Significance
Deep sequencing of bar-coded Listeria monocytogenes enabled determination of the pathogen’s dissemination routes and founding population sizes. The gallbladder, which is seeded by very few L. monocytogenes cells, becomes the source for pathogen shedding in the feces. The low complexity of shed populations suggests that genetic drift may be a powerful force in L. monocytogenes evolution. Innate immune factors and microbiota differentially regulate pathogen establishment and proliferation at distal sites. Sequence tag-based analysis of microbial populations is a powerful approach for investigating the impact of host factors on pathogen population dynamics.
Keywords: Listeria monocytogenes, STAMP, pathogen dissemination, pathogen transmission, population dynamics
Abstract
Listeria monocytogenes is a common food-borne pathogen that can disseminate from the intestine and infect multiple organs. Here, we used sequence tag-based analysis of microbial populations (STAMP) to investigate L. monocytogenes population dynamics during infection. We created a genetically barcoded library of murinized L. monocytogenes and then used deep sequencing to track the pathogen’s dissemination routes and quantify its founding population (Nb) sizes in different organs. We found that the pathogen disseminates from the gastrointestinal tract to distal sites through multiple independent routes and that Nb sizes vary greatly among tissues, indicative of diverse host barriers to infection. Unexpectedly, comparative analyses of sequence tags revealed that fecally excreted organisms are largely derived from the very small number of L. monocytogenes cells that colonize the gallbladder. Immune depletion studies suggest that distinct innate immune cells restrict the pathogen’s capacity to establish replicative niches in the spleen and liver. Finally, studies in germ-free mice suggest that the microbiota plays a critical role in the development of the splenic, but not the hepatic, barriers that prevent L. monocytogenes from seeding these organs. Collectively, these observations illustrate the potency of the STAMP approach to decipher the impact of host factors on population dynamics of pathogens during infection.
Some pathogens are able to disseminate from their sites of inoculation to reach distant organs and proliferate to high numbers. During dissemination, pathogens must circumvent host defense mechanisms that restrict access to niches permissive for pathogen replication, which constitute “bottlenecks” constraining pathogen establishment. Understanding pathogen dissemination routes and the extent, timing, and nature of host bottlenecks provides valuable understanding of host–pathogen interactions but can be challenging to investigate experimentally (1). Although dissemination routes and population bottlenecks can, in principle, be determined by meticulous counting of the number of organisms at multiple sites over time, obtaining sufficient spatial and temporal resolution for such studies is often not possible.
Experimental approaches that rely on inoculation of a population of distinguishable, rather than clonal, organisms have been developed to facilitate quantification of population bottlenecks (2–7). Sequence tag-based analysis of microbial populations (STAMP) (8) uses organisms that can be distinguished based on short-sequence barcodes integrated at a neutral position within their genome [also known as wild-type (wt) isogenic tags]. Barcoded organisms from infected animals are enumerated through deep sequencing, and STAMP combines this information with a mathematical framework from classical population genetics to quantify the founding population (Nb) at each infected site (i.e., the number of organisms that survive host bottlenecks and contribute to subsequent population expansion). STAMP enables determination of the Nb with high accuracy and over a wide dynamic range, and the STAMP tags enable assessment of the relatedness among pathogen populations at different sampling sites.
Listeria monocytogenes is a gram-positive, food-borne pathogen that can spread from the gastrointestinal (GI) tract, where it can cause gastroenteritis, to distal sites, including the spleen, liver, gallbladder (GB), brain, and placenta, which can result in meningitis, septic abortion, and other manifestations of systemic infection (9–12). A variety of pathogen factors that enable the organism to grow within host cells and to spread from cell to cell have been identified; additionally, studies of host–pathogen interactions have yielded considerable insight into the host response to L. monocytogenes (13–17). However, there is relatively little knowledge of L. monocytogenes population dynamics during infection. Portnoy and coworkers (7) and Bakardjiev et al. (10) studied orogastrically infected guinea pigs to identify routes by which L. monocytogenes disseminates from the intestine to the liver, mesenteric lymph nodes (MLNs), and spleen, and they have characterized how L. monocytogenes traffics between maternal organs and the placenta (7, 10). However, none of these studies measured Nb sizes in these organs.
Here, we used STAMP to map L. monocytogenes dissemination routes and quantify Nb sizes following orogastric (OG) and i.v. inoculation of mice. For both infection protocols, we found that Nb size was highest for the liver and spleen, suggesting that a subset of the OG inoculum escapes from the GI tract before constriction of the L. monocytogenes Nb there. A markedly lower number of founders gave rise to the L. monocytogenes populations in the GB. Unexpectedly, comparative analyses of sequence tags revealed that this organ is the principal source for fecally excreted L. monocytogenes. Moreover, using immune depletion and germ-free (GF) mice, we show the potency of the STAMP approach for investigating the impact of host factors on pathogen population dynamics.
Results
L. monocytogenes Nb Sizes Vary Among Different Organs.
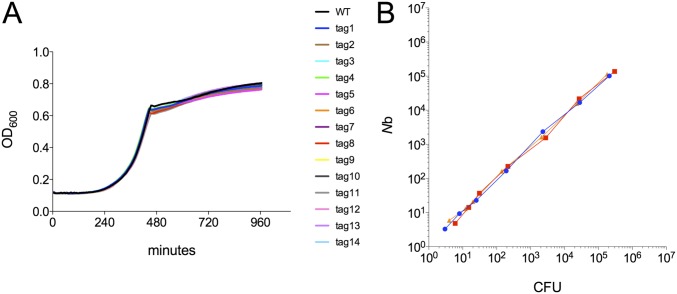
A library of barcoded but otherwise isogenic L. monocytogenes strains was constructed to enable quantitation of the bottlenecks the pathogen confronts within different tissues during infection and to track its dissemination through the body. Each of the strains in the library harbors 1 of 200 unique short (∼30 bp) sequence tags inserted into a neutral site (18) on the chromosome. The in vitro growth kinetics of a subset of the tagged strains were indistinguishable from the in vitro growth kinetics of the untagged wt strain (Fig. S1A), strongly suggesting that the barcodes do not influence L. monocytogenes growth. Based on mathematical modeling, this number of barcodes is sufficient to determine Nb sizes as great as 105 with high accuracy (8). Moreover, we experimentally established that we could measure Nb sizes over a wide range with this library using STAMP by comparing the frequencies of tags in an inoculum with the frequencies recovered after exposure to distinct bottlenecks. We plated defined fractions of an in vitro culture of the library (i.e., imposed population bottlenecks) and compared the number of colony-forming units (CFUs) observed on the plates with the calculated number of founders ascertained using STAMP (Fig. S1B). There was an excellent correlation between these values (R2 = 0.98) over a range of at least four orders of magnitude (Fig. S1B), indicating that this library could be used to estimate bottleneck sizes ranging from as low as three cells to as high as 5 × 105 cells.
Fig. S1.
Validation of the STAMP approach for determination of Nb sizes. (A) In vitro growth of wt and barcoded L. monocytogenes. (B) Correspondence of Nb sizes was determined based on CFUs (x axis) and analyses of barcode frequencies using STAMP (y axis). The three colors indicate three independent experiments.
