Abstract
Background
This study evaluated the antibacterial activity of some plants used in folklore medicine to treat diarrhoea in the Eastern Cape Province, South Africa.
Methods
The acetone extracts of Acacia mearnsii De Wild., Aloe arborescens Mill., A. striata Haw., Cyathula uncinulata (Schrad.) Schinz, Eucomis autumnalis (Mill.) Chitt., E. comosa (Houtt.) Wehrh., Hermbstaedtia odorata (Burch. ex Moq.) T.Cooke, Hydnora africana Thunb, Hypoxis latifolia Wight, Pelargonium sidoides DC, Psidium guajava L and Schizocarphus nervosus (Burch.) van der Merwe were screened against Staphylococcus aureus, Escherichia coli, Enterococcus faecalis, multi-resistant Salmonella enterica serovar Isangi, S. typhi, S. enterica serovar Typhimurium, Shigella flexneri type 1b and Sh. sonnei phase II. A qualitative phytochemical screening of the plants extracts was by thin layer chromatography. Plants extracts were screened for antibacterial activity using serial dilution microplate technique and bioautography.
Results
The TLC fingerprint indicated the presence of terpenoids and flavonoids in the herbs. Most of the tested organisms were sensitive to the crude acetone extracts with minimum inhibitory concentration (MIC) values ranging from 0.018–2.5 mg/mℓ. Extracts of A. striata, C. uncinulata, E. autumnalis and P. guajava were more active against enteropathogens. S. aureus and Sh. flexneri were the most sensitive isolates to the crude extracts but of significance is the antibacterial activity of A. arborescens and P. guajava against a confirmed extended spectrum betalactamase positive S. enterica serovar Typhimurium.
Conclusion
The presence of bioactive compounds and the antibacterial activity of some of the selected herbs against multidrug resistant enteric agents corroborate assertions by traditional healers on their efficacies.
Keywords: Medicinal plants, Diarrhoea, Antibacterial, Bioautography, Phytochemical
Background
Gastrointestinal and enteric diseases remain a problem worldwide with the greatest burden of diseases in the sub-Saharan Africa and Asia [1, 2].These diseases cause nearly 19% of the 10 million worldwide deaths of children younger than 5 years old [3]. The diseases continue to be important causes of morbidity and incur substantial health-care costs [4, 5]. Severe acute bacterial gastroenteritis is caused majorly by Shigella, but Salmonella spp., some E. coli pathotypes, Campylobacter and Vibrio spp. play an important role in the epidemiology of diarrhoea, especially in certain areas of the globe [6, 7].
Treatment failures of enteric diseases, particularly, the emerging multidrug resistant enteric bacteria is a big challenge. Multiple antibiotic resistance is on the increase among clinical isolates of bacteria [8, 9] and the emergence of resistance to the 3rd and 4th generation beta-lactam drugs has complicated therapy. The burden of resistance to extended-spectrum cephalosporin and other beta-lactam drugs among the Enterobacteriaceae is enormous both in the hospital and community [10, 11]. Pharmaceutical industries have produced a number of new drugs in the last three decades. An estimated 122 drugs from 94 plant species active against other diseases have been discovered through ethnobotanical leads [12, 13]. Some of these include Ephedrine (bronchodilator) derived from Ephedra sinica, Quinine (antimalarial) from Cinchona ledgerian [14], the antimalarial compound Artemisin, derived from Artemisia annua L [15] and several antitumor compounds [16, 17]. Despite these big strides, resistance to antimicrobials by microorganisms is still on the increase. This is due mainly to the remarkable genetic plasticity of the microorganisms [18], inappropriate use, high selective pressures of use or under-use through inaccessibility, poor quality drugs, inadequate dosing, poor patient compliance and the increased mobility of the world population [19]. The incorporation of antimicrobials as growth promoting additives in animal feed is also a contributory factor to the emergence of drug-resistant bacteria [20, 21]. The sub-therapeutic use leads to bacterial exposure to sublethal concentration of drugs over a period of time leading to selection of resistance strains [22].
The rate at which new drugs are developed is not keeping pace with the changing virulence and drug resistant patterns of microbes. According to Kunin’s [18] review of the book by Owen and Lautenbach [23], antimicrobial resistance is the inevitable result of Darwinian evolution — natural selection and survival of the fittest. While there is a continuous modification of strategies by microbes in the ‘no victor no vanquish’ fight for survival, developing resistance to antibiotics is outpacing the pharmaceutical industry’s ability to develop new ones [24]. Drug resistance and development according to Ridley [25] takes 10 to 15 years and hence the quest for new drugs should be a continuous process [26, 27].
Medicinal plants have been acknowledged as potential sources of new compounds of therapeutic value and as sources of lead compounds for drug design and development [13, 28]. Various studies have documented the use of medicinal plants in various parts of the world including developed countries [27, 29, 30]. In contrast to other diseases, no antimicrobial with the potential for economic use has yet been discovered from plants. There are several possible reasons for this situation [31]. In South Africa, a sizeable number of both the rural and urban dwellers rely on traditional medicine for their primary health care [32, 33]. Some of the documented use of plants in stomach related ailments includes that of Iwu [34] in which immature fruits of Olea europaea subsp. Africana (Wild Olive), was reported to be used as astringents against diarrhoea. Pentanisia prunelloides (Klotzsch ex Eckl. & Zeyh) Walp is being used for a range of ailments and the root serves as enema for stomach pain [35]. However, many plants are yet to be explored scientifically and moreover, the need to find a lasting solution to the problem of infectious diseases with lingering treatment failures necessitated further exploration of natural products to uncover new grounds in drug production. This study screened and evaluated selected medicinal plants used in ethnomedicine in the Oliver R. Tambo municipality of Eastern Cape South Africa for their antibacterial activities against some enteropathogenic bacteria.
