Abstract:
Patients receiving extracorporeal membrane oxygenation (ECMO) are at risk of circuit thrombosis due to constant contact between blood and the extracorporeal components. Unfractionated heparin has traditionally been used in this setting as a systemic form of anticoagulation to prevent thrombosis of the circuit. However, if a patient develops heparin-induced thrombocytopenia (HIT), an alternative anticoagulant would be required while the patient is maintained on ECMO. Unfortunately, the pharmacokinetic changes induced by ECMO and critical illness may potentially affect optimal drug dosing. In addition, other modalities, such as continuous renal replacement therapy, may further complicate dosing strategies. We report the case of a 27-year-old man with severe acute respiratory distress syndrome who developed HIT while on venovenous ECMO with continuous venovenous hemofiltration. We describe the successful use of an argatroban infusion in this setting at much higher doses than what has previously been reported in the adult literature.
Keywords: argatroban, extracorporeal circulation, extracorporeal membrane oxygenation, renal replacement therapy, thrombocytopenia
Heparin-induced thrombocytopenia (HIT) is an immune-mediated complication of unfractionated or low-molecular-weight heparin therapy. IgG antibodies are formed against the heparin–platelet factor 4 (PF4) complex, resulting in widespread platelet activation, thrombin generation, and release of prothrombotic substances. Patients are thrown into a paradoxical hypercoagulable state, placing them at an elevated risk of developing thromboembolic events (1).
Extracorporeal membrane oxygenation (ECMO) is a potentially life-saving modality for patients requiring advanced respiratory and/or cardiac circulatory support (2). While patients are receiving ECMO, blood comes into contact with the synthetic nonendothelial extracorporeal circuit, leading to altered coagulation. To prevent thrombosis of the circuit and other components, systemic anticoagulation with unfractionated heparin has traditionally been used (3). In patients who develop HIT while on ECMO, or have a documented history of HIT, an alternative form of anticoagulation may be required to maintain circuit patency and prevent thrombotic complications (4).
Argatroban is a parenteral direct thrombin inhibitor approved for the treatment of HIT (5). The use of argatroban in ECMO patients with HIT could theoretically serve a dual role: treatment of HIT and prevention of thrombus formation in the extracorporeal circuit. However, critically ill patients on ECMO often have altered pharmacokinetics that potentially impact optimal dosing and monitoring of argatroban. The addition of concomitant continuous renal replacement therapy (CRRT) may further affect dosing. Available literature on the use of argatroban in this setting is limited.
PATIENT DESCRIPTION
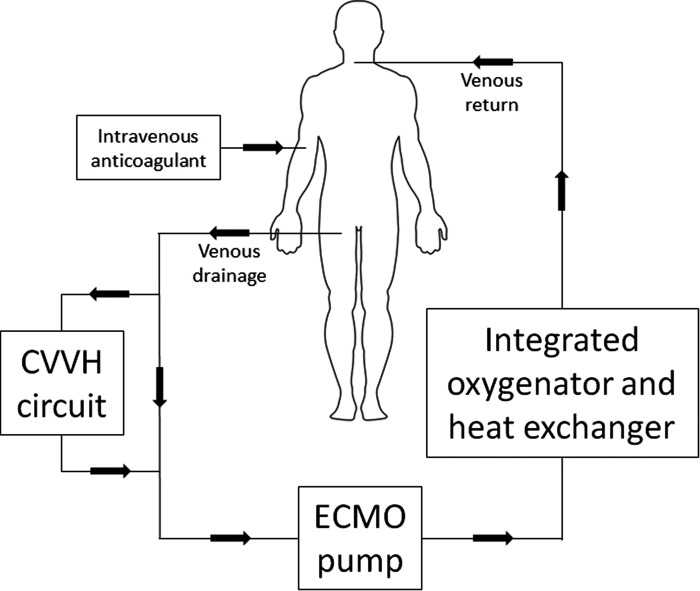
