Abstract
Trefoil factor (TFF) peptides, containing a 40-amino acid motif, including six conserved cysteine residues that form intramolecular disulfide bonds, are a family of mucin-associated secretory molecules mediating many physiological roles that maintain and restore gastrointestinal (GI) mucosal homeostasis. TFF peptides play important roles in response to GI mucosal injury and inflammation. In response to acute GI mucosal injury, TFF peptides accelerate cell migration to seal the damaged area from luminal contents, whereas chronic inflammation leads to increased TFF expression to prevent further progression of disease. Although much evidence supports the physiological significance of TFF peptides in mucosal defenses, the molecular and cellular mechanisms of TFF peptides in the GI epithelium remain largely unknown. In this review, we summarize the functional roles of TFF1, 2, and 3 and illustrate their action mechanisms, focusing on defense mechanisms in the GI tract.
Keywords: GI mucosal defense, TFF signaling, homeostasis, mucin, cell migration, immune system
INTRODUCTION
There are three mammalian trefoil factor (TFF) peptides: TFF1 (original name: pS2) (1), TFF2 (original name; pancreatic spasmolytic polypeptide, PSP) (2), and TFF3 (original name; intestinal trefoil factor, ITF) (3). The current nomenclature TFF was unified in 1996 at the Conférence Philippe Laudat (4). Members of the trefoil peptide family share a conserved 40-amino acid sequence termed the trefoil, or P-type domain, that produces a defining three clover-leaf structure comprised of covalent loops stabilized through internal disulfide bonds at Cys1-Cys5, Cys2-Cys4, and Cys3-Cys6 (5). This conformation increases resistance to degradation by protease, acid, and heat (2, 6) and results in structurally and functionally stable TFF peptides in mammalian tissues. Both TFF1 and TFF3 contain a single trefoil domain, whereas TFF2 has two trefoil domains. Additionally, TFF1 and TFF3 contain a free cysteine residue in the C-terminal shown to form covalent dimers with TFF peptides or other proteins. This dimerization may be critical for TFF biological properties and activities (7–9).
The TFF genes were cloned in various species, and the genomic organization of rodent Tff peptides was shown to be similar to that of human (10, 11). TFF1 was originally described as a breast cancer-associated gene regulated by estrogen in the MCF-7 human breast cancer cell line (1, 12); therefore, TFF peptides were initially considered as cancer-related genes. This concept is still valid today. Many researchers report TFF peptide involvement in cancer development as well as cancer progression/suppression in numerous organs, including the gastrointestinal (GI) tract (6). However, because TFF peptides are widely expressed in normal tissues, TFF peptides clearly have functional significance in healthy tissues. Although much current research explores the role of TFF peptides in cancer (6, 13), this review seeks to emphasize the physiological significance of TFF peptides outside the oncogenic implications. Furthermore, this review focuses on the role of TFF peptides in the GI tract, where TFF peptides are detected at high levels both in mucus-secretory cells and in the mucus layer overlaying the GI epithelium.
Location of Trefoil Factor Peptides in the Gastrointestinal Tract and Lessons from Knockout Mouse Models
In the healthy organism, TFF peptides are distributed in numerous tissues, including the brain, kidney, liver, pancreas, respiratory tract, and salivary gland (14, 15), but the most abundant expression is found in the normal GI tract where the three TFF isoforms are found to have a differential distribution. Examination of mice with genetic deletion of Tff peptides can reveal functions of the peptides that may be due to deletion of the target protein or to the adaptive regulation of another protein affected by the deleted gene product. Such mice can be useful to reveal at least a subset of the functional networks that are controlled by a TFF isoform and its downstream effectors.
TFF1 is predominantly expressed in gastric foveolar cells and surface epithelial cells throughout the stomach and is detected in the upper ducts of Brunner’s glands in both rodents and humans (16–18). Additionally, TFF1 was also detected in the gastric juice (16). Although Tff1 mRNA was not detected in the esophagus, small intestine, and colon in mice (18), it is present in these organs in humans (19, 20). Interestingly, Tff1 knockout (KO) mice develop antral and pyloric gastric hyperplasia and dysplasia (21), which can be explained by a lack of a proliferative brake in the absence of Tff1 (22). Because downregulation of TFF1 is also found in human gastric cancers (Reference 4a), both observations suggest that TFF1 is a candidate tumor suppressor. Tff1 KO mice also show decreased numbers of parietal cells and decreased Tff2 protein expression in the gastric corpus (24), suggesting that TFF1 plays important roles in the census of differentiated cells of the stomach epithelium. Interestingly, Tff1 KO mice also have abnormally small intestinal villi. This abnormality is usually associated with reduced cell production in crypts, either by decreased proliferation or increased apoptosis. It is not clear how these observations might be reconciled with proposed TFF1 functions in the stomach or if it reveals divergent functions of the peptide in the two tissues.
TFF2 is expressed in similar cellular locations in both rodents and humans, namely in epithelial cell types deep within the gastric glands of the corpus and antral stomach regions, in gastric mucous neck cells, and in the duodenal Brunner’s gland (17, 18, 25). Negligible expression of TFF2 was detected in the esophagus, small intestine, and colon. Tff2 KO mouse stomach exhibits decreased proliferation activity in the epithelium and decreased gastric mucosal thickness (26). Additionally, basal gastric acid secretion is increased in Tff2 KO mice (26), consistent with findings that administration of TFF2 inhibits acid secretion (25). No other major pathological or morphological changes were found in the GI epithelium of unchallenged Tff2 KO mice (26, 27). Interestingly, Tff2 mRNA transcript expression marks progenitor cells (preneck cells) in the gastric corpus; from these cells arise mucus neck and parietal and zymogenic cells but not pit- or enterochromaffin-like cell lineages (28). Results suggest the potential role of TFF2 in cell differentiation, but this was not examined directly.
TFF3 is predominantly expressed within goblet cells in the small intestine and colon, whereas no expression was detected in liver, pancreas, or stomach in rodents (3). Human TFF3 was confirmed to have high homology to rat Tff3 and is highly expressed in goblet cells in human small intestine and colon (29, 30). Tff3 KO mouse colon has an increase of both epithelial proliferation and apoptosis, resulting in normal crypt appearance (31, 32). No obvious change in any tissue morphology was reported in Tff3 KO mice. Results suggest a potential role of TFF3 in colonic epithelial homeostasis.
As discussed below, additional functions of TFF peptides in mucosal protection are revealed in animals and tissues that are stressed, suggesting that this family of peptides plays complementary roles during normal and pathological situations.
Hormonal Regulation of Trefoil Factor Peptides
Based on the original identification of TFF1 as an estrogen-regulated gene, estrogen was presumed to physiologically regulate TFF1 peptide production and secretion in the GI tract. However, even early evidence suggested that TFF1 is unlikely to be regulated by estrogen or its receptors in the GI tract (16). The TFF1 gene sequence contains DNA enhancer elements responsive to 12-O-tetradecanolylphorbol-13-acetate (TPA), epidermal growth factor (EGF), c-Ha-ras oncoprotein, and the c-Jun protein in MCF-7 (human breast cancer) cells (33). EGF regulates the transcription of all TFF genes. In vitro induction of TFF transcription in GI cell lines suggests a pathway mediated by epidermal growth factor receptor (EGF-R) and the Ras/mitogen-activated protein kinase (MEK)/mitogen-activated protein kinase (MAPK) signaling pathway (34). Individual TFF peptides can also induce expression of other TFF peptides through EGF-R in vitro (34). Reports show that TFF2 and TFF3 upregulation are inhibited in transforming growth factor α (Tgfα, one of the EGF-R ligands) KO mice (35), suggesting EGF-R involvement in the regulation of TFF peptides.
Gastrin (produced in G cells in the gastric antrum) regulates gastric acid secretion and proliferation and also regulates TFF expression. Gastrin activates TFF1 transcription via Raf, MEK, and the extracellular signal regulated kinase (Erk)-dependent pathway mediated by the gastrin cholecystokinin (CCK)2 receptor but not the EGF-R or Ras-pathway (13). Similarly, TFF2 transcription is activated by gastrin/CCK2 through a guanine-cytosine (GC)-rich DNA-binding site and a protein kinase C (PKC)-, MEK1-, and phosphoinositide-3-kinase (PI3K)-dependent but EGF-R-independent pathway (36). Both Tff1 and Tff2 expressions are reduced in gastrin KO mice and increased in hypergastrinemic transgenic mice (13, 36). Treatment with the proton-pump inhibitor, omeprazole is also known to induced hypergastrenia, and interestingly omeprazole-treated mouse stomach was found to exhibited increase Tff1 secretion. (37).
Recently, a decreased number of periodic acid-schiff positive goblet cells, accompanied with reduction of Tff3 and Muc2 expression, was observed in vasoactive intestinal polypeptide (Vip, a peptide hormone) KO mice, and exogenous administration of recombinant VIP increased Tff3/Muc2 production by the goblet cells (38). This provides the first evidence that TFF3 production is regulated by hormones and that the beneficial effects of VIP may in part be mediated by TFF3.
Trefoil Factor Binding Sites
It is presumed that TFF actions are mediated by specific membrane receptors, yet unequivocal demonstration of such receptors remains elusive. Intravenously administered 125I-TFF2 rapidly distributes to the basolateral domain of gastric neck cells, parietal cells, and the pyloric glands to the Paneth cells in the small intestine and crypt cells in the colon (39). Over a longer time, radioactivity appears at the luminal surface or mucus layer (39). 125I-TFF1 or 125I-TFF3 yield a similar distribution as TFF2 (40). These suggest the presence of a basolateral binding site in the specific GI epithelial cells. Although it is difficult to resolve high-affinity binding in such experiments, results suggest that all TFF peptides bind to similar basolateral sites but do not necessarily bind to sites where endogenous TFF1- or TFF3-expressing cells are present. Results support the presence of receptor-like binding at cell membranes, at a location distinct from their site of synthesis/storage in the GI tract. The results also suggest that despite physiologic secretion of TFF peptides directly into the luminal compartment, there is the possibilty of trans-epithelial transit of the peptides that may be part of their bioactivity.
Binding studies have identified a limited number of candidates for TFF receptors. A possible binding site for TFF was identified in small intestinal crypt and gastric mucus neck cells. The putative receptor is a 50 kDa membrane glycoprotein for TFF3 (41) or CRP-ductin (also named Muclin) for TFF2 (42). Using a solid-phase ELISA assay, it was found that TFF3 dimer, but not monomeric TFF3 nor (glycosylated or nonglycosylated) TFF2, binds to DMBT1gp340 (Deleted in Malignant Brain Tumors 1/glycoprotein340). DMBT1gp340 is the human homologue of rodent CRP-ductin, and is known to be upregulated in epithelial cells in inflammatory bowel disease (IBD). However, the radioactive peptide binding sites and DMBT1 do not colocalize in the tissue [e.g., DMBT1 is expressed in epithelial cells adjacent to TFF3-producing goblet cells (19)]. The specificity of interaction between TFF3 and DMBT1gp340 is still unclear. Any DMBT1gp340 + TFF3 binding complex could also have roles in immune defense since DMBT1gp340 binds to bacteria and is known to act as an anti-inflammatory molecule downstream of innate immune receptors (Reference 8a).
In addition to binding studies, functional assays are also used to propose potential TFF receptors. Unfortunately, such data are often unable to distinguish rigorously between a protein required in TFF signaling versus a direct binding partner of the TFF peptide. The strongest work in this regard implicates the C-X-C chemokine receptor (CXCR)4 as a TFF2 receptor. In this study, work with lymphocytic cells provided evidence of TFF2 competing for binding with an anti-CXCR4 antibody and functionally competing with the best-established ligand of CXCR4 (CXC ligand 12, CXCL12, also known as stromal cell-derived factor, SDF-1) (43). More discussion of CXCR4 and other receptors involved with TFF signaling is found in later sections that evaluate how such proteins participate in specific TFF actions.
It should be noted that TFF peptide is detected in both serum and the luminal fluid of the GI tract (44). It is widely assumed that the route of TFF action is via luminal secretion of the peptide from the epithelial cells lining the GI tract, and results suggest that even systemic TFF peptides can be secreted into the gastric lumen in parallel with mucus secretion from mucous cells (45). It is also worth noting that, at the concentrations applied experimentally, TFF peptides are promiscuous. The same physiologic effect can be elicited by any one of multiple TFF isoforms added as exogenous peptides (46). Given the lack of information about binding affinities for any of the peptides at their receptors, it is currently not possible to determine if this observation reflects physiologically relevant overlap among TFF receptor specificity.
