Significance
The Arctic is warming rapidly, causing permafrost soils to thaw. Vast stocks of nitrogen (>67 billion tons) in the permafrost, accumulated thousands of years ago, could now become available for decomposition, leading to the release of nitrous oxide (N2O) to the atmosphere. N2O is a strong greenhouse gas, almost 300 times more powerful than CO2 for warming the climate. Although carbon dynamics in the Arctic are well studied, the fact that Arctic soils store enormous amounts of nitrogen has received little attention so far. We report that the Arctic may become a substantial source of N2O when the permafrost thaws, and that N2O emissions could occur from surfaces covering almost one-fourth of the entire Arctic.
Keywords: Arctic soils, nitrogen, greenhouse gases, climate change, tundra
Abstract
Permafrost in the Arctic is thawing, exposing large carbon and nitrogen stocks for decomposition. Gaseous carbon release from Arctic soils due to permafrost thawing is known to be substantial, but growing evidence suggests that Arctic soils may also be relevant sources of nitrous oxide (N2O). Here we show that N2O emissions from subarctic peatlands increase as the permafrost thaws. In our study, the highest postthaw emissions occurred from bare peat surfaces, a typical landform in permafrost peatlands, where permafrost thaw caused a fivefold increase in emissions (0.56 ± 0.11 vs. 2.81 ± 0.6 mg N2O m−2 d−1). These emission rates match those from tropical forest soils, the world’s largest natural terrestrial N2O source. The presence of vegetation, known to limit N2O emissions in tundra, did decrease (by ∼90%) but did not prevent thaw-induced N2O release, whereas waterlogged conditions suppressed the emissions. We show that regions with high probability for N2O emissions cover one-fourth of the Arctic. Our results imply that the Arctic N2O budget will depend strongly on moisture changes, and that a gradual deepening of the active layer will create a strong noncarbon climate change feedback.
Arctic land areas are predicted to warm up to 5.6–12.4 °C by the end of this century (1), likely leading to widespread permafrost degradation (2–5) and substantial changes in ecosystem functioning (6). With thawing, a vast pool of immobile C stored in permafrost (7) becomes available for decomposition and remobilization, triggering greenhouse gas emissions of carbon dioxide (CO2) and methane (CH4). Thus, gaseous C release from thawing permafrost is being studied extensively to evaluate the magnitude of the permafrost–carbon feedback to the climate (8, 9). Often overlooked, however, is the fact that permafrost soils are also large N reservoirs, with a conservative estimate of 67 billion tons of total N in the upper 3 m (10). Thus, the permafrost N stocks are more than 500 times larger than the annual N load added as fertilizer to soils globally (11, 12). Upon thawing, organically bound N is subject to mineralization, leading to a release of mineral N. Mineral N forms, predominantly ammonium (NH4+) and nitrate (NO3−), fuel nitrification and denitrification, respectively, the two dominant processes generating the strong greenhouse gas nitrous oxide (N2O) in soils (13).
Mounting evidence shows that Arctic soils may produce (14, 15) and release (16, 17) substantial amounts of N2O. In previous studies, we identified patches of bare peat in permafrost peatlands as hot spots for N2O emissions in subarctic tundra (16, 17). Furthermore, an increase in growing season temperature without causing permafrost thaw not only increases N2O emissions from these hot spots, but also triggers N2O emissions from vegetated tundra peatlands (18), which cover large areas of the Arctic. This highlights the important role that peatlands may play in promoting Arctic N2O emissions in the future.
So far, soil moisture, soil organic matter (SOM) content, C/N ratio, and plant growth have been identified as the key regulators of Arctic N2O emissions (17, 18). The great unknown, however, is how permafrost thaw will affect N2O emissions by potentially unlocking the vast N stocks currently stored in Arctic soils (10). Evidence for an N2O pulse from thawing permafrost is scarce, and up to now based solely on laboratory incubations with external N input (15). Field experiments, or mesocosm studies at near-field conditions, are lacking.
To assess whether permafrost thaw will increase N2O release to the atmosphere, we measured N2O fluxes from 16 intact peat mesocosms (diameter, 10 cm; length, 80 cm) during a 33-wk experiment. The mesocosms were collected in a typical subarctic permafrost peatland (68°89′N, 21°05′E) in Finnish Lapland (SI Appendix, Fig. S1) and originated from vegetated and naturally bare parts of a palsa mound (17, 19) (SI Appendix, Fig. S2), a reported N2O source (17). Some of the bare peat surfaces exhibited a sporadic lichen cover, but vascular plants, with roots penetrating into the peat profile, were absent. The mesocosms included living plants, when present, and the full peat profile from the surface of the active layer (soil layer above the permafrost subjected to seasonal thawing; 0–∼65 cm) to the upper permafrost layer (∼65–∼80 cm). On these bare and vegetated surfaces, we applied two distinct moisture treatments, representing possible postthaw conditions: an unaltered water table level (>55 cm below the surface; “dry” scenario), simulating gradual active layer deepening, and an artificially raised water table level (5–10 cm below the surface; “wet” scenario), simulating conditions after palsa collapse. Transport and storage of mesocosms took place in mild freezing temperatures (−5 °C minimum), imitating natural winter conditions. After a 5-mo preincubation period, the mesocosms were set up in a climate-controlled growth chamber (air temperature 10 °C) and then sequentially thawed, stepwise, by lowering the level of a saltwater bath (−4 °C) (SI Appendix, Figs. S3 and Fig. S4 and Table S1).