A 27-year-old man with severe acute respiratory distress syndrome was transferred to a tertiary-care academic medical center for initiation of ECMO. Cannulation for venovenous ECMO (VV-ECMO) was performed via the right internal jugular and right femoral veins (QUADROX-iD Adult Diffusion Membrane Oxygenator with BIOLINE Coating [Maquet Cardiopulmonary AG, Hirrlingen, Germany]; ROTAFLOW Console [Maquet Cardiopulmonary AG]; Carmeda BioActive Surface or Trillium BioSurface [Medtronic, Inc., Minneapolis, MN]). Upon cannulation, subcutaneous heparin prophylaxis was discontinued and a heparin drip was started to target an activated clotting time of 160–180 seconds. On day 5 of ECMO, continuous venovenous hemofiltration (CVVH) was initiated at an ultrafiltration rate of 3,200 mL/h due to anuria and worsening acid–base status. The CVVH circuit applied the CAR-505 cartridge with a polyethersulfone membrane with a 1.6-m2 surface area in conjunction with the NxStage System One Machine (NxStage Medical, Inc., Lawrence, MA) and was connected directly to the ECMO circuit prior to the pump (Figure 1). On day 6, microthrombi began forming on the pre-membrane side of the oxygenator. On day 9, platelets decreased from a baseline of 186 × 103/μL to 111 × 103/μL after 11 days of heparin exposure: 2 days at the outside hospital and 9 days at our institution. The heparin anti-platelet factor 4 (anti-PF4) enzyme-linked immunosorbent assay was sent, but returned negative with an optical density (OD) of .146. Despite transfusions, on day 12, platelets decreased to 38 × 103/μL and on day 14, the patient developed a large right-sided hemothorax with acute hemodynamic instability, prompting an immediate exploratory thoracotomy and evacuation. During the procedure, a large clot burden was also evacuated from the pleural space. The circuit was exchanged on day 15 due to an increased clot and fibrin burden on the pre-membrane side of the oxygenator. On day 18, a repeat anti-PF4 returned positive with an OD of 1.392. On day 19, a third anti-PF4 was also positive with an OD of 1.590 and a serotonin-release assay (SRA) was sent for confirmation of HIT.
Figure 1.
Diagram of the ECMO circuit with the incorporation of CVVH.
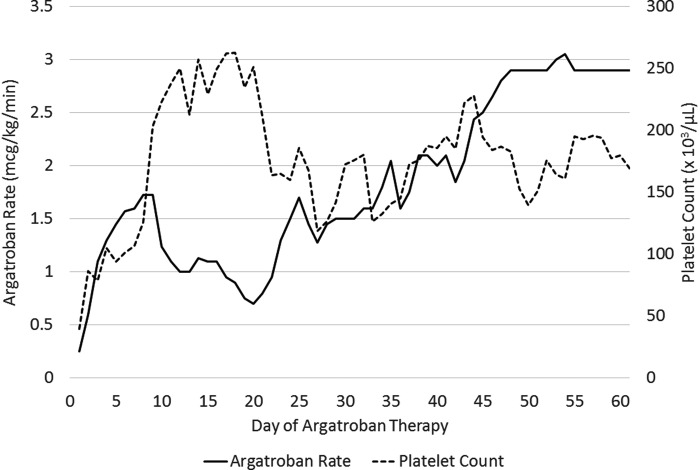
The heparin drip was discontinued and argatroban was initiated at .2 mcg/kg/min, using a dosing weight of 88.6 kg, to target an activated partial thromboplastin time (aPTT) of 50–60 seconds. The oxygenator was exchanged for a non-heparin-coated device (QUADROX-iD Adult Diffusion Membrane Oxygenator with SOFTLINE Coating) and all heparin-coated polyvinyl chloride tubing was switched out. On day 2 of argatroban therapy, the CVVH ultrafiltration rate was decreased to 1,600 mL/h due to an improving metabolic and electrolyte status. Platelets increased to 100 × 103/μL 3 days after starting argatroban. The SRA returned positive 4 days after starting argatroban, confirming the diagnosis of HIT. Platelets continued to recover and reached above 150 × 103/μL 7 days after starting argatroban (Figure 2). The aPTT goal was decreased to 40–45 seconds on days 2 to 12 of argatroban therapy based on bloody chest tube output and subsequently increased to 50–55 seconds on days 13 to 26. After the bleeding completely resolved, the aPTT was liberated to 50–70 seconds on day 27 of argatroban. After 31 days on argatroban, the ECMO circuit was exchanged and moderate fibrin formation was noted within the old circuit. A repeat anti-PF4 was sent after 37 days on argatroban, which was negative with an OD of .139. CVVH was discontinued on day 54 of argatroban as the patient was showing signs of renal recovery. After 60 days on argatroban, a total of 79 days of VV-ECMO support at our institution, the patient was transferred to another facility for a lung transplant evaluation.