TREFOIL FACTOR PEPTIDES ENHANCE GASTROINTESTINAL MUCOSAL DEFENSES
Numerous studies have demonstrated a mucosal protective effect of TFF peptides in the GI tract. It is a shared feature that within the gastrointestinal epithelium of mutant mice lacking any one of the TFF proteins there is an increased susceptibility to damage in injury models (26, 32). In complementary studies, exogenous TFF peptides opposed the generation of injury by numerous agents. Both approaches suggest that TFF peptides contribute to mucosal defenses in the normal tissue. Oral administration or luminal application of TFF2 was shown to inhibit gastric injury [induced by nonsteroidal anti-inflammatory drugs (NSAIDs) or ethanol] in rats and human cell lines, respectively (47–50). Mice with ubiquitous Tff2 KO show increased susceptibility to NSAID injury in the stomach (26).
Oral administration of TFF3 decreased the intestinal damage in chemotherapy- and radiotherapy-induced mucositis in mice (51). Enteral adminisration of monomer and dimer TFF3 prevent the induction of ischemia-reperfusion-induced intestinal injury in immature rats (52). TFF3 inhibits the platelet-activating factor-induced downregulation of claudin-1 and ZO-1 and transepithelial leakage of lucifer yellow in the human intestinal Caco-2 cell line (53). TFF3 also increases transepithelial electrical resistance in vitro in Caco-2, HT29/B6, and MDCK cells (53, 54). These studies suggest that TFF3 enhances intestinal barrier function via regulation of tight junctions to decrease paracellular permeability of the intestinal epithelium.
TFF3 is principally expressed with MUC2 in intestinal goblet cells where it participates in mucosal regeneration and repair. Administration of dextran sulfate sodium (DSS) generates severe colonic damage in Tff3 KO mice in comparison with wild-type (WT) mice, although the protective mucin is still present within goblet cells in untreated Tff3 KO mice (32). Observations show that the administration of TFF3 peptide prevents acetic acid-induced colonic mucosal injury in mice (32). Luminal administration of TFF2 and TFF3 dimer improves DSS-induced colitis in rats, whereas the TFF3 monomer reportedly has no effect on DSS-induced colitis in rats (55). In Vip KO mice, there is less production of goblet cells and low secretion of Tff3/Muc2, whereas colonic mucosal permeability and susceptibility to dinitrobenzene sulfonic acid-induced damage are increased. These effects can be reversed by stimulation of Tff3/Muc2 production following VIP administration, implicating TFF3 in intestinal barrier protection (38).
As noted previously, TFF functions are redundant among isoforms and species, potentially due to their high sequence homology, and/or they are acting through shared mechanisms. In Tff2 KO mice, exogenous TFF3 can inhibit gastric injury induced by indomethacin or ethanol in rats (49) and rescue the delayed gastric epithelial repair in these mutant mice lacking Tff2 (27). Tff2 KO mice also show an increased susceptibility to DSS-induced colonic injury (57).
Although luminal application of TFF peptides most consistently elicits a beneficial effect on mucosal defenses, other administration routes are more controversial. Intravenous, intraperitoneal or subuctaneous administration of TFF2 protect against ethanol- or indomethacin- induced gastric mucosal injury (47, 49, 50). Subcutaneous injection of TFF3 prevents the induction of ischemia-reperfusion-induced intestinal injury (52). Subcutaneous administration of TFF1 (Reference 2a) or TFF2 (Reference 6a) promoted rat gastric ulcer healing induced by indomethacin and restraint. It has been noted that TFF1 dimer had greater protective effect than its monomer (Reference 2a). In aggregate, these outcomes suggest that basolateral TFF receptors/binding sites or transepithelial transit of the TFF peptide to the luminal surface can underly beneficial effects of the exogenous TFF peptides. Such beneficial effects are not always observed. In contrast, subcutaneous injection of TFF2 aggravated duodenal ulcer induced by mercaptamine (Reference 6a). Subcutaneous injection of TFF2 or TFF3 dimer also aggravated DSS-induced colitis (55), although subcutaneous injection of TFF2 at a much lower concentration showed a protective effect on DSS-induced colitis (Reference 7a). Transgenic overexpression of Tff1 in the mouse jejunum increases mucosal resistance against NSAID-induced small intestinal mucosal injury (56). Application of exogenous TFF may result in nonphysiologic concentrations at the site of action, but the effect of such high concentrations may be offset by low cross-species reactivity of peptides or low bioactivity of recombinant TFF protein. For these reasons, controversy remains about the issues of TFF redundancy and the epithelial polarity of TFF action.
Trefoil Factor Peptides in the Mucus Layer
Most regions of the gastrointestinal epithelium are covered by a protective mucus layer, maintained by the continuous secretion of mucin proteins. For example, the gastroduodenal epithelium has a tightly associated mucus–bicarbonate layer to prevent injury or self-digestion from strong gastric acid or secreted proteases (58). The best-established endogenous regulators of mucin and bicarbonate secretion are prostaglandins (PGs), a class of eicosanoids produced by cyclooxygenase (COX)-1 metabolism of arachidonic acid (58). It is known that NSAIDs, which are COX inhibitory drugs, generate gastrointestinal mucosal injury in human and experimental animals (59).
It is unlikely that TFF peptides directly stimulate PG production and bicarbonate secretion (27) or that PG is required to stimulate Tff secretion. Tff peptides upregulate in response to NSAID-induced gastric injury, a condition in which mucosal PG production is inhibited (60). Acting through pathways that appear to be independent of PG/COX-1 regulation, exogenous TFF peptides prevent the generation of injury induced by NSAIDs, with conflicting reports about whether this action of TFF peptides occurs without (47–50) or with an inhibitory effect on gastric acid secretion (25, 26). Topical (but not intravenous) application of TFF2 reduced proton permeation through gastric mucus layers and inhibited surface epithelial cell acidification. In this in vivo action, TFF2 did not affect gastric mucosal blood flow or mucus gel thickness (61). Interestingly, inhibition of gastric acid secretion by omeprazole, a proton pump inhibitor (PPI), increases Tff1 secretion into the rat gastric lumen without affecting Tff1 synthesis in the tissue, whereas there is no effect on Tff2 secretion (37). This suggests that TFF1 is a candidate to mediate at least part of the gastric protective mechanisms of PPIs.
Each TFF peptide is associated with specific mucins in the different regions of the GI tract. In all cases the TFF is stored and secreted from the same cells as the mucin protein. TFF1 colocalizes with MUC5AC in the gastric surface epithelium, TFF2 with MUC6 in the gastric pit to deep gland and neck cells, and TFF3 with MUC2 in the intestine (3, 62–64). In some cases it is clear that the TFF is cosecreted with the mucin, such as TFF3 being cosecreted with MUC2 from the goblet cell (10).
Direct interaction of TFF peptides with mucins is hypothesized to enhance mucosal defense. TFF1 has been detected in three different forms: monomer, dimer, and complexed with other molecules. Most TFF1 peptide in the human gastric mucus layer is covalently complexed with unknown molecules via disulfide bond (65). Use of a cesium chloride gradient was able to separate the TFF1 complex from mucins, whereas TFF1 dimer remained tightly associated with mucins (65). Addition of TFF2 or TFF3 dimer to mucin solution increases the viscosity and elasticity of the mucin solution, resulting in a gel-like structure, although the monomers of TFF1 and TFF3 only have minimal effect (66). Likewise, the TFF2 or TFF3 dimer in the presence of mucin glycoproteins shows enhanced protection of the integrity of intestinal epithelial monolayers of the human colonic cancer-derived T84 cell line against insults (taurocholic acid, oleic acid, Phytohemagglutinin, Clostridium difficile toxin A, etc.) (67). It is reported that the trefoil domain of Tff1 can directly interact with the von Willebrand factor C (Vwfc)1 and Vwfc2 cysteine-rich domains in Muc2 and Muc5ac (68). TFF3 forms a disulfide-linked heterodimer with immunoglobulin G (IgG) Fc binding protein, which is a constituent of intestinal mucus secreted by goblet cells (69). Thus, TFF peptides are thought to enhance protection of the GI mucosa against injury through interactions with mucins.
Immediately following epithelial cellular injury, exfoliated damaged cells form a mucoid cap over the repairing epithelium, which has been found to contain mucus, HCO3−, and plasma proteins such as fibrin. This elaborated structure is 5–10 times thicker than the mucus gel layer and is not firmly attached to the gastrointestinal epithelial surface (70). It is believed to function as a scab that protects the damaged mucosa against further insult and promotes healing. TFF2 protein was identified in the mucoid cap, but the functional significance of this observation to either the structure or function of the mucoid cap is unknown (71).
TREFOIL FACTOR PEPTIDES AND CELL MIGRATION
Several studies support the involvement of TFF peptides in cell migration, most deriving from the response of a damaged epithelium attempting to restore epithelial continuity. In the simplest models, where no cell proliferation is needed to restore a small breach in the epithelium, the epithelial restitution occurs rapidly following injury and coordinates the removal of damaged cells and the migration of healthy epithelial cells into the breach (Figure 1). Efficient restitution is physiologically important to limit fluid and electrolyte loss, and prevent luminal antigens and bacteria from accessing the host tissue and immune cells. In the case of more extensive damage (e.g., peptic ulceration), restitution is supplemented by proliferation because additional cells are needed to span the larger damage area, and both epithelial and non-epithelial cells are involved in the tissue remodeling. As discussed below, extensive damage brings additional complications of inflammatory responses, and this is a function in which TFF peptides also play a role.
Figure 1.
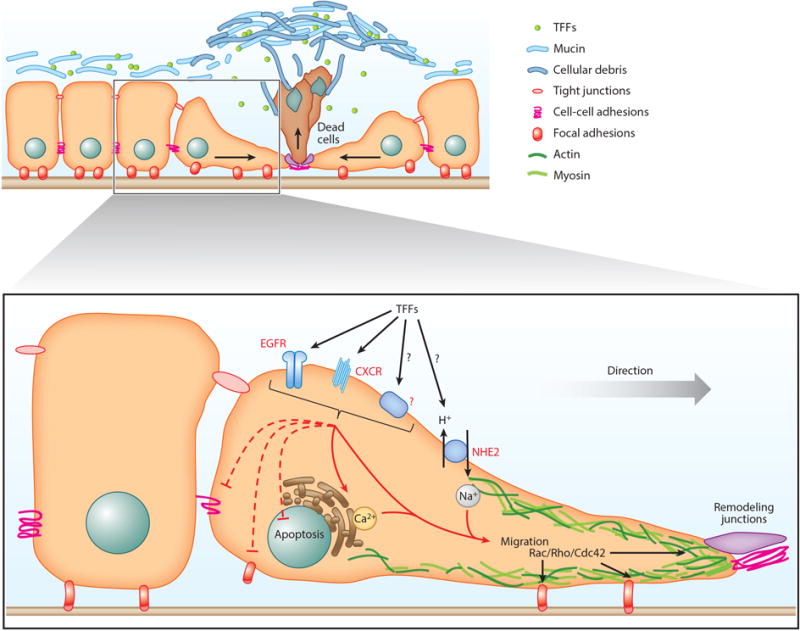
Candidate TFF pathways in epithelial repair. In response to acute epithelial injury, neighboring healthy epithelial cells start to rapidly migrate toward the damaged site, resulting in force to extrude injured cells and simultaneously cover the denuded area. TFF peptides, mucus, and cellular debris form a mucoid cap to cover the injured area to protect against further injury and potentially partake in promoting recovery directly. TFF peptides facilitate GI epithelial restitution by stimulating cell migration and inhibiting apoptosis of the migrating cells. The motogenic effect of TFF peptides can be mediated (directly or indirectly) by EGFR, CXCRs, or other receptors. This is followed by the stimulation of the Rho-family GTPase to promote cell migration by altering the cytoskeleton and tight junctions as well as cell adhesions. Either upstream or downstream of the above signaling cascades, TFF peptides can also stimulate calcium mobilization resulting in enhanced cell migration. TFF peptides may directly or indirectly affect the NHE2 exchanger, as NHE2 activity is crucial for TFF-driven cell migration (at least in the stomach). Abbreviations: CXCR, C-X-C chemokine receptor; EGFR, epidermal growth factor receptor; GI, gastrointestinal; NHE, sodium hydrogen exchanger; TFF, trefoil factor.
In the case of focal damage to the epithelium, the process of restitution can be incredibly rapid. Specifically, in the normal mammalian stomach in vivo, laser injury to 3–5 epithelial cells is followed by cell exfoliation and restitution within 10 minutes (27, 46). In this model, the Tff2 KO mice show a delay of restitution, which can be rescued by the exogenous application of recombinant TFF (46). Secreted TFF peptides are able to upregulate their own gene expression within minutes as comfirmed by an increase of TFF transcription in GI cell lines treated with TFF2 or TFF3 (34). These in vivo and in vitro studies suggest that TFF peptides are rapid responders to damage. In this setting, TFF peptides act as “motogens” (substance that stimulate cell motility) to promote migration of viable cells at the transition zone toward the area of damage to cover the denuded mucosa without proliferation (72). GI epithelial restitution is an intrinsic function of epithelial cells that can occur in the absence of systemic signals, as restitution can be demonstrated in vitro in cell lines (72, 73) and recently in gastrointestinal organoids (74).