Methods
Plant material
Various plants parts used in the treatment of diarrhoea and related diseases in Oliver R. Tambo District municipality in the Eastern Cape province of South Africa were collected. These were Acacia mearnsii De Wild. (AC), Aloe arborescens Mill., (AA), Aloe striata Haw. (AS), Cyathula uncinulata (Schrad.) Schinz (CU), Eucomis autumnalis (Mill.) Chitt. (E1 & E3), E. comosa (Houtt.) Wehrh. (E2), Hermbstaedtia odorata (Burch. ex Moq.) T.Cooke (MB), Hydnora africana Thunb. (UM), Hypoxis latifolia Wight (HY), Pelargonium sidoides DC. (PE), Psidium guajava L. (PS), Schizocarphus nervosus (Burch.) van der Merwe, (SC). Samples of the plants were identified by the Kei Herbarium curator, Dr. Immelman, Walter Sisulu University, South Africa and voucher specimens deposited were tagged (Jaca 10 to Jaca 21). The traditional uses and the common names of the plants species selected are presented in Table 1.
Table 1.
Names, plant parts and Traditional usage of herbs investigated
| Plant Name | Common name | Plant part | Traditional usage | References |
|---|---|---|---|---|
|
Acacia mearnsii De Wild. Family: Leguminosae |
Blackwood Black Wattle |
Bark | Diarrhoea, dysentery, sore throat, coughs, children fever, tooth ache | [35] |
|
Aloe arborescens
Family: Xanthorrhoeaceae |
Aloe | Leaves | Vomiting, Skin ailments, diarrhoea, urinary complaints, rheumatism, tuberculosis | [35, 62] |
|
Aloe striata
Family: Xanthorrhoeaceae |
Aloe | Leaves | Treatment of constipation | [63] |
|
Cyathula uncinulata
(Schrad.) Schinz Family: Amaranthaceae |
NA | Leaves | Antidiarrhoea, philter or medicine for love | [64] |
|
Eucomis autumnalis (Mill.) Chitt.
Family: Asparagaceae |
Common Pineapple Flower | Bulb | Decoctions of bulb and roots for colic, flatulence | [65] |
|
Eucomis comosa (Houtt.) Wehrh.
Family: Asparagaceae |
Pineapple lily | Bulb | Help teething in children and to treat rheumatism | [66] |
|
Hermbstaedtia odorata Wild Cockscomb Family: Amaranthaceae |
Rooi-aarbossie | Leaves | Cleansing stomach wash alone or with Acaccia xanthophloea and Cappa | [35] |
|
Hydnora africana
Thunb. Family: Hydnoraceae |
Warty Jackal Food, Jakkalskos Kanip |
Tuber | Diarrhoea, plant dried ground raw for dysentery, amenorrhoea, swollen glands | [66] |
| Hypoxis colchicifolia Baker. Family: Hypoxidaceae | African potato | Tuber | Headaches, dizziness, mental disorders, to treat cancers, inflammation, HIV, diarrhoea | [67, 68] |
|
Pelargonium sidoides DC. Family: Geraniaceae |
Rose-scented Pelargonium | Root | Gonorrhoea, diarrhoea, dysentery, root decoction severe diarrhoea, stomach ailment in children | [35] |
|
Psidium guajava L. Family: Myrtaceae |
Guava | Leaves | Leaves used for diarrhoea, Infusion of leaves for bloody diarrhoea, infusion as enema for severe diarrhoea |
[35] |
|
Schizocarphus nervosus (Burch.) van der Merwe Family: Asparagaceae |
White Scilla | Corms | Rheumatic fever, dysentery. All purpose herb. | [69, 70] |
Key: NA not available
Plant preparation and extraction
Plant parts were washed with distilled water, air-dried and milled into a fine powder with a Wiley Grinder. Fifty gram portion of ground dried material was soaked overnight in 500 ml of acetone on an orbital shaker. The samples were suction-filtered through a Whattman No 1 filter paper using a Buchner funnel. The extract was concentrated at 45 °C using a Rotavapor (Eyela N-1100, Rikakikai, China). The concentrated extract was transferred to a pre-weighed glass vial and allowed to dry at room temperature under a stream of air. Working stock solutions of extracts were obtained by re-dissolving in acetone to yield 10 mg/ml solutions. Plant materials were kept in air-tight containers while extracts were kept at 4 °C in the dark for further analysis.
Phytochemical analysis of extracts
Thin layer chromatography (TLC) plates (Merck, silica gel 60 F254) were used to separate the extracts into chemical constituents. The TLC plates were prepared in duplicate and developed under saturated conditions in different mobile solvent systems according to Kotze and Eloff [36]: Ethylacetate: methanol: water (EMW) (40:5.4:4) polar/neutral; Chloroform: ethylacetate: formic acid (CEF) (20:16:4) intermediate polarity/acidic and Benzene: ethanol: ammonium hydroxide (BEA) (36:4:0.4) non-polar/basic. An aliquot of 10 μℓ of extract (representing 100 μg of the extract) was placed in a line c, 0.8 cm long and was separated by TLC using Merck, Kieselgel 60 F254 TLC plates in a closed, saturated TLC tank. For the qualitative evaluation of a given substance, the Rf value (retention factor) of chromatograms was used as the parameter for comparison. The Rf value of a substance is the ratio of the distance moved by the compound from its origin to the movement of the solvent from the origin. The TLC plates were then sprayed with the Vanillin-sulphuric acid spray reagent (0.1 g vanillin, 28 ml methanol, 1 ml sulphuric acid) for the detection of higher alcohols, phenols, steroids and essential oils [37]. The plates were heated at 105 °C until the colours of chromatograms were optimally developed.
Determination of minimum inhibitory concentration
The minimum inhibitory concentration (MIC) values of plant extracts against the enteric pathogens were determined using a serial dilution microplate method [38]. The test organisms used were obtained from the Enteric Diseases and Respiratory Unit of National Institute of Communicable Diseases, Johannesburg and were confirmed to be extended spectrum beta-lactamase (ESBL) positive as provided elsewhere [9]. These are S. typhi, S. enterica serovar Typhimurium, Shigella flexneri type 1b and Sh. sonnei phase II and typed culture of Escherichia coli (ATCC 25922), Enterococcus faecalis (ATCC 29212), Staphylococcus aureus (ATCC 29213).A 0.5 McFarland’s standard suspension of bacteria inoculum (1 X 10 8 CFU/ml) was prepared in Mueller Hinton Broth. A series of two-fold dilutions of extracts (10 mg/ml), acetone (negative control) and gentamycin (positive control) in a microtitre plate was seeded with 100 μl of the inoculum and incubated at 37 °C for 18 h. An hour before the end of incubation, 40 μl of 0.2 mg/ml INT (p-iodonitrotetrazolium violet) solution was added to each well and colour development was observed at 2 h and after further incubation for 24 h. The lowest concentration where growth is inhibited was recorded as the minimum inhibitory concentration (MIC). This was indicated by a well with a decreased colour or no clear colour after incubation with INT.
