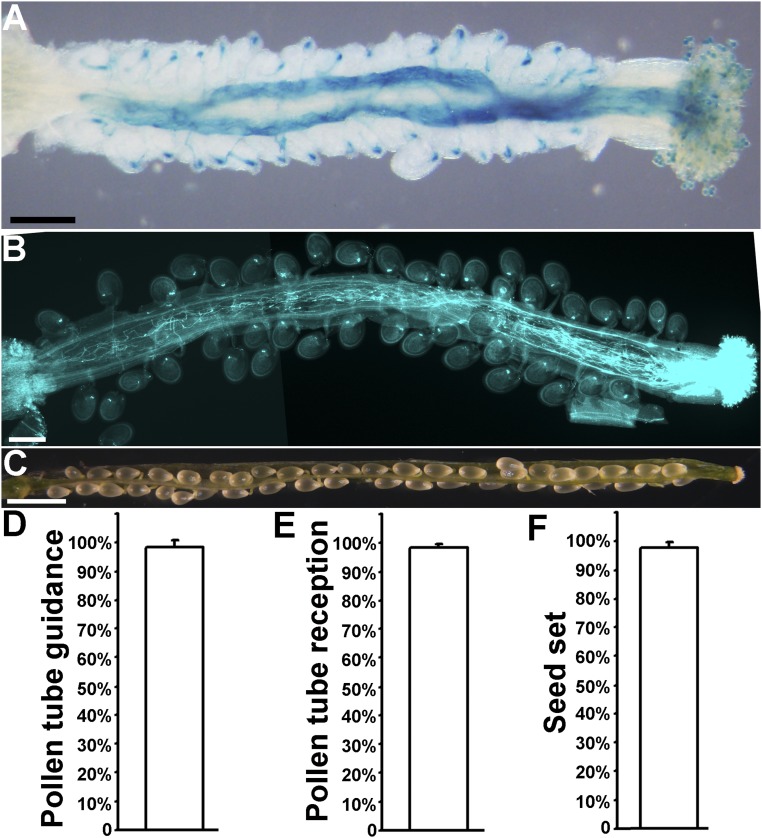
Significance
Double fertilization of angiosperms is preceded by the death of gametophytic cells: Two synergid cells degenerate to create a microenvironment for fertilization, whereas the pollen tube bursts to discharge sperm cells. This process is called pollen tube reception, in which a few synergid surface proteins have been identified, but intracellular activities involved are obscure. We report here that vacuolar acidification, mediated by V-ATPases and adaptor protein 1, might be an important mechanism for synergid degeneration during pollen tube reception. The study provides insights into a cell-death mechanism specifically adopted by the plant phylum.
Keywords: adaptor protein 1, pollen tube reception, vacuolar trafficking, vacuolar acidification, cell death
Abstract
Double fertilization in angiosperms requires the delivery of immotile sperm through pollen tubes, which enter embryo sacs to initiate synergid degeneration and to discharge. This fascinating process, called pollen tube reception, involves extensive communications between pollen tubes and synergids, within which few intracellular regulators involved have been revealed. Here, we report that vacuolar acidification in synergids mediated by AP1G and V-ATPases might be critical for pollen tube reception. Functional loss of AP1G or VHA-A, encoding the γ subunit of adaptor protein 1 or the shared component of two endomembrane V-ATPases, respectively, impaired synergid-controlled pollen tube reception and caused partial female sterility. AP1G works in parallel to the plasma membrane-associated receptor FERONIA in synergids, suggesting that synergid-mediated pollen tube reception requires proper sorting of vacuolar cargos by AP1G. Although AP1G did not mediate the targeting of V-ATPases, AP1G loss of function or the expression of AP1G-RNAi compromised vacuolar acidification mediated by V-ATPases, implying their genetic interaction. We propose that vacuolar acidification might represent a distinct cell-death mechanism specifically adopted by the plant phylum, which is critical for synergid degeneration during pollen tube reception.
Double fertilization in angiosperm is preceded by fine-tuned communication between male and female gametophytes (1, 2). The female gametophyte (FG), i.e., the embryo sac, is formed inside the ovules and contains an egg cell, a central cell, two synergid cells, and three antipodal cells (3). Upon landing on a receptive pistil, a pollen grain forms a long cylindrical extension, called a pollen tube, which extends inside female sporophytic tissues. To deliver immotile sperm, pollen tubes perceive attractive signals sent out by the FG, change their growth axis, and finally enter the embryo sacs through the micropyle (1–4). A process called “pollen tube reception” instructs the cessation and discharge of the penetrating pollen tube, leading to sperm release and fertilization (1–4).
Studies have uncovered key female factors controlling pollen tube reception, including FERONIA (FER) (5–7), LORELEI (LRE) (8, 9), and early nodulin-like proteins (ENODLs) (10) and NORTIA (NTA) (11). These synergid receptors likely operate in the same genetic pathway (10–12) whose functional loss caused the failure of pollen tube discharge and led to reduced female fertility (5–9, 11). Male ligands are yet to be identified. However, recent studies showed that the production of reactive oxygen species (ROS) and Ca2+ spiking are downstream events of FER-controlled pollen tube reception (13–16).
Synergid degeneration, a form of programmed cell death (PCD), is a key step during pollen tube reception. Between the two synergid cells, a receptive synergid cell is the one that succeeds in interacting with the pollen tube and induces it to burst and undergoes cell death first. The other synergid cell that continues to persist and then undergoes cell death orchestrated by the fertilized egg cell and the central cell is the persistent synergid (3, 11, 17). In Arabidopsis, the degeneration of the receptive synergid usually accompanies the discharge of the incoming pollen tube (18). Synergid degeneration can also be initiated without pollen tube discharge (17). The degeneration and removal of the persistent synergid are mediated by ethylene signaling and the central cell (19, 20).
We report here that vacuolar acidification mediated by AP1G and V-ATPases might be critical for synergid-controlled pollen tube reception. AP1 is a tetrameric protein complex regulating protein sorting at the trans-Golgi network/early endosome (TGN/EE), the convergent compartment for anterograde and retrograde trafficking pathways. AP1γ (AP1G) is encoded by AP1G1 and AP1G2. Single mutants of either AP1G1 or AP1G2 did not have a discernible phenotype. However, functional loss of both genes resulted in partial female sterility due to the failure of pollen tube discharge and synergid degeneration. Although AP1G mediates protein sorting at the TGN/EE both to the plasma membrane (PM) and the tonoplast, AP1G loss of function caused mistargeting of tonoplast proteins but not that of FER. In addition, AP1G and FER are genetically independent in controlling pollen tube reception. These results suggested that AP1G functions in synergids through mediating vacuolar trafficking. We further showed that abolishing the activity of V-ATPases compromised vacuolar acidification and caused a similar defect in pollen tube reception. Finally, we demonstrate that AP1G is critical for synergid vacuolar acidification, possibly by mediating the activities of V-ATPases. Our results suggested that vacuolar acidification, contributed by AP1G and V-ATPases, is involved in synergid degeneration and pollen tube reception. Vacuolar acidification-mediated cell degeneration may represent a distinct cell-death mechanism adopted specifically by the plant kingdom.
Results
Functional Loss of AP1G Reduces Fertility.
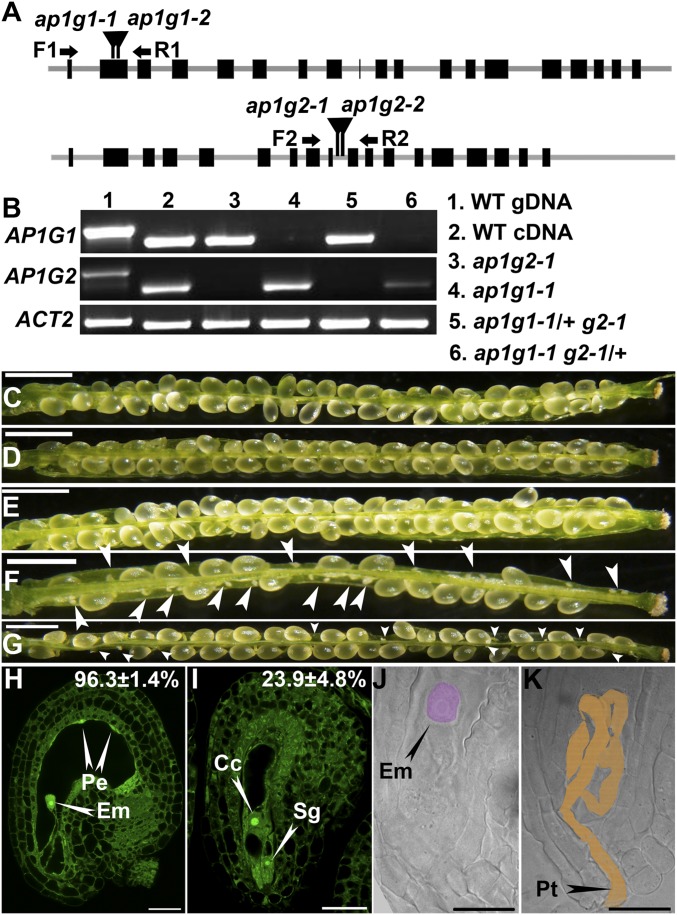
To determine the function of AP1Gs in planta, we analyzed mutants for AP1G1 and AP1G2 (Fig. 1A). Single mutants showed no discernible phenotype (Fig. 1 D and E and Fig. S1). We thus crossed the null alleles, ap1g1-1 and ap1g2-1, to generate a double knockout line. Plants with the genotype ap1g1-1/ap1g1-1;AP1G2/ap1g2-1 (ap1g1 g2/+) but not those with the genotype AP1G1/ap1g1-1;ap1g2-1/ap1g2-1 (ap1g1/+ g2) were dwarf and bushy (Fig. S1), possibly due to haplo-insufficiency, because the expression of AP1G1 is higher than that of AP1G2 in most tissues (21). A significant portion of ap1g1/+ g2 or ap1g1 g2/+ ovules did not grow, compared with that in wild type (WT) or in the single mutants (Fig. 1 C–G). To determine whether these ovules were unfertilized or defective in embryogenesis, we analyzed these ovules 24 h after pollination (HAP) by confocal laser scanning microscopy (CLSM) and after whole-mount ovule clearing. Almost all wild-type ovules and approximately 75% of ap1g1/+ g2 ovules contained an embryo and peripheral endosperm (Fig. 1 H and J and Table S1), indicative of fertilization. However, approximately 25% ap1g1/+ g2 ovules contained a central cell and egg cell at 24 HAP (Fig. 1I and Table S1), indicative of failed fertilization. The unfertilized ap1g1/+ g2 ovules contained entangled pollen tubes (Fig. 1K), whereas they ceased growth and discharge contents in WT.