All TFF peptides are shown to be motogens, (47, Reference 1a, Reference 2a, Reference 3a). Both TFF monomers and dimers stimulate cell migration, however the TFF1 dimer shows a greater effect than TFF1 monomer on cell migration in wounded monolayers of the human colonic cell line HT29 (Reference2a). In contrast, results suggest that neither an intact trefoil domain nor dimerization of TFF3 is required to promote cell migration (91). However, much remains unknown on the underlying mechanism of TFF-driven restitution. In a more general sense, numerous factors are shown in vitro and in vivo to regulate epithelial restitution/cell migration, including luminal acid, calcium, epithelial cell microfilaments, prostaglandins, secreted growth factors such as EGF, hepatocyte growth factor (HGF), transforming growth factor (TGF), and TFF peptides (27, 46, 72, 73, 75). It is a limitation that most of the TFF effects on cell migration are studied in vitro using cancer cell lines. Although not an ideal model, due to the altered regulation of proliferation and cell migration (invasion), a variety of GI cancer cell lines have provided the bulk of information about the TFF signaling cascades that relate to cell migration. Fortunately, some outcomes were confirmed by in vivo studies of normal tissues.
Restitution generally involves the following: (a) the orderly dissociation of cell–cell and cell–substratum contacts to liberate viable cells for motion from their site of origin, (b) reorganization of the cytoskeleton, including the formation of leading edge lamellipodia to promote cell movement, and (c) signal production to promote cell migration and prevent cell death via anoikis, a form of apoptosis activated when cells detach from their natural substratum (76) (Figure 1). An overlapping array of molecular signals is involved in each step of this process.
Cell Adhesion
The cell–cell adhesion promoted by the Zonula adherens complex is established and maintained by epithelial cadherin (E-cadherin) (also known as CAM 120/80). Cadherin binding between adjacent cells is gated by extracellular Ca2+, which strengthens extracellular cadherin-binding domains to promote intercellular adhesion (77). E-cadherin normally associates with β-catenin, forming the E-cadherin/β-catenin complex that plays an essential role in maintaining epithelial integrity. Disruption of the E-cadherin/β-catenin complex results in loss of cell–cell adhesion (78). In HT29 colonic carcinoma cells in vitro, TFF3 disorders the E-cadherin/β-catenin complex by inducing β-catenin tyrosine phosphorylation, resulting in subsequent destabilization of the adherens junctions and disruption of cell–cell adhesion (78). Its also reported that TFF3-induced E-cadherin degradation in HT29 cells was blocked by inhibiton of the ERK and JAK/STAT3 pathways (Reference 3a). Because restitution of the gastric epithelium in vivo was shown to be dependent on both Tff and extracellular calcium mobilization, there is some evidence for multiple signals that would impact cell–cell adhesion (46, 79). It is also well documented in cultured colonic carcinoma cells that TFF3 increases claudin-mediated transepithelial resistance (54), implicating a role of TFF in regulating tight junctional permeability that would be intimately associated with the fidelity of cell–cell adhesion.
Disruption of cell–substratum adhesion is another critical step required for cell migration. Integrins are transmembrane surface receptors that mediate cell–cell and cell–extracellular matrix interactions. Stimulation of integrins activate focal adhesion kinase (FAK) and initiates a cascade of signals through Ras to ERK/MAPK (76). It was demonstrated in vitro in nontransformed intestinal epithelial cells that autophosphorylation of FAK at tyrosine 397 promotes the association of FAK with PI3K, thereby disrupting the focal adhesion complex (80). Evidence suggests that TFF peptides do not directly affect integrin action (81). In contrast, TFF peptides are hypothesized to activate FAK via tetraspanins, proteins that are integrin-binding and scaffolding partners. TFF2 and TFF3 in vitro promote the formation of the tetraspanins/integrin complex and through this complex are thought to regulate various integrin adhesion and motility functions (80, 82).
Cytoskeletal Changes
Rho-family GTPases are known to regulate cytoskeletal changes and are considered to be initiating signals for cell migration (83). Rho triggers the formation of contractile stress fibers and focal adhesion complexes, and the Rho-family member Rac induces rapid actin polymerization and lamellipodial protrusions (83, 84). Several studies have found that TFF peptides are able to activate Rho-family members. TFF stimulation in vitro in the presence of active Src kinase was shown to promote Src localization to focal adhesions and activate Rho-family members (85). Similarly, all TFF peptides were shown in vitro in premalignant colonic epithelial cells to require activated Src/RhoA to induce cellular invasion (86, 87). Further support of TFF-mediated actin cytoskeleton rearrangement was demonstrated in the Caco-2 cells by Xu et al. (88), who showed that TFF3 was capable of restricting platelet-activating factor-induced disruption of the F-actin cytoskeleton. These studies point to the role of TFF in activating actin cytoskeleton changes during cell migration.
Cell-Survival Signaling
When GI epithelial cells detach from neighboring cells and the substratum, anoikis is actively triggered as a mechanism of programmed cell death. To promote migration that allows restitution, factors must be present to inhibit anoikis and promote cell survival. The presence of these factors ensures that migrating cells are able to reach the site of damage and reestablish the epithelial barrier. TFF peptides were demonstrated to regulate cell survival via ERK/MAPK, PI3K/Akt, phospholipase C (PLC)/PKC, β-catenin, and EGF signaling pathway (34, 78, 87, 89–91). Sun et al. (92) showed that in cultured GES-1 cells (normal human gastric epithelial cells), exogenous addition of TFF3 activated the PI3K/Akt pathway known to be involved in gastric epithelial repair. Given the previously noted promiscuity of TFF peptide action, results support the concept that TFF peptides are involved in GI mucusal repair, although TFF3 is normally found only in the intestine and not the stomach. Interestingly, in vitro PI3K/Akt also mediates TFF3 antiapoptotic function, which leads to the activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), a known downstream target of the PI3K/Akt pathway (31, 91). Thus, studies support the role of TFF peptides as motogenic factors that simultaneously stimulate pro-survival pathways necessary for restitution.
Trefoil Factor Cellular Signaling Required for Cell Movement
A number of signaling cascades are activated during TFF-driven cell migration and restitution. When blockade of these pathways is used to slow migration, it is generally unknown if the effect is on one of the aforementioned functions that prepare cells to migrate efficiently, or if there is a separate effect of the TFF that directly promotes cell movement into the damage area.
Many of the signaling pathways assigned to the cell migration function strongly overlap with those examined for other TFF functions. For instance, in intestinal epithelial cells and colonic cancer cells, TFF3 increases the phosphorylation of ERK1/2, paralleling TFF3’s motogenic effect (34, 91, Reference3a). In vitro, TFF3 is shown to be dependent on ERK1/2 activation (89). This is speculated to be an important regulator of cell motility, leading to phosphorylation of the myosin light kinase chain (91, 93). TFFs’ effect on the cell scattering of epithelial cells (a form of undirected cell spreading that models migration) is dependent on various pathways, including PI3K/Akt, PLC, and Src/RhoA (86, 89). In vivo murine data suggest that the Tff pathway for the regulation of gastric epithelial restitution does not require Cox activity (27). In various gastrointestinal cell types, there is a linkage between increased TFF expression and increased ERK1/2. There is also in vitro evidence that TFF2-dependent cell motility is dependent on active ERK signaling in bronchial epithelial cells (89). Together these findings suggest a pivitol role of ERK activation in the downstream signals of TFF peptides that lead to cell migration.
In addition to some familiar signaling pathways described above, analysis of TFF-dependent migration has also revealed some unique pathways. Pharmacologic inhibition in vitro suggests the involvement of PKC in TFF-dependent cell migration (89). It is hypothesized that PKC, along with Ras/GTP, activates the Raf cascade, which leads to the phosphorylation of MEK-1 and ERK1/2 (89). As outlined below, a group of membrane receptors are also implicated as part of the cell migration signaling pathways.
Trefoil Factor Stimulation of Epidermal Growth Factor Receptors
Studies have demonstrated the stimulation of Egf-r and MAPK (Erk1 and Erk2) in the healing gut mucosa (94). TFF2 and TFF3 peptides signal through the ErbB pathways, but it is entirely unknown if this is due to direct interaction of TFF peptides with EGF-R/ErB or if there is an intermediate EGF family ligand involved. There is evidence in vitro that TFF3 can act on EGF-R to activate several downstream signaling pathways, including MAPK and PI3K/Akt (90, 92). In vitro in colonic cancer and in nontransformed intestinal epithelial cell lines, MAPK activation is primarily responsible for the TFF3-mediated initiation of healing (91). Incubation of the human gastric cancer cell lines AGS and KATO-III with recombinant TFF2 lead to the phosphorylation of EGF-R and the activation of MAPKs in vitro (34). In the stomach in vivo, Egf-r signaling was shown to inhibit parietal cell activity and secretion (94, 95), demonstrating the presence of the appropriate receptors but not shedding light on the involvement with TFF in this tissue. The stomach tissue of Tff2 KO mice has no change in the two major gastric Egf-R ligands (heparin-binding-Egf and Tgf-α), although these mice have slowed gastric repair and increased acid secretion (26), so results suggest that engagement of TFF2 peptide may be required for full action of EGF signaling or EGF-R in the stomach.
Trefoil Factor Family Stimulation via CXC Receptors
TFF2 peptide in vitro and in vivo stimulates G protein-coupled C-X-C chemokine receptor 4 (CXCR4)-dependent signaling in immune and epithelial cells (43, 46). This TFF2 action has a role in both stimulating epithelial restitution and modulating immune response. TFF2 is also expressed by immune cells such as lyophocytes, monocytes, macropahges, etc. and is capable of influencing immune cell chemotaxis and cytokine release.. In these immune cells, TFF2 directly or indirectly activates CXCR4, resulting in chemotaxis. TFF2 peptide was shown in cultured lymphocytic cells to stimulate Akt signaling via CXCR4 (43). TTF2-expressing lymphoblastic Jurkat cells were shown to migrate more efficiently when compared with empty vector controls and competitively inhibit SDF-1α-mediated migration (43). The implications of this for GI physiology of the mucosal immune system remain untested.
Similar to evidence in immune cells, TFF2 peptide has also been shown to directly activate CXCR4 in the epithelial cells. This was clearly demonstrated using the gastric epithelial cell line AGS which does not endogenously express CXCR4. Unmodified AGS cells were shown to be unresponssive to TFF2 (43). However, stably CXCR4 transfected AGS cells responded to TFF2 treatment with robust phosphorylation of ERK1/2 and protein kinase B/AKT, a pattern which mirrored the Jurkat cell pathway (43). CXCR4 activation required high concentrations of recombinant TFF both in vivo and in vitro, consistent with previous reports of high levels of TFF in normal physiological conditions (43, 89). During ulcer healing, in vivo epithelial levels of Cxcr4 and Tff2 are increased, which suggests that any activity via this pathway would be amplified during wound healing (112, Reference 5a).
Another feature of TFF2-driven CXCR4 activation is Ca2+ mobilization, which was originally observed in the Jurkat lymphocytic cell line (43). More recently, activation of CXCR4 in the Caco-2 cell line was found to stimulate the release of intracellular Ca2+ and enhance intestinal epithelial restitution through reorganization of the actin cytoskeleton via the calcium-dependent focal adhesion kinase Pyk2 (97). Because gastric epithelial repair is associated with second messenger-stimulated Ca+2 mobilization in vivo (79), and a Cxcr4 antagonist (AMD3100) blocks Tff2-dependent gastric epithelial repair (27), these results most closely support a role of CXCR4 in the stomach response to TFF2.
CXCR4 is best established as a receptor for the chemokine CXCL12. It was shown in colon carcinoma cell lines and intestinal epithelial cell lines that CXCL12 activation of CXCR4 stimulates the restitution of wounded epithelium and migration of epithelial cells in human T84 colonic epithelial cells (98). This activation requires PI3K signaling to direct cell migration while also stimulating ERK1/2 phosphorylation that is required for cell motility (98). CXCL12 binding to CXCR4 may also play a role in enhancing epithelial barrier integrity as CXCL12 regulates E-cadherin sheet migration during restitution. Results indicate that CXCL12-CXCR4 stimulates E-cadherin localization and monolayer tightening through Rho-associated protein kinase activation and F-actin reorganization (99). The parallelism between actions of CXCL12 and TFF peptides supports the hypothesis that they act via the same receptor. However, in the lymphocytic Jurkat cells, it was noted that only CXCL12 was able to activate ERK1/2, suggesting that at a minimum the receptor does not always respond equally to the two potential ligands (43).