Figure 2.
Dosage requirements and platelet counts during argatroban therapy.
The argatroban infusion rate frequently fluctuated throughout the treatment course (Figure 2). The mean (range) argatroban rate was 1.4 (.9–1.8) mcg/kg/min when the aPTT goal was 40–45 seconds, 1.2 (.7–1.8) mcg/kg/min when the aPTT goal was 50–55 seconds, and 2.1 (1.3–3.1) mcg/kg/min when the aPTT was liberated to 50–70 seconds. Due to escalating argatroban requirements, despite maintaining an aPTT goal of 50–70 seconds, a dilute thrombin time was sent on day 43 of argatroban therapy. The result was 73.2 seconds, which reflected an appropriate level of anticoagulation. In regard to hepatic function, liver function tests remained within normal limits the majority of the hospitalization. Transient elevations in liver markers were noted, but they quickly resolved without any major interventions. The patient did not develop a systemic thromboembolism while on argatroban therapy at our facility.
DISCUSSION
Herein, we have described the successful utilization of argatroban in a patient with confirmed HIT concomitantly receiving VV-ECMO and CVVH. The usual dosage for treatment of HIT ranges from 2 to 10 mcg/kg/min, titrated to an aPTT 1.5–3x above baseline. The majority of available literature suggests a much lower dosage requirement for adult patients on ECMO (6–12). The cases are briefly summarized in Table 1. Beiderlinden et al. reported an average dose of .15 mcg/kg/min achieved target aPTT values in this patient population. One patient who received a starting dose of 2 mcg/kg/min experienced excessive anticoagulation and severe bleeding (11). Contrary to previous literature, our patient required much higher infusion rates to maintain a target aPTT.
Table 1.
| Primary author | ECMO Modality | Reported Details of ECMO Circuit/Components during Argatroban Therapy | CRRT | Goal Coagulation Parameter | Initial Argatroban Rate | Therapeutic Argatroban Rate | Duration of Argatroban Therapy | Bleeding or Thrombotic Events during Argatroban Therapy |
|---|---|---|---|---|---|---|---|---|
| Ratzlaff (2016) | VV (n = 1) | Cannulas, circuit, and oxygenator with heparin-free SOFTLINE coating | No | aPTT: 60–90 seconds | .1–.3 mcg/kg/min | 1.5–1.7 mcg/kg/min | 11 days | No bleeding or thrombosis reported |
| Phillips (2014) | VV (n = 1) | Not reported | No | aPTT: 60 seconds | .1 mcg/kg/min | .65 mcg/kg/min | 7 days | No bleeding or thrombosis reported |
| ACT: 170–200 seconds | ||||||||
| Kuhl (2013) | VA (n = 1) | Not reported | Yes | Not reported | Not reported | Not reported | 5 days | • Hemorrhagic lung edema |
| • Left ventricular thrombus despite therapeutic aPTTs | ||||||||
| Dolch (2010) | VV (n = 1) | Bio-Medicus 540 Bio-Console (Medtronic, Inc.) | No | aPTT: 45–60 seconds | .35 mcg/kg/min | .25–1.63 mcg/kg/min | 95 days, then stopped for 4 days during peri-operative lung transplant period, then resumed for another 13 days since still required ECMO due to graft failure | • Mean of .44 units of PRBC transfused per day during the first 95 days |
| • Mean of .6 units of PRBC transfused per day during the last 13 days | ||||||||
| Jostra QUADROX D oxygenator (Maquet Cardiopulmonary AG) | .02 mcg/kg/min when resumed after lung transplant | .02 mcg/kg/min achieved goal aPTTs after lung transplant, likely due to liver failure | • No thrombosis reported | |||||
| Cornell (2007)* | VA (n = 1) | Servo-regulated roller-pump system and silicone membrane lung | No | ACT: 210–230 seconds | .2 mcg/kg/min | Max rate: .2 mcg/kg/min | 88 hours | • Acute intrathoracic bleed |