Fig. 1.
AP1G loss of function reduced fertility. (A) Schematic illustration of T-DNA insertions within AP1Gs (AP1G1 and AP1G2). Filled boxes indicate coding regions. Arrows indicate the binding regions for RT-PCR primers. (B) Transcript analysis showing that both ap1g1-1 and ap1g2-1 are null mutants. ACT2 (ACTIN2) was used as the internal control. (C–G) Seed set analyses in self-fertilized WT (C), ap1g1-1 (D), ap1g2-1 (E), the trans-heterozygous mutant ap1g1 g2/+ (F), and ap1g1/+ g2 (G). Results are means ± SD (n = 100). Seed sets of ap1g1/+ g2 and ap1g1 g2/+ are significantly different from WT or either single mutant (one-way ANOVA, Tukey–Kramer test, P < 0.05). Arrowheads point at unfertilized ovules. (H and I) CLSM of ovules at 24 HAP either from WT (H) or from ap1g1/+ g2 (I). Data shown on the top right indicate the average percentage of the displayed category. Results shown are means ± SEs (SEM) of 10 replicates. Wild type and ap1g1/+ g2 are significantly different (t test, P < 0.05). (J and K) Differential interference contrast images showing an embryo (pseudocolored pink) at 24 HAP from WT (J) or an embryo sac from ap1g1/+ g2 with an overgrown pollen tube (K, pseudocolored light brown). Cc, central cell; Em, embryo; Pe, peripheral endosperm; Pt, pollen tube; Sg, synergid. (Scale bars: C–G, 500 µm; H and I, 25 µm; J and K, 20 µm.)
Fig. S1.
ProUBQ10:GFP-AP1G1 or ProUBQ10:GFP-AP1G2 restored the fertility of the ap1g1 g2 double mutant. (A) Wild-type (WT), homozygous single mutants, trans-heterozygous mutants of AP1G, and the complementation lines are shown at vegetative (Upper) and reproductive (Lower) stages. Representatives from 30 to 200 plants of different genotypes are shown. (B–D) A representative silique from WT (B), from ProUBQ10:GFP-AP1G1;ap1g1 g2 (C), or from ProUBQ10:GFP-AP1G2;ap1g1 g2 (D). (E) Transcriptional analysis by RT-PCRs. Exogenous genes were driven by the constitutive promoter ProUBQ10. Inflorescences were used for RNA extractions. ACT2 was used as the internal control. (F) Aniline blue-stained pistils pollinated with WT pollen at 48 HAP. The ap1g1 g2 double mutant (aabb) was transformed with the homozygous ProUBQ10:GFP-AP1G1 (A;aabb) or ProUBQ10:GFP-AP1G2 (B;aabb). Two overlapping high-magnification images were taken for one pistil. The images were then overlaid with Photoshop (Adobe Systems) to show the whole pistil. (Scale bars: B–D, 500 µm; F, 200 µm.)
Table S1.
Summary of quantitative studies of the FG defects in various genetic materials used in this study
| Experiment | Genotype of female | Ratio, % | Note |
| Seed set | WT | 98.6 ± 1.59 | Seed set of self-fertilized plants. |
| ap1g1-1 | 97.9 ± 1.81 | ||
| ap1g2-1 | 98.6 ± 1.56 | ||
| ap1g1 g2/+ | 47.9 ± 1.07 | ||
| ap1g1/+ g2 | 73.6 ± 8.97 | ||
| fer-4/+ ap1g1/+ g2 | 35.8 ± 6.2 | ||
| fer-4/+ | 85.5 ± 3.09 | ||
| CLSM of ovules at 24 HAP | WT | 96.3 ± 1.4 | Fertilized, based on the presence of embryo/endosperm. |
| ap1g1/+ g2 | 23.9 ± 4.8 | Unfertilized, based on the presence of FG nuclei. | |
| Pollination with ProLAT52:GUS at 9 HAP | WT | 97.46 ± 3.29 | Ratio of pollen tube guidance. |
| ap1g1/+ g2 | 89.87 ± 9.64 | ||
| vha-A/+ | 85.62 ± 9.36 | ||
| Atvpe | 98.1 ± 2.47 | ||
| Aniline blue staining at 48 HAP | WT | 92.3 ± 6.5 | Ratio of fertilized and developed ovules. |
| ap1g1/+ g2 | 65.2 ± 11.6 | ||
| fer-4/+ ap1g1/+ g2 | 23.1 ± 6.5 | ||
| fer-4/+ | 74.2 ± 5.8 | ||
| vha-A/+ | 71.54 ± 9.75 | ||
| Atvpe | 98.19 ± 1.41 | ||
| Pollination with ProLAT52:DsRed at 12 HAP | WT | 91.1 ± 3.47 | Ratio of ovules containing a bursting pollen tube among all ovules examined. |
| ap1g1/+ g2 | 62.1 ± 8.5 |
No double homozygous plants were obtained from self-pollinated ap1g1/+ g2 or ap1g1 g2/+ (Table S2). Segregation ratios from reciprocal crosses showed that ap1g1 g2 was male gametophytic lethal and partially defective in female gametophytic transmission (Table S2). Because of the haplo-insufficient phenotype of ap1g1 g2/+ in vegetative tissue development (Fig. S1), most of our following studies used ap1g1/+ g2 (hereafter as ap1g/+) to avoid the influence of poor vegetative growth. However, either gene driven by the ProUBQ10 promoter fully complemented the mutant phenotype (Fig. S1), indicating that the two genes are functionally interchangeable.
Table S2.
Segregation analysis of the AP1G mutations by PCR-based genotyping
| Female x Male | Genotypes | Expected ratio | Observed ratio |
| ap1g1-1+/− ap1g2-1 × ap1g1-1+/− ap1g2-1 | ap1g1-1 +/+:+/−:−/− (ap1g2-1) | 1:2:1 | 131:50:0* |
| ap1g1-1+/− ap1g2-1 × WT | ap1g1-1 +/+:+/− (ap1g2-1 +/−) | 1:1 | 109:69† |
| WT × ap1g1-1+/− ap1g2-1 | ap1g1-1 +/+:+/− (ap1g2-1 +/−) | 1:1 | 106:0† |
| ap1g1-1 ap1g2-1+/− × ap1g1-1 ap1g2-1 +/− | ap1g2-1 +/+:+/−:−/− (ap1g1-1) | 1:2:1 | 162:84:0* |
| ap1g1-1 ap1g2-1+/− × WT | ap1g2-1 +/+:+/− (ap1g1-1 +/−) | 1:1 | 108:27† |
| WT × ap1g1-1 ap1g2-1+/− | ap1g2-1 +/+:+/− (ap1g1-1 +/−) | 1:1 | 144:0† |
Significantly different from the segregation ratio 1:2:1 (χ2, P < 0.01).
Significantly different from the segregation ratio 1:1 (χ2, P < 0.01).
Impaired Pollen Tube Reception in the FG of ap1g/+.
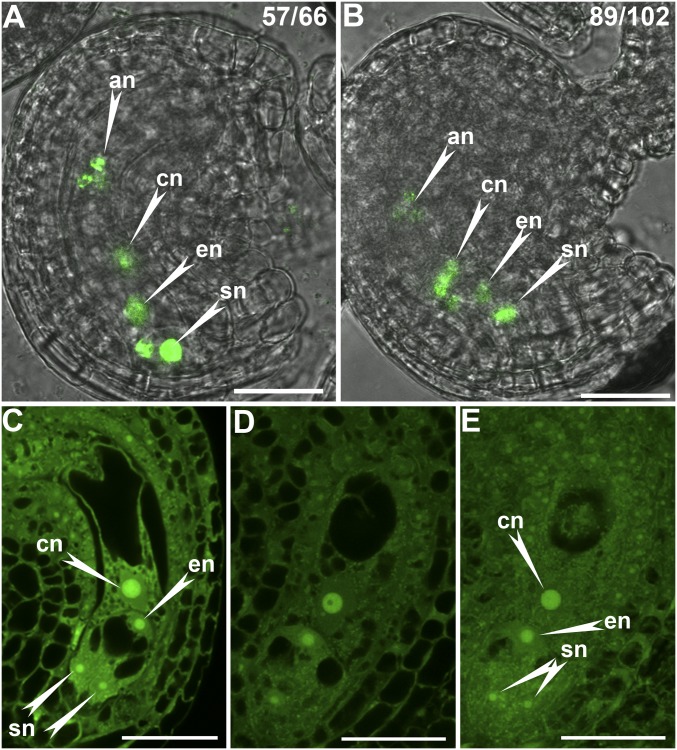
To determine the cause of reduced female transmission of ap1g, we first introduced ProES1:NLS-YFP into WT or ap1g/+ and examined the development of the FG. Because ProES1 is active in all cells of the FGs (22), the nucleus-targeting YFP (NLS-YFP) driven by ProES1 should show FGs with seven nuclei. CLSM on unfertilized mature ovules at flower stage 12 indicated that the position of synergid cells and the organization of FG were comparable between WT and ap1g/+ (Fig. S2), indicating that AP1Gs were not essential for FG development.
Fig. S2.
