Significance
Ecosystems’ feedback to climate change remains a source of uncertainty in global models that project future climate conditions. That uncertainty rests largely on how much soil carbon will be lost as microbial respiration and how that loss varies across ecosystems. Although there has been a large emphasis on microbial temperature responses, how soil microorganisms respond to changes in moisture remains poorly understood. Here we show that historical rainfall controls soil respiration responses to current moisture. This finding was robust, with historical climate repeatedly limiting current respiration regardless of alterations to soil moisture, rainfall, or the arrival of new taxa. This study highlights the importance that legacies in microbial responses to climate change can have in future ecosystem responses.
Keywords: microbial, climate change, precipitation, legacies
Abstract
Ecosystem carbon losses from soil microbial respiration are a key component of global carbon cycling, resulting in the transfer of 40–70 Pg carbon from soil to the atmosphere each year. Because these microbial processes can feed back to climate change, understanding respiration responses to environmental factors is necessary for improved projections. We focus on respiration responses to soil moisture, which remain unresolved in ecosystem models. A common assumption of large-scale models is that soil microorganisms respond to moisture in the same way, regardless of location or climate. Here, we show that soil respiration is constrained by historical climate. We find that historical rainfall controls both the moisture dependence and sensitivity of respiration. Moisture sensitivity, defined as the slope of respiration vs. moisture, increased fourfold across a 480-mm rainfall gradient, resulting in twofold greater carbon loss on average in historically wetter soils compared with historically drier soils. The respiration–moisture relationship was resistant to environmental change in field common gardens and field rainfall manipulations, supporting a persistent effect of historical climate on microbial respiration. Based on these results, predicting future carbon cycling with climate change will require an understanding of the spatial variation and temporal lags in microbial responses created by historical rainfall.
Ecosystem models generally assume that aggregate function in soil microbial communities will respond to environmental change instantaneously and uniformly. However, microbial responses to altered environmental conditions can diverge from expectations if taxa are environmentally sorted or locally adapted and resistant to compositional or genetic shifts (1). Such historical contingencies mean that microbial processes are not predictable based on contemporary environmental conditions. This may be one reason why current models that incorporate microbial processes predict, at best, 50% of the existing spatial variation in soil carbon (C) stocks (2). Historical contingencies are supported by observed spatial variation in the temperature sensitivity of soil microbial respiration, decomposition, and C use efficiency across boreal to tropical sites (3, 4), albeit with no indication of mechanism.
Soil moisture constrains microbial processes (5, 6), but the lack of consensus on microbial moisture dependence and sensitivity has led to the use of diverse moisture functions in biogeochemical models, resulting in widely varying predictions (7). Moisture functions are largely based on abiotic soil properties that affect access to C and nutrients via diffusion to the cell surface; biotic contributions are generally limited to the C uptake rate, which is assumed to be proportional to the respiration rate (8, 9). However, microbial adaptation to stress and species sorting based on environmental conditions (10, 11) can both generate historical contingencies across a gradient of moisture availability. We propose that divergent model predictions could be resolved by accounting for variation in microbial respiration caused by historical contingencies, which should be found in ecosystems with different rainfall regimes.
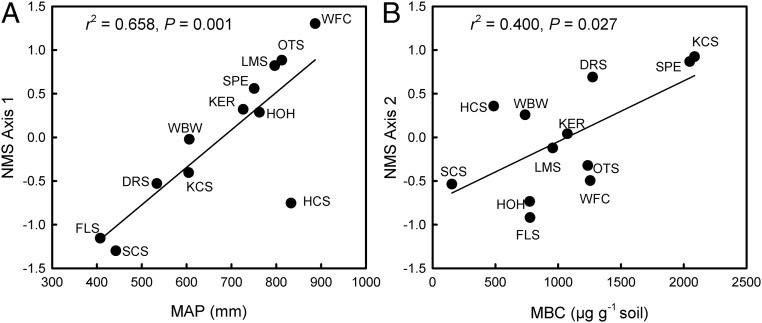
Previous studies of soil microbial respiration responses to environmental factors have compared distant regions where many conditions other than climate covary. To avoid this problem, we investigated respiration–moisture responses using soils from savanna grasslands across a steep precipitation gradient on a single geological formation (SI Appendix, Fig. S1 and Table S1). Savanna and grassland ecosystems cover 35% of Earth’s terrestrial surface and contain 28% of soil C (12), and therefore play an important role in the global C cycle. Across the gradient from east to west, mean annual precipitation (MAP) declines from ∼87 to 41 cm, whereas mean annual temperature varies by ∼2 °C north to south. Additional site characteristics are uncorrelated with position on the gradient, including soil pH, C, N, P, Ca, Mg, percentage clay, microbial biomass C, and vegetation cover. However, based on nonmetric multidimensional scaling (NMS) ordination of 454 pyrosequencing data, bacteria community composition along NMS axis 1 (representing 72% of the variation in composition) covaries with MAP across the gradient, with the least similar communities located at the driest and wettest endpoints (Fig. 1A). To a much lesser extent, bacteria community composition correlates with microbial biomass C along NMS axis 2, which captured 21% of the community variation (Fig. 1B). This regional pattern is consistent with local adaptation and/or species sorting along historical rainfall gradients, with secondary variation caused by site factors that affect microbial biomass. The lack of collinearity between rainfall and soil conditions, coupled with the close relationship between bacteria and rainfall, makes this an ideal system in which to test the independent effects of historical climate on soil microbial function.
