Identifying factors that regulate the balance between stem cell self-renewal and differentiation into specialized cells is key to understanding tissue homeostasis and regeneration. Long before the identification of the four factors sufficient to define stemness, a single gene named bag of marbles (bam) was shown to be necessary and sufficient to control the transition from stem cell to differentiated cell in the germ-line lineage of Drosophila females (1). In the absence of bam, germ-line stem cells (GSCs) cannot differentiate into cystoblasts and accumulate as round cells forming a bam in the germarium at the tip of the ovary (2). In contrast, ectopic expression of bam in GSCs is sufficient to induce their differentiation and the subsequent loss of all germ-line cells (3). Bam thus acts as an ON and OFF switch of the stem cell vs. differentiated fate. This switch is controlled by a short-range Dpp signal (bone morphogenetic protein-like) sent from neighboring somatic cells, which directly inhibits bam transcription in GSCs (4, 5). The GSC daughter cell that is not in contact with these somatic cells does not receive enough Dpp signal, and can thus express bam and differentiate into a cystoblast (Fig. 1). During the window of bam expression, cystoblasts go through four rounds of incomplete divisions to form a cyst of 16 interconnected germ cells. This simple model provides a paradigm in the stem cell field to explain both how asymmetric divisions balance self-renewal and differentiation, and how external signals regulate asymmetric divisions. Two decades of intense research have refined this model to include many other signaling pathways (6). However, despite being at the heart of it, the biochemical activity of Bam remained mysterious. The lack of clear homologs at the sequence level has not helped. In an exciting research article published in PNAS, Ji et al. convincingly show that Bam and ovarian tumor (Otu) proteins associate and deubiquitinate cyclin A (CycA) (7). The resulting stabilization of CycA is sufficient to explain the loss of GSCs when expressing Bam ectopically. Thus, a long-standing question in the stem cell field has been clarified.
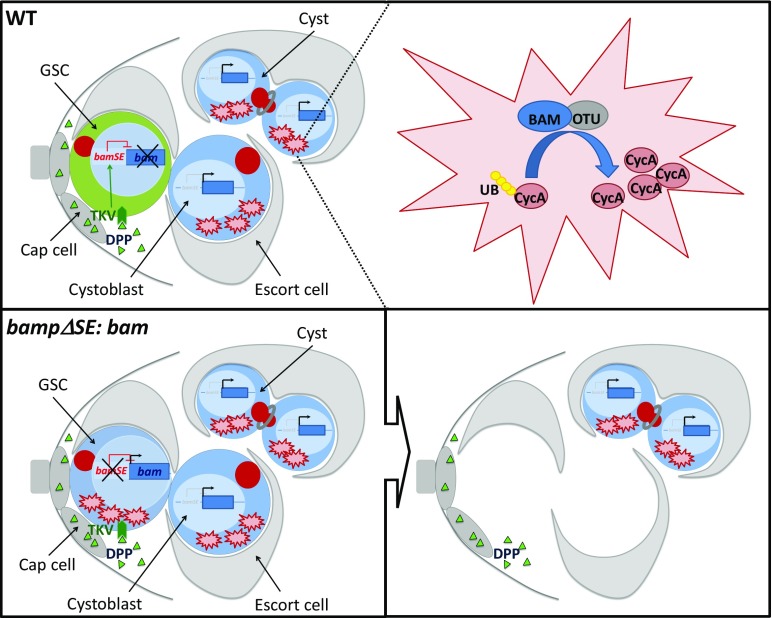
Fig. 1.
In WT GSCs (green), DPP/TKV signaling represses bam transcription (blue square) through the silencer element bamSE (in red). In cyst cells (blue), Bam can associate with Otu and regulate cyst divisions. When bamSE is mutated (Lower), bam becomes expressed in GSC. It can then bind to Otu in GSCs to deubiquitinate and stabilize CycA, which leads to GSC loss.
