Abstract
Background:
This research was to examine the effects of synbiotic intake on minerals, liver enzymes, and blood pressure in patients with type 2 diabetes (T2D).
Methods:
This randomized, cross-over clinical trial was performed among 62 diabetic patients. Persons were randomly assigned to intake either a synbiotic (n = 62) or a control food (n = 62) for 6 weeks. A 3-week washout period was applied following which persons were crossed over to the alternate intervention arm for an additional 6 weeks. The synbiotic was consisted of Lactobacillus sporogenes (1 × 107 CFU), 0.04 g inulin (HPX) as prebiotic. Persons were asked to consume the synbiotic and control foods 27 g a day. Blood pressure was measured, and blood samples were taken at baseline and after 6-week intervention to assess calcium, magnesium, iron, alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase, and total bilirubin.
Results:
The consumption of a synbiotic food, compared to the control food, resulted in a significant rise of calcium (0.66 vs. −0.14 mg/dL, P = 0.03) and iron (5.06 vs. −9.98 mg/dL, P = 0.03). The decrease of total bilirubin (0.08 vs. −0.04 mg/dL; P = 0.009) was also seen in the synbiotic group compared with the control group.
Conclusions:
Overall, synbiotic in T2D patients had beneficial effects on calcium, iron, and total bilirubin concentrations.
Keywords: Blood pressure, liver enzymes, serum minerals, synbiotic, type 2 diabetes
Introduction
Metabolic complications in patients with type 2 diabetes (T2D) are associated with changes in serum minerals.[1,2] Insulin resistance in patients with T2D is also established as factor of elevated levels of liver enzymes, hypertension, dyslipidemia, and atherosclerotic cardiovascular diseases.[3,4] Increased activities of liver enzymes including alkaline phosphatase (ALP), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) are considered as early surrogate markers of nonalcoholic fatty liver disease.[5,6] Earlier studies reported that the elevated values of these enzymes are related to metabolic syndrome and cardiovascular disease.[7,8] Furthermore, high blood pressure is associated with thrombosis in vessels,[9] heart failure, aortic aneurysms, and atherosclerosis.[10]
Various strategies for the management of diabetes complications have been suggested including the use of fortified food supplements,[11,12] supplementation of micronutrients[13] as well as physical activity and weight loss,[14] consumption of various medications, including rosiglitazone and metformin[15] and incretin-based therapies.[16] Recently, few trials have documented that intake of synbiotics might result in increased mineral absorption, metabolism, and bone composition,[17] as well as decreased activities of liver enzymes[18] and might help controlling blood pressure in animal models and nondiabetic patients.[19] Synbiotics are nutritional supplements that are composed of probiotics and prebiotics.[20] Pro- and pre-biotics might result in decreased activity of liver enzymes through the maintenance of colonization resistance[21] and stimulating growth of beneficial probiotic bacteria in the gut.[22] Furthermore, released bioactive peptides[23] and changes in the gut microbiota composition by pro- and pre-biotics[24] may lead to the beneficial effects on blood pressure.
The objective of our study was to determine the effects of synbiotic intake on minerals, liver enzymes (ALP, AST, and ALT), and blood pressure in Iranian patients with T2D.
Methods
Participants
This randomized, cross-over clinical trial was carried out in Kashan, Iran, from July 2011 to January 2012. On the basis of sample size formula suggested for randomized clinical trials,[25] we considered the Type I error of 5% (α = 0.05) and Type II error of 20% (β = 0.2; power = 80%) and plasma calcium levels as a key variable, and we reached the sample size of 31 persons for each group. Diagnosis of T2D was done based on the criteria of the American Diabetes Association.[26] Persons were not included pregnant, using insulin or vitamin supplements, chronic or acute inflammatory disease, and allergies, using probiotic, antibiotics, or glucocorticoids during 8 weeks before intervention. The Ethical Committee of Kashan University of Medical Sciences approved the study and informed written consent was obtained from all persons.
