SUMMARY
Endophthalmitis is a severe eye infection that may result in permanent loss of useful vision in the affected eye. Most cases are exogenous and occur as a complication of cataract surgery, an intravitreal injection, or penetrating ocular trauma. Endogenous endophthalmitis results from hematogenous seeding of the eye by bacteria or fungi, but bacteremia or fungemia may be transient and patients may present without symptoms of systemic infection. Nearly all endophthalmitis patients present with decreased vision, and some also have eye pain. Eye examination usually reveals a hypopyon and intraocular inflammation. Diagnosis is clinical, supported by cultures of the vitreous and/or aqueous or by blood cultures in some endogenous cases. Molecular diagnostic techniques have been used in research laboratories for pathogen identification in endophthalmitis and offer the possibility of rapid diagnosis, including in culture-negative cases. Intravitreal injection of antibiotics is the most important component of treatment; some cases also benefit from surgical debridement of the vitreous by a vitrectomy. The visual outcome depends partly on the pathogen: coagulase-negative staphylococcal endophthalmitis has a better prognosis than does streptococcal endophthalmitis, for example. Endophthalmitis is a medical emergency, and prompt diagnosis and treatment are essential for saving vision.
KEYWORDS: endophthalmitis, bacterial endophthalmitis, fungal endophthalmitis, endophthalmitis prophylaxis, Candida endophthalmitis, bleb-related endophthalmitis, keratitis-related endophthalmitis, posttraumatic endophthalmitis, postoperative endophthalmitis
INTRODUCTION
Endophthalmitis is one of the most devastating eye infections and may lead to irreversible blindness in the infected eye within hours or days of symptom onset. The term “endophthalmitis” refers to infection of the vitreous and/or aqueous by bacteria or fungi. Intraocular infections by viruses or parasites are usually considered types of uveitis rather than endophthalmitis.
Endophthalmitis may be either exogenous, in which microbes on the ocular surface or from an external source are introduced into the eye, or endogenous, arising from hematogenous seeding of pathogens during bacteremia or fungemia. Most cases of endophthalmitis are exogenous. Exogenous endophthalmitis is further divided into several categories, primarily by risk factor, such as postcataract, posttraumatic, and bleb related. It is important to identify the category of endophthalmitis, as this influences the typical presentation, microbiology, and visual outcome (Table 1).
TABLE 1.
Major categories of endophthalmitis
| Category | Risk factor | Relative frequency (% of all endophthalmitis cases) | Major pathogens |
|---|---|---|---|
| Acute postcataract | Cataract surgery | 40–80 | Coagulase-negative staphylococci (70% of cases), Staphylococcus aureus (10%), streptococci (9%) |
| Postinjection | Intravitreal injection | 0–50 | Coagulase-negative staphylococci, streptococci |
| Posttraumatic | Penetrating eye trauma | 2–15 | Coagulase-negative staphylococci, Bacillus, streptococci, Gram-negative bacilli, fungi |
| Bleb related | Filtering bleb (for glaucoma) | 0–5 | Streptococcus pneumoniae and other streptococci, enterococci, Haemophilus influenzae |
| Keratitis related | Corneal infection | 0–10 | Fungi (Aspergillus, Fusarium) in 50%, S. aureus, streptococci, Pseudomonas |
| Endogenous | Bacteremia or fungemia | 0–20 | Klebsiella pneumoniae (especially in East Asian nations), Candida, streptococci, S. aureus, Escherichia coli |
Endophthalmitis is rare, and the incidence varies by category. The rate of endophthalmitis after cataract surgery is approximately 0.1%, for example, while the rate after penetrating eye trauma is 1 to 18%. Postoperative and posttraumatic endophthalmitis are the major types of endophthalmitis seen worldwide, with postoperative (primarily postcataract) cases accounting for 40 to 80% and posttraumatic cases comprising 2 to 15% of all endophthalmitis cases seen at centers in Brazil, England, Israel, Iran, India, Australia, and South Korea (1–7). Regional differences exist: posttraumatic endophthalmitis accounted for 40 to 60% of all endophthalmitis cases treated in some centers in Egypt, India, and China (7–9). The time period included in a study also influences the frequency of various types of endophthalmitis. The U.S. Food and Drug Administration (FDA) approved intravitreal anti-vascular endothelial growth factor (anti-VEGF) medications to treat neovascular age-related macular degeneration in 2004, and since then, there has been a rapid increase in the use of these and other intravitreal injections. Some centers report that postinjection endophthalmitis is now more common than postoperative endophthalmitis (3, 10).