Trefoil Factor Stimulation and Sodium Hydrogen Exchangers
Sodium hydrogen exchangers (NHEs) are membrane transporters that mediate numerous functions, including cellular Na+ uptake and intracellular pH and cell volume regulation (95, 100). Interestingly, the Nhe2 isoform expressed in the GI epithelium was also implicated in repair of the mouse and pig intestinal epithelium (101, 102) and repair of the mouse gastric epithelium (46). In the mouse stomach, addition of recombinant TFF rescued the slowed restitution in Tff2 KO mice, and this effect was inhibited by the addition of an Nhe2 inhibitor. Furthermore, Xue et al. (46) demonstrated that Nhe2 KO mice have delayed restitution and repair of microscopic lesions, despite that TFF2 peptide was upregulated in the Nhe2 KO stomach. Because this delayed repair in the Nhe2 KO mice was not rescued by recombinant TFF (46), Nhe2 was implicated in vivo as an important downstream effector of Tff peptides, acting as a mediator of Tff2 action to promote gastric epithelial restitution (46). It was possible to exclude a role of the NHE2 exchanger in controlling luminal pH as a means to promote gastric cell migration, but there are no further insights into the mechanisms whereby NHE2 accelerates restitution. Furthermore, the potential interaction of TFF and NHE2 in the porcine or murine intestine remains untested.
TREFOIL FACTOR AND GASTROINTESTINAL DISEASES
TFF expression dramatically changes in response to injury, and these changes are sustained during healing. Selective TFF peptides upregulate at sites of damage; however, the expression of TFF peptides in injured GI tissues is not always a mere amplification of the expression patterns in the normal GI tract. It was reported that following injury, TFF1 and TFF2 are strongly expressed in the intestine, whereas TFF3 is upregulated in the stomach (103, 104). NO derived from iNos regulates Tff3 production in NSAID-induced small intestinal injury (105) and inflammatory cytokines regulate TFF expression (131–133, 139).
Gastric Ulcer Healing
Gastric ulcer healing is a prolonged and complex process that requires cellular proliferation and tissue remodeling of both mucosa and blood flow in addition to cell migratory responses (106, 107). TFF3 upregulation is observed in mouse ulcer models and human ulcer patients, often associated with intestinal metaplasia (30, 35, 62, 108). TFF1 and TFF2 proteins are both upregulated and appear in the deep gland of the ulcer-regenerated epithelium colocalized with MUC6 and Griffonia simplicifolia lectin (GSII) staining. This unusual distribution is sustained for at least one month in experimental animal models, far beyond the time required to restore a continuous epithelium (30, 109–112). The TFF2-expressing lineage arises coincident with the loss of parietal cells and is referred to as spasmolytic polypeptide expressing metaplasia (SPEM) (113–115). SPEM is derived from transdifferentiation of chief cells, but upregulation of Muc6/GSII also appears in the Tff2 KO mice (112, 115), suggesting that the TFF2 increase is a consequence of mucus metaplasia/hyperplasia, and TFF2 is not required for generation of at least some of the observed changes. It should be noted that Tff1 expression is decreased in Tff2 KO mice, so there are only limited parallels between the injured stomach and the Tff2 KO stomach (26). Gastric ulcer healing is dramatically delayed in Tff2 KO mice (112), and effects to slow cell migration may be exacerbated by losing the effects of TFF peptides on proliferation or cell survival (22, 26, 31). It should also be noted that in this complex healing environment, many growth factors are known to upregulate and promote healing (106, 107). In this constellation of bioactive peptides it is difficult to define which is the master signal for repair. Interestingly, in injury models, Tff upregulation can precede the upregulation of other growth factors, such as Egf and Tgfα (104), suggesting that TFF peptides have enhanced potential to initiate healing.
Gastric ulcer healing is strongly inhibited by NSAIDs, whereas healing is facilitated by the administration of PGs (59, 106). As described, PGs and TFF peptides have separate actions and can act somewhat independently to elicit gastrointestinal mucosal cytoprotection. Because the cytoprotective mechanisms of PGs include enhancing mucus secretion (75) as well as Egf-dependent proliferation (116), PG action can complement TFF action. There is still some potential for cross talk between these mechanisms. Aspirin-induced injury does not alter Tff1 or Tff2 mRNA and protein in rat stomach (117), but the more severe damage caused by repetitive administration of aspirin causes an increase of Tff2 and Tgfα (60). Indomethacin or aspirin also increases TFF2 mRNA (with no effect to TFF1 and TFF3) in MKN45 cell, a human gastric cancer cell line (118, 119). Because neither arachidonic acid nor PG affect TFF2 expression, indomethacin-induced upregulation of TFF2 appears independent of PG action (118). The COX-independent actions of NSAIDs are well established to be mediated by several transcriptional factors, including peroxisome proliferator-activated receptor γ (PPARγ) (120). Pparγ is expressed in normal gastric epithelium and Pparγ agonist also accelerates ulcer healing (121, 122). PPARγ activator, 15-deoxy-Delta12,14-prostaglandin J2 stimulates TFF1 and TFF2 gene expression (but has no effect on TFF3) in MKN45 cells (123).
Helicobacter pylori
Helicobacter pylori is a bacterial pathogen that causes chronic gastritis and gastric cancer. In the stomach, H. pylori selectively interacts with TFF1 and MUC5AC to colonize (63, 124) (Figure 2). The H. pylori core oligosaccharide portion (rough form) of lipopolysaccharide (LPS) specifically binds to TFF1 dimer (but not its monomer) most favorably in pH 5.0–6.0 conditions. H. pylori is also capable of interacting with TFF3 dimer at >pH 7.0, but not with TFF2; even glycosylated TFF2 was shown not to interact with H. pylori (63, 125). The C-terminal of TFF1 can also specifically bind copper ions, which enhances H. pylori binding to TFF1 (126). However, MUC5AC is downregulated late in the gastric epithelium after H. pylori infection or ulceration (112, 127). This could be explained by the observation that H. pylori preferentially colonizes initially in the injury or ulcerated site of the stomach, whereas this preferential colonization is not observed when H. pylori is inoculated 30 days after ulcer induction (110, 112).
Figure 2.
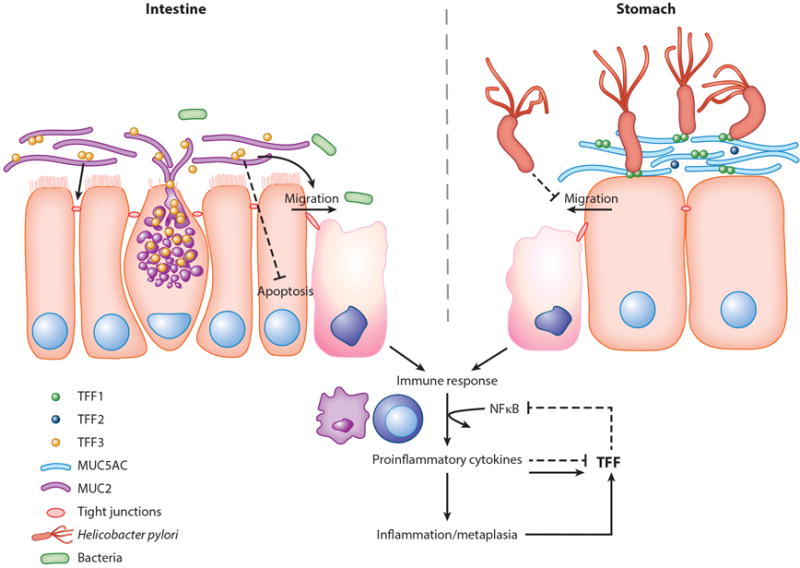
Candidate TFF pathways in GI inflammation. Goblet cells produce and secrete TFF3 along with MUC2 into the lumen within the intestine, whereas gastric mucus cells secrete TFF1, which is present with MUC5AC. Although TFF2/MUC6 is also present within the stomach, Helicobacter pylori is shown to primarily interact with MUC5AC/TFF1 in the gastric lumen. The response to chronic inflammation within both the stomach and the intestine acts in a similar manner where cytokine stimulation causes TFF upregulation or downregulation, or metaplasia leads to the increase of TFF-secreting cell differentiation and results in a negative feedback to the immune response. Abbreviations: GI, gastrointestinal; MUC, mucin; NF-κB, nuclear factor kappa- B; TFF, trefoil factor.
Chronic H. pylori infection induces inflammation/mucus metaplasia that is accompanied by the alteration of TFF expression. This may be either an increase or decrease of TFF peptides, dependsing upon the stimulus and/or progression of inflammation. The process of H. pylori-induced inflammation involves many cytokines, including tumor necrosis factor (TNF)α, IL-1β, IL-6, and activation of NF-κB, among others. In vitro, TFF1 strongly suppress H. pylori-induced activation of NF-kB and resultant target proinflammatory cytokine production (128). H. pylori infected stomach in Tff1 KO mice showed an enhanced inflammation and invasive gastric adenocarcinoma (128). TFF2 specifically binds to O-linked α1,4-GlcNAc-capped MUC6 glycan, which is speculated to have a potential antibiotic activity against H. pylori (64). H. pylori infection for 12 and 15 months in normal mouse stomach reduced antral expression of Tff2 by increased methylation at Tff2 promoter site, mirroring the findings in human samples (130). The role of Tff2 was further established in Tff2 KO mice as H. pylori was shown to promote the progression of gastritis to dysplasia (129). Using a related organism, Helicobacter felis infection develops severe inflammation, including mucus metaplasia, intestinal metaplasia, and dysplasia in Tff2 KO mice, with a higher serum level of H. felis-specific IgGs compared to WT mice, suggesting a strong Th1-polarized T-cell response (57).
In this inflammatory environment, the role of TFF in immune cells is likely of higher importance to disease status (Figure 2). H. pylori-induced pro-inflammatory cytokines, Tnfα or Il-1β production, are markedly enhanced in Tff1 KO mice (128), suggesting Tff1 plays an anti-inflammatory role. The expression of Tff2 is observed in splenocytes and peritoneal macrophages, and both splenic T cells and peritoneal macrophages from Tff2 KO mice exhibit enhanced activities, such as greater Il-1β-stimulated Il-6 secretion in Tff2 KO peritoneal macrophages (57). In WT animals, evidence suggests that TFF2 functions acts as a damper of immune cell cytokine responses and will help prevent progression of inflammation. Proinflammatory cytokines also differentially affect TFF expression. Activation of TNFα/NF-κB is reported to increase TFF1 transcription in AGS and MKN48 gastric carcinoma cell lines (131). In contrast, IL-1β or IL-6 repress TFF promoter activity in the HT-29 (colorectal adenocarcinoma) and KATO-III (gastric carcinoma) cell lines via activation of NF-κB or C/EBPβ, respectively (132). More recently, it was demonstrated that exposure of TNFα and IL-1β to human gastric cancer sample ex-vivo and MKN48 cells in vitro downregulates TFF1 protein expression (133). H. pylori causes qualitatively different changes in TFF1, TFF2, and TFF3 expression in tissue and gastric carcinoma cell lines with differential TFF expression patterns in each stage of inflammation (6, 134), so the the in vivo profile of TFF abundance is complicated by alteration of cell types during inflammation and the action of proinflammatory cytokines.
Intestinal Diseases
Different types of intestinal injury produce a characteristic TFF expression profile. Methotrexate-induced intestinal damage causes an initial decrease in Tff3 protein expression, but Tff3 mRNA rebounds even before goblet cell/Tff3 repopulation (135). In response to acetic acid-induced colonic epithelial injury, Tff1 mRNA is upregulated in the initial injury phase and is not detected in the healing phase. In contrast, Tff3 mRNA is decreased in the initial phase and upregulated in the healing phase (136). Tff3 is also increased in response to DSS-induced colitis in mice, however, there was no detection of Tff1 and Tff2 (137). Regeneration of colonic epithelium following injury was impaired in Tff3 KO or Vip KO (with less Tff3/Muc2) mice (32, 38). Colonic luminal application of recombinant TFF3 improved mucosal healing of acetic acid-induced injury in Tff3 KO mice (32) and after ischemia-reperfusion-induced injury in rats (52). The beneficial effect of TFF3 for mucosal healing can be in part mediated by its anti-apoptotic function via EGF-R/PI3K and activation of the NF-κB pathway in intestinal tissues (31, 138). Overall, results suggest a significant involvement of TFF3 in regeneration of mucosal integrity after injury, in addition to mucosal protection of healthy tissue.
