Abstract
Background and Aims:
Local infiltration of the surgical wound is one of the important components of multimodal analgesia for post-operative pain relief. This study determines the post-operative analgesic effect of addition of dexmedetomidine to bupivacaine for local infiltration of the surgical wound.
Methods:
Sixty women belonging to American Society of Anesthesiologists’ Grade 1 or 2 posted for abdominal hysterectomy were randomly allocated to Group I (control group) where patients received wound infiltration with 30 mL 0.25% bupivacaine at the end of surgery, or Group II, where patients received wound infiltration with 1.0 μg/kg dexmedetomidine diluted in 30 mL 0.25% bupivacaine. The primary objective of the study was to assess post-operative pain scores. Number of patients requiring rescue analgesia and total morphine consumption during 24 h after surgery were also recorded. Statistical significance for analgesic requirement was determined by one-way analysis of variance.
Results:
Pain scores were lower at rest for 12 h and on cough for 6 h in Group II (<0.01). All patients in Group I required supplemental morphine compared to only 3 patients in Group II (P < 0.003). Post-operative analgesia requirement was significantly less in patients receiving dexmedetomidine in wound infiltration compared to patients receiving bupivacaine alone (P < 0.001).
Conclusions:
Wound infiltration of dexmedetomidine with bupivacaine provides superior pain relief compared to bupivacaine alone.
Keywords: Analgesia, dexmedetomidine, hysterectomy
INTRODUCTION
Abdominal hysterectomy, the second most common surgery in females between age of 25 and 50 years after caesarean section, is associated with moderate to severe post-operative pain.[1] The post-operative pain not only delays recovery but also can lead to chronic pain. A multimodal approach to pain is the current standard in perioperative pain management. Epidural analgesia may be considered by some to be gold standard for pain management after abdominal surgeries.[2] Nevertheless, concerns remain regarding complications after neuraxial blocks specifically in older patients.[3] Thus, there is considerable interest in alternative methods for analgesia requiring minimal post-operative monitoring. Post-operative wound infiltration with local anaesthetics is an attractive method because of its simplicity, safety, and low cost.[4] Local anaesthetic infiltration with added adjuvants can improve the quality and duration of analgesia. The added adjuvants are epinephrine, ketorolac, opioids, clonidine, etc.[5] Dexmedetomidine, a potent α2 adrenoceptor agonist, is approximately eight times more selective towards α2 adrenoceptor than clonidine.[6] When dexmedetomidine is given intravenously, it has a significant opioid sparing effect as well as decreased requirement of anaesthetic agents.[6,7] Dexmedetomidine also has been used as an adjunct to local anaesthetics for various nerve blocks.[8] The current study was designed to test the hypothesis that dexmedetomidine when added as an adjuvant to local anaesthetic for post-operative wound infiltration after abdominal hysterectomy effectively reduces morphine consumption in first 24 hours of post operative period.
METHODS
The study was approved by Institutional Ethics Review Committee. The clinical trial is also registered with Clinical Trials Registry of India (CTRI/2016/09/007245). Sixty women posted for elective abdominal hysterectomy under general anaesthesia between January 2016 and November 2016 belonging to American Society of Anesthesiologists’ (ASA) physical status (I or II) aged 30–60 years were selected for the study. Patients with morbid obesity, Raynaud's disease, hepatorenal insufficiency, those receiving adrenoceptor agonists or antagonists or narcotics before the operation were excluded from the study. All patients underwent a pre-operative assessment on the day before surgery and written informed consent was obtained for participation in the study. They were pre-medicated with oral midazolam 0.5 mg/kg 2 h before surgery. Patients were randomly allocated into two groups using a computer-generated random number table. Group I (control group) patients received wound infiltration with 30 mL 0.25% bupivacaine at the end of surgery. Group II patients received 30 mL 0.25% bupivacaine with 1 μg/kg dexmedetomidine at the end of surgery. The person who prepared the study drugs did not participate in the data collection. Anaesthesia was induced with propofol 2–3 mg/kg intravenous (IV) and fentanyl 2 μg/kg IV followed by incremental doses of 1 μg/kg IV hourly. Tracheal intubation was facilitated by vecuronium 0.1 mg/kg IV. Anaesthesia was maintained with isoflurane and 60% nitrous oxide in oxygen. Patients were monitored using Datex Ohmeda GE B40 cardiac monitor. Intraoperative monitoring included electrocardiogram leads II and V5, non-invasive blood pressure at 5 min intervals, oxygen saturation, end-tidal carbon dioxide and nasopharyngeal temperature. Patient's lungs were ventilated by intermittent positive pressure ventilation using a circle system to maintain normocapnia. Heart rate (HR) and mean arterial pressure (MAP) were maintained within 20% of the pre-operative value. Hypotension (MAP <20% of the baseline or <60 mmHg) was treated with infusion of normal saline and if required injection mephentermine 3–6 mg boluses IV. Bradycardia (HR <40 beats/min) was treated with IV atropine 40 μg/kg bolus. All patients received paracetamol 20 mg/kg IV and ondansetron 0.1 mg/kg IV ½ h before the completion of surgery. At the end of surgery, residual neuromuscular block was antagonised with appropriate dose of neostigmine and glycopyrrolate IV. Tracheal extubation was performed on meeting the standard criteria for extubation. Post-operative analgesia was provided with tramadol 1.5 mg/kg IV every 8 h.