Study design
Persons were asked to record their dietary intakes for 3 dayS. At the end of run-in period, patients were matched one-by-one according to age, body mass index (BMI), gender, and the dosage and kind of medications. A 3-week washout period was applied following which persons were crossed over to the alternate intervention arm for an additional 6 weeks. A trained nutritionist at diabetes clinic did the randomized allocation sequence and assigned participants to the groups. Compliance with the consumption of foods was monitored through phone interviews. The dietary records were based on 3-day food diaries in each phase; we used Nutritionist IV software (First Databank, San Bruno, CA, USA) modified for Iranian foods. Physical activity was described as metabolic equivalents in hours per day.[27]
Synbiotic and control foods
The synbiotic food (Sekkeh Gaz Company, Isfahan, Iran) consisted of Lactobacillus sporogenes (1 × 107 CFU), 0.04 g inulin (HPX) with 0.38 g isomalt, 0.36 g sorbitol, and 0.05 g stevia as sweetener per 1 g. Persons were requested to take the synbiotic and control foods three times a day in a 9 g package. Due to existence of limited data about the appropriate dosage of L. sporogenes and inulin for patients with T2DM, we used the above-mentioned dosage of L. sporogenes and inulin based on a previous study in pregnant women.[28]
Assessment of variables
Anthropometric measurements (Seca, Hamburg, Germany) were assessed at baseline and after the 6-week trial. Fasting blood samples (10 mL) were taken at baseline and after a 6-week trial at Kashan reference laboratory. Serum samples were analyzed to determine calcium, magnesium, iron, ALP, AST, ALT, and total bilirubin values. Serum calcium, magnesium, iron, ALP, AST, ALT, and total bilirubin values were assayed using mentioned kits (Pars Azmun Inc., Tehran, Iran). All inter- and intra-assay coefficient of variations mentioned markers were <5%.
Statistical analysis
To ensure the normal distribution of variables, Histogram and Kolmogorov–Smirnov test were applied. For nonnormally distributed variables, log-transformation was applied. Descriptive statistics (means, standard error of the means, and range) for general characteristics of the study participants were reported. Data on dietary intakes were compared by paired t-test. For each dependent variable, we computed the changes from baseline by subsidizing the baseline value from the end-of-trial value. With-in and between-group changes in dependent variables were compared by the use of paired samples t-test. We also assessed if the carryover and treatment effects were significant. The carryover effect was tested for by computing the average of the two treatments and comparing the two treatment orders using t-test. As we found no evidence of a carryover effect, the participants with the two different orders were combined, and the treatment effect was tested for using the paired t-test since each patient had each treatment. P < 0.05 was considered as statistically significant. All statistical analyses were done using the Statistical Package for Social Science version 17 (SPSS Inc., Chicago, Illinois, USA).
Results
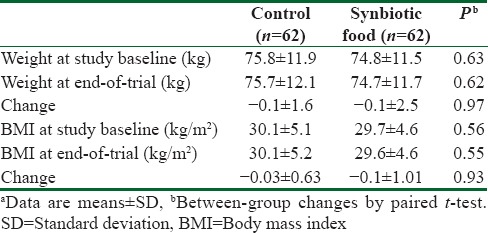
At baseline, we recruited 85 patients; however, 15 subjects were excluded from the study because of not living in Kashan (n = 10) and not meeting inclusion criteria (n = 5). Then, 70 patients with T2D aged 35–70 years were randomly allocated to intake either a synbiotic (n = 35) or a control food (n = 35) for 6 weeks. Among individuals in the synbiotic and control groups, 4 persons were excluded from the study. We found no significant alterations in weight and BMI at study baseline as well as after intervention between individuals consumed synbiotic food and those in the control groups [Table 1].
Table 1.
General characteristics of the study participantsa
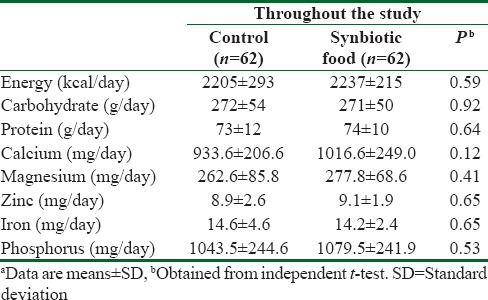
No statistically significant difference was observed between the two groups in terms of dietary intakes of energy, calcium, magnesium, zinc, iron, and phosphorus [Table 2]. Within-group differences in dietary intakes were also not significant.