Trefoil Factor and Immune Function in the Intestine
TFF3 may regulate the immune response indirectly via epithelial cells or directly via immune cells (Figure 2). Recombinant human TFF3 improves 2,4,6-trinitrobenzene sulphonic acid (TNBS)-induced colitis via inhibiting Toll-like receptor (Tlr)4/Nf-κb signaling and Tnfα production in the mouse colonic epithelium (140). In a rat necrotizing enterocolitis model, TFF3 improves inflammation by inhibiting the increase of Nf-κb, Il-1β, and Il-6, while enhancing the expression of Il-10, an anti-inflammatory cytokine (141). TFF3 inhibits IL-6 and IL-8 secretion induced by LPS and stimulates defensin production in the HT29 cell line in vitro (142). In addition to dampening pro-inflammatory cytokine amounts, TFF peptides themselves have been demonstrated to be regulated by cytokines or TLR signaling. The proinflammatory TNFα downregulates TFF3 in the HT29 cell line, mediated by NF-κB activation (139). Additionally, activated Nf-κb expression was detected in both immune and epithelial cells paralleled a reduction in Tff3 expression in a rat model of TNBS-induced colitis (139). Activation of TLR2 leads to TFF3 production in the goblet cell, and it was demonstrated in colitis conditions that the Tlr2 antiapoptotic effect is mainly mediated by TFF3 (143). The involvement of TLR2 in TFF3 production was also demonstrated in TLR2-transfected Caco-2 cells. Although the antiapoptotic effect of Tlr2 is absent in Tff3 KO mice, activation of Tlr2 improved restoration of Zo-1-associated barrier integrity during the recovery phase of DSS-induced colitis in Tff3 KO mice, suggesting that TLR2 acts via a TFF3-independent pathway as well. Additionally, recombinant TFF3 dramatically improved DSS-induced colitis in Tlr2 KO mice (143). The decrease of transepithelial resistance and increase of cytokines, such as IL-6 and IL-8, and TNFα secretion induced by IL-1β is reversed by overexpression of TLR2 or TFF3. This effect is accompanied by an increased expression of the tight junction proteins ZO-1, OCCLUDIN, and CLAUDIN-1 and is reversed if TLR2 is knocked down in the Caco-2 cell (144). Effects were mediated by the PI3K/Akt pathway (144), consistent with other findings that the protective effect of TFF3 in gastric mucosal epithelial cell is mediated by activation of this signaling pathway (92). Thus, TFF3 seems able to inhibit inflammatory cytokines in addition to other cellular effects that enhance mucosal defenses.
TFF2 also promotes intestinal mucosal healing in the setting of inflammation (55, 145). The increase of heterogeneous nuclear ribonucleoprotein 1 (hnRNP A1) and Tff2 expression is observed in anti-CD3 antibody-induced T cell-mediated murine enteritis. It was demonstrated that hnRNP A1 positively affects mucosal repair mediated by interacting with Tff2 and enhancing Tff2 anti-apoptotic function (146). Tff2 upregulation, but not Tff3, is observed in dinitrobenzene sulfonic acid-induced rat colitis, and further administration of recombinant human TFF2 facilitates healing (145). Administration of engineered TFF2-secreting Lactococcus lactis improves the spontaneous chronic colitis in Il-10 KO mice (147). TFF2 reduces colonic inflammation by inhibiting NO production derived from iNOS in monocytes (148). Following DSS-induced colitis, Tff2 KO mice, but not Tff1 KO mice, show severe weight loss accompanied with an altered inflammatory cytokine expression profile, such as further upregulation of Il-6 and downregulation of Il-33, when compared with WT mice (149). Interestingly, the peritoneal macrophages from Tff2 KO mice show downregulation of Il-6, Il-1β, Il-10, Il-19, and Il-33 compared to WT mice. Additionally, LPS stimulation enhanced an increase of Il-6 and Il-1β production and reduced levels of Il-10 and Il-19 production, whereas Il-33 sustained a low level in the peritoneal macrophages from Tff2 KO mice (149). As TFF3 expression is altered in the Tff2 KO mice, it is possible that some of these effects are mediated by TFF3. In overview, data suggest that TFF peptides regulate immune cells in colitis as another route to promote tissue repair when damage is extensive and the inflammatory response is part of the healing environment.
CONCLUDING REMARKS
The GI epithelium is continually exposed to insults. Bacteria, frequent cell renewal, chemical stressors, ingested medicines, and physical stresses all contribute to the need for tissue repair. Although much of the TFF signaling pathway remains unclear, studies have revealed unequivocal benefits of the TFF peptides to healthy and damaged GI tissues. Since TFF peptides were proposed to be luminal surveillance peptides by Playford in 1995 (150), TFF peptides have been shown to physiologically contribute to epithelial repair, consolidate the mucus layer in collaboration with mucin, and generally prepare for an epithelial emergency on either a small or large scale. The most abundant literature focuses on the compelling role of TFF peptides in the processes of re-epithelialization and in regulation of inflammation and the immune system. As the signaling mechanisms of the TFF family continue to be revealed, there is much to learn about the distinct and shared pathways among this small family of protective peptides.
Acknowledgments
This work was supported by the National Institutes of Health (R01-DK102551) to M.H.M.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Literature Cited
- 1.Masiakowski P, Breathnach R, Bloch J, Gannon F, Krust A, Chambon P. Cloning of cDNA sequences of hormone-regulated genes from the MCF-7 human breast cancer cell line. Nucleic Acids Res. 1982;10:7895–903. doi: 10.1093/nar/10.24.7895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jorgensen KH, Thim L, Jacobsen HE. Pancreatic spasmolytic polypeptide (PSP): I. Preparation and initial chemical characterization of a new polypeptide from porcine pancreas. Regul Pept. 1982;3:207–19. doi: 10.1016/0167-0115(82)90126-4. [DOI] [PubMed] [Google Scholar]
- 3.Suemori S, Lynch-Devaney K, Podolsky DK. Identification and characterization of rat intestinal trefoil factor: tissue- and cell-specific member of the trefoil protein family. PNAS. 1991;88:11017–21. doi: 10.1073/pnas.88.24.11017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright NA, Hoffmann W, Otto WR, Rio MC, Thim L. Rolling in the clover: trefoil factor family (TFF)-domain peptides, cell migration and cancer. FEBS Lett. 1997;408:121–23. doi: 10.1016/s0014-5793(97)00424-9. [DOI] [PubMed] [Google Scholar]
- 5.Thim L. A new family of growth factor-like peptides. ‘Trefoil’ disulphide loop structures as a common feature in breast cancer associated peptide (pS2), pancreatic spasmolytic polypeptide (PSP), and frog skin peptides (spasmolysins) FEBS Lett. 1989;250:85–90. doi: 10.1016/0014-5793(89)80690-8. [DOI] [PubMed] [Google Scholar]
- 6.Xiao P, Ling H, Lan G, Liu J, Hu H, Yang R. Trefoil factors: gastrointestinal-specific proteins associated with gastric cancer. Clin Chim Acta. 2015;450:127–34. doi: 10.1016/j.cca.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Chinery R, Bates PA, De A, Freemont PS. Characterisation of the single copy trefoil peptides intestinal trefoil factor and pS2 and their ability to form covalent dimers. FEBS Lett. 1995;357:50–54. doi: 10.1016/0014-5793(94)01297-e. [DOI] [PubMed] [Google Scholar]
- 8.Williams MA, Westley BR, May FE, Feeney J. The solution structure of the disulphide-linked homodimer of the human trefoil protein TFF1. FEBS Lett. 2001;493:70–74. doi: 10.1016/s0014-5793(01)02276-1. [DOI] [PubMed] [Google Scholar]
- 9.Muskett FW, May FE, Westley BR, Feeney J. Solution structure of the disulfide-linked dimer of human intestinal trefoil factor (TFF3): the intermolecular orientation and interactions are markedly different from those of other dimeric trefoil proteins. Biochemistry. 2003;42:15139–47. doi: 10.1021/bi030182k. [DOI] [PubMed] [Google Scholar]
- 10.Kjellev S. The trefoil factor family—small peptides with multiple functionalities. Cell Mol Life Sci. 2009;66:1350–69. doi: 10.1007/s00018-008-8646-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seib T, Blin N, Hilgert K, Seifert M, Theisinger B, et al. The three human trefoil genes TFF1, TFF2, and TFF3 are located within a region of 55 kb on chromosome 21q22.3. Genomics. 1997;40:200–2. doi: 10.1006/geno.1996.4511. [DOI] [PubMed] [Google Scholar]
- 12.Jakowlew SB, Breathnach R, Jeltsch JM, Masiakowski P, Chambon P. Sequence of the pS2 mRNA induced by estrogen in the human breast cancer cell line MCF-7. Nucleic Acids Res. 1984;12:2861–78. doi: 10.1093/nar/12.6.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan ZE, Wang TC, Cui G, Chi AL, Dimaline R. Transcriptional regulation of the human trefoil factor, TFF1, by gastrin. Gastroenterology. 2003;125:510–21. doi: 10.1016/s0016-5085(03)00908-9. [DOI] [PubMed] [Google Scholar]
- 14.Hertel SC, Chwieralski CE, Hinz M, Rio MC, Tomasetto C, Hoffmann W. Profiling trefoil factor family (TFF) expression in the mouse: identification of an antisense TFF1-related transcript in the kidney and liver. Peptides. 2004;25:755–62. doi: 10.1016/j.peptides.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann W, Jagla W, Wiede A. Molecular medicine of TFF-peptides: from gut to brain. Histol Histopathol. 2001;16:319–34. doi: 10.14670/HH-16.319. [DOI] [PubMed] [Google Scholar]
- 16.Rio MC, Bellocq JP, Daniel JY, Tomasetto C, Lathe R, et al. Breast cancer-associated pS2 protein: synthesis and secretion by normal stomach mucosa. Science. 1988;241:705–8. doi: 10.1126/science.3041593. [DOI] [PubMed] [Google Scholar]
- 17.Hanby AM, Poulsom R, Singh S, Elia G, Jeffery RE, Wright NA. Spasmolytic polypeptide is a major antral peptide: Distribution of the trefoil peptides human spasmolytic polypeptide and pS2 in the stomach. Gastroenterology. 1993;105:1110–16. doi: 10.1016/0016-5085(93)90956-d. [DOI] [PubMed] [Google Scholar]
- 18.Lefebvre O, Wolf C, Kédinger M, Chenard MP, Tomasetto C, et al. The mouse one P-domain (pS2) and two P-domain (mSP) genes exhibit distinct patterns of expression. J Cell Biol. 1993;122:191–98. doi: 10.1083/jcb.122.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madsen J, Sorensen GL, Nielsen O, Tornøe I, Thim L, et al. A variant form of the human deleted in malignant brain tumor 1 (DMBT1) gene shows increased expression in inflammatory bowel diseases and interacts with dimeric trefoil factor 3 (TFF3) PLOS ONE. 2013;8:e64441. doi: 10.1371/journal.pone.0064441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madsen J, Nielsen O, Tornøe I, Thim L, Holmskov U. Tissue localization of human trefoil factors 1, 2, and 3. J Histochem Cytochem. 2007;55:505–13. doi: 10.1369/jhc.6A7100.2007. [DOI] [PubMed] [Google Scholar]
- 21.Lefebvre O, Chenard MP, Masson R, Linares J, Dierich A, et al. Gastric mucosa abnormalities and tumorigenesis in mice lacking the pS2 trefoil protein. Science. 1996;274:259–62. doi: 10.1126/science.274.5285.259. [DOI] [PubMed] [Google Scholar]
- 22.Bossenmeyer-Pourié C, Kannan R, Ribieras S, Wendling C, Stoll I, et al. The trefoil factor 1 participates in gastrointestinal cell differentiation by delaying G1-S phase transition and reducing apoptosis. J Cell Biol. 2002;157:761–70. doi: 10.1083/jcb200108056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karam SM, Tomasetto C, Rio MC. Trefoil factor 1 is required for the commitment programme of mouse oxyntic epithelial progenitors. Gut. 2004;53:1408–15. doi: 10.1136/gut.2003.031963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jørgensen KD, Diamant B, Jørgensen KH, Thim L. Pancreatic spasmolytic polypeptide (PSP): III. Pharmacology of a new porcine pancreatic polypeptide with spasmolytic and gastric acid secretion inhibitory effects. Regul Pept. 1982;3:231–43. doi: 10.1016/0167-0115(82)90128-8. [DOI] [PubMed] [Google Scholar]
