Abstract
Iron (Fe) is an essential mineral nutrient and a metal cofactor required for many proteins and enzymes involved in the processes of DNA synthesis, respiration, and photosynthesis. Iron limitation can have detrimental effects on plant growth and development. Such effects are mediated, at least in part, through the generation of reactive oxygen species (ROS). Thus, plants have evolved a complex regulatory network to respond to conditions of iron limitations. However, the mechanisms that couple iron deficiency and oxidative stress responses are not fully understood. Here, we report the discovery that an Arabidopsis thaliana monothiol glutaredoxin S17 (AtGRXS17) plays a critical role in the plants ability to respond to iron deficiency stress and maintain redox homeostasis. In a yeast expression assay, AtGRXS17 was able to suppress the iron accumulation in yeast ScGrx3/ScGrx4 mutant cells. Genetic analysis indicated that plants with reduced AtGRXS17 expression were hypersensitive to iron deficiency and showed increased iron concentrations in mature seeds. Disruption of AtGRXS17 caused plant sensitivity to exogenous oxidants and increased ROS production under iron deficiency. Addition of reduced glutathione rescued the growth and alleviates the sensitivity of atgrxs17 mutants to iron deficiency. These findings suggest AtGRXS17 helps integrate redox homeostasis and iron deficiency responses.
Keywords: iron deficiency, oxidative stress, redox homeostasis, glutaredoxin, Arabidopsis
Introduction
Iron is an essential mineral nutrient for plants (Guerinot and Yi, 1994; Briat et al., 2015). It serves as a metal cofactor required for hundreds of metabolic enzymes in the energy-yielding electron transfer reactions of respiration and photosynthesis (Briat et al., 2007; Balk and Schaedler, 2014). Perturbations in iron homeostasis can lead to cytotoxicity in the plant cell, reduction of growth and organ development, and eventually chlorosis and reduced crop yield (Connolly and Guerinot, 2002; Briat et al., 2007). Therefore, iron sensing and uptake from the soil, translocation within the plant, and intracellular storage and trafficking are tightly regulated in plants (Curie and Briat, 2003; Hell and Stephan, 2003; Jeong and Guerinot, 2009; Conte and Walker, 2011; Kobayashi and Nishizawa, 2012).
The iron deficiency response is thought to be controlled by a complex regulatory network involving multiple signaling pathways and its interplay with hormones (Hindt and Guerinot, 2012; Xia et al., 2015). Early studies indicate that adaptation to iron deficiency requires remodeling of the photosynthetic apparatus to minimize the photooxidative damage caused by reactive oxygen species (ROS) (Moseley et al., 2002). Genome-wide analyses of both transcript and protein expression profiles reveal significant changes in the expression of genes and/or proteins involved in antioxidant and oxidative stress response pathways (O’Rourke et al., 2007; Forner-Giner et al., 2010; Urzica et al., 2012; Zamboni et al., 2012; Lopez-Millan et al., 2013). In particular, expression of genes encoding glutaredoxins (Grxs) and thioredoxins (Trxs) is significantly enhanced under iron deficiency (Urzica et al., 2012). Furthermore, hydrogen peroxide (H2O2) production is increased in roots of plants grown under iron deficiency (Le et al., 2016). Nonetheless, the role of ROS and the function of redox-regulatory proteins in iron deficiency response regulation has not been well defined.
Grx and Trx enzyme systems help to control cellular redox potential in plants (Meyer et al., 2009). Grxs are ubiquitous small heat-stable disulfide oxidoreductases conserved in both prokaryotes and eukaryotes (Lillig et al., 2008) and are important in redox regulation and stress response (Sanchez-Riego et al., 2013; Considine and Foyer, 2014). There is growing evidence that plant Grxs have diverse functions in transcriptional regulation of defense responses and flower development (Xing et al., 2005; Ndamukong et al., 2007; Werner and Schmülling, 2009; La Camera et al., 2011), antioxidative stress (Cheng et al., 2006; Cheng, 2008; Laporte et al., 2012), redox signaling (Zaffagnini et al., 2012), hormonal regulation and environmental adaptation (Sundaram and Rathinasabapathi, 2010; Cheng et al., 2011). Monothiol Grxs are first identified in yeast (ScGrx3, -4, and -5) and bacteria (Grx4) that have a single cysteine residue in the putative active motif (Rodriguez-Manzaneque et al., 1999; Fernandes et al., 2005). This group of Grxs is conserved across species and accumulating evidence suggests they play a unique function in regulating iron homeostasis (Herrero and de la Torre-Ruiz, 2007; Lillig et al., 2008; Rouhier et al., 2010; Stroher and Millar, 2012). Yeast ScGrx5 encodes a mitochondrial monothiol Grx, which is required for biogenesis of iron-sulfur clusters (Rodriguez-Manzaneque et al., 2002; Uzarska et al., 2013), whereas ScGrx3 and ScGrx4, through interactions with iron-regulatory transcription factors, like Aft1 and Aft2, and coactivators like Fra/BolA proteins, globally modulate iron uptake, intracellular sensing, and trafficking in yeast cells (Ojeda et al., 2006; Pujol-Carrion et al., 2006; Kumanovics et al., 2008; Mercier and Labbé, 2009; Muhlenhoff et al., 2010; Li et al., 2011; Li and Outten, 2012; Vachon et al., 2012; Tamayo et al., 2016). In plants, Arabidopsis and Poplar monothiol Grxs, such as AtGRXS14 (AtGRXcp), AtGRXS15 (AtGRX4), AtGRXS16 and AtGRXS17, bind a Fe-S cluster and are able to complement yeast ScGrx5 function in Fe-S cluster assembly when expressed in yeast mutant cells (Cheng et al., 2006; Bandyopadhyay et al., 2008; Cheng, 2008; Li et al., 2010; Liu et al., 2013; Knuesting et al., 2015). However, the function of plant monothiol Grxs in iron regulation and stress responses in planta remains to be explored.
Arabidopsis AtGRXS17 is one of four “CGFS” type monothiol Grxs in Arabidopsis (Lemaire, 2004) with one Trx-like domain at its N-terminal region and three “CGFS” containing Grx domains at its C-terminus (Cheng et al., 2006; Herrero and de la Torre-Ruiz, 2007). Our previous studies indicate that AtGRXS17 is essential for post-embryonic growth and hormonal responses in plants under elevated temperature (Cheng et al., 2011). Meanwhile, ectopic expression of AtGRXS17 enhances stress tolerance (Wu et al., 2012; Hu et al., 2015). AtGRXS17 interacts with plant BolA proteins in an in vitro study (Couturier et al., 2014) and AtGRXS17 appears to be able to bind an iron-sulfur (Fe-S) cluster (Knuesting et al., 2015), suggesting that AtGRXS17 plays an important role in iron homeostasis. In the present report, we utilize yeast expression studies and reverse genetics in Arabidopsis to study the function of AtGRXS17 under iron deficiency stress. Our findings demonstrate that AtGRXS17 plays an important role in protecting plants from iron deficiency induced oxidative damage.
Materials and Methods
Reagents
All chemicals were purchased from Sigma–Aldrich (St. Louis, MO, United States) unless stated otherwise. Murashige and Skoog (MS) medium was purchased from Caisson Laboratories Inc (North Logan, UT, United States). AtGRXS17 polyclonal antibody was made in-house using the full-length Arabidopsis AtGRXS17 recombinant protein. This antibody does not cross react with other AtGRXs. Rabbit polyclonal antiserum against rubisco large subunit (form I and II) was purchased from Agrisera (Agrisera AB, Sweden).
Plasmid DNA, Yeast Transformation, and Iron Content Assay
Yeast strains, expression plasmids, and the transformation protocol were described previously (Wu et al., 2012) (See detailed description in Supplementary Materials). Yeast cells were grown in nutrient-enriched medium (YPD) overnight, harvested, washed twice with distilled water, then dried for metal ion measurement as previously described (Cheng et al., 2006).