Results and Discussion
N2O Fluxes.
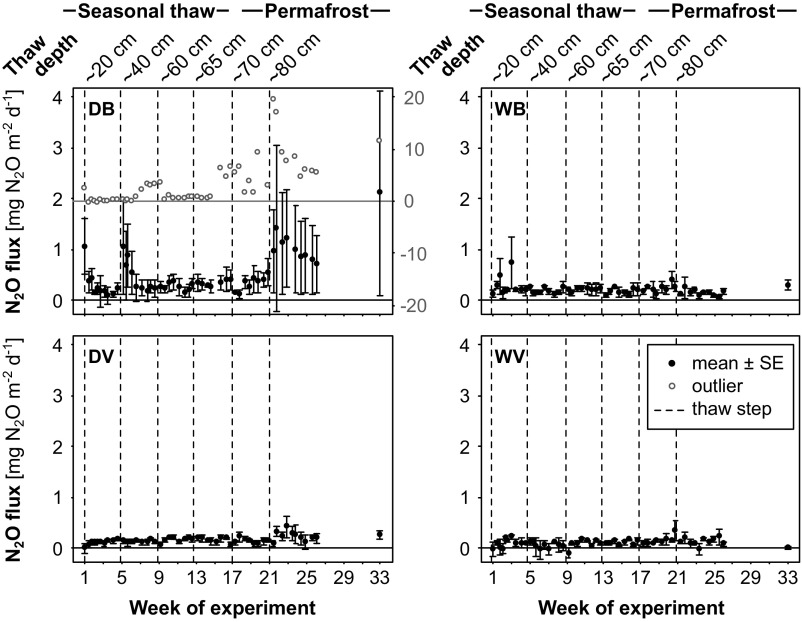
During thawing of the active layer, before the permafrost table was reached, all mesocosms released N2O. The largest emissions occurred from bare mesocosms under the dry scenario (Figs. 1 and 2). These emissions matched in situ N2O fluxes measured from bare patches in subarctic peatlands, where the absence of vascular plants increases the N available for microbial processes (17, 18). Thawing of permafrost caused a fivefold increase in N2O emissions from these bare, dry mesocosms compared with levels measured during active layer thawing (mean ± SE, 0.56 ± 0.11 vs. 2.81 ± 0.6 mg of N2O m−2 d−1; P < 0.001). The postthaw emissions matched in situ fluxes from tropical forest soils (20), the largest terrestrial N2O source (21). Fluxes from vegetated mesocosm were smaller, but thawing of permafrost almost doubled the emissions from vegetated, dry mesocosms as well (0.14 ± 0.01 vs. 0.20 ± 0.03 mg of N2O m−2 d−1; P = 0.034) (Figs. 1 and 2 and SI Appendix, Table S6). The high postthaw N2O emissions persisted for several weeks after initiation of thawing.
Fig. 1.
N2O fluxes from the peat mesocosms. N2O fluxes, measured two to three times per week, from bare and vegetated mesocosms with natural (dry) and artificially raised (5–10 cm below surface; wet) water tables, referred to as follows: DB, dry bare; DV, dry vegetated; WB, wet bare; and WV, wet vegetated. Flux data are shown as mean ± SE (closed black circles). n = 3 for DB, n = 4 for DV, WB, and WV. One replicate of DB (DB 2) with high N2O fluxes was removed from the calculated mean, and is shown separately on a secondary y-axis (open gray circles). Dashed black lines indicate thawing steps. Week 1: thawing down to 20 cm; week 5: thawing down to 40 cm; week 9: thawing down to 5 cm above the maximum seasonal thaw depth; week 13: thawing down to the maximum seasonal thaw depth; week 17: thawing down to 5 cm below the maximum seasonal thaw depth; week 21: thawing of the full core (15 cm below the maximum seasonal thaw depth). Flux rates of individual replicates are provided in SI Appendix, Figs. S7–S10.
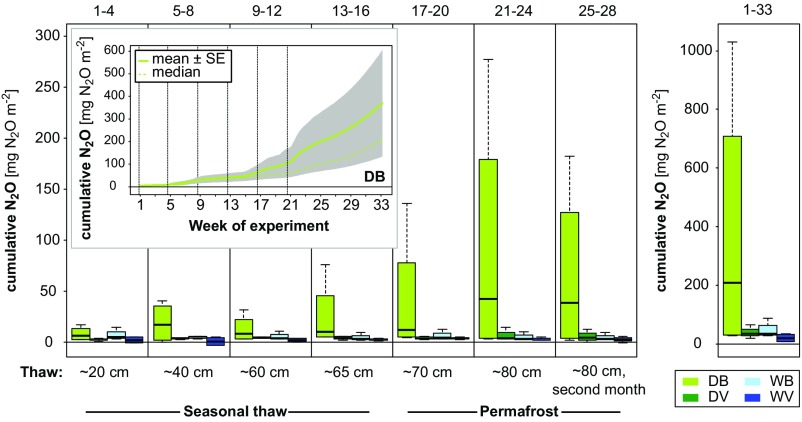
Fig. 2.
