitudes. Endocr Rev. 2015;36:149–173. doi: 10.1210/er.2014-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem. 1994;269:23757–23763. [PubMed] [Google Scholar]
- 43.McClain DA, et al. Decreased serum glucose and glycosylated hemoglobin levels in patients with Chuvash polycythemia: A role for HIF in glucose metabolism. J Mol Med (Berl) 2013;91:59–67. doi: 10.1007/s00109-012-0961-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Julian CG, et al. Potential role for elevated maternal enzymatic antioxidant status in Andean protection against altitude-associated SGA. J Matern Fetal Neonatal Med. 2012;25:1233–1240. doi: 10.3109/14767058.2011.636102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sergueeva AI, et al. Elevated homocysteine, glutathione and cysteinylglycine concentrations in patients homozygous for the Chuvash polycythemia VHL mutation. Haematologica. 2008;93:279–282. doi: 10.3324/haematol.11851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lahiri S, Milledge JS. Sherpa physiology. Nature. 1965;207:610–612. doi: 10.1038/207610a0. [DOI] [PubMed] [Google Scholar]
- 47.Moore LG, Charles SM, Julian CG. Humans at high altitude: Hypoxia and fetal growth. Respir Physiol Neurobiol. 2011;178:181–190. doi: 10.1016/j.resp.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moore LG, Young D, McCullough RE, Droma T, Zamudio S. Tibetan protection from intrauterine growth restriction (IUGR) and reproductive loss at high altitude. Am J Hum Biol. 2001;13:635–644. doi: 10.1002/ajhb.1102. [DOI] [PubMed] [Google Scholar]
- 49.Smith C. The effect of maternal nutritional variables on birthweight outcomes of infants born to Sherpa women at low and high altitudes in Nepal. Am J Hum Biol. 1997;9:751–763. doi: 10.1002/(SICI)1520-6300(1997)9:6<751::AID-AJHB8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 50.Jawerbaum A, Capobianco E. Review: Effects of PPAR activation in the placenta and the fetus: Implications in maternal diabetes. Placenta. 2011;32:S212–S217. doi: 10.1016/j.placenta.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 51.Bogacka I, Kurzynska A, Bogacki M, Chojnowska K. Peroxisome proliferator-activated receptors in the regulation of female reproductive functions. Folia Histochem Cytobiol. 2015;53:189–200. doi: 10.5603/fhc.a2015.0023. [DOI] [PubMed] [Google Scholar]
- 52.Roberts LD, et al. The contrasting roles of PPARδ and PPARγ in regulating the metabolic switch between oxidation and storage of fats in white adipose tissue. Genome Biol. 2011;12:R75. doi: 10.1186/gb-2011-12-8-r75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rassaf T, Bryan NS, Kelm M, Feelisch M. Concomitant presence of N-nitroso and S-nitroso proteins in human plasma. Free Radic Biol Med. 2002;33:1590–1596. doi: 10.1016/s0891-5849(02)01183-8. [DOI] [PubMed] [Google Scholar]