Abstract
Background and Aims:
Caudal epidural analgesia is commonly practised regional block technique in children undergoing infraumbilical surgeries but has a short duration of action after single shot local anaesthetic injection. The aim of this study was to compare ropivacaine 0.25% with dexmedetomidine and tramadol in caudal anaesthesia in paediatric infraumbilical surgeries.
Methods:
In a randomised, prospective, double-blinded study, sixty children (1–8 years) belonging to American Society of Anesthesiologists’ physical status I or II scheduled for infraumbilical surgeries were included. They were randomly assigned into two groups: Group ropivacaine with tramadol (RT) (n = 30) received 0.25% ropivacaine 1 mL/kg with 2 mg/kg of tramadol, and Group ropivacaine with dexmedetomidine (RD) (n = 30) received 0.25% ropivacaine 1 mL/kg with dexmedetomidine 2 μg/kg. The primary outcome variable was the duration of analgesia, and the secondary outcome variables included motor block, sedation score, time from caudal block to skin incision, emergence time and adverse effects.
Results:
The mean duration of analgesia was 654.20 ± 78.38 min in Group RT, while in Group RD, it was 780.29 ± 71.21 min (P = 0.0001). The difference between the mean sedation score and mean emergence time between the two groups were statistically significant (P = 0.0001 and 0.0411, respectively). No significant difference was observed in the incidence of haemodynamic changes or side effects.
Conclusion:
Caudal dexmedetomidine with ropivacaine prolongs post-operative analgesia compared to caudal tramadol with ropivacaine.
Keywords: Anaesthesia, analgesia, caudal block, dexmedetomidine, emergence agitation, ropivacaine, tramadol
INTRODUCTION
Caudal block is one of the most popular and commonly used regional anaesthetic procedures in paediatric patients for most surgeries below the umbilicus. The block can be practised by a single-shot injection or as a continuous infusion through a caudal epidural catheter. For continuous infusion, use of a caudal catheter is usually not preferred due to high risk of catheter contamination from faecal soiling.[1] Ropivacaine, the S-enantiomer of the amide local anaesthetic, is suitable for day-care surgery in children as it produces differential neural blockade, with less motor blockade, cardiovascular and neurological toxicity.[2]
To extend the duration of post-operative analgesia provided by the ‘single shot’ caudal technique, various additives, such as tramadol, ketamine, ephedrine, morphine, fentanyl and clonidine with local anaesthetics, have been investigated. Tramadol, a synthetic 4-phenyl-piperidine analogue of codeine, is a racemic mixture of two enantiomers, both of which contribute to the analgesic activity through different mechanisms enhancing inhibitory effects on pain transmission in the spinal cord. The (+) enantiomer has moderate affinity for the opioid μ-receptor, which is greater than that of the (−) enantiomer. In addition, the (+) enantiomer stimulates the pre-synaptic release of serotonin and inhibits serotonin reuptake, and the (−) enantiomer is a norepinephrine reuptake inhibitor. The complementary and synergistic actions of the two enantiomers improve the analgesic efficacy and tolerability profile of the two. Tramadol has a striking lack of respiratory depressant effect despite having analgesic potency approximately equal to that of pethidine.[3,4]
To improve the quality and duration of analgesia in recent years, studies are being conducted to evaluate dexmedetomidine as an adjuvant in regional anaesthesia. Dexmedetomidine, a stereoisomer of medetomidine, is a highly selective α2-adrenergic receptor agonist with eight times more specificity for α2 adrenoceptors than clonidine (ratios of α2:α1 activity, 1620:1 for dexmedetomidine and 220:1 for clonidine).[5] It provides better perioperative haemodynamic stability than many other adjuvants now in use and good quality of intraoperative and prolonged post-operative analgesia with minimal side effects. It has sympatholytic, analgesic and sedative effects and is remarkably free from side effects except for manageable hypotension and bradycardia.[6] We hypothesised that adding dexmedetomidine to the caudal ropivacaine would prolong the duration of analgesia in comparison to tramadol-ropivacaine (primary outcome) and duration of the motor block, post-operative sedation as well as the incidence of any side effect (secondary outcome) in paediatric patients undergoing infraumbilical surgeries.