Plant Materials and Growth Conditions
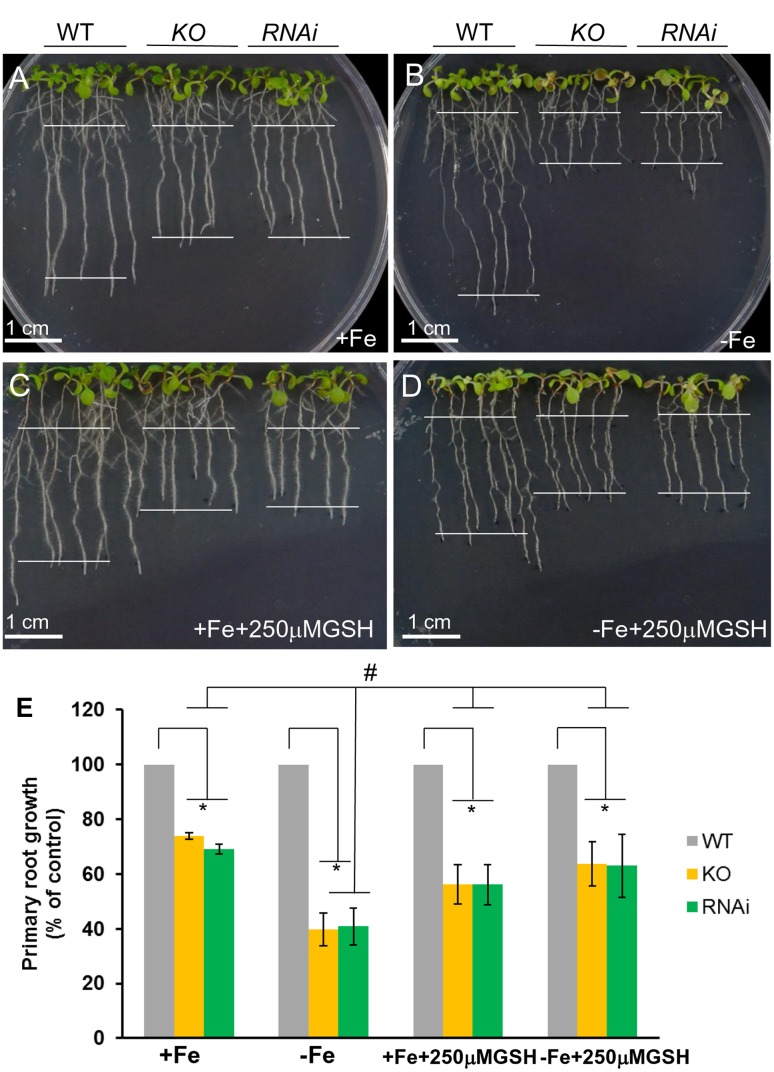
Wild type (ecotype Columbia, Col-0), atgrxs17 KO, and AtGRXS17 RNAi lines were described previously (Cheng et al., 2011) (See detailed description in Supplementary Materials). For growth assays, wild type and mutant seeds were surface-sterilized, germinated, and grown on one-half strength MS medium (plus 0.5% sucrose), which consists of 50 μM Fe, solidified with 0.8% agar or ½ MS medium supplemented with various concentrations of H2O2. Iron sufficient and deficient medium were made following the previous report with minor modification (Connolly et al., 2002; Barberon et al., 2014). In brief, 1 L of synthetic medium (SM) was made containing 0.47 g Ca(NO3)2.4H2O, 0.1307 g K2SO4, 0.1602 g MgSO4.7H2O, 0.0136 g KH2PO4, 0.5 g MES, 1 mL of 1000× micronutrients (0.01 mM H3BO3, 0.1 μM MnSO4, 0.05 μM CuSO4, 0.05 μM ZnSO4, and 5 nM Na2MO4). The pH was adjusted to 6 with 1 M NaOH. To make iron-sufficient medium, 50 μM (final concentration) FeEDTA was added to the SM plus 0.5% sucrose. For iron-deficient medium, 300 μM (final concentration) Ferrozine was added into the SM plus 0.5% sucrose as well. For iron stress assays, wild type, atgrxs17 KO, and AtGRXS17 RNAi seeds were germinated and grown on ½ MS medium for 5 days, then transferred and grown on iron sufficient or deficient medium for 6 days before measuring primary root length of seedlings or for 11 days before fresh weight of seedlings was measured. For iron deficiency stress rescue experiments, 250 μM (final concentration) GSH was added into the iron sufficient or iron deficient medium.
Plant Mineral Ion Concentration Measurement
Wild type, atgrxs17 KO, and AtGRXS17 RNAi seeds were germinated and grown in soil (Sunshine Mix, Sun Gro Horticulture, Agawam, MA, United States) in a controlled greenhouse or growth chamber at 22°C. Mature leaves were collected from 5-week-old plants, while seeds were harvested from mature plants. Four independent experiments were done for each treatment of each genotype. Elemental analysis was performed using inductively coupled plasma–optical emission spectroscopy as described previously (Farnham et al., 2011).
Ferric Chelate Reductase Assay
Ferric chelate reductase assays were performed as previously described (Grusak et al., 1990). In brief, wild type, atgrxs17 KO, and AtGRXS17 RNAi seeds were germinated and grown on ½ MS medium for 10 days at 22°C under 16 h light and 8 h dark. Seedlings were transferred and grown on iron sufficient or deficient medium for 3 additional days, then rinsed twice with distilled water and the entire root system of each seedling was submerged in 1 mL of assay solution [100 μM Fe(III)-EDTA and 300 μM Na2-BDPS] for 1hr at room temperature in the dark. The amount of Fe(II)-BPDS3 was measured by reading the absorbance at 562 nM. The Fe(III)-reductase activity was calculated as μmol Fe(II) per gram root fresh weight per hour. Six samples were measured for each genotype and three independent experiments were conducted.
RNA Isolation, cDNA Synthesis, and qRT-PCR Analysis
Wild-type seeds were germinated and grown on ½ MS medium for 10 days at 22°C under 16 h light and 8 h night. Seedlings were transferred and grown on iron sufficient or deficient medium for growth of 6 or 24 h. Twenty seedlings for each treatment were pooled for RNA isolation. Three independent experiments were conducted. Total RNA was extracted from wild-type seedlings and purified RNA samples underwent reverse transcription to yield cDNA. qRT-PCR was performed using the SYBR Green-based system on the Bio-Rad CFX96TM. Primers were used for AtGRXS17: tgctgtgccttatttcgtcttc (forward) and tctgcaccctcaagtgtatcca (reverse); and for ACTIN1serving as the internal control: gtgctcgactctggagatggtgtg (forward) and cggcgattccagggaacattgtgg (reverse).
Western Blot Analysis
Wild type, atgrxs17 KO, and AtGRXS17 RNAi seeds were germinated and grown on ½ MS medium for 14 days and then transferred onto iron deficient or sufficient medium for additional 3 days before being harvested. Seedling tissue homogenates (20 μg per lane) were run on SDS–PAGE gel and western blot analysis was conducted following an established procedure (Liu et al., 2013) (See detailed description in Supplementary Materials). Rabbit antiserum against AtGRXS17 was used at dilution of 1:500 and Anti-RbcL antibody was used at 1:2500 dilution.
