Abstract
Cardiovascular diseases burden is increasing due to aging populations and represents one of the major health issues worldwide. Dietary habits have been extensively studied in the cardiovascular field despite the difficulty in the quantification of the assumption of each single food and the observation that several foods affect cardiovascular risk with opposite effects. Moreover, some older findings have been reverted by more recent studies. Red meat has been widely studied in this context, and it has been suggested to increase cardiovascular risk primarily by causing dyslipidemia. Our aim is to review the relationship between red meat assumption and cardiovascular risk and to present novel findings regarding their link.
Keywords: Cardiovascular disease, cardiovascular risk, dyslipidemia, fat, lipid profile, red meat
Introduction
Dietary habits vary widely worldwide according to geographical, cultural, religious, and socioeconomic background. Dietary habits have been shown to influence significantly several biologic pathways, including lipoprotein and cholesterol metabolism, blood pressure, insulin resistance, energy expenditure and energy effective utilization, inflammation and, all together, cardiovascular risk.[1]
Despite conflicting opinions regarding its conduction, the Seven Countries Study addressed for the first time the correlation between lifestyle, diet and cardiovascular diseases (CVDs) in several geographical areas. It showed that a lifestyle approach can lower the individual risk of developing heart disease.[2,3,4] A later study showed a lower incidence of CVDs in initially healthy middle-aged adults that adhere to the Mediterranean diet.[5] Epidemiologic studies of the Seventh-Day Adventists confirmed a lower risk of fatal ischemic heart disease for Adventists, which represent a cohort of people with a healthy lifestyle.[6] Studies based on this low-risk cohort showed that traditional cardiovascular risk factors operate as usual,[7] but several foods have a role in the determination of cardiovascular risk.[8] The Adventists studies reported an effect of meat in increasing the risk of fatal heart disease in men (relative risk [RR] 2.31; P = 0.025), but not in women.[8,9] Since then, several studies examined the role of red meat in determining cardiovascular risk.
In this review, we discuss the impact of red meat in the determination of cardiovascular risk.
Red Meat and Cardiovascular Risk
Red meat consumption is considered a dietary risk factor for CVD. Most of the risk of red meat intake has been related to saturated fat and cholesterol content. This led to the large-scale suggestion of lowering meat consumption and preferring lean meats.[10] However, there is increasing evidence challenging this view. The daily intake of saturated fat has been demonstrated to be related to the incidence of CVD if adjusted for the overall background diet and for carbohydrate consumption.[11,12,13] Other ingredients used in the processation and preservation of red meat, such as sodium or other preservatives, can account for most of the risk.[14] Moreover, heme iron may increase the risk of diabetes[15,16,17] and L-carnitine may be metabolized by gut bacteria to pro-atherosclerotic compounds.[18]
Since dietary habits affect a wide range of intermediate biologic pathways and of biomarkers (including low-density lipoprotein cholesterol [LDL-C]), neither one nor even several surrogate outcomes can predict clinical outcomes.[1,19] Many studies have proposed comprehensive methods for weighting the effects of dietary habits on chronic disease end-points, mainly based on evidence derived from the literature.[20,21,22] Similarly, to most of the other lifestyle risk factors for CVD (e.g., smoking, physical activity, obesity, consumption of salt, dietary cholesterol, fruits, vegetables, nuts, and whole grains), the effects of meat consumption on cardiovascular risk have not been investigated in ad hoc randomized trial.
The investigation of the role of single foods is difficult in large studies, both due to the variability in the assumption and the variability of the food itself. Thus, it is difficult to have a reliable quantification of each food in large and long-lasting studies (which are often need when end-points are cardiovascular outcomes). In fact, large studies usually choose to use more or less broad categories of food. The different kind of meats has potentially important nutritional differences. Recent literature suggests that red and processed meats have some important nutritional differences. Among these differences, the most significant in determining cardiovascular risk are the content of calories, specific fats, sodium, iron, or additives (such as nitrites) content, or specific preparation methods (such as high-temperature commercial cooking).
Charring and blackening of meat may lead to the production of heterocyclic amines and polycyclic aromatic hydrocarbons that might increase the risk of cancer.[23] However, the effects of cooking methods on cardiovascular risk have not been studied so far.
Meats are often broadly classified into red (e.g., beef, pork, lamb) or white (e.g., chicken, turkey, rabbit) meats based on the contents of fat, cholesterol, and iron. Both red and white meat can be either preserved, typically by the addition of high levels of salt and/or chemical preservatives (processed meat) or eaten without such preservatives (unprocessed meat). It is evident that even the category of red meat is made of several different sub-categories.