| • Required 99 mL/kg PRBC, 36 mL/kg platelets, and 7 mL/kg of FFP during entire ECMO run | ||||||||
| • No thrombosis reported | ||||||||
| VA (n = 1) | No | ACT: 210–230 seconds | 2 mcg/kg/min | Max rate: 2 mcg/kg/min | 163 hours | • Required 52 mL/kg PRBC, 29 mL/kg platelets, 3 mL/kg FFP, and 2 mL/kg cryoprecipitate during entire ECMO run | ||
| • No thrombosis reported | ||||||||
| VV (n = 1) | No | ACT: 210–230 seconds | .98 mcg/kg/min | Max rate: .98 mcg/kg/min | 6 hours | • Bleeding from cannula, catheter, oral, abdominal, and bladder sites (started before argatroban, but worsened during argatroban) | ||
| • Required 130 mL/kg PRBC, 114 mL/kg platelets, and 5 mL/kg FFP during entire ECMO run | ||||||||
| • No thrombosis reported | ||||||||
| Beider-linden (2007) | VV (n = 9) | Bio-Medicus Cannula (Medtronic GmbH, Dusseldorf, Germany) | Yes (n = 8) | aPTT: 50–60 seconds | 2 mcg/kg/min (n = 1) | Mean rate: .15 mcg/kg/min | Mean: 4 days | • Patient initiated on 2 mcg/kg/min developed severe bleeding requiring transfusions |
| Circuit with Super Tygon tubing (Medtronic GmbH) | .2 mcg/kg/min (n = 8) | • No bleeding reported in patients initiated on .2 mcg/kg/min | ||||||
| I-3500–2A oxygenator (Medtronic GmbH) | • No thromboses reported | |||||||
| CAPS roller pump (Stockert Instrumente GmbH, Munich, Germany) or | ||||||||
| Bio-Medicus centrifugal pump (Medtronic GmbH) | ||||||||
| Johnston (2002) | VA (n = 1) | Non-heparin-coated circuit and membrane | No | aPTT: 80–90 seconds | 10 mg bolus, then 2 mcg/kg/h† | 2 mcg/kg/h† | 5 days | • No bleeding or thrombosis reported |
| ACT: 200–400 seconds | • Received an unspecified number of blood products to maintain hematocrit and platelet counts |
ACT, activated clotting time; FFP, fresh frozen plasma; PRBC, packed red blood cells; VA, venoarterial.
Authors report the case of one pediatric patient, which was not included in this table.
Authors report the argatroban rate in mcg/kg/h.
ECMO can significantly alter pharmacokinetics of medications, leading to challenges in achieving optimal dosing. Circuits may sequester drug molecules to various extents and the amount of sequestration may depend on specific drug properties including ionization, lipophilicity, and protein binding. The large surface area found on ECMO circuits, tubing, and membranes can increase a drug's volume of distribution (Vd) considerably (13). As ECMO technology continues to develop, newer circuits, tubing, and components may cause various degrees of pharmacokinetic alterations. Therefore, if the technology differs, it may be difficult to compare current experiences to past published literature. Although we cannot definitively estimate the extent of our circuit's effect on argatroban pharmacokinetics, there is a possibility it may have led to some degree of alteration in our patient.
In addition to ECMO altering pharmacokinetic parameters, critically ill patients have numerous factors that may further complicate dosing strategies. Our patient received frequent transfusions, fluid boluses, and albumin administration due to tachycardia and chatter from the ECMO circuit, likely leading to fluid alterations and further altering the Vd. Despite argatroban only having moderate protein binding, serum albumin concentrations may still affect argatroban requirements. Hypoalbuminemia may lead to lower dosage requirements due to higher free drug concentrations (14). Conversely, our patient's albumin levels were consistently at the upper limit of normal or hyperalbuminemic at times, which may have led to higher argatroban requirements.