Functional loss of AP1G did not compromise the patterning of female gametophytes. (A and B) CLSM of a representative immature ovule from ProES1:NLS-YFP (A) or from ProES1:NLS-YFP;ap1g1/+ g2 plants (B). On top is the ratio of displayed ovules among all ovules examined. Left images are merges of fluorescent and bright-field images. (C and D) A single optical section of mature ovules from WT (C) or ap1g1/+ g2 plants (D). (E) Maximum projection of Z-stack images captured for the ovule shown in D. An, antipodal nuclei; cn, central cell nuclei; en, egg cell nuclei; sn, synergid nuclei. (Scale bars: 25 µm.)
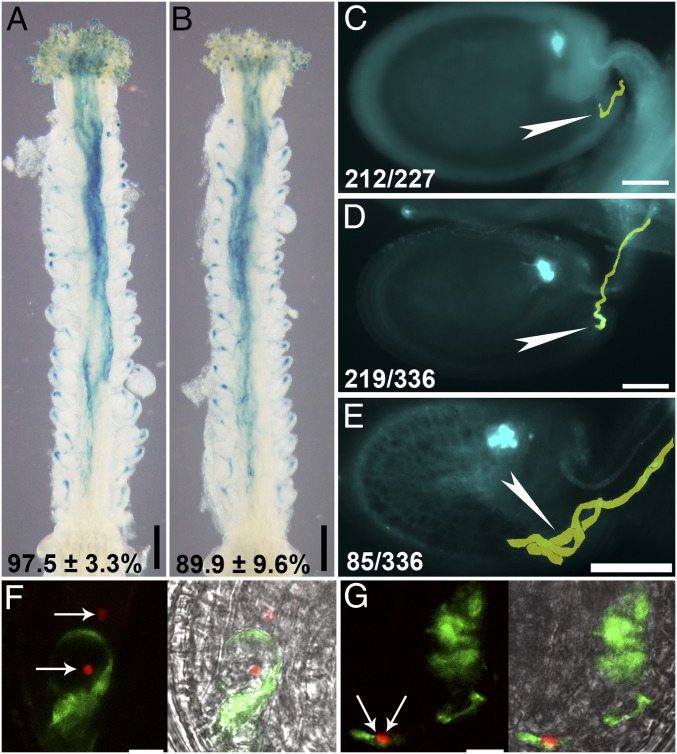
Next, we hand-pollinated emasculated wild-type or ap1g/+ pistils with ProLAT52:GUS pollen; histochemical GUS staining showed a slight reduction of pollen tube attraction in ap1g/+ pistils (Fig. 2 A and B and Table S1). Aniline blue staining of WT or ap1g/+ pistils at 48 HAP showed that a significant portion (36.3 ± 10.8%) of ap1g/+ ovules were unfertilized (Table S1), in contrast to that in WT (Table S1). Overgrown pollen tubes were present in unfertilized ovules (85 of 117; Fig. 2E), indicating impaired pollen tube reception (5–7, 9, 11, 23, 24).
Fig. 2.
Female gametophytes of ap1g1 g2 are compromised in pollen tube reception. (A and B) Representative histochemical GUS analysis of wild-type (A) or ap1g1+/− g2 (B) pistils emasculated and hand-pollinated with ProLAT52:GUS pollen at 12 HAP. Results shown at the bottom are means ± SD, n = 25. (C–E) Representative aniline blue-stained WT ovule (C), ap1g1+/− g2 ovule with normal pollen tube reception (D), ap1g1+/− g2 ovule with a tangled pollen tube (E) at 48 HAP. Pollen tubes are pseudocolored in yellow. Arrowheads point at the micropyle. At the bottom: displayed ovules/all ovules examined. (F and G) CLSM of a wild-type (F) or ap1g1 g2 (G) embryo sac invaded by a ProLAT52:GFP;ProHTR10:HTR10-mRFP pollen tube. The right images are merges of GFP, RFP, and BF channels. Arrows in F and G indicate the position of sperm cells. (Scale bars: A and B, 200 µm; C–E, 50 µm; F and G, 10 µm.)
To verify the impaired pollen tube reception, we hand-pollinated emasculated wild-type or ap1g/+ pistils with ProLAT52:GFP;ProHTR10:HTR10-mRFP pollen, in which sperm nuclei are labeled with mRFP and pollen tubes express cytoplasmic GFP (25). Pollen tubes discharged their content, that is, cytoplasm and sperm cells (67 of 79; Fig. 2F) upon arrival in WT. However, 18 of 75 ap1g/+ FGs contained a nondischarging pollen tube (Fig. 2G). As a result, sperm cells were trapped way back in pollen tubes rather than being released into the FG (Fig. 2G). These results demonstrated a critical role of AP1G in FG-controlled pollen tube reception.
Pollen Tube Arrival–Induced Synergid Degeneration Is Impaired by AP1G Loss of Function.
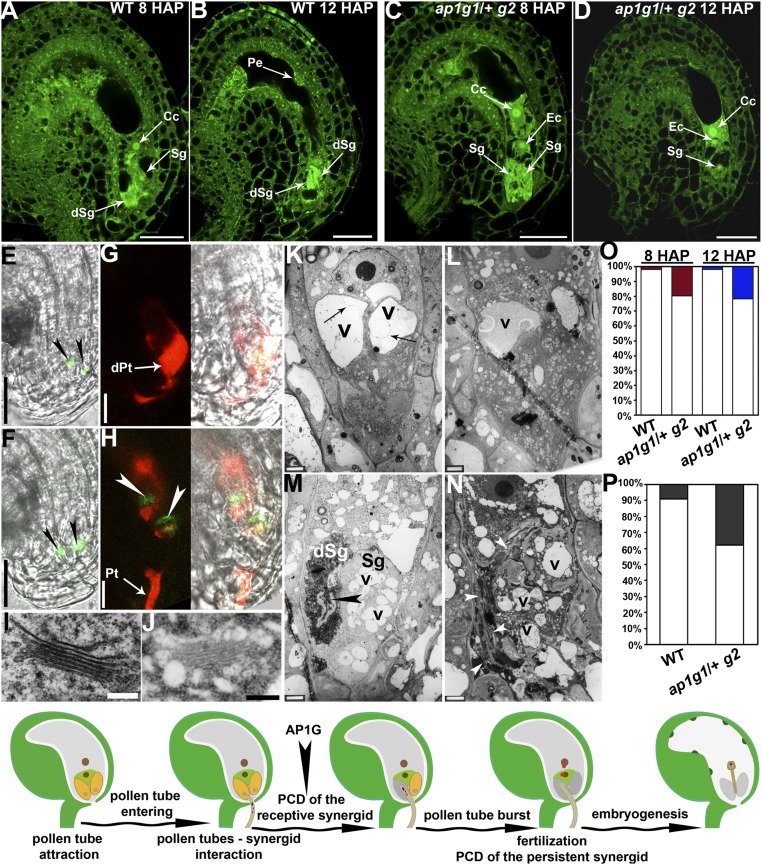
To determine how AP1G loss of function caused abnormal tube reception, we first used CLSM to examine wild-type and ap1g/+ pistils at 8 HAP or 12 HAP. As reported (18, 26), the receptive synergid degenerated at 8 HAP, whereas the persistent synergid degenerated at 12 HAP in most wild-type ovules (Fig. 3 A, B, and O). The appearance of peripheral endosperm also indicated successful fertilization (Fig. 3B). By contrast, a notable portion of ap1g/+ ovules contained two synergids at 8 HAP or one synergid at 12 HAP (Fig. 3 C, D, and O). Accordingly, autofluorescence from degenerating synergids as seen in WT at a comparable stage was undetectable and peripheral endosperm was not present in those ap1g/+ ovules (Fig. 3D). These results supported a role of AP1G in synergid degeneration induced by pollen tube arrival.
Fig. 3.
Synergid degeneration induced by pollen tube arrival is compromised by AP1G loss of function. (A–D) CLSM images of ovules from WT (A and B) or ap1g1/+ g2 (C and D) emasculated and hand-pollinated with wild-type pollen at 8 HAP (A and C) or 12 HAP (B and D). Image shown in C is projection of three confocal images taken at different focal planes to show the presence of different nuclei. (E–H) CLSM image of ovules from ProDD39:NLS-YFP (E and G) or ProDD39:NLS-YFP; ap1g1/+ g2 (F and H) before pollination (E and F) or pollinated with a ProLAT52:DsRed pollen tube at 8 HAP (G and H). The transgene ProDD39:NLS-YFP is present as homozygous single copy in both WT and ap1g1/+ g2. Arrowheads indicate synergid nuclei. Images in E and F are merges of GFP and bright-field (BF) channels. Images shown in G and H are merges of GFP/RFP channels and merges of GFP/RFP/BF channels. (I and J) TEM of a Golgi stack in a wild-type (I) or a presumble ap1g1 g2 synergid (J). (K–N) TEM of a wild-type (K and M) or a presumble ap1g1 g2 (L and N) embryo sac before pollination (K and L) or upon pollen tube entrance (M and N). Arrows in K indicate invaginated or convoluted vacuolar membrane inside the central vacuole of a synergid. Arrowhead in M indicates a discharging pollen tube. Arrowheads in N point at an overgrown pollen tube. (O) Quantitative analyses of synergid degeneration by CLSM. Percentage of ovules containing one synergid degenerated at 8 HAP or two synergids degenerated at 12 HAP is shown with open columns, whereas filled columns indicate the percentage of ovules containing two intact synergids at 8 HAP (red) or one intact synergid at 12 HAP (blue). (P) Percentage of discharging (empty columns) or nondischarging (filled columns) ProLAT52:DsRed pollen tubes at 12 HAP. In total, 25 pistils were analyzed for each genotype and all showed similar results. (Q) A cartoon model illustrating the steps leading to fertilization. Arrowhead indicates the timing of AP1G action in these processes. Cc, central cell; dSg, degenerated synergid; dPt, discharged pollen tube; Ec, egg cell; Pe, peripheral endosperm; Pt, pollen tube; Sg, synergid; v, vacuoles. (Scale bars: A–F, 25 µm; G and H, 10 µm; I and J, 200 nm; K–N, 2 µm.)