- 26.Farrell JJ, Taupin D, Koh TJ, Chen D, Zhao CM, et al. TFF2/SP-deficient mice show decreased gastric proliferation, increased acid secretion, and increased susceptibility to NSAID injury. J Clin Invest. 2002;109:193–204. doi: 10.1172/JCI12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xue L, Aihara E, Podolsky DK, Wang TC, Montrose MH. In vivo action of trefoil factor 2 (TFF2) to speed gastric repair is independent of cyclooxygenase. Gut. 2010;59:1184–91. doi: 10.1136/gut.2009.205625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quante M, Marrache F, Goldenring JR, Wang TC. TFF2 mRNA transcript expression marks a gland progenitor cell of the gastric oxyntic mucosa. Gastroenterology. 2010;139:2018–27.e2. doi: 10.1053/j.gastro.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Podolsky DK, Lynch-Devaney K, Stow JL, Oates P, Murgue B, et al. Identification of human intestinal trefoil factor. Goblet cell-specific expression of a peptide targeted for apical secretion. J Biol Chem. 1993;268:12230. [PubMed] [Google Scholar]
- 30.Hauser F, Poulsom R, Chinery R, Rogers LA, Hanby AM, et al. hP1.B, a human P-domain peptide homologous with rat intestinal trefoil factor, is expressed also in the ulcer-associated cell lineage and the uterus. PNAS. 1993;90:6961–65. doi: 10.1073/pnas.90.15.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taupin DR, Kinoshita K, Podolsky DK. Intestinal trefoil factor confers colonic epithelial resistance to apoptosis. PNAS. 2000;97:799–804. doi: 10.1073/pnas.97.2.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mashimo H, Wu DC, Podolsky DK, Fishman MC. Impaired defense of intestinal mucosa in mice lacking intestinal trefoil factor. Science. 1996;274:262–65. doi: 10.1126/science.274.5285.262. [DOI] [PubMed] [Google Scholar]
- 33.Nunez AM, Berry M, Imler JL, Chambon P. The 5′ flanking region of the pS2 gene contains a complex enhancer region responsive to oestrogens, epidermal growth factor, a tumour promoter (TPA), the c-Ha-ras oncoprotein and the c-jun protein. EMBO J. 1989;8:823–29. doi: 10.1002/j.1460-2075.1989.tb03443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taupin D, Wu DC, Jeon WK, Devaney K, Wang TC, Podolsky DK. The trefoil gene family are coordinately expressed immediate-early genes: EGF receptor- and MAP kinase-dependent interregulation. J Clin Invest. 1999;103:R31–38. doi: 10.1172/JCI3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cook GA, Yeomans ND, Giraud AS. Temporal expression of trefoil peptides in the TGF-alpha knockout mouse after gastric ulceration. Am J Physiol. 1997;272:G1540–49. doi: 10.1152/ajpgi.1997.272.6.G1540. [DOI] [PubMed] [Google Scholar]
- 36.Tu S, Chi AL, Lim S, Cui G, Dubeykovskaya Z, et al. Gastrin regulates the TFF2 promoter through gastrin-responsive cis-acting elements and multiple signaling pathways. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1726–37. doi: 10.1152/ajpgi.00348.2006. [DOI] [PubMed] [Google Scholar]
- 37.Kang B, Alderman BM, Nicoll AJ, Cook GA, Giraud AS. Effect of omeprazole-induced achlorhydria on trefoil peptide expression in the rat stomach. J Gastroenterol Hepatol. 2001;16:1222–27. doi: 10.1046/j.1440-1746.2001.02609.x. [DOI] [PubMed] [Google Scholar]
- 38.Wu X, Conlin VS, Morampudi V, Ryz NR, Nasser Y, et al. Vasoactive intestinal polypeptide promotes intestinal barrier homeostasis and protection against colitis in mice. PLOS ONE. 2015;10:e0125225. doi: 10.1371/journal.pone.0125225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poulsen SS, Thulesen J, Nexø E, Thim L. Distribution and metabolism of intravenously administered trefoil factor 2/porcine spasmolytic polypeptide in the rat. Gut. 1998;43:240–47. doi: 10.1136/gut.43.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poulsen SS, Thulesen J, Hartmann B, Kissow HL, Nexø E, Thim L. Injected TFF1 and TFF3 bind to TFF2-immunoreactive cells in the gastrointestinal tract in rats. Regul Pept. 2003;115:91–99. doi: 10.1016/s0167-0115(03)00145-9. [DOI] [PubMed] [Google Scholar]
- 41.Tan XD, Hsueh W, Chang H, Wei KR, Gonzalez-Crussi F. Characterization of a putative receptor for intestinal trefoil factor in rat small intestine: identification by in situ binding and ligand blotting. Biochem Biophys Res Commun. 1997;237:673–77. doi: 10.1006/bbrc.1997.7144. [DOI] [PubMed] [Google Scholar]
- 42.Thim L, Mortz E. Isolation and characterization of putative trefoil peptide receptors. Regul Pept. 2000;90:61–8. doi: 10.1016/s0167-0115(00)00110-5. [DOI] [PubMed] [Google Scholar]
- 43.Dubeykovskaya Z, Dubeykovskiy A, Solal-Cohen J, Wang TC. Secreted trefoil factor 2 activates the CXCR4 receptor in epithelial and lymphocytic cancer cell lines. J Biol Chem. 2009;284:3650–62. doi: 10.1074/jbc.M804935200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aikou S, Ohmoto Y, Gunji T, Matsuhashi N, Ohtsu H, et al. Tests for serum levels of trefoil factor family proteins can improve gastric cancer screening. Gastroenterology. 2011;141:837–45.e7. doi: 10.1053/j.gastro.2011.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kjellev S, Nexø E, Thim L, Poulsen SS. Systemically administered trefoil factors are secreted into the gastric lumen and increase the viscosity of gastric contents. Br J Pharmacol. 2006;149:92–99. doi: 10.1038/sj.bjp.0706840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xue L, Aihara E, Wang TC, Montrose MH. Trefoil factor 2 requires Na/H exchanger 2 activity to enhance mouse gastric epithelial repair. J Biol Chem. 2011;286:38375–82. doi: 10.1074/jbc.M111.268219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Playford RJ, Marchbank T, Chinery R, Evison R, Pignatelli M, et al. Human spasmolytic polypeptide is a cytoprotective agent that stimulates cell migration. Gastroenterology. 1995;108:108–16. doi: 10.1016/0016-5085(95)90014-4. [DOI] [PubMed] [Google Scholar]
- 48.McKenzie C, Marchbank T, Playford RJ, Otto W, Thim L, Parsons ME. Pancreatic spasmolytic polypeptide protects the gastric mucosa but does not inhibit acid secretion or motility. Am J Physiol. 1997;273:G112–17. doi: 10.1152/ajpgi.1997.273.1.G112. [DOI] [PubMed] [Google Scholar]
- 49.Babyatsky MW, deBeaumont M, Thim L, Podolsky DK. Oral trefoil peptides protect against ethanol- and indomethacin-induced gastric injury in rats. Gastroenterology. 1996;110:489–97. doi: 10.1053/gast.1996.v110.pm8566596. [DOI] [PubMed] [Google Scholar]
- 50.McKenzie C, Thim L, Parsons ME. Topical and intravenous administration of trefoil factors protect the gastric mucosa from ethanol-induced injury in the rat. Aliment Pharmacol Ther. 2000;14:1033–40. doi: 10.1046/j.1365-2036.2000.00796.x. [DOI] [PubMed] [Google Scholar]
- 51.Beck PL, Wong JF, Li Y, Swaminathan S, Xavier RJ, et al. Chemotherapy- and radiotherapy-induced intestinal damage is regulated by intestinal trefoil factor. Gastroenterology. 2004;126:796–808. doi: 10.1053/j.gastro.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 52.Carrasco R, Pera M, May FE, Westley BR, Martinez A, Morales L. Trefoil factor family peptide 3 prevents the development and promotes healing of ischemia-reperfusion injury in weanling rats. J Pediatr Surg. 2004;39:1693–700. doi: 10.1016/j.jpedsurg.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 53.Xu LF, Teng X, Guo J, Sun M. Protective effect of intestinal trefoil factor on injury of intestinal epithelial tight junction induced by platelet activating factor. Inflammation. 2012;35:308–15. doi: 10.1007/s10753-011-9320-x. [DOI] [PubMed] [Google Scholar]
- 54.Meyer zum Büschenfelde D, Tauber R, Huber O. TFF3-peptide increases transepithelial resistance in epithelial cells by modulating claudin-1 and -2 expression. Peptides. 2006;27:3383–90. doi: 10.1016/j.peptides.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 55.Poulsen SS, Kissow H, Hare K, Hartmann B, Thim L. Luminal and parenteral TFF2 and TFF3 dimer and monomer in two models of experimental colitis in the rat. Regul Pept. 2005;126:163–71. doi: 10.1016/j.regpep.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 56.Playford RJ, Marchbank T, Goodlad RA, Chinery RA, Poulsom R, Hanby AM. Transgenic mice that overexpress the human trefoil peptide pS2 have an increased resistance to intestinal damage. PNAS. 1996;93:2137–42. doi: 10.1073/pnas.93.5.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kurt-Jones EA, Cao L, Sandor F, Rogers AB, Whary MT, et al. Trefoil family factor 2 is expressed in murine gastric and immune cells and controls both gastrointestinal inflammation and systemic immune responses. Infect Immun. 2007;75:471–80. doi: 10.1128/IAI.02039-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Laine L, Takeuchi K, Tarnawski A. Gastric mucosal defense and cytoprotection: bench to bedside. Gastroenterology. 2008;135:41–60. doi: 10.1053/j.gastro.2008.05.030. [DOI] [PubMed] [Google Scholar]
- 59.Wallace JL. Prostaglandins, NSAIDs, and gastric mucosal protection: Why doesn’t the stomach digest itself? Physiol Rev. 2008;88:1547–65. doi: 10.1152/physrev.00004.2008. [DOI] [PubMed] [Google Scholar]
- 60.Konturek PC, Brzozowski T, Pierzchalski P, Kwiecien S, Pajdo R, et al. Activation of genes for spasmolytic peptide, transforming growth factor alpha and for cyclooxygenase (COX)-1 and COX-2 during gastric adaptation to aspirin damage in rats. Aliment Pharmacol Ther. 1998;12:767–77. doi: 10.1046/j.1365-2036.1998.00371.x. [DOI] [PubMed] [Google Scholar]
- 61.Tanaka S, Podolsky DK, Engel E, Guth PH, Kaunitz JD. Human spasmolytic polypeptide decreases proton permeation through gastric mucus in vivo and in vitro. Am J Physiol. 1997;272:G1473–80. doi: 10.1152/ajpgi.1997.272.6.G1473. [DOI] [PubMed] [Google Scholar]
- 62.Longman RJ, Douthwaite J, Sylvester PA, Poulsom R, Corfield AP, et al. Coordinated localisation of mucins and trefoil peptides in the ulcer associated cell lineage and the gastrointestinal mucosa. Gut. 2000;47:792–800. doi: 10.1136/gut.47.6.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ruchaud-Sparagano MH, Westley BR, May FE. The trefoil protein TFF1 is bound to MUC5AC in human gastric mucosa. Cell Mol Life Sci. 2004;61:1946–54. doi: 10.1007/s00018-004-4124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hanisch FG, Bonar D, Schloerer N, Schroten H. Human trefoil factor 2 is a lectin that binds α-GlcNAc-capped mucin glycans with antibiotic activity against Helicobacter pylori. J Biol Chem. 2014;289:27363–75. doi: 10.1074/jbc.M114.597757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Newton JL, Allen A, Westley BR, May FE. The human trefoil peptide, TFF1, is present in different molecular forms that are intimately associated with mucus in normal stomach. Gut. 2000;46:312–20. doi: 10.1136/gut.46.3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thim L, Madsen F, Poulsen SS. Effect of trefoil factors on the viscoelastic properties of mucus gels. Eur J Clin Invest. 2002;32:519–27. doi: 10.1046/j.1365-2362.2002.01014.x. [DOI] [PubMed] [Google Scholar]
- 67.Kindon H, Pothoulakis C, Thim L, Lynch-Devaney K, Podolsky DK. Trefoil peptide protection of intestinal epithelial barrier function: cooperative interaction with mucin glycoprotein. Gastroenterology. 1995;109:516–23. doi: 10.1016/0016-5085(95)90340-2. [DOI] [PubMed] [Google Scholar]
- 68.Tomasetto C, Masson R, Linares JL, Wendling C, Lefebvre O, et al. pS2/TFF1 interacts directly with the VWFC cysteine-rich domains of mucins. Gastroenterology. 2000;118:70–80. doi: 10.1016/s0016-5085(00)70415-x. [DOI] [PubMed] [Google Scholar]
- 69.Albert TK, Laubinger W, Muller S, Hanisch FG, Kalinski T, et al. Human intestinal TFF3 forms disulfide-linked heteromers with the mucus-associated FCGBP protein and is released by hydrogen sulfide. J Proteome Res. 2010;9:3108–17. doi: 10.1021/pr100020c. [DOI] [PubMed] [Google Scholar]