An effective categorization for the study of the impact on cardiovascular risk could be the division into two main groups: “fresh” unprocessed meat and preserved-processed meat (including sausage, salami, hot dogs, bacon, and processed deli or luncheon meats).
A 2010 meta-analysis based on three prospective cohort studies and one case–control study including a total of 56311 participants and 769 events showed no significant association between unprocessed red meat and cardiovascular risk (RR 1.00 per 100 g serving/day, 95% confidence interval [CI] 0.81–1.23).[14,24,25,26,27]
The same meta-analysis included six observational studies that evaluated processed meat consumption for a total of 614,062 participants and 21308 events.[14,24,25,27,28,29] It showed that each additional 50 g serving/day of processed meats was associated with a 42% higher risk (RR 1.42, 95% CI 1.07–1.89) of cardiovascular events.
These results should also take into account the smaller serving size for processed (50 g) compared to unprocessed red meats (100 g). The authors of the meta-analysis showed that considering a 100 g serving/day of processed meats, it was associated with a 2-fold increase in the risk of CVD (RR 2.02, 95% CI 1.14–3.57).
The reported RR refers to a daily dose of either unprocessed (100 g) or processed (50 or 100 g, as previously discussed) red meat, thus seven servings in a week. The RR for only one weekly serving of both processed and unprocessed red meat is around 1.0.
In the next sections, we will discuss several contributors of the associations between red meat and CVD.
Red Meat and Lipid Profile
Red meat is historically referred to increase CVD due to its saturated fatty acids (SFAs) content.[2] Since then, the dietary guidance of developed countries suggested to limit red meat consumption. Moreover, the 2013 American College of Cardiology/American Heart Association Guidelines on Lifestyle management to reduce cardiovascular risk supported low-fat meals, including vegetables, fruits, fish, legumes and nuts and restricted the consumption of sweetened drinks and red meats.[30] Nevertheless, the association between meat content of SFA and cardiovascular risk is still inconsistent.
The first issue is the difficulty in measuring the precise level of fatty acids derived from meat consumption. One possibility to assess fats contained in red meat could be measuring biochemical markers on plasma lipid fraction, red cell and platelet membranes, or subcutaneous adipose tissue. This method could be useful also to distinguish the degree of fat modification due to red meat preparation. However, in this case, fatty acids levels should be considered carefully because saturated and monounsaturated fatty acids (MUFAs) can be produced also endogenously. Moreover, prospective observational studies on specific SFA showed that the effect of each single SFA could be difficult to separate thus suggesting a “class effect.” However, stearic acid, which is contained in red meat, was most strongly associated with risk of CHD.[31]
Another issue is to assess if red meat is really rich in SFA. Lean red meat contains less than 1.5 g SFA/100 g of visible fat [Figure 1]. The main lipid contained in lean meat tissue is phospholipid, which is the major element of cell membranes. Membrane structures contain also high levels of polyunsaturated fatty acids. The second portion of lipids in lean meat is represented by triacylglycerol (TAG). Red meat TAG has an increased level of SFA and MUFA.[32]
Figure 1.

Meats rich in visible fat and preservatives (red box on the left) have been demonstrated to increase cardiovascular risk while lean fresh red meat (green box on the right) has not
The still-open question is to understand whether SFA contained in red meat affect serum lipid profile. In fact, each saturated fats showed different effects on serum LDL-C fractions or lipid fractions.[33] Some studies, for example, refer that LDL levels increased significantly with butter and dairy fats (high in 14:0, myristic acid), to a lesser degree with beef fat (containing palmitic acid, 16:0; stearic acid, 18:00), and only slightly with cocoa butter (containing largely stearic acid).[34] However, recent literature reported limitations in both the observational data and methods used to evaluate the impact of SFA on cardiovascular risk.[35] Some other studies addressed this issue. For example, the introduction of lean beef in a study about AHA diet was useful to diminish total cholesterol (total-C) and LDL-C.[36] In several studies, low-fat red meat-based regimens reduced total-C and LDL-C similarly to lean red meat ones.[37,38,39,40] Compared to lean white meat in various diets, beef, in particular, has been referred to be equally effective for reducing total-C, LDL-C, and triglycerides.[41] When compared to diets based on vegetable sources of proteins, regimens based on the same levels of animal proteins did not show significant differences in lipids profile and physical property.[42,43,44] Moreover, the restriction of red meat intake from plant protein-based dietary patterns could lead to reduced intake of high-quality protein and essential nutrients. Thus, the choice of lean cuts of red meat should be encouraged.[45] In a 76-week crossover study of dyslipidemic people consuming lean red meat or white meat, those who ate red meat demonstrated better long-term compliance to their dietary pattern.[40]
The mechanisms of SFA action on lipid profile were deeply investigated. One postulated mechanism relates to the formation of oxidated-LDL (ox-LDL). SFA are usually resistant to oxidation. However, the presence of heme group in red meat acts as a catalyzer to promote to the formation of ox-LDL.