To confirm that synergid degeneration was not properly executed upon pollen tube arrival by AP1G loss of function, we expressed NLS-YFP under a synergid-specific promoter ProDD39 (27). Synergid nuclei were labeled in both unfertilized wild-type and ap1g/+ ovules (Fig. 3 E and F). Pollination with ProLAT52:DsRed pollen at 12 HAP led to pollen tube discharge and undetectable NLS-YFP signals in WT (Fig. 3 G and P), indicative of synergid death. By contrast, ovules from the ProDD39:NLS-YFP;ap1g/+ plants contained a nondischarging, overgrowing pollen tube wrapping around two synergid nuclei (17 of 72; Fig. 3 H and P).
To gain further evidence for the role of AP1Gs in synergid degeneration, we performed transmission electron microscopy (TEM) on mature pistils before pollination or at 8 HAP. In mature wild-type embryo sacs, synergid cells contained a large central vacuole distal to the micropyle, in which few electron-dense materials were present (Fig. 3K). In embryo sacs with distorted Golgi morphology (Fig. 3J) indicative of the ap1g identity (28), synergid cells also contained a central vacuole distal to the micropyle (Fig. 3L). However, unlike those in WT (Fig. 3K), the central vacuoles were filled with electron-dense materials (Fig. 3L), suggesting defective vacuolar degradation. In addition, numerous small vacuoles were present in addition to the central vacuoles of the presumable ap1g synergids (Fig. 3L), but not in those of WT (Fig. 3K). At 8 HAP, pollen tube arrival in wild-type embryo sacs was accompanied with the degeneration of the receptive synergid (Fig. 3M). At the same time, vacuoles became fragmented with reduced membrane integrity of the persistent synergid (Fig. 3M), indicating an immediate cell death. Consistent with the results obtained by using fluorescent pollen tubes (Fig. 3P), nondischarging pollen tubes were observed in the presumable ap1g embryo sacs after pollination (Fig. 3N) based on the Golgi morphology (Fig. 3J). No synergid degeneration occurred in these embryo sacs (Fig. 3N). Fragmented vacuoles filled with electron-dense materials were observed in synergid cells, for which membrane integrity was well preserved (Fig. 3N), unlike that of WT (Fig. 3M). These results indicated that functional loss of AP1Gs compromised synergid degeneration upon pollen tube arrival, which is likely crucial for pollen tube discharge (18).
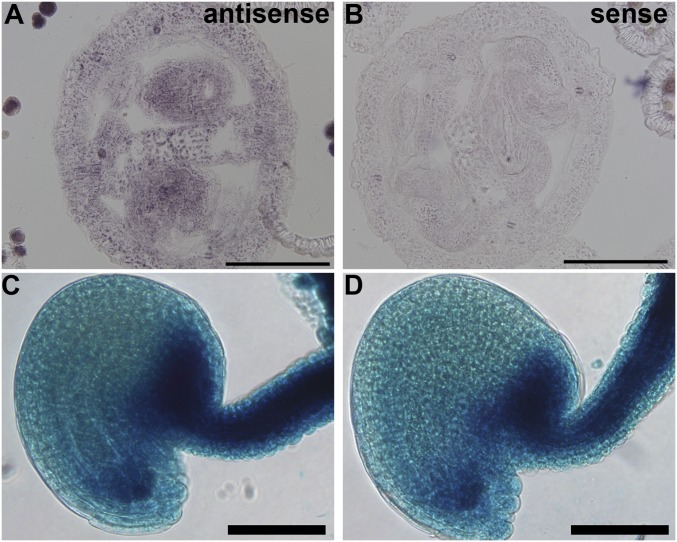
To determine whether the role of AP1G in synergid degeneration was cell-autonomous, we confirmed their expression in ovules and synergid cells by RNA in situ hybridization and by histochemical GUS staining of their promoter-reporter lines. Both AP1G1 and AP1G2 were expressed in ovules, especially at the micropylar region where synergid cells are located (Fig. S3).
Fig. S3.
AP1Gs are expressed in synergid cells. (A and B) RNA in situ hybridization with antisense (A) or sense (B) probe for AP1Gs. (C and D) Representative histochemical GUS staining of ProAP1G1:GUS (C) or ProAP1G2:GUS (D) transgenic plants. (Scale bars: A and B, 100 µm; C and D, 50 µm.)
Mistargeting of Tonoplast Proteins by AP1G Functional Loss.
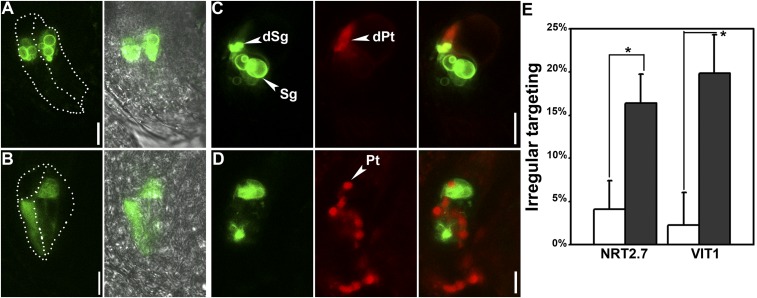
Because AP1G1 was reported to regulate vacuolar trafficking of tonoplast proteins (29), we attempted to examine whether AP1Gs regulated vacuolar trafficking of tonoplast proteins in synergids. To this purpose, we introduced ProDD39:GFP-NRT2.7 into WT and ap1g/+. Synergid-specific expression of the tonoplast-associated NRT2.7 (30) would allow us to examine the potential defects on the vacuolar trafficking of tonoplast proteins in ap1g synergids. In unpollinated mature wild-type ovules, GFP-NRT2.7 was localized at the tonoplast of the vacuoles (Fig. 4A). Upon pollen tube arrival and discharge, GFP-NRT2.7 signals formed as large aggregates in the degenerating synergid (Fig. 4B). By contrast, GFP-NRT2.7 was not associated with the tonoplast in a substantial portion of ap1g/+ ovules (Fig. 4A and Fig. S4). Because AP1G loss of function did not impair vacuolar biogenesis in synergids (Fig. 3), this result suggested impaired vacuolar delivery of NRT2.7. Upon pollination, in the ap1g/+ FGs that contained a nondischarging pollen tube, GFP did not show tonoplast association (Fig. 4B). A previously confirmed AP1G1-cargo, vacuolar ion transporter1 (VIT1) (29), showed the same pattern of distribution in the synergids of ap1g/+ (Fig. S4). These results indicated that vacuolar targeting of some tonoplast proteins in synergids was impaired by AP1G loss of function.
Fig. 4.
Targeting of tonoplast proteins but not FERONIA the key plasma membrane receptor is interfered in synergids by AP1G loss of function. (A and B) CLSM of ProDD39:GFP-NRT2.7 in synergids of WT or ap1g1/+ g2. Pistils from ProDD39:GFP-NRT2.7 or ProDD39:GFP-NRT2.7;ap1g1/+ g2 plants were either emasculated at maturation for visualization (A) or emasculated and pollinated with ProLAT52:DsRed pollen and visualized at 8 HAP (B). Merges of GFP, RFP, and BF channels are shown at the right side in B. Dotted lines illustrate synergid cells. (C–F) CLSM of ProDD39:FER-YFP in synergids of WT (C and D) or ap1g1/+ g2 (E and F). Pistils from ProDD39:FER-YFP or ProDD39:FER-YFP;ap1g1/+ g2 plants were either emasculated at maturation for visualization (C and E) or emasculated and pollinated with ProLAT52:DsRed pollen and visualized at 8 HAP (D and F). Merges of GFP, RFP, and BFA channels are shown at the right side in D and F. Insets in C and E are their corresponding close-ups at the micropylar region. dPt, discharged pollen tube; Pt, pollen tube. (Scale bars: A and B, 10 µm; D and F and C and E, Inset, 25 µm.)
Fig. S4.
Targeting of tonoplast proteins is interfered in synergids by AP1G loss of function. (A–D) CLSM of ProDD39:VIT1-GFP in synergids of WT (A and C) or ap1g1/+ g2 (B and D). Pistils from ProDD39:VIT1-GFP or ProDD39:VIT1-GFP;ap1g1/+ g2 plants were either emasculated at maturation for visualization (A and B) or emasculated and pollinated with ProLAT52:DsRed pollen and visualized at 8 HAP (C and D). Merges of GFP and RFP channels are shown at Right. Dotted lines illustrate synergid cells. dPt, discharged pollen tube; dSg, degenerating synergid; Pt, pollen tube; Sg, synergid. (E) Percentage of ovules in which GFP-NRT2.7 and VIT1-GFP were not associated with the tonoplast in synergids. Results are means ± SEM of three independent experiments involving 150 ovules. Open columns indicate WT, whereas filled columns indicate ap1g1/+ g2. Asterisk indicates significant difference (t test, P < 0.01). (Scale bars: 10 µm.)
To exclude the possibility that defective vacuolar trafficking interfered with cellular homeostasis and, thus, impaired the targeting of PM receptors such as FER, we introduced ProDD39:FER-YFP into both WT and ap1g/+. In unpollinated ovules, FER distributed comparably in WT and ap1g/+ (Fig. 4 C and E). Pollination with ProLAT52:DsRed pollen at 12 HAP showed no differences between WT and ap1g/+ in FER distribution (Fig. 4 D and F), suggesting that AP1G loss of function did not affect the distribution of key PM proteins such as FER.