Dahua Chen and colleagues identified Bam binding partners by immunoprecipitation and mass-spectrometry analysis. Bam strongly interacted with free ubiquitin, suggesting that Bam could bind proteins bearing a ubiquitin chain, which can be marked for degradation. Previous work by the authors had shown that ubiquitin-mediated degradation of CycA was necessary for GSC maintenance (8). Expression of a nondegradable CycA in GSCs leads to their loss by differentiation, a phenotype identical to the ectopic expression of Bam (8). In PNAS, Ji et al. (7) discover that CycA can be coimmunoprecipitated with Bam from S2 cells and ovarian extracts. They further show that increasing the levels of Bam leads to a corresponding increase in CycA levels, and that decreasing the levels of Bam leads to a decrease in CycA levels. The authors propose that Bam could act as a deubiquitinating enzyme (Dub) for CycA. Furthermore, ectopic expression of bam in the GSC could be enhanced by coexpression of cycA, and suppressed by cycA reduction. However, Ji et al. could not demonstrate any deubiquitinating activity for Bam. Ji et al. then searched for potential Bam partners that could provide this activity by screening an RNAi library targeting deubiquitinating enzymes (DUBs). The authors found that Otu down-regulation led to an increase of CycA ubiquitination and demonstrate a direct DUB activity for Otu. In a series of elegant experiments, Ji et al. demonstrate that Bam, Otu, and CycA are part of the same complex, that Otu binding to CycA is dependent on Bam, and that CycA stabilization by Bam requires Otu Dub activity. These biochemical conclusions are supported by genetic interactions in vivo using alleles and knockdowns for bam, otu, and cycA. Altogether, the results presented in the Ji et al. article propose a novel model for the biochemical function of Bam when ectopically expressed in GSCs.
The biochemical activity of Bam described here is in contrast with its previously proposed functions in protein degradation and inhibiting mRNA translation (9–11). Indeed, Bam was shown to form a complex with Bgcn, a protein related to the RNA-interacting DExH-box polypeptides. This complex was proposed to inhibit the translation of the stem cell promoting factor nanos. Nanos promotes GSC fate, whereas Bam promotes differentiation. These antagonistic activities were shown to depend on the repression of nanos mRNA translation by the Bam/Bgcn complex, possibly via nanos 3′UTR (9). In this model, Bam would thus promote differentiation by inhibiting stem cell-promoting factors, which is different from inhibiting self-renewal by stabilizing differentiation factors, such as CycA. How to reconcile both models? Here, it is important to have in mind in which cell types these proteins are endogenously expressed to distinguish gain- vs. loss-of-function experiments. Bam and Nanos proteins are expressed in nonoverlapping reciprocal domains, with Nanos expressed in GSCs and 16-cell cysts, whereas Bam protein is found exclusively during the four mitotic divisions (9). Bgcn and Otu are expressed throughout the germarium. When ectopically expressed in GSCs, Bam could associate with both Bgcn and Otu, which would lead to the inhibition of nanos translation and the stabilization of CycA, respectively. Both would lead to GSC differentiation and germ-line loss in these gain-of-function experiments. Similarly, in its endogenous window of expression, from cystoblasts to eight-cell cysts, Bam could also associate with both Bgcn and Otu. The Bam/Bgcn complex would inhibit nanos translation, allowing differentiation, although the Bam/Otu complex could regulate CycA levels and the number of cyst divisions (see below). Bam could thus have several biochemical activities, depending on which cofactor it binds: Otu for deubiquitination, Bgcn for mRNA translation, and Csn4 for deneddylation (9–11). These pathways could act in parallel, and each interaction could be regulated by posttranslational modifications, such as ubiquitination, as uncovered by Ji et al. (7).
With the Ji et al. (7) work, Bam can be dubbed a regulator of the cell cycle. It could shed new light on the role of Bam in regulating the number of cyst divisions in both Drosophila males and females within its endogenous domain of expression. Indeed, in both testis and ovaries, differentiating germ-line cysts go through four rounds of divisions. However, in bam mutant testis, cysts undergo several rounds of extra divisions and fail to enter meiosis (12). An elegant model suggests that increasing levels of Bam protein during the four mitoses would limit the number of divisions above a specific threshold (13). Increasing artificially the levels of Bam limits the number of divisions to three and leads to the formation of cysts made of eight cells only (13). In addition, Bam was shown to associate with Bgcn, as in ovaries, and to inhibit the translation of Mei-P26 mRNA (14). The repression of Mei-P26 translation would limit the number of divisions and induce the transition to meiosis. The results by Ji et al. (7) in ovaries open the possibility that, in addition to regulating translation with Bgcn, Bam could regulate CycA levels with Otu in males as well. Remarkably, Ji et al. show that overexpressing Bam in ovaries induces the formation of cysts with 32 cells, suggesting an extra round of divisions. The number of 32-cell cysts increased by coexpressing CycA, indicating that the Bam-Otu-CycA regulatory axis could regulate the number of divisions both in males and females. However, one discrepancy remains, as overexpressing Bam in testis induces 8-cell cysts, whereas 32-cell cysts are formed in ovaries. This difference could be technical, as a stabilized form of Bam was overexpressed in testis under its own promoter
Altogether, the results presented in the Ji et al. article propose a novel model for the biochemical function of Bam when ectopically expressed in GSCs.