However, despite the majority of the studies support the idea that red meat SFA are not associated with an increase in cardiovascular risk, there are also some studies that partially call into question this finding. For example, a multiethnic study published in 2012 showed that red meat SFA was associated with an increased cardiovascular risk. This study showed that a greater consumption of dairy SFA was associated with lower cardiovascular risk (hazard ratio [HR] 0.79, 95% CI 0.68–092 and HR 0.62, 0.47–0.82 for + 5 g/die and + 5% of energy from dairy SFA, respectively). In contrast, a larger intake of red meat SFA was associated to a slightly increase with borderline statistical significance cardiovascular risk (HR 1.26, 95% CI 1.02–1.54 and HR 1.48, 95% CI 0.98–2.23 for + 5 g/die and + 5% of energy from meat SFA, respectively).[46]
Red Meat and Iron
Iron is a fundamental element for human health, and its deficiency is one of the main dietary deficiencies worldwide.[47] Red meat is one the most important source of heme iron, which is the absorbable form of this metal.[48] The iron content of red meat is estimated to vary from 5.8 mg in beef liver, braised (3 oz) to 2.9 mg in lean sirloin, broiled (3 oz).[49]
Recent dietary guidelines recommend both children and young adults consuming red meat to prevent iron deficiency. However, iron intake is also associated with increasing reactive oxygen species production, which is thought to be key elements in the pathogenesis of CVD [Figure 2].[50] Nevertheless, the evidence of the association between heme iron intake and CVD risk is restricted to a few papers. The first hypothesis about the relationship between iron and cardiovascular risk was made by Jerome Sullivan in 1981. In his study, he argued that the incidence of CVDs was higher in men and postmenopausal women rather than in premenopausal women because in premenopausal period menstruation reduces total iron deposits. Two large studies showed a different association of dietary iron and CVD in men and in women. In 1994, 4 years prospective study analyzed if iron intake led to the significant risk of coronary disease in 44,933 US men of 40–75 years old with no previous CVDs. Participants were asked about their food frequency consumption at baseline, and 844 incident cases of coronary disease were reported. However, this study showed that neither total nor heme iron intake was associated with coronary heart disease (CHD) risk. Nevertheless, a higher number of fatal coronary disease or nonfatal myocardial infarction (MI) in men in the first quintile of iron intake than in those in the last quintile (RR = 1.42; 95% CI: 1.02–1.98). In conclusion, authors reported an increased risk of MI among men with a higher intake of heme iron, which mostly derives from meat intake.[26] In the same year, another study found an inverse association between iron serum level and women's MI risk. It also reported an inverse association between serum iron and CHD in both sexes. According to the same authors, dietary iron could not explain increasing MI and CHD risk.[51] These results should be however compared to most recent studies. In 2012, Zhang et al. showed a positive association between iron intake from diet and mortality due to total and ischemic stroke and CVD in men. Dietary iron showed no association with these end-points.[52]
Figure 2.
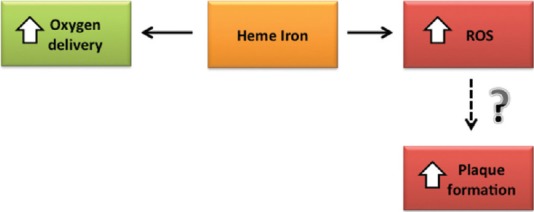
Heme iron not only increases oxygen delivery but also increases reactive oxygen species formation. Reactive oxygen species might increase atherosclerotic plaque formation
Red Meat and L-carnitine Gut Metabolism
The inconsistent connection between red meat SFA, iron, and CVD suggested investigating the role of other environmental elements linking meat consumption to cardiovascular risk. Recent literature provided a key role of gut microbiota metabolism of red meat on this risk. Gut microflora is made of different species of microorganisms, which are influenced by dietary patterns; for example, a high animal proteins and fats consumption relate to a predominant level of gut Bacteroides species. Human microbiome participates in both saccharolytic and proteolytic food digestion, but it also influences immune function, metabolism, vitamins activation, and pathologic conditions such as insulin resistance and metabolic syndrome. Saccharolytic microflora is thought to be the main part of a balanced microflora and dietary habits have a deep impact on its composition. Nevertheless, the association of the different genera of human bacteria to a precise dietary pattern is complex and still poorly defined. Recent literature referred that changes in gut flora are deeply affected by long-term diet modifications.[53]