Bioautographic assay
The bioautography procedure as described by Begue and Kline [39] and refined for plant extracts by Masoko and Eloff [40] was used to identify bioactive chromatograms of plant extracts. The duplicate of TLC plates prepared were dried overnight under a stream of air to remove residual TLC solvents which may be harmful to bacteria. A 10 ml of overnight broth culture of test bacteria in Mueller Hinton broth (Merck) was centrifuged at 5300 x g for 20 min. The supernatant was discarded and the pellet was re-suspended in 2–4 ml of fresh broth and adjusted to make 0.5 McFarland standards which is equivalent to1.0 × 10−7 cfu/mL [41]. The dried chromatographic plates were sprayed with the test bacteria until they were completely wet in a Laminar flow cabinet (Labotec, SA). The plates were incubated overnight at 37 °C in a clean chamber at 100% relative humidity. After overnight incubation, plates were sprayed with a 2 mg/ml solution of INT (p-iodonitrotetrazolium violet, Sigma Chemicals). Plates were incubated and monitored for colour development at 2 h and further incubated overnight. Inhibition of growth of tested organisms was indicated by clear or yellow zones on chromatogram an indication of where reduction of INT to the coloured formazan did not take place.
Results
Phytochemical screening
The medium polar (CEF) and polar eluents (EMW) gave the best separation of compounds indicating that these extracts contained mainly relatively polar compounds. The TLC plates were photographed under short and long-wave UV light. Spraying with vanillin-sulphuric acid revealed the presence of different chemical constituents of the plant extracts indicated by the different coloured compounds (Fig. 1a–c). Characteristic green and blue fluorescence indicating presence of flavonoids according to the TLC evaluation scheme of Wagner et al. [42] were revealed under long-wave UV light (366 nm) may whereas spots of quenching of fluorescence as dark zones against light green fluorescent background with short-wavelength UV light (254 nm) indicated the presence of aromatic compounds (figures not shown).
Fig. 1.
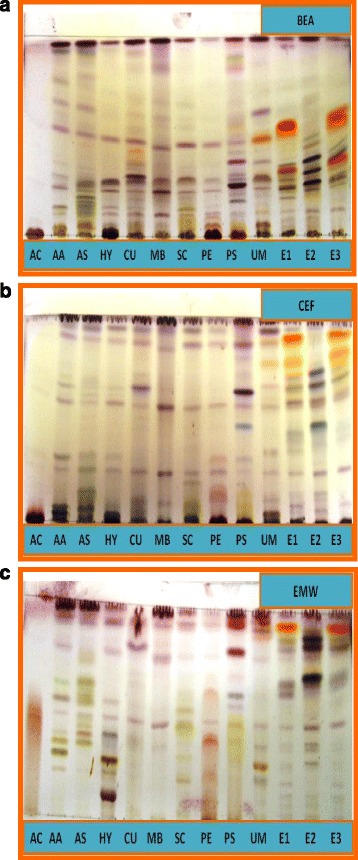
a TLC separation of components of the different plant extracts with BEA as eluent and vanillin-sulphuric acid spray reagent. b TLC separation of components of the different plant extracts with CEF as eluent and vanillin-sulphuric acid spray reagent. c TLC separation of components of the different plant extracts with EMW as eluent and vanillin-sulphuric acid spray reagent
Bioautography assay
The bioautography results revealed the different compounds present in the extracts that were responsible for the antibacterial activity. E. autumnalis, E. comosa and P. guajava extracts contained one or two major bands of bioactive compounds (Fig. 2). There were two major antibacterial compounds from PS with Rf values of 0.64 and 0.79, two from EA with Rf values of 0.65 and 0.89, and one from EC with Rf values of 0.68 that inhibited the growth of S. enterica serovar Typhimurium with CEF used as the solvent system.
Fig. 2.
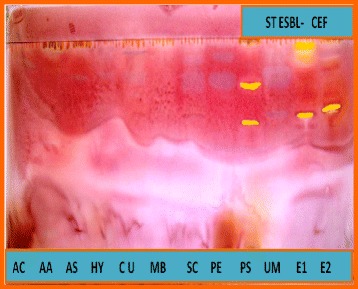
Bioautograms of components of the different plant extracts with CEF as eluent sprayed with S. Typhimurium
Active antibacterial compound was shown as clear or yellowish spot of inhibition of the growth of test organism by bioautogram. The pinkish contrasting area of bacterial growth indicated non-reduction of the tetrazolium salt by the microorganism in the presence of this compound to yield the pinkish or purplish formazan product seen in the background [39].
Minimum inhibitory concentration
The MIC values of the plant extracts ranged from 0.018 mg/ml to 2.5 mg/ml after 24 h of incubation. The average MIC values varied for the different bacterial species with the lowest value (0.018) against S. aureus and Shigella flexneri (Table 2). Of all the crude plant extracts evaluated, A. arborescence, C. uncinulata, E. autumnalis and P. guajava had considerable antibacterial activities with MICs between 0.018 and 0.078 mg/ml. However only, P. guajava showed bioautograms (Fig. 2) of bioactive fractions of these plants in combination with lowest MIC. The other two plants with bioactive fractions are E. autumnalis and E. comosa. The MIC values obtained were comparable to that of the reference antibiotic (gentamicin). Of particular interest is the low MIC of A. arborescence against S. aureus and Shigella flexneri. The activities of A. arborescence and P. guajava against extended spectrum beta-lactamase positive S. enterica serovar Typhimurium is also of significance.
Table 2.