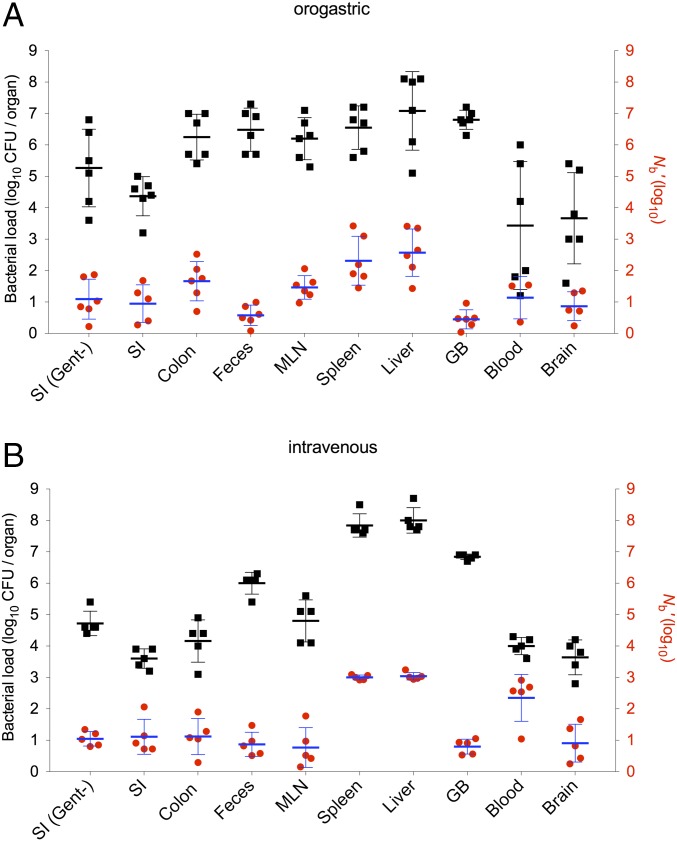
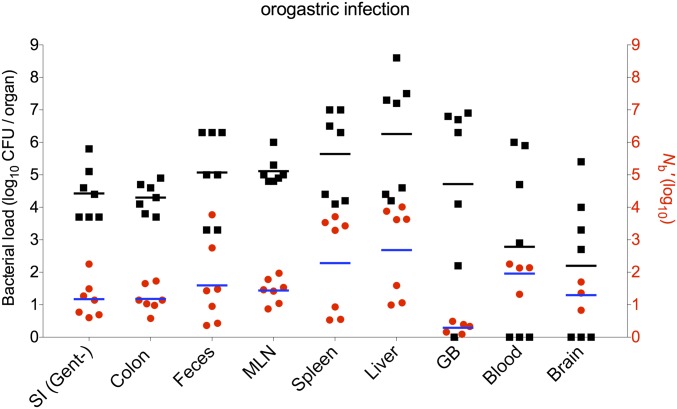
The barcoded library was created in L. monocytogenes 10403S InlAm, a so-called “murinized” version of a human clinical isolate. This strain expresses a variant of InlA, a surface protein critical for invasion of intestinal epithelial cells, which interacts more efficiently with the murine rather than the human E-cadherin receptor (19). OG inoculation of 10403S InlAm into adult mice results in a disease that mimics human food-borne listerosis, with dissemination of the pathogen from the GI tract, although large inocula are required (19). Consistent with previous reports, 72 h after BALB/c mice were orogastrically inoculated with the barcoded library (3 × 109 CFUs per mouse), all mice exhibited signs of infection (e.g., ruffled fur, reduced activity) and the pathogen could be isolated from the small intestine (SI), colon, and feces as well as from sites beyond the GI tract, such as the MLNs, liver, spleen, and GB (Fig. 1A). L. monocytogenes was isolated from the blood and brain as well (Fig. 1A), but there was greater variability in the recovery of the pathogen from the latter two tissues than from other sites. The number of CFUs recovered from brain samples was highly correlated with CFU numbers from blood samples (R2 = 0.81), consistent with the model that blood-borne organisms seed the brain.
Fig. 1.
Recovered CFU and Nb′ sizes in different tissues following OG or i.v. inoculation of L. monocytogenes. BALB/c mice were orogastrically inoculated with 3 × 109 CFUs of L. monocytogenes (10403S InlAm) (A) or injected i.v. with 6 × 104 CFUs (B). Bacterial loads (CFU, black squares) and Nb sizes (Nb′, red circles) in different tissues were determined 3 d after OG infection or 2 d after i.v. infection. CFU values are expressed per organ for the SI, colon, MLN, spleen, liver, GB, and brain; they are expressed per milliliter for blood and per pellet for feces. Each black square and red circle represents data from one mouse. Geometric means (horizontal lines) and SD (whiskers) are shown. SI (Gent−), gentamicin-negative (untreated SI tissues).
We enumerated CFUs recovered from each of these sites and estimated the size of the Nb by comparing the frequencies of tags in the inoculum with the frequencies in organisms recovered from the different samples (yielding Nb) and adjusting it with the calibration curve (yielding Nb′) (8). In general, similar numbers of L. monocytogenes CFUs were recovered from the colon, feces, MLN, spleen, liver, and GB at 3 d postinfection (dpi), with geometric mean values of ∼4 × 106 (Fig. 1A), suggesting that these sites all have a fairly similar capacity to carry L. monocytogenes. In contrast, Nb′ values from these samples displayed more variation (from a mean of 3 in the GB to a mean of 373 in the liver; Fig. 1A), suggesting that the magnitude of host restrictions for establishing infection in these locations varies. Unexpectedly, Nb′ values were lower in the GI tract [SI (9) and colon (46)] compared with inner organs [spleen (205) and liver (373); P < 0.01 for SI vs. spleen or liver, but values for colon vs. spleen or liver did not reach statistical significance; Fig. 1A], although the latter sites presumably are colonized by bacteria initially disseminated from the GI tract. The fact that Nb′ values for the spleen and liver exceed Nb′ values for the GI tract suggests that a subset of the inoculum must have escaped from the GI tract, presumably via the portal vein, lymphatics, or systemic circulation (7), before restriction of the population there. The Nb′ values for the SI at 3 dpi predominantly reflect intracellular L. monocytogenes because they were similar for tissue treated with gentamicin, which kills extracellular organisms, and untreated SI tissues (Fig. 1A). The fact that even the largest calculated Nb′ values were at least six orders of magnitude lower than the inoculum size of 3 × 109 CFUs per mouse indicates that there is an extremely severe bottleneck for L. monocytogenes to establish infection in this model; the different Nb sizes in the tested host tissues reveal that additional tissue-specific bottlenecks constrain the capacity of L. monocytogenes to access different host organs.
The lowest Nb′ values were found in the GB. Even though there was Nb′ ≤ 9 in the GB samples (mean Nb′ = 3, Nb′ range: 1–9), the numbers of L. monocytogenes CFUs recovered from these samples were comparable to the numbers found in other tissues (Fig. 1A). Thus, host barriers appear to restrict access of L. monocytogenes to the GB severely; however, once the pathogen reaches this site, presumably by passing through the hepatic duct and then ascending through the cystic duct, it is capable of replicating to high densities. The GB is known to be relatively deficient in its capacity to recruit innate immune effector cells such as neutrophils (20), likely accounting for the high density of organisms found there. Notably, despite the relatively large Nb′ value observed in the colon (mean = 46), the Nb′ value in the feces was very low and similar to the Nb′ value in the GB, suggesting that fecal organisms are not principally derived from intracellular organisms replicating within colonic tissue.
Historically, many studies have used i.v. inoculation of L. monocytogenes as a model to investigate in vivo pathogen–host interactions. In contrast to OG inoculation, the i.v. route introduces a high number of bacteria into the blood at the same time. It bypasses gut barriers that may prevent L. monocytogenes dissemination, and the pathogen can directly seed the spleen and liver. We measured Nb′ and CFU values 2 d after i.v. inoculation of 6 × 104 CFUs. At this dose and time, the mice exhibit similar signs of disease observed 3 d after OG inoculation. Although Nb′ and CFU values were generally similar for i.v. and OG inoculation (Fig. 1 A vs. B), there were some differences dependent upon the route of inoculation. For example, Nb′ values tended to be higher and less varied in the spleen and liver after i.v. vs. OG inoculation, consistent with the pathogen’s direct and immediate access to these organs after i.v. inoculation. Not surprisingly, Nb′ values were also higher in blood after i.v. injection compared with OG inoculation, and CFU counts had a lower variance. Similarly, CFU counts recovered from brain samples also exhibited lower variance with the i.v. infection route, consistent with hematogenous seeding of the brain. The i.v. infection route did not increase the particularly low Nb′ values that were observed in fecal, GB, and brain samples with OG inoculation, suggesting that escape from the intestine is not the primary impediment to colonization of these sites (Fig. 1 A and B).
The GB Is the Reservoir for Fecal Shedding of L. monocytogenes.
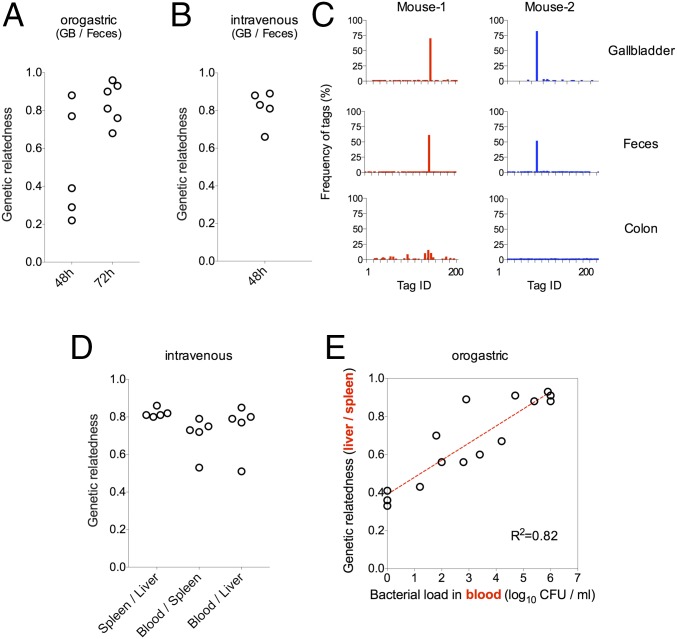
Besides enabling calculation of Nb sizes, the sequence tags facilitate comparisons of the genetic relatedness among populations from different samples, which can be calculated by comparing tag frequencies within populations. The most striking finding from these analyses was the high degree of genetic relatedness between populations resident in the GB and the feces after either OG (3 dpi) or i.v. inoculation, which was far higher than the relatedness of GB or fecal populations to populations from any other tissue (Fig. 2 A and B, respectively, and Fig. S2 A–C). Animals were often found to contain the same single predominant tagged strain in samples from both sites (consistent with the low Nb′ values for these sites); however, the identity of this tag differed among mice, indicating that a selective advantage does not account for a particular strain’s predominance within GB and fecal samples (Fig. 2C). Notably, there was far less correspondence between fecal populations and populations within colonic tissue, and colonic samples were typically not dominated by a single tagged strain (Fig. 2C). Collectively, these data provide further support for the idea that L. monocytogenes replicating intracellularly within colonic tissue is not the predominant source of the pathogen shed in feces; instead, the GB serves as the reservoir for bacteria likely to be fecally transmitted. The similarity between the populations in the GB and feces was not as marked 2 d after OG inoculation, likely because some of the fecal bacteria are still derived from the inoculum (Fig. 2A and Fig. S3).