Table 2.
Dietary intakes of study participants at run-in period and throughout the studya
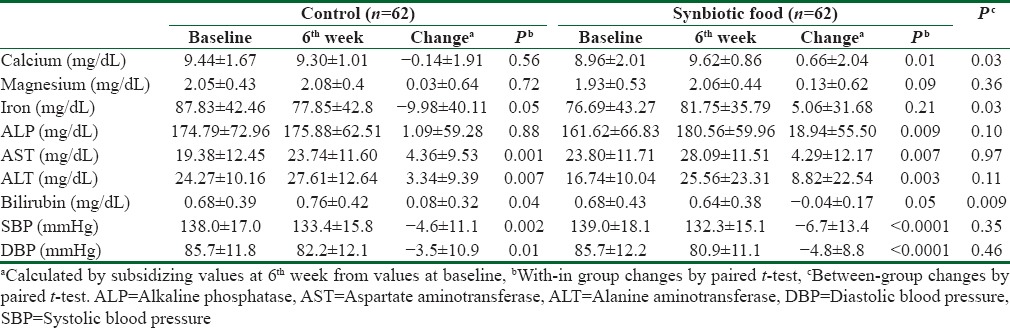
Intake of synbiotic food did not significantly affect fasting plasma glucose levels compared with control food (P = 0.11). Consumption of synbiotic food, compared to the control food, resulted in a significant increase in calcium (0.66 vs. −0.14 mg/dL, P = 0.03) and iron (5.06 vs. −9.98 mg/dL, P = 0.03) [Table 3]. Decreased values of total bilirubin (0.08 vs. −0.04 mg/dL; P = 0.009) were seen after synbiotic intake compared with the control. We did see no significant effect of synbiotic food intake on magnesium, ALP, AST, and ALT values. Despite a significant within-group reduction of systolic blood pressure (SBP) (−6.69; P < 0.0001, −4.62 mmHg; P = 0.002, respectively) and diastolic blood pressure (DBPs) (−4.77; P < 0.0001, −3.52 mmHg; P = 0.01, respectively), by synbiotic and control foods consumption, the between-group differences were not significant.
Table 3.
Means±standard deviation of serum minerals, liver enzymes and blood pressure at baseline and after the intervention
Discussion
This cross-over clinical trials revealed that the consumption of a synbiotic food for 6 weeks among patients with T2D resulted in a significant increase in serum calcium and iron concentrations and decreased total bilirubin levels. We did not find any significant effect of synbiotic food intake on serum magnesium, ALP, AST, and ALT levels as well as on SBP and DBP. Patients with T2D are very susceptible to abnormal serum minerals, increased activity of liver enzymes, and elevated blood pressure. Elevated activity of liver enzymes and high blood pressure among patients with T2D would result in several disorders, including the development of insulin resistance, vascular diseases, and stroke.[9,29,30]
The current study showed that synbiotic food intake significantly increased serum calcium and iron levels after 6 weeks among patients with T2D, but it did not affect serum magnesium concentrations. Earlier studies have reported beneficial effects of synbiotics, pro-, and pre-biotics on serum minerals. The majority of these studies were performed in animal models. The findings of our study were concurrent with several studies in animal studies. In a study by Naughton et al.[25] consumption of synbiotics containing Lactobacillus GG, Bifidobacterium lactis (Bb12), and oligofructose-enriched inulin for 21 days resulted in the increased levels of plasma calcium in aged rats. A significant increase of serum calcium levels with consumption of a probiotic preparation was also seen in broiler chickens.[31] Similar findings were also reported after consumption of Enterococcus faecium M74 preparation (2 × 109 CFU/g) in broiler chickens.[32] Furthermore, the effect of inulin ingestion on mineral absorption have been investigated in both growing and adult animals[33] and in humans.[34] Few studies have shown no significant effect,[35] whereas few found that intake of inulin induced the absorption of calcium[36,37] or magnesium.[38] Ingestion of 40 g/d of inulin has also led to increased calcium absorption among young men.[39] Several mechanisms might explain the favorable effects of synbiotics on increased levels of serum calcium and iron. Consumption of synbiotic might result in absorption of calcium and iron. In addition, the production of short-chain fatty acid (SCFA) by probiotics might improve the bioavailability of these trace elements.[40] Furthermore, fermentation of inulin in the gut can increase the SCFA production.[41] Increases in SCFA production have been associated with increased absorption of calcium and iron.