Average Minimum Inhibitory Concentration values of plant extracts at 2 h and 24 h
| Bacteria Codes | Plant Extracts and Antibiotic Codes | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AC | AA | AS | E1 | E2 | HY | IQ | MB | SC | PE | PS | UM | GENT | |
| (mg/ml) | |||||||||||||
| EC 2H | 1.25 | 2.5 | 2.5 | 0.625 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 1.25 | 0.156 | 2.5 | 0.039 |
| EC 24H | 1.25 | 0.625 | 0.625 | 0.312 | 0.625 | 2.5 | 1.25 | 2.5 | 2.5 | 1.25 | 0.312 | 1.25 | 0.078 |
| EF 2H | 0.625 | 1.25 | 2.5 | 0.312 | 2.5 | 2.5 | 1.25 | 2.5 | 2.5 | 0.312 | 0.078 | 2.5 | 0.625 |
| EF 24H | 0.625 | 0.089 | 0.039 | 0.156 | 0.078 | 2.5 | 0.625 | 2.5 | 0.312 | 0.625 | 0.156 | 0.312 | 0.625 |
| SA 2H | 0.312 | 2.5 | 2.5 | 0.078 | 0.312 | 1.25 | 0.625 | 2.5 | 2.5 | 0.156 | 0.078 | 2.5 | 0.078 |
| SA 24H | 0.312 | 0.018 | 0.018 | 0.312 | 0.156 | 0.312 | 0.156 | 0.078 | 0.156 | 0.312 | 0.156 | 0.078 | 0.078 |
| SI 2H | 1.25 | 0.156 | 0.312 | 0.156 | 0.312 | 2.5 | 0.625 | 1.25 | 1.25 | 1.25 | 0.312 | 1.25 | 0.625 |
| SI 24H | 1.25 | 1.25 | 0.625 | 1.25 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| ST 2H | 0.039 | 0.078 | 0.312 | 0.078 | 0.078 | 0.625 | 0.625 | 1.25 | 0.312 | 0.312 | 0.156 | 0.625 | 0.156 |
| ST 24H | 0.312 | 0.156 | 1.25 | 0.625 | 0.312 | 2.5 | 1.25 | 2.5 | 1.25 | 0.312 | 0.312 | 1.25 | 1.25 |
| STE- 2H | 1.25 | 2.5 | 2.5 | 0.156 | 0.625 | 2.5 | 1.25 | 2.5 | 1.25 | 1.25 | 0.312 | 1.25 | 2.5 |
| STE- 24H | 1.25 | 0.078 | 0.078 | 0.156 | 0.625 | 1.25 | 1.25 | 2.5 | 0.625 | 1.25 | 0.312 | 0.625 | 2.5 |
| STE+ 2H | 1.25 | 1.25 | 2.5 | 0.156 | 0.312 | 2.5 | 0.625 | 1.25 | 1.25 | 1.25 | 0.078 | 1.25 | 0.078 |
| STE+ 24H | 1.25 | 0.078 | 0.312 | 0.312 | 0.625 | 2.5 | 1.25 | 2.5 | 1.25 | 1.25 | 0.312 | 0.625 | 0.156 |
| SHF 2H | 0.312 | 0.078 | 0.156 | 0.625 | 0.625 | 0.312 | 0.625 | 1.25 | 1.25 | 0.625 | 0.078 | 1.25 | 0.078 |
| SHF 24H | 0.625 | 0.018 | 0.078 | 0.078 | 0.312 | 1.25 | 1.25 | 2.5 | 0.625 | 0.625 | 0.312 | 0.312 | 0.078 |
| SHS 2H | 0.625 | 0.156 | 0.312 | 0.312 | 1.25 | 2.5 | 0.625 | 1.25 | 2.5 | 1.25 | 0.156 | 2.5 | 0.156 |
| SHS 24H | 0.625 | 0.039 | 0.039 | 0.039 | 0.312 | 1.25 | 0.312 | 1.25 | 0.625 | 0.625 | 0.312 | 0.312 | 0.156 |
| Legend 1: Bacteria codes | |||||||||||||
| Code | Isolates | ||||||||||||
| EC 2H | Escherichia coli at 2 h | ||||||||||||
| EC 24H | Escherichia coli at 24 h | ||||||||||||
| EF 2H | Enterococcus faecalis at 2 h | ||||||||||||
| EF 24H | E. faecalis at 24 h | ||||||||||||
| SA 2H | Staphylococcus aureus at 2 h | ||||||||||||
| SA 24H | S. aureus at 24 h | ||||||||||||
| SI 2H | Salmonella isangi at 2 h | ||||||||||||
| SI 24H | S. isangi at 24 h | ||||||||||||
| ST 2H | Salmonella Typhimurium at 2 h | ||||||||||||
| ST 24H | S. Typhimurium at 24 h | ||||||||||||
| STE- 2H | S. Typhimurium (ESBL negative) at 2 h | ||||||||||||
| STE- 24H | S. Typhimurium (ESBL negative) at 24 h | ||||||||||||
| STE+ 2H | S. Typhimurium (ESBL positive) at 2 h | ||||||||||||
| STE+ 24H | S. Typhimurium (ESBL positive) at 24 h | ||||||||||||
| SHF 2H | Shigella flexneri at 2 h | ||||||||||||
| SHF 24H | Sh. flexneri at 24 h | ||||||||||||
| SHS 2H | Sh. sonnei at 2 h | ||||||||||||
| SHS 24H | Sh. sonnei at 24 h | ||||||||||||
| Legend 2: Plant codes | |||||||||||||
| Code | Plant Extract/ Control | ||||||||||||
| AC | Acacia mearnsii | ||||||||||||
| AA | Aloe arborescence | ||||||||||||
| AS | Aloe striata | ||||||||||||
| CU | Cyathula uncinulata | ||||||||||||
| E1 | Eucomis autumnalis | ||||||||||||
| E2 | Eucomis comosa | ||||||||||||
| HY | Hypoxis latifolia | ||||||||||||
| MB | Hermbstaedtia odorata | ||||||||||||
| SC | Scilla nervosa | ||||||||||||
| PE | Pelargonium sidoides | ||||||||||||
| PS | Psidium guajava | ||||||||||||
| UM | Hydnora africana | ||||||||||||
| GENT | Gentamycin | ||||||||||||
Discussion
The presence of compounds such as flavanoids and triterpenoids as indicated by the TLC plates are in accordance with some other studies and perhaps are responsible for antibacterial activities as previously described [43–45]. An active flavonoid compound, quercetin-3-O-a-l-arabinopyranoside (guaijaverin) isolated from Psidium guajava has been reported with high potential antiplaque agent by inhibiting the growth of Streptococcus mutans [46], giving credence to the use of P. guajava as toothpaste in folklore practices to maintain oral hygiene. Similarly, a total of seven homoisoflavonoids of varying sub-classes including a novel benzylidene type and two spirocyclic nortriterpenoids were isolated from three species of Eucomis by Koorbanally et al. [47]. Several reports on the biological activity of homoisoflavonoids indicated their anti-inflammatory, antibacterial, antihistaminic, antimutagenic and angioprotective qualities, and value as potent phosphodiesterase inhibitors [48, 49].