ROS Production Measurement
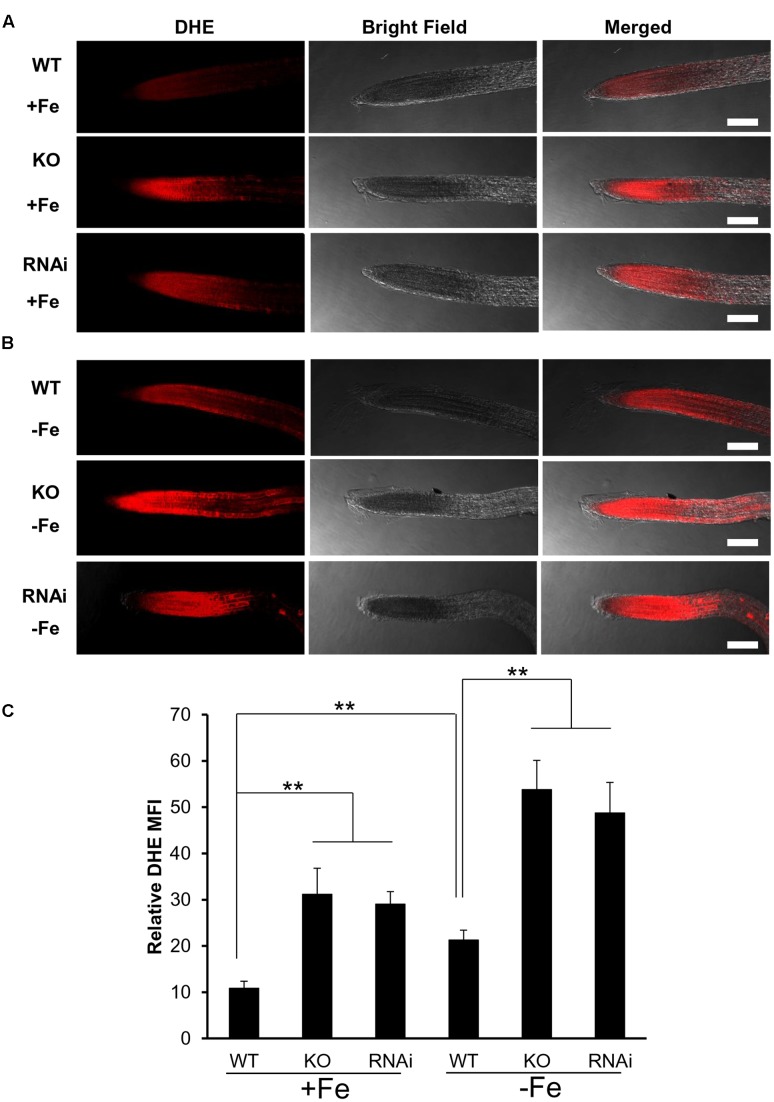
Wild type, atgrxs17 KO, and AtGRXS17 RNAi seeds were germinated and grown on ½ MS medium for 5 days, then transferred and grown on iron sufficient or deficient medium for 3 days before seedlings were collected for measurement of ROS production in roots. Seedlings were transferred from agar plates into Eppendorf tubes containing 1 mL of cold PBS and were washed twice with 1 mL cold PBS. For ROS measurement, the roots were stained with 10 μM Dihydroethidium (DHE) (Camacho-Cristóbal et al., 2015) for 45 min to 1 h, washed once with PBS and left in PBS before imaging with a confocal microscope at 582 nm (excitation at 543 nm) for Texas Red. The mean fluorescence intensity (MFI) of root tips from six to ten randomly selected seedlings was quantified using ImageJ software.
Results
AtGRXS17 Is Able to Suppress the Yeast grx3grx4 Iron Accumulation Phenotype
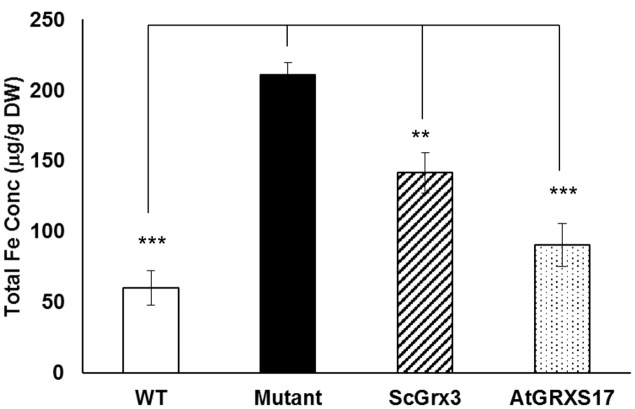
Arabidopsis AtGRXS17 suppresses the sensitivity of yeast grx3grx4 cells to oxidative stress (Wu et al., 2012). In yeast, ScGrx3 and ScGrx4 play a critical role in iron uptake, trafficking, mitochondrial iron dynamics and homeostasis (Ojeda et al., 2006; Pujol-Carrion et al., 2006; Muhlenhoff et al., 2010). Disruption of both ScGrx3 and ScGrx4 results in the accumulation of iron in the cell (Pujol-Carrion et al., 2006) and expression of yeast ScGrx3 could rescue, at least in part, the accumulation of free iron in the grx3grx4 mutant (Figure 1). When expressed in grx3grx4, AtGRXS17 was able to suppress the iron accumulation phenotype of grx3grx4 cells (Figure 1). These results suggest that AtGRXS17 may function in iron regulation in plants.
FIGURE 1.
AtGRXS17 suppresses yeast grx3grx4 iron accumulation phenotypes. Yeast wild type and grx3grx4 cells expressing empty vector and plasmid DNA as indicated were grown in YPD medium overnight and whole cell iron concentrations were measured by inductively coupled plasma optical emission spectrometry. Results represent the mean value of three independent replications. Student’s t-test, ∗∗p < 0.01, ∗∗∗p < 0.001.
AtGRXS17 Expression Is Induced by Iron Deficiency Stress
To understand the physiological function of AtGRXS17 in iron regulation in plants, the responsiveness of endogenous AtGRXS17 to iron limiting conditions was examined by qRT-PCR. As shown in Supplementary Figure S1A, the level of AtGRXS17 mRNA was increased about threefolds under iron deficiency conditions. IRT1 was used as a control to indicate iron status as this gene induced under this condition (Connolly et al., 2002). AtGRXS17 protein levels were also increased in plants grown under iron deficiency compared to iron sufficient condition (Supplementary Figure S1B). The results suggest that AtGRXS17 may play an important role in response to iron deficiency in plants.
AtGRXS17 Loss-of-function Seedlings Are Sensitive to Iron Deficiency Stress
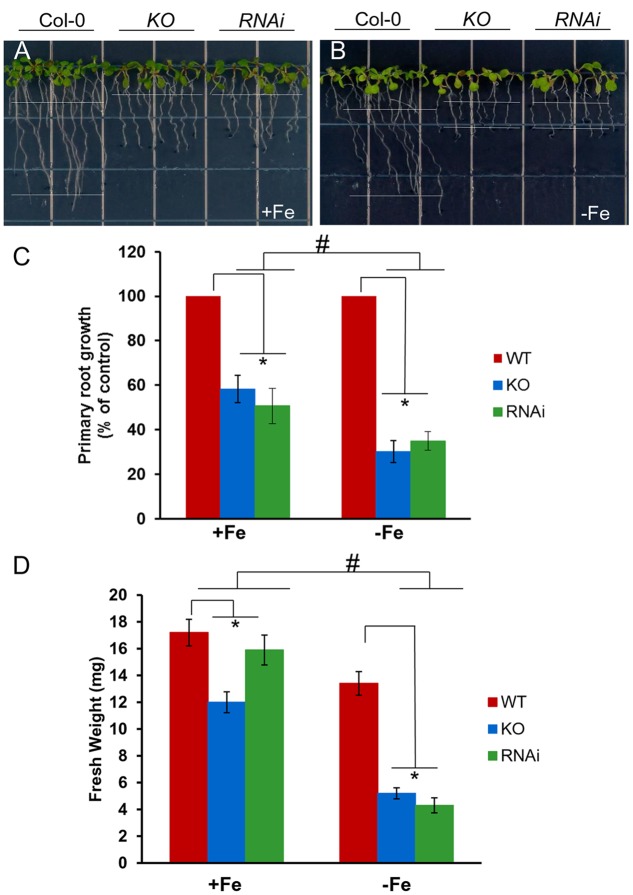
To test the function of AtGRXS17 in planta, atgrxs17 KO and AtGRXS17 RNAi lines were generated (Supplementary Figure S1C). AtGRXS17 loss-of-function seedlings (KO and RNAi lines) displayed strong growth inhibition of primary roots under iron deficiency (about 35% of wild-type root growth) compared to those grown on iron sufficient medium (about 60% of wild-type root growth) (Figures 2A–C). The overall growth of mutant seedlings as measured by fresh weight was decreased under iron deficiency compared to those grown on iron sufficient medium (Figure 2D). These findings indicate that AtGRXS17 helps to maintain plant growth under iron deficiency stress.
FIGURE 2.