Our findings indicated that taking synbiotic in persons with T2D decreased total bilirubin levels, but it did not affect serum ALP, AST, and ALT levels. In a study by Kirpich et al.,[42] supplementation with Bifidobacterium bifidum (0.9 × 108 CFU/g) and Lactobacillus plantarum 8PA3 (0.9 × 109 CFU/g) for 6 weeks was associated with a significant reduction in serum total bilirubin, ALT, AST, GGT, and lactate dehydrogenase levels in human alcohol-induced liver injury. Treatment with fructooligosaccharides was also resulted in a decreased ALT activity compared to controls in rats.[43] However, no significant effects of probiotics (Ecologic 641, containing four Lactobacillus and two Bifidobacillus strains) were observed on serum bilirubin, ALP, ALT, AST, GGT, prothrombin, albumin, or bile salts in primary sclerosing cholangitis patients for 3 months.[44] Furthermore, consumption of E. faecium M74 (2 × 109 CFU) in experimental chickens led to a significant rise in serum bilirubin levels after 42 days.[32] The exact mechanisms by which synbiotics might affect total bilirubin concentrations are unknown. Earlier studies have suggested the probiotic-inflammation relationship. Segawa et al.[45] found the inhibition of TNF-α by Lactobacillus brevis. Biological membranes[46] and decreased insulin sensitivity[47] and/or promoting pro-inflammatory cytokines[46] might mediate the effects on serum bilirubin levels.
We did not find any significant effect of the synbiotic food consumption on blood pressure among patients with T2D. In consistent with our findings, no significant effects on blood pressure (systolic and diastolic) were seen with supplementation of Lactobacillus salivarius Ls-33 (10 CFU) among obese adolescents after 12 weeks.[48] Consumption of milk fermented with Lactobacillus helveticus resulted in a significant decrease in SBP and a nonsignificant decrease in DBP compared with placebo among patients with mild hypertension after 4 weeks.[49] Furthermore, long-chain inulin, oligofructose, or an oligofructose-enriched inulin did not affect the SBP for 15 weeks.[50] However, several other studies have reported the decrease of blood pressure through probiotics.[23,51] Supplementation with long-chain inulin and oligofructose-enriched inulin prevented fructose-induced elevated blood pressure in rats after 4 weeks.[52] The different findings of our study with other studies may be due to the choice, dosage of bacterial strain and inulin, different participants of the study as well as the intervention time.
Few limitations should be considered in our study. Duration of intervention was just 6 weeks. Long-term trials might reach much more beneficial effects on dependent variables. In addition, further studies are recommended to assess the effects of synbotics supplementation on the expressed levels of related variables to liver enzymes to explore the plausible mechanism and confirm our findings. In this trial, due to funding limitations, we did not characterize the microbiota and thus cannot establish whether taking synbiotic over 6 weeks changed its composition. However, it would be prudent to do this in future studies with a higher CFU treatment of the chosen probiotics product formulation.
Conclusions
Overall, synbiotic intake in T2D patients had beneficial effects on serum calcium, iron, and total bilirubin concentrations after 6 weeks; however, it did not affect serum magnesium concentrations, liver enzymes, and blood pressure.
