Abstract:
This literature review summarizes recommendations and challenges encountered when establishing cardiac surgery centers in developing countries and common heart diseases encountered abroad. Cardiac surgery is not widely available in most developing countries, and most patients have no choice but to live in morbid conditions. The ideal continuous treatment for these patients would be provided by a local, sustainable cardiac surgery center. A collaborative effort from international volunteers, nongovernmental organizations, local governments, and private benefactors is necessary to facilitate adequate cardiac care in developing countries.
Keywords: cardiac surgery, developing nations, medical volunteerism, cardiovascular disease
Disease prevention and surveillance is challenging in developing countries, given their reduced literacy rates, sanitation, and governance. According to the World Bank, a developing country is defined as having a Gross National Income per capita per year of $11,905 USD or less (1). The World Health Organization (WHO) reported that nearly 44% of countries have less than one physician per 1,000 people, emphasizing the global lack of access to health care. The WHO reports, “The African Region suffers more than 24% of the global burden of disease but has access to only 3% of health workers and less than 1% of the world's financial resources (2).” Timely treatment of cardiovascular diseases is particularly arduous, given the scanty health care, financial, and human resources available. As such, cardiac surgery is not commonly available in most developing countries, and many patients have no choice but to live in morbid conditions (3,4). Where cardiac programs do exist abroad, low caseloads are common. It is typical for developing nations' hospitals to run collaborative mission programs sponsored by international or domestic nongovernmental organizations (NGOs) (3).
Several options exist for patients in need of cardiovascular surgery abroad. These options include sending patients to foreign countries for surgery, foreign surgeons visiting and operating locally on a short-term basis, or developing a local, permanent cardiac center. Sending patients abroad for surgery is the least preferred method as its high cost benefits only a small percentage of wealthy patients. Visiting surgeons, although a better alternative, only aid a small number of patients for a short time. Visiting surgeons also face challenges associated with poor operating room (OR) conditions and lack of trained personnel, which have the potential to negatively effect surgical results. Therefore, the ideal option is to develop local cardiac surgery institutions to provide continuous treatment for patients in developing countries (5–8).
This paper reviews common practices and recommendations for establishing sustainable cardiac surgery centers in developing countries, a brief overview of existing cardiac programs serving developing nations, challenges encountered in inaugurating said programs, and prevalent heart diseases effecting patients in developing nations.
METHODS
A literature search of the PubMed database was performed using the following search terms: “open heart surgery,” “cardiothoracic (CT) surgery,” “developing country,” “cardiovascular,” “international,” “bypass,” “perfusion,” “health-care resources,” and “obesity.” A total of 2305,698 articles were narrowed down to 635,241 articles by selecting only free, full-text, peer-reviewed, human species articles published between 2005 and 2015. Articles were sorted by most relevant, and the first 400 article abstracts were reviewed for objectives pertinent to the literature review topic for final inclusion selection.
ESTABLISHING CARDIOVASCULAR SURGICAL CENTERS ABROAD
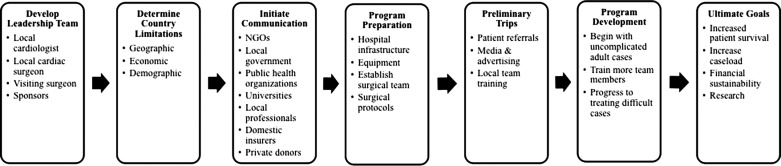
Each developing nation presents unique conditions that should be addressed before setting up a cardiac surgical center. These conditions may include geographic, economic, and demographic limitations, some of which are summarized in Table 1. Starting a new program abroad, particularly when it involves a highly specialized service like heart surgery, requires extensive preparation and financial commitment (4,9). Although there is no specific protocol for success when instigating a cardiovascular program in a developing country, the following recommendations should be considered. Figure 1 provides an overview of this process.
Table 1.
Challenges in developing cardiac centers abroad.
| Medical personnel/training | • Lack of expertise in diagnosis and treatment and the country's healthcare system as a whole (2,4,18) |
| • Training local surgeons on unfamiliar heart diseases restricted to a particular geographic region (2,4) | |
| • Local surgical team members lacked incentive and work ethic due to low salaries (18) | |
| • Poor retention of highly trained medical personnel (10) | |
| • Noncompliance with aseptic technique among the native healthcare team members (18) | |
| Equipment | • Lack of reliable medical records system (18) |
| • Low quality equipment and maintenance (2,18) | |
| Hospital infrastructure | • Lack of hospital infrastructure and capacity (2,18) |
| • Lack of research facilities (2,18) | |
| Communication | • Language barriers between the international and local team (11) |
| • Lack of cooperation between domestic and international donors (4) | |
| Patients | • Poor health and literacy of patients resulting in them not abiding by a physician's instructions or understanding operative risks (18) |
| • Poor patient follow-up (2,9,19) | |
| Miscellaneous | • Poor local governance and lack of commitment to cardiac care from politicians (2,18) |
| • Problems with growth and expansion of the program due to the region's isolated location (18) | |
| • Poor financial sustainability (2,4,18) | |
| • Late surgical interventions with poor prognosis due to late diagnosis (2,9,19) | |
| • Time delay between diagnosis and surgery (2,9,19) |
Figure 1.