- 70.Sellers LA, Allen A, Bennett MK. Formation of a fibrin based gelatinous coat over repairing rat gastric epithelium after acute ethanol damage: interaction with adherent mucus. Gut. 1987;28:835–43. doi: 10.1136/gut.28.7.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ota H, Hayama M, Momose M, El-Zimaity HM, Matsuda K, et al. Co-localization of TFF2 with gland mucous cell mucin in gastric mucous cells and in extracellular mucous gel adherent to normal and damaged gastric mucosa. Histochem Cell Biol. 2006;126:617–25. doi: 10.1007/s00418-006-0197-y. [DOI] [PubMed] [Google Scholar]
- 72.Svanes K, Ito S, Takeuchi K, Silen W. Restitution of the surface epithelium of the in vitro frog gastric mucosa after damage with hyperosmolar sodium chloride. Morphologic and physiologic characteristics. Gastroenterology. 1982;82:1409–26. [PubMed] [Google Scholar]
- 73.Svanes K, Takeuchi K, Ito S, Silen W. Effect of luminal pH and nutrient bicarbonate concentration on restitution after gastric surface cell injury. Surgery. 1983;94:494–500. [PubMed] [Google Scholar]
- 74.Schumacher MA, Aihara E, Feng R, Engevik A, Shroyer NF, et al. The use of murine-derived fundic organoids in studies of gastric physiology. J Physiol. 2015;593:1809–27. doi: 10.1113/jphysiol.2014.283028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Szabo S. “Gastric cytoprotection” is still relevant. J Gastroenterol Hepatol. 2014;29(Suppl 4):124–32. doi: 10.1111/jgh.12735. [DOI] [PubMed] [Google Scholar]
- 76.Taupin D, Podolsky DK. Trefoil factors: initiators of mucosal healing. Nat Rev Mol Cell Biol. 2003;4:721–32. doi: 10.1038/nrm1203. [DOI] [PubMed] [Google Scholar]
- 77.Kim SA, Tai CY, Mok LP, Mosser EA, Schuman EM. Calcium-dependent dynamics of cadherin interactions at cell-cell junctions. PNAS. 2011;108:9857–62. doi: 10.1073/pnas.1019003108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu D, El-Hariry I, Karayiannakis AJ, Wilding J, Chinery R, et al. Phosphorylation of β-catenin and epidermal growth factor receptor by intestinal trefoil factor. Lab Investig. 1997;77:557–63. [PubMed] [Google Scholar]
- 79.Aihara E, Hentz CL, Korman AM, Perry NP, Prasad V, et al. In vivo epithelial wound repair requires mobilization of endogenous intracellular and extracellular calcium. J Biol Chem. 2013;288:33585–97. doi: 10.1074/jbc.M113.488098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen HC, Appeddu PA, Isoda H, Guan JL. Phosphorylation of tyrosine 397 in focal adhesion kinase is required for binding phosphatidylinositol 3-kinase. J Biol Chem. 1996;271:26329–34. doi: 10.1074/jbc.271.42.26329. [DOI] [PubMed] [Google Scholar]
- 81.Efstathiou JA, Pignatelli M. Modulation of epithelial cell adhesion in gastrointestinal homeostasis. Am J Pathol. 1998;153:341–47. doi: 10.1016/S0002-9440(10)65576-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Buda A, Jepson MA, Pignatelli M. Regulatory function of trefoil peptides (TFF) on intestinal cell junctional complexes. Cell Commun Adhesion. 2012;19:63–68. doi: 10.3109/15419061.2012.748326. [DOI] [PubMed] [Google Scholar]
- 83.Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 84.Machesky LM, Hall A. Role of actin polymerization and adhesion to extracellular matrix in Rac- and Rho-induced cytoskeletal reorganization. J Cell Biol. 1997;138:913–26. doi: 10.1083/jcb.138.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Timpson P, Jones GE, Frame MC, Brunton VG. Coordination of cell polarization and migration by the Rho family GTPases requires Src tyrosine kinase activity. Curr Biol. 2001;11:1836–46. doi: 10.1016/s0960-9822(01)00583-8. [DOI] [PubMed] [Google Scholar]
- 86.Emami S, Le Floch N, Bruyneel E, Thim L, May F, et al. Induction of scattering and cellular invasion by trefoil peptides in src- and RhoA-transformed kidney and colonic epithelial cells. FASEB J. 2001;15:351–61. doi: 10.1096/fj.00-0355com. [DOI] [PubMed] [Google Scholar]
- 87.Emami S, Rodrigues S, Rodrigue CM, Le Floch N, Rivat C, et al. Trefoil factor family (TFF) peptides and cancer progression. Peptides. 2004;25:885–98. doi: 10.1016/j.peptides.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 88.Xu LF, Xu C, Mao ZQ, Teng X, Ma L, Sun M. Disruption of the F-actin cytoskeleton and monolayer barrier integrity induced by PAF and the protective effect of ITF on intestinal epithelium. Arch Pharmacal Res. 2011;34:245–51. doi: 10.1007/s12272-011-0210-4. [DOI] [PubMed] [Google Scholar]
- 89.Graness A, Chwieralski CE, Reinhold D, Thim L, Hoffmann W. Protein kinase C and ERK activation are required for TFF-peptide-stimulated bronchial epithelial cell migration and tumor necrosis factor-α-induced interleukin-6 (IL-6) and IL-8 secretion. J Biol Chem. 2002;277:18440–46. doi: 10.1074/jbc.M200468200. [DOI] [PubMed] [Google Scholar]
- 90.Baus-Loncar M, Giraud AS. Multiple regulatory pathways for trefoil factor (TFF) genes. Cell Mol Life Sci. 2005;62:2921–31. doi: 10.1007/s00018-005-5480-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kinoshita K, Taupin DR, Itoh H, Podolsky DK. Distinct pathways of cell migration and antiapoptotic response to epithelial injury: structure-function analysis of human intestinal trefoil factor. Mol Cell Biol. 2000;20:4680–90. doi: 10.1128/mcb.20.13.4680-4690.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sun Z, Liu H, Yang Z, Shao D, Zhang W, et al. Intestinal trefoil factor activates the PI3K/Akt signaling pathway to protect gastric mucosal epithelium from damage. Int J Oncol. 2014;45:1123–32. doi: 10.3892/ijo.2014.2527. [DOI] [PubMed] [Google Scholar]
- 93.Klemke RL, Cai S, Giannini AL, Gallagher PJ, de Lanerolle P, Cheresh DA. Regulation of cell motility by mitogen-activated protein kinase. J Cell Biol. 1997;137:481–92. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hansson HA, Hong L, Helander HF. Changes in gastric EGF, EGF receptors and acidity during healing of gastric ulcer in the rat. Acta Physiol Scand. 1990;138:241–42. doi: 10.1111/j.1748-1716.1990.tb08840.x. [DOI] [PubMed] [Google Scholar]
- 95.Furukawa O, Matsui H, Suzuki N, Okabe S. Epidermal growth factor protects rat epithelial cells against acid-induced damage through the activation of Na+/H+ exchangers. J Pharmacol Exp Ther. 1999;288:620–26. [PubMed] [Google Scholar]
- 97.Agle KA, Vongsa RA, Dwinell MB. Calcium mobilization triggered by the chemokine CXCL12 regulates migration in wounded intestinal epithelial monolayers. J Biol Chem. 2010;285:16066–75. doi: 10.1074/jbc.M109.061416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Smith JM, Johanesen PA, Wendt MK, Binion DG, Dwinell MB. CXCL12 activation of CXCR4 regulates mucosal host defense through stimulation of epithelial cell migration and promotion of intestinal barrier integrity. Am J Physiol Gastrointest Liver Physiol. 2005;288:G316–26. doi: 10.1152/ajpgi.00208.2004. [DOI] [PubMed] [Google Scholar]
- 99.Hwang S, Zimmerman NP, Agle KA, Turner JR, Kumar SN, Dwinell MB. E-cadherin is critical for collective sheet migration and is regulated by the chemokine CXCL12 protein during restitution. J Biol Chem. 2012;287:22227–40. doi: 10.1074/jbc.M112.367979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yanaka A, Suzuki H, Shibahara T, Matsui H, Nakahara A, Tanaka N. EGF promotes gastric mucosal restitution by activating Na+/H+ exchange of epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2002;282:G866–76. doi: 10.1152/ajpgi.00150.2001. [DOI] [PubMed] [Google Scholar]
- 101.Moeser AJ, Nighot PK, Ryan KA, Simpson JE, Clarke LL, Blikslager AT. Mice lacking the Na+/H+ exchanger 2 have impaired recovery of intestinal barrier function. Am J Physiol Gastrointest Liver Physiol. 2008;295:G791–97. doi: 10.1152/ajpgi.00538.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Moeser AJ, Nighot PK, Ryan KA, Wooten JG, Blikslager AT. Prostaglandin-mediated inhibition of Na+/H+ exchanger isoform 2 stimulates recovery of barrier function in ischemia-injured intestine. Am J Physiol Gastrointest Liver Physiol. 2006;291:G885–94. doi: 10.1152/ajpgi.00380.2005. [DOI] [PubMed] [Google Scholar]
- 103.Wright NA, Poulsom R, Stamp G, Van Noorden S, Sarraf C, et al. Trefoil peptide gene expression in gastrointestinal epithelial cells in inflammatory bowel disease. Gastroenterology. 1993;104:12–20. doi: 10.1016/0016-5085(93)90830-6. [DOI] [PubMed] [Google Scholar]
- 104.Alison MR, Chinery R, Poulsom R, Ashwood P, Longcroft JM, Wright NA. Experimental ulceration leads to sequential expression of spasmolytic polypeptide, intestinal trefoil factor, epidermal growth factor and transforming growth factor alpha mRNAs in rat stomach. J Pathol. 1995;175:405–14. doi: 10.1002/path.1711750408. [DOI] [PubMed] [Google Scholar]
- 105.Riaño A, Ortiz-Masià D, Velazquez M, Calatayud S, Esplugues JV, Barrachina MD. Nitric oxide induces HIF-1alpha stabilization and expression of intestinal trefoil factor in the damaged rat jejunum and modulates ulcer healing. J Gastroenterol. 2011;46:565–76. doi: 10.1007/s00535-011-0374-1. [DOI] [PubMed] [Google Scholar]
- 106.Tarnawski A, Stachura J, Krause WJ, Douglass TG, Gergely H. Quality of gastric ulcer healing: a new, emerging concept. J Clin Gastroenterol. 1991;13(Suppl 1):S42–47. doi: 10.1097/00004836-199112001-00007. [DOI] [PubMed] [Google Scholar]
- 107.Okabe S, Amagase K. An overview of acetic acid ulcer models–-the history and state of the art of peptic ulcer research. Biol Pharm Bull. 2005;28:1321–41. doi: 10.1248/bpb.28.1321. [DOI] [PubMed] [Google Scholar]
- 108.Nam KT, Lee HJ, Mok H, Romero-Gallo J, Crowe JE, Jr, et al. Amphiregulin-deficient mice develop spasmolytic polypeptide expressing metaplasia and intestinal metaplasia. Gastroenterology. 2009;136:1288–96. doi: 10.1053/j.gastro.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ulaganathan M, Familari M, Yeomans ND, Giraud AS, Cook GA. Spatio-temporal expression of trefoil peptide following severe gastric ulceration in the rat implicates it in late-stage repair processes. J Gastroenterol Hepatol. 2001;16:506–12. doi: 10.1046/j.1440-1746.2001.02469.x. [DOI] [PubMed] [Google Scholar]
- 110.Aihara E, Closson C, Matthis AL, Schumacher MA, Engevik AC, et al. Motility and chemotaxis mediate the preferential colonization of gastric injury sites by Helicobacter pylori. PLOS Pathog. 2014;10:e1004275. doi: 10.1371/journal.ppat.1004275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kikuchi M, Nagata H, Watanabe N, Watanabe H, Tatemichi M, Hibi T. Altered expression of a putative progenitor cell marker DCAMKL1 in the rat gastric mucosa in regeneration, metaplasia and dysplasia. BMC Gastroenterol. 2010;10:65. doi: 10.1186/1471-230X-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Aihara E, Matthis AL, Karns RA, Engevik KA, Jiang P, et al. Epithelial regeneration after gastric ulceration causes prolonged cell type alterations. Cell Mol Gastroenterol Hepatol. 2016;2:625–47. doi: 10.1016/j.jcmgh.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nozaki K, Ogawa M, Williams JA, Lafleur BJ, Ng V, et al. A molecular signature of gastric metaplasia arising in response to acute parietal cell loss. Gastroenterology. 2008;134:511–22. doi: 10.1053/j.gastro.2007.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nomura S, Baxter T, Yamaguchi H, Leys C, Vartapetian AB, et al. Spasmolytic polypeptide expressing metaplasia to preneoplasia in H. felis-infected mice. Gastroenterology. 2004;127:582–94. doi: 10.1053/j.gastro.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 115.Ogawa M, Nomura S, Varro A, Wang TC, Goldenring JR. Altered metaplastic response of waved-2 EGF receptor mutant mice to acute oxyntic atrophy. Am J Physiol Gastrointest Liver Physiol. 2006;290:G793–804. doi: 10.1152/ajpgi.00309.2005. [DOI] [PubMed] [Google Scholar]
- 116.Sasaki E, Tominaga K, Watanabe T, Fujiwara Y, Oshitani N, et al. COX-2 is essential for EGF induction of cell proliferation in gastric RGM1 cells. Dig Dis Sci. 2003;48:2257–62. doi: 10.1023/b:ddas.0000007860.87503.09. [DOI] [PubMed] [Google Scholar]