METHODS
After the Institutional Ethics Committee approval, written informed consent was obtained from the parents of all children in this randomised, prospective double-blinded study. This study was conducted in sixty children of American Society of Anesthesiologists’ physical status I or II, aged 1–8 years, undergoing elective infraumbilical surgeries. Patients having a history or evidence of infection at the back, allergy to drugs, bleeding/coagulation disorder, history of developmental delay, sepsis, pre-existing neurological or spinal diseases and allergy to the study drugs were excluded from the study.
During a pre-operative visit on the day before surgery, all parents were explained about the anaesthetic technique and the perioperative course. Patients were randomly assigned into two groups (thirty patients in each group) using the Web site Randomization.com (http://www.randomization.com); the allocation ratio was 1:1, and the group identification paper was put in sequentially numbered, sealed and opaque envelope to hide allocation.
An anaesthesiologist not participating in the study kept the table of random numbers and prepared the drugs as per patient's body weight in syringes. Group ropivacaine with tramadol (RT) patients received 0.25% ropivacaine 1 mL/kg with 2 mg/kg of tramadol, making the volume to 0.5 mL and Group ropivacaine with dexmedetomidine (RD) patients received 0.25% ropivacaine 1 mL/kg with dexmedetomidine 2 μg/kg, making the volume to 0.5 mL. The tramadol used as adjuvant was preservative-free preparation (Supridol™, Neon Laboratories Ltd., Mumbai, Maharashtra, India). On receiving the patient in the operation theatre, electrocardiogram (ECG), pulse oximeter (SpO2) and non-invasive blood pressure (NIBP) were monitored and baseline parameters recorded. Pre-medication was done with intravenous (IV) midazolam 0.05 mg/kg through already secured venous access. Induction of anaesthesia was achieved with 50% nitrous oxide (N2O) and 8% sevoflurane in oxygen in spontaneous ventilation. After appropriate-sized laryngeal mask airway was inserted, sevoflurane concentration was reduced to 3% with 50% oxygen and 50% N2O. Thereafter, patients were placed in a lateral position and the skin of the back over the sacrum was disinfected using povidone-iodine solution, and under aseptic precautions, single-dose caudal epidural injection was performed using a 25-gauge needle. Needle position was confirmed by the pop sensed during penetration of the sacrococcygeal ligament, which was followed by the whoosh test[7] using 0.5 ml of air. After negative aspiration of blood or cerebrospinal fluid, caudal medication was given as per the group assigned. Caudal block was performed by an anaesthesiologist who was blinded to the drug that was to be administered in the caudal epidural space. The intraoperative monitoring and post-operative observation were done by an experienced anaesthesiologist who was unaware of the content of the syringes. The time of caudal block was recorded, and the surgery was allowed to start 10 min after caudal injection. The inhaled concentration of sevoflurane was adjusted to achieve haemodynamic changes within 20% of the baseline values. No other analgesics, sedatives or narcotics were used intraoperatively. Time taken for the administration of block was noted. Anaesthesia was maintained with sevoflurane and oxygen 50% and N2O 50%. All the patients were monitored by a standard protocol in a uniform pattern during anaesthesia and surgery. Continuous monitoring of vital parameters - heart rate (HR), ECG, respiratory rate, NIBP, SpO2 - was done, and values were recorded before and after pre-medication, induction, caudal block, after incision and thereafter every 10 min until the surgery was over. At the end of surgery, all anaesthetic drugs were discontinued. Total time of surgery was recorded. Any side effects such as breath holding/apnoea, hypotension, involuntary movements, nausea and vomiting were noted. The occurrence of intraoperative Hypotension (fall in blood pressure > 20% from baseline) requiring a fluid bolus and bradycardia (fall in heart rate > 20% from baseline) requiring atropine was recorded. The primary outcome was the duration of analgesia, defined as the time period between administration of block until the time face, legs, activity, cry, consolability (FLACC) score reached ≥4.[8] Secondary outcomes were the duration of the motor block, post-operative sedation by 5-point sedation score, and emergence time.