PATHOGENESIS
The source of pathogens in exogenous endophthalmitis is the ocular surface (e.g., in postoperative, postinjection, keratitis-related, bleb-related, or device-related endophthalmitis) or the environment (e.g., in posttraumatic endophthalmitis). In endogenous endophthalmitis, the source of infection is either a transient focus (e.g., an indwelling central venous catheter) or an ongoing one (e.g., a liver abscess).
The likelihood that a patient will develop endophthalmitis depends on host factors, inoculum size, and pathogen factors. Bacteria that colonize the conjunctiva, such as coagulase-negative staphylococci, may be cultured from the aqueous at the end of surgery in approximately one-third of cataract surgery cases (11, 12), yet only 1 in 500 to 1 in 1,000 cataract surgeries result in endophthalmitis, probably because of the immune system's ability to clear small inocula. The constant turnover of the aqueous every 100 min likely helps; communication with the vitreous, which does not regenerate, during cataract surgery increases the risk of postoperative endophthalmitis 6-fold (13). Large numbers of pathogens introduced into the eye can overwhelm host defenses; outbreaks resulting from use of a contaminated solution during surgery, for example, typically result in attack rates of 80 to 100%.
Pathogen factors also play a role in pathogenesis. Bacteremia or fungemia rarely results in endogenous endophthalmitis (incidence of <1%) unless the organism is hypermucoviscous (serotype K1 or K2) Klebsiella pneumoniae. That organism has been associated with liver abscess in East Asian centers, and as many as 7% of cases develop endophthalmitis (14). Experimental models with mice have confirmed that eyes injected with Klebsiella with the hypermucoviscosity (HMV) phenotype have greater retinal function loss and inflammation than eyes injected with HMV-negative strains (15). The magA (mucoviscosity-associated gene) region has been associated with production of capsular type K1, the predominate type causing liver abscess and metastatic complications such as endophthalmitis. A bacteriophage that specifically infects K1 serotype Klebsiella strains has been isolated and may have future implications in diagnosis (16). In posttraumatic endophthalmitis, Bacillus cereus and other Bacillus species are major pathogens and cause a fulminant endophthalmitis with very poor visual prognosis. Factors that play a role in this destruction include membrane-damaging toxins such as hemolysins, sphingomyelinases, and phospholipases, virulence factors regulated by the quorum sensing-dependent transcriptional regulator PlcR, rapid intraocular growth, and bacterial motility within the eye (17–20). Neutrophils themselves cause damage to the retina, and chemokine CXCL1 and other factors contribute to neutrophil recruitment and the damaging inflammation seen during Bacillus endophthalmitis (21). In bleb-related endophthalmitis, Streptococcus pneumoniae is an important pathogen and causes a severe endophthalmitis; the pneumococcal capsule, pneumolysin, and autolysin all appear to contribute to pathogenesis (22, 23).
Biofilms may play a role in cases of endophthalmitis related to implants such as glaucoma drainage devices, keratoprostheses (artificial corneas), and possibly intraocular lenses (IOLs). Intraocular lenses are placed during cataract surgery, and the IOL material may affect biofilm formation. A study comparing Staphylococcus epidermidis adhesion and biofilm formation on 4 types of IOLs (polymethylmethacrylate, silicone, and hydrophilic and hydrophobic acrylic) found that biofilm growth occurred on all types but that there was a significant difference between the types (hydrophilic acrylic had the least bacterial binding, and silicone had the most) (24). The role of biofilms in postcataract endophthalmitis is still unclear, however, since biofilms apparently occur on IOLs in uninfected eyes: 19% of IOLs in eyes donated after death for corneal transplant had bacterial biofilms (25).