Conceptual model for developing a sustainable cardiac program abroad.
Initial Organization
Establishing heart surgery centers abroad can be initially difficult due to competition between the few available providers in the region for scarce resources. Therefore, form a leadership team consisting of a local cardiologist and cardiac surgeon, a visiting cardiac surgeon (preferably with prior experience volunteering abroad), and the sponsors. The visiting surgeon should mentor the team and oversee the program's progress by visiting the program periodically. Focusing the leadership team around the patrons and native cardiologists allows coordination of patient referrals and resources to ensure long-term sustainability as the program matures (5). It is helpful if the local government can include their public health organizations and aid in the secure transportation of equipment (4). Preliminary communication should also be made with local professionals, universities, and insurers (if applicable), as these entities are true enablers of success in developing nations (10).
The initial program preparation includes upgrading the physical infrastructure including the OR and intensive care unit (ICU), acquiring required medical equipment, training local health-care personnel, and recruiting patients (4). To help eliminate mistakes, protocols for all aspects of care should be written to ensure a standardized procedure prior to the first mission. Protocols should be made available to the whole health-care team and translated for native team members. Any clinical mistakes that occur should be thoroughly discussed to avoid recurrence, and monthly morbidity and mortality meetings should take place as the program develops. Because many developing countries lack standardized patient databases or charting systems, tracking patients in a Microsoft Excel (Microsoft Corp., Redmond, WA) document is recommended (9).
Brief trips abroad by NGOs and the lead cardiac surgeon prior to the mission can help determine the local need, establish direct contact with local professionals, and act as preliminary diagnostic visits. These trips are also beneficial in determining the existing state of possible hospital facilities, major regional cardiomyopathies, and can provide training opportunities for local health-care personnel (10).
Hospital Infrastructure
The cardiac surgical center should be modeled using examples from existing facilities available in developed countries, yet adapted to fit the needs of local culture (10). The facilities and required financial support will differ depending on the location of the center. Stand-alone facilities, upgrades to existing heart programs, or the development of an entirely new department in a general hospital all may require varied levels of financial support (9). Depending on local conditions, initial areas of focus may include the OR, ICU for pediatrics and adults (7), admission facilities, nurse's station, pharmacist's station, equipment storage, and hospital management and administration offices (4). The OR should have a powerful emergency electrical generator given the high-risk nature and electrical requirements of cardiac surgery. In addition, gas lines should be routed into the OR and ICU. The OR space must be sufficient to accommodate the surgical team, heart–lung machine (HLM), and anesthesia, so a space of at least 8 × 10 m with large entrances for the HLM is suggested (9). The ICU should have ample room for ventilators, intraaortic balloon pumps, and monitoring equipment (4). The ICU should be located next to the OR to minimize transfer times and avoid contact from visitors who are always present in developing countries, a cultural difference from the West (9).
Equipment
State of the art equipment assists in the delivery of optimal care and should be used whenever available (10). Required equipment includes at least one HLM, proper tubing and cannulas for the HLM, cardioplegia and priming solutions, cardiac monitors, multiple ventilators, infusion pumps, pneumatic sternal saws, synthetic graft patches, prosthetic replacement valves, suture materials, chest drainage catheters, drugs, and standard surgical instrument trays. Despite fiscal limitations, all efforts should be made to procure a HLM with at least three roller heads and room for add-ons. Equipment is typically funded initially by domestic or international NGOs. Purchasing supplies in bulk, although cost effective, can be difficult to clear through customs, safely store, and license. However, developing countries may face delays in ordering and transporting supplies on an as-needed basis (9). Developing nations struggle with cardiovascular drug availability due to political controversy, insufficient human resources, and/or inadequate funding. Furthermore, many developing countries lack effective health insurance systems that cover the cost of the population's drugs, thus rendering them unaffordable to patients (11). The minimum support services required by a cardiac center are 24-hour laboratory facilities, a blood bank providing fresh frozen plasma, and sterilization facilities (4,9).