After surgery, patients were shifted to the post-anaesthesia care unit (PACU) for further observation and monitoring. Adverse events such as nausea, vomiting, hypotension, bradycardia, respiratory depression and urinary retention were monitored for 24 h and treated accordingly. Post-operative respiratory depression was defined as respiratory rate <10/min or decrease in SpO2 of <95% requiring supplementary oxygen. Fall in blood pressure (BP) and HR by >20% from the pre-operative values was defined as hypotension or bradycardia, respectively, and was treated by fluid bolus, mephentermine, or atropine, as necessary. Nausea and vomiting were treated with IV ondansetron. Post-operative pain status and degree of sedation were evaluated and recorded. Using the paediatric observational FLACC pain score with its 0–10 score range, each patient's pain intensity was assessed every hour till 6 h, every 3 h till 12 h and every 6 h till 24 h until the first dose of rescue analgesia was given. Rescue analgesia was with paracetamol suppository 15 mg/kg, given when the FLACC score was ≥4. The number of doses of rescue medication required and the time to first administration of rescue medication were also noted. Pain was assessed by the nursing staff blinded to the group of the patients. Motor block was assessed in the PACU on awakening by using a modified Bromage scale[9] that consisted of 4 points: 0 = full motor strength (flexion of knees and feet), 1 = flexion of knees, 2 = little movement of feet only, 3 = no movement of knees or feet. However, younger children who could not move their legs on command were stimulated by tapping on the legs and feet. Level of sedation was assessed by Ramsay sedation scale[10] at 15 min, 30 min, and 60 min after extubation and thereafter hourly until the Ramsay sedation score became 1 in all patients. Duration of post-operative sedation was deemed from the time of extubation until Ramsay sedation score was 2 or less. The times recorded were anaesthesia time (time from induction of anaesthesia to the end of surgery, when sevoflurane was discontinued), time from caudal block to skin incision, time from caudal block to end of surgery and emergence time (time from the end of surgery to opening the eyes on calling). Anaesthetic emergence was considered as delayed if the time elapsed from the end of surgery to exiting the operating theatre was greater than 20 min. The criteria for transferring the patient from operating room to PACU were being awake, moving all limbs, patent airway and normal respiratory pattern, normal oxygen saturation with no need for mandible support, stable hemodynamics, normothermia, and pain free.
Failure of the caudal block was defined as any increase in HR or mean arterial pressure (MAP) more than 20% of the pre-incision values. Failure of the caudal block was not reported in any patient.
Sample size was calculated using Power Analysis and Sample Size 15.0 software PASS (NCSS, Kaysville, UT, USA). The primary endpoint of the study was the time to FLACC score ≥4 after the administration of the study drug. Before the study, the number of subjects required in each group was determined using a power calculation with data obtained from a pilot study. The expected mean duration of analgesia for the dexmedetomidine and tramadol groups was 770.21 (91.19) and 580.02 (40.38) min, respectively. This indicated that a sample size of 24 subjects would be required in each group in order to detect a difference of 240 min in the duration of analgesia between the groups with α = 0.05 and β = 0.20 and power of 80%. We therefore recruited 30 subjects in each group to replace any dropouts. Data thus collected were entered into a computer-based SpreadSheet for analysis using SPSS statistical software (version 20.0) (IBM Corporation, NY, USA). Numerical variables (e.g., age, weight, HR and BP) were presented as mean and standard deviation and categorical variables (e.g., sex and adverse events) were presented as frequency (%). Student's t-test was used for numerical values and Chi-square test used for categorical values. The value P < 0.05 was considered statistically significant.