Fig. 1.
NMS of 454 pyrosequencing data from the gradient sites captured 93% of the variation in bacteria community composition, with 72% in the first axis and 21% in the second axis. (A) Along NMS axis 1, historical rainfall (MAP) explained 66% of the variation in bacteria community composition (P = 0.001). (B) On NMS axis 2, microbial biomass C (MBC) accounted for 40% of the variation (P = 0.027). No other environmental variables contributed to the regression models.
We examined the moisture responses of soil respiration using three complementary experiments: (i) a reciprocal moisture laboratory incubation with and without litter addition (12 mo), (ii) a field reciprocal transplant experiment (18 mo), and (iii) a field rainfall manipulation (18 mo). The long-term laboratory incubation experiment allowed us to quantify two aspects of soil respiration responses to water availability, moisture dependence (the magnitude of the response to soil moisture) and moisture sensitivity (the slope of respiration versus soil moisture), which we expected to vary with historical rainfall. Furthermore, we used the litter treatments to test the hypothesis that moisture responses were independent of resource availability. The field reciprocal transplant experiment was conducted to examine whether historical contingencies observed in the laboratory could be replicated in the field, more specifically to test the premise that microbial acclimatization to novel moisture conditions was constrained by potential dispersal of microbes from the surrounding environment. Finally, the objective of the field rainfall manipulation experiment was to examine whether microbial functional legacies of past rainfall regimes could be altered by directly manipulating precipitation.
Results and Discussion
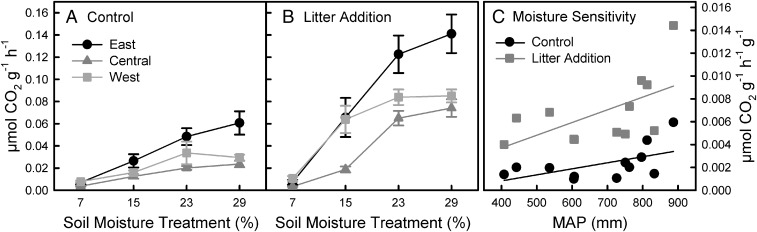
We found regional variation in the moisture dependence of respiration (P = 0.006) using soils from the gradient (Fig. 1) that were maintained at four moisture levels for 1 y in laboratory microcosms. At 29% moisture, respiration rates doubled in soils from historically wetter eastern vs. historically drier western and central sites, but this pattern collapsed as moisture decreased to 7% (Fig. 2 A and B). Maximum respiration rates were achieved for eastern soils at or above 23% moisture, whereas western and central sites reached maximum respiration at 15% moisture (Fig. 2 A and B). Within regions, site-level moisture responses also varied somewhat (SI Appendix, Fig. S2 and Table S2), likely due to contributions from local edaphic factors. Historical climate can interact with local resource availability or stoichiometry to drive microbial respiration (13) and thus we added litter to half of the microcosms to ensure responses were not C limited. Litter addition stimulated respiration by ∼2.2 times (P < 0.001), except at the lowest 7% soil moisture treatment where litter had no effect (P < 0.001; SI Appendix, Table S2). Regardless of magnitude, the relationship among regions remained unchanged by the addition of litter (P = 0.100; Fig. 2 A and B) and was consistent throughout the experiment (SI Appendix, Figs. S3 and S4), suggesting that historical climate effects on the moisture–respiration relationship occur independent of C availability and can be persistent.
Fig. 2.