Team
Surgical team compositions may differ as local team experience differs. Most commonly, a visiting surgical team consists of one CT surgeon, three surgical assistants, one anesthetist, one perfusionist, two OR nurses, and two ICU nurses (4,9). As the program progresses, international team members may be added, including cardiologists, an anesthesiologist, a second perfusionist, and a pediatrician (5). Physician assistants, nurse assistants, and medical students should be considered once the program has started gaining local independence (9). Bioengineers are also recommended, as the equipment will require proper maintenance over time (8). In hopes of developing relationships with potential donors and increasing public awareness, an individual in public relations should join the team and serve as an enthusiastic advocate for the program (5). The international health-care team should be composed of professionals who have experience volunteering abroad as well as novice volunteers (8). International health-care team organization requires 3 months of planning prior to the mission. All international health-care team volunteers should have appropriate licensure, diplomas, references, and familiarity to educate domestic team members (12). More information about volunteering abroad can be found in Table 2.
Table 2.
Cardiac surgery programs serving developing countries.
| Organization | Countries Served | Website | Contact |
|---|---|---|---|
| CardioStart International | Nepal, Dominican Republic, India, Myanmar, Brazil, Peru, Ghana, Uganda, Vietnam | www.cardiostart.org | Janine Henson: info@cardiostart.org |
| Caribbean Heart Menders | Jamaica, Dominican Republic, Costa Rica, Panama | www.caribbeanheartmenders.org | Barbara Davis-Sears: info@caribbeanheartmenders.com |
| Chain of Hope | Mozambique, Uganda, Egypt, Jamaica, Ethiopia, El Salvador, Panama, Haiti, Trinidad, Vietnam, Mauritius, Zimbabwe, Malawi, Kenya, Burundi, Yemen, Sudan, Cameroon, Gambia, Senegal, Morocco, Kosovo, Iraq, Lebanon, Palestinian Territories, Eritrea, Somalia | www.chainofhope.org | Lucy Ossack: lucy@chainofhope.org |
| Children's Heartlink | Brazil, China, Ecuador, India, Malaysia, Ukraine, Vietnam | www.childrensheartlink.org | Bistra Zheleva: bistra@childrensheartlink.org |
| Earthmed | Mongolia | www.earthmed.org | Lou Schonder: lschonder@earthmed.org |
| Global Heart Network | Nigeria, India, Lebanon, Senegal | www.globalheartnetwork.net | Annabel Lavielle: info@globalheartnetwork.net |
| Heart Care International | Mexico, Peru, El Salvador, Guatemala, Dominican Republic | www.heartcareintl.org | Nick Mellas: heartcarei@aol.com |
| Heart to Heart | Russia | www.heart-2-heart.org | Josie Everett: contact@heart-2-heart.org |
| Heart to Heart Mission | Dominican Republic | www.heartmission.com | Bryan Lich: bvlich@perfusion.com |
| Hearts for Haiti | Haiti, Dominican Republic | www.openheartshaiti.org | Jason Chmel: jchmel@memorialcare.org |
| Hearts with Hope | Peru | www.heartswithhope.org | Chris Pierce: cpierce@heartswithhope.org |
| International Children's Heart Foundation | Ethiopia, Egypt, Libya, Nigeria, Sudan, Morocco, Iraq, Palestine, Kuwait, Paraguay, Colombia, Guyana, Ecuador, Peru, Brazil, Bolivia, Kazakhstan, China, India, Pakistan, Uzbekistan, Dominican Republic, Jamaica, Nicaragua, Haiti, Honduras, Serbia, Russia, Ukraine, Belarus, Bosnia, Macedonia, Croatia | www.babyheart.org | info@babyheart.org |
| London Cardiac Outreach Team | Peru, China, Mongolia | N/A | Peter Allen: peter.allen@lhsc.on.ca |
| Open Heart International | Cambodia, Fiji, Myanmar, Papua New Guinea, Rwanda, Tanzania, Tonga, Solomon Islands, Philippines, Vietnam, Mongolia, China, Vanuatu | www.ohi.org.au | Hayden Dando: hayden@ausbm.net |
| Palestine Children's Relief Fund | Syria, Palestinian Territories, Iraq | www.pcrf.net | Steve Scosbee: pcrf1@pcrf.net |
| Save a Child's Heart | Israel | www.saveachildsheart.org | info@saveachildsheartus.org |
| Team Heart | Rwanda | www.teamheart.org | Cecilia Patton Bolman: teamheartinfo@gmail.com |
| VOOM Foundation | Nigeria | www.voomfoundation.org | Dr Vincent Ohaju: Vincent.Ohaju@essentiahealth.org |
| The William Novick Global Cardiac Alliance | 32 countries total including Iraq, China, Libya, Macedonia, Pakistan, Russia, Ukraine, Iran, Honduras, Ecuador, Nigeria | www.cardiac-alliance.org | www.cardiac-alliance.org/volunteer |
Visit AmSECT's Perfusion Without Borders webpage for more perfusion-specific resources: http://amsect.societyhq.com/perfwob.iphtml.