Some studies, in particular, showed that omnivorous gut bacteria can produce higher level of trimethylamine-N-oxide (TMAO) than vegetarians when their diet is enriched in L-carnitine.[54] TMAO is the oxidized form of an intermediate product which derives from choline, phosphatidylcholine and L-carnitine hepatic metabolism.[55] It has been recently referred to increase both atherogenesis and CVD risk.[56] Carnitine comes from the Latin carnus as this amino acid mostly derives from red meat ingestion. Together these, data could give a new explanation on the possible association between red meat and cardiovascular risk. However, it is not clear if meat ingestion is really effective in increasing TMAO levels. Some authors, in fact referred that only seafood, which contain TMAO itself, has measurable impact on trimethylamine and its N-oxide form.[57] Moreover, the pro-atherosclerotic mechanisms of TMAO are still under debate, and main results come from mice models. TMAO have been recently referred to promote atherosclerosis by lowering reverse cholesterol transport (RCT) and increasing foam cells production.[54] RCT is the process of cholesterol returning to the liver for excretion.[58] In their study, Koeth et al. showed that dietary carnitine and direct supplementation with TMAO both reduced RCT (P < 0.05) in mice only in case of intact gut microbiome.[54] The main issue regards the molecular mechanisms, which link microflora and TMAO synthesis to RCT reduction. In the intestines, TMAO is referred to diminish cholesterol uptake by reducing Niemann-Pick C1-like 1, which take cholesterol from the lumen into the enterocytes.[59] However, the intestinal metabolism of the cholesterol cannot justify the decrease of RCT. Of note, in the liver, TMAO has been referred to reduce the expression of Cyp7a1, a fundamental bile enzyme in the metabolism, and transport of cholesterol. The downregulation of this enzyme is associated with the reduction in bile acid pool size and the enhancement in atherosclerosis.[60,61,62] These results show that TMAO would be capable to influence various functional pathways, which together interact in reducing RCT. Less is known about TMAO direct or indirect effects on other molecules to explain its action. Foam cells are generated when macrophages reach and surround fatty deposits on the blood vessel walls giving them a “foamy” way of looking.[63] According to recent literature, TMAO can upregulate the expression of the scavengers receptors cluster of differentiation 36 and scavenger receptor A which promote macrophages deposition of cholesterol.[64,65]
Preservatives
Recent literature suggests a key role for preservatives in the increased cardiovascular risk associated with red meat consumption. As previously discussed in the red meat and cardiovascular risk paragraph, the consumption of a 100 g serving/day of processed meats doubled the risk of CVD.
Sodium is one of the most common preservatives in processed red meat.[66] Processed meat has four times the sodium content of unprocessed red meat and 1.5 times more nitrates. Dietary sodium has been largely demonstrated to increases blood pressure, peripheral vascular resistance, and to lower arterial compliance.[67] A recent large prospective study showed an association between processed red meat and hypertension in women, but no association for unprocessed red meat consumption.[68]
Moreover, to its action on blood pressure and thus its indirect role in the increased cardiovascular events, sodium intake has been demonstrated to independently increase the risk of CVD.[69] The sodium contained in a daily 50 g serving of processed meat would predict most of the observed 42% higher risk seen in cohort studies.[70]
However, there are other preservatives involved in the increase of cardiovascular risk with preserved meat. For example, nitrates and their byproducts such as peroxynitrite have been experimentally demonstrated to promote endothelial dysfunction and atherosclerosis development and have been also used as biomarkers of endothelial dysfunction. Nitrates can also induce insulin resistance, which in turn can lead to CVDs and represent biomarker of impaired glucose metabolism.[71,72,73] In addition, streptozotocin, which is a nitrosamine-related compound, has been associated to the onset of diabetes.[74]