Fig. 2.
Genetic relatedness of L. monocytogenes populations isolated from different tissues. Genetic relatedness of L. monocytogenes populations in the indicated samples 48 h and/or 72 h after OG inoculation (A) and 48 h after i.v. inoculation (B and D). (C) Relative frequencies of barcodes in the GB, feces, and colon of two representative mice. (E) Correlation between the L. monocytogenes burden in the blood (x axis) and the genetic relatedness of L. monocytogenes populations in the spleen and liver (y axis) 48 and 72 h after OG inoculation of mice. The Pearson correlation coefficient value was used to quantify correlation. Each open circle in A, B, D, and E represents data from one mouse.
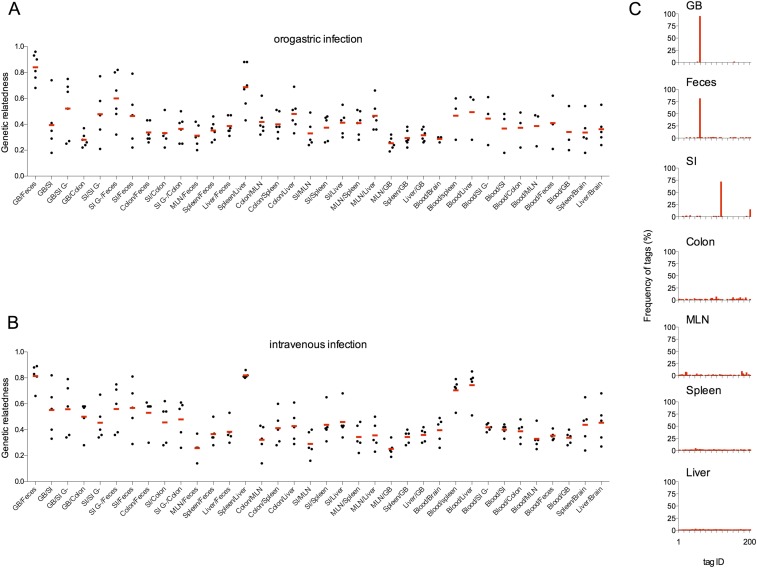
Fig. S2.
Genetic relatedness of L. monocytogenes populations isolated from different tissues after OG and i.v. inoculation. Pairwise comparisons of the genetic relatedness of L. monocytogenes populations in the indicated tissues 72 h after OG inoculation (A) or 48 h after i.v. inoculation (B) are shown. Each black circle represents data from one mouse, and the red bars represent the arithmetic means. (C) Relative frequencies of barcodes in the GB, feces, SI, colon, MLN, spleen, and liver of a representative mouse.
Fig. S3.
Recovered CFUs (black squares) and Nb (Nb′, red circles) in the indicated tissues 2 d following OG inoculation of BALB/c mice with L. monocytogenes. Each black square and red circle represents data from one mouse. Geometric means (horizontal lines) are shown. SI (Gent−), gentamicin-negative (untreated SI tissues).
Additional pairwise comparisons between L. monocytogenes populations in samples from different sites showed that the genetic relatedness between samples from different sites was generally low for animals inoculated by the OG route (Fig. S2A). This finding suggests that L. monocytogenes may arrive at sites distal to the GI tract through multiple independent paths, rather than seeding a primary niche from which bacteria subsequently spread. These observations are consistent with episodic spread of the population from the OG inoculum through the portal vein, and perhaps other routes, to the liver and via lymphatics and systemic circulation to the MLN and spleen as proposed by Melton-Witt et al. (7). In contrast, after i.v. inoculation, the L. monocytogenes recovered from blood, spleen, and liver samples were all closely related (Fig. 2D), consistent with bacterial injection into the bloodstream and subsequent filtration and sequestration by the spleen and liver. In animals inoculated by the OG route, bacterial populations in the liver and spleen tended to become more related as the bacterial load in the blood increased (Fig. 2E). However, in the absence of high-grade bacteremia (bacterial load < 1,000 CFU/mL; Fig. 2E), the samples’ relative lack of relatedness suggests that they are seeded by independent processes. These findings suggest a model in which the liver and spleen are seeded with distinct founders at an early stage of the infection; subsequently, as the pathogen burden increases, the blood enables exchange of bacteria between the two organs. Furthermore, following OG inoculation, it is likely that pathogen arrival at the liver and spleen is progressive, because Nb′ values tended to increase from day 2 to day 3 following inoculation (Fig. 1A vs. Fig. S3).
We investigated whether L. monocytogenes chemotaxis and/or motility modulates the pathogen’s capacity to reach or proliferate within colonized sites by performing infections with a ΔcheA L. monocytogenes strain, which is defective in both chemotaxis and motility (Fig. 3A). Following OG inoculation of mice with the barcoded ΔcheA mutant, Nb and CFU values from the spleen, liver, and GB samples were very similar to the values obtained with inoculation of the wt strain (Fig. 1A vs. 3B). Furthermore, when the barcoded ΔcheA and wt libraries were coinoculated orogastrically, the ΔcheA mutant was found to have a competitive index of ∼1 relative to the wt strain (i.e., no colonization deficit) (Fig. 3C), and ΔcheA and wt strains were equally likely to be the dominant strain isolated from GB samples (Fig. 3D). Together, these observations all suggest that neither chemotaxis nor motility modulates the capacity of L. monocytogenes to reach or colonize the GB, liver, spleen, and MLN.
Fig. 3.
Chemotaxis and motility are not required for L. monocytogenes to breach intestinal barriers and reach distal infection sites. (A) Motility of wt (WT) and ΔcheA L. monocytogenes in 0.3% agar plates. (B) CFU and Nb sizes for the indicated tissues at 3 dpi of mice inoculated orogastrically with 3 × 109 CFUs of ΔcheA L. monocytogenes. Each black square and red circle represents data from one mouse. Geometric means (horizontal lines) and SD (whiskers) are shown. (C) Competitive indexes for the indicated tissues from mice inoculated orogastrically with a 1:1 mixture of barcoded ΔcheA and wt L. monocytogenes. Each black circle represents data from one mouse. (D) Relative frequencies of L. monocytogenes barcodes in the GB of infected mice; data are from two litters of mice (n = 9) that were infected on different days.
Different Host Innate Immune Factors Contribute to Restricting L. monocytogenes Nb Sizes in the Spleen and Liver.
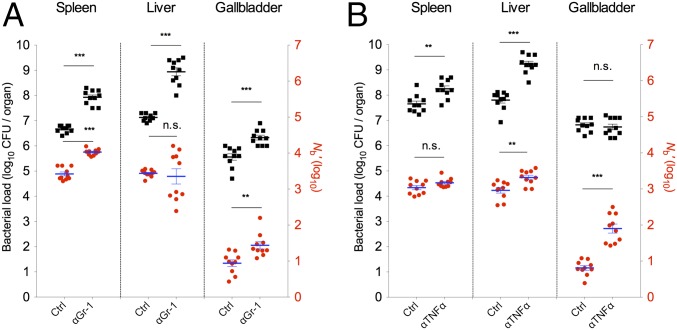
To begin to explore the host factors that control L. monocytogenes Nb sizes in various tissues, we treated mice with antibodies against components of the innate immune response before inoculation of the pathogen. Inoculation via the i.v. route, which is known to deliver an initial synchronous bolus of the pathogen to the liver (∼80% of inoculum) and spleen (∼20% of inoculum) (21), was used for these experiments because this infection route has been used for similar immune depletion studies in the past (22). Prior studies have established that after i.v. inoculation, there are two stages of L. monocytogenes growth in the spleen and liver: a lag phase (first 6 h), followed by an exponential expansion phase (21). During the lag phase, there is no net proliferation of the pathogen in the spleen, whereas in the liver, there is a net reduction in the L. monocytogenes cells during this stage (23). Most (∼80%) of the L. monocytogenes cells that reach the liver are thought to be captured and subsequently inactivated by Kupffer cells during the lag phase, whereas the remaining L. monocytogenes cells enter hepatocytes, where they can replicate (23–25).