Loss of AtGRXS17 impairs seedlings growth under iron deficiency stress. (A–C), Wild type control, atgrxs17 KO, and AtGRXS17 RNAi seeds were germinated and grown on ½ MS medium (0.5% Sucrose) for 5 days and then transferred onto Fe sufficient or deficient medium as indicated for 6 additional days of growth at 22°C. In (C), primary root growth was measured and the growth rate was calculated relative to wild type controls. Statistical analysis using a two-way ANOVA. n ≥ 18. ∗p < 0.05, significance between WT controls and atgrxs17 mutants; #p < 0.05, significance between +Fe and –Fe treatments. (D) Wild type control, atgrxs17 KO, and AtGRXS17 RNAi seeds were germinated on ½ MS medium (0.5% Suc) for 5 days and then transferred onto iron sufficient or deficient medium as indicated for 11 days of growth at 22°C. Fresh weight was measured to compare mutants to wild type controls. Statistical analysis using a two-way ANOVA. n ≥ 12. ∗p < 0.05, significance between WT controls and atgrxs17 mutants; #p < 0.05, significance between +Fe and –Fe treatments.
atgrxs17 Plants Accumulate More Iron in Seeds
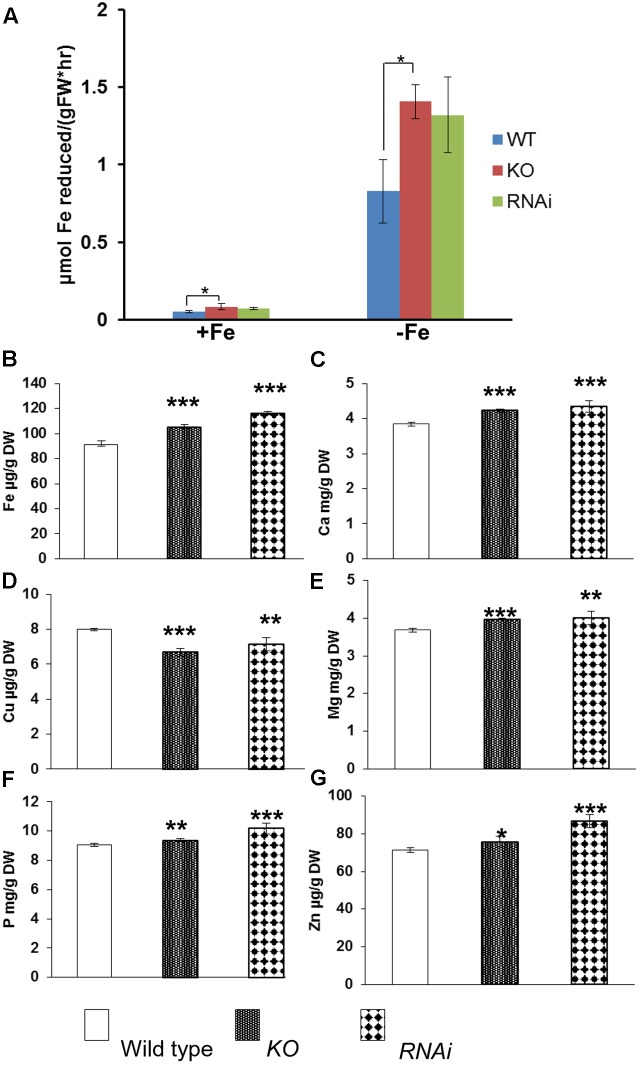
To determine whether disruption of AtGRXS17 affects ferric chelate reductase, both wild type control and mutant seedlings were grown on iron sufficient or deficient medium for 3 days. Root ferric chelate reductase activities of both mutant and wild-type seedlings were increased under iron deficiency compared to those under iron sufficient medium (Figure 3A). Compared to wild type controls, atgrxs17 KO and AtGRXS17 RNAi seedlings had higher reductase activities under both iron sufficient and deficient conditions, in which the increase of reductase activity in atgrxs17 KO seedlings was significant (Figure 3A). When grown in soil under normal growth conditions, AtGRXS17 loss-of-function plants demonstrate subtle growth defects (Cheng et al., 2011; Knuesting et al., 2015). When mature leaves from 5-week-old mutant and wild type control plants grown under normal growth conditions were collected and iron concentrations were examined, total iron concentration in leaves of mutant plants was indistinguishable from wild type controls (data not shown). However, measurement of iron concentrations of dry seeds from mutant and wild-type plants indicated that AtGRXS17 loss-of-function plants demonstrated significantly higher iron concentrations in seeds compared to wild type controls (Figure 3B). Furthermore, mutant plants showed higher concentrations of other mineral ions, such as Ca, Mg, Zn, and P, while exhibiting decreased concentrations of Cu in seeds (Figures 3C–G).
FIGURE 3.
Iron reductase assay and metal ion concentrations in wild type, atgrxs17 KO, and AtGRXS17 RNAi seeds. (A), Wild type, atgrxs17 KO, and AtGRXS17 RNAi seeds were germinated and grown on ½ MS medium for 10 days, then transferred to iron sufficient or deficient medium for 3 days. Root ferric reductase activity was measured. Student’s t-test, n = 6, ∗p < 0.05. (B–G), Wild type, atgrxs17 KO, and AtGRXS17 RNAi seeds were germinated and grown in soil until mature. Seeds were harvested and dried. Whole seed Fe and other mineral concentrations were measured by inductively coupled plasma optical emission spectroscopy. Results represent the mean value of three independent replications. Student’s t-test, ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
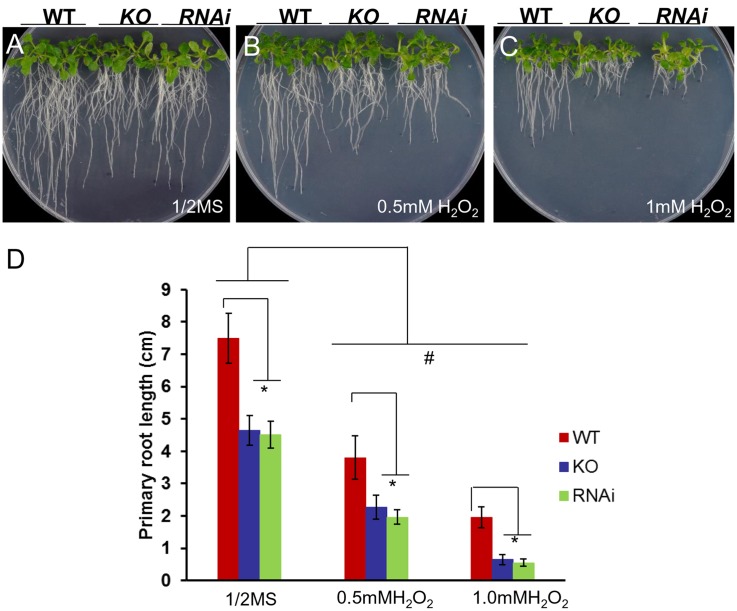
atgrxs17 Seedlings Are Sensitive to Oxidative Stress and Increase ROS Production under Iron Deficiency Stress
When measuring H2O2 accumulation in atgrxs17 KO seedlings by DAB staining, the root tips and the junction areas (between the hypocotyl and the root) display more intense staining than controls (Cheng et al., 2011). When grown on medium containing H2O2, the primary root growth of atgrxs17 KO and AtGRXS17 RNAi seedlings was significantly inhibited compared to wild type controls (Figure 4), suggesting that AtGRXS17 loss-of-function plants are more sensitive to external oxidative stress. Previous studies have reported that iron deficiency induces gene expression response to oxidative stress in various species (O’Rourke et al., 2007; Zamboni et al., 2012) and a rapid increase in hydrogen peroxide (H2O2) production (Le et al., 2016). To ascertain whether iron deficiency stress induces ROS production and how AtGRXS17 affects this process, wild type control and mutant roots were stained with DHE, which enables the detection of ROS by fluorescence microscopy. Under iron sufficient condition, ROS production was increased in mutant root tips compared to wild type controls (Figures 5A,C), which is consistent with our previous report (Cheng et al., 2011). As expected, iron deficiency caused a significant increase of red fluorescence (ROS levels) in both wild type and mutant roots compared to that under iron sufficient condition (Figures 5B,C), in which enhancement of ROS production in atgrxs17 KO and RNAi roots had expanded from the root tips to the elongation zone of the roots (Figures 5A,B). Thus, these findings indicate that AtGRXS17 plays a role in controlling oxidative stress induced by iron deficiency stress.