The minimum inhibitory concentration values of plant extracts against bacteria were notably lower at 24 h readings in most cases. The variations in MIC observed at 2 h and 24 h suggest that antibacterial activity of the extracts may not only be dose-dependent but also time-dependent. Whereas bacteriostatic effect may be noticeable in some plant extract-bacterial assay within 1 to 2 h some bacteriostatic or bactericidal effects are not apparent until incubation time of about 18 to 24 h. The decrease in MIC at 24 h compared with 2 h suggests that the action of the extract is bactericidal against the particular microbe as against increase in MIC overtime which is an indication of an initial bacteriostatic action of extract against the bacteria as observed for most plants against S. enterica serovar Typhimurium. Several studies have similarly reported time-dependent antibacterial activity of some medicinal plants [50–52]. It is surprising that there were no correlation between the MIC values and presence of antibacterial compounds after bioautography. For instance A. arborescens and A. striata had impressive MIC values but no active compounds in bioautography. This observation may be attributable to volatility, decomposition or instability of bioactive components during the course of the bioassay as previously reported [53]. For the extracts showing bioactive compounds (PS, E1 and E2), the antibacterial activity resided mostly in intermediate polarity compounds (Figs. 2a). It has been reported that bioautography allows easy localization of activity even in complex matrix as that derived from natural products [54]. Developed chromatogram comparison under identical conditions and visualized with the use of suitable chromogen reagent can provide useful information about nature of active compounds [55] and can guide isolation of active compound.
Presence of bioautogram is known to guide the chromatographic fractionation of biologically active compound, quercetin-3-O-a-l-arabinopyranoside (guaijaverin), from crude methanol extract of P. guajava [46]. The absence of bioautogram in some plants extracts has been adduced to instability or volatility of the bioactive chromatograms [53]. In most cases plant extracts have been reportedly shown to be more active against Gram-positive (GP) pathogens [56], a similar observation was found in this study but in addition, most of the extracts had substantial activity against the selected Gram-negative (GN) enteric bacteria as previously reported [57]. Pelargonium sidoides gave a moderate antibacterial activity in particular against E. faecalis, S. aureus and Shigella species. Similarly, anti-infective properties of the commercially important Pelargonium have been investigated [58]. H. latifolia did not show good antibacterial activity against most of the tested bacteria and this is in harmony with the findings on H. decumbens [59] even though the sterols in Hypoxis spp. had been reported to reduce plasma viral loads and stabilized CD4 cell counts in HIV positive patients [60], while an aqueous extract of Hypoxis hemerocallidea (H. rooperi) (62.5 μg/ml) inhibited some microorganisms [61].
Conclusions
This study showed that the crude extracts of some of the herbs used in traditional medicine as remedy for stomach related ailments in our area of study have potential as antibacterial agents. A. arborescens, A. striata, C. uncinulata, E. autumnalis, E. comosa and P. guajava are particularly promising in the context of this study because of their bioactivities against ESBL positive bacteria and since the activities of the crude extracts compared reasonably well with gentamicin. This gives scientific credence to the use of these plants although we did not use the same extractant as traditional leaders. Also, the bioautograms are useful in bioassay-guided isolation of active compounds. Based on our results it may be useful to isolate and characterize the compounds present in Eucomis autumnalis and in Psidium guajava.
Acknowledgements
We are grateful to the traditional healers and community members around O. R. Tambo Municipality in the Eastern Cape of South Africa who provided information about the local use of the plants in this study. We are also grateful to Dr. Immelman, L. Kambizi, Tuli Jaca and David Wopula of the Department of Botany, Walter Sisulu University, Mthatha, South Africa. We appreciate the South African Medical Research Council for financial support.
Funding
The field work, collection of plants and laboratory analysis were supported by the Institutional Research Grant of Walter Sisulu University, Mthatha, South Africa.
Availability of data and materials
All data generated or analysed during this study are included in this published article [and its supplementary information files]. The plants used in this study were identified at the Kei Herbarium, Walter Sisulu University, Mthatha, South Africa where voucher specimens have been deposited.
Authors’ contributions
MAB participated in the design of the study, carried out field work, prepared the extracts, participated in the bioassay and drafted the manuscript. CLO conceived of the study, participated in the design and coordination of the study, supervised the study and revised the manuscript. BBS was involved in aspect of phytochemical analysis and bioautographic assay. JNE was involved in aspect of laboratory coordination and helped to revise the manuscript. AIO assisted with the concept and design of the study, provided technical advice and reviewed manuscript. Authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Bacteria codes
- EC 2H
Escherichia coli at 2h,
- EC 24H
Escherichia coli at 24h
- EF 2H
Enterococcus faecalis at 2h
- EF 24H
E. faecalis at 24h
- SA 2H
Staphylococcus aureus at 2h
- SA 24H
S. aureus at 24h
- SI 2H
Salmonella isangi at 2h
- SI 24H
S. isangi at 24h
- ST 2H
Salmonella Typhimurium at 2h
- ST 24H
S. Typhimurium at 24h
- STE- 2H
S. Typhimurium (ESBL negative) at 2h
- STE- 24H
S. Typhimurium (ESBL negative) at 24h
- STE+ 2H
S. Typhimurium (ESBL positive) at 2h
- STE+ 24H
S. Typhimurium (ESBL positive) at 24h
- SHF 2H
Shigella flexneri at 2h
- SHF 24H
Sh. flexneri at 24h
- SHS 2H
Sh. sonnei at 2h
- SHS 24H
Sh. sonnei at 24h.
Plant codes
- AC
Acacia mearnsii
- AA
Aloe arborescence
- AS
Aloe striata
- CU
Cyathula uncinulata
- E1, E3
Eucomis autumnalis
- E2
Eucomis comosa
- HY
Hypoxis latifolia
- MB
Hermbstaedtia odorata
- PE
Pelargonium sidoides
- PS
Psidium guajava
- SC
Schizocarphus nervosus
- UM
Hydnora Africana
Other abbreviation
- CFU/ml
colony forming units/ml
Contributor Information
Mary A. Bisi-Johnson, Email: jumokade@yahoo.co.uk
Chikwelu L. Obi, Email: c355251@yahoo.com
Babatunde B Samuel, Email: Bolorundurosamuel@yahoo.com.