Soil respiration by region of origin and soil moisture for (A) control and (B) litter addition treatments and (C) moisture sensitivity of soil respiration as a function of increasing MAP and litter addition in the laboratory incubation reciprocal moisture experiment. There were significant interactions of region by moisture (P < 0.001) and moisture by litter (P < 0.001) (A and B). Soils originating from wetter eastern regions respired significantly more than drier central or western regions except at 7% moisture, where western soils respired more than eastern. Respiration was elevated with litter, except at 7% moisture. Means ±1 SE (n = 8) are plotted, with values averaged over 12 dates. MAP explained 61% of the variation in moisture sensitivity (C) (P < 0.001).
The moisture sensitivity of soil respiration was affected by MAP and litter treatment [r2 = 0.611, P < 0.001, Akaike information criterion (AIC) = −293.3]. Sensitivity quadrupled as MAP increased by 480 mm (Fig. 2C). Moisture sensitivity tripled when litter was added compared with control microcosms, but there was no significant interaction of litter with MAP (P = 0.303), supporting independent climate- and resource-based selection. These results further underscore the potential for regional spatial variation in the respiration–moisture relationship to affect ecosystem C cycling. Moreover, no soil properties were retained in the final model, suggesting that biotic drivers were principally responsible for the patterns observed. Microbial moisture sensitivity was also more important than abiotic diffusion constraints when modeling respiration pulses (Birch effects) in response to drying–rewetting cycles (14). Our work focused on microbial respiration responses to stable moisture treatments; in other systems, responses to variable moisture conditions that induce multiple stress events may depend on different aspects of historical climate and have different outcomes (15).
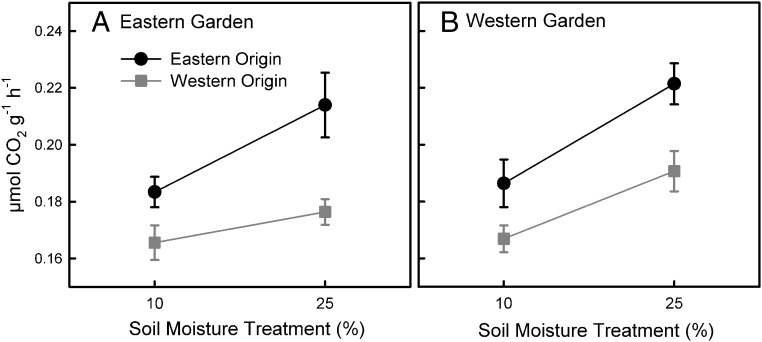
Because this initial experiment was carried out in laboratory microcosms, there were no inputs of new taxa from dispersal. We postulated that microbial dispersal would reduce legacy effects under a changing moisture regime (16). To separate historical and current climate conditions in the field, we transplanted soil cores from the wetter eastern and drier western regions of the rain gradient into common gardens located in each region. We focused on the eastern and western regions because the central region and western region rarely differed in the long-term incubation. All soil cores in the experiment were open to passive dispersal. After 18 mo in the field, region of origin remained the primary determinant of soil respiration responses to controlled moisture in the laboratory (P = 0.004; SI Appendix, Table S3), with soils from wetter eastern sites respiring ∼13% more on average than those from drier western sites (Fig. 3). Respiration also depended to a much smaller extent on the four-way interaction of date, garden location, moisture treatment, and site (P = 0.018; SI Appendix, Table S3), reflecting site differences in the response to transplantation and moisture.
Fig. 3.
Respiration rates for soils originating from historically wetter eastern sites and drier western sites incubated at high and low soil moisture after 18 mo transplanted in the (A) eastern and (B) western common gardens. Region of soil origin was the only significant factor affecting respiration (P = 0.004). Data are means ±1 SE (n = 18), with values averaged over four time points taken across 8 wk in the laboratory. Note that the y axes do not start at zero.