RESULTS
The two groups were comparable with respect to age, sex, weight and duration of surgery. The type of surgeries included inguinal hernia, hypospadias repair, orchidopexy and orchidectomy [Table 1]. Group RT had a mean duration of analgesia of 654. 20 ± 78.38 min and Group RD had a mean duration of analgesia of 780.29 ± 71.21 min (P = 0.0001). This shows the duration of analgesia was significantly prolonged by the addition of dexmedetomidine to ropivacaine.
Table 1.
Demographic profile, caudal block characteristic and sedation of patients
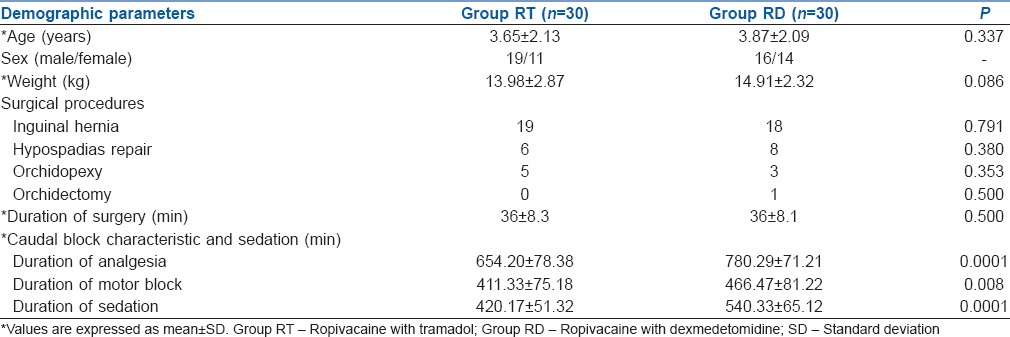
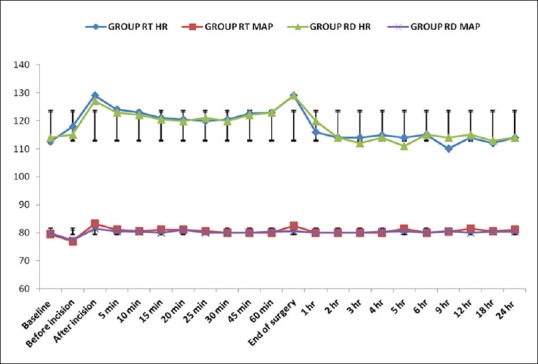
There was no significant difference between the two groups for pre- and post-operative HR and MAP [Figure 1]. During the first 4 h after operation, all patients in both groups had adequate analgesia (FLACC score <4), and then the number of patients with adequate analgesia declined rapidly in Group RT as compared to Group RD and the difference was statistically significant. At 6 h post-operative, 47% of the patients in Group RT achieved a FLACC score of ≥4 as compared to 0% patients in Group RD, whereas 80% of the patients in Group RD compared to 100% of the patients in Group RT achieved a FLACC score of ≥4 at 18 h post-operative [Figure 2]. Mean time to first rescue analgesic in the RD group was 889 ± 98.6 min which was significantly longer than in Group RT 698 ± 38.4 min (P = 0.001).
Figure 1.
Haemodynamic parameters - trends of heart rate (bpm) and mean blood pressure (mmHg) in both groups at different time intervals. Values were not significantly different between the two groups at all time points. Data are presented as mean ± standard deviation
Figure 2.