Patients were observed for 24 h after operation in the post-anaesthesia care unit (PACU) by an anaesthesiologist who was not aware of the patient's group assignment. The primary objective was to assess pain at rest and at cough by visual analogue scale (VAS 0–10: 0 - no pain, 10 - worst imaginable pain) at the time of arrival in the PACU and then at 2, 4, 6, 12 and 24 h after operation. Rescue analgesia was given with morphine 3 mg IV boluses on demand or whenever VAS score was ≥4. The number of patients requiring rescue analgesia and total morphine consumption during the first 24 h after operation was recorded. The level of sedation was assessed using four-point sedation scale (0–3, 0 - awake and oriented, 1 - drowsy but responding to commands, 2 - sleepy but easy to arouse [by loud command or glabellar tap], 3 - deep sleep, difficult to arouse). The incidence and severity of nausea and vomiting were assessed by 4-point categorical scales (0 - none, 1 - mild, 2 - moderate, 3 - severe). Metoclopramide 10 mg IV was given for severe nausea or vomiting. Any other adverse effect was also recorded. Patient's satisfaction with the technique was assessed at 24 h after operation on an 11-point satisfaction score (0 - unsatisfied, 10 - most satisfied).
Sample size was calculated on the basis of previous study.[9] At 95% significance level and 80% power, assuming 30% reduction in morphine consumption, 27 patients were required in each group. To minimise the effects of data loss, a total of sixty patients were enrolled. The data from the present study were systematically collected, compiled and statistically analysed by Statistical Package for Social Sciences Version 15.0 software (Chicago, USA) Statistical significance for analgesic requirement was determined by one-way analysis of variance (ANOVA). The pain scores, sedation scores and patient satisfaction scores (non-parametric data) were compared by Kruskal–Wallis anova followed by Mann–Whitney U-test for inter-group differences. ASA physical status, sex ratio and need for rescue analgesia in recovery room were analysed using Chi-square test and Fisher's exact test. Bonferroni correction was used for multiple comparisons. Comparisons of HR and arterial pressure were made using ANOVA, followed by Student–Neumans–Keul test for in-between group comparisons. Differences were considered statistically significant if P < 0.05.
RESULTS
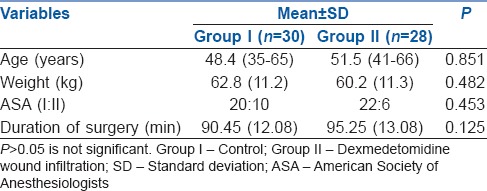
In total, 58 patients completed the study out of sixty recruited. Two patients were excluded from the analysis (both underwent extended hysterectomy) as shown in consort chart [Figure 1]. Both groups were similar with respect to patient characteristics, ASA physical status and duration of surgery [Table 1].
Figure 1.