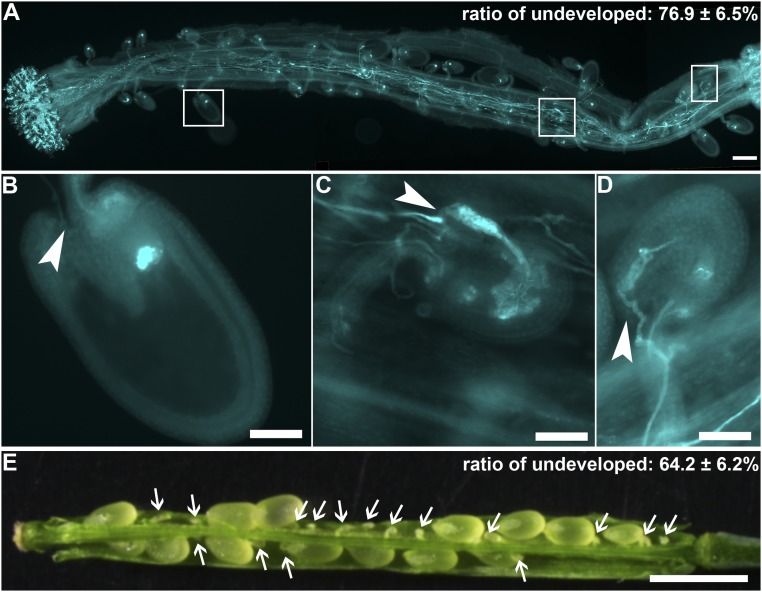
To provide further evidence that FER pathway was not affected in ap1g/+, we introduced a null mutant of FER, fer-4 (31), into ap1g/+. We were able to obtain fer-4/+ ap1g/+ plants in which both fer-4 and ap1g1 are heterozygous, whereas ap1g2 was homozygous (Fig. S5). Self-fertilized fer-4/+ ap1g/+ plants produced a significantly reduced seed set compared with either fer-4/+ or ap1g/+ (Table S1). In addition, aniline blue staining of the fer-4/+ ap1g/+ pistils at 48 HAP showed that more than 75% of ovules were undeveloped, significantly different from either single mutant (Fig. S5 and Table S1). Tangled pollen tubes were detected in undeveloped ovules (Fig. S5), indicative of defective pollen tube reception. The reduced seed set and pollen tube reception of fer-4/+ ap1g/+ was a combination of fer-4 (31) and AP1G loss of function (Fig. S5 and Table S1), strongly suggesting that AP1G and FER act independently in pollen tube reception.
Fig. S5.
AP1G-mediated and FER-mediated pathways are genetically independent. (A) A representative fer-4/+ ap1g1/+ g2/g2 pistil emasculated and hand-pollinated with WT pollen and examined by aniline blue staining at 48 HAP. On the top right is the ratio of undeveloped ovules. Four overlapping high-magnification images were taken for one pistil. The images were then overlaid with Photoshop (Adobe Systems) to show the whole pistil. Results shown are means ± SD (n = 15 pistils) (B–D) Close-up of rectangle boxes showing in A from left to right, showing an developed ovule (B) and two representative ovules with tangled pollen tubes inside (C and D). Arrowheads point at the micropyle. (E) A representative fer-4/+ ap1g1/+ g2/g2 silique. Arrowheads point at undeveloped seeds. (Scale bars: A, 200 µm; B–D, 50 µm; E, 500 µm.)
Functional Loss of V-ATPases Phenocopies That of AP1G in Defective Pollen Tube Reception.
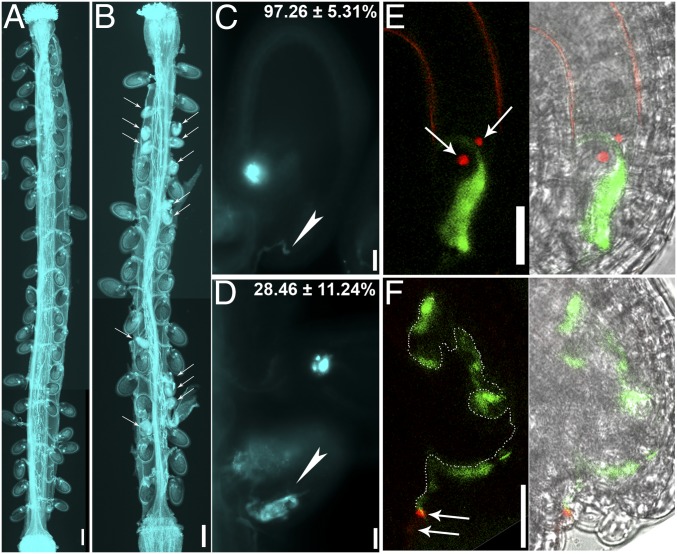
Above results suggested that vacuolar trafficking from the TGN/EE was impaired by AP1G loss of function in synergids. Arabidopsis VHA-A is the single gene encoding the catalytic subunit of V-ATPases located at the TGN/EE and the tonoplast (32). Its mutation caused complete male gametophytic lethality and partial female transmission defect (32), resembling that of AP1G. In addition, functional loss of V-ATPases and AP1 both resulted in distorted Golgi morphology (28, 32, 33). We thus hypothesized that V-ATPases might be involved in synergid degeneration during pollen tube reception. To test this hypothesis, we analyzed the FG defects of the heterozygous mutant of VHA-A, vha-A/+ (Fig. S6). Histochemical GUS staining of vha-A/+ pistils pollinated with the ProLAT52:GUS pollen showed a slight reduction of pollen tube attraction in vha-A/+ (Fig. S7 and Table S1). A significant portion of vha-A/+ ovules were unfertilized and contained entangled pollen tubes at 48 HAP (Fig. 5B). Finally, a notable portion of vha-A/+ embryo sacs (15 of 83) were penetrated by a nondischarging ProLAT52:GFP;ProHTR10:HTR10-mRFP pollen tube containing unreleased sperm cells (Fig. 5 D and F), suggesting that V-ATPases are critical for synergid-controlled pollen tube reception.
Fig. S6.
Genetic characterization of a VHA-A mutant allele. (A) Schematic illustration of the T-DNA insertion within VHA-A. Filled boxes indicate coding regions. (B) Relative transcript abundance of VHA-A in WT and in vha-A+/− by real-time quantitative PCRs. Results shown are means ± SEM of three biological replicates. Asterisk indicates significant difference (t test, P < 0.05). (C) SEM of pollen grains from vha-A +/−. (D and E) Mature pollen grains from WT (D) or from vha-A+/− (E) were stained with neutral red for acidic vacuoles. Percentage of pollen grains with vacuolar neutral red staining is shown on top of the image. Results are means ± SD (n = 200). (F and G) Mature pollen grains from WT (F) or from vha-A+/− (G) were stained with BCECF-AM for acidic vacuoles. Arrowhead points at a defective pollen grain with no fluorescent signals. Fluorescent images are merged with bright-field images at the side. (Scale bars: 10 µm.)
Fig. S7.

Pollen tube guidance is slightly reduced in the mutant of VHA-A. Representative histochemical GUS analysis of WT pistil (A) or vha-A/+ (B) pistils emasculated and hand-pollinated with ProLAT52:GUS pollen at 9 HAP. Two overlapping high-magnification images were taken for one pistil. The images were then overlaid with Photoshop (Adobe Systems) to show the whole pistil. Bars = 500 µm. (Scale bars: 200 µm.)
Fig. 5.
Pollen tube reception is compromised in the mutant of VHA-A. (A and B) Aniline blue staining of wild-type pistil (A) or vha-A/+ pistil (B) emasculated and hand-pollinated with wild-type pollen at 48 HAP. Arrows indicate unfertilized ovules. Two to three overlapping high-magnification images were taken for one pistil. The images were then overlaid with Photoshop (Adobe Systems) to show the whole pistil. (C and D) Representative aniline blue staining of wild-type (C) or vha-A/+ (D) ovules at 48 HAP. Arrowheads point at micropyle in C and a tangled pollen tube in D. Percentage of the displayed category (fertilized in C; defective in pollen tube reception in D) is shown on top right of the image. Results are means ± SEM. Approximately 200 ovules were analyzed in four independent experiments. (E and F) An ovule from WT (E) or vha-A/+ plants (F) pollinated with ProLAT52:GFP;ProHTR10:HTR10-mRFP pollen at 12 HAP. Images shown on the left are merges of RFP and GFP channel images, whereas on the right are merges of bright-field image and fluorescence image. Dotted line in F indicates the growing pollen tube in the embryo sac. Arrows point at sperm cells. (Scale bars: A and B, 200 µm; C–F, 20 µm.)
Because several plant-specific PCD processes are executed by vacuolar processing enzymes (VPE) that are delivered by vacuolar trafficking and mediate vacuolar rupture (34), we thus tested whether it also participated in pollen tube arrival–induced synergid degeneration. To this purpose, we obtained a quadruple mutant of VPEs (atvpe), in which all four Arabidopsis VPE-coding genes were mutated (35). However, pollination assays and seed set analysis showed that atvpe did not show any abnormalities in pollen tube reception or fertility (Fig. S8), suggesting that synergid degeneration does not involve VPE-mediated vacuolar rupture.
Fig. S8.
VPE-mediated vacuolar rupture does not contribute to pollen tube reception. (A) Representative histochemical GUS analysis of the quadruple VPE mutant (atvpe) pistils emasculated and hand-pollinated with ProLAT52:GUS pollen at 9 HAP. (B) Aniline blue staining of atvpe pistil emasculated and hand-pollinated with WT pollen at 48 HAP. Two overlapping high-magnification images were taken for one pistil. The images were then overlaid with Photoshop (Adobe Systems) to show the whole pistil. (C) A representative silique in self-fertilized atvpe. (D–F) Quantitative analysis of pollen tube guidance from ProLAT52:GUS pollination assay (D), pollen tube reception from aniline blue staining assay (E), or seed sets from silique analysis (F). Results are means ± SEM of six replicate experiments. (Scale bars: A and B, 200 µm; C, 1 mm.)
Vacuolar Acidification in Synergids Is Mediated by V-ATPases and AP1G.