Financial support and sponsorship
The present study was supported by a grant from the Vice chancellery for Research, Kashan University of Medical Sciences, Iran.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Aguilar MV, Saavedra P, Arrieta FJ, Mateos CJ, González MJ, Meseguer I, et al. Plasma mineral content in type-2 diabetic patients and their association with the metabolic syndrome. Ann Nutr Metab. 2007;51:402–6. doi: 10.1159/000108108. [DOI] [PubMed] [Google Scholar]
- 2.Shakeri H, Hadaegh H, Abedi F, Tajabadi-Ebrahimi M, Mazroii N, Ghandi Y, et al. Consumption of synbiotic bread decreases triacylglycerol and VLDL levels while increasing HDL levels in serum from patients with type-2 diabetes. Lipids. 2014;49:695–701. doi: 10.1007/s11745-014-3901-z. [DOI] [PubMed] [Google Scholar]
- 3.Kuzhandai velu V, Jyothirmayi B, Kumar JS. Insulin resistance and alanine amino transaminase (ALT) levels in first degree relatives of type 2 diabetes mellitus. Diabetes Metab Syndr. 2011;5:143–7. doi: 10.1016/j.dsx.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 4.Zheng JQ, Wang K, Pei D, Chen YL, Chang YL, Hsu CH, et al. Improvement of abnormal liver enzymes after rosiglitazone treatment in Chinese type 2 diabetes. Indian J Pharmacol. 2012;44:372–6. doi: 10.4103/0253-7613.96340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liverdisease. N Engl J Med. 2010;363:1341–50. doi: 10.1056/NEJMra0912063. [DOI] [PubMed] [Google Scholar]
- 6.Patel DA, Srinivasan SR, Xu JH, Chen W, Berenson GS. Persistent elevation of liver function enzymes within the reference range is associated with increased cardiovascular risk in young adults: The Bogalusa Heart Study. Metabolism. 2007;56:792–8. doi: 10.1016/j.metabol.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Nannipieri M, Gonzales C, Baldi S, Posadas R, Williams K, Haffner SM, et al. Liver enzymes, the metabolic syndrome, and incident diabetes: The Mexico City diabetes study. Diabetes Care. 2005;28:1757–62. doi: 10.2337/diacare.28.7.1757. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen QM, Srinivasan SR, Xu JH, Chen W, Hassig S, Rice J, et al. Elevated liver function enzymes are related to the development of prediabetes and type 2 diabetes in younger adults: The Bogalusa heart study. Diabetes Care. 2011;34:2603–7. doi: 10.2337/dc11-0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: Epidemiology, pathophysiology, and management. JAMA. 2002;287:2570–81. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- 10.Singer DR, Kite A. Management of hypertension in peripheral arterial disease: Does the choice of drugs matter? Eur J Vasc Endovasc Surg. 2008;35:701–8. doi: 10.1016/j.ejvs.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Shakur YA, Garriguet D, Corey P, O’Connor DL. Folic acid fortification above mandated levels results in a low prevalence of folate inadequacy among Canadians. Am J Clin Nutr. 2010;92:818–25. doi: 10.3945/ajcn.2010.29696. [DOI] [PubMed] [Google Scholar]
- 12.Martini LA, Catania AS, Ferreira SR. Role of vitamins and minerals in prevention and management of type 2 diabetes mellitus. Nutr Rev. 2010;68:341–54. doi: 10.1111/j.1753-4887.2010.00296.x. [DOI] [PubMed] [Google Scholar]
- 13.Liu J, Bao W, Jiang M, Zhang Y, Zhang X, Liu L. Chromium, selenium, and zinc multimineral enriched yeast supplementation ameliorates diabetes symptom in streptozocin-induced mice. Biol Trace Elem Res. 2012;146:236–45. doi: 10.1007/s12011-011-9248-x. [DOI] [PubMed] [Google Scholar]