Mice were treated with an antibody to Gr-1, which is expressed on myelomonocytic cells (MMCs), including neutrophils and monocytes, at doses known to deplete cells bearing this marker (26). This treatment leads to an accelerated course of disease (22); therefore, tissue samples for analyses were obtained 24 h after infection. Consistent with previous reports, more L. monocytogenes CFUs were recovered from the spleen and liver (22), as well as from the GB of anti–Gr-1–treated mice, than from control animals (Fig. 4A). The Nb′ values were also elevated in the spleen and GB (Fig. 4A), suggesting that Gr-1–bearing cells (or some process that requires these cells) restrict the size of the Nb′ in these two organs and likely restrain the pathogen’s subsequent replication in these sites as well. In the spleen, Nb′ values in anti–Gr-1–treated animals were elevated to values that are close to the predicted fraction of the inoculum initially captured by this organ (∼20% of the inoculum), suggesting that the anti–Gr-1 treatment ablated the spleen’s capacity to hinder L. monocytogenes in establishing a replicative niche. In contrast, Nb′ values in the livers of control and anti–Gr-1–treated mice did not differ, even though ∼60-fold more L. monocytogenes CFUs were recovered from the livers of treated mice (Fig. 4A). This discrepancy suggests that MMCs do not play a major role in modulating L. monocytogenes’ initial access to and establishment within hepatocytes, but that these cells restrict the subsequent expansion of the L. monocytogenes population, perhaps by killing L. monocytogenes released from lysed hepatocytes during the later stage of L. monocytogenes proliferation (21, 23).
Fig. 4.
Modulation of the capacity of L. monocytogenes to establish and proliferate in the spleen, liver, and GB following treatment with monoclonal anti–Gr-1 or anti–TNF-α antibodies. Control (Ctrl) and anti–Gr-1 mAb-treated mice (A) or anti–TNF-α–treated mice (B) were infected i.v. with 6 × 104 CFUs of L. monocytogenes. CFU (black squares) and Nb′ (red circles) values for the indicated tissues were determined 1 dpi in the anti–Gr-1–treated mice and 2 dpi in the anti–TNF-α–treated mice. The data are from two litters of mice (n = 10) that were infected on different days. Each black square and red circle represents data from one mouse. Geometric means (horizontal lines) and SEM (whiskers) are shown. The Mann–Whitney test was used to assess significance: *P < 0.05, **P < 0.01, ***P < 0.001. n.s., no significance.
Mice were also treated with antibody to TNF-α, a cytokine that promotes the activity of macrophages (including Kupffer cells in the liver), monocytes, and neutrophils (27–29). For these experiments, tissue samples were collected 2 d after i.v. inoculation of L. monocytogenes. Similar to anti–Gr-1–treated mice, the numbers of L. monocytogenes CFUs recovered from the spleens and livers of anti–TNF-α–treated mice were elevated compared with control animals (Fig. 4B). However, in contrast to anti–Gr-1 treatment, administration of antibody to TNF-α elevated Nb′ values in the livers, but not in the spleens, of treated mice (Fig. 4B). The elevation of hepatic Nb′ values in response to TNF-α inactivation, but not in response to anti–Gr-1 treatment, which depletes monocytes and neutrophils, suggests that the pathogen’s capacity to establish infection in the liver may be restricted by Kupffer cells. These cells are known to produce antimicrobial products (e.g., reactive oxygen species, superoxide) in response to TNF-α stimulation (30), and their activity is reduced by anti–TNF-α treatment (30). Taken together, the observations from the antibody depletion experiments suggest that different innate immune cell types restrict the capacity of L. monocytogenes to establish infection in the spleen and liver.
In addition to its effect on liver and spleen colonization, pretreatment with anti–TNF-α mAb significantly increased the Nb′ values for GBs (Fig. 4B) of mice inoculated i.v. with L. monocytogenes. However, inactivation of TNF-α did not change the bacterial burden in the GB (Fig. 4B), suggesting that TNF-α activity restricts the capacity of L. monocytogenes to access/establish in this organ but not its subsequent proliferation to 107 CFUs, which may represent the maximal carrying capacity of the GB for L. monocytogenes.
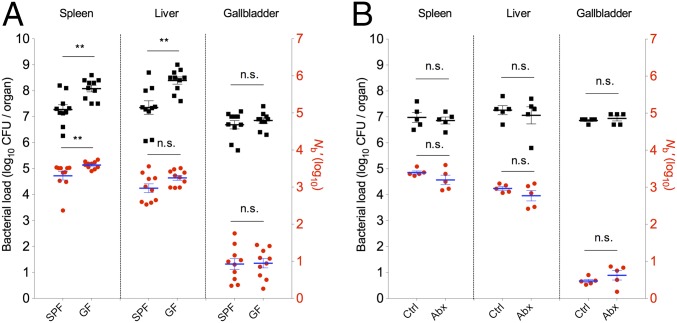
Microbiota Augments Splenic but Not Hepatic Barriers to the Pathogen.
The intestinal microbiota can play a direct role in protecting the host from intestinal pathogens (31). In addition, the microbiota exerts indirect yet critical influences on pathogen–host interactions by modulating the development of several host organ systems, including the innate and adaptive immune systems (32). We compared L. monocytogenes CFU and Nb′ values from the spleen, liver, and GB from conventional [specific pathogen-free (SPF)] and GF Swiss Webster mice following i.v. inoculation of the pathogen. Elevated CFUs were recovered from the spleens and livers of GF mice relative to SPF mice, as observed previously in C57BL/6 mice (33), but no increase was observed in CFUs recovered from the GB (Fig. 5A). The elevated L. monocytogenes CFU values in spleens from GF mice were accompanied by elevated Nb′ values in this tissue (Fig. 5A), whereas hepatic GF samples only showed an increase in CFUs. Thus, the alterations seen in GF livers and spleens parallel the alterations observed in anti–Gr-1–treated animals (compare Figs. 4A and 5A), raising the possibility that the absence of microbiota impairs development of neutrophils and/or monocytes that are targeted by the anti–Gr-1 treatment. Depletion of gut microbiota by oral administration of broad-spectrum antibiotics for 17 d, which resulted in a 2 × 105-fold reduction in amplifiable 16S rRNA sequences in feces, did not alter splenic or hepatic bacterial loads or Nb′ values (Fig. 5B), consistent with reports that the microbiota must be present during an early critical period to shape innate immune development (34). Previous studies have suggested that the intestinal microbiota enhances the bactericidal activity of bone marrow-derived neutrophils (35) and promotes the differentiation of granulocyte and monocyte progenitors in the bone marrow and spleen (33), which might underlie the distinct CFU and Nb′ values we observed for spleens of GF and SPF animals. However, the similarity of Nb′ values in livers from GF and SPF mice suggests that the gut microbiota does not markedly shape the capacity of Kupffer cells or any other hepatic innate immune cells that restrict the establishment of L. monocytogenes in the liver. Taken together, our experiments suggest that the microbiota exerts distinct roles in the development of splenic and hepatic innate immune cells.
Fig. 5.