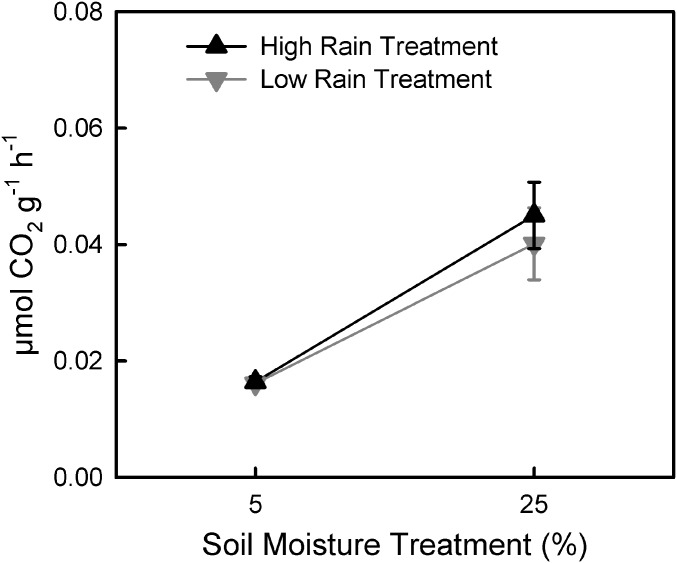
An important question about historical contingencies is whether and how quickly they can be overcome when the environment changes. We did not see such a shift in the field reciprocal transplant common gardens, but drought across the gradient during that time period may have limited opportunities for moisture-based selection. Thus, we also tested respiration–moisture responses for soils from a field experiment on the eastern end of the gradient. Rainfall was manipulated for 18 mo, using rainfall exclusion shelters and irrigation (SI Appendix, Fig. S5). The field rainfall treatments varied by ∼1,000 mm (326 vs. 1,331 mm), representing the driest and wettest years observed in the local historical record. When the respiration–moisture response for these soils was tested under controlled conditions, respiration depended on the laboratory moisture treatment (P < 0.001; SI Appendix, Table S4), but was unaffected by the field rainfall treatments (P = 0.629; SI Appendix, Table S4 and Fig. 4). The lack of functional change in both the field common gardens and field rainfall manipulation suggests that local microbial communities are highly resistant to change. We know this is the case for the soils reciprocally transplanted in the gradient common gardens, where bacteria community composition primarily reflected the site of origin rather than garden treatment (SI Appendix, Table S5).
Fig. 4.
The moisture response of soil respiration rates in the field rainfall manipulation experiment was significantly affected by current soil moisture (P < 0.001). Field rain treatments, which were maintained at high (1,331 mm⋅y−1) or low (326 mm⋅y−1) levels, had no effect on respiration. Means ±1 SE (n = 8) are plotted with values averaged over 8 wk in laboratory microcosms.
In ecosystem C models, environmental dependency is often linked to C use efficiency (17), which we did not measure here. We can, however, consider the relationship between microbial production and respiration by using microbial biomass C as a covariate in our statistical models. Across the rain gradient sites, microbial biomass C varied by four times, but was independent of historical mean annual rainfall (r2 = 0.048, P = 0.490). Microbial biomass C did not significantly covary with respiration in any of the experiments testing moisture dependence (SI Appendix, Tables S3 and S4) and instead generally reflected site or garden differences (SI Appendix, Tables S1 and S6–S9). This observation supports a decoupling between microbial physiological and biomass responses to water availability, meaning that microbial moisture responses may not be predictable from the size of the biomass pool. However, the chloroform-fumigation direct-extraction method used here can lyse living, dead, and dormant cells and thus this relationship needs to be further investigated.
Greater moisture sensitivity of respiration for soils from historically wetter sites contrasts with our previous findings that enzyme activities were more sensitive to moisture in historically drier sites on this same gradient (18). Long-term selection in drought-prone environments likely affects microbial metabolism and extracellular enzymes in different ways. In drier, more stressful environments, we might expect decreased moisture sensitivity of microbial respiration if C is instead allocated to survival mechanisms. In the same conditions, increased moisture sensitivity of enzymes can support maximizing resource availability during brief water pulses. If this is the case, water inputs should cause a more rapid rise in extracellular enzyme activities that release resources compared with intracellular activities that affect respiration in drought-prone habitats. Additionally, because enzymes can persist in the environment while stabilized on soil minerals (19), enzymatic responses to environmental change may occur over different time scales than respiratory responses of the microbial biomass.
Variation in the ecological mechanisms underlying microbial responses to climate may explain why experimental manipulations do not consistently generate legacy effects (20, 21). In our system, we argue that differential local microbial adaptation or prior species sorting combined with resistance to species turnover are at play, reflecting variation in local site climatic history. Biotic legacies observed elsewhere support this idea; for example, the effect of site of origin on soil respiration persisted after a 17-y reciprocal transplant across an elevation gradient (22). However, results of reciprocal transplants can be mixed and may depend on whether the soils remain within their existing climate envelope (23). High resistance has also been found in long-term climate change experiments. In temperate forests, microbial acclimation to warming was only evident after 15–18 y based largely on reductions in microbial biomass and substrate depletion (24, 25), whereas the microbial community shifted between 12 and 20 y as the organic horizon came to resemble the mineral horizon (26). A similar time frame was also required to see effects of warming on microbial communities in subarctic heath (27). Although most studies of microbial responses to environmental change are short term, long-term moisture manipulations are needed for insight into the time scales and mechanisms by which microbial moisture responses and moisture sensitivity can shift.