- 14.Blumenthal JA, Babyak MA, Hinderliter A, Watkins LL, Craighead L, Lin PH, et al. Effects of the DASH diet alone and in combination with exercise and weight loss on blood pressure and cardiovascular biomarkers in men and women with high blood pressure: The ENCORE study. Arch Intern Med. 2010;170:126–35. doi: 10.1001/archinternmed.2009.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iliadis F, Kadoglou NP, Hatzitolios A, Karamouzis M, Alevizos M, Karamitsos D. Metabolic effects of rosiglitazone and metformin in Greek patients with recently diagnosed type 2 diabetes. In Vivo. 2007;21:1107–14. [PubMed] [Google Scholar]
- 16.Yerram P, Whaley-Connell A. Novel role for the incretins in blood pressure regulation. Curr Opin Nephrol Hypertens. 2012;21:463–8. doi: 10.1097/MNH.0b013e328356bccd. [DOI] [PubMed] [Google Scholar]
- 17.Scholz-Ahrens KE, Ade P, Marten B, Weber P, Timm W, Açil Y, et al. Prebiotics, probiotics, and synbiotics affect mineral absorption, bone mineral content, and bone structure. J Nutr. 2007;137(3 Suppl 2):838S–46S. doi: 10.1093/jn/137.3.838S. [DOI] [PubMed] [Google Scholar]
- 18.Sohail MU, Rahman ZU, Ijaz A, Yousaf MS, Ashraf K, Yaqub T, et al. Single or combined effects of mannan-oligosaccharides and probiotic supplements on the total oxidants, total antioxidants, enzymatic antioxidants, liver enzymes, and serum trace minerals in cyclic heat-stressed broilers. Poult Sci. 2011;90:2573–7. doi: 10.3382/ps.2011-01502. [DOI] [PubMed] [Google Scholar]
- 19.Hord NG. Eukaryotic-microbiota crosstalk: Potential mechanisms for health benefits of prebiotics and probiotics. Annu Rev Nutr. 2008;28:215–31. doi: 10.1146/annurev.nutr.28.061807.155402. [DOI] [PubMed] [Google Scholar]
- 20.de Vrese M, Schrezenmeir J. Probiotics, prebiotics, and synbiotics. Adv Biochem Eng Biotechnol. 2008;111:1–66. doi: 10.1007/10_2008_097. [DOI] [PubMed] [Google Scholar]
- 21.Xing HC, Li LJ, Xu KJ, Shen T, Chen YB, Sheng JF, et al. Protective role of supplement with foreign Bifidobacterium and Lactobacillus in experimental hepatic ischemia-reperfusion injury. J Gastroenterol Hepatol. 2006;21:647–56. doi: 10.1111/j.1440-1746.2006.04306.x. [DOI] [PubMed] [Google Scholar]
- 22.Hashem MA, Mohamed MH. Haemato-biochemical and pathological studies on aflatoxicosis and treatment of broiler chicks in Egypt. Vet Ital. 2009;45:323–37. [PubMed] [Google Scholar]
- 23.Donkor ON, Henriksson A, Vasiljevic T, Shah N. [alpha]-Galactosidase and proteolytic activities of selected probiotic and dairy cultures in fermented soymilk. Food Chem. 2007;104:10–20. [Google Scholar]
- 24.Cloetens L, Ulmius M, Johansson-Persson A, Akesson B, Onning G. Role of dietary beta-glucans in the prevention of the metabolic syndrome. Nutr Rev. 2012;70:444–58. doi: 10.1111/j.1753-4887.2012.00494.x. [DOI] [PubMed] [Google Scholar]
- 25.Naughton V, McSorley E, Naughton PJ. Changes in calcium status in aged rats fed Lactobacillus GG and Bifidobacterium lactis and oligofructose-enriched inulin. Appl Physiol Nutr Metab. 2011;36:161–5. doi: 10.1139/H10-088. [DOI] [PubMed] [Google Scholar]