CLINICAL FEATURES
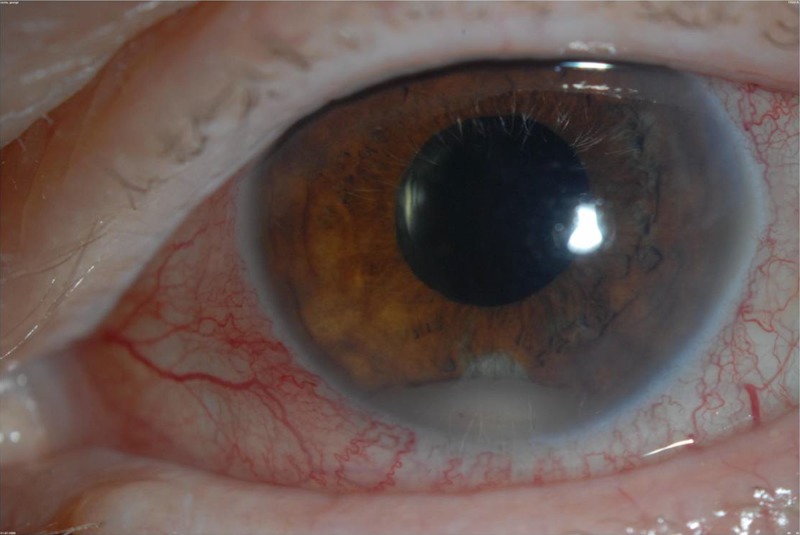
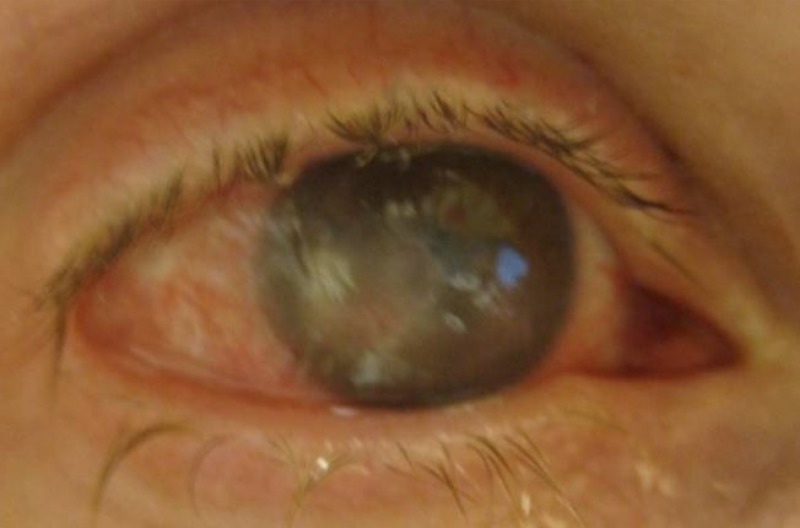
The most common symptom of endophthalmitis is decreased vision, affecting nearly all patients. Eye pain or discomfort and a red eye are also common although not universal complaints. Systemic symptoms such as fever are absent in exogenous but often present in endogenous endophthalmitis cases (26). On examination of the eye, a hypopyon is seen in most cases (e.g., in 80% of postcataract cases) (27), and this represents a layer of white blood cells in the anterior chamber (Fig. 1). Funduscopic examination reveals intraocular inflammation, and this often obscures the view of the retina (a view of retinal vessels is obscured in 80% of postcataract endophthalmitis cases, for example) (28).
FIG 1.
A hypopyon is seen as a layer of white blood cells in the aqueous in this eye with endogenous S. aureus endophthalmitis. (Republished from reference 66 with permission of Springer.)