(i.e., only in dividing cyst cells) (13). In contrast, Ji et al. (7) used a heat-shock promoter to overexpress Bam in both GSCs and dividing cysts. Thirty-two–cell cysts could be the consequence of the formation of stem-cysts with four rounds of divisions and 16-cell cysts could also arise from stem-cyst followed by only three divisions, as in males (15). Further research is required to reconcile both sets of results in a single model for Bam activity. Alternatively, Bam could have different functions in males and females or the regulation of the number of divisions could be different in testis and ovaries.
Interestingly, the acetyltransferase Gcn5 was recently shown to be required for the maintenance of GSCs by promoting ubiquitination of CycA (16). Gcn5 is mainly known for acetylating histones but was shown to target APC2 and CycA. APC2 is a subunit of the anaphase-promoting complex (APC), which is an E3-ubiquitin ligase known to target CycA and other cyclins, such as for degradation. Acetylation could somehow promote ubiquitination of CycA and its degradation in GSCs. Interestingly, the decrease of CycA ubiquitination in the Gcn5 mutant seems independent of Bam levels. It thus remains unclear how ubiquitination and deubiquitination of CycA are coordinated. Nevertheless, the results show that the regulation of CycA levels is critical for proper maintenance of GSC fate.
In contrast to CycA, the levels of CycB (another B-type cyclin) need to be maintained for female GSC self-renewal (17). Indeed, loss-of-function of cycB induces a loss of GSC with no visible defects in somatic cells. In contrast, its overexpression has no detectable phenotype in GSCs. In addition, neither the loss nor the gain of the third Drosophila B-type cyclin, CycB3, has any effect on GSC maintenance and differentiation (8). Therefore, the three B-type cyclins work in specific ways to maintain the balance between GSC self-renewal and differentiation. The G1/S cyclin E is also essential for the maintenance of female GSCs (18). Taken together, these data show that the levels of CycA and several other cyclins are critical to ensure the proper balance between self-renewal and differentiation in stem cells. How can cyclin levels regulate stem cell fate? In mammalian cells, self-renewal is associated with a short G1 phase, which becomes longer as stem cells differentiate (19). Because cells are more responsive to extracellular cues in G1, a short G1 would favor self-renewal by shortening the time window during which stem cells can respond to differentiation signals. In Drosophila female GSCs, the G1 phase is also short and most of the cell cycle is spent in G2 (18). In addition, recent work in human embryonic stem cells shows that the maintenance of their pluripotency is actively controlled in S and G2 phases, through the ATM and Cyclin B1 pathways (20). Because Drosophila GSCs exhibit an extended G2 phase, their self-renewal ability could similarly be maintained during this phase. The specific and opposite roles of CycA and CycB in the GSC remain to be elucidated. They cannot substitute for each other in GSCs and may thus have specific targets in GSCs, or may even act more specifically on the ability to receive or transduce self-renewal signals (17, 21). The Ji et al. (7) work demonstrates that the essential but not conserved differentiation factor Bam regulates the stability of CycA, and therefore the GSC ability to self-renew. The conservation of this cyclin within the animal kingdom suggests that CycA ubiquitination may control the maintenance of other types of stem cells.
Acknowledgments
The authors’ work is supported by the CNRS, Institut Curie, and grants from the Association pour la Recherche sur le Cancer (PJA 20141202045), Agence Nationale de Recherches (AbsCyStem), and Fondation pour la Recherche Médicale (DEQ20160334884).
Footnotes
The authors declare no conflict of interest.
See companion article on page 6316.