- 26.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012;35(Suppl 1):S64–71. doi: 10.2337/dc12-s064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: An update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 Suppl):S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 28.Taghizadeh M, Hashemi T, Shakeri H, Abedi F, Sabihi SS, Alizadeh SA, et al. Synbiotic food consumption reduces levels of triacylglycerols and VLDL, but not cholesterol, LDL, or HDL in plasma from pregnant women. Lipids. 2014;49:155–61. doi: 10.1007/s11745-013-3867-2. [DOI] [PubMed] [Google Scholar]
- 29.Hanley AJ, Wagenknecht LE, Festa A, D’Agostino RB, Haffner SM. Alanine aminotransferase and directly measured insulin sensitivity in a multiethnic cohort: The Insulin Resistance Atherosclerosis Study. Diabetes Care. 2007;30:1819–27. doi: 10.2337/dc07-0086. [DOI] [PubMed] [Google Scholar]
- 30.Weyer C, Tataranni PA, Bogardus C, Pratley RE. Insulin resistance and insulin secretory dysfunction are independent predictors of worsening of glucose tolerance during each stage of type 2 diabetes development. Diabetes Care. 2001;24:89–94. doi: 10.2337/diacare.24.1.89. [DOI] [PubMed] [Google Scholar]
- 31.Capcarova M, Hascik P, Kolesarova A, Kacaniova M, Mihok M, Pal G. The effect of selected microbial strains on internal milieu of broiler chickens after peroral administration. Res Vet Sci. 2011;91:132–7. doi: 10.1016/j.rvsc.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 32.Capcarova M, Weiss J, Hrncar C, Kolesarova A, Pal G. Effect of Lactobacillus fermentum and Enterococcus faecium strains on internal milieu, antioxidant status and body weight of broiler chickens. J Anim Physiol Anim Nutr (Berl) 2010;94:e215–24. doi: 10.1111/j.1439-0396.2010.01010.x. [DOI] [PubMed] [Google Scholar]
- 33.Younes H, Coudray C, Bellanger J, Demigné C, Rayssiguier Y, Rémésy C. Effects of two fermentable carbohydrates (inulin and resistant starch) and their combination on calcium and magnesium balance in rats. Br J Nutr. 2001;86:479–85. doi: 10.1079/bjn2001430. [DOI] [PubMed] [Google Scholar]
- 34.Coudray C, Feillet-Coudray C, Tressol JC, Gueux E, Thien S, Jaffrelo L, et al. Stimulatory effect of inulin on intestinal absorption of calcium and magnesium in rats is modulated by dietary calcium intakes short- and long-term balance studies. Eur J Nutr. 2005;44:293–302. doi: 10.1007/s00394-004-0526-7. [DOI] [PubMed] [Google Scholar]
- 35.López-Huertas E, Teucher B, Boza JJ, Martínez-Férez A, Majsak-Newman G, Baró L, et al. Absorption of calcium from milks enriched with fructo-oligosaccharides, caseinophosphopeptides, tricalcium phosphate, and milk solids. Am J Clin Nutr. 2006;83:310–6. doi: 10.1093/ajcn/83.2.310. [DOI] [PubMed] [Google Scholar]
- 36.van den Heuvel EG, Schoterman MH, Muijs T. Transgalactooligosaccharides stimulate calcium absorption in postmenopausal women. J Nutr. 2000;130:2938–42. doi: 10.1093/jn/130.12.2938. [DOI] [PubMed] [Google Scholar]
- 37.Griffin IJ, Davila PM, Abrams SA. Non-digestible oligosaccharides and calcium absorption in girls with adequate calcium intakes. Br J Nutr. 2002;87(Suppl 2):S187–91. doi: 10.1079/BJNBJN/2002536. [DOI] [PubMed] [Google Scholar]
- 38.Tahiri M, Tressol JC, Arnaud J, Bornet F, Bouteloup-Demange C, Feillet-Coudray C, et al. Five-week intake of short-chain fructo-oligosaccharides increases intestinal absorption and status of magnesium in postmenopausal women. J Bone Miner Res. 2001;16:2152–60. doi: 10.1359/jbmr.2001.16.11.2152. [DOI] [PubMed] [Google Scholar]