Jacobus N. Eloff, Email: kobus.eloff@up.Ac.Za
Anthony I. Okoh, Email: aokoh@ufh.ac.za
References
- 1.Crump JA, Mintz ED. Global trends in typhoid and paratyphoid fever. Clin Infect dis. 2010;50:241–246. doi: 10.1086/649541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wain J, Hendriksen RS, Mikoleit ML, Keddy KH, Ochiai RL. Typhoid fever. Lancet. 2015;385:1136–1145. doi: 10.1016/S0140-6736(13)62708-7. [DOI] [PubMed] [Google Scholar]
- 3.WHO. World Health Statistics. 2015. 1. Health status indicators. 2. World health. 3. Health services - statistics. 4. Mortality. 5. Life expectancy. 6. Demography. 7. Statistics. 2015. [ONLINE] http://apps.who.int/iris/bitstream/10665/170250/1/9789240694439_eng.pdf?ua=1&ua=1. World Health Organization. WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland.
- 4.WGO. World Gastroenterology Organisation. 2008. World Gastroenterology Organisation practice guideline: Acute diarrhoea. 2008. [ONLINE] http://www.worldgastroenterology.org/guidelines/global-guidelines/acute-diarrhea/acute-diarrhea-english. Accessed 17 Feb 2014.
- 5.Burke RM, Smith ER, Dahl RM, Rebolledo PA, Calderón MC, Cañipa B, et al. The economic burden of pediatric gastroenteritis to Bolivian families: a cross-sectional study of correlates of catastrophic cost and overall cost burden. BMC pub Health. 2014;14:642. https://bmcpublichealth.biomedcentral.com/articles/10.1186/1471-2458-14-642 [DOI] [PMC free article] [PubMed]
- 6.Diniz-Santos DR, Santana JS, Barretto JR, Andrade MGM, Silva LR. Epidemiological and microbiological aspects of acute bacterial diarrhoea in children from Salvador, Bahia. Brazil. Braz J Infect Dis. 2005;9(1):77–83. doi: 10.1590/S1413-86702005000100013. [DOI] [PubMed] [Google Scholar]
- 7.Humphries RM, Linscott AJ. Laboratory diagnosis of bacterial gastroenteritis. Clin Microbiol rev. 2015;28(1):3–31. doi: 10.1128/CMR.00073-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Obi CL, Ramalivhana J, Momba MNB, Onabolu B, Igumbor JO, Lukoto M, et al. Antibiotic resistance profiles and relatedness of enteric bacterial pathogens isolated from HIV/AIDS patients with and without diarrhoea and their household drinking water in rural communities in Limpopo Province South Africa. Afr J Biotechnol. 2007;6(8):1035–47.
- 9.Bisi-Johnson MA, Obi CL. Detection of Carbapenem resistance in Salmonella species from a tertiary Hospital in Eastern Cape, South Africa. British Microbiol res J. 2015;10(3):1–6. doi: 10.9734/BMRJ/2015/18586. [DOI] [Google Scholar]
- 10.Bradford PA. Extended-spectrum β-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin Microbiol rev. 2001;14:933–951. doi: 10.1128/CMR.14.4.933-951.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sonda T, Kumburu H, van Zwetselaar M, et al. Meta-analysis of proportion estimates of extended-Spectrum-Beta-lactamase-producing Enterobacteriaceae in East Africa hospitals. Antimicrob res Infect Control. 2016;5:18. doi: 10.1186/s13756-016-0117-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao PF, Watanabe K. Introduction of the World Health Organization project of the international classification of traditional medicine. Zhong xi Yi Jie He Xue Bao. 2011;9:1161–1164. doi: 10.3736/jcim20111101. [DOI] [PubMed] [Google Scholar]
- 13.Gu R, Wang Y, Long B, Kennelly E, Wu S, Liu B, et al. Prospecting for bioactive constituents from traditional medicinal plants through ethnobotanical approaches. Biol Pharmacol Bull. 2014;37(6):903–15. [DOI] [PubMed]
- 14.El-Baz M. 2007. Yesterday's plants - Today's medicines http://ezinearticles.com/?Yesterdays-Plants---Todays-Medicines&id=484879 Accessed august 2015.
- 15.Camacho MR, Croft SL, Phillipson JD. Natural products as sources of antiprotozoal drugs. Curr Opinion Anti-Infective Invest Drugs. 2000;2:47–62. [Google Scholar]
- 16.Soares AM, Ticli FK, Marcussi S, Lourenco MV, Januario AH, Sampaio SV, et al. Medicinal plants with inhibitory properties against snake venoms. Curr med Chem. 2005;12:2625–41. [DOI] [PubMed]
- 17.Plengsuriyakarn T, Viyanant V, Eursitthichai V, Picha P, Kupradinun P, Itharat A, et al. Anticancer activities against cholangiocarcinoma, toxicity and pharmacological activities of Thai medicinal plants in animal models. BMC Complem Alternat med. 2012;12:23. [DOI] [PMC free article] [PubMed]
- 18.Kunin CM. Antimicrobial resistance: problem pathogens and clinical countermeasures. N Engl J med. 2009;360:550–551. doi: 10.1056/NEJMbkrev0807044. [DOI] [Google Scholar]
- 19.Okeke IN, Aboderin OA, Byarugaba DK, Ojo KK, Opintan JA. 2007. Growing problem of multidrug-resistant enteric pathogens in Africa. Emerg Infect Dis 13(11) available from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3375797/. [DOI] [PMC free article] [PubMed]
- 20.WHO. World Health Organization. 2005. Health and the Millennium Development Goals. MDGs, Health and Development Policy World Health Organization Publications of the World Health Organization can be obtained from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland.
- 21.USDA (United States Department of Agriculture). 2016. USDA’s Response to Antibiotic Resistance Audit Report 50601–0004-31. United States Department of Agriculture Office of Inspector General Washington, D.C. 20250. March 2016. Available at https://www.usda.gov/oig/webdocs/50601-0004-31.pdf Accessed June 2016.