Conclusions
Red meat has been associated for a long time to an increased the risk of CVDs. However, recent findings demonstrated that despite the presence of heme iron and carnitine, red meat does not significantly increase cardiovascular risk when it is assumed in recommended doses. Visible fat and preservatives are the major issues in the link between red meat and increased cardiovascular risk, thus leading to a significant causal role for preserved red meats, especially if they are consumed daily. Despite some other links have been advocated, there is still debate regarding their role.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Mozaffarian D. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. Ch. 48. Philadelphia: Elsevier; 2012. Nutrition and cardiovascular diseases. [Google Scholar]
- 2.Keys A, Aravanis C, Blackburn HW, Van Buchem FS, Buzina R, Djordjevic BD, et al. Epidemiological studies related to coronary heart disease: Characteristics of men aged 40-59 in seven countries. Acta Med Scand Suppl. 1966;460:1–392. [PubMed] [Google Scholar]
- 3.Menotti A, Lanti M, Kromhout D, Blackburn H, Jacobs D, Nissinen A, et al. Homogeneity in the relationship of serum cholesterol to coronary deaths across different cultures: 40-year follow-up of the Seven Countries Study. Eur J Cardiovasc Prev Rehabil. 2008;15:719–25. doi: 10.1097/HJR.0b013e328315789c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coronary heart disease in seven countries. I. The study program and objectives. Circulation. 1970;41(4 Suppl):I1–8. [PubMed] [Google Scholar]
- 5.Martínez-González MA, García-López M, Bes-Rastrollo M, Toledo E, Martínez-Lapiscina EH, Delgado-Rodriguez M, et al. Mediterranean diet and the incidence of cardiovascular disease: A Spanish cohort. Nutr Metab Cardiovasc Dis. 2011;21:237–44. doi: 10.1016/j.numecd.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Phillips RL, Kuzma JW, Beeson WL, Lotz T. Influence of selection versus lifestyle on risk of fatal cancer and cardiovascular disease among Seventh-day Adventists. Am J Epidemiol. 1980;112:296–314. doi: 10.1093/oxfordjournals.aje.a112996. [DOI] [PubMed] [Google Scholar]
- 7.Fraser GE, Strahan TM, Sabaté J, Beeson WL, Kissinger D. Effects of traditional coronary risk factors on rates of incident coronary events in a low-risk population. The Adventist Health Study. Circulation. 1992;86:406–13. doi: 10.1161/01.cir.86.2.406. [DOI] [PubMed] [Google Scholar]
- 8.Fraser GE, Sabaté J, Beeson WL, Strahan TM. A possible protective effect of nut consumption on risk of coronary heart disease. The Adventist Health Study. Arch Intern Med. 1992;152:1416–24. [PubMed] [Google Scholar]
- 9.Snowdon DA, Phillips RL, Fraser GE. Meat consumption and fatal ischemic heart disease. Prev Med. 1984;13:490–500. doi: 10.1016/0091-7435(84)90017-3. [DOI] [PubMed] [Google Scholar]
- 10.U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2010. 7th Edition. Washington, DC: U.S. Government Printing Office; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am J Clin Nutr. 2010;91:535–46. doi: 10.3945/ajcn.2009.27725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mente A, de Koning L, Shannon HS, Anand SS. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med. 2009;169:659–69. doi: 10.1001/archinternmed.2009.38. [DOI] [PubMed] [Google Scholar]
- 13.Micha R, Mozaffarian D. Saturated fat and cardiometabolic risk factors, coronary heart disease, stroke, and diabetes: A fresh look at the evidence. Lipids. 2010;45:893–905. doi: 10.1007/s11745-010-3393-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Micha R, Wallace SK, Mozaffarian D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: A systematic review and meta-analysis. Circulation. 2010;121:2271–83. doi: 10.1161/CIRCULATIONAHA.109.924977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajpathak S, Ma J, Manson J, Willett WC, Hu FB. Iron intake and the risk of type 2 diabetes in women: A prospective cohort study. Diabetes Care. 2006;29:1370–6. doi: 10.2337/dc06-0119. [DOI] [PubMed] [Google Scholar]
- 16.Lee DH, Folsom AR, Jacobs DR., Jr Dietary iron intake and Type 2 diabetes incidence in postmenopausal women: The Iowa Women's Health Study. Diabetologia. 2004;47:185–94. doi: 10.1007/s00125-003-1307-1. [DOI] [PubMed] [Google Scholar]