Argatroban is hepatically metabolized via hydroxylation and aromatization. Hepatic dysfunction and diminished liver perfusion may result in drug accumulation. Previous literature has reported decreased argatroban requirements in critical illness and/or multi-organ dysfunction (14–18). This was not the case for our patient, despite the presence of critical illness. Throughout the hospital course, the patient did not experience any major liver injury or sustained cardiac disturbances except for tachycardic episodes. Robust baseline hepatic function and increased hepatic perfusion from an augmented cardiac output may have caused an intrinsic increase in argatroban metabolism, although this could not be objectively proven. Numerous metabolic and hormonal changes are expected in the critically ill patient (19,20), but whether or not augmented hepatic clearance actually occurs in the setting of critical illness and ECMO remains unclear and more pharmacokinetic studies are needed.
Argatroban does not require a dosage adjustment in patients with renal dysfunction or in patients undergoing renal replacement therapy (5). However, up to 22% of unchanged drug is renally eliminated, so one can make the argument that there is a potential effect of renal dysfunction on dosing (21). The available literature on the effect of CRRT on argatroban is controversial and conflicting (14,22–24). When our patient's CVVH ultrafiltration rate was decreased to 1,600 mL/h, the argatroban requirements did not decrease along with it. In addition, when CVVH was finally discontinued, there was no adjustment in the infusion rate needed. Our patient's overall infusion rates were higher than expected, but CVVH as the culprit for increased argatroban requirements is less likely. Additional studies evaluating argatroban removal during CRRT are needed before a definitive conclusion can be formed.
This report presents a case of successful use of argatroban as an anticoagulant during concomitant VV-ECMO and CVVH in a critically ill adult patient with confirmed HIT. The patient did not develop a systemic thromboembolism or a serious adverse bleeding event while on the argatroban infusion at our medical center. Contrary to previous published reports of adult ECMO patients on argatroban, our patient had higher dosage requirements to maintain a target aPTT. The alterations induced by VV-ECMO, CVVH, and/or critical illness may have affected the drug's distribution, metabolism, and clearance. Additional studies are needed to further assess pharmacokinetic alterations and optimal argatroban dosing in this setting.
REFERENCES
- 1. Greinacher A. Clinical practice. Heparin-induced thrombocytopenia. N Engl J Med. 2015;373:252–61. [DOI] [PubMed] [Google Scholar]
- 2. Brodie D., Bacchetta M.. Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med. 2011;365:1905–14. [DOI] [PubMed] [Google Scholar]
- 3. Extracorporeal Life Support Organization ELSO anticoagulation guideline. 2014. Available at: http://www.elso.org/Portals/0/Files/elsoanticoagulationguideline8-2014-table-contents.pdf.
- 4. Welp H., Ellger B., Scherer M., Lanckohr C., Martens S., Gottschalk A.. Heparin-induced thrombocytopenia during extracorporeal membrane oxygenation. J Cardiothorac Vasc Anesth. 2014;28:342–4. [DOI] [PubMed] [Google Scholar]
- 5. Argatroban [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2016. [Google Scholar]
- 6. Ratzlaff R. A., Ripoll J. G., Kassab L. L., Diaz-Gomez J. L.. Acute oxygenator failure: A new presentation of heparin-induced thrombocytopenia in a patient undergoing venovenous extracorporeal membrane oxygenation support. BMJ Case Rep. 2016. doi:10.1136/bcr-2016-218179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Phillips M. R., Khoury A. I., Ashton R. F., Cairns B. A., Charles A. G.. The dosing and monitoring of argatroban for heparin-induced thrombocytopenia during extracorporeal membrane oxygenation: A word of caution. Anaesth Intensive Care. 2014;42:97–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kuhl T., Wendt S., Langebartels G., Kroner A., Wahlers T.. Recurrent left atrial and left ventricular thrombosis due to heparin-induced thrombocytopenia: Case report and short review. Thorac Cardiovasc Surg. 2013;61:537–40. [DOI] [PubMed] [Google Scholar]