Total N2O emissions. Cumulative N2O fluxes for each 4-wk thawing step, as well as total emissions for the entire duration of the experiment (33 wk). Emissions are shown for bare and vegetated mesocosms with natural (dry) and artificially raised (5–10 cm below surface, wet) water tables, referred to as follows: DB, dry bare; DV, dry vegetated; WB, wet bare; WV, wet vegetated. Boxplots show upper and lower quartiles, median (thick black line), and smallest and largest value (dashed lines). n = 4. (Inset) Cumulative N2O fluxes (mean and median; n = 4) during the entire experiment for DB.
This significant increase in N2O emissions under simulated in situ conditions indicates the potential for a substantial noncarbon feedback to the climate when permafrost thaws. Notably, this thaw-induced N2O pulse occurred only in the dry scenario, whereas peatland collapse, simulated by a raised water table in the wet scenario, did not result in obvious emission peaks (Fig. 1). This means that relatively dry conditions (water-filled pore space 55–85%; SI Appendix, Fig. S6), associated with gradual active layer deepening, trigger substantial postthaw N2O release, whereas high N2O emissions are unlikely to occur when the permafrost peatland collapses completely. The observation of postthaw N2O release from vegetated surfaces is important, because these surfaces cover much larger areas in the Arctic than bare peat.
Soil Processes Regulating N2O Emissions to the Atmosphere.
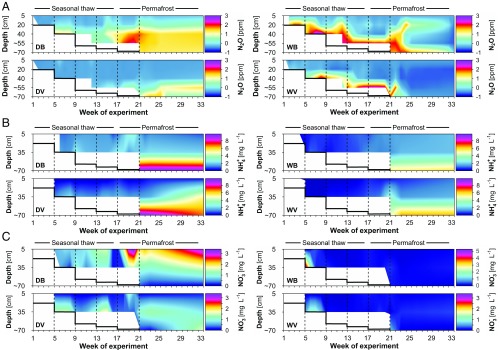
The escape of N2O to the atmosphere after permafrost thaw can be attributed to two processes (13) (SI Appendix, Fig. S5): direct release of (old) N2O, trapped during permafrost aggradation, and microbial production of N2O by nitrification (aerobic oxidation of NH4+ to NO3−) or denitrification (anaerobic respiration using NO3− as an electron acceptor). The production of N2O depends on the supply of the mineral N species NH4+ and NO3−, either by direct release from permafrost or through postthaw organic matter mineralization and nitrification. Furthermore, the amount of net atmospheric N2O release will strongly depend on the reduction of N2O to dinitrogen (N2) during denitrification, a process that reduces the amount of N escaping from the soil as N2O. To gain insight into these soil processes triggering the postthaw N2O release, we complemented our flux observations with detailed measurements of N2O, NO3−, and NH4+ throughout the peat profile.
In the dry mesocosms, peak emissions after permafrost thaw were preceded by an immediate accumulation of N2O in the deeper soil layers (bare, up to 160 ppm vs. the atmospheric concentration of 0.3 ppm; Fig. 3A and SI Appendix, Tables S7 and S8), where moisture conditions (water-filled pore space >90%; SI Appendix, Table S2) were favorable for N2O production via denitrification (SI Appendix, Fig. S14). N2O also accumulated within the wet mesocosms, especially in the lower active layer (∼40 cm; bare, up to 836 ppm; Fig. 3A), and at the active layer–permafrost interface. After permafrost thaw, however, gas concentrations in the active layer of the wet mesocosms were close to ambient, indicating effective N2O to N2 reduction during upward diffusion. Thus, complete denitrification likely explains the absence of emission peaks under wet conditions. Importantly, we show that despite the large N2O production potential and availability of mineral N forms in thawing permafrost, observed in other studies as well (15, 22–24), the emissions to the atmosphere can still be negligible when the active layer is water-saturated.
Fig. 3.
Depth distribution of gas and nutrients along the soil profile. (A) Soil profile concentration of N2O, either in the gas phase or dissolved in soil pore water, depending on the wetness of the mesocosm (atmospheric concentration of N2O, 0.3 ppm). (B and C) Concentrations of ammonium (NH4+, shown as NH4+–N) (B) and nitrate (NO3−, shown as NO3−–N) (C) in the soil pore water. Data are shown for bare and vegetated mesocosms with natural (dry) and artificially raised (5–10 cm below the surface; wet) water tables, referred to as follows: DB, dry bare; DV, dry vegetated; WB, wet bare; WV, wet vegetated. Contour plots were created by linear interpolation between measurement points. The number of measurement points was 27 for N2O and 15 for NO3− and NH4+. The white areas indicate no data available owing to frozen soil conditions. The thick black line indicates thaw depths, and the dashed lines indicate thawing steps. Week 1: thawing down to ∼20 cm; week 5: thawing down to ∼40 cm; week 9: thawing down to 5 cm above the maximum seasonal thaw depth; week 13: thawing down to the maximum seasonal thaw depth; week 17: thawing down to 5 cm below the maximum seasonal thaw depth; week 21: thawing of the full core (15 cm below the maximum seasonal thaw depth). Note the logarithmic scaling of color legends for N2O soil gas, and the deviating scaling of WB for NO3−. N2O concentrations of individual replicates are provided in SI Appendix, Figs. S12 and S13.