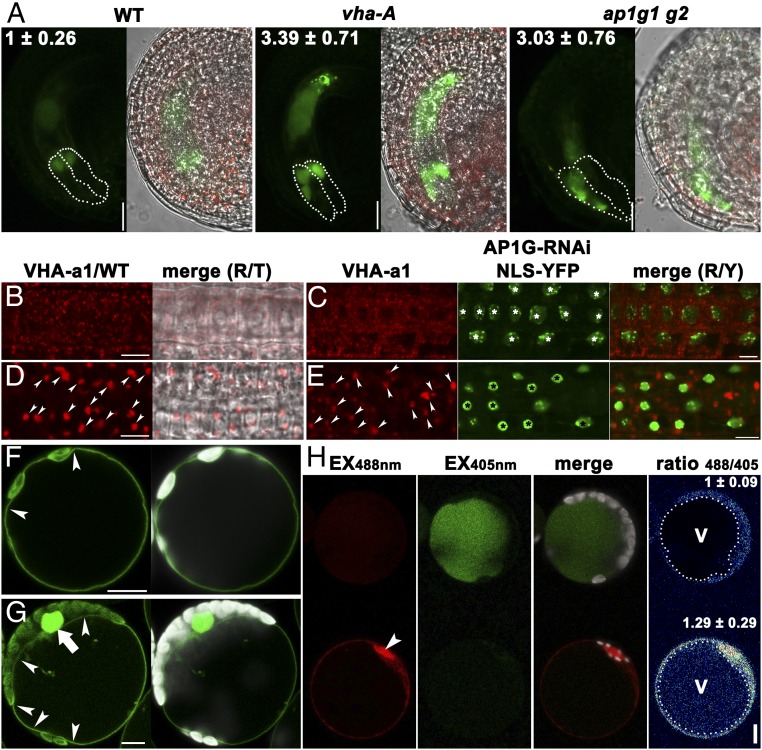
The strikingly similar defects of the vha-A/+ and ap1g/+ mutants in pollen tube reception implied their genetic interaction. Because the sole function of V-ATPases is to regulate vacuolar acidification, we introduced a vacuolar pH probe, Aleu-PR (36), whose intensity negatively correlates with vacuolar acidification. By using the same confocal parameters, we found that Aleu-PR showed faint signals in the synergid vacuoles of WT (Fig. 6A), indicating strong vacuolar acidity. By contrast, half of the vha-A/+ embryo sacs contained intense Aleu-PR signals (Fig. 6A), indicating impaired vacuolar acidification. It was the same case for ap1g/+ (Fig. 6A) in which half of the FG were of the ap1g1 g2 genotype, whereas the other half was of the AP1G1 g2 genotype. To provide further evidence that AP1G loss of function impaired vacuolar acidification, we transformed ProUBQ10:AP1G-RNAi;ProUBQ10NLS-YFP in Arabidopsis protoplasts stably expressing Aleu-PR. Consistent with the results obtained from synergid vacuoles, vacuoles of the protoplasts expressing AP1G-RNAi;NLS-YFP showed a significantly higher 488 nm/405 nm value (36) than that of WT (Fig. 6H), indicating compromised vacuolar acidification by the expression of AP1G-RNAi.
Fig. 6.
AP1G is critical for vacuolar acidification. (A) CLSM of unfertilized mature ovules from the ProUBQ10:Aleu-PR (WT), ProUBQ10:Aleu-PR;vha-A/+ (vha-A), or ProUBQ10:Aleu-PR;ap1g1/+ g2 (ap1g1 g2) transgenic plants. Numbers on the top are arbitrary intensity of Aleu-PR fluorescence in the vacuoles of synergids (highlighted with dotted lines). Results are means ± SD (n = 30). (B–E) CLSM of root epidermal cells from VHA-a1-RFP (B and D) or VHA-a1-RFP;ProUBQ10:AP1G-RNAi; ProUBQ10:NLS-YFP (C and E) transgenic plants with BFA treatment (D and E) or without (B and C). Arrowheads in D and E point at BFA compartments. Asterisks in C and E point at the nuclei labeled by NLS-YFP, indicative of AP1G-RNAi expression. (F and G) CLSM of an Arabidopsis leaf protoplast from the Pro35S:VHA-a3-GFP transgenic plants (F) or from the ProUBQ10:AP1G-RNAi;ProUBQ10:NLS-YFP transgenic plants (G). Arrowheads point at the tonoplast. The arrow indicates the nucleus labeled by NLS-YFP, indicative of AP1G-RNAi expression. (H) Mesophyll protoplasts from the ProUBQ10:Aleu-PR transgenic plants. Emission intensities were shown in red (excitation at 488 nm, Ex488nm) or green (excitation at 405 nm, Ex405nm). The bottom one was transformed with ProUBQ10:AP1G-RNAi;ProUBQ10:NLS-YFP as indicated by the NLS-YFP–labeled nucleus (false-colored red, pointed at by an arrowhead). Pseudocolor image in the Right panel indicates the 488/405 nm ratio of fluorescence intensity within the vacuoles (highlighted with dotted circles). On the top right are means ± SD (n = 30). v, vacuole. The expression of AP1G-RNAi resulted in a significant difference in vacuolar pH (t test, P < 0.01). (Scale bars: 10 µm.)
VHA-A is a shared component for V-ATPases at the TGN/EE and at the tonoplast, which are specified by the presence of TGN/EE-associated VHA-a1 and tonoplast-associated VHA-a2 or VHA-a3, respectively (32). To determine whether AP1G affected the targeting of V-ATPases, we examined the effect of AP1G-RNAi on the subcellular targeting of VHA-a1 and VHA-a3 by using root epidermal cells or protoplasts because synergid signals were difficult to image. As reported (37), VHA-a1 was distributed at cytosolic vesicles (Fig. 6B) sensitive to BFA (Fig. 5D), indicative of its TGN/EE identity. Expression of AP1G-RNAi;NLS-YFP did not detectably alter the TGN/EE distribution of VHA-a1 (Fig. 6 C and D). Similarly, the tonoplast-specific targeting of VHA-a3 was not affected by AP1G-RNAi;NLS-YFP (Fig. 6 F and G). These results suggested that AP1G was not involved in the subcellular targeting of V-ATPases.
Discussion
We show here that AP1G is crucial for synergid-controlled pollen tube reception. The ap1g/+ FGs were penetrated by nondischarging pollen tubes, a typical feature of defective pollen tube reception. CLSM of ovules at different HAP showed that synergid degeneration was impaired by AP1G loss of function, which is further supported by that fact that nondischarging pollen tubes always correlated with the failure of synergid degeneration. However, synergid degeneration could be initiated without pollen tube discharge (17), which explains the comparable ratio of ovules with two intact synergids at 8 HAP and one intact synergid at 12 HAP within ap1g/+ pistils. The impaired degeneration of the remaining synergid in ap1g/+ might be a secondary effect due to the failure of pollen tube discharge (23, 24). Although as a fertilization recovery strategy after defective sperm cell release, nondegenerating synergids keep sending out attractive signals for pollen tubes (26, 38), no supernumerary pollen tubes were observed in ap1g/+ FGs likely due to reduced pollen tube guidance.
We propose that AP1G mediates synergid degeneration during pollen tube reception through V-ATPases. First, functional loss of V-ATPases resulted in defective pollen tube reception, similar to that of AP1G. Second, ap1g synergids have a vacuolar pH comparable to that of vha-A. Third, genetic interference of AP1G by RNAi impaired vacuolar acidification in mesophyll protoplasts. Because V-ATPases are directly responsible for vacuolar acidification (39), whereas AP1G functions through mediating protein sorting at the TGN/EE to the tonoplast, it is more feasible that V-ATPases are genetically downstream of AP1G.
The next obvious question was how AP1G regulates V-ATPases to mediate vacuolar acidification. A recent study indicated that vacuolar acidification is collectively contributed by the TGN/EE-associated and the tonoplast-associated V-ATPases (40). Thus, the effect of VHA-A loss of function could be from the TGN/EE and the vacuoles (39). Tonoplast targeting of NRT2.7 and VIT1 was disrupted by AP1G functional loss, implying the key role of vacuolar trafficking in pollen tube reception. However, our results showed that the subcellular localization of both VHA-a1 and VHA-a3 was not detectably affected by genetic interference of AP1G. An alternative, which requires further investigation, is that AP1G may affect the activity of V-ATPases by mediating the targeting of some regulatory proteins along the vacuolar trafficking route.
Because of its specific feature compared with metazoan lysosomes, vacuole participates in plant cell death in two ways. One is through vacuolar membrane collapse to release hydrolytic enzymes in the cytosol, whereas the other is through fusion of the tonoplast and the PM (34). Because the quadruple mutant of VPE, which encode key proteins involved in vacuolar rupture-mediated plant PCD (34, 35), was comparably with WT during pollen tube reception, vacuolar rupture during pollen tube arrival as detected by TEM and CLSM is more likely an effect rather than the cause of synergid degeneration. Then, how vacuolar acidification may initiate synergid cell death is an intriguing question. Proton homeostasis affects endomembrane trafficking (39). Thus, defective vacuolar acidification may have compromised vesicular trafficking, leading to impaired cell death of synergids. However, the distribution of FER as the key receptor was not affected, suggesting that post-Golgi secretion was not compromised by defective vacuolar acidification. A more likely scenario is that proton homeostasis affects ROS and Ca2+ spiking, which have recently been shown to mediate pollen tube reception (13–16).
Materials and Methods
Details on materials and methods, including the plant material and chemicals, plasmid construction, confocal imaging, and other analyses and tools used are provided in SI Materials and Methods.
SI Materials and Methods
Plant Materials and Growth Conditions.
The T-DNA insertion lines SALK_144321 (ap1g1-1), SALK_043364 (ap1g1-2), SALK_032500 (ap1g2-1), SALK_032502 (ap1g2-2), vha-A (GK-233F07), and the quadruple mutant atvpe (CS67918) were obtained from the Arabidopsis Biological Resource Center (www.Arabidopsis.org). fer-4 (31) was kindly provided by Alice Cheung, University of Massachusetts. Columbia-0 was used as the WT. Plants were grown as described (41). Flowers at stage 12 were emasculated and left to grow for 16 h before pollination assays.