- 39.Coudray C, Bellanger J, Castiglia-Delavaud C, Rémésy C, Vermorel M, Rayssignuier Y. Effect of soluble or partly soluble dietary fibres supplementation on absorption and balance of calcium, magnesium, iron and zinc in healthy young men. Eur J Clin Nutr. 1997;51:375–80. doi: 10.1038/sj.ejcn.1600417. [DOI] [PubMed] [Google Scholar]
- 40.Silva MR, Dias G, Ferreira CL, Franceschini SC, Costa NM. Growth of preschool children was improved when fed an iron-fortified fermented milk beverage supplemented with Lactobacillus acidophilus. Nutr Res. 2008;28:226–32. doi: 10.1016/j.nutres.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 41.Sauer J, Richter KK, Pool-Zobel BL. Products formed during fermentation of the prebiotic inulin with human gut flora enhance expression of biotransformation genes in human primary colon cells. Br J Nutr. 2007;97:928–37. doi: 10.1017/S0007114507666422. [DOI] [PubMed] [Google Scholar]
- 42.Kirpich IA, Solovieva NV, Leikhter SN, Shidakova NA, Lebedeva OV, Sidorov PI, et al. Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: A pilot study. Alcohol. 2008;42:675–82. doi: 10.1016/j.alcohol.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neyrinck AM, Alexiou H, Delzenne NM. Kupffer cell activity is involved in the hepatoprotective effect of dietary oligofructose in rats with endotoxic shock. J Nutr. 2004;134:1124–9. doi: 10.1093/jn/134.5.1124. [DOI] [PubMed] [Google Scholar]
- 44.Vleggaar FP, Monkelbaan JF, van Erpecum KJ. Probiotics in primary sclerosing cholangitis: A randomized placebo-controlled crossover pilot study. Eur J Gastroenterol Hepatol. 2008;20:688–92. doi: 10.1097/MEG.0b013e3282f5197e. [DOI] [PubMed] [Google Scholar]
- 45.Segawa S, Wakita Y, Hirata H, Watari J. Oral administration of heat-killed Lactobacillus brevis SBC8803 ameliorates alcoholic liver disease in ethanol-containing diet-fed C57BL/6N mice. Int J Food Microbiol. 2008;128:371–7. doi: 10.1016/j.ijfoodmicro.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 46.Larson-Meyer DE, Newcomer BR, Heilbronn LK, Volaufova J, Smith SR, Alfonso AJ, et al. Effect of 6-month calorie restriction and exercise on serum and liver lipids and markers of liver function. Obesity (Silver Spring) 2008;16:1355–62. doi: 10.1038/oby.2008.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171–6. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gobel RJ, Larsen N, Jakobsen M, Molgaard C, Michaelsen KF. Probiotics to obese adolescents; RCT examining the effects on inflammation and metabolic syndrome. J Pediatr Gastroenterol Nutr. 2012;55:673–8. doi: 10.1097/MPG.0b013e318263066c. [DOI] [PubMed] [Google Scholar]
- 49.Aihara K, Kajimoto O, Hirata H, Takahashi R, Nakamura Y. Effect of powdered fermented milk with Lactobacillus helveticus on subjects with high-normal blood pressure or mild hypertension. J Am Coll Nutr. 2005;24:257–65. doi: 10.1080/07315724.2005.10719473. [DOI] [PubMed] [Google Scholar]
- 50.Rault-Nania MH, Gueux E, Demougeot C, Demigné C, Rock E, Mazur A. Inulin attenuates atherosclerosis in apolipoprotein E-deficient mice. Br J Nutr. 2006;96:840–4. doi: 10.1017/bjn20061913. [DOI] [PubMed] [Google Scholar]
- 51.Korhonen H. Milk-derived bioactive peptides: From science to applications. J Funct Foods. 2009;1:177–87. [Google Scholar]
- 52.Rault-Nania MH, Demougeot C, Gueux E, Berthelot A, Dzimira S, Rayssiguier Y, et al. Inulin supplementation prevents high fructose diet-induced hypertension in rats. Clin Nutr. 2008;27:276–82. doi: 10.1016/j.clnu.2008.01.015. [DOI] [PubMed] [Google Scholar]