Modulation of host barriers to L. monocytogenes infection by the microbiota. (A) GF and SPF Swiss Webster mice were inoculated i.v. with 6 × 104 L. monocytogenes CFUs. CFU (black squares) and Nb′ (red circles) values in the indicated tissues were determined 2 d later. Data are from two litters of mice (n = 10) that were infected on different days. Each black square and red circle represents data from one mouse. (B) Antibiotic-treated (Abx) Swiss Webster mice and nontreated control mice were inoculated i.v. with 6 × 104 L. monocytogenes CFUs. CFU (black squares) and Nb′ (red circles) values in the indicated tissues were determined 2 d later. Each black square and red circle represents data from one mouse. Geometric means (horizontal lines) and SEM or SD (whiskers, A and B, respectively) are shown. The Mann–Whitney test was used to assess significance: *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
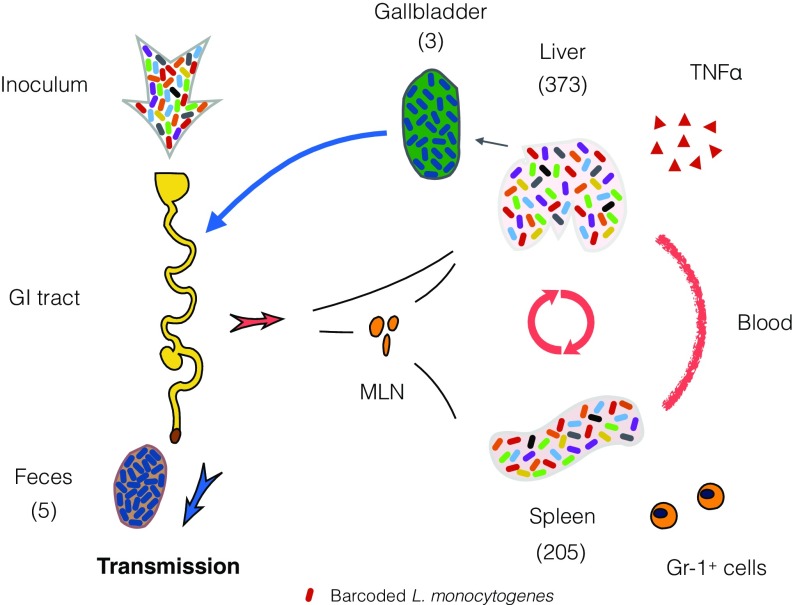
We took advantage of STAMP, using barcoded but otherwise isogenic L. monocytogenes, to quantify the effects of host barriers on this food-borne pathogen’s capacity to establish a replicative niche (i.e., defined Nb) in various host organs following OG or i.v. inoculation. We found that L. monocytogenes populations with distinct composition and complexity arise within the spleen, liver, GB, and other tissues due to organ-specific restrictions imposed by innate immune factors and the microbiota, as well as by the existence of multiple dissemination pathways (Fig. S4). The i.v. infection route did not increase the particularly low Nb′ values that were observed in fecal, GB, and brain samples with OG inoculation, suggesting that escape from the intestine is not the primary barrier to colonization of these sites. Surprisingly, Nb′ values in the SI and colon were similar regardless of the route of inoculation. Thus, the number of niches available for L. monocytogenes replication in intestinal tissue does not appear to be primarily determined by the route (intestinal tract vs. blood) through which the pathogen reaches these sites. Collectively, our observations illustrate the power of the STAMP approach to decipher the impact of host factors on population dynamics of pathogens during infection.
Fig. S4.
Schematic of L. monocytogenes population dynamics following OG inoculation of barcoded organisms. L. monocytogenes populations with distinct composition and complexity arise within the spleen, liver, GB, and other tissues due to organ-specific restrictions imposed by innate immune factors, as well as by the existence of multiple dissemination pathways (mean Nb values are shown in parentheses). Very few organisms establish infection in the GB, but the founder(s) ultimately replicate to very high numbers. Organisms released from the GB through the bile become the principal source of L. monocytogenes excreted in the feces.
Besides enabling quantification of Nb sizes, STAMP facilitates comparative analyses of the genetic diversity of populations recovered from different host organs. Prior studies by Contag and coworkers (12) revealed that the GB is routinely colonized in mice infected with L. monocytogenes, but did not discern the full ramifications of this finding. Here, by comparing the barcodes of L. monocytogenes recovered from the GB and feces, we discovered that the GB is the principal source for L. monocytogenes shed in the feces. Organisms recovered from the GB and feces usually contained the same single highly dominant tag, although the identity of the tag differed among animals. Although fecal-oral transmission is not an important route of L. monocytogenes transmission in humans, it is thought to be important in ruminants in the wild (36). Thus, if the GBs of ruminant animals are colonized with L. monocytogenes in a pauciclonal manner as we have observed in mice, then it is likely that pauciclonal fecal-oral transmission of L. monocytogenes occurs in the wild. With such a restrictive bottleneck limiting transmission of the pathogen, it is likely that genetic drift is a potent force in shaping L. monocytogenes evolution in the wild. Furthermore, our findings suggest an unexpected similarity between L. monocytogenes and Salmonella enterica serotype Typhi, for which the GB is the reservoir for S. typhi transmitted among humans (37).
Both MMCs and TNF-responsive cells limit the capacity of L. monocytogenes to establish a replicative niche in the GB; however, additional studies are required to define host and pathogen factors further that account for the profound restriction of the L. monocytogenes Nb size in this organ. We speculate that a very small pauciclonal L. monocytogenes population seeds the GB early in infection and that this population receives unfettered access to niches in which rapid replication is possible. This initial Nb′ value may be derived from the first pathogen cells to lyse out of infected hepatocytes and migrate through the bile to the GB. Subsequently, after the initial replication of these founders, resources for replication of “late arrivers” to the GB are restricted and they are at a competitive disadvantage. This scenario, which is similar to the “priority effect” proposed by Lam and Monack (5) to explain the clonality of Salmonella enterica serovar Typhimurium observed during chronic colonization of the murine colon, assumes that there are a relatively finite number of niches optimal for L. monocytogenes replication in the GB. It is also possible that the growth of the L. monocytogenes founders in the GB induces expression of pathogen factors that antagonize growth of the late arrivers. Further studies to elucidate the molecular mechanisms that account for the highly restricted size of the founding L. monocytogenes population in the GB are warranted. Indeed, deeper understanding of the L. monocytogenes factors that facilitate its replication in the GB could contribute to the creation of safer live-attenuated L. monocytogenes strains for use as vaccine vectors and in cancer therapeutics (38).
Materials and Methods
The STAMP protocol used here was similar to the STAMP protocol described by Abel et al. (8). For most in vivo studies, 8-wk-old female BALB/c mice were orogastrically inoculated with 3 × 109 CFUs of barcoded L. monocytogenes 10403S InlAm; 48 h and 72 h after inoculation, animals were euthanized and organs (proximal SI, colon, MLN, spleen, liver, GB, blood, and brain), as well as fecal pellets, were collected and homogenized. All of the homogenates/samples were plated on BHI-streptomycin plates for CFU enumeration and Nb analysis. For Nb analysis, L. monocytogenes colonies were washed off plates, genomic DNA was extracted, and the region harboring the 30-bp barcodes was amplified using primer PLM30 and primer PLM6-P29 (Table S1). The purified PCR products were combined in equimolar concentrations and sequenced on an Illumina MiSeq machine using primer PLM49. Reaper-12–340 was used to discard sequence reads with low quality (≤Q30) and trim the sequence following the barcode. The trimmed sequences were clustered with QIIME (version 1.6.0) using pick_otus.py with a sequence similarity threshold of 0.9. Then, Nb was calculated and adjusted by the calibration curve to yield Nb′ using an R script (8). Genetic distance was estimated using the Cavalli–Sforza chord distance method (39) as described by Abel et al. (8). Genetic relatedness is 1 − genetic distance. The animal protocol used for this study was reviewed and approved by Harvard Medical Area Standing Committee on Animals. All methods are described in detail in SI Materials and Methods.
Table S1.