References
- 1.McKearin DM, Spradling AC. bag-of-marbles: A Drosophila gene required to initiate both male and female gametogenesis. Genes Dev. 1990;4:2242–2251. doi: 10.1101/gad.4.12b.2242. [DOI] [PubMed] [Google Scholar]
- 2.McKearin D, Ohlstein B. A role for the Drosophila bag-of-marbles protein in the differentiation of cystoblasts from germline stem cells. Development. 1995;121:2937–2947. doi: 10.1242/dev.121.9.2937. [DOI] [PubMed] [Google Scholar]
- 3.Ohlstein B, McKearin D. Ectopic expression of the Drosophila Bam protein eliminates oogenic germline stem cells. Development. 1997;124:3651–3662. doi: 10.1242/dev.124.18.3651. [DOI] [PubMed] [Google Scholar]
- 4.Chen D, McKearin D. Dpp signaling silences bam transcription directly to establish asymmetric divisions of germline stem cells. Curr Biol. 2003;13:1786–1791. doi: 10.1016/j.cub.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 5.Song X, et al. Bmp signals from niche cells directly repress transcription of a differentiation-promoting gene, bag of marbles, in germline stem cells in the Drosophila ovary. Development. 2004;131:1353–1364. doi: 10.1242/dev.01026. [DOI] [PubMed] [Google Scholar]
- 6.Fuller MT, Spradling AC. Male and female Drosophila germline stem cells: two versions of immortality. Science. 2007;316:402–404. doi: 10.1126/science.1140861. [DOI] [PubMed] [Google Scholar]
- 7.Ji S, et al. Bam-dependent deubiquitinase complex can disrupt germ-line stem cell maintenance by targeting cyclin A. Proc Natl Acad Sci USA. 2017;114:6316–6321. doi: 10.1073/pnas.1619188114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen D, et al. Effete-mediated degradation of Cyclin A is essential for the maintenance of germline stem cells in Drosophila. Development. 2009;136:4133–4142. doi: 10.1242/dev.039032. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Minor NT, Park JK, McKearin DM, Maines JZ. Bam and Bgcn antagonize Nanos-dependent germ-line stem cell maintenance. Proc Natl Acad Sci USA. 2009;106:9304–9309. doi: 10.1073/pnas.0901452106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan L, et al. Protein competition switches the function of COP9 from self-renewal to differentiation. Nature. 2014;514:233–236. doi: 10.1038/nature13562. [DOI] [PubMed] [Google Scholar]
- 11.Shen R, Weng C, Yu J, Xie T. eIF4A controls germline stem cell self-renewal by directly inhibiting BAM function in the Drosophila ovary. Proc Natl Acad Sci USA. 2009;106:11623–11628. doi: 10.1073/pnas.0903325106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gönczy P, Matunis E, DiNardo S. bag-of-marbles and benign gonial cell neoplasm act in the germline to restrict proliferation during Drosophila spermatogenesis. Development. 1997;124:4361–4371. doi: 10.1242/dev.124.21.4361. [DOI] [PubMed] [Google Scholar]
- 13.Insco ML, Leon A, Tam CH, McKearin DM, Fuller MT. Accumulation of a differentiation regulator specifies transit amplifying division number in an adult stem cell lineage. Proc Natl Acad Sci USA. 2009;106:22311–22316. doi: 10.1073/pnas.0912454106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Insco ML, et al. A self-limiting switch based on translational control regulates the transition from proliferation to differentiation in an adult stem cell lineage. Cell Stem Cell. 2012;11:689–700. doi: 10.1016/j.stem.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathieu J, et al. Aurora B and cyclin B have opposite effects on the timing of cytokinesis abscission in Drosophila germ cells and in vertebrate somatic cells. Dev Cell. 2013;26:250–265. doi: 10.1016/j.devcel.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu T, et al. Gcn5 determines the fate of Drosophila germline stem cells through degradation of Cyclin A. FASEB J. 2017;31:2185–2194. doi: 10.1096/fj.201601217R. [DOI] [PubMed] [Google Scholar]
- 17.Wang Z, Lin H. The division of Drosophila germline stem cells and their precursors requires a specific cyclin. Curr Biol. 2005;15:328–333. doi: 10.1016/j.cub.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 18.Ables ET, Drummond-Barbosa D. Cyclin E controls Drosophila female germline stem cell maintenance independently of its role in proliferation by modulating responsiveness to niche signals. Development. 2013;140:530–540. doi: 10.1242/dev.088583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh AM, Dalton S. The cell cycle and Myc intersect with mechanisms that regulate pluripotency and reprogramming. Cell Stem Cell. 2009;5:141–149. doi: 10.1016/j.stem.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzales KA, et al. Deterministic restriction on pluripotent state dissolution by cell-cycle pathways. Cell. 2015;162:564–579. doi: 10.1016/j.cell.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Lilly MA, de Cuevas M, Spradling AC. Cyclin A associates with the fusome during germline cyst formation in the Drosophila ovary. Dev Biol. 2000;218:53–63. doi: 10.1006/dbio.1999.9570. [DOI] [PubMed] [Google Scholar]