Local surgeons should be trained one-on-one by a visiting surgeon to ensure apt expertise is achieved. As a program cultivates, the quantity of surgeons should increase per the caseload. It is recommended that one experienced surgeon and two native training surgeons perform up to 150 cases per year, two experienced surgeons and four native training surgeons perform 150–240 cases per year, and three experienced surgeons and five native training surgeons perform 300–400 cases per year. Additional native surgeons should be trained as staff turnover is inevitable and long-term sustainability is the objective (9).
Local Training
Domestic health-care team members should be trained prior to the first mission trip with follow-up sessions occurring during each mission thereafter. Didactic lectures and hands-on clinical experience should be organized and conducted by experienced visiting professionals (4,8). Continuing education classes should be performed periodically to assess local staff skills (4,10). The local government can also aid in registration and licensure for resident medical personnel once training has been completed (4).
To reduce postoperative (post-op) complications, three key points should be stressed when training domestic teams: using a surgical safety checklist, effective communication between the surgical team despite educational and cultural barriers between team members, and using sterile technique and hand hygiene practices to reduce surgical site infections (13). As the domestic surgical team members begin to match the skills of their international counterparts, full responsibilities should be transferred over to them. With time, the international team should steadily decrease their presence giving the local team independence (8,12).
Patient Referrals
Some surgical teams may encounter complications with patient referrals if faced with resistance from local cardiologists. Payments to domestic cardiologists or general practitioners might be vital to ensure early program growth. Thereby, it is helpful for surgical centers to have their own diagnostic equipment and support from native cardiologists. A new surgical center cannot build credibility and a solid reputation within a community without a steady referral system. Affordability and accessibility are two main factors for acceptance by communities in developing nations (9).
Patient Selection
Regardless of the surgical team's experience, it is recommended to begin operating on less morbid, simple patient cases when initiating a program. Adult cases with physically fit individuals lacking comorbidities are best. Pediatric cases should be considered once pediatric HLM requirements are met (4,5,9). Gradually increase surgeries in volume and complexity as the team gains experience.
Early referral and diagnosis in patients of all ages should become common practice over time (6). Many patients in developing countries are diagnosed so late, that poor results are inevitable (9). A local cardiologist and the visiting CT surgeon should conduct patient screenings. A thorough clinical history, physical examination, transthoracic echocardiogram, and blood tests should be carefully assessed before surgery (4). Surgeons should be honest in their assessment and communicate those odds transparently with the patient and family. Specifically, the surgeon should discuss all therapeutic options, acute and chronic outcomes, and the responsibilities and expectations of the patient and family after surgery as regular follow-up visits are necessary. Patient education and compliance is of extreme importance in developing countries (9).
Although there is controversy regarding the value and indications for open heart surgery in the elderly, there is overwhelming evidence suggesting that surgical intervention provides consistent, positive outcomes that improve their quality of life (QOL) (14). Age, although an important factor in post-op morbidity and mortality, is still a questionable criterion for cardiac surgery. Recent evidence suggests age should not be a sole exclusion factor for determining a patient's condition for cardiac surgery. Given the strong outcomes of the elderly undergoing cardiac surgery, the health-care resources allotted to these patients are justified in developing nations (14). Above all, surgical cases should be selected and performed based on what intervention will provide the best outcome for the patient (5).
Postsurgical Complications
Post-op complications unique to the developing world include patients with poor general health, nutritional deficiencies, and endemic infectious comorbidities, which all increase surgical risk (3). It is crucial for family members to understand and acknowledge the post-op home care for their loved one undergoing surgery to prevent complications and poor outcomes. It is critical for adequate discharge counseling given the poor literacy rates and possible language barriers (16).
Research
The current lack of cardiovascular research in developing countries is due to little local expertise and poor funding (11). An important component of a sustainable cardiac center in a developing country is its ability to conduct research. Research will ensure appropriate clinical outcomes, increase the data available on neglected diseases, and revolutionize diagnosis and treatment of local populations (15). It is recommended for developing countries to use a developed country as a research partner when initiating research management and facilities. Research aimed at understanding underlying pathological mechanisms based on geography, toxins, cultural practices, and country-specific conditions should be investigated further (15). Research in developing countries can also benefit other areas of health care, including epidemiology (10,15), increase public awareness, and influence political decisions.