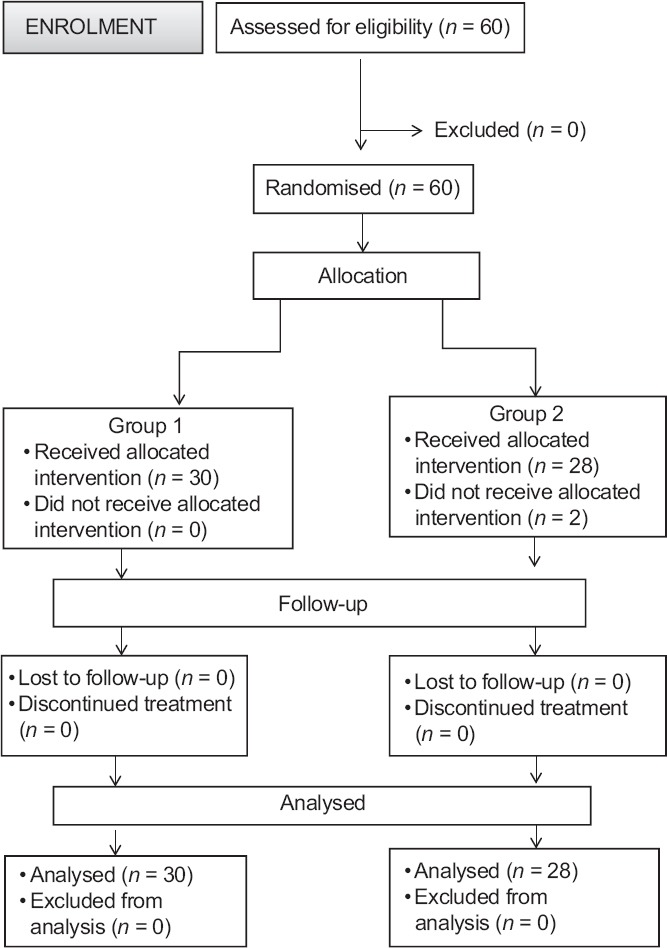
Consort diagram
Table 1.
Comparison of demographic data of both the group
Group II had significantly lower pain scores at rest for first 12 h i.e., at 2, 4, 6 and 12 h [Figure 2] and on cough for 6 h after operation when compared with patients in Group I.
Figure 2.
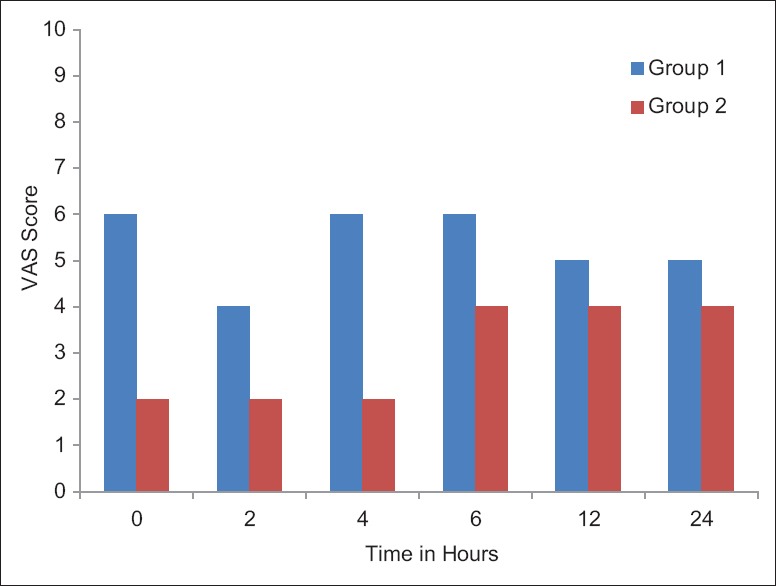
Comparison of visual analogue scale score of both the group at rest. Group I - Control; Group II - Dexmedetomidine wound infiltration
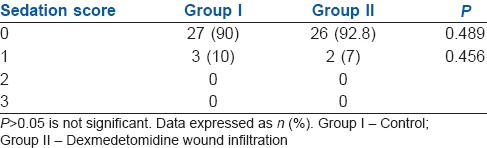
The 24 h morphine consumption was also less in Group II when compared with Group I. All the patients in Group I (100%) required supplemental morphine, while only 14 patients in Group II (50%) required it and this was statistically significant (P < 0.002) [Table 2]. Sedation scores in both the groups were comparable [Table 3].
Table 2.
Comparison of total morphine consumption in both the groups
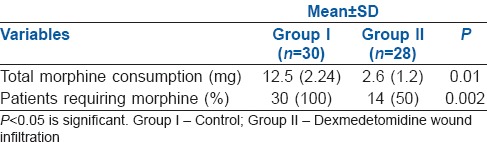
Table 3.
Comparison of the sedation score in both the groups
The incidence of post-operative hypotension was negligible in both the groups. No other side effect was recorded in any group. Patients in Group II were more satisfied than those in Group I (satisfaction score, median [interquartile range], 6.00[1] and 8.00[1] for Groups I and II, respectively, P < 0.0001). Intraoperative HR and MAP were comparable among groups.