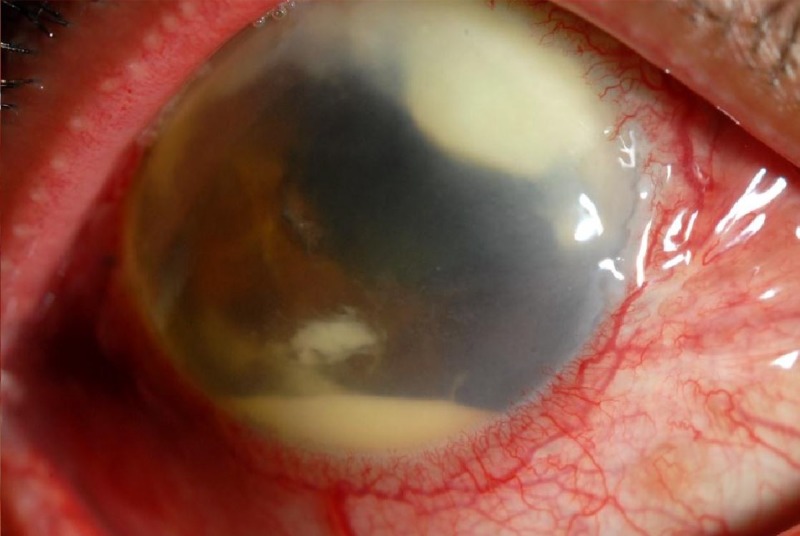
The pace of symptoms and type of intraocular inflammation can be clues to a bacterial versus fungal etiology. Bacterial endophthalmitis usually presents acutely, often within days of an inciting event such as cataract surgery. Fungal endophthalmitis typically has a subacute presentation with symptoms worsening over days to weeks. The intraocular inflammation in fungal endophthalmitis tends to occur in “clumps” within the aqueous and/or vitreous (Fig. 2), while intraocular inflammation is typically diffuse in bacterial endophthalmitis.
FIG 2.
Fungal endophthalmitis from Scedosporium. Note the “clumped” appearance of the intraocular inflammation. (Republished from reference 66 with permission of Springer.)
DIAGNOSIS
Endophthalmitis is a clinical diagnosis supported by culture of the vitreous and/or aqueous and also by blood cultures in endogenous endophthalmitis. Negative cultures do not exclude the diagnosis, since 20 to 30% of endophthalmitis cases are culture negative. Molecular diagnostic techniques have demonstrated a pathogen in many culture-negative cases, and these techniques may play a larger role in endophthalmitis diagnosis in the future.
Sampling and Culture Techniques
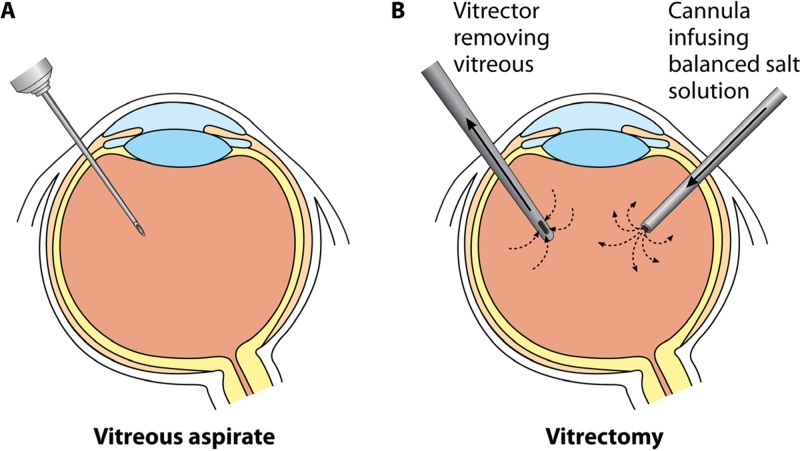
Both the aqueous and vitreous may be sampled by the ophthalmologist. The aqueous is liquid, approximately 0.3 ml, and continuously regenerated, with a turnover time of 100 min. In contrast, the vitreous is a gel of approximately 4 ml in volume; it is present at birth and not regenerated. Small samples of the aqueous (e.g., 0.1 ml) or vitreous (0.2 to 0.3 ml) may be obtained for culture by needle aspiration in the ophthalmologist's office (Fig. 3A), although some vitreous aspirates are “dry taps” because of the difficulty of aspirating a gel. Alternatively, some but not all of the vitreous may be removed surgically via a vitrectomy in the operating room (Fig. 3B). This procedure is performed with a vitrector, a mechanized instrument that rapidly cuts (e.g., 5,000 cuts per minute) and aspirates the vitreous while eye turgor is maintained by simultaneous replacement with balanced salt solution. During this procedure, an undiluted vitreous “biopsy specimen” may be obtained for culture at the start of the case. The remaining vitreous sample is diluted by the continuously infused balanced salt solution, and these collected vitreous “washings” are also sent for culture. Microbiology laboratories of eye specialty hospitals generally handle these specimens in the following way: (i) a 5-ml sample of washings is centrifuged, and the resulting pellet is used for Gram and calcofluor staining, and (ii) the rest of the washings (50 to 100 ml) are sterilely vacuum filtered through a 0.45-μm filter, and the filter paper is then divided and placed on agar plates for culture.
FIG 3.
Vitreous sampling by needle aspirate (A) or vitrectomy (B).
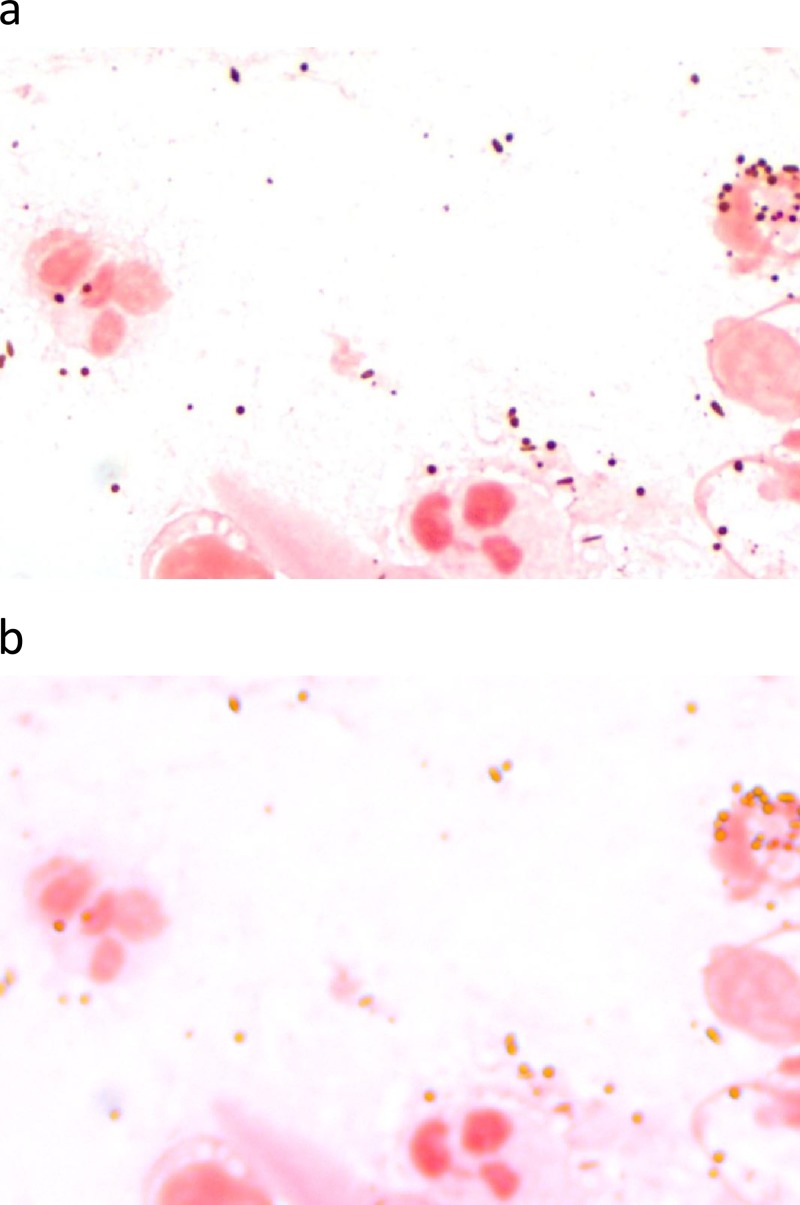
Gram stains of intraocular fluids are positive in approximately 50% of bacterial endophthalmitis cases. Caution must be exercised to avoid mistaking pigment granules for Gram-positive cocci (Fig. 4a), although pigment granules are hyperrefractile (Fig. 4b) and typically much larger than cocci or are football shaped. Pigment granules are melanin granules that may be released from the iris pigment epithelium or retinal pigment epithelium during intraocular inflammation.