Sustainability
As of 2011, the estimated cost of open heart surgery in developing countries was between $6,230 and $11,200 USD depending on the procedure, which is much less than seeking surgical care overseas ($20,000–$40,000 USD per traveler). Procedure expenses could be further reduced by ∼$1,000 USD if consumables and cardiac prostheses costs were reduced (15). Lack of local cardiac centers contributes to millions of dollars lost annually to international cardiac programs. Therefore, initiating local cardiac programs will ultimately benefit patients, benefactors, and the country's economy (15). Because most developing countries lack an efficient health insurance system, affordability is one of the most critical aspects of local cardiac centers. Therefore, the patient's bill must reflect an amount for the center to break even. Patients of lower socioeconomic status might need sponsorship by the NGO or private donor (9).
Initial contributions from NGOs, local government, private investors, and insurance companies are necessary for startup expenses. As a cardiac center's success expands over time, the fiscal responsibilities should shift to the local contributors entirely. Surgical centers will benefit from staff retention as well, so good working conditions, compensation, and training opportunities are of significant importance to the center's success (4,10). From a financial standpoint, the survival of a cardiac center depends on fulfilling the needs of the community by delivering quality care and publicizing its success. The program should be advertised in the media, press, and online to increase public awareness. Financial analysis and cost containment procedures should be periodically assessed (9).
Measurement of Success
The primary goal of the surgical center should be to provide each patient with the best possible care to give them the best chance of survival (5). The increase in number of treated patients, decrease in mortality rates, and increase in trained personnel over time can attest to the success of a surgical center in a developing country (6).
A secondary, long-term goal is that of program development and sustainability. A program should strive to have domestic NGO funding increase over time and have the program evaluated and approved by domestic health insurance programs to ensure financial sustainability. Each country's unique circumstances will dictate the amount of time required to ensure the program's success and definite sustainability (5). Additionally, developing pediatric specialty services may require longer commitment, often exceeding 5 years before a surgical center reaches its independence (12).
COMMON HEART DISEASES IN DEVELOPING COUNTRIES
Congenital Heart Disease
Congenital heart disease (CHD) is caused by genetic alterations, consanguinity, viral infections, fetal exposure to drugs and radiation, and other environmental factors. Family members with siblings or parents with CHD are twice as likely to have a child with CHD (18). Although the diagnosis and treatment of CHD has made significant progress over the last few decades, this progress has not extended into the developing world (19). About 133 million babies are born worldwide every year, and about 1 million of those neonates will suffer from CHD. Nine out of 10 newborns with CHD will live in a developing country where cardiac care is subpar or unavailable entirely (18,19). Seventy percent of those infants in a developing nation will require medical or surgical intervention within their first year of life, and 30% of those children will die without treatment in that first year (18). Infants with a patent ductus arteriosus have a 30% risk of mortality before reaching one year of age and a 42% chance of death before age 45. Those with a large atrial septal defect have a 5–15% risk of mortality before age 30. Twenty-five percent of babies with tetralogy of Fallot will die within the first year of life, and 40%, 70%, and 95% will die by ages 3, 10, and 40, respectively. Infants with transposition of the great arteries have a mortality risk of 45%, 85%, and 90% by 1 month, 6 months, and 1 year of age, respectively. Neonates with hypoplastic left heart syndrome will die within 1–2 weeks after birth. Although these mortality rates are significant alone, they do not reflect the surviving children and adults with CHD in all corners of the world that endure a decreased QOL as they suffer from bacterial endocarditis, polycythemia, cyanosis, coagulopathy, arrhythmias, secondary pulmonary vascular disease, congestive heart failure (CHF), and neurological complications (3,18,19). Unfortunately, adults with CHD often have accelerated forms of pulmonary hypertension leading to irreversible pulmonary changes, myocardial fibrosis, and other significant heart malformations (3,10,20).
Rheumatic Heart Valve Disease
Although an increase in education and access to antibiotic treatment has reduced rheumatic heart valve disease (RHVD) incidence, as of 2007, an estimated 15 million people still suffer from RHVD worldwide (10), with 1.4 million people dying annually (21). RHVD has the highest prevalence (15–20 per 1,000 people) in sub-Saharan Africa (11). RHVD occurs in 30–45% of patients with rheumatic fever (22), and is a preventable disease altogether with rheumatic fever prophylaxis (3). Rheumatic fever is more common in subtropical climates with crowded populations, low socioeconomic populations, and a high prevalence of streptococcal infections. RHVD elicits an autoimmune response triggered by β-hemolytic group A streptococci, which induces pericardial, myocardial, and endocardial inflammation. This inflammation leads to heart valve damage and CHF requiring risky, palliative heart valve surgery (3,11,20,21) or heart transplantation (22). Further deterioration of heart function post-op is common (22). RHVD is most prevalent in youths, particularly in females of childbearing age, and requires surgical intervention early in life (3,11,20,21).