Based on this series of 12- to 18-mo experiments, we demonstrate that microbial respiration is effectively locally specialized to moisture, although the mechanism could be either prior selection on community assembly or microbial physiology. We also demonstrate high resistance to change. This combination of local specialization and resistance can generate historical contingencies in the respiration–moisture relationship. The magnitude and persistence of such functional legacies are expected to vary across ecosystems, with substantial implications for ecosystem C models. If historical contingencies in soil respiration prove to be widespread, then moisture functions in C models should be both spatially and temporally dynamic, whereas current models use static moisture functions (2, 7, 8, 28). One possibility for scaling up local respiration–moisture relationships is the development of functional types defined to capture these dynamics (29). Such efforts will require an improved understanding of the spatial and temporal scales at which historical contingencies occur and persist and how those will impact microbial C cycling in the face of a changing climate.
Methods
Rainfall Gradient Characterization.
The gradient sites are located across the Edwards Plateau in central Texas, where MAP ranges from ∼86 cm in the east to ∼46 cm in the west, and decreases by ∼10 cm every ∼100 km (1981–2010; Prism Climate Group, Oregon State University, prism.oregonstate.edu). Rainfall variability is also high throughout the gradient, with the coefficient of variation for mean monthly precipitation no less than 0.8 at any site. Soils are rocky, calcareous Mollisols. Sample locations within sites were selected to minimize differences in topography (<2% slope) and the plant community (native grassland with <50% woody plant cover).
In October 2008, we sampled 12 sites across the gradient (SI Appendix, Table S1). At each site, we identified a 20 × 20-m plot that met our criteria and collected ∼15 L of soil by sampling to a depth of 10 cm at a minimum of 10 separate points from randomly generated locations in the plot. Soils from within each site were combined, transported to the laboratory on ice, and sieved to 2 mm to homogenize within 24 h of collection. Subsamples were taken for analysis of soil pH, organic C, total inorganic nitrogen (TIN), microbial biomass C and N, soil moisture, and microbial community composition (SI Appendix, SI Methods); the remaining soils were air dried in the laboratory to ∼5% gravimetric soil moisture for use in the long-term reciprocal moisture experiment described below. From each 20 × 20-m plot, we also collected standing dead leaf litter from the dominant grasses. Litter from all sites was combined, dried to constant weight at 60 °C, ground to 2-mm particle size to homogenize, and quantified for C content by combustion; the litter C content was used to determine the amount of litter added in the laboratory reciprocal moisture experiment (see below).
Laboratory Reciprocal Soil Moisture Experiment.
To separate the effects of historical and current moisture on microbial respiration, we exposed soils collected as described above from different precipitation regimes to a range of soil moistures reflective of the gradient moisture conditions. For this purpose, we binned the gradient into three precipitation regions (east, central, and west) with four sites in each region based on the top, middle, and bottom third values of mean annual precipitation (MAP; 1981–2010, PRISM Climate Group, Oregon State University, prism.oregonstate.edu). In the east, central, and west regions, MAP ranged from 80 to 90, 61 to 76, and 40 to 60 cm, respectively. To create the reciprocal design, soils (60 g) were added to microcosms (500-mL glass canning jars) and incubated at four soil moisture treatments (7%, 15%, 23%, and 29%) that were selected on the basis of the range of soil moisture measured across the precipitation gradient. To examine potential interactions between water and substrate availability, we added 0.68 g of litter to half of the experimental microcosms, representing an approximate 10% increase over baseline soil C content. The full factorial design included three regions, four sites per region, four moisture treatments, two litter treatments, and 2 microcosms per treatment combination, for 192 total microcosms. Note that site was the level of replication in this design, and the two microcosms per site per treatment were averaged for all analyses. For 12 mo, microcosms were incubated at 24 °C in the dark and moisture treatments maintained within ±1% via weekly weighing. To measure CO2 flux from soils, air samples were collected from the headspace of each microcosm at the initial setup and 1, 2, 3, 4, 6, 8, 12, 16, 20, 24, and 52 wk after the start of the incubation experiment.
Field Reciprocal Transplant Common Gardens.