Primers used in this study
| Primers | 5′ to 3′ sequence |
| PLM1 | AAAGCTGGTACCGGGCCCCCCCTCGAGGTCGACGGGCGACACGGAAATGTTGAA |
| PLM2 | TGCCACCTGCAGATCTGCAGGTCGACGGATCCCAAGCTTCTTCTAGACAGGAAACAGCTATGACNNNNNNNNNNNNNNNNNNNNNNNNNNNNNNACTGGCCGTCGTTTTACACCTCAGCGGAGAAGAACACT |
| PLM3 | AGTCAAAACAAACTAGCGATCGAATTCCCGGGAGAGCTCACCGCACAGATGCGTAAGGAG |
| PLM4 | ACGTCAATACGACTCACTATAGGGCGAATTGGAGCTAACACTTAACGGCTGACATGG |
| PLM5 | CTGCAGATCTGCAGGTCGACGGATCCCAAGC |
| PLM6 | CAAGCAGAAGACGGCATACGAGATCGTGATGTGACTGGAGTTCAGACGTGTGCTCTTCCGATCTTGTCTCATGAGCGGATACA |
| PLM7 | CAAGCAGAAGACGGCATACGAGATACATCGGTGACTGGAGTTCAGACGTGTGCTCTTCCGATCTTGTCTCATGAGCGGATACA |
| PLM8 | CAAGCAGAAGACGGCATACGAGATGCCTAAGTGACTGGAGTTCAGACGTGTGCTCTTCCGATCTTGTCTCATGAGCGGATACA |
| PLM9 | CAAGCAGAAGACGGCATACGAGATTGGTCAGTGACTGGAGTTCAGACGTGTGCTCTTCCGATCTTGTCTCATGAGCGGATACA |
| PLM10 | CAAGCAGAAGACGGCATACGAGATCACTGTGTGACTGGAGTTCAGACGTGTGCTCTTCCGATCTTGTCTCATGAGCGGATACA |
| PLM11 | CAAGCAGAAGACGGCATACGAGATATTGGCGTGACTGGAGTTCAGACGTGTGCTCTTCCGATCTTGTCTCATGAGCGGATACA |
| PLM12 | CAAGCAGAAGACGGCATACGAGATGATCTGGTGACTGGAGTTCAGACGTGTGCTCTTCCGATCTTGTCTCATGAGCGGATACA |
| PLM13 | CAAGCAGAAGACGGCATACGAGATTCAAGTGTGACTGGAGTTCAGACGTGTGCTCTTCCGATCTTGTCTCATGAGCGGATACA |
| PLM14 | CAAGCAGAAGACGGCATACGAGATCTGATCGTGACTGGAGTTCAGACGTGTGCTCTTCCGATCTTGTCTCATGAGCGGATACA |
| PLM15 | CAAGCAGAAGACGGCATACGAGATAAGCTAGTGACTGGAGTTCAGACGTGTGCTCTTCCGATCTTGTCTCATGAGCGGATACA |
| PLM16 | CAAGCAGAAGACGGCATACGAGATGTAGCCGTGACTGGAGTTCAGACGTGTGCTCTTCCGATCTTGTCTCATGAGCGGATACA |
| PLM17 | CAAGCAGAAGACGGCATACGAGATTACAAGGTGACTGGAGTTCAGACGTGTGCTCTTCCGATCTTGTCTCATGAGCGGATACA |
| PLM18 | CAAGCAGAAGACGGCATACGAGATTGTTGACTGTGACTGGAGTTCAGACGTGTGCTCTTCCGATCTTGTCTCATGAGCGGATACA |
| PLM19 | CAAGCAGAAGACGGCATACGAGATACGGAACTGTGACTGGAGTTCAGACGTGTGCTCTTCCGATCTTGTCTCATGAGCGGATACA |
| PLM20 | CAAGCAGAAGACGGCATACGAGATTCTGACATGTGACTGGAGTTCAGACGTGTGCTCTTCCGATCTTGTCTCATGAGCGGATACA |
| PLM21 | CAAGCAGAAGACGGCATACGAGATCGGGACGGGTGACTGGAGTTCAGACGTGTGCTCTTCCGATCTTGTCTCATGAGCGGATACA |
| PLM22 | CAAGCAGAAGACGGCATACGAGATGTGCGGACGTGACTGGAGTTCAGACGTGTGCTCTTCCGATCTTGTCTCATGAGCGGATACA |
| PLM23 | CAAGCAGAAGACGGCATACGAGATCGTTTCACGTGACTGGAGTTCAGACGTGTGCTCTTCCGATCTTGTCTCATGAGCGGATACA |
| PLM24 | CAAGCAGAAGACGGCATACGAGATAAGGCCACGTGACTGGAGTTCAGACGTGTGCTCTTCCGATCTTGTCTCATGAGCGGATACA |
| PLM25 | CAAGCAGAAGACGGCATACGAGATTCCGAAACGTGACTGGAGTTCAGACGTGTGCTCTTCCGATCTTGTCTCATGAGCGGATACA |
| PLM26 | CAAGCAGAAGACGGCATACGAGATTACGTACGGTGACTGGAGTTCAGACGTGTGCTCTTCCGATCTTGTCTCATGAGCGGATACA |
| PLM27 | CAAGCAGAAGACGGCATACGAGATATCCACTCGTGACTGGAGTTCAGACGTGTGCTCTTCCGATCTTGTCTCATGAGCGGATACA |
| PLM28 | CAAGCAGAAGACGGCATACGAGATATATCAGTGTGACTGGAGTTCAGACGTGTGCTCTTCCGATCTTGTCTCATGAGCGGATACA |
| PLM29 | CAAGCAGAAGACGGCATACGAGATAAAGGAATGTGACTGGAGTTCAGACGTGTGCTCTTCCGATCTTGTCTCATGAGCGGATACA |
| PLM30 | AATGATACGGCGACCACCGAGATCTACACTCTTTCCCTACACGACGCTCTTCCGATCTTGTAAAACGACGGCCAGT |
| PLM49 | ACGCTCTTCCGATCTTGTAAAACGACGGCCAGT |
| CheAupF | TTGCATGCCTGCAGGTCGACTCTAGAGGTCTTTCTGCTGGAACAATC |
| CheAupR | GCTATCCACCTCCATTTCTC |
| CheAdownF | GAGAAATGGAGGTGGATAGCGGAGGGACCATGATGGAACA |
| CheAdownR | TCGAGCTCGGTACCCGGGGATCCTAGCTTGTTTGGCGATTGCC |
| 16SrRNAF | GTGSTGCAYGGYTGTCGTCA |
| 16SrRNAR | ACGTCRTCCMCACCTTCCTC |
| pIgRF | TTTGCTCCTGGGCCTCCAAGTT |
| pIgRR | AGCCCGTGACTGCCACAAATCA |
SI Materials and Methods
Animals.
BALB/c mice were purchased from Charles River Laboratories. Eight-week-old female BALB/c mice were used for all experiments shown in Figs. 1–4. Due to lack of availability of GF BALB/c mice, 8-wk-old female GF Swiss Webster mice were purchased from the Harvard Digestive Disease Center Gnotobiotic and Microbiology Core and used for the GF experiments shown in Fig. 5. Age- and gender-matched murine pathogen-free Swiss Webster mice were purchased from Taconic Biosciences and were used as controls. Eight-week-old female Swiss Webster mice (Taconic Biosciences) were used for gut microbiota depletion experiments in Fig. 5.
Bacterial Strains and Growth Conditions.
All Listeria monocytogenes strains used in this study are derivatives of L. monocytogenes 10403S InlAm (made by Hélène Marquis, Cornell University, Ithaca, NY), a strain where internalin A contains two amino acid substitutions that increase its capacity to bind murine E-cadherin (19). The ΔcheA strain was constructed through standard allelic exchange using shuttle plasmid pKSV-7 oriT (40). Escherichia coli was grown in LB (Difco) at 37 °C supplemented with antibiotics when necessary. L. monocytogenes was grown in brain heart infusion (BHI; BD Biosciences) broth at 37 °C supplemented with antibiotics when necessary. Antibiotics were used at the following final concentrations: ampicillin (Amp, 50 μg/mL), chloramphenicol (Cm, 7.5 μg/mL for L. monocytogenes and 20 μg/mL for E. coli), and streptomycin (Sm, 200 μg/mL). To test the growth kinetics of L. monocytogenes strains, 5 μL of 40× diluted overnight culture was inoculated into 200 μL of BHI broth. The Bioscreen C growth curve analysis system was used to measure optical density of the cultures at 5-min intervals. To test the motility of L. monocytogenes strains, wt and ΔcheA strains were spotted on a soft agar plate (1% tryptone, 0.25% NaCl, and 0.3% agar) and incubated at 30 °C for 16 h.
Construction of the Library of Barcoded L. monocytogenes.
Construction of the barcoded L. monocytogenes library followed a similar protocol as described by Abel et al. (8). A DNA fragment encoding the CcdB toxin was amplified from pSoA160 (8) with primers PLM1 and PLM4 (Table S1) and inserted into SacI- and SalI-digested pPL2, a plasmid that is capable of integrating into the phage attachment site in the genome of L. monocytogenes 10403S InlAm strain (18), yielding plasmid pTZ1. An ∼300-bp fragment was amplified from pPL2 using PLM3 and PLM2, which contains a 30-bp random sequence, and inserted into BamHI- and SacI-digested pTZ1 using isothermal assembling, resulting in plasmid pTZ2.mix. Subsequently, the set of pTZ2.mix plasmids was introduced to E. coli SM10λpir and then transferred to L. monocytogenes 10403S InlAm via conjugation (18). Briefly, L. monocytogenes and Sm10λpir were cultured to exponential growth phase (OD600 of 0.6∼0.8) at 30 °C. One microliter of donor and recipient cultures was pelleted, washed three times with BHI broth, and mixed. One hundred microliters of the mixtures was spotted on 0.45-μm HA-type filters (Millipore) on an LB agar plate and kept at 37 °C for 2 h. The mixtures were washed off filters using 2 mL of BHI, and 50-μL aliquots were spread on BHI plates with Sm and Cm. Plates were first kept at 30 °C overnight, and then moved to 37 °C for 48–72 h. The SmR CmR colonies were pooled, resulting in L. monocytogenes 10403S InlAm. mix. To build a defined barcoded L. monocytogenes library, individual colonies from L. monocytogenes 10403S InlAm. mix were arrayed and their barcode sequences were determined using Sanger sequencing. Two hundred colonies, each of which contained a unique barcode, were mixed and grown to an OD600 of 1.1, resulting in the Lm-STAMP-200 library, which was saved in 1-mL aliquots and used for the animal infection experiments.