The little research conducted on RHVD in the developing world suggests the most frequent lesion is mitral valve regurgitation, but is often multivalvular (3,11,21,22). The second most common valve involved is the aortic valve. Tricuspid regurgitation (3), infective endocarditis, CHF (20,22), pulmonary hypertension (3,21,22), atrial fibrillation (22), right ventricle dilation (3,22), and left ventricle dysfunction (21) are also common due to late diagnosis. In addition, RHVD is one of the major nonobstetric causes of maternal death in developing countries (21,22). RHVD in the developed world, in contrast, is seen in middle-aged adults in the form of thickened valve leaflets leading to mitral valve stenosis with or without regurgitation (3).
Much of RHVD morbidity and mortality can be prevented by existing therapies. These include surgical intervention, the lifelong use of penicillin for secondary prophylaxis to rheumatic fever and endocarditis, use and monitoring of oral anticoagulation therapy to prevent thromboembolic complications, and contraception for female patients of childbearing age (3,21). Patients in developing countries lack access and adherence to many of these treatments. Nearly half of patients do not use prophylactic medication for rheumatic fever and endocarditis. Only ∼58% of patients in developing nations are prescribed oral anticoagulation therapy, and there is little monitoring of international normalized ratio (INR) levels to test its effectiveness. Only ∼25% of patients in developing countries have INR levels in the therapeutic range (21). In addition, little to no reproductive counseling exists for women in developing countries (3,21). Without use of prophylactic medications, post-op complications are higher (20).
Endomyocardial Fibrosis
Endomyocardial fibrosis (EMF) is a restrictive cardiomyopathy that occurs when fibrous tissue forms on the mural endocardium of the ventricles causing atrioventricular valve insufficiency and decreased ventricle sizes leading to increased filling resistance. It can affect one or both ventricles. Cardiac failure and death occur shortly thereafter, as medical therapy is unsuccessful without surgical intervention. Surgical intervention involves complete resection of the ventricular endocardium and valve replacement or repair, but is also associated with high morbidity and mortality (∼10% initially and 13% in the following two post-op years) as the patient is unlikely to wean from CPB (3,20).
EMF's etiology is currently unknown (3,20), but it is most prevalent in developing countries with a tropical or subtropical climate. Other factors such as ethnicity, socioeconomic status, diet, age, infection, and eosinophilia have been associated with the EMF disease processes (23). EMF is most common in children and young adults (3,23). Both genders show peak incidence at 11–15 years of age, but women show a second peak incidence at 26–30 years of age (23). The prognosis is generally poor, with mortality seen frequently within 2 years after diagnosis (11,23). The best outcomes occur with early diagnosis and surgical intervention before the ventricles become hypoplastic (3).
Chagas Disease
In 2007, the WHO estimated that 16–18 million people were infected with Chagas disease and an additional 90 million people were at risk for infection (10). Chagas disease is caused by the protozoan parasite, Trypanosoma cruzi, and is a major public health concern in Central and South America. The disease is transmitted to humans via a bite by the reduviid bug, which lives in the walls and roofs of houses (20).
Clinical manifestation is characterized by three phases: acute, latent, and chronic. Acute phase presentation occurs after an immune response provokes fever, muscle pain, hepatosplenomegaly, myocarditis, and occasionally meningoencephalitis. The acute phase resolves over several months and the patient enters an asymptomatic latent period that can last up to 30 years. As of 2007 research data, about 30% of patients proceed to a chronic phase, which presents with CHF, dilation, and hypertrophy of all cardiac chambers, bradyarrhythmias or heart block, and occasionally left ventricular (LV) apex aneurysms. Surgical intervention via heart transplantation, aneurysm repair, or cardiomyoplasty with the latissimus dorsi muscle, and/or pacemaker implantation is necessary in the chronic phase (20).
Dilated Cardiomyopathy
The prevalence of dilated cardiomyopathy (DCM) in developed countries is 37 per 100,000 people, but is one of the leading causes of CHF in Africa and other developing countries as of 2005. Patients present with impaired systolic function, LV dilation, low arterial pressure, ventricular arrhythmias, and possible atrioventricular valve incompetence. Although it can occur at any age, it is most common in adults, and is twice as prevalent in men than women. Mortality is expected within the first 5 years of the first symptom. The etiology of DCM is currently unknown, but it is thought to be influenced by factors such as genetics, untreated hypertension, infectious diseases associated with myocarditis, autoimmune processes, nutritional deficiencies, alcohol, pregnancy, and environmental toxins (23).