- 17.Zhao Z, Li S, Liu G, Yan F, Ma X, Huang Z, et al. Body iron stores and heme-iron intake in relation to risk of type 2 diabetes: A systematic review and meta-analysis. PLoS One. 2012;7:e41641. doi: 10.1371/journal.pone.0041641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;11:576–85. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Institute of Medicine of the National Academies. Evaluation of Biomarkers and Surrogate Endpoints in Chronic Disease. Institute of Medicine of the National Academies: Institute of Medicine of the National Academies. 2010 [PubMed] [Google Scholar]
- 20.Micha R, Kalantarian S, Wirojratana P, Byers T, Danaei G, Elmadfa I, et al. Estimating the global and regional burden of suboptimal nutrition on chronic disease: Methods and inputs to the analysis. Eur J Clin Nutr. 2012;66:119–29. doi: 10.1038/ejcn.2011.147. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. Diet, Nutrition and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation. World Health Organization Technical Report Series. Vol. 7. Geneva: World Health Organization; 2003. p. 916. [PubMed] [Google Scholar]
- 22.Hill AB. The environment and disease: Association or causation? Proc R Soc Med. 1965;58:295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turesky RJ, Le Marchand L. Metabolism and biomarkers of heterocyclic aromatic amines in molecular epidemiology studies: Lessons learned from aromatic amines. Chem Res Toxicol. 2011;24:1169–214. doi: 10.1021/tx200135s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whiteman D, Muir J, Jones L, Murphy M, Key T. Dietary questions as determinants of mortality: The OXCHECK experience. Public Health Nutr. 1999;2:477–87. doi: 10.1017/s136898009900066x. [DOI] [PubMed] [Google Scholar]
- 25.Burke V, Zhao Y, Lee AH, Hunter E, Spargo RM, Gracey M, et al. Health-related behaviours as predictors of mortality and morbidity in Australian Aborigines. Prev Med. 2007;44:135–42. doi: 10.1016/j.ypmed.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 26.Ascherio A, Willett WC, Rimm EB, Giovannucci EL, Stampfer MJ. Dietary iron intake and risk of coronary disease among men. Circulation. 1994;89:969–74. doi: 10.1161/01.cir.89.3.969. [DOI] [PubMed] [Google Scholar]
- 27.Martínez-González MA, Fernández-Jarne E, Serrano-Martínez M, Marti A, Martinez JA, Martín-Moreno JM. Mediterranean diet and reduction in the risk of a first acute myocardial infarction: An operational healthy dietary score. Eur J Nutr. 2002;41:153–60. doi: 10.1007/s00394-002-0370-6. [DOI] [PubMed] [Google Scholar]
- 28.Liu J, Stampfer MJ, Hu FB, Ascherio A, Manson J, Willett WC, et al. Dietary iron and red meat intake and risk of coronary heart disease in postmenopausal women. Am J Epidemiol. 2003;157:S100. [Google Scholar]
- 29.Sinha R, Cross AJ, Graubard BI, Leitzmann MF, Schatzkin A. Meat intake and mortality: A prospective study of over half a million people. Arch Intern Med. 2009;169:562–71. doi: 10.1001/archinternmed.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2960–84. doi: 10.1016/j.jacc.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Willett WC. Dietary fats and coronary heart disease. J Intern Med. 2012;272:13–24. doi: 10.1111/j.1365-2796.2012.02553.x. [DOI] [PubMed] [Google Scholar]
- 32.Sinclair AJ, Slattery WJ, O’Dea K. The analysis of polyunsaturated fatty acids in meat by capillary gas-liquid chromatography. J Sci Food Agric. 1982;33:771–6. [Google Scholar]
- 33.Hunter JE, Zhang J, Kris-Etherton PM. Cardiovascular disease risk of dietary stearic acid compared with trans, other saturated, and unsaturated fatty acids: A systematic review. Am J Clin Nutr. 2010;91:46–63. doi: 10.3945/ajcn.2009.27661. [DOI] [PubMed] [Google Scholar]
- 34.Denke MA, Grundy SM. Effects of fats high in stearic acid on lipid and lipoprotein concentrations in men. Am J Clin Nutr. 1991;54:1036–40. doi: 10.1093/ajcn/54.6.1036. [DOI] [PubMed] [Google Scholar]
- 35.McAfee AJ, McSorley EM, Cuskelly GJ, Moss BW, Wallace JM, Bonham MP, et al. Red meat consumption: An overview of the risks and benefits. Meat Sci. 2010;84:1–13. doi: 10.1016/j.meatsci.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 36.Beauchesne-Rondeau E, Gascon A, Bergeron J, Jacques H. Plasma lipids and lipoproteins in hypercholesterolemic men fed a lipid-lowering diet containing lean beef, lean fish, or poultry. Am J Clin Nutr. 2003;77:587–93. doi: 10.1093/ajcn/77.3.587. [DOI] [PubMed] [Google Scholar]