Face, legs, activity, cry, consolability score: Number of patients with adequate caudal analgesia (<4) in both groups at different time intervals. +Statistically significant compared with Group ropivacaine with tramadol
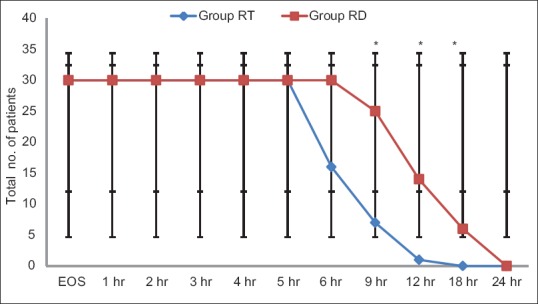
More patients in Group RT needed two or three doses of rescue analgesics compared to patients in Group RD. The rescue analgesia requirement was compared between the two groups (P = 0.0001), with three children not requiring rescue analgesia for 24 h post-operatively in Group RD compared to all children requiring rescue analgesia for 24 h post-operatively in Group RT. Using caudal dexmedetomidine 2 μg/kg with sevoflurane anaesthesia, we found the mean emergence time of RT group was 4.0 ± 1.4 min and that of the RD group was 4.9 ± 1.9 min (P = 0.0411). Post-operative sedation scores between the two groups are shown in Table 2.
Table 2.
Ramsay sedation score during observation period
Three children in Group RT and two in Group RD had vomited in the post-operative period. No child had respiratory depression in the post-operatively. There was no significant difference between the two groups as regards the incidence of side effects such as shivering (P = 1.0), post-operative nausea and vomiting (P = 0.642) and hypotension (P = 1.0).
DISCUSSION
In this study, we found that the use of dexmedetomidine, as an additive to ropivacaine in caudal epidural analgesia, prolonged the duration of analgesia following infraumbilical surgeries as compared with caudal tramadol in children.
Tramadol, a centrally acting synthetic analgesic with low affinity for opioid receptors, appears to modify the transmission of pain impulses by the inhibition of monoamine reuptake. Few studies have shown that in caudal epidural block, addition of tramadol to ropivacaine showed significant prolongation of post-operative analgesia as compared to ropivacaine alone.[3,11,12] Studies have established the safety of tramadol as caudal adjuvant without significant adverse effects.[13,14]
Dexmedetomidine enhances the effects of local anaesthetics without increasing the incidence of side effects.[15,16] Dexmedetomidine in comparison to other sedatives has minimal respiratory effects in adults and children which make it a good adjuvant. Sedation caused by dexmedetomidine can be easily reversed with slight stimulation and do not cause respiratory depression even at high doses.[17] Furthermore, respiratory rate, oxygen saturation and carbon dioxide tension are generally maintained during dexmedetomidine sedation in children.[18,19]
We observed that the duration of analgesia (FLACC <4) without the need rescue analgesic was significantly longer in the group receiving ropivacaine-dexmedetomidine mixture than the group receiving ropivacaine with tramadol. These results are similar to those reported in a study where dexmedetomidine as an adjuvant with 0.25% ropivacaine caudally was used and observed that the duration of analgesia was significantly higher in the group receiving ropivacaine-dexmedetomidine mixture than the group receiving ropivacaine alone.[20] Similarly, in another study, dexmedetomidine and clonidine, both in a dose of 2 μg/kg as an adjuvant with 0.25% bupivacaine, were given caudally.[19] They found that the duration of analgesia was significantly higher in the group receiving bupivacaine-dexmedetomidine mixture or bupivacaine-clonidine mixture than the group receiving bupivacaine alone.
A study with caudal dexmedetomidine (2 μg/kg) with 0.25% ropivacaine (1 mL/kg) for paediatric lower abdominal surgeries achieved significant post-operative pain relief consequently a better quality of sleep, prolonged duration of arousable sedation and lower incidence of emergence agitation following sevoflurane anaesthesia.[20]
Sedation scores were generally <2 and the level of sedation was decreased significantly in RT group in the present study. In support of these findings, improved sedation and pain scores have also been observed with dexmedetomidine as intrathecal adjuvant.[21]
The ropivacaine-dexmedetomidine group required significantly less number of rescue analgesics as compared to the ropivacaine-tramadol group in this study. These results are similar to a study conducted on the effect of dexmedetomidine on bupivacaine in the caudal block in paediatric patients.[22]
The pre-, intra- and post-operative haemodynamic variables between the groups were comparable and were not statistically significant and therapeutic interventions were not required. Post-operative side effects, including vomiting, shivering and hypotension, were recorded in the PACU more with tramadol rather than dexmedetomidine but were statistically non-significant.