- 22.Burt SA, Reinders RD. Antibacterial activity of selected plant essential oils against Escherichia coli O157:H7. Lett Appl Microbiol. 2003;36(3):162–167. doi: 10.1046/j.1472-765X.2003.01285.x. [DOI] [PubMed] [Google Scholar]
- 23.Owens RC, Lautenbach E. 2008. Editors, Antimicrobial Resistance: Problem Pathogens and Clinical Countermeasures (infectious disease and therapy. 48.); 492, illustrated. New York, Informa healthcare ISBN: 978-0-8247-2941-7.
- 24.PCAST. White house report to the president on combating antibiotic resistance. Executive Office f the President’s Council of Advisors on Science and Technology PCAST Antibiotic Resistant Working Group September 2014 Available at https://obamawhitehouse.archives.gov/sites/default/files/microsites/ostp/PCAST/pcast_carb_report_sept2014.pdf. Assessed June 2016.
- 25.Ridley RG. Introduction. Antimalarial drug resistance: ramifications, explanations and challenges. Microbes Infect. 2002;4:155–6. doi: 10.1016/S1286-4579(01)01536-2. [DOI] [PubMed] [Google Scholar]
- 26.Hostettmann K, Wolfender J, Terreaux C. Modern screening techniques for plant extracts. Pharm Biol. 2001;39(1):18–32. doi: 10.1076/phbi.39.7.18.5867. [DOI] [PubMed] [Google Scholar]
- 27.Tugume P, Kakudidi EK, Buyinza M, Namaalwa J, Kamatenesi M, Mucunguzi P, et al. Ethnobotanical survey of medicinal plant species used by communities around Mabira central Forest reserve, Uganda. J Ethnobiol Ethnomed. 2016;12:5. [DOI] [PMC free article] [PubMed]
- 28.Newman DJ, Cragg GM, Snader KM. Natural products as sources of new drugs over the period 1981-2002. J Nat Prod. 2003;66:1022–1037. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- 29.Van Wyk BE. A broad review of commercially important southern African medicinal plants. J Ethnopharmacol. 2008;119:342–355. doi: 10.1016/j.jep.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 30.WHO. World Health Organization. 2008. Traditional medicine. Fact Sheet N°134 2008 Dec http://www.who.int/mediacentre/factsheets/2003/fs134/en/ Accessed March 2016.
- 31.Eloff JN, McGaw LJ. Using African plant biodiversity to combat microbial infections, Gurib-Fakim A (ed). Novel Plant Bioresources: Applications in Food Medicine and Cosmetics. John Wiley DOI: 10.1002/9781118460566; ch12 163-173. 2014.
- 32.Fennell CW, Lindsey KL, McGaw LJ, Sparg SG, Stafford GI, Elgorashi EE, et al. Assessing African medicinal plants for efficacy and safety: pharmacological screening and toxicology. J Ethnopharmacol. 2004;94:205–17. [DOI] [PubMed]
- 33.Bisi-Johnson MA, Obi CL, Kambizi L, Nkomo M. A survey of indigenous herbal diarrhoeal remedies of O.R. Tambo district, Eastern Cape Province, South Africa. Afr J Biotechnol. 2010;9(8):1245–1254. doi: 10.5897/AJB09.1475. [DOI] [Google Scholar]
- 34.Iwu MM. Handbook of African medicinal plants. Inc.: CRC Press; 1993. [Google Scholar]
- 35.Hutchings A, Scott AH, Lewis G, Cunningham A. Zulu Medicinal Plants. Natal University Press, Pietermaritzburg, ISBN 0–86980–923-7. 1996.
- 36.Kotze M, Eloff JN. Extraction of antibacterial compounds from Combretum microphyllum (Combretaceae) S Afr J bot. 2002;68:62–67. doi: 10.1016/S0254-6299(16)30456-2. [DOI] [Google Scholar]
- 37.Stahl E. Thin Layer Chromatography. Springer-Verlag Berlin, Heidenberg, New York. (2nd edn) 1967. Reprint 1988.
- 38.Eloff JN. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta med. 1998;64:711–714. doi: 10.1055/s-2006-957563. [DOI] [PubMed] [Google Scholar]
- 39.Begue WJ, Kline RM. The use of tetrazolium salts in bioautographic procedures. J Chromatogr. 1972;64:182–184. doi: 10.1016/S0021-9673(00)92965-0. [DOI] [PubMed] [Google Scholar]
- 40.Masoko P, Eloff JN. The diversity of antifungal compounds of six South African Terminalia species (Combretaceae) determined by bioautography. Afr J Biotechnol. 2005;4:1425–1431. [Google Scholar]
- 41.CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fourth Informational. Supplement. CLSI document M100-S24. 2014.
- 42.Wagner H, Bladt S. Plant Drug Analysis: A Thin Layer Chromatography Atlas. 2. Berlin Heidelberg: Springer-Verlag; 1996. p. 204. [Google Scholar]
- 43.Abbas FA, Massarany SM, Khan S, Al-howiriny TA, Mossa JS, Abourashed EA. Phytochemical and biological studies on Saudi Commiphora opobalsamum L. Nat Prod res. 2007;21:383–391. doi: 10.1080/14786410600942025. [DOI] [PubMed] [Google Scholar]
- 44.Da Silva VC, Giannini MJSM, Carbone V, Piacente S, Pizza C, Bolzani VDS, et al. New antifungal terpenoid glycosides from Alibertia edulis (Rubiaceae). Helv Chim Acta. 2008;91:1355–62.
- 45.Martinez MA, Mattana CM, Satorres SE, Sosa A, Fusco MR, Laciar AL, et al. Screening phytochemical and antibacterial activity of three San Luis native species belonging at the Fabaceae family. Pharmacol Online. 2014;3:1–6. http://pharmacologyonline.silae.it/files/archives/2014/vol3/PhOL_2014_3_A001_Martinez_1_001.pdf ISSN: 1827–8620.
- 46.Prabu GR, Gnanamani A, Sadulla S. Guaijaverin – a plant flavonoid as potential antiplaque agent against Streptococcus mutans. J Appl Microbiol 2006 101: 487–495 ISSN 1364–5072. [DOI] [PubMed]
- 47.Koorbanally C, Crouch NR, Mulholland DA. The phytochemistry and ethnobotany of the southern African genus Eucomis (Hyacinthaceae: Hyacinthoideae) Phytochemistry Adv Res 2006 69–85.