FIGURE 4.
atgrxs17 KO and AtGRXS17 RNAi seedlings are sensitive to oxidative stress. Wild type, atgrxs17 KO, and AtGRXS17 RNAi seeds were germinated and vertically grown on ½ MS medium (A) and the same medium supplemented with 0.5 mM (B) and 1 mM (C) H2O2 for 10 days. (D) The length of primary roots was recorded. Statistical analysis using a two-way ANOVA. n ≥ 18. ∗p < 0.05, significance between WT controls and atgrxs17 mutants; #p < 0.05, significance between ½ MS medium and H2O2 treatments.
FIGURE 5.
atgrxs17 seedlings increase ROS production under iron deficiency stress. Wild type control, atgrxs17 KO, and AtGRXS17 RNAi seeds were germinated and vertically grown on ½ MS medium for 5 days, then transferred to and grown on iron sufficient or deficient medium for 3 days. Seedlings were stained with DHE to monitor ROS levels. Shown are representative images for iron sufficient (A) and deficient (B) treatments. Scale Bars = 50 μm. (C) The intensity of ROS signals were captured by confocal microscope and analyzed with Image J. Student’s t-test, n = 6–10, ∗∗p < 0.01.
Attenuation of atgrxs17 Seedling Sensitivity to Iron Deficiency Stress by Reduced GSH
To determine whether AtGRXS17 loss-of-function seedling sensitivity to iron-deficiency stress was due to disruption of redox balance, atgrxs17 KO and AtGRXS17 RNAi seedlings were tested on iron sufficient and deficient conditions with addition of reduced GSH. When grown on iron sufficient and deficient conditions without addition of reduced GSH, the growth of primary roots of KO and RNAi seedlings was inhibited under iron deficient condition compared to that under iron sufficient condition (Figures 6A,B,E), while grown on iron sufficient and deficient conditions with addition of reduced GSH, the growth of primary roots of atgrxs17 KO and AtGRXS17 RNAi seedlings was indistinguishable under iron deficiency compared to that under iron sufficient condition (Figures 6C–E). These findings indicate AtGRXS17 modulates iron deficiency stress responses through mediation of redox homeostasis in plants.
FIGURE 6.
atgrxs17 seedling sensitivities to iron deficiency stress are rescued by reduced glutathione (GSH). Wild type control, atgrxs17 KO, and AtGRXS17 RNAi seeds were germinated on ½ MS medium for 5 days and then transferred to and grown on Fe sufficient (A) or deficient (B) medium without or on Fe sufficient (C) or deficient (D) with addition of 250 μM reduced GSH as indicated for 6 days of growth at 22°C. In (E), primary root length was measured and the growth rate was calculated relative to wild type controls. Statistical analysis using a two-way ANOVA. n ≥ 18. ∗p < 0.05, significance between WT controls and atgrxs17 mutants; #p < 0.05, significance between +Fe and –Fe treatments in the presence of reduced GSH.
Discussion
Our genetic studies presented here offer insight into the relationship between redox regulation and iron homeostasis in plants. This work provides evidence that AtGRXS17 is involved in regulation of iron homeostasis in plants and helps to alleviate iron deficiency stress through mediating redox balance.
In yeast mutant cells, AtGRXS17 suppressed iron accumulation phenotypes (Figure 1). Interestingly, the suppression of iron accumulation in double mutant cells by AtGRXS17 appears to be stronger than ScGrx3 alone (Figure 1). This observation could be due to the fact that ScGrx3 and ScGrx4 functions are not completely overlapping. It has been shown that cytosolic and nuclear ScGrx3/ScGrx4 have distinct functions in iron regulation and homeostasis (Ojeda et al., 2006; Pujol-Carrion et al., 2006; Muhlenhoff et al., 2010). When ectopically expressed in yeast, AtGRXS17 is found both in the nucleus and the cytoplasm (Wu et al., 2012). It is possible that AtGRXS17 could rescue the yeast grx3grx4 phenotype better due to its dual cellular localization in yeast cells.
In yeast, deletion of both ScGrx3 and ScGrx4 results in growth defects and enhanced sensitivity to oxidative stress caused by iron accumulation (Pujol-Carrion et al., 2006; Wu et al., 2012). Similarly, AtGRXS17 loss-of-function plants displayed root and vegetative growth retardation under normal conditions (Figures 2A,C,D) (Cheng et al., 2011; Knuesting et al., 2015) and increased sensitivity to oxidative stress (Figure 4). This finding indicates that AtGRXS17 and its yeast orthologs have conserved functions. However, there is functional divergene between the plant and yeast genes as yeast grx3grx4 cells use an iron chelator to alleviate oxidative stress (Pujol-Carrion et al., 2006). Meanwhile AtGRXS17 loss-of-function seedlings were hypersensitive to iron deficiency (in the presence of iron chelator) (Figure 2).
Root iron reductase activity was significantly induced in wild type controls and mutants under iron deplete medium compared to that under iron sufficient medium (Figure 3A). It appears that atgrxs17 KO and AtGRXS17 RNAi seedlings has higher root iron reductase activities than wild type controls under both iron sufficient and deficient conditions (Figure 3A). The increase in root iron reductase activity, especially under iron sufficient medium, may not result in increased iron uptake and accumulation in mutant plants. In agreement with this, no difference in iron accumulation in mature leaves between AtGRXS17 loss-of-function plants and wild type controls was observed when plants were grown in soil. However, iron concentration in mature seeds of mutant plants was slightly, but significantly increased compared to wild type controls (Figure 3B). This suggests that AtGRXS17 may modulate iron distribution within a plant. It is also possible that a reduction in seed yield of these mutant plants could have contributed to the elevated seed iron levels through a concentrating process (i.e., the same total partitioning of iron to seeds, but to a smaller pool of seeds). Higher concentrations of calcium, magnesium, phosphorus, and zinc were also seen in seeds of soil-grown mutant plants (Figures 3C,E–G). Unfortunately, seed yield was not measured in the current study. Previous research has shown that seeds comprise about 30% of whole-shoot iron content and about 15% of shoot mass at maturity in Arabidopsis (Waters and Grusak, 2008). Thus, even a moderate lowering of seed production in the mutants could explain a portion of the increased seed iron concentration in these plants. Previous studies have shown that AtGRXS17 loss-of-function plants display subtle growth defects (Cheng et al., 2011; Knuesting et al., 2015). Furthermore, when seedlings were grown on iron sufficient medium, the growth of AtGRXS17 mutant primary roots was slower than that of wild type controls (Figures 2A,C, 6A,E). Whether those growth defects are attributed to altered iron accumulation/distribution is not clear in the current study. We posit that the increased ROS production/oxidative stress (Figures 5A,C) are the causal factors for inhibition of root growth.
Iron deficiency causes cellular oxidative stress and induces antioxidant defense genes (pathways) including Grxs in plants and green algae (Thimm et al., 2001; Connolly and Guerinot, 2002; O’Rourke et al., 2007; Lan et al., 2011; Urzica et al., 2012; Lopez-Millan et al., 2013; Le et al., 2016). Reduced GSH contents are significantly decreased in Arabidopsis plants under iron deficiency, whereas ROS levels are drastically increased (Ramirez et al., 2013). Furthermore, addition of reduced GSH can alleviate the detrimental effects of iron deficiency through controlling ROS production/oxidative stress caused by iron deficiency (Ramirez et al., 2013). Our data revealed an increase of AtGRXS17 expression (both mRNA and protein levels) in plants under iron deficiency (Supplementary Figure S1), while disruption of AtGRXS17 significantly inhibited plant growth under the same condition (Figure 2). This finding demonstrates that AtGRXS17 is required for plant survival under iron deficiency stress. Our studies support the notion that AtGRXS17 may alleviate the iron deficiency stress through mediating redox balance. First, atgrxs17 KO and AtGRXS17 RNAi seedlings are sensitive to oxidative stress (Figure 4); Second, ROS production is significantly increased in mutant roots under iron deficiency (Figures 5B,C), which is a contributing factor to cause cell damage and impair plant growth. Third, although the intracellular GSH levels in both mutant seedlings and wild type controls were not measured in the current study, addition of reduced GSH is able to suppress, at least in part, the growth defects of mutant seedlings under iron deficiency (Figure 6). Furthermore, overexpression of AtGRXS17 enhances antioxidant enzymatic activities in transgenic tomato plants (Wu et al., 2012). Taken together, these results indicate that AtGRXS17 is crucial for protecting plants from iron deficiency induced oxidative damage.