There are few limitations of our study. First, as for all additives in regional anaesthesia, the analgesic role of dexmedetomidine and tramadol through systemic absorption cannot be completely excluded from our study design. Hence, comparing the potential local effects to a systemic administration particularly when additives are used is difficult since we did not have a control group with IV dexmedetomidine and IV tramadol. Second, ultrasound guidance for caudal block administration should be considered in cases where the detection of sacral anatomy is difficult, especially by palpation. Third, the study could not stabilise the severity of surgical trauma which may lead to variability in pain severity.
CONCLUSION
Caudal ropivacaine 0.25% with dexmedetomidine 2 μg/kg provided longer duration of analgesia and reduced requirement for rescue analgesic in the post-operative period compared to caudal ropivacaine 0.25% with tramadol 2 mg/kg. Thus, dexmedetomidine with ropivacaine can be used as an alternative to tramadol with ropivacaine for paediatric infraumbilical surgeries through the caudal route as a safe and effective agent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Dobereiner EF, Cox RG, Ewen A, Lardner DR. Evidence-based clinical update: Which local anesthetic drug for pediatric caudal block provides optimal efficacy with the fewest side effects? Can J Anaesth. 2010;57:1102–10. doi: 10.1007/s12630-010-9386-1. [DOI] [PubMed] [Google Scholar]
- 2.Doctor TP, Dalwadi DB, Abraham L, Shah N, Chadha IA, Shah BJ. Comparison of ropivacaine and bupivacaine with fentanyl for caudal epidural in pediatric surgery. Anesth Essays Res. 2013;7:212–5. doi: 10.4103/0259-1162.118965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krishnadas A, Suvarna K, Hema VR, Taznim M. A comparison of ropivacaine, ropivacaine with tramadol and ropivacaine with midazolam for post-operative caudal epidural analgesia. Indian J Anaesth. 2016;60:827–32. doi: 10.4103/0019-5049.193672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yildiz TS, Ozdamar D, Bagus F, Solak M, Toker K. Levobupivacaine-tramadol combination for caudal block in children: A randomized, double-blinded, prospective study. Paediatr Anaesth. 2010;20:524–9. doi: 10.1111/j.1460-9592.2010.03296.x. [DOI] [PubMed] [Google Scholar]
- 5.Kaur M, Singh PM. Current role of dexmedetomidine in clinical anesthesia and intensive care. Anesth Essays Res. 2011;5:128–33. doi: 10.4103/0259-1162.94750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu HH, Wang HT, Jin JJ, Cui GB, Zhou KC, Chen Y, et al. Does dexmedetomidine as a neuraxial adjuvant facilitate better anesthesia and analgesia? A systematic review and meta-analysis. PLoS One. 2014;9:e93114. doi: 10.1371/journal.pone.0093114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewis MP, Thomas P, Wilson LF, Mulholland RC. The ‘whoosh’ test. A clinical test to confirm correct needle placement in caudal epidural injections. Anaesthesia. 1992;47:57–8. doi: 10.1111/j.1365-2044.1992.tb01957.x. [DOI] [PubMed] [Google Scholar]
- 8.Merkel SI, Voepel-Lewis T, Shayevitz JR, Malviya S. The FLACC: A behavioral scale for scoring postoperative pain in young children. Pediatr Nurs. 1997;23:293–7. [PubMed] [Google Scholar]