FIG 4.
Gram stain of an intraocular sample demonstrating abundant pigment granules but no organisms. Pigment granules usually appear purple on Gram stain (a) and can be mistaken for Gram-positive cocci unless the fine-focus knob is rotated back and forth, which shows the hyperrefractile (coppery) color of the pigment granules (b).
Cultures are positive in approximately 90% of vitrectomy specimens, 50 to 70% of vitreous aspirates, and 40% of aqueous aspirates. Aqueous aspirates usually have higher yields in endophthalmitis cases in which inflammation is greatest in the aqueous, such as cases secondary to corneal infection.
Molecular Diagnostic Techniques
PCR testing of intraocular fluids has the potential to rapidly identify pathogens in endophthalmitis cases, including culture-negative cases. However, currently PCR testing for bacteria or fungi in aqueous or vitreous samples is available mainly in research laboratories. Several types of PCR assays have been applied to intraocular fluids in endophthalmitis (29). The pan-bacterial conventional PCR technique can take 2 to 3 days and involves amplification of the bacterial 16S rRNA gene followed by sequencing of the amplified DNA. Pan-bacterial real-time PCR combines PCR amplification of target DNA with simultaneous detection of the amplified PCR products; this shortens the turnaround time. Multiplex real-time PCR increases the cost-effectiveness of the testing and allows simultaneous detection of multiple DNA targets in a single reaction. Quantitative real-time PCR can increase specificity for endophthalmitis by defining a cutoff threshold, below which contamination rather than true infection is likely. Use of PCR for endophthalmitis diagnosis was initially reported in 1994 (30) and most studies since then have used conventional PCR. Chiquet and colleagues note that PCR and conventional culture methods are actually complementary (29). They found that the sensitivity of pan-bacterial PCR of 100 specimens from acute postcataract endophthalmitis cases was similar to that of culture, but combining the two techniques allowed identification of bacterial species in 87% of cases, including 25% of cases with negative cultures (31). Most PCR studies of endophthalmitis have focused on bacterial pathogens, but fungi may also be detected by targeting the common ribosomal 18S/28S DNA sequence.
Recently, another molecular method, with the acronym BRiSK (biome representational in silico karyotyping) has been applied by the Van Gelder laboratory to identify any DNA-based microbe in a sample (32). This technique isolates a 33-base-pair (bp) sequence from every 4,000 bp of the starting DNA in a sample, sequences these 33-bp “tags,” and compares the tags to a large database (GenBank, National Center for Biotechnology Information) of all known DNA sequences. The BRiSK technique was applied along with culture and 16S PCR to intraocular samples from 21 postprocedural endophthalmitis cases (14 culture positive) and 7 controls in one study (33). Control cases were negative for bacteria by all 3 methods. Of culture-positive endophthalmitis cases, PCR and BRiSK identified the same or a similar species as culture in 71% but were falsely negative in 29%. Of the 7 culture-negative cases, 2 were positive for bacteria by BRiSK but negative by PCR. Of interest, BRiSK identified a small DNA virus, torque teno virus (TTV), in 57% of the culture-positive and 100% of the culture-negative endophthalmitis cases but none of the controls. The significance of this finding is unknown, and this virus appears to be ubiquitous in the general population. A study from Russia found that 94% of healthy individuals had 1,000 TTV genome copies per 1 ml of blood (34).