A reciprocal transplant experiment was established along the precipitation gradient to examine soil microbial responses to new rainfall regimes when open to dispersal from the surrounding region. The experiment consisted of two common garden plots located at a historically wetter eastern site (MAP = 89 cm; Ladybird Johnson Wildflower Center) and a historically drier site 300 km to the west (MAP = 63 cm; MOR Ecolab) (SI Appendix, Table S1). Soils from three eastern sites and three western sites were incubated at each common garden in PVC cores, creating a full factorial combination of home region and transplanted treatments. As a control treatment, six cores containing soils sampled on site were also incubated in each garden. Soils were collected in July 2011, sieved to 2 mm, packed into cores at approximately natural bulk density (150 g at ∼0.95 g⋅cm3), and transported to each common garden site within a week of collection. Cores were made of PVC pipe 5-cm diameter × 15-cm depth with 38-µm mesh bottoms to allow for drainage while preventing root in-growth from below. The cores were buried in a 6 × 3-m grid at 50-cm intervals, arranged in random order with respect to treatment.
Each factorial combination of common garden and soil origin treatments contained 12 experimental replicates, yielding a total of 72 cores at each common garden site in addition to the six control cores (n = 78 per garden, 156 for the entire experiment). Cores remained in the field for 18 mo until harvest in December 2012, at which time cores were removed from the ground intact and transported to the laboratory on ice where microbial biomass C was measured (SI Appendix, SI Methods). Soils from each core were sieved to 2 mm and homogenized. To examine how the common garden environments affected soil microbial responses to water availability, soil subsamples from each field core were randomly assigned to dry (10%) or wet (25%) soil moisture treatments, and soil respiration was monitored for 8 wk (SI Appendix, SI Methods).
Field Rainfall Manipulation Experiment.
In November 2013, we sampled an experimental rainfall common garden located at the Ladybird Johnson Wildflower Center (30) (SI Appendix, Fig. S5). Soils were sampled from two rainfall treatments, high (1,331 mm⋅y−1) and low (326 mm⋅y−1), that were implemented beginning in May 2012, after a year of establishment at the local mean of 850 mm. Soils were collected from four plots per treatment (two 2.5-cm diameter × 10-cm deep cores per plot), combined within treatments, sieved to 2 mm, and air dried to ∼5% soil moisture. To examine how rainfall treatments affected soil microbial responses to water availability, soil subsamples were added to microcosms that were maintained at 5% or 25% soil moisture and soil respiration was measured over 8 wk (SI Appendix, SI Methods).
Statistical Analyses.
For the gradient soils, we characterized the changes in bacterial community composition among sites using nonmetric multidimensional scaling in PC Ord (v6.19) (31). To examine potential drivers of bacteria community composition, the resulting two NMS axes were then used as the dependent variables. We used stepwise multiple regressions for each NMS axis with independent variables related to climate (soil moisture, 1-mo rainfall, 6-mo rainfall, MAP, historical mean maximum, and minimum annual temperature) and soils (microbial biomass C and N, soil C, TIN, percentage clay, and pH). Regression statistics were carried out in SPSS (v23, IBM). Error terms reported in text and figures are ±1 SE.
In the laboratory reciprocal moisture incubation, soil respiration across all 12 sampling dates was analyzed with repeated measures ANOVA as a function of region of origin, soil moisture treatment, litter treatment, and their interactions; in addition, site nested in region was included as a random factor. Greenhouse–Geisser corrections were used when sphericity was violated. When main effects were significant (P < 0.05), post hocs were performed with Ryan–Einot–Gabriel–Welsch multiple range tests. Significant interactions were tested by examining each level of one factor across levels of the other factor using one-way ANOVA and post hocs as above.
We calculated the moisture sensitivity of respiration as the linear slope of the relationship between respiration (µmol CO2 g−1 soil h−1) and moisture (g water) for each site origin and litter treatment. Data from the first 8 wk of sampling were included to limit any effect of declining respiration caused by nonrenewal of labile C in microcosms. Stepwise multiple regression with AIC was used to examine how moisture sensitivity varied with litter and climate and soil characteristics of the site of origin (MAP, mean annual minimum and maximum temperatures, microbial biomass C and N, soil percentage C, TIN, percentage clay, and pH). Site variables were examined for collinearity; all had variance inflation factors of less than 2. To examine differences in slopes, ANCOVA was used to test for an interaction between litter and significant continuous variables. All ANOVA and regression statistics were carried out in SPSS (v23).