- 117.Alderman BM, Ulaganathan M, Judd LM, Howlett M, Parker LM, et al. Insights into the mechanisms of gastric adaptation to aspirin-induced injury: a role for regenerating protein but not trefoil peptides. Lab Investig. 2003;83:1415–25. doi: 10.1097/01.lab.0000092231.54761.cd. [DOI] [PubMed] [Google Scholar]
- 118.Koitabashi A, Shimada T, Fujii Y, Hashimoto T, Hosaka K, et al. Indometacin up-regulates TFF2 expression in gastric epithelial cells. Aliment Pharmacol Ther. 2004;20(Suppl 1):171–76. doi: 10.1111/j.1365-2036.2004.01991.x. [DOI] [PubMed] [Google Scholar]
- 119.Azarschab P, Al-Azzeh E, Kornberger W, Gott P. Aspirin promotes TFF2 gene activation in human gastric cancer cell lines. FEBS Lett. 2001;488:206–10. doi: 10.1016/s0014-5793(00)02422-4. [DOI] [PubMed] [Google Scholar]
- 120.Lehmann JM, Lenhard JM, Oliver BB, Ringold GM, Kliewer SA. Peroxisome proliferator-activated receptors alpha and gamma are activated by indomethacin and other non-steroidal anti-inflammatory drugs. J Biol Chem. 1997;272:3406–10. doi: 10.1074/jbc.272.6.3406. [DOI] [PubMed] [Google Scholar]
- 121.Wada K, Nakajima A, Takahashi H, Yoneda M, Fujisawa N, et al. Protective effect of endogenous PPARgamma against acute gastric mucosal lesions associated with ischemia-reperfusion. Am J Physiol Gastrointest Liver Physiol. 2004;287:G452–58. doi: 10.1152/ajpgi.00523.2003. [DOI] [PubMed] [Google Scholar]
- 122.Konturek PC, Brzozowski T, Kania J, Konturek SJ, Kwiecien S, et al. Pioglitazone, a specific ligand of peroxisome proliferator-activated receptor-gamma, accelerates gastric ulcer healing in rat. Eur J Pharmacol. 2003;472:213–20. doi: 10.1016/s0014-2999(03)01932-0. [DOI] [PubMed] [Google Scholar]
- 123.Shimada T, Koitabashi A, Kuniyoshi T, Hashimoto T, Yoshiura K, et al. Up-regulation of TFF expression by PPARgamma ligands in gastric epithelial cells. Aliment Pharmacol Ther. 2003;18(Suppl 1):119–25. doi: 10.1046/j.1365-2036.18.s1.14.x. [DOI] [PubMed] [Google Scholar]
- 124.Clyne M, Dillon P, Daly S, O’Kennedy R, May FE, et al. Helicobacter pylori interacts with the human single-domain trefoil protein TFF1. PNAS. 2004;101:7409–14. doi: 10.1073/pnas.0308489101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Reeves EP, Ali T, Leonard P, Hearty S, O’Kennedy R, et al. Helicobacter pylori lipopolysaccharide interacts with TFF1 in a pH-dependent manner. Gastroenterology. 2008;135:2043–54.e2. doi: 10.1053/j.gastro.2008.08.049. [DOI] [PubMed] [Google Scholar]
- 126.Montefusco S, Esposito R, D’Andrea L, Monti MC, Dunne C, et al. Copper promotes TFF1-mediated Helicobacter pylori colonization. PLOS ONE. 2013;8:e79455. doi: 10.1371/journal.pone.0079455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schmitz JM, Durham CG, Ho SB, Lorenz RG. Gastric mucus alterations associated with murine Helicobacter infection. J Histochem Cytochem. 2009;57:457–67. doi: 10.1369/jhc.2009.952473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Soutto M, Chen Z, Katsha AM, Romero-Gallo J, Krishna US, et al. Trefoil factor 1 expression suppresses Helicobacter pylori-induced inflammation in gastric carcinogenesis. Cancer. 2015;121:4348–58. doi: 10.1002/cncr.29644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fox JG, Rogers AB, Whary MT, Ge Z, Ohtani M, et al. Accelerated progression of gastritis to dysplasia in the pyloric antrum of TFF2−/− C57BL6 × Sv129 Helicobacter pylori-infected mice. Am J Pathol. 2007;171:1520–28. doi: 10.2353/ajpath.2007.070249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Peterson AJ, Menheniott TR, O’Connor L, Walduck AK, Fox JG, et al. Helicobacter pylori infection promotes methylation and silencing of trefoil factor 2, leading to gastric tumor development in mice and humans. Gastroenterology. 2010;139:2005–17. doi: 10.1053/j.gastro.2010.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Koike T, Shimada T, Fujii Y, Chen G, Tabei K, et al. Up-regulation of TFF1 (pS2) expression by TNF-α in gastric epithelial cells. J Gastroenterol Hepatol. 2007;22:936–42. doi: 10.1111/j.1440-1746.2007.04861.x. [DOI] [PubMed] [Google Scholar]
- 132.Dossinger V, Kayademir T, Blin N, Gött P. Down-regulation of TFF expression in gastrointestinal cell lines by cytokines and nuclear factors. Cell Physiol Biochem. 2002;12:197–206. doi: 10.1159/000066279. [DOI] [PubMed] [Google Scholar]
- 133.Cobler L, Mejias-Luque R, Garrido M, Pera M, Badia-Garrido E, de Bolós C. Activation of the NF-kB pathway downregulates TFF-1 in gastric carcinogenesis. Virchows Arch. 2013;463:497–507. doi: 10.1007/s00428-013-1469-2. [DOI] [PubMed] [Google Scholar]
- 134.Matsuda K, Yamauchi K, Matsumoto T, Sano K, Yamaoka Y, Ota H. Quantitative analysis of the effect of Helicobacter pylori on the expressions of SOX2, CDX2, MUC2, MUC5AC, MUC6, TFF1, TFF2, and TFF3 mRNAs in human gastric carcinoma cells. Scand J Gastroenterol. 2008;43:25–33. doi: 10.1080/00365520701579795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xian CJ, Howarth GS, Mardell CE, Cool JC, Familari M, et al. Temporal changes in TFF3 expression and jejunal morphology during methotrexate-induced damage and repair. Am J Physiol. 1999;277:G785–95. doi: 10.1152/ajpgi.1999.277.4.G785. [DOI] [PubMed] [Google Scholar]
- 136.Itoh H, Tomita M, Uchino H, Kobayashi T, Kataoka H, et al. cDNA cloning of rat pS2 peptide and expression of trefoil peptides in acetic acid-induced colitis. Biochem J. 1996;318(Pt 3):939–44. doi: 10.1042/bj3180939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kjellev S, Thim L, Pyke C, Poulsen SS. Cellular localization, binding sites, and pharmacologic effects of TFF3 in experimental colitis in mice. Dig Dis Sci. 2007;52:1050–59. doi: 10.1007/s10620-006-9256-4. [DOI] [PubMed] [Google Scholar]
- 138.Chen YH, Lu Y, De Plaen IG, Wang LY, Tan XD. Transcription factor NF-κB signals antianoikic function of trefoil factor 3 on intestinal epithelial cells. Biochem Biophys Res Commun. 2000;274:576–82. doi: 10.1006/bbrc.2000.3176. [DOI] [PubMed] [Google Scholar]
- 139.Loncar MB, Al-azzeh ED, Sommer PS, Marinovic M, Schmehl K, et al. Tumour necrosis factor α and nuclear factor κB inhibit transcription of human TFF3 encoding a gastrointestinal healing peptide. Gut. 2003;52:1297–303. doi: 10.1136/gut.52.9.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Teng X, Xu LF, Zhou P, Sun HW, Sun M. Effects of trefoil peptide 3 on expression of TNF-α, TLR4, and NF-κB in trinitrobenzene sulphonic acid induced colitis mice. Inflammation. 2009;32:120–29. doi: 10.1007/s10753-009-9110-x. [DOI] [PubMed] [Google Scholar]
- 141.Shi L, Zhou PH, Xi JL, Yu HG, Zhang BH. Recombinant human trefoil factor 3 ameliorates bowel injury: its anti-inflammatory effect on experimental necrotizing enterocolitis. Int J Pept. 2014;2014:634135. doi: 10.1155/2014/634135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Barrera GJ, Sanchez G, Gonzalez JE. Trefoil factor 3 isolated from human breast milk downregulates cytokines (IL8 and IL6) and promotes human beta defensin (hBD2 and hBD4) expression in intestinal epithelial cells HT-29. Bosn J Basic Med Sci. 2012;12:256–64. doi: 10.17305/bjbms.2012.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Podolsky DK, Gerken G, Eyking A, Cario E. Colitis-associated variant of TLR2 causes impaired mucosal repair because of TFF3 deficiency. Gastroenterology. 2009;137:209–20. doi: 10.1053/j.gastro.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lin N, Xu LF, Sun M. The protective effect of trefoil factor 3 on the intestinal tight junction barrier is mediated by toll-like receptor 2 via a PI3K/Akt dependent mechanism. Biochem Biophys Res Commun. 2013;440:143–49. doi: 10.1016/j.bbrc.2013.09.049. [DOI] [PubMed] [Google Scholar]
- 145.Tran CP, Cook GA, Yeomans ND, Thim L, Giraud AS. Trefoil peptide TFF2 (spasmolytic polypeptide) potently accelerates healing and reduces inflammation in a rat model of colitis. Gut. 1999;44:636–42. doi: 10.1136/gut.44.5.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ando K, Fujiya M, Konishi H, Ueno N, Inaba Y, et al. Heterogeneous nuclear ribonucleoprotein A1 improves the intestinal injury by regulating apoptosis through trefoil factor 2 in mice with anti-CD3-induced enteritis. Inflamm Bowel Dis. 2015;21:1541–52. doi: 10.1097/MIB.0000000000000401. [DOI] [PubMed] [Google Scholar]
- 147.Vandenbroucke K, Hans W, Van Huysse J, Neirynck S, Demetter P, et al. Active delivery of trefoil factors by genetically modified Lactococcus lactis prevents and heals acute colitis in mice. Gastroenterology. 2004;127:502–13. doi: 10.1053/j.gastro.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 148.Giraud AS, Pereira PM, Thim L, Parker LM, Judd LM. TFF-2 inhibits iNOS/NO in monocytes, and nitrated protein in healing colon after colitis. Peptides. 2004;25:803–9. doi: 10.1016/j.peptides.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 149.Judd LM, Chalinor HV, Walduck A, Pavlic DI, Dabritz J, et al. TFF2 deficiency exacerbates weight loss and alters immune cell and cytokine profiles in DSS colitis, and this cannot be rescued by wild-type bone marrow. Am J Physiol Gastrointest Liver Physiol. 2015;308:G12–24. doi: 10.1152/ajpgi.00172.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Playford RJ. Peptides and gastrointestinal mucosal integrity. Gut. 1995;37:595–97. doi: 10.1136/gut.37.5.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reference 1a.Dignass A, Lynch-Denaney K, Kindon H, Thim L, Podolsky DK. Trefoild peptides promote epithelial migraition through a transforming growth factor beta-independent pathway. J Clin Invest. 1994;94:376–83. doi: 10.1172/JCI117332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reference 2a.Marchbank T, Westley BR, May FE, Calnan DP, Playford RJ. Dimerization of human pS2 (TFF1) plays a key role in its protective/healing effects. J Phathol. 1998;185:15–58. doi: 10.1002/(SICI)1096-9896(199806)185:2<153::AID-PATH87>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Reference 3a.Le J, Zhang DY, Zhao Y, Qiu W, Wang P, Sun Y. ITF promotes migrartion of intestinal epithelial cells through crosstalk between the ERK and JAK/STAT3 pathways. Sci Rep. 2016;6:33014. doi: 10.1038/srep33014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reference 4a.Carvalho R, Kayademir T, Soares P, Canedo P, Sousa S, Oliveira C, Leistenschneider P, Seruca R, Gott P, Blin N, Carneiro F, Machado JC. Loss of heterozygosity and promoter methylation, but not mutation, may underlie loss of TFF1 in gastric carcinoma. Lab Invest. 2002;82:1319–26. doi: 10.1097/01.lab.0000029205.76632.a8. [DOI] [PubMed] [Google Scholar]
- Reference 5a.Sato T, Amano H, Eshima K, Minamino T, Abe T, et al. NSAID, aspirin delays gastric ulcer healing with reduced accumulation of CXCR4(+)VEGFR1(+) cells to the ulcer granulation tissues. Biomed Pharmacother. 2013;67:607–13. doi: 10.1016/j.biopha.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Reference 6a.Poulsen SS, Thulesen J, Christensen L, Nexø E, Thim L. Metabolism of oral trefoil factor 2 (TFF2) and the effect of oral and parenteral TFF2 on gastric and duodenal ulcer healing in the rat. Gut. 1999;45:516–22. doi: 10.1136/gut.45.4.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reference 7a.FitzGerald AJ, Pu M, Marchbank T, Westley BR, May FEB, et al. Synergistic effects of systemic trefoil factor family 1 (TFF1) peptide and epidermal growth factor in a rat model of colitis. Peptides. 2004;25:793–801. doi: 10.1016/j.peptides.2003.12.022. [DOI] [PubMed] [Google Scholar]
- Reference 8a.Rosenstiel P, Sina C, End C, Renner M, et al. Regulation of DMBT1 via NOD2 and TLR4 in intestinal epithelial cells modulates bacterial recognition and invasion. J Immunol. 2007;178:8203–11. doi: 10.4049/jimmunol.178.12.8203. [DOI] [PubMed] [Google Scholar]