- 48.Della Logia R, Del Negro P, Tubaro A, Barone G, Parilli M. Homoisoflavanones as anti-inflammatory principles of Muscari comosum. Planta med. 1989;55:587–588. doi: 10.1055/s-2006-962120. [DOI] [Google Scholar]
- 49.Amschler G, Frahm AW, Hatzelmann A, Killian U, Müller-Doblies D, Müller-Doblies U. Constituents of Veltheimia viridifolia; I. Homoisoflavanones of the Bbulbs. Planta Med. 1996;62(6):534–539. doi: 10.1055/s-2006-957964. [DOI] [PubMed] [Google Scholar]
- 50.Witkowska AM, Hickey DK, Alonso-Gomez M, Wilkinson M. Evaluation of antimicrobial activities of commercial herb and spice extracts against selected Food-borne bacteria. J Food res. 2013;2(4):37–54. doi: 10.5539/jfr.v2n4p37. [DOI] [Google Scholar]
- 51.Saritha K, Rajesh A, Manjulatha K, Setty OH, Yenugu S. Mechanism of antibacterial action of the alcoholic extracts of Hemidesmus indicus (L.) R. Br. ex Schult, Leucas aspera (Wild.), Plumbago zeylanica L., and Tridax procumbens (L.) R. Br. ex Schult. Front Microbiol 2015 6:577. doi: 10.3389/fmicb.2015.00577 [DOI] [PMC free article] [PubMed]
- 52.Djeussi DE, Noumedem JAK, Seukep JA, Fankam AG, Voukeng IK, et al. Antibacterial activities of selected edible plants extracts against multidrug-resistant gram-negative bacteria. BMC com Alternative med. 2013;13:164. doi: 10.1186/1472-6882-13-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suleiman MM, McGaw LJ, Naidoo V, Eloff JN. Detection of antimicrobial compounds by bioautography of different extracts of leaves of selected South African tree species. Afr J Tradit Complement Altern Med. 2010;7(1):64–78. doi: 10.4314/ajtcam.v7i1.57269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hamburger MO, Cordell GA. A direct bioautographic TLC assay for compounds possessing antibacterial activity. J Nat Prod. 1987;50:19–22. doi: 10.1021/np50049a003. [DOI] [PubMed] [Google Scholar]
- 55.Valgas C. Machado de Souza S, Smânia EFA, Smânia a. Jr. screening methods to determine antibacterial activity of natural products. Braz J Microbiol. 2007;38:369–380. doi: 10.1590/S1517-83822007000200034. [DOI] [Google Scholar]
- 56.Vlietinck AJ, Van Hoof L, Totte J, Lasure A, Vanden Berghe D, Rwangabo PC, et al. Screening of hundred Rwandese medicinal plants for antimicrobial and antiviral properties. J Ethnopharmcol. 1995;46:31–47. [DOI] [PubMed]
- 57.Eloff JN, Famakin JO, Katarere DRP. Combretum woodii Combretaceae leaf extracts have high activity against gram-negative and gram-positive bacteria. Afr J Biotechnol. 2005;4(10):1161–1166. [Google Scholar]
- 58.Lalli JYY, Van Zyl RL, Van Vuuren SF, Viljoen AM. In vitro biological activities of South African Pelargonium (Geraniaceae) species. S Afr J bot. 2008;74:153–157. doi: 10.1016/j.sajb.2007.08.011. [DOI] [Google Scholar]
- 59.Chiappeta ADA, De Mello JF. Higher plants with biological activity. Plants of Pernambuco. II. III. Rev Inst Antibiotic Univ fed Pernambuco Recife. 1984;11:99–111. [Google Scholar]
- 60.Bouic PJD, Clark A, Lamprecht J. The effects of B-Sitosterol (BSS) and BSitosterol glucoside (BSSG) mixture on selected immune parameters of Marathon runners: inhibition of post Marathon immune suppression and inflammation. Int J Sports med. 1999;20:258–262. doi: 10.1055/s-2007-971127. [DOI] [PubMed] [Google Scholar]
- 61.Steenkamp V, Gouws MC, Gulumian M, Elgorashi EE, Van Staden J. Studies on antibacterial, anti-inflammatory and antioxidant activity of herbal remedies used in the treatment of benign prostatic hyperplasia and prostatitis. J Ethnopharmacol. 2006;103:71–75. doi: 10.1016/j.jep.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 62.Castleman M. The healing herbs. Emmaus, PA (ed). Rodale Press; 42–44. 1991.
- 63.Haller JS. A drug for all seasons, medical and pharmacological history of aloe. Bull N Y Acad med. 1990;66:647–659. [PMC free article] [PubMed] [Google Scholar]
- 64.Yamada T. A report on the ethnobotany of the Nyindu of the Eastern part of the former Zaire. Afr Study Monogr. 1999;20(1):1–72. [Google Scholar]
- 65.Cunningham AB. Ethics, ethnobiological research and biodervisty. WWF-World Wide Fund for Nature (formerly World Wildlife Fund), Gland, Switzerland. The healing herbs. Emmaus, PA, Rodale Press 42–44. 1993.
- 66.Watt JM, Breyer-Brandwijk MG. The medicinal and poisonous plants of southern and Eastern Africa. 2. London: Livingstone; 1962. [Google Scholar]
- 67.Singh Y. Hypoxis: yellow stars of horticulture, folk remedies and conventional medicine. Veld Flora. 1999;85:123–125. [Google Scholar]
- 68.Van Wyk BE, Van Oudtshoorn B, Gericke N. Medicinal Plants of South Africa [2nd improved impression, 2000]. Briza Publications, Pretoria, 1997. ISBN 1–875093–09-5.
- 69.Rood B. Uit die veldapteek. Cape Town: Tafelberg Publishers; 1994. [Google Scholar]
- 70.Silayo A, Ngadjui BT, Abegaz BM. Homoisoflavonoids and stilbenes from the bulbs of Scilla nervosa subsp. rigidifolia. Phytochemistry. 1999;52(5):947–955. doi: 10.1016/S0031-9422(99)00267-8. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article [and its supplementary information files]. The plants used in this study were identified at the Kei Herbarium, Walter Sisulu University, Mthatha, South Africa where voucher specimens have been deposited.