- 9.Chipde S, Banjare M, Arora K, Saraswat M. Prospective randomized controlled comparison of caudal bupivacaine and ropivacaine in pediatric patients. Ann Med Health Sci Res. 2014;4(Suppl 2):S115–8. doi: 10.4103/2141-9248.138025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramsay MA, Luterman DL. Dexmedetomidine as a total intravenous anesthetic agent. Anesthesiology. 2004;101:787–90. doi: 10.1097/00000542-200409000-00028. [DOI] [PubMed] [Google Scholar]
- 11.Kamal M, Mohammed S, Meena S, Singariya G, Kumar R, Chauhan DS. Efficacy of dexmedetomidine as an adjuvant to ropivacaine in pediatric caudal epidural block. Saudi J Anaesth. 2016;10:384–89. doi: 10.4103/1658-354X.177325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Günes Y, Seçen M, Ozcengiz D, Gündüz M, Balcioglu O, Isik G. Comparison of caudal ropivacaine, ropivacaine plus ketamine and ropivacaine plus tramadol administration for postoperative analgesia in children. Paediatr Anaesth. 2004;14:557–63. doi: 10.1111/j.1460-9592.2004.01220.x. [DOI] [PubMed] [Google Scholar]
- 13.Doda M, Mukherjee S. Postoperative analgesia in children – Comparative study between caudal bupivacaine and bupivacaine plus tramadol. Indian J Anaesth. 2009;53:463–6. [PMC free article] [PubMed] [Google Scholar]
- 14.Solanki NM, Engineer SR, Jansari DB, Patel RJ. Comparison of caudal tramadol versus caudal fentanyl with bupivacaine for prolongation of postoperative analgesia in pediatric patients. Saudi J Anaesth. 2016;10:154–60. doi: 10.4103/1658-354X.168807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishii H, Kohno T, Yamakura T, Ikoma M, Baba H. Action of dexmedetomidine on the substantia gelatinosa neurons of the rat spinal cord. Eur J Neurosci. 2008;27:3182–90. doi: 10.1111/j.1460-9568.2008.06260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ibacache ME, Muñoz HR, Brandes V, Morales AL. Single-dose dexmedetomidine reduces agitation after sevoflurane anesthesia in children. Anesth Analg. 2004;98:60–3. doi: 10.1213/01.ANE.0000094947.20838.8E. [DOI] [PubMed] [Google Scholar]
- 17.Afonso J, Reis F. Dexmedetomidine: Current role in anesthesia and intensive care. Rev Bras Anestesiol. 2012;62:118–33. doi: 10.1016/S0034-7094(12)70110-1. [DOI] [PubMed] [Google Scholar]
- 18.Petroz GC, Sikich N, James M, van Dyk H, Shafer SL, Schily M, et al. A phase I, two-center study of the pharmacokinetics and pharmacodynamics of dexmedetomidine in children. Anesthesiology. 2006;105:1098–110. doi: 10.1097/00000542-200612000-00009. [DOI] [PubMed] [Google Scholar]
- 19.El-Hennawy AM, Abd-Elwahab AM, Abd-Elmaksoud AM, El-Ozairy HS, Boulis SR. Addition of clonidine or dexmedetomidine to bupivacaine prolongs caudal analgesia in children. Br J Anaesth. 2009;103:268–74. doi: 10.1093/bja/aep159. [DOI] [PubMed] [Google Scholar]
- 20.Anand VG, Kannan M, Thavamani A, Bridgit MJ. Effects of dexmedetomidine added to caudal ropivacaine in paediatric lower abdominal surgeries. Indian J Anaesth. 2011;55:340–6. doi: 10.4103/0019-5049.84835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jarineshin H, Fekrat F, Kargar Kermanshah A. Treatment of postoperative pain in pediatric operations: Comparing the efficiency of bupivacaine, bupivacaine-dexmedetomidine and bupivacaine-fentanyl for caudal block. Anesth Pain Med. 2016;6:e39495. doi: 10.5812/aapm.39495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saadawy I, Boker A, Elshahawy MA, Almazrooa A, Melibary S, Abdellatif AA, et al. Effect of dexmedetomidine on the characteristics of bupivacaine in a caudal block in pediatrics. Acta Anaesthesiol Scand. 2009;53:251–6. doi: 10.1111/j.1399-6576.2008.01818.x. [DOI] [PubMed] [Google Scholar]