Toward explaining the origin of the N2O, our data suggest that direct release of old N2O or nutrients from permafrost plays a role, given that high emission pulses occurred directly after thawing. However, SOM mineralization from freshly thawed soil (24) seemed to be the key in providing an N source for N2O producers in peat; after thawing the permafrost, NH4+ accumulated at the active layer–permafrost interface (Fig. 3B), indicating sustained mineralization until the end of the experiment, thereby demonstrating the potential for longer-term N2O emissions after permafrost thaws. The NO3− pool showed only minor changes (Fig. 3C), indicating rapid turnover and consumption of NO3−. The dry mesocosms displayed a larger nutrient accumulation than the wet ones (Fig. 3 B and C), owing to oxygen-limited nitrification and mineralization processes under wet conditions.
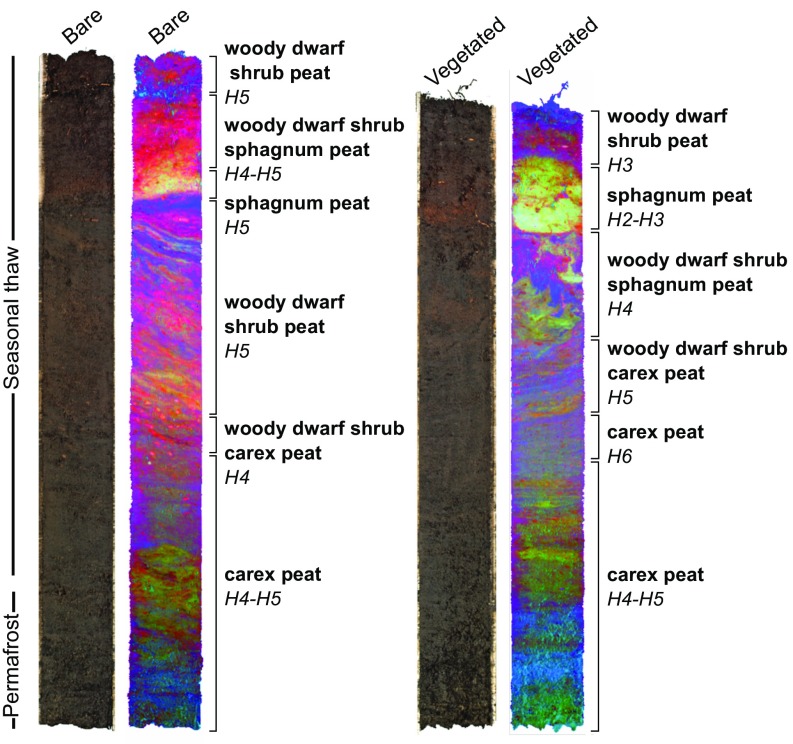
Hyperspectral imaging of two intact mesocosms (bare and vegetated), coupled with peat quality analyses, add support to the foregoing conclusions above. The image analysis identified a complex stratified structure of the peat profile (Fig. 4) typical of uplifted permafrost peatlands (25), reflecting the peatland transition from wet fen to uplifted permafrost bog. The lower half of the profile consisted of minerotrophic fen peat, with a high potential for N2O release (26). In fact, the permafrost part displayed by far the highest concentrations of dissolved N (SI Appendix, Fig. S11 and Tables S4 and S5). During the course of the experiment, the highest N2O pulses were observed when these layers thawed. Notably, N2O peaks also occurred when thawing the surface soil (Fig. 1), demonstrating a high potential for N2O production in the active layer as well.
Fig. 4.
RGB image and hyperspectral false-color images showing the peat type and the spatial variability within the peat mesocosms. The false-color image is combined from the three main components of principal component analysis of hyperspectral shortwave infrared (1,000–2,500 nm) imaging. The cores used for hyperspectral imaging (one bare and one vegetated) were not used during the experiment and thus were not subjected to sequential thawing. These unaltered cores were sampled at the same time as the mesocosms used in the experiment, and represent the original peat structure and chemistry under natural conditions. The average length of the cores was 80 cm, the average maximum seasonal thaw depth was 65 cm, and the length of the permafrost part was 15 cm. Owing to the presence of ice lenses in the bare core, the permafrost part was compacted after thawing. The degree of peat humification is indicated on the von Post scale, ranging from H1 (completely undecomposed) to H10 (completely decomposed).
Statistical analysis using linear mixed-effects models indicated that N2O production in the active layer might have added to the postthaw N2O release. The concentrations of N2O and NH4+ in the active layer and permafrost, as well as their interactions, were identified as significant model components explaining the N2O release (SI Appendix, Table S9). Nonetheless, the highest and most sustained N2O release was observed only after the permafrost part was completely thawed.