In the field common gardens, respiration was analyzed with repeated measures ANOVA and as a function of common garden location (eastern vs. western), region of origin (eastern vs. western), moisture treatment (high or low), and site of soil origin nested in region; initial and final microbial biomasses were included as covariates. Site of soil origin was treated as a random factor nested within region, and F ratios were constructed accordingly (32). Microbial biomass measured on field soils and at the end of the moisture incubation were analyzed using ANOVA with the same model as above, but without date. Post hoc tests were performed as above.
In the experimental field rainfall manipulation, soil respiration was analyzed across the eight sampling dates in the laboratory incubation with repeated measures ANOVA as a function of field rain treatment, field plant community, laboratory soil moisture treatment, and their interactions; in addition, microbial biomass measured at the harvest of the incubation experiment was included as a covariate. Microbial biomass C was analyzed with ANOVA using the same model, but without date and without covariates.
Supplementary Material
Acknowledgments
We thank M. Blizard, E. Brault, J. Du, J. Dula, L. Fredlund, N. Johnson, K. Myers, B. Narang, and T. Reid for assistance with fieldwork and labwork. Site access was kindly permitted by the Texas Parks and Wildlife Department, the Texas Ecolab Program, and the Texas Historical Commission. Previous drafts of the manuscript were improved by comments from R. Phillips, C. Averill, and D. Hillis. This material is based upon work supported by Texas Ecolab, the Department of Energy National Institute for Climate Change Research (08-SC-NICCR-1071), and the University of Texas startup funds (to C.V.H.). B.G.W. was supported by National Science Foundation Graduate Research Fellowship DGE-1110007 and Doctoral Dissertation Improvement Grant DEB-1210361.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the NCBI Sequence Read Archive (accession no. PRJNA379880).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1620811114/-/DCSupplemental.
References
- 1.Hawkes CV, Keitt TH. Resilience vs. historical contingency in microbial responses to environmental change. Ecol Lett. 2015;18:612–625. doi: 10.1111/ele.12451. [DOI] [PubMed] [Google Scholar]
- 2.Wieder WR, Bonan GB, Allison SD. Global soil carbon projections are improved by modelling microbial processes. Nat Clim Chang. 2013;3:909–912. [Google Scholar]
- 3.Karhu K, et al. Temperature sensitivity of soil respiration rates enhanced by microbial community response. Nature. 2014;513:81–84. doi: 10.1038/nature13604. [DOI] [PubMed] [Google Scholar]
- 4.Manzoni S, Taylor P, Richter A, Porporato A, Ågren GI. Environmental and stoichiometric controls on microbial carbon-use efficiency in soils. New Phytol. 2012;196:79–91. doi: 10.1111/j.1469-8137.2012.04225.x. [DOI] [PubMed] [Google Scholar]
- 5.Henry HAL. Soil extracellular enzyme dynamics in a changing climate. Soil Biol Biochem. 2012;47:53–59. [Google Scholar]
- 6.Brzostek ER, et al. The effect of experimental warming and precipitation change on proteolytic enzyme activity: Positive feedbacks to nitrogen availability are not universal. Glob Change Biol. 2012;18:2617–2625. [Google Scholar]
- 7.Sierra CA, Trumbore SE, Davidson EA, Vicca S, Janssens I. Sensitivity of decomposition rates of soil organic matter with respect to simultaneous changes in temperature and moisture. J Adv Model Earth Syst. 2015;7:335–356. [Google Scholar]
- 8.Manzoni S, Moyano F, Kätterer T, Schimel J. Modeling coupled enzymatic and solute transport controls on decomposition in drying soils. Soil Biol Biochem. 2016;95:275–287. [Google Scholar]
- 9.Moyano FE, Manzoni S, Chenu C. Responses of soil heterotrophic respiration to moisture availability: An exploration of processes and models. Soil Biol Biochem. 2013;59:72–85. [Google Scholar]
- 10.Fierer N, et al. Cross-biome metagenomic analyses of soil microbial communities and their functional attributes. Proc Natl Acad Sci USA. 2012;109:21390–21395. doi: 10.1073/pnas.1215210110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennett AF, Lenski RE, Mittler JE. Evolutionary adaptation to temperature. I. Fitness responses of Escherichia coli to changes in its thermal environment. Evolution. 1992;46:16–30. doi: 10.1111/j.1558-5646.1992.tb01981.x. [DOI] [PubMed] [Google Scholar]