- 37.Davidson MH, Hunninghake D, Maki KC, Kwiterovich PO, Jr, Kafonek S. Comparison of the effects of lean red meat vs lean white meat on serum lipid levels among free-living persons with hypercholesterolemia: A long-term, randomized clinical trial. Arch Intern Med. 1999;159:1331–8. doi: 10.1001/archinte.159.12.1331. [DOI] [PubMed] [Google Scholar]
- 38.Scott LW, Dunn JK, Pownall HJ, Brauchi DJ, McMann MC, Herd JA, et al. Effects of beef and chicken consumption on plasma lipid levels in hypercholesterolemic men. Arch Intern Med. 1994;154:1261–7. [PubMed] [Google Scholar]
- 39.Li D, Siriamornpun S, Wahlqvist ML, Mann NJ, Sinclair AJ. Lean meat and heart health. Asia Pac J Clin Nutr. 2005;14:113–9. [PubMed] [Google Scholar]
- 40.Hunninghake DB, Maki KC, Kwiterovich PO, Jr, Davidson MH, Dicklin MR, Kafonek SD. Incorporation of lean red meat into a National Cholesterol Education Program Step I diet: A long-term, randomized clinical trial in free-living persons with hypercholesterolemia. J Am Coll Nutr. 2000;19:351–60. doi: 10.1080/07315724.2000.10718931. [DOI] [PubMed] [Google Scholar]
- 41.Maki KC, Van Elswyk ME, Alexander DD, Rains TM, Sohn EL, McNeill S. A meta-analysis of randomized controlled trials that compare the lipid effects of beef versus poultry and/or fish consumption. J Clin Lipidol. 2012;6:352–61. doi: 10.1016/j.jacl.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 42.Campbell WW, Barton ML, Jr, Cyr-Campbell D, Davey SL, Beard JL, Parise G, et al. Effects of an omnivorous diet compared with a lactoovovegetarian diet on resistance-training-induced changes in body composition and skeletal muscle in older men. Am J Clin Nutr. 1999;70:1032–9. doi: 10.1093/ajcn/70.6.1032. [DOI] [PubMed] [Google Scholar]
- 43.Wiebe SL, Bruce VM, McDonald BE. A comparison of the effect of diets containing beef protein and plant proteins on blood lipids of healthy young men. Am J Clin Nutr. 1984;40:982–9. doi: 10.1093/ajcn/40.5.982. [DOI] [PubMed] [Google Scholar]
- 44.Yamashita T, Sasahara T, Pomeroy SE, Collier G, Nestel PJ. Arterial compliance, blood pressure, plasma leptin, and plasma lipids in women are improved with weight reduction equally with a meat-based diet and a plant-based diet. Metabolism. 1998;47:1308–14. doi: 10.1016/s0026-0495(98)90297-9. [DOI] [PubMed] [Google Scholar]
- 45.Huth PJ, Fulgoni VL, Keast DR, Park K, Auestad N. Major food sources of calories, added sugars, and saturated fat and their contribution to essential nutrient intakes in the U.S. diet: Data from the National Health and Nutrition Examination Survey (2003-2006) Nutr J. 2013;12:116. doi: 10.1186/1475-2891-12-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Oliveira Otto MC, Mozaffarian D, Kromhout D, Bertoni AG, Sibley CT, Jacobs DR, Jr, et al. Dietary intake of saturated fat by food source and incident cardiovascular disease: The Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr. 2012;96:397–404. doi: 10.3945/ajcn.112.037770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Savva SC, Kafatos A. Is red meat required for the prevention of iron deficiency among children and adolescents? Curr Pediatr Rev. 2014;10:177–83. [PubMed] [Google Scholar]
- 48.Sharma S, Sheehy T, Kolonel LN. Contribution of meat to Vitamin B12, iron and zinc intakes in five ethnic groups in the USA: Implications for developing food-based dietary guidelines. J Hum Nutr Diet. 2013;26:156–68. doi: 10.1111/jhn.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.American Dietetic Association and Roberta Larson Duyff. Complete Food and Nutrition Guide. 2nd ed. Houghton Mifflin Harcourt; 2002. [Google Scholar]
- 50.Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol. 2011;301:H2181–90. doi: 10.1152/ajpheart.00554.2011. [DOI] [PubMed] [Google Scholar]
- 51.Liao Y, Cooper RS, McGee DL. Iron status and coronary heart disease: Negative findings from the NHANES I epidemiologic follow-up study. Am J Epidemiol. 1994;139:704–12. doi: 10.1093/oxfordjournals.aje.a117060. [DOI] [PubMed] [Google Scholar]