Peripartum Cardiomyopathy
Peripartum cardiomyopathy (PPCM) is a disorder characterized by LV dysfunction (ejection fraction below 45%) that presents anywhere from the last month of pregnancy to the first 5 months postpartum leading to CHF in previously healthy women. Although there is low incidence of PPCM in developed countries (1 in 15,000 deliveries), it is rampant in developing countries, particularly Africa (1 in 1,000 deliveries) (23,24). Zaria, Nigeria, has the highest incidence of PPCM in the world (1 in 100 deliveries). The prevalence is thought to be caused by excessive vasodilation, hypervolemia, and hypertension due to traditional practices of consuming a lake salt while lying on a heated mud bed in a humid room with frequent hot baths for 40 days postpartum in an attempt to stimulate lactation (23). PPCM is a condition associated with high morbidity and mortality as diagnosis is delayed in the developing world in half of patients (24). Although the etiology of PPCM remains unknown, socioeconomic and genetic factors such as preeclampsia, twin pregnancies, and African decent are thought to influence incidence and prognosis (23,24).
Impact of the Rise in Obesity on Global Cardiovascular Health
Obesity and its concurrent comorbidities have become a global public health concern in recent years due to developing countries converting to a Western diet (11,25). Obesity has reached 40–60% of adult populations in developed countries and is as high as 35% in developing countries (26). While endemic infectious diseases remain critical in developing countries, noncommunicable diseases related to obesity are increasing. Notably, sub-Saharan Africa's main cause of death has shifted from acquired immunodeficiency syndrome to cardiovascular diseases in recent years (11).
Obesity promotes the atherosclerotic process, enhances oxidative stress, and induces inflammatory disease states, which can lead to increased mortality after coronary artery bypass graft (CABG) surgery. There has been an increase in patients requiring CABG surgery in developing countries in recent years. Most experts agree waist circumference is the best measurement for central obesity rather than body mass index. Intra-abdominal fat is resistant to insulin, leading to hyperlipidemia, diabetes mellitus, and hypertension. Females are at higher risk of obesity and wound infections are more prevalent in obese post-op patients (18,25,26). Furthermore, lack of proper nutrition and inactive lifestyle during pregnancy has been shown to cause epigenetic programming, or the causation of a genetic predisposition to inadequate metabolic responses in newborns. Epigenetic programing could lead to increased morbidity and mortality in individuals with CHD (18).
SUMMARY
A large gap exists for access to cardiovascular services in developing countries (3). In developed countries such as North America, Australia, and Europe, the average number of cardiac surgical cases performed was 860 per 1 million people as of 2008. On the other spectrum, in developing countries such as South America, the Russian Federation, Asia, and Africa, the average number of cardiac surgical cases performed was 60 per 1 million people. Therefore, 93% of people who require cardiac surgery living in developing countries, an estimated 4.5 billion people total, do not have access to treatment (17). For example, as of 2010 data from South America, 138 cardiac centers exist, equating to one center for every 2.9 million people, performing an average of 42 operations per million people (18). The WHO defines optimal pediatric cardiac care access in a developed country as one surgical center per 2 million people performing 300–500 pediatric cases annually. Although there is no statistical data available to define the need for pediatric cardiac services in the developing world, one can infer the need to be much greater. It is an unfortunate reality that developing countries with populations upwards of 15–70 million people have no pediatric cardiac surgical centers (10).
The need for cardiac surgical centers in developing countries and underserved areas of the world is critical to help ease this gap in care. The enormity of this issue necessitates a collaborative effort from international volunteers, NGOs, local governments, and private benefactors to facilitate adequate cardiac care for the world's population and to overcome the many challenges associated with establishing cardiac programs abroad. Successful cardiac programs will benefit local patients and economies, and international volunteer teams will provide long-term educational commitment and support until the centers reach their full independence. For more information on how to volunteer abroad, see Table 2 or AmSECT's Perfusion Without Borders website. Further research at these cardiac centers may uncover underlying pathophysiological mechanisms of regional cardiac diseases, which will benefit the global community and reduce morbidity and mortality over time.
REFERENCES
- 1. The International Statistical Institute Developing countries. Available at: http://www.isi-web.org/component/content/article/5-root/root/8759. Accessed December 5, 2014.
- 2. The World Health Organization Density of physicians (total number per 1000 population, latest available year). Available at: http://www.who.int/gho/health_workforce/physicians_density/en/. Accessed December 5, 2014.