Imaging of Female Gametophytes and Fluorescent Microscopy.
Whole-mount ovule clearing, aniline blue staining of pistils, pharmacological treatments, RNA in situ hybridization, CLSM sections of ovules, and TEMs were performed as described (41, 42). Histochemical GUS analysis of ProLAT52:GUS-pollinated pistils and aniline blue staining of pollinated pistils were performed as described (43). The fluorescence of Aleu-PR in synergids or in protoplasts was imaged by using a Zeiss LSM880 META CLSM. The excitation/emission was 488 nm/512 nm. Signal intensity was quantified by using ImageJ software. The ratio between 488 nm/405 nm was performed as described (36). Quantitative studies of the FG defects in various genetic materials used in this study are listed in Table S1 in addition to the main text and figures.
RNA Extraction and RT-PCRs.
T-DNA insertions were determined by PCRs using the following primers: ZP728/ZP729 for AP1G1, ZP1/ZP728 for the AP1G1 mutant copy in ap1g1-1 and ap1g1-2,ZP722/ZP723 for AP1G2, ZP1/ZP722 for the AP1G2 mutant copy in ap1g2-1 and ap1g2-2, ZP8/ZP2592 for VHA-A, ZP2591/ZP2592 for vha-A. Total RNAs were isolated by using a Qiagen RNeasy plant mini kit according to the manufacturer’s instructions. Oligo(dT)-primed cDNAs were synthesized by using SuperScript III reverse transcriptase with on-column DNase digestion (Invitrogen). For transcript analysis of the complementation lines, ProUBQ10:GFP-AP1G1;ap1g or ProUBQ10:GFP-AP1G2;ap1g, the endogenous AP1G1 and AP1G2 were amplified with the primer pair ZP3248/ZP729 and ZP3250/ZP723, respectively. The exogenous AP1G1 and AP1G2 were amplified with the primer pair ZP1648/ZP729 and ZP1648/ZP723, respectively. Primers to amplify ACTIN2 were as described (41). All primers are listed in Table S2.
Plasmid Construction.
All constructs were generated by using the Gateway technology (Invitrogen) unless noted otherwise. The pENTR/D/TOPO vector (Invitrogen) was used to generate all entry vectors. The full-length CDS of AP1G1 and AP1G2 were cloned by using the primer pair ZP1360/ZP2157 and ZP1364/ZP2158, respectively. ProES1 was cloned by using the primer pair ZP2501/ZP2502. ProUBQ10 was cloned with the primer pair ZP2719/ZP2720. The coding sequence of VHA-a3 was cloned with the primer pair ZP1741/ZP1742. ProDD39 was cloned with the primer pair ZP2398/ZP2399 or ZP2400/ZP2401 for generating the expression vector ProDD39:NLS-YFP or for the destination vectors ProDD39:GFP-GW and ProDD39:GW-GFP. The entry vectors for FERONIA, NRT2.7, VHA-a3, and VIT1 were cloned by using the primer pair ZP610/ZP611, ZP975/ZP976, ZP1742/ZP1743, and ZP3599/ZP3600 from a mixed Arabidopsis cDNA library. The destination vector for ProUBQ10:GFP-AP1G1 and ProUBQ10:GFP-AP1G2 was generated by replacing the Pro35S in pB7WGF2 (44) with ProUBQ10. The destination vector for ProDD39:GFP-NRT2.7, ProDD39: GFP-VIT1, ProDD39:FER-YFP, and ProUBQ10:VHA-a3-YFP was generated by replacing the Pro35S in pB7WGF2, pB7FWG2, or pB7YWG2 (44) with ProDD39 or ProUBQ10. Expression vectors were generated by LR reactions using Gateway LR Clonase (Invitrogen). A 440-bp fragment of the AP1G1 coding sequence (from 1,160 bp to 1,600 bp starting from the start codon) was amplified with the primer pair ZP1331/ZP1332. The resultant PCR products were subcloned into the RNAi vector pTCK303 (45) to obtain the ProUBQ10:AP1G-RNAi construct. ProUBQ10 was then replaced with ProDD39 to generate ProDD39:AP1G-RNAi. The fragment of ProUBQ10:NLS-YFP was amplified with the primer pair ZP4432/ZP4433. The resultant PCR products were subcloned into the the ProUBQ10:AP1G-RNAi construct. ProUBQ10 was then replaced with Pro35S promoter to generate the Pro35S:AP1G-RNAi;ProUBQ10:NLS-YFP construct. All primers are listed in Table S3.
Table S3.
Oligos used in this study
| Application | Oligo | 5′-3′ sequences |
| Genotyping PCR | ZP1 | ATTTTGCCGATTTCGGAAC |
| ZP722/F2 | TCTATGAGTGTGTTGAAACAATC | |
| ZP723/R2 | TGCATGCCACACATCATCTT | |
| ZP728/F1 | CCATTCTCTTCCGGCACG | |
| ZP729/R1 | CTTGCGTATATTTGGATCACG | |
| ZP8 | ATATTGACCATCATACTCATTGC | |
| ZP2591 | TTCTGGGGTTTGGACAAAAAGCTTG | |
| ZP2592 | AAACGGTAGAAAAGATCTCCTAAGCG | |
| RT-PCR | ZP1648 | ATGGTGAGCAAGGGCGAGGAG |
| ZP3248 | CACAGACCTCTCTTTTCTCTCTTCATG | |
| ZP3250 | CGAGCATCGTCCATCGAGATCCAAATC | |
| ZP4393 | GAGGGAAGACGATCTTAATGAAATTG | |
| ZP4394 | GCCTGGTTGGCTAGGTTGTAGAA | |
| Cloning AP1G1 | ZP1364 | CACCATGAATCCATTCTCTTCCGGC |
| ZP2158 | TCATAACCCACGTGGGAAATTG | |
| Cloning AP1G2 | ZP1360 | CACCATGAATCCCTTTTCTTCTGGTACTC |
| ZP2157 | TCACAACCCGCGAGGG | |
| Cloning | ZP610 | CACCATGAAGATCACAGAGGGACG |
| FERONIA | ZP611 | ACGTCCCTTTGGATTCATGA |
| Cloning | ZP3599 | CACCATGTCGTCGGAGGAAGATAAGATTAC |
| VIT1 | ZP3600 | CTAATGTTGCACAACTTTAGCCAAAC |
| Cloning | ZP975 | CACCATGGAGCCATCTCAACGCAA |
| NRT2.7 | ZP976 | TCAACAAACGGGACGTAGACTAC |
| Cloning | ZP1275 | CACCATGGCTGCAGCTGGAAACA |
| Rab5-DN | ZP1276 | CTAAGCACAACAAGATGAGCTCAC |
| Generating Pro35S:AP1G-RNAi;ProUBQ10:NLS-YFP | ZP2719 | CACCATAAGCTTGTCGACGAGTCAGTAATAAACG |
| ZP2720 | ACTAGTGACGTCTGTTAATCAGAAAAACTC | |
| ZP4432 | ATATGTTTAAACATAAGCTTGTCGACGAGTCAGTAATAAACG | |
| ZP4433 | ATATAGGCCTTCACTGGATTTTGGTTTTAGGAATTAGAA | |
| Cloning | ZP2398 | CACCCTAGAATAAGTGTTGGTTTTGACATG |
| ProDD39 | ZP2399 | GGTTTCCTCCTATAATAATCGAATGAGC |
| ZP2400 | ATATGAGCTCCTAGAATAAGTGTTGGTTTTGACATG | |
| ZP2401 | ATATACTAGTGGTTTCCTCCTATAATAATCGAATGAGC | |
| Cloning | ZP2501 | CACCGACCACAATAAGTGTAATGCGTTAAA |
| ProES1 | ZP2502 | TAAAATCGCCGTTTACAAAAAGAG |
| Cloning | ZP1742 | CTCGTCTTCGTTTGCCGTGAA |
| VHA-a3 | ZP1741 | CACCATCTCTCCTCTCCTCTCGTTTGATTC |
| AP1G1 | ZP1331 | ATATGGATCCGAGCTCGGAGCTAATTGATTATTTGGAAATC |
| -RNAi | ZP1332 | ATATGGTACCACTAGTCCAATGCCATTGCTTTTGTA |
Accession Numbers.
Sequence data from this article can be found in the GenBank databases under the following accession numbers: AP1G1, At1g60070; AP1G2, At1g23900; FERONIA, At3g51550; DD39, At4g20050; ES1, At5g40260; NRT2.7, At5g14570; VIT1, AT2G01770; VHA-A, AT1G78900; ALPHAVPE-3, AT2G25940; BETAVPE-5, AT1G62710; DELTAVPE-1, AT3G20210; GAMMAVPE-1, AT4G32940; VHA-a1, AT2G28520; VHA-a3, AT4G39080.
Acknowledgments
We thank Profs. Gregory Copenhaver for ProLAT52:DsRed, Frédéric Berger for ProHTR10:HTR10-mRFP, Liwen Jiang for Aleu-PR, Alice Cheung for fer-4, Lei Ge for the VHA-a1:RFP lines, and Prof. Sheila McCormick for the English editing of the manuscript.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1617967114/-/DCSupplemental.