DISCUSSION
In this study, we found statistically significant reduction in post-operative morphine requirement when the patients were given post-operative wound infiltration with bupivacaine and dexmedetomidine combination. Furthermore, complications such as hypotension, sedation and bradycardia associated with IV dexmedetomidine were negligible when dexmedetomidine was given as local infiltration.
In this study, we hypothesised that post-operative local infiltration of dexmedetomidine at incisional site would decrease the requirement of other analgesics in post-operative period and may prove to be a significant contribution in multimodal analgesia. Various research done so far has shown good results for the use of dexmedetomidine in IV sedation (Intensive Care Unit and operative patients), spinal,[10] epidural,[11] caudal anaesthesia[12] and Bier's block.[5,13]
Peripherally, α2-agonists produce analgesia by reducing the release of norepinephrine and causing α2-receptor-independent inhibitor effect on nerve fibre action potential. Infiltration of dexmedetomidine in surgical wound may be useful to avoid the adverse hemodynamic effects of IV administration while still providing post-operative analgesia.[14] Various animal studies have reported potent antinociceptive effect of dexmedetomidine on peripheral administration along with its safety. Dexmedetomidine enhanced duration of bupivacaine anaesthesia and analgesia of sciatic nerve block in rats without any evidence of histopathological damage to the nerve.[15,16] In another study, dexmedetomidine added to ropivacaine increased the duration of sciatic nerve blockade in rats, most likely due to the blockade of hyperpolarisation-activated cation current (i.e., a direct effect on the peripheral nerve activity).[17] When dexmedetomidine and clonidine were added to lignocaine for nerve block, it enhanced the local anaesthetic action of lignocaine through peripheral α-2A adrenoceptors.[18] In the present study, patients who received dexmedetomidine in wound infiltration with bupivacaine after abdominal hysterectomy had reduced post-operative pain score and morphine requirement when compared with the control group. This was similar to few other studies using local infiltration of dexmedetomidine for various surgeries with no delay in psychomotor recovery or increase in post-operative clinically significant adverse effect.[18,19,20]
The main limitation of our study is that we did not compare dexmedetomidine infiltration with IV dexmedetomidine. Further studies are required to see that prolonged analgesic effect of dexmedetomidine infiltration is not due to its intravascular absorption rather due to peripheral effect.
CONCLUSIONS
Wound infiltration of bupivacaine with dexmedetomidine 1.0 μg/kg provides superior pain relief compared to wound infiltration with bupivacaine alone.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Perniola A, Gupta A, Crafoord K, Darvish B, Magnuson A, Axelsson K. Intraabdominal local anaesthetics for postoperative pain relief following abdominal hysterectomy: A randomized, double-blind, dose-finding study. Eur J Anaesthesiol. 2009;26:421–9. doi: 10.1097/EJA.0b013e3283261b53. [DOI] [PubMed] [Google Scholar]
- 2.Hein A, Rösblad P, Gillis-Haegerstrand C, Schedvins K, Jakobsson J, Dahlgren G. Low dose intrathecal morphine effects on post-hysterectomy pain: A randomized placebo-controlled study. Acta Anaesthesiol Scand. 2012;56:102–9. doi: 10.1111/j.1399-6576.2011.02574.x. [DOI] [PubMed] [Google Scholar]
- 3.Cook TM, Counsell D, Wildsmith JA. Royal College of Anaesthetists Third National Audit Project. Major complications of central neuraxial block: Report on the Third National Audit Project of the Royal College of Anaesthetists. Br J Anaesth. 2009;102:179–90. doi: 10.1093/bja/aen360. [DOI] [PubMed] [Google Scholar]
- 4.Bray DA, Jr, Nguyen J, Craig J, Cohen BE, Collins DR., Jr Efficacy of a local anesthetic pain pump in abdominoplasty. Plast Reconstr Surg. 2007;119:1054–9. doi: 10.1097/01.prs.0000252536.56982.34. [DOI] [PubMed] [Google Scholar]