AtGRXS17, similar to ScGrx3/ScGrx4, is a Fe-S cluster binding protein (Knuesting et al., 2015) and is postulated to mediate iron or iron-sulfur cluster transfer processes (Philpott, 2012; Inigo et al., 2016). Disruption of both ScGrx3 and ScGrx4 in yeast drastically alters iron sensing, intracellular trafficking, and mitochondrial iron distribution through their bound iron-sulfur clusters (Muhlenhoff et al., 2010). Whether AtGRXS17 modulates iron deficiency responses through its bound cluster is yet to be determined. Interestingly, Arabidopsis BolA protein, an interacting partner of AtGRXS17, might play a role in iron metabolism and redox regulation independent of its iron-sulfur binding ability (Qin et al., 2015). We envision AtGRXS17 playing a myriad of roles in planta; however, studies directed at clarifying other AtGRXS17 functions require additional inquiry.
Recent advances have indicated that the interaction among multiple phytohormones, such as auxin, ethylene, and nitric oxide (NO), plays an important role in iron deficiency responses in plants (Schmidt et al., 2000; Seguela et al., 2008; Romera et al., 2011; Hindt and Guerinot, 2012). For example, recent reports indicate that auxin can regulate plant responses to iron deficiency through a NO-mediated signaling pathway (Chen et al., 2010; Jin et al., 2011). Furthermore, decreased auxin concentrations and polar auxin transport in auxin transporter mutants trigger up-regulation of iron deficient responsive genes (Xu et al., 2014). Our previous study demonstrates that AtGRXS17 is crucial for auxin response and function in temperature stress (Cheng et al., 2011). Whether AtGRXS17 mediates its effects on the iron deficiency response via modulation of auxin response pathways remains to be further investigated.
Conclusion
AtGRX7 is part of the ensemble of plant genes that sense and respond to fluctuations in iron availability. Using heterologous expression and reverse genetic approaches, this work establishes that AtGRXS17 functions under iron limiting conditions to modulate plant growth, iron accumulation, and redox balance.
Author Contributions
NC and MG designed the study and wrote the paper. HY, JY, JD, YS, ST, TR, D-LW, JL, SP, and NC performed and analyzed the experiments. PN, EC, and KH provided technical assistance and analysis and interpretation of data. All authors reviewed the results and approved the final version of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the United States Department of Agriculture, Agricultural Research Service through Cooperative Agreement Number 58-6250-0-008. The contents of this publication do not necessarily reflect the views or policies of the US Department of Agriculture, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. EC was supported by NSF IOS 1456881. JL was supported in part by the Natural Science Foundation of China (31371401).
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2017.01045/full#supplementary-material
References
- Balk J., Schaedler T. A. (2014). Iron cofactor assembly in plants. Annu. Rev. Plant Biol. 65 125–153. 10.1146/annurev-arplant-050213-035759 [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay S., Gama F., Molina-Navarro M. M., Gualberto J. M., Claxton R., Naik S. G., et al. (2008). Chloroplast monothiol glutaredoxins as scaffold proteins for the assembly and delivery of [2Fe-2S] clusters. EMBO J. 27 1122–1133. 10.1038/emboj.2008.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberon M., Dubeaux G., Kolb C., Isono E., Zelazny E., Vert G. (2014). Polarization of IRON-REGULATED TRANSPORTER 1 (IRT1) to the plant-soil interface plays crucial role in metal homeostasis. Proc.Natl. Acad. Sci.U.S.A. 111 8293–8298. 10.1073/pnas.1402262111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briat J. F., Curie C., Gaymard F. (2007). Iron utilization and metabolism in plants. Curr. Opin. Plant Biol. 10 276–282. 10.1016/j.pbi.2007.04.003 [DOI] [PubMed] [Google Scholar]
- Briat J.-F., Dubos C., Gaymard F. (2015). Iron nutrition, biomass production, and plant product quality. Trends Plant Sci. 20 33–40. 10.1016/j.tplants.2014.07.005 [DOI] [PubMed] [Google Scholar]
- Camacho-Cristóbal J. J., Martín-Rejano E. M., Herrera-Rodríguez M. B., Navarro-Gochicoa M. T., Rexach J., González-Fontes A. (2015). Boron deficiency inhibits root cell elongation via an ethylene/auxin/ROS-dependent pathway in Arabidopsis seedlings. J. Exp. Bot. 66 3831–3840. 10.1093/jxb/erv186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. W., Yang J. L., Qin C., Jin C. W., Mo J. H., Ye T., et al. (2010). Nitric oxide acts downstream of auxin to trigger root ferric-chelate reductase activity in response to iron deficiency in Arabidopsis. Plant Physiol. 154 810–819. 10.1104/pp.110.161109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng N. H. (2008). AtGRX4, an Arabidopsis chloroplastic monothiol glutaredoxin, is able to suppress yeast grx5 mutant phenotypes and respond to oxidative stress. FEBS Lett. 582 848–854. 10.1016/j.febslet.2008.02.006 [DOI] [PubMed] [Google Scholar]
- Cheng N. H., Liu J. Z., Brock A., Nelson R. S., Hirschi K. D. (2006). AtGRXcp, an Arabidopsis chloroplastic glutaredoxin, is critical for protection against protein oxidative damage. J. Biol. Chem. 281 26280–26288. 10.1074/jbc.M601354200 [DOI] [PubMed] [Google Scholar]
- Cheng N.-H., Liu J.-Z., Liu X., Wu Q., Thompson S. M., Lin J., et al. (2011). Arabidopsis monothiol glutaredoxin, AtGRXS17, is critical for temperature-dependent postembryonic growth and development via modulating auxin response. J. Biol. Chem. 286 20398–20406. 10.1074/jbc.M110.201707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly E. L., Fett J. P., Guerinot M. L. (2002). Expression of the IRT1 metal transporter is controlled by metals at the levels of transcript and protein accumulation. Plant Cell 14 1347–1357. 10.1105/tpc.001263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly E. L., Guerinot M. (2002). Iron stress in plants. Genome Biol. 3:r1024 10.1186/gb-2002-3-8-reviews1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Considine M. J., Foyer C. H. (2014). Redox regulation of plant development. Antioxid. Redox Signal. 21 1305–1326. 10.1089/ars.2013.5665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte S. S., Walker E. L. (2011). Transporters contributing to iron trafficking in plants. Mol. Plant 4 464–476. 10.1093/mp/ssr015 [DOI] [PubMed] [Google Scholar]
- Couturier J., Wu H. C., Dhalleine T., Pegeot H., Sudre D., Gualberto J. M., et al. (2014). Monothiol glutaredoxin-BolA interactions: redox control of Arabidopsis thaliana BolA2 and SufE1. Mol. Plant 7 187–205. 10.1093/mp/sst156 [DOI] [PubMed] [Google Scholar]
- Curie C., Briat J. F. (2003). Iron transport and signaling in plants. Annu. Rev. Plant Biol. 54 183–206. 10.1146/annurev.arplant.54.031902.135018 [DOI] [PubMed] [Google Scholar]
- Farnham M. W., Keinath A. P., Grusak M. A. (2011). Mineral concentration of Broccoli florets in relation to year of cultivar release. Crop Sci. 51 2721–2727. 10.2135/cropsci2010.09.0556 [DOI] [Google Scholar]
- Fernandes A. P., Fladvad M., Berndt C., Andresen C., Lillig C. H., Neubauer P., et al. (2005). A novel monothiol glutaredoxin (Grx4) from Escherichia coli can serve as a substrate for thioredoxin reductase. J. Biol. Chem. 280 24544–24552. 10.1074/jbc.M500678200 [DOI] [PubMed] [Google Scholar]