Role of Soil Moisture and Vegetation in Regulating Arctic N2O Emissions.
Undoubtedly, SOM quality is an important factor underlying N2O release from thawing permafrost peatlands. However, even if soil quality is optimal, moisture conditions at times of permafrost thaw crucially govern the rates of N2O emitted from tundra. Whether Arctic soils become wetter or drier following permafrost thaw depends largely on local hydrology and drainage conditions (27). Incorporating such postthaw landscape changes into ecosystem models still causes large uncertainties in the projections on the future C balance (9); however, it is known that even areas facing abrupt thaw and ground subsidence, initially leading to water-saturated soil conditions, often show improved drainage in the long term (27, 28). Our findings imply that thawing of permafrost causes a considerable release of N2O under drier conditions when oxygen is sufficiently available for mineralization and nitrification. These conditions prevail in permafrost peatlands, where the active layer is gradually deepening, but also in previously inundated peatlands, where permafrost thaw promotes drainage.
Field studies show that the presence of vegetation reduces N2O emissions (16–18), also observed here, by lowering the mineral N supply in the rooting zone of vascular plants (Fig. 3 B and C). Nonetheless, it is surprising that only a sparse vegetation cover so effectively reduced N2O release even after thawing the permafrost layer (Fig. 1), located at ∼65 cm depth, since differences in peat quality and moisture content between bare and vegetated mesocosms were minor (Fig. 4 and SI Appendix, Fig. S6 and Tables S2 and S3). Interestingly, not only plant uptake, but also microbial N immobilization, seemed to limit the available substrate for N2O production in vegetated mesocosms (SI Appendix, Fig. S11), indicating the importance of the rhizosphere in regulating microbial functioning. Although N immobilization was most pronounced in the top soil layer, the permafrost also displayed large microbial N pools (SI Appendix, Fig. S11), suggesting that leaching from the top soil enhanced N immobilization at depth. Importantly, the presence of vegetation decreased, but did not prevent, thawing-induced N2O release—a mechanism that requires further investigation.
Potential Pan-Arctic N2O Emissions from Thawing Permafrost.
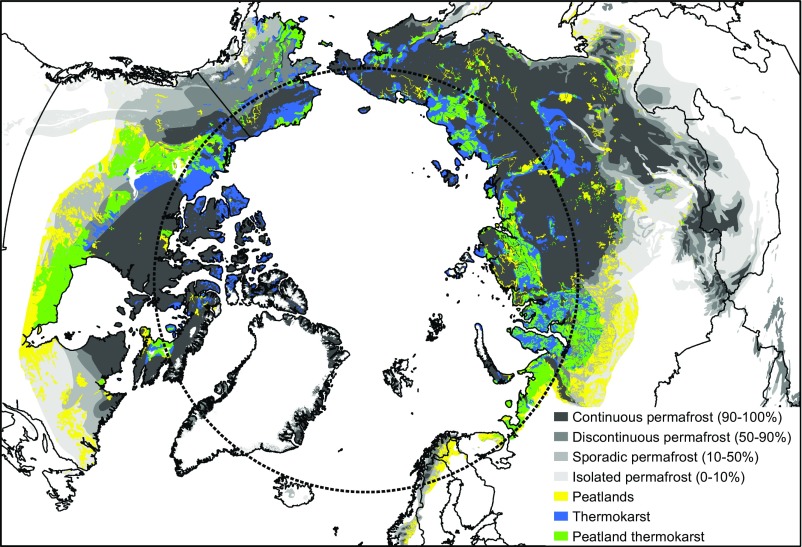
To understand the spatial relevance of postthaw N2O emissions, we produced a vulnerability map of the Arctic regions most prone to N2O emissions from thawing permafrost (Fig. 5). The results of this study and previous work on Arctic N2O emissions (16–18) show that high N2O emissions occur particularly from N-rich soil deposits, such as peatlands, or soils with a disrupted vegetation cover. Vegetation growth is disrupted particularly in thermokarst-affected soils, where the exposure of old permafrost N stocks and coexisting aerobic and anaerobic microhabitats also create favorable conditions for N2O production (14) and release. Our geographic information system (GIS)-based approach revealed that areas with a high abundance of peatlands (>15%) and landscapes vulnerable for thermokarst (>30%) cover as much as one-fourth of the northern circumpolar permafrost region (SI Appendix, Table S10). Peatlands affected by thermokarst, the most probable Arctic N2O hot spots, cover more than 10% of the Arctic, corresponding to an area of 1.9 million km2.
Fig. 5.
Vulnerability map of Arctic regions with high potential for N2O emissions due to permafrost thaw. Shown are permafrost distribution across the Arctic (33) and surfaces with high potential for N2O emissions: peatlands and thermokarst (current thermokarst landforms and areas with high susceptibility to future thermokarst development). Peatlands include histel and histosol landcover classes with >15% coverage (7, 34), and thermokarst includes areas with high (30–60%) and very high (60–100%) estimated thermokarst coverage (35).