- 12.Smith P. Do grasslands act as a perpetual sink for carbon? Glob Change Biol. 2014;20:2708–2711. doi: 10.1111/gcb.12561. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Ziegler SE, Lane CS, Billings SA. Legacies of native climate regime govern responses of boreal soil microbes to litter stoichiometry and temperature. Soil Biol Biochem. 2013;66:204–213. [Google Scholar]
- 14.Evans S, Dieckmann U, Franklin O, Kaiser C. Synergistic effects of diffusion and microbial physiology reproduce the Birch effect in a micro-scale model. Soil Biol Biochem. 2016;93:28–37. [Google Scholar]
- 15.Tiemann LK, Billings SA. Changes in variability of soil moisture alter microbial community C and N resource use. Soil Biol Biochem. 2011;43:1837–1847. [Google Scholar]
- 16.Lawrence D, Bell T, Barraclough TG. The effect of immigration on the adaptation of microbial communities to warming. Am Nat. 2016;187:236–248. doi: 10.1086/684589. [DOI] [PubMed] [Google Scholar]
- 17.Geyer KM, Kyker-Snowman E, Grandy AS, Frey SD. Microbial carbon use efficiency: Accounting for population, community, and ecosystem-scale controls over the fate of metabolized organic matter. Biogeochemistry. 2016;127:173–188. [Google Scholar]
- 18.Averill C, Waring BG, Hawkes CV. Historical precipitation predictably alters the shape and magnitude of microbial functional response to soil moisture. Glob Change Biol. 2016;22:1957–1964. doi: 10.1111/gcb.13219. [DOI] [PubMed] [Google Scholar]
- 19.Stursova M, Sinsabaugh RL. Stabilization of oxidative enzymes in desert soil may limit organic matter accumulation. Soil Biol Biochem. 2008;40:550–553. [Google Scholar]
- 20.Rousk J, Smith AR, Jones DL. Investigating the long-term legacy of drought and warming on the soil microbial community across five European shrubland ecosystems. Glob Change Biol. 2013;19:3872–3884. doi: 10.1111/gcb.12338. [DOI] [PubMed] [Google Scholar]
- 21.Schindlbacher A, Schnecker J, Takriti M, Borken W, Wanek W. Microbial physiology and soil CO2 efflux after 9 years of soil warming in a temperate forest: No indications for thermal adaptations. Glob Change Biol. 2015;21:4265–4277. doi: 10.1111/gcb.12996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bond-Lamberty B, et al. Soil respiration and bacterial structure and function after 17 years of a reciprocal soil transplant experiment. PLoS One. 2016;11:e0150599. doi: 10.1371/journal.pone.0150599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waldrop MP, Firestone MK. Response of microbial community composition and function to soil climate change. Microb Ecol. 2006;52:716–724. doi: 10.1007/s00248-006-9103-3. [DOI] [PubMed] [Google Scholar]
- 24.Bradford MA, et al. Thermal adaptation of soil microbial respiration to elevated temperature. Ecol Lett. 2008;11:1316–1327. doi: 10.1111/j.1461-0248.2008.01251.x. [DOI] [PubMed] [Google Scholar]
- 25.Frey SD, Drijber R, Smith H, Melillo J. Microbial biomass, functional capacity, and community structure after 12 years of soil warming. Soil Biol Biochem. 2008;40:2904–2907. [Google Scholar]
- 26.DeAngelis KM, et al. Long-term forest soil warming alters microbial communities in temperate forest soils. Front Microbiol. 2015;6:104. doi: 10.3389/fmicb.2015.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rinnan R, Michelsen A, Bååth E, Jonasson S. Fifteen years of climate change manipulations alter soil microbial communities in a subarctic heath ecosystem. Glob Change Biol. 2007;13:28–39. [Google Scholar]
- 28.Todd-Brown K, Hopkins F, Kivlin S, Talbot J, Allison S. A framework for representing microbial decomposition in coupled climate models. Biogeochemistry. 2012;109:19–33. [Google Scholar]
- 29.Bond-Lamberty B, et al. Estimating heterotrophic respiration at large scales: Challenges, approaches, and next steps. Ecosphere. 2016;7:e01380. [Google Scholar]
- 30.Kim S, Williams A, Kiniry JR, Hawkes CV. Simulating diverse native C4 perennial grasses with varying rainfall. J Arid Environ. 2016;134:97–103. [Google Scholar]
- 31.McCune B, Mefford MJ. 2011. PC-ORD Multivariate Analysis of Ecological Data. (MjM Software, Gleneden Beach, Oregon), 6.19.
- 32.Underwood AJ. Experiments in Ecology: Their Logical Design and Interpretation Using Analysis of Variance. Cambridge Univ Press; Cambridge: 1997. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.