- 5.Demiraran Y, Ilce Z, Kocaman B, Bozkurt P. Does tramadol wound infiltration offer an advantage over bupivacaine for postoperative analgesia in children following herniotomy? Paediatr Anaesth. 2006;16:1047–50. doi: 10.1111/j.1460-9592.2006.01910.x. [DOI] [PubMed] [Google Scholar]
- 6.Ravipati P, Reddy PN, Kumar C, Pradeep P, Pathapati RM, Rajashekar ST. Dexmedetomidine decreases the requirement of ketamine and propofol during burns debridement and dressings. Indian J Anaesth. 2014;58:138–42. doi: 10.4103/0019-5049.130813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts SB, Wozencraft CP, Coyne PJ, Smith TJ. Dexmedetomidine as an adjuvant analgesic for intractable cancer pain. J Palliat Med. 2011;14:371–3. doi: 10.1089/jpm.2010.0235. [DOI] [PubMed] [Google Scholar]
- 8.Yoshitomi T, Kohjitani A, Maeda S, Higuchi H, Shimada M, Miyawaki T. Dexmedetomidine enhances the local anesthetic action of lidocaine via an alpha-2A adrenoceptor. Anesth Analg. 2008;107:96–101. doi: 10.1213/ane.0b013e318176be73. [DOI] [PubMed] [Google Scholar]
- 9.Bharti N, Dontukurthy S, Bala I, Singh G. Postoperative analgesic effect of intravenous (i.v.) clonidine compared with clonidine administration in wound infiltration for open cholecystectomy. Br J Anaesth. 2013;111:656–61. doi: 10.1093/bja/aet130. [DOI] [PubMed] [Google Scholar]
- 10.Gupta R, Verma R, Bogra J, Kohli M, Raman R, Kushwaha JK. A comparative study of intrathecal dexmedetomidine and fentanyl as adjuvants to bupivacaine. J Anaesthesiol Clin Pharmacol. 2011;27:339–43. doi: 10.4103/0970-9185.83678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bajwa SJ, Bajwa SK, Kaur J, Singh G, Arora V, Gupta S, et al. Dexmedetomidine and clonidine in epidural anaesthesia: A comparative evaluation. Indian J Anaesth. 2011;55:116–21. doi: 10.4103/0019-5049.79883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abosedira MA. Adding clonidine or dexmedetomidine to lignocaine during Biers block: A comparative study. J Med Sci. 2008;8:660–4. [Google Scholar]
- 13.Neogi M, Bhattacharjee DP, Dawn S, Chatterjee N. A comparative study between clonidin and dexmedetomidine used as adjuncts to ropivacaine for caudal analgesia in paediatric patients. J Anaesthesiol Clin Pharmacol. 2010;26:149–53. [Google Scholar]
- 14.Grewal A. Dexmedetomidine: New avenues. J Anaesthesiol Clin Pharmacol. 2011;27:297–302. doi: 10.4103/0970-9185.83670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brummett CM, Norat MA, Palmisano JM, Lydic R. Perineural administration of dexmedetomidine in combination with bupivacaine enhances sensory and motor blockade in sciatic nerve block without inducing neurotoxicity in rat. Anesthesiology. 2008;109:502–11. doi: 10.1097/ALN.0b013e318182c26b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brummett CM, Amodeo FS, Janda AM, Padda AK, Lydic R. Perineural dexmedetomidine provides an increased duration of analgesia to a thermal stimulus when compared with a systemic control in a rat sciatic nerve block. Reg Anesth Pain Med. 2010;35:427–31. doi: 10.1097/AAP.0b013e3181ef4cf0. [DOI] [PubMed] [Google Scholar]
- 17.Brummett CM, Hong EK, Janda AM, Amodeo FS, Lydic R. Perineural dexmedetomidine added to ropivacaine for sciatic nerve block in rats prolongs the duration of analgesia by blocking the hyperpolarization-activated cation current. Anesthesiology. 2011;115:836–43. doi: 10.1097/ALN.0b013e318221fcc9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheung CW, Ng KF, Choi WS, Chiu WK, Ying CL, Irwin MG. Evaluation of the analgesic efficacy of local dexmedetomidine application. Clin J Pain. 2011;27:377–82. doi: 10.1097/AJP.0b013e318208c8c5. [DOI] [PubMed] [Google Scholar]
- 19.Kang H, Kim BG. Intravenous lidocaine for effective pain relief after inguinal herniorrhaphy: A prospective, randomized, double-blind, placebo-controlled study. J Int Med Res. 2011;39:435–45. doi: 10.1177/147323001103900211. [DOI] [PubMed] [Google Scholar]
- 20.Esmaoglu A, Yegenoglu F, Akin A, Turk CY. Dexmedetomidine added to levobupivacaine prolongs axillary brachial plexus block. Anesth Analg. 2010;111:1548–51. doi: 10.1213/ANE.0b013e3181fa3095. [DOI] [PubMed] [Google Scholar]