We refrain from presenting a pan-Arctic N2O emission budget, owing to uncertainties associated with the abundance of bare soils, wet vs. dry peatlands, and deep soil N stocks in the Arctic. However, N2O emissions from bare soils in permafrost peatlands have been estimated to presently be as high as 0.1 Tg N y−1 (16). With increasing postthaw emissions from bare as well as vegetated surfaces, future pan-Arctic N2O emissions likely exceed this estimate. At a global scale, this puts Arctic N2O emissions from thawing permafrost in the range of emissions from fossil fuel combustion, industrial processes, and biomass burning, the second-largest anthropogenic N2O sources after agriculture (12, 29).
Relevance of Results and Long-Term Implications for Future N2O Release from Permafrost.
With the current active layer deepening of ∼1 cm y−1 in northern Scandinavia (30), thawing of the upper 15 cm of permafrost in a typical permafrost peatland provides a realistic near-term estimate for future N2O release under different thawing scenarios. Our observed fluxes cannot be taken as the N2O release of one growing season with increased thaw depth; rather, thawing the upper 15 cm of permafrost would take ∼15 y under in situ conditions, and warming of the soil column would occur more gradually. As our results show, however, only part of the released N2O was trapped N2O directly released from permafrost, whereas organic matter mineralization at the active layer–permafrost interface continuously stimulated N2O production. Thus, gradual thawing of permafrost, even at rates of ∼1 cm y−1, and subsequent warming of the soil would provide a sustained N source, continuously fueling N2O production at depth for decades or even centuries, depending on the thickness of the organic layer. This potential for long-term persistence in N2O production may be a unique property of permafrost peatlands, not sufficiently acknowledged so far.
Our results suggest that the magnitude of future N2O emissions depends mainly on landscape changes that alter soil moisture conditions, as well as on changes in vegetation coverage and growth. Recent reports indicate a browning trend for the Arctic (31), seemingly reversing the enhanced plant growth referred to as Arctic greening predicted by the majority of carbon models (32). Arctic browning, triggered by factors such as winter warming, extreme weather events, tundra fires, and thermokarst development (31), may be an important driver promoting Arctic N2O emissions in the future. In any case, our results show that N2O emissions in the Arctic are likely substantial and underestimated at present, and show high potential to increase with permafrost thaw.
Conclusions
Here we present strong evidence for a substantial thawing-induced N2O release from Arctic peatlands. We also show that vegetated peat soils may turn from a negligible to a small but significant N2O source, with significant implications for pan-Arctic emission budgets. Furthermore, the positive climate change feedback of N2O will be stronger under aerobic conditions than under anaerobic conditions. Because N2O has an almost 300 times stronger global warming potential than CO2 on a 100-y time horizon (12), a postthaw N2O release would further enhance the radiative forcing stemming from C gases (8, 9).
Materials and Methods
Study Site.
Peat mesocosms for this study were collected from a palsa mire (68°89’N, 21°05’E; SI Appendix, Fig. S1) located in the subarctic permafrost zone in Finnish Lapland. Underlain by discontinuous permafrost, the palsa complex was uplifted by frost heave ∼3 m above the surrounding mire area. The vegetation cover on the palsa surface is dominated by dwarf shrubs and herbaceous plants, such as Empetrum nigrum subsp. hermaphroditum, Vaccinium vitis-idaea L., Betula nana L., Rubus chamaemorus L., lichens, and mosses (e.g., Dicranum spp., Polytrichum spp., Pleurozium spp.) in the wetter areas. Patches of bare peat, naturally free of vascular plants, are scattered among the vegetated areas. More details are provided in SI Appendix.
Sampling and Transport of Peat Mesocoms.
We sampled 16 intact peat mesocosms from vegetated and naturally bare (absent of vascular plants) parts of the palsa. A steel corer (∼1 m length) with a removable steel cap was hammered into the soil using a pneumatic drill (SI Appendix, Fig. S2). The soil cores were collected within plastic tubes (polypropylene, 10 cm diameter; SI Appendix, Fig. S3), which were inserted into the steel corer before drilling. A chain connected to a pulley and tripod was used to retrieve the peat cores. The sampling occurred at maximum seasonal thaw depth at the end of September 2012. The peat cores were frozen immediately after sampling. Great care was taken to keep the cores frozen at gentle minus temperatures (−5 °C minimum) at all times during the transport and the 5-mo preincubation period (= artificial winter), until the start of the experiment in March 2013. Details of mesocosm collection are provided in SI Appendix.
Climate Chamber Setup and Replication.
The cores were set up in a climate-controlled chamber (BDR16 Reach-in plant growth chamber; Conviron, Winnipeg, Canada), providing constant humidity and air temperature (+10 °C) and the ability to regulate the light level. To simulate thawing, we placed the mesocosms in two replicate saltwater baths (water temperature −3 to −4 °C), and sequentially thawed the peat mesocosms from top to bottom, by gradually lowering the saltwater level, during six thawing stages (SI Appendix, Figs. S3 and S4 and Table S1).