- 3. Mocumbi A. O. The challenges of cardiac surgery for African children. Cardiovasc J Afr. 2012;23:165–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Oludara M., Nwiloh J., Fabamwo A., et al. Commencing open heart surgery in resource limited countries: Lessons from the LASUTH experience. Pan Afr Med J. 2014;19:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fenton K. N., Castillo S. H., Claro C. D., et al. Teamwork and program organization in developing countries. World J Pediatr Congenit Heart Surg. 2011;2:219–24. [DOI] [PubMed] [Google Scholar]
- 6. Stolf N. Congenital heart surgery in a developing country. Circulation. 2007;116:1874–5. [DOI] [PubMed] [Google Scholar]
- 7. Larrazabal L., Jenkins K., Gauvreau K.. Improvement in congenital heart surgery in a developing country: The Guatemalan experience. Circulation. 2007;116:1882–7. [DOI] [PubMed] [Google Scholar]
- 8. Novick W., Stidham G., Karl T., et al. Are we improving after 10 years of humanitarian paediatric cardiac assistance? Cardiol Young. 2005;15:379–84. [DOI] [PubMed] [Google Scholar]
- 9. Ghosh P. Setting up an open heart surgical program in a developing country. Asian Cardiovasc Thorac Ann. 2005;13:299–301. [DOI] [PubMed] [Google Scholar]
- 10. Yacoub M. Establishing pediatric cardiovascular services in the developing world: A wake-up call. Circulation. 2007;116:1876–8. [DOI] [PubMed] [Google Scholar]
- 11. Mocumbi A. O. Lack of focus on cardiovascular disease in sub-Saharan Africa. Cardiovasc Diagn Ther. 2012;2:74–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Novick W., Stidham G., Karl T., et al. Paediatric cardiac assistance in developing and transitional countries: The impact of a fourteen year effort. Cardiol Young. 2008;18:316–23. [DOI] [PubMed] [Google Scholar]
- 13. Jenkins K., Castaneda A., Cherian K., et al. Reducing mortality and infections after congenital heart surgery in the developing world. Pediatrics. 2014;134:e1422–30. [DOI] [PubMed] [Google Scholar]
- 14. Hariharan S., Fakoory M. T., Harris A., et al. Outcome of elderly patients undergoing open-heart surgery in a developing country. Int J Clin Pract. 2005;59:953–7. [DOI] [PubMed] [Google Scholar]
- 15. Falase B., Sanusi M., Majekodunmi A., et al. The cost of open heart surgery in Nigeria. Pan Afr Med J. 2013;14:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Staveski S. L., Zhelva B., Paul R., et al. Pediatric cardiac surgery Parent Education Discharge Instruction (PEDI) program: A pilot study. World J Pediatr Congenit Heart Surg. 2015;6:18–25. [DOI] [PubMed] [Google Scholar]
- 17. Akomea-Agyin C., Galukande M., Mwambu T., et al. Pioneer human open heart surgery using cardiopulmonary by pass in Uganda. Afr Health Sci. 2008;8:259–60. [PMC free article] [PubMed] [Google Scholar]
- 18. Sandoval N., Kreutzer C., Jatene M., et al. Pediatric cardiovascular surgery in South America: Current status and regional differences. World J Pediatr Congenit Heart Surg. 2010;1:321–7. [DOI] [PubMed] [Google Scholar]
- 19. Tchervenkov C., Jacobs J., Bernier P., et al. The improvement of care for pediatric and congenital cardiac disease across the world: A challenge for the World Society for Pediatric and Congenital Heart Surgery. Cardiol Young. 2008;18:63–9. [DOI] [PubMed] [Google Scholar]
- 20. Moraes C. R. Some aspects of cardiac surgery in the tropics. Eur J Cardiothorac Surg. 1990;4:235–7. [DOI] [PubMed] [Google Scholar]
- 21. Zühlke L., Engel M. E., Karthikeyan G., et al. Characteristics, complications, and gaps in evidence-based interventions in rheumatic heart disease: The Global Rheumatic Heart Disease Registry (the REMEDY study). Eur Heart J. 2015;36:1115–22a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chi N., Chou N., Yu Y., et al. Heart transplantation in endstage rheumatic heart disease. Circ J. 2014;78:1900–7. [DOI] [PubMed] [Google Scholar]
- 23. Sliwa K., Damasceno A., Mayosi B., et al. Epidemiology and etiology of cardiomyopathy in Africa. Circulation. 2005;112:3577–83. [DOI] [PubMed] [Google Scholar]
- 24. Hilfiker-Kleiner D., Sliwa K.. Pathophysiology and epidemiology of peripartum cardiomyopathy. Nat Rev Cardiol. 2014;11:364–70. [DOI] [PubMed] [Google Scholar]
- 25. Ardeshiri M., Faritous Z., Ojaghi Haghighi Z., et al. Effect of obesity on mortality and morbidity after coronary artery bypass grafting surgery in Iranian patients. Anesth Pain Med. 2014;4:e18884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dişcigil G., Ozkisacik E. A., Badak M. I., et al. Obesity and open-heart surgery in a developing country. Anadolu Kardiyol Derg. 2008;8:22–6. [PubMed] [Google Scholar]