References
- 1.Johnson MA, Preuss D. Plotting a course: Multiple signals guide pollen tubes to their targets. Dev Cell. 2002;2:273–281. doi: 10.1016/s1534-5807(02)00130-2. [DOI] [PubMed] [Google Scholar]
- 2.Kessler SA, Grossniklaus U. She’s the boss: Signaling in pollen tube reception. Curr Opin Plant Biol. 2011;14:622–627. doi: 10.1016/j.pbi.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Drews GN, Yadegari R. Development and function of the angiosperm female gametophyte. Annu Rev Genet. 2002;36:99–124. doi: 10.1146/annurev.genet.36.040102.131941. [DOI] [PubMed] [Google Scholar]
- 4.Berger F, Hamamura Y, Ingouff M, Higashiyama T. Double fertilization - caught in the act. Trends Plant Sci. 2008;13:437–443. doi: 10.1016/j.tplants.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Escobar-Restrepo JM, et al. The FERONIA receptor-like kinase mediates male-female interactions during pollen tube reception. Science. 2007;317:656–660. doi: 10.1126/science.1143562. [DOI] [PubMed] [Google Scholar]
- 6.Huck N, Moore JM, Federer M, Grossniklaus U. The Arabidopsis mutant feronia disrupts the female gametophytic control of pollen tube reception. Development. 2003;130:2149–2159. doi: 10.1242/dev.00458. [DOI] [PubMed] [Google Scholar]
- 7.Rotman N, et al. Female control of male gamete delivery during fertilization in Arabidopsis thaliana. Curr Biol. 2003;13:432–436. doi: 10.1016/s0960-9822(03)00093-9. [DOI] [PubMed] [Google Scholar]
- 8.Tsukamoto T, Qin Y, Huang Y, Dunatunga D, Palanivelu R. A role for LORELEI, a putative glycosylphosphatidylinositol-anchored protein, in Arabidopsis thaliana double fertilization and early seed development. Plant J. 2010;62:571–588. doi: 10.1111/j.1365-313X.2010.04177.x. [DOI] [PubMed] [Google Scholar]
- 9.Capron A, et al. Maternal control of male-gamete delivery in Arabidopsis involves a putative GPI-anchored protein encoded by the LORELEI gene. Plant Cell. 2008;20:3038–3049. doi: 10.1105/tpc.108.061713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hou Y, et al. Maternal ENODLs are required for pollen tube reception in Arabidopsis. Curr Biol. 2016;26:2343–2350. doi: 10.1016/j.cub.2016.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kessler SA, et al. Conserved molecular components for pollen tube reception and fungal invasion. Science. 2010;330:968–971. doi: 10.1126/science.1195211. [DOI] [PubMed] [Google Scholar]
- 12.Li C, et al. Glycosylphosphatidylinositol-anchored proteins as chaperones and co-receptors for FERONIA receptor kinase signaling in Arabidopsis. eLife. 2015;4:4. doi: 10.7554/eLife.06587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duan Q, et al. Reactive oxygen species mediate pollen tube rupture to release sperm for fertilization in Arabidopsis. Nat Commun. 2014;5:3129. doi: 10.1038/ncomms4129. [DOI] [PubMed] [Google Scholar]
- 14.Denninger P, et al. Male-female communication triggers calcium signatures during fertilization in Arabidopsis. Nat Commun. 2014;5:4645. doi: 10.1038/ncomms5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ngo QA, Vogler H, Lituiev DS, Nestorova A, Grossniklaus U. A calcium dialog mediated by the FERONIA signal transduction pathway controls plant sperm delivery. Dev Cell. 2014;29:491–500. doi: 10.1016/j.devcel.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Hamamura Y, et al. Live imaging of calcium spikes during double fertilization in Arabidopsis. Nat Commun. 2014;5:4722. doi: 10.1038/ncomms5722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leydon AR, et al. Pollen tube discharge completes the process of synergid degeneration that is initiated by pollen tube-synergid interaction in Arabidopsis. Plant Physiol. 2015;169:485–496. doi: 10.1104/pp.15.00528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sandaklie-Nikolova L, Palanivelu R, King EJ, Copenhaver GP, Drews GN. Synergid cell death in Arabidopsis is triggered following direct interaction with the pollen tube. Plant Physiol. 2007;144:1753–1762. doi: 10.1104/pp.107.098236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maruyama D, et al. Rapid elimination of the persistent synergid through a cell fusion mechanism. Cell. 2015;161:907–918. doi: 10.1016/j.cell.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 20.Völz R, Heydlauff J, Ripper D, von Lyncker L, Groß-Hardt R. Ethylene signaling is required for synergid degeneration and the establishment of a pollen tube block. Dev Cell. 2013;25:310–316. doi: 10.1016/j.devcel.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Zimmermann P, Hirsch-Hoffmann M, Hennig L, Gruissem W. GENEVESTIGATOR. Arabidopsis microarray database and analysis toolbox. Plant Physiol. 2004;136:2621–2632. doi: 10.1104/pp.104.046367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pagnussat GC, Alandete-Saez M, Bowman JL, Sundaresan V. Auxin-dependent patterning and gamete specification in the Arabidopsis female gametophyte. Science. 2009;324:1684–1689. doi: 10.1126/science.1167324. [DOI] [PubMed] [Google Scholar]
- 23.Leydon AR, et al. Three MYB transcription factors control pollen tube differentiation required for sperm release. Curr Biol. 2013;23:1209–1214. doi: 10.1016/j.cub.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang Y, et al. MYB97, MYB101 and MYB120 function as male factors that control pollen tube-synergid interaction in Arabidopsis thaliana fertilization. PLoS Genet. 2013;9:e1003933. doi: 10.1371/journal.pgen.1003933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ingouff M, Hamamura Y, Gourgues M, Higashiyama T, Berger F. Distinct dynamics of HISTONE3 variants between the two fertilization products in plants. Curr Biol. 2007;17:1032–1037. doi: 10.1016/j.cub.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 26.Beale KM, Leydon AR, Johnson MA. Gamete fusion is required to block multiple pollen tubes from entering an Arabidopsis ovule. Curr Biol. 2012;22:1090–1094. doi: 10.1016/j.cub.2012.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steffen JG, Kang IH, Macfarlane J, Drews GN. Identification of genes expressed in the Arabidopsis female gametophyte. Plant J. 2007;51:281–292. doi: 10.1111/j.1365-313X.2007.03137.x. [DOI] [PubMed] [Google Scholar]
- 28.Park M, et al. Arabidopsis μ-adaptin subunit AP1M of adaptor protein complex 1 mediates late secretory and vacuolar traffic and is required for growth. Proc Natl Acad Sci USA. 2013;110:10318–10323. doi: 10.1073/pnas.1300460110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X, et al. Trans-Golgi network-located AP1 gamma adaptins mediate dileucine motif-directed vacuolar targeting in Arabidopsis. Plant Cell. 2014;26:4102–4118. doi: 10.1105/tpc.114.129759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chopin F, et al. The Arabidopsis ATNRT2.7 nitrate transporter controls nitrate content in seeds. Plant Cell. 2007;19:1590–1602. doi: 10.1105/tpc.107.050542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duan Q, Kita D, Li C, Cheung AY, Wu H-M. FERONIA receptor-like kinase regulates RHO GTPase signaling of root hair development. Proc Natl Acad Sci USA. 2010;107:17821–17826. doi: 10.1073/pnas.1005366107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dettmer J, et al. Essential role of the V-ATPase in male gametophyte development. Plant J. 2005;41:117–124. doi: 10.1111/j.1365-313X.2004.02282.x. [DOI] [PubMed] [Google Scholar]
- 33.Strompen G, et al. Arabidopsis vacuolar H-ATPase subunit E isoform 1 is required for Golgi organization and vacuole function in embryogenesis. Plant J. 2005;41:125–132. doi: 10.1111/j.1365-313X.2004.02283.x. [DOI] [PubMed] [Google Scholar]
- 34.Hara-Nishimura I, Hatsugai N. The role of vacuole in plant cell death. Cell Death Differ. 2011;18:1298–1304. doi: 10.1038/cdd.2011.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuroyanagi M, et al. Vacuolar processing enzyme is essential for mycotoxin-induced cell death in Arabidopsis thaliana. J Biol Chem. 2005;280:32914–32920. doi: 10.1074/jbc.M504476200. [DOI] [PubMed] [Google Scholar]
- 36.Shen J, et al. Organelle pH in the Arabidopsis endomembrane system. Mol Plant. 2013;6:1419–1437. doi: 10.1093/mp/sst079. [DOI] [PubMed] [Google Scholar]
- 37.Viotti C, et al. Endocytic and secretory traffic in Arabidopsis merge in the trans-Golgi network/early endosome, an independent and highly dynamic organelle. Plant Cell. 2010;22:1344–1357. doi: 10.1105/tpc.109.072637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kasahara RD, et al. Fertilization recovery after defective sperm cell release in Arabidopsis. Curr Biol. 2012;22:1084–1089. doi: 10.1016/j.cub.2012.03.069. [DOI] [PubMed] [Google Scholar]
- 39.Schumacher K, Krebs M. The V-ATPase: Small cargo, large effects. Curr Opin Plant Biol. 2010;13:724–730. doi: 10.1016/j.pbi.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 40.Kriegel A, et al. Job sharing in the endomembrane system: Vacuolar acidification requires the combined activity of V-ATPase and V-PPase. Plant Cell. 2015;27:3383–3396. doi: 10.1105/tpc.15.00733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou LZ, et al. Protein S-ACYL Transferase10 is critical for development and salt tolerance in Arabidopsis. Plant Cell. 2013;25:1093–1107. doi: 10.1105/tpc.112.108829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang JG, et al. HAPLESS13, the Arabidopsis μ1 adaptin, is essential for protein sorting at the trans-Golgi network/early endosome. Plant Physiol. 2013;162:1897–1910. doi: 10.1104/pp.113.221051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li S, et al. Arabidopsis COBRA-LIKE 10, a GPI-anchored protein, mediates directional growth of pollen tubes. Plant J. 2013;74:486–497. doi: 10.1111/tpj.12139. [DOI] [PubMed] [Google Scholar]
- 44.Karimi M, Inzé D, Depicker A. GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci. 2002;7:193–195. doi: 10.1016/s1360-1385(02)02251-3. [DOI] [PubMed] [Google Scholar]
- 45.Guo J, Wang F, Song J, Sun W, Zhang XS. The expression of Orysa;CycB1;1 is essential for endosperm formation and causes embryo enlargement in rice. Planta. 2010;231:293–303. doi: 10.1007/s00425-009-1051-y. [DOI] [PubMed] [Google Scholar]