POSTOPERATIVE ENDOPHTHALMITIS
Acute Postcataract Endophthalmitis
Acute postcataract endophthalmitis is the major type of endophthalmitis seen worldwide. Most cases are bacterial, and approximately 75% present within 1 week postoperatively. Patients complain of decreased vision (95% of cases), red eye (80%), and eye pain (75%) (35). Fever is absent. The source of infection in most cases is the patient's resident ocular surface or lid skin flora, as demonstrated by several studies. A study of 105 patients with coagulase-negative staphylococcal postcataract endophthalmitis found that intraocular isolates were identical to simultaneous lid skin isolates in 68% of cases (36). In a study from Australia of 98 patients undergoing cataract surgery, one patient developed postoperative Staphylococcus epidermidis endophthalmitis, and the isolate was identical to the patient's preoperative conjunctival isolate by pulsed-field gel electrophoresis (37). Occasionally a contaminated solution or material used during surgery is the source of pathogens, as in a recent outbreak of postoperative Fusarium oxysporum endophthalmitis following use of contaminated viscoelastic material (38).
Approximately 30% of postcataract endophthalmitis cases are culture negative. Of culture-positive cases, Gram-positive cocci comprise approximately 95% of isolates, with coagulase-negative staphylococci the primary pathogens (70% of cases) (35). Other pathogens include Staphylococcus aureus (10%), streptococci (9%), mixed Gram-positive bacteria (5%), and Gram-negative bacilli (6%). Fungal postoperative endophthalmitis is rare except in tropical regions such as India, where 10 to 20% of cases are due to fungi (39, 40).
The treatment of acute postoperative endophthalmitis includes the intravitreal injection of antibiotics, with a vitrectomy performed first in severe or rapidly worsening cases. Both vancomycin and ceftazidime are injected empirically for suspected bacterial cases. In patients allergic to ceftazidime or cases known to be due to ceftazidime-resistant Gram-negative bacilli, amikacin may be injected instead of ceftazidime, although intravitreal aminoglyocosides are otherwise avoided because of the known but very rare complication of macular infarction. If the eye fails to improve or worsens over the first 48 h, a second injection of antibiotics may be performed, usually in conjunction with a vitrectomy if this procedure was not performed on admission. Performing a vitrectomy on admission improves visual outcomes in eyes with severe inflammation. This was demonstrated in the Endophthalmitis Vitrectomy Study (EVS), a large prospective trial published in 1995. In the EVS, patients were randomized to receive vitrectomy plus intravitreal antibiotics on presentation (vitrectomy cohort) or only intravitreal antibiotics (tap/biopsy cohort) (35). Patients with the worst vision (light perception only) did best if treated with immediate vitrectomy: 20% of the vitrectomy cohort were left with severe vision loss versus 47% of the tap/biopsy cohort. Vitrectomy also helps to clear the infection rapidly. Approximately 10% of patients in the EVS required a second procedure during the first week, usually for ongoing inflammation, and cultures were still positive in 71% of the tap/biopsy cohort but only 13% of the vitrectomy cohort (41).