- 9. Dolch M. E., Frey L., Hatz R., et al. . Extracorporeal membrane oxygenation bridging to lung to transplant complicated by heparin-induced thrombocytopenia. Exp Clin Transplant. 2010;8:329–32. [PubMed] [Google Scholar]
- 10. Cornell T., Wyrick P., Fleming G., et al. . A case series describing the use of argatroban in patients on extracorporeal circulation. ASAIO J. 2007;53:460–3. [DOI] [PubMed] [Google Scholar]
- 11. Beiderlinden M., Treschan T., Gorlinger K., Peters J.. Argatroban in extracorporeal membrane oxygenation. Artif Organs. 2007;31:461–5. [DOI] [PubMed] [Google Scholar]
- 12. Johnston N., Wait M., Huber L.. Argatroban in adult extracorporeal membrane oxygenation. J Extra Corpor Technol. 2002;34:281–4. [PubMed] [Google Scholar]
- 13. Shekar K., Fraser J. F., Smith M. T., Roberts J. A.. Pharmacokinetic changes in patients receiving extracorporeal membrane oxygenation. J Crit Care. 2012;27:741. [DOI] [PubMed] [Google Scholar]
- 14. Keegan S. P., Gallagher E. M., Ernst N. E., Young E. J., Mueller E. W.. Effects of critical illness and organ failure on therapeutic argatroban dosage requirements in patients with suspected or confirmed heparin-induced thrombocytopenia. Ann Pharmacother. 2009;43:19–27. [DOI] [PubMed] [Google Scholar]
- 15. Saugel B., Phillip V., Moessmer G., Schmid R. M., Huber W.. Argatroban therapy for heparin-induced thrombocytopenia in ICU patients with multiple organ dysfunction syndrome: A retrospective study. Crit Care. 2010;14:R90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smythe M. A., Koerber J. M., Forsyth L. L., Priziola J. L., Balasubramaniam M., Mattson J. C.. Argatroban dosage requirements and outcomes in intensive care versus non-intensive care patients. Pharmacotherapy. 2009;29:1073–81. [DOI] [PubMed] [Google Scholar]
- 17. Begelman S. M., Baghdasarin S. B., Singh I. M., Militello M. A., Hursting M. J., Bartholomew J. R.. Argatroban anticoagulation in intensive care patients: Effects of heart failure and multiple organ system failure. J Intensive Care Med. 2008;23:313–20. [DOI] [PubMed] [Google Scholar]
- 18. Beiderlinden M., Treschan T. A., Gorlinger K., Peters J.. Argatroban anticoagulation in critically ill patients. Ann Pharmacother. 2007;41:749–54. [DOI] [PubMed] [Google Scholar]
- 19. Smith B. S., Yogaratnam D., Levasseur-Franklin K. E., Forni A., Fong J.. Introduction to drug pharmacokinetics in the critically ill patient. Chest. 2012;141:1327–36. [DOI] [PubMed] [Google Scholar]
- 20. Boucher B. A., Wood G. C., Swanson J. M.. Pharmacokinetic changes in critical illness. Crit Care Clin. 2006;22:255–71. [DOI] [PubMed] [Google Scholar]
- 21. Abel E. E., Kane-Gill S. L., Seybert A. L., Kellum J. A.. Direct thrombin inhibitors for management of heparin-induced thrombocytopenia in patients receiving renal replacement therapy: Comparison of clinical outcomes. Am J Health Syst Pharm. 2012;69:1559–67. [DOI] [PubMed] [Google Scholar]
- 22. Hursting M. J., Murray P. T.. Argatroban anticoagulation in renal dysfunction: A literature analysis. Nephron Clin Pract. 2008;109:c80–94. [DOI] [PubMed] [Google Scholar]
- 23. Reddy B. V., Grossman E. J., Trevino S. A., Hursting M. J., Murray P. T.. Argatroban anticoagulation in patients with heparin-induced thrombocytopenia requiring renal replacement therapy. Ann Pharmacother. 2005;39:1601–5. [DOI] [PubMed] [Google Scholar]
- 24. Tang I. Y., Cox D. S., Patel K., et al. . Argatroban and renal replacement therapy in patients with heparin-induced thrombocytopenia. Ann Pharmacother. 2005;39:231–6. [DOI] [PubMed] [Google Scholar]