- 52.Zhang W, Iso H, Ohira T, Date OC, Tanabe N, Kikuchi S, et al. Associations of dietary iron intake with mortality from cardiovascular disease: The JACC study. J Epidemiol. 2012;22:484–93. doi: 10.2188/jea.JE20120006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–8. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–85. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bain MA, Fornasini G, Evans AM. Trimethylamine: Metabolic, pharmacokinetic and safety aspects. Curr Drug Metab. 2005;6:227–40. doi: 10.2174/1389200054021807. [DOI] [PubMed] [Google Scholar]
- 56.Brown JM, Hazen SL. Metaorganismal nutrient metabolism as a basis of cardiovascular disease. Curr Opin Lipidol. 2014;25:48–53. doi: 10.1097/MOL.0000000000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang AQ, Mitchell SC, Smith RL. Dietary precursors of trimethylamine in man: A pilot study. Food Chem Toxicol. 1999;37:515–20. doi: 10.1016/s0278-6915(99)00028-9. [DOI] [PubMed] [Google Scholar]
- 58.Spady DK. Reverse cholesterol transport and atherosclerosis regression. Circulation. 1999;100:576–8. doi: 10.1161/01.cir.100.6.576. [DOI] [PubMed] [Google Scholar]
- 59.Altmann SW, Davis HR, Jr, Zhu LJ, Yao X, Hoos LM, Tetzloff G, et al. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303:1201–4. doi: 10.1126/science.1093131. [DOI] [PubMed] [Google Scholar]
- 60.Charach G, Rabinovich A, Argov O, Weintraub M, Rabinovich P. The role of bile acid excretion in atherosclerotic coronary artery disease. Int J Vasc Med. 2012;2012:949672. doi: 10.1155/2012/949672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Charach G, Rabinovich PD, Konikoff FM, Grosskopf I, Weintraub MS, Gilat T. Decreased fecal bile acid output in patients with coronary atherosclerosis. J Med. 1998;29:125–36. [PubMed] [Google Scholar]
- 62.Lu Y, Feskens EJ, Boer JM, Müller M. The potential influence of genetic variants in genes along bile acid and bile metabolic pathway on blood cholesterol levels in the population. Atherosclerosis. 2010;210:14–27. doi: 10.1016/j.atherosclerosis.2009.10.035. [DOI] [PubMed] [Google Scholar]
- 63.Jones NL, Reagan JW, Willingham MC. The pathogenesis of foam cell formation: Modified LDL stimulates uptake of co-incubated LDL via macropinocytosis. Arterioscler Thromb Vasc Biol. 2000;20:773–81. doi: 10.1161/01.atv.20.3.773. [DOI] [PubMed] [Google Scholar]
- 64.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kunjathoor VV, Febbraio M, Podrez EA, Moore KJ, Andersson L, Koehn S, et al. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J Biol Chem. 2002;277:49982–8. doi: 10.1074/jbc.M209649200. [DOI] [PubMed] [Google Scholar]
- 66.The Third National Health and Nutrition Examination Survey, NHANES III. National Center for Health Statistics Centers for Disease Control and Prevention. Hyattsville, Maryland: 2013. [Google Scholar]
- 67.Sacks FM, Campos H. Dietary therapy in hypertension. N Engl J Med. 2010;362:2102–12. doi: 10.1056/NEJMct0911013. [DOI] [PubMed] [Google Scholar]
- 68.Lajous M, Bijon A, Fagherazzi G, Rossignol E, Boutron-Ruault MC, Clavel-Chapelon F. Processed and unprocessed red meat consumption and hypertension in women. Am J Clin Nutr. 2014;100:948–52. doi: 10.3945/ajcn.113.080598. [DOI] [PubMed] [Google Scholar]
- 69.Singh GM, Danaei G, Farzadfar F, Stevens GA, Woodward M, Wormser D, et al. The age-specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: A pooled analysis. PLoS One. 2013;8:e65174. doi: 10.1371/journal.pone.0065174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Micha R, Michas G, Mozaffarian D. Unprocessed red and processed meats and risk of coronary artery disease and type 2 diabetes – An updated review of the evidence. Curr Atheroscler Rep. 2012;14:515–24. doi: 10.1007/s11883-012-0282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Förstermann U. Oxidative stress in vascular disease: Causes, defense mechanisms and potential therapies. Nat Clin Pract Cardiovasc Med. 2008;5:338–49. doi: 10.1038/ncpcardio1211. [DOI] [PubMed] [Google Scholar]
- 72.McGrowder D, Ragoobirsingh D, Dasgupta T. Effects of S-nitroso-N-acetyl-penicillamine administration on glucose tolerance and plasma levels of insulin and glucagon in the dog. Nitric Oxide. 2001;5:402–12. doi: 10.1006/niox.2001.0360. [DOI] [PubMed] [Google Scholar]
- 73.Portha B, Giroix MH, Cros JC, Picon L. Diabetogenic effect of N-nitrosomethylurea and N-nitrosomethylurethane in the adult rat. Ann Nutr Aliment. 1980;34:1143–51. [PubMed] [Google Scholar]
- 74.Gajdosík A, Gajdosíková A, Stefek M, Navarová J, Hozová R. Streptozotocin-induced experimental diabetes in male Wistar rats. Gen Physiol Biophys. 1999;18:54–62. [PubMed] [Google Scholar]