- Forner-Giner M. A., Llosa M. J., Carrasco J. L., Perez-Amador M. A., Navarro L., Ancillo G. (2010). Differential gene expression analysis provides new insights into the molecular basis of iron deficiency stress response in the citrus rootstock Poncirus trifoliata (L.) Raf. J. Exp. Bot. 61 483–490. 10.1093/jxb/erp328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grusak M. A., Welch R. M., Kochian L. V. (1990). Does iron deficiency in Pisum sativum enhance the activity of the root plasmalemma iron transport protein? Plant Physiol. 94 1353–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerinot M. L., Yi Y. (1994). Iron: nutritious, noxious, and not readily available. Plant Physiol. 104 815–820. 10.1104/pp.104.3.815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hell R., Stephan U. W. (2003). Iron uptake, trafficking and homeostasis in plants. Planta 216 541–551. [DOI] [PubMed] [Google Scholar]
- Herrero E., de la Torre-Ruiz M. A. (2007). Monothiol glutaredoxins: a common domain for multiple functions. Cell Mol. Life Sci. 64 1518–1530. 10.1007/s00018-007-6554-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindt M. N., Guerinot M. L. (2012). Getting a sense for signals: regulation of the plant iron deficiency response. Biochim. Biophys. Acta 1823 1521–1530. 10.1016/j.bbamcr.2012.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Wu Q., Sprague S. A., Park J., Oh M., Rajashekar C. B., et al. (2015). Tomato expressing Arabidopsis glutaredoxin gene AtGRXS17 confers tolerance to chilling stress via modulating cold responsive components. Hortic. Res. 2:15051 10.1038/hortres.2015.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inigo S., Durand A. N., Ritter A., Le Gall S., Termathe M., Klassen R., et al. (2016). Glutaredoxin GRXS17 associates with the cytosolic iron-sulfur cluster assembly pathway. Plant Physiol. 172 858–873. 10.1104/pp.16.00261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong J., Guerinot M. L. (2009). Homing in on iron homeostasis in plants. Trends Plant Sci. 14 280–285. 10.1016/j.tplants.2009.02.006 [DOI] [PubMed] [Google Scholar]
- Jin C. W., Du S. T., Shamsi I. H., Luo B. F., Lin X. Y. (2011). NO synthase-generated NO acts downstream of auxin in regulating Fe-deficiency-induced root branching that enhances Fe-deficiency tolerance in tomato plants. J. Exp. Bot. 62 3875–3884. 10.1093/jxb/err078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuesting J., Riondet C., Maria C., Kruse I., Becuwe N., Konig N., et al. (2015). Arabidopsis glutaredoxin S17 and its partner, the nuclear factor Y subunit C11/negative cofactor 2α, contribute to maintenance of the shoot apical meristem under long-day photoperiod. Plant Physiol. 167 1643–1658. 10.1104/pp.15.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T., Nishizawa N. K. (2012). Iron uptake, translocation, and regulation in higher plants. Annu. Rev. Plant Biol. 63 131–152. 10.1146/annurev-arplant-042811-105522 [DOI] [PubMed] [Google Scholar]
- Kumanovics A., Chen O. S., Li L., Bagley D., Adkins E. M., Lin H., et al. (2008). Identification of FRA1 and FRA2 as genes involved in regulating the yeast iron regulon in response to decreased mitochondrial iron-sulfur cluster synthesis. J. Biol. Chem. 283 10276–10286. 10.1074/jbc.M801160200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Camera S., L’Haridon F., Astier J., Zander M., Abou-Mansour E., Page G., et al. (2011). The glutaredoxin AtGRXS13 is required to facilitate Botrytis cinerea infection of Arabidopsis thaliana plants. Plant J. 68 507–519. 10.1111/j.1365-313X.2011.04706.x [DOI] [PubMed] [Google Scholar]
- Lan P., Li W., Wen T. N., Shiau J. Y., Wu Y. C., Lin W., et al. (2011). iTRAQ protein profile analysis of Arabidopsis roots reveals new aspects critical for iron homeostasis. Plant Physiol. 155 821–834. 10.1104/pp.110.169508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laporte D., Olate E., Salinas P., Salazar M., Jordana X., Holuigue L. (2012). Glutaredoxin GRXS13 plays a key role in protection against photooxidative stress in Arabidopsis. J. Exp. Bot. 63 503–515. 10.1093/jxb/err301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le C. T., Brumbarova T., Ivanov R., Stoof C., Weber E., Mohrbacher J., et al. (2016). Zinc Finger of Arabidopsis thaliana 12 (ZAT12) interacts with Fer-like iron deficiency-induced transcription factor (FIT) linking iron deficiency and oxidative stress responses. Plant Physiol. 170 540–557. 10.1104/pp.15.01589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire S. D. (2004). The glutaredoxin family in oxygenic photosynthetic organisms. Photosynth. Res. 79 305–318. 10.1023/B:PRES.0000017174.60951.74 [DOI] [PubMed] [Google Scholar]
- Li H., Mapolelo D. T., Dingra N. N., Keller G., Riggs-Gelasco P. J., Winge D. R., et al. (2011). Histidine 103 in Fra2 Is an iron-sulfur cluster ligand in the [2Fe-2S] Fra2-Grx3 complex and is required for in vivo iron signaling in yeast. J. Biol. Chem. 286 867–876. 10.1074/jbc.M110.184176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Outten C. E. (2012). Monothiol CGFS glutaredoxins and BolA-like proteins: [2Fe-2S] binding partners in iron homeostasis. Biochemistry 51 4377–4389. 10.1021/bi300393z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Cheng N., Hirschi K. D., Wang X. (2010). Structure of Arabidopsis chloroplastic monothiol glutaredoxin AtGRXcp. Acta Crystallogr. D Biol. Crystallogr. 66 725–732. 10.1107/S0907444910013119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillig C. H., Berndt C., Holmgren A. (2008). Glutaredoxin systems. Biochim. Biophys. Acta 1780 1304–1317. 10.1016/j.bbagen.2008.06.003 [DOI] [PubMed] [Google Scholar]
- Liu X., Liu S., Feng Y., Liu J. Z., Chen Y., Pham K., et al. (2013). Structural insights into the N-terminal GIY-YIG endonuclease activity of Arabidopsis glutaredoxin AtGRXS16 in chloroplasts. Proc. Natl. Acad. Sci. U.S.A. 110 9565–9570. 10.1073/pnas.1306899110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Millan A. F., Grusak M. A., Abadia A., Abadia J. (2013). Iron deficiency in plants: an insight from proteomic approaches. Front. Plant Sci. 4:254 10.3389/fpls.2013.00254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier A., Labbé S. (2009). Both Php4 function and subcellular localization are regulated by iron via a multistep mechanism involving the glutaredoxin Grx4 and the exportin Crm1. J. Biol. Chem. 284 20249–20262. 10.1074/jbc.M109.009563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer Y., Buchanan B. B., Vignols F., Reichheld J. P. (2009). Thioredoxins and glutaredoxins: unifying elements in redox biology. Annu. Rev. Genet. 43 335–367. 10.1146/annurev-genet-102108-134201 [DOI] [PubMed] [Google Scholar]
- Moseley J. L., Allinger T., Herzog S., Hoerth P., Wehinger E., Merchant S., et al. (2002). Adaptation to Fe-deficiency requires remodeling of the photosynthetic apparatus. EMBO J. 21 6709–6720. 10.1093/emboj/cdf666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlenhoff U., Molik S., Godoy J. R., Uzarska M. A., Richter N., Seubert A., et al. (2010). Cytosolic monothiol glutaredoxins function in intracellular iron sensing and trafficking via their bound iron-sulfur cluster. Cell Metab. 12 373–385. 10.1016/j.cmet.2010.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndamukong I., Abdallat A. A., Thurow C., Fode B., Zander M., Weigel R., et al. (2007). SA-inducible Arabidopsis glutaredoxin interacts with TGA factors and suppresses JA-responsive PDF1.2 transcription. Plant J. 50 128–139. 10.1111/j.1365-313X.2007.03039.x [DOI] [PubMed] [Google Scholar]