One-half of the cores from vegetated and bare parts of the palsa were left under natural soil moisture conditions, and the water table level of the other half of the cores was artificially raised to 5–10 cm below the surface. The four treatments that we used in this study, each with four replicates, are referred to as dry bare (DB), dry vegetated (DV), wet bare (WB), and wet vegetated (WV). Details are provided in SI Appendix.
N2O Fluxes.
For gas flux measurements, the peat mesocosms were permanently covered with 6-mm transparent Plexiglas chambers (diameter, 120 mm; height, 250 mm; volume, 2.8 L), fixed around the plastic tubes with the peat monoliths with two rubber rings. A layer of distilled water on top of the rubber rings further ensured the gas tightness of the chambers. N2O samples were taken manually two to three times per week from each mesocosm. More information is provided in SI Appendix.
Soil Profile Concentration of N2O.
To determine the concentration of N2O along the soil profile, we installed five soil gas collectors horizontally in each core at the following depths: 5, 20, and 40 cm below the soil surface; 10 cm above the measured thaw depth (∼55 cm); and 5 cm below the measured thaw depth (∼70 cm) (SI Appendix).
Nutrient Profile in Soil Pore Water.
For soil water sampling, we used Rhizon pore water samplers (Rhizosphere, Wageningen, The Netherlands) installed at depths of 5–10 cm, 35–40 cm below the surface, and 0–5 cm below the maximum seasonal thaw depth (∼65–70 cm). Amounts of NO3− and NH4+ in the pore water were determined using spectrophotometric methods, as described in detail in SI Appendix.
Soil Analyses and Statistical Methods.
Detailed descriptions of soil analyses, hyperspectral imaging of peat profiles, GIS mapping, and statistical analyses are provided in SI Appendix.
Supplementary Material
Acknowledgments
We thank Igor Marushchak, Timo Oksanen, Maxim Dorodnikov, Hanne Säppi, Tatiana Trubnikova, Ville Närhi, and Kateřina Diáková for their help with practical work, and two anonymous reviewers for their valuable comments that helped improve the manuscript. This study was funded by the Nordic Center of Excellence DEFROST. We gratefully acknowledge financial support from the Academy of Finland project CryoN (decision no. 132045), the European Union FP7-ENV project PAGE21 (contract no. 282700), and the Joint Programming Initiative Climate project “Constraining Uncertainties in the Permafrost-Climate Feedback” (COUP; decision no. 291691). C.V. received personal funding from the University of Eastern Finland’s doctoral program in Environmental Physics, Health, and Biology, and travel support from European Cooperation in Science and Technology (COST) Action ABBA (ES0804), Nordic Network for Stable Isotope Research (NordSIR), and NORDFLUX.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1702902114/-/DCSupplemental.
References
- 1.Christensen JH, et al. Climate phenomena and their relevance for future regional climate change: Supplementary material. In: Stocker TF, et al., editors. Climate Change 2013—The Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge Univ Press; Cambridge, UK: 2013. pp. 14SM-1–14SM-62. [Google Scholar]
- 2.Borge AF, Westermann S, Solheim I, Etzelmüller B. Strong degradation of palsas and peat plateaus in Northern Norway during the last 60 years. Cryosphere. 2017;11:1–16. [Google Scholar]
- 3.Jones BM, et al. Presence of rapidly degrading permafrost plateaus in south-central Alaska. Cryosphere. 2016;10:2673–2692. [Google Scholar]
- 4.Christiansen HH, et al. The thermal state of permafrost in the Nordic area during the international polar year 2007–2009. Permafr Periglac Process. 2010;21:156–181. [Google Scholar]
- 5.Romanovsky VE, et al. Thermal state of permafrost in Russia. Permafr Periglac Process. 2010;21:136–155. [Google Scholar]
- 6.Grosse G, Goetz S, McGuire AD, Romanovsky VE, Schuur EA. Changing permafrost in a warming world and feedbacks to the Earth system. Environ Res Lett. 2016;11:040201. [Google Scholar]
- 7.Hugelius G, et al. Estimated stocks of circumpolar permafrost carbon with quantified uncertainty ranges and identified data gaps. Biogeosciences. 2014;11:6573–6593. [Google Scholar]
- 8.Schädel C, et al. Potential carbon emissions dominated by carbon dioxide from thawed permafrost soils. Nat Clim Chang. 2016;6:950–953. [Google Scholar]
- 9.Schuur EAG, et al. Climate change and the permafrost carbon feedback. Nature. 2015;520:171–179. doi: 10.1038/nature14338. [DOI] [PubMed] [Google Scholar]
- 10.Harden JW, et al. Field information links permafrost carbon to physical vulnerabilities of thawing. Geophys Res Lett. 2012;39:L15704. [Google Scholar]