To introduce the barcodes into the ΔcheA strain, an ∼300-bp fragment was amplified from genomic DNA of Lm-STAMP-200 using PLM3 and PLM5 and ligated into BamHI- and SacI-digested pTZ1, resulting in plasmid pTZ200.mix. The pTZ200.mix was introduced to SM10λpir and transferred to the ΔcheA strain via conjugation as described above.
Animal Experiments.
A 1-mL aliquot of the Lm-STAMP-200 library was cultured in 10 mL of BHI broth with Sm and Cm for 3 h at 37 °C. Bacteria were pelleted by centrifugation (3.000 × g for 10 min), washed twice with 20 mL of PBS, resuspended in PBS, and adjusted to 1.5 × 1010 CFU/mL. For OG inoculation experiments, a protocol slightly modified from Disson et al. (41) was used. Briefly, animals were fasted for 8 h, lightly sedated by inhalation of isoflurane, and orogastrically inoculated with 3 × 109 CFUs of barcoded L. monocytogenes (200 μL) suspended in a 300-μL mixture of 200 mM calcium carbonate using a 1-mL syringe attached to p50 polyethylene tubing. For i.v. inoculations, the barcoded L. monocytogenes library was prepared as described above and diluted to 6 × 105 CFU/mL in PBS, and 100 μL (6 × 104 CFUs) was injected into the tail vein.
Forty-eight hours and 72 h after OG inoculation and 48 h after i.v. infection, animals were euthanized and organs [proximal SI (proximal one-third), colon, MLN, spleen, liver, GB, blood, and brain] as well as fecal pellets were collected. The MLN, spleen, liver, and brain were homogenized in 5 mL of sterile PBS using a beat-beater (BioSpec Products, Inc.). GBs and fecal pellets were collected in Eppendorf tubes containing 1 mL of PBS. GBs were ruptured with a 23-gauge needle (Becton Dickinson), and fecal pellets were homogenized with wooden sticks. Blood was collected into an Eppendorf tube and mixed with heparin (final concentration of 100 U/mL; Sigma). The proximal SI was evenly divided into two parts and randomly assigned to either a gentamicin treatment group or an untreated group. Untreated SI was homogenized in 5 mL of sterile PBS. The colons and the SIs in the gentamicin-treated group were cut open longitudinally, put in DMEM with 100 μg/mL gentamicin for 2 h to kill the extracellular bacteria, transferred to 100 × 15-mm Petri dishes, washed with 25 mL of PBS five times, and homogenized in 5 mL of sterile PBS. For Nb analysis, 600 μL of samples from the colon, MLN, spleen, liver, GB, and blood, and three replicates of the inoculum (200 μL each) were independently spread on three BHI-Sm plates. For the brain and proximal SI, 3-mL samples were spread on three 150 × 15-mm BHI-Sm plates. Plates were kept at 37 °C for 48 h before bacteria were harvested. For CFU enumeration, all of the homogenates/samples were serially diluted, plated on BHI-Sm plates, and kept at 37 °C for 48 h. The CFUs were counted and expressed as per organ for the proximal SI, colon, MLN, spleen, liver, GB, and brain; per milliliter for blood and in vitro cultures; and per pellet for feces.
Depletion of Gr-1 Cells and TNF-α by mAb.
Eight-week-old female BALB/c mice were injected i.p. with 200 μg of either anti–TNF-α (MP6-XT22; BioLegend) or anti–Gr-1 (RB6-8C5; eBioscience) mAb 5 h before i.v. infection. Control mice were injected i.p. with 200 μL of PBS or untreated, yielding equivalent results. Control and mAb-treated mice were infected i.v. with 6 × 104 CFUs of barcoded L. monocytogenes via tail veins. The bacteria burden (CFUs) and Nb′ value of the spleen, liver, and GB of control and mAb-treated mice were analyzed at 24 h postinjection (PI) for anti–Gr-1 mAb treatment and 48 h PI for anti–TNF-α mAb treatment as described above. The resource equation (42) was used to estimate the number of animals needed in the control and treated groups for these experiments.
Antibiotic Treatment of Mice.
To deplete the gut microbiota, 8-wk-old female Swiss Webster mice (Taconic Biosciences) were given a mixture of Amp (Sigma), neomycin sulfate (Sigma), and Sm (Life Technologies), each at 1 mg/mL, and vancomycin (0.5 mg/mL; Sigma) in their drinking water for 17 d (33). Drinking water with antibiotics was provided ad libitum and changed every other day. Depletion of gut microbiota was confirmed by quantification of bacterial 16S genes in fecal samples normalized to the mouse pIgR using quantitative PCR (43). Antibiotics were withdrawn from the water 2 d before infection. Mice that were given water free of antibiotics were used as nontreated controls. Antibiotic-treated and nontreated mice were inoculated i.v. with 6 × 104 CFUs of barcoded L. monocytogenes. The bacterial burden (CFUs) and Nb′ value of the spleen, liver, and GB were analyzed 48 h later.
Calculation of Nb and Genetic Distance.
Calculation of Nb′ and genetic relatedness was determined as described by Abel et al. (8). Briefly, L. monocytogenes colonies were washed off from BHI plates with PBS. Genomic DNA was then extracted (Wizard Genomic DNA Purification Kit; Promega) from ∼1 × 1010 bacteria. The region that harbors the 30-bp barcodes was amplified from genomic DNA using primer PLM30 and primer PLM6-P29. The PCR products were purified (MinElute; Qiagen) and quantified (Qubit dsDNA HS Assay Kit; Life Technologies). The purified PCR products were combined in equimolar concentrations and sequenced on an Illumina MiSeq machine (Miseq Reagent Kit V2, 50-cycle; Illumina) using primer PLM49. Reaper-12–340 was used to discard sequence reads with low quality (≤Q30) and to trim the sequence following the barcode. The trimmed sequences were clustered with QIIME (version 1.6.0) using pick_otus.py with a sequence similarity threshold of 0.9. Then, Nb was calculated and adjusted by the calibration curve to yield Nb′ using an R script (8). When the number of recovered CFUs was below 500 (or 1,500 in gentamicin-treated samples), Nb determination was not performed. Genetic distance was estimated using the Cavalli–Sforza chord distance method (39) as described by Abel et al. (8). Genetic relatedness is 1 − genetic distance.
Calibration Curve.
A calibration curve was generated as described by Abel et al. (8). Briefly, Lm-STAMP-200 culture was prepared as described above. Three independent replicates (from three aliquots of Lm-STAMP-200) were performed in parallel. For each sample, a defined number of bacteria was obtained by an independent serial dilution of Lm-STAMP-200 culture, plated on BHI-Sm plates, and kept at 37 °C for 48 h. CFUs were counted, and colonies were washed off the plates for Nb analysis as described above. The resulting correlation curve was used to calibrate the in vivo data.
Infection with ΔcheA Strain and in Vivo Competition Experiment.
The inoculum of barcoded ΔcheA L. monocytogenes was prepared as described above. Eight-week-old female BALB/c mice were orogastrically inoculated with 3 × 109 CFUs of the ΔcheA library. Mice were killed at 72 h PI; bacterial burden (CFUs) and Nb size of the spleen, liver, and GB were analyzed as described above. For competition experiments, equal amounts (CFUs) of uniquely barcoded wt (42 barcodes) and ΔcheA (41 barcodes) strains were mixed and used to infect mice orogastrically as described above. At 72 h PI, the competitive index was determined by calculating the ratio of the total number of reads from wt barcodes to the total number of reads from ΔcheA barcodes from the tissue samples, divided by the ratio of barcodes in the inoculum.
Statistics.
Prism 7 (GraphPad software) was used for statistical analysis. Mann–Whitney tests were used for comparison of data between two groups, and the Pearson correlation coefficient value was used to quantify correlation. Data from all animals are displayed in the figures, and no outliers were removed before analyses were carried out.
Acknowledgments
We thank members of the M.K.W. laboratory for helpful discussion. This work is supported by NIH Grant R37-AI-042347 (to M.K.W.), the Howard Hughes Medical Institute (M.K.W.), Research Council of Norway (NFR) Grant 249979 (to S.A.), and Helse-Nord Grant 14796 (to S.A.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1702077114/-/DCSupplemental.