- Ojeda L., Keller G., Muhlenhoff U., Rutherford J. C., Lill R., Winge D. R. (2006). Role of glutaredoxin-3 and glutaredoxin-4 in the iron regulation of the Aft1 transcriptional activator in Saccharomyces cerevisiae. J. Biol. Chem. 281 17661–17669. 10.1074/jbc.M602165200 [DOI] [PubMed] [Google Scholar]
- O’Rourke J. A., Charlson D. V., Gonzalez D. O., Vodkin L. O., Graham M. A., Cianzio S. R., et al. (2007). Microarray analysis of iron deficiency chlorosis in near-isogenic soybean lines. BMC Genomics 8:476 10.1186/1471-2164-8-476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpott C. C. (2012). Coming into view: eukaryotic iron chaperones and intracellular iron delivery. J. Biol. Chem. 287 13518–13523. 10.1074/jbc.R111.326876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol-Carrion N., Belli G., Herrero E., Nogues A., de la Torre-Ruiz M. A. (2006). Glutaredoxins Grx3 and Grx4 regulate nuclear localisation of Aft1 and the oxidative stress response in Saccharomyces cerevisiae. J. Cell Sci. 119 4554–4564. 10.1242/jcs.03229 [DOI] [PubMed] [Google Scholar]
- Qin L., Wang M., Zuo J., Feng X., Liang X., Wu Z., et al. (2015). Cytosolic BolA plays a repressive role in the tolerance against excess iron and MV-induced oxidative stress in plants. PLoS ONE 10:e0124887 10.1371/journal.pone.0124887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez L., Bartoli C. G., Lamattina L. (2013). Glutathione and ascorbic acid protect Arabidopsis plants against detrimental effects of iron deficiency. J. Exp. Bot. 64 3169–3178. 10.1093/jxb/ert153 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Manzaneque M. T., Ros J., Cabiscol E., Sorribas A., Herrero E. (1999). Grx5 glutaredoxin plays a central role in protection against protein oxidative damage in Saccharomyces cerevisiae. Mol. Cell Biol. 19 8180–8190. 10.1128/MCB.19.12.8180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Manzaneque M. T., Tamarit J., Belli G., Ros J., Herrero E. (2002). Grx5 is a mitochondrial glutaredoxin required for the activity of iron/sulfur enzymes. Mol. Biol. Cell 13 1109–1121. 10.1091/mbc.01-10-0517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romera F. J., Garcia M. J., Alcantara E., Perez-Vicente R. (2011). Latest findings about the interplay of auxin, ethylene and nitric oxide in the regulation of Fe deficiency responses by strategy I plants. Plant Signal. Behav. 6 167–170. 10.4161/psb.6.1.14111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouhier N., Couturier J., Johnson M. K., Jacquot J. P. (2010). Glutaredoxins: roles in iron homeostasis. Trends Biochem. Sci. 35 43–52. 10.1016/j.tibs.2009.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Riego A. M., Lopez-Maury L., Florencio F. J. (2013). Glutaredoxins are essential for stress adaptation in the cyanobacterium Synechocystis sp. PCC 6803. Front. Plant Sci. 4:428 10.3389/fpls.2013.00428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt W., Tittel J., Schikora A. (2000). Role of hormones in the induction of iron deficiency responses in Arabidopsis roots. Plant Physiol. 122 1109–1118. 10.1104/pp.122.4.1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seguela M., Briat J. F., Vert G., Curie C. (2008). Cytokinins negatively regulate the root iron uptake machinery in Arabidopsis through a growth-dependent pathway. Plant J. 55 289–300. 10.1111/j.1365-313X.2008.03502.x [DOI] [PubMed] [Google Scholar]
- Stroher E., Millar A. H. (2012). The biological roles of glutaredoxins. Biochem. J. 446 333–348. 10.1042/BJ20112131 [DOI] [PubMed] [Google Scholar]
- Sundaram S., Rathinasabapathi B. (2010). Transgenic expression of fern Pteris vittata glutaredoxin PvGrx5 in Arabidopsis thaliana increases plant tolerance to high temperature stress and reduces oxidative damage to proteins. Planta 231 361–369. 10.1007/s00425-009-1055-7 [DOI] [PubMed] [Google Scholar]
- Tamayo E., Benabdellah K., Ferrol N. (2016). Characterization of three new glutaredoxin genes in the arbuscular mycorrhizal fungus Rhizophagus irregularis: putative role of RiGRX4 and RiGRX5 in iron homeostasis. PLoS ONE 11:e0149606 10.1371/journal.pone.0149606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimm O., Essigmann B., Kloska S., Altmann T., Buckhout T. J. (2001). Response of Arabidopsis to iron deficiency stress as revealed by microarray analysis. Plant Physiol. 127 1030–1043. 10.1104/pp.010191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urzica E. I., Casero D., Yamasaki H., Hsieh S. I., Adler L. N., Karpowicz S. J., et al. (2012). Systems and trans-system level analysis identifies conserved iron deficiency responses in the plant lineage. Plant Cell 24 3921–3948. 10.1105/tpc.112.102491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzarska M. A., Dutkiewicz R., Freibert S. A., Lill R., Muhlenhoff U. (2013). The mitochondrial Hsp70 chaperone Ssq1 facilitates Fe/S cluster transfer from Isu1 to Grx5 by complex formation. Mol. Biol. Cell 24 1830–1841. 10.1091/mbc.E12-09-0644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vachon P., Mercier A., Jbel M., Labbe S. (2012). The monothiol glutaredoxin Grx4 exerts an iron-dependent inhibitory effect on Php4 function. Eukaryot. Cell 11 806–819. 10.1128/EC.00060-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters B. M., Grusak M. A. (2008). Whole-plant mineral partitioning throughout the life cycle in Arabidopsis thaliana ecotypes Columbia, Landsberg erecta, Cape Verde Islands, and the mutant line ysl1ysl3. New Phytol. 177 389–405. [DOI] [PubMed] [Google Scholar]
- Werner T., Schmülling T. (2009). Cytokinin action in plant development. Curr. Opin. Plant Biol. 12 527–538. 10.1016/j.pbi.2009.07.002 [DOI] [PubMed] [Google Scholar]
- Wu Q., Lin J., Liu J.-Z., Wang X., Lim W., Oh M., et al. (2012). Ectopic expression of Arabidopsis glutaredoxin AtGRXS17 enhances thermotolerance in tomato. Plant Biotechnol. J. 10 945–955. 10.1111/j.1467-7652.2012.00723.x [DOI] [PubMed] [Google Scholar]
- Xia X.-J., Zhou Y.-H., Shi K., Zhou J., Foyer C. H., Yu J.-Q. (2015). Interplay between reactive oxygen species and hormones in the control of plant development and stress tolerance. J. Exp. Bot. 66 2839–2856. 10.1093/jxb/erv089 [DOI] [PubMed] [Google Scholar]
- Xing S., Rosso M. G., Zachgo S. (2005). ROXY1, a member of the plant glutaredoxin family, is required for petal development in Arabidopsis thaliana. Development 132 1555–1565. 10.1242/dev.01725 [DOI] [PubMed] [Google Scholar]
- Xu Y., Zhang S., Guo H., Wang S., Xu L., Li C., et al. (2014). OsABCB14 functions in auxin transport and iron homeostasis in rice (Oryza sativa L.). Plant J. 79 106–117. 10.1111/tpj.12544 [DOI] [PubMed] [Google Scholar]
- Zaffagnini M., Bedhomme M., Marchand C. H., Couturier J. R., Gao X. H., Rouhier N., et al. (2012). Glutaredoxin s12: unique properties for redox signaling. Antioxid. Redox Signal. 16 17–32. 10.1089/ars.2011.3933 [DOI] [PubMed] [Google Scholar]
- Zamboni A., Zanin L., Tomasi N., Pezzotti M., Pinton R., Varanini Z., et al. (2012). Genome-wide microarray analysis of tomato roots showed defined responses to iron deficiency. BMC Genomics 13:101 10.1186/1471-2164-13-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.