- 11.Bouwman L, et al. Exploring global changes in nitrogen and phosphorus cycles in agriculture induced by livestock production over the 1900-2050 period. Proc Natl Acad Sci USA. 2013;110:20882–20887. doi: 10.1073/pnas.1012878108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.IPCC . In: Climate change 2013—The physical science basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM, editors. Cambridge Univ Press; Cambridge, UK: 2013. [Google Scholar]
- 13.Butterbach-Bahl K, Baggs EM, Dannenmann M, Kiese R, Zechmeister-Boltenstern S. Nitrous oxide emissions from soils: How well do we understand the processes and their controls? Philos Trans R Soc Lond B Biol Sci. 2013;368:20130122. doi: 10.1098/rstb.2013.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abbott BW, Jones JB. Permafrost collapse alters soil carbon stocks, respiration, CH4, and N2O in upland tundra. Glob Change Biol. 2015;21:4570–4587. doi: 10.1111/gcb.13069. [DOI] [PubMed] [Google Scholar]
- 15.Elberling B, Christiansen HH, Hansen BU. High nitrous oxide production from thawing permafrost. Nat Geosci. 2010;3:332–335. [Google Scholar]
- 16.Repo ME, et al. Large N2O emissions from cryoturbated peat soil in tundra. Nat Geosci. 2009;2:189–192. [Google Scholar]
- 17.Marushchak ME, et al. Hot spots for nitrous oxide emissions found in different types of permafrost peatlands. Glob Change Biol. 2011;17:2601–2614. [Google Scholar]
- 18.Voigt C, et al. Warming of subarctic tundra increases emissions of all three important greenhouse gases—carbon dioxide, methane, and nitrous oxide. Glob Change Biol. 2016 doi: 10.1111/gcb.13563. [DOI] [PubMed] [Google Scholar]
- 19.Seppälä M. Synthesis of studies of palsa formation underlining the importance of local environmental and physical characteristics. Quat Res. 2011;75:366–370. [Google Scholar]
- 20.Werner C, Butterbach-Bahl K, Haas E, Hickler T, Kiese R. A global inventory of N2O emissions from tropical rainforest soils using a detailed biogeochemical model. Global Biogeochem Cycles. 2007;21:GB3010. [Google Scholar]
- 21.Zhuang Q, Lu Y, Chen M. An inventory of global N2O emissions from the soils of natural terrestrial ecosystems. Atmos Environ. 2012;47:66–75. [Google Scholar]
- 22.Keuper F, et al. A frozen feast: Thawing permafrost increases plant‐available nitrogen in subarctic peatlands. Glob Change Biol. 2012;18:1998–2007. [Google Scholar]
- 23.Salmon VG, et al. Nitrogen availability increases in a tundra ecosystem during five years of experimental permafrost thaw. Glob Change Biol. 2016;22:1927–1941. doi: 10.1111/gcb.13204. [DOI] [PubMed] [Google Scholar]
- 24.Finger RA, et al. Effects of permafrost thaw on nitrogen availability and plant–soil interactions in a boreal Alaskan lowland. J Ecol. 2016;104:1542–1554. [Google Scholar]
- 25.Zoltai S, Tarnocai C. Perennially frozen peatlands in the western Arctic and subarctic of Canada. Can J Earth Sci. 1975;12:28–43. [Google Scholar]
- 26.Martikainen PJ, Nykänen H, Crill P, Silvola J. Effect of a lowered water table on nitrous oxide fluxes from northern peatlands. Nature. 1993;366:51–53. [Google Scholar]
- 27.Liljedahl AK, et al. Pan-arctic ice-wedge degradation in warming permafrost and its influence on tundra hydrology. Nat Geosci. 2016;9:312–318. [Google Scholar]
- 28.Avis CA, Weaver AJ, Meissner KJ. Reduction in areal extent of high-latitude wetlands in response to permafrost thaw. Nat Geosci. 2011;4:444–448. [Google Scholar]
- 29.Wagner-Riddle C, et al. Globally important nitrous oxide emissions from croplands induced by freeze-thaw cycles. Nat Geosci. 2017 doi: 10.1038/ngeo2907. [DOI] [Google Scholar]
- 30.Åkerman HJ, Johansson M. Thawing permafrost and thicker active layers in sub‐arctic Sweden. Permafr Periglac Process. 2008;19:279–292. [Google Scholar]
- 31.Phoenix GK, Bjerke JW. Arctic browning: Extreme events and trends reversing arctic greening. Glob Change Biol. 2016;22:2960–2962. doi: 10.1111/gcb.13261. [DOI] [PubMed] [Google Scholar]
- 32.Qian H, Joseph R, Zeng N. Enhanced terrestrial carbon uptake in the northern high latitudes in the 21st century from the Coupled Carbon Cycle Climate Model Intercomparison Project model projections. Glob Change Biol. 2010;16:641–656. [Google Scholar]
- 33.Brown J, Ferrians O, Jr, Heginbottom J, Melnikov E. 2014. Circum-Arctic map of permafrost and ground-ice conditions. National Snow and Ice Data Center. nsidc.org/data/docs/fgdc/ggd318_map_circumarctic/. Accessed May 7, 2017.
- 34.Hugelius G, et al. The Northern Circumpolar Soil Carbon Database: Spatially distributed datasets of soil coverage and soil carbon storage in the northern permafrost regions. Earth Syst Sci Data. 2013;5:3–13. [Google Scholar]
- 35.Olefeldt D, et al. Circumpolar distribution and carbon storage of thermokarst landscapes. Nat Commun. 2016;7:13043. doi: 10.1038/ncomms13043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.