References
- 1.Abel S, Abel zur Wiesch P, Davis BM, Waldor MK. Analysis of bottlenecks in experimental models of infection. PLoS Pathog. 2015;11:e1004823. doi: 10.1371/journal.ppat.1004823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes PD, Bergman MA, Mecsas J, Isberg RR. Yersinia pseudotuberculosis disseminates directly from a replicating bacterial pool in the intestine. J Exp Med. 2006;203:1591–1601. doi: 10.1084/jem.20060905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grant AJ, et al. Modelling within-host spatiotemporal dynamics of invasive bacterial disease. PLoS Biol. 2008;6:e74. doi: 10.1371/journal.pbio.0060074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaiser P, Slack E, Grant AJ, Hardt WD, Regoes RR. Lymph node colonization dynamics after oral Salmonella Typhimurium infection in mice. PLoS Pathog. 2013;9:e1003532. doi: 10.1371/journal.ppat.1003532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lam LH, Monack DM. Intraspecies competition for niches in the distal gut dictate transmission during persistent Salmonella infection. PLoS Pathog. 2014;10:e1004527. doi: 10.1371/journal.ppat.1004527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim CH, et al. Independent bottlenecks characterize colonization of systemic compartments and gut lymphoid tissue by Salmonella. PLoS Pathog. 2014;10:e1004270. doi: 10.1371/journal.ppat.1004270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melton-Witt JA, Rafelski SM, Portnoy DA, Bakardjiev AI. Oral infection with signature-tagged Listeria monocytogenes reveals organ-specific growth and dissemination routes in guinea pigs. Infect Immun. 2012;80:720–732. doi: 10.1128/IAI.05958-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abel S, et al. Sequence tag-based analysis of microbial population dynamics. Nat Methods. 2015;12:223–226. doi: 10.1038/nmeth.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lecuit M, et al. A transgenic model for listeriosis: Role of internalin in crossing the intestinal barrier. Science. 2001;292:1722–1725. doi: 10.1126/science.1059852. [DOI] [PubMed] [Google Scholar]
- 10.Bakardjiev AI, Theriot JA, Portnoy DA. Listeria monocytogenes traffics from maternal organs to the placenta and back. PLoS Pathog. 2006;2:e66. doi: 10.1371/journal.ppat.0020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Disson O, Lecuit M. Targeting of the central nervous system by Listeria monocytogenes. Virulence. 2012;3:213–221. doi: 10.4161/viru.19586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardy J, et al. Extracellular replication of Listeria monocytogenes in the murine gall bladder. Science. 2004;303:851–853. doi: 10.1126/science.1092712. [DOI] [PubMed] [Google Scholar]
- 13.Disson O, Lecuit M. In vitro and in vivo models to study human listeriosis: Mind the gap. Microbes Infect. 2013;15:971–980. doi: 10.1016/j.micinf.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 14.Portnoy DA, Auerbuch V, Glomski IJ. The cell biology of Listeria monocytogenes infection: The intersection of bacterial pathogenesis and cell-mediated immunity. J Cell Biol. 2002;158:409–414. doi: 10.1083/jcb.200205009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agaisse H, et al. Genome-wide RNAi screen for host factors required for intracellular bacterial infection. Science. 2005;309:1248–1251. doi: 10.1126/science.1116008. [DOI] [PubMed] [Google Scholar]
- 16.Cossart P. Illuminating the landscape of host-pathogen interactions with the bacterium Listeria monocytogenes. Proc Natl Acad Sci USA. 2011;108:19484–19491. doi: 10.1073/pnas.1112371108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Witte CE, et al. Innate immune pathways triggered by Listeria monocytogenes and their role in the induction of cell-mediated immunity. Adv Immunol. 2012;113:135–156. doi: 10.1016/B978-0-12-394590-7.00002-6. [DOI] [PubMed] [Google Scholar]
- 18.Lauer P, Chow MY, Loessner MJ, Portnoy DA, Calendar R. Construction, characterization, and use of two Listeria monocytogenes site-specific phage integration vectors. J Bacteriol. 2002;184:4177–4186. doi: 10.1128/JB.184.15.4177-4186.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wollert T, et al. Extending the host range of Listeria monocytogenes by rational protein design. Cell. 2007;129:891–902. doi: 10.1016/j.cell.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez-Escobedo G, Gunn JS. Gallbladder epithelium as a niche for chronic Salmonella carriage. Infect Immun. 2013;81:2920–2930. doi: 10.1128/IAI.00258-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conlan JW. Early host-pathogen interactions in the liver and spleen during systemic murine listeriosis: An overview. Immunobiology. 1999;201:178–187. doi: 10.1016/S0171-2985(99)80057-6. [DOI] [PubMed] [Google Scholar]
- 22.Carr KD, et al. Specific depletion reveals a novel role for neutrophil-mediated protection in the liver during Listeria monocytogenes infection. Eur J Immunol. 2011;41:2666–2676. doi: 10.1002/eji.201041363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gregory SH, Sagnimeni AJ, Wing EJ. Bacteria in the bloodstream are trapped in the liver and killed by immigrating neutrophils. J Immunol. 1996;157:2514–2520. [PubMed] [Google Scholar]
- 24.Broadley SP, et al. Dual-track clearance of circulating bacteria balances rapid restoration of blood sterility with induction of adaptive immunity. Cell Host Microbe. 2016;20:36–48. doi: 10.1016/j.chom.2016.05.023. [DOI] [PubMed] [Google Scholar]
- 25.Zeng Z, et al. CRIg functions as a macrophage pattern recognition receptor to directly bind and capture blood-borne gram-positive bacteria. Cell Host Microbe. 2016;20:99–106. doi: 10.1016/j.chom.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Waite JC, et al. Dynamic imaging of the effector immune response to listeria infection in vivo. PLoS Pathog. 2011;7:e1001326. doi: 10.1371/journal.ppat.1001326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roca FJ, Ramakrishnan L. TNF dually mediates resistance and susceptibility to mycobacteria via mitochondrial reactive oxygen species. Cell. 2013;153:521–534. doi: 10.1016/j.cell.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiong H, et al. Innate lymphocyte/Ly6C(hi) monocyte crosstalk promotes Klebsiella pneumoniae clearance. Cell. 2016;165:679–689. doi: 10.1016/j.cell.2016.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 30.Nakashima H, et al. Superoxide produced by Kupffer cells is an essential effector in concanavalin A-induced hepatitis in mice. Hepatology. 2008;48:1979–1988. doi: 10.1002/hep.22561. [DOI] [PubMed] [Google Scholar]
- 31.Caballero S, Pamer EG. Microbiota-mediated inflammation and antimicrobial defense in the intestine. Annu Rev Immunol. 2015;33:227–256. doi: 10.1146/annurev-immunol-032713-120238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature. 2016;535:75–84. doi: 10.1038/nature18848. [DOI] [PubMed] [Google Scholar]
- 33.Khosravi A, et al. Gut microbiota promote hematopoiesis to control bacterial infection. Cell Host Microbe. 2014;15:374–381. doi: 10.1016/j.chom.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gensollen T, Iyer SS, Kasper DL, Blumberg RS. How colonization by microbiota in early life shapes the immune system. Science. 2016;352:539–544. doi: 10.1126/science.aad9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clarke TB, et al. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med. 2010;16:228–231. doi: 10.1038/nm.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nightingale KK, et al. Ecology and transmission of Listeria monocytogenes infecting ruminants and in the farm environment. Appl Environ Microbiol. 2004;70:4458–4467. doi: 10.1128/AEM.70.8.4458-4467.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gunn JS, et al. Salmonella chronic carriage: Epidemiology, diagnosis, and gallbladder persistence. Trends Microbiol. 2014;22:648–655. doi: 10.1016/j.tim.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le DT, et al. Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J Clin Oncol. 2015;33:1325–1333. doi: 10.1200/JCO.2014.57.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cavalli-Sforza LL, Edwards AW. Phylogenetic analysis. Models and estimation procedures. Am J Hum Genet. 1967;19:233–257. [PMC free article] [PubMed] [Google Scholar]
- 40.Whiteley AT, Pollock AJ, Portnoy DA. The PAMP c-di-AMP is essential for Listeria monocytogenes growth in rich but not minimal media due to a toxic increase in (p)ppGpp. Cell Host Microbe. 2015;17:788–798. doi: 10.1016/j.chom.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Disson O, et al. Modeling human listeriosis in natural and genetically engineered animals. Nat Protoc. 2009;4:799–810. doi: 10.1038/nprot.2009.66. [DOI] [PubMed] [Google Scholar]
- 42.Mead R. The Design of Experiments. Cambridge Univ Press; Cambridge, UK: 1988. [Google Scholar]
- 43.Reikvam DH, et al. Depletion of murine intestinal microbiota: Effects on gut mucosa and epithelial gene expression. PLoS One. 2011;6:e17996. doi: 10.1371/journal.pone.0017996. [DOI] [PMC free article] [PubMed] [Google Scholar]