Systemic antibiotics alone are not effective in treating bacterial endophthalmitis, and their value as adjunctive therapy (in addition to intravitreal antibiotics and vitrectomy) in postoperative and other types of exogenous bacterial endophthalmitis is unknown. The EVS attempted to answer this question by randomizing patients with postcataract endophthalmitis to receive intravenous (i.v.) antibiotics or not. Unfortunately, the antibiotics chosen in the EVS, i.v. amikacin plus ceftazidime, have minimal activity against staphylococci, the bacteria responsible for 80% of culture-positive cases in the study. In addition, systemic amikacin does not penetrate the blood-eye barrier. Therefore, the only conclusion one can draw from the EVS is that systemic amikacin and ceftazidime did not provide benefit. Whether antibiotics that treat the majority of endophthalmitis pathogens and achieve good intraocular levels would be beneficial as adjunctive therapy remains unknown. No other randomized prospective study has been performed since the EVS to address this question.
Chronic Postcataract Endophthalmitis
Chronic postcataract endophthalmitis is rare, and most cases are due to fungi or indolent bacteria such as Propionibacterium acnes. Diagnosis should be suspected in patients with persistent inflammation postoperatively or in a patient misdiagnosed with “uveitis” postoperatively even if onset of inflammation is weeks later. A pattern of apparent response to topical corticosteroids followed by relapse each time corticosteroids are tapered is common, particularly in chronic bacterial postcataract endophthalmitis. A clue to diagnosis in chronic fungal endophthalmitis is the “clumped” appearance of the intraocular inflammation, while a clue to chronic P. acnes endophthalmitis is the presence of a white plaque on the posterior lens capsule. Diagnosis by needle aspirate alone is often negative, and vitrectomy may be required. In P. acnes endophthalmitis, aspirate of the white capsular plaque is often the sample most likely to yield a positive culture.
Treatment of postcataract fungal endophthalmitis nearly always requires removal of the IOL in addition to an intraocular injection of an antifungal agent (either amphotericin or voriconazole), vitrectomy, and a systemic azole. For systemic therapy, fluconazole is given for susceptible Candida species and voriconazole for susceptible molds and fluconazole-resistant but voriconazole-susceptible Candida species. Treatment of chronic P. acnes endophthalmitis does not include systemic therapy but does usually require a combination of intraocular antibiotic injections plus surgery (vitrectomy, capsulectomy, and exchange or removal of the IOL), since intraocular injections alone result in relapse in 70% of cases (42).
Postvitrectomy Endophthalmitis
Postvitrectomy endophthalmitis is less common than postcataract endophthalmitis, but the microbiology is similar, with the majority of cases caused by coagulase-negative staphylococci. Vitrectomies are performed for various retinal conditions (e.g., retinal tears or detachment, vitreous hemorrhage), and most recent studies report an incidence of endophthalmitis of 0.02 to 0.06% (43). Methods for diagnosing and treating postvitrectomy endophthalmitis are the same as those for postcataract endophthalmitis.
Postkeratoplasty Endophthalmitis
Postkeratoplasty endophthalmitis refers to endophthalmitis following corneal transplant (keratoplasty). Endophthalmitis occurs in approximately 0.2% of keratoplasty cases in the acute postoperative period but in up to 0.7% if later cases are included (44, 45). The microbiology varies; a study from the United Kingdom of postkeratoplasty endophthalmitis, including cases developing long after surgery, found that 31% of cases were due to fungi (mostly Candida) and the remainder due to Pseudomonas, streptococci, staphylococci, and mycobacterial species (45). A review of 31 culture-positive cases in the literature reported similar results, with Candida causing 33% of cases, streptococci 27%, staphylococci 20%, enterococci 10%, and Pseudomonas 10% (46). The rim of the donor cornea, left over after the central core is removed for transplantation, is often sent for surveillance culture, but the value of doing this has been controversial. However, this information might be important for Candida: an eye that receives a cornea whose donor rim culture subsequently grows Candida has a 3% chance of developing Candida endophthalmitis (46). It is unknown whether such eyes should be treated prophylactically with an antifungal agent or just followed closely by the ophthalmologist. Treatment of postkeratoplasty endophthalmitis may require replacement of the infected cornea in addition to intracameral (into the aqueous) and/or intravitreal antibiotics and vitrectomy (as needed).
POSTINJECTION ENDOPHTHALMITIS
The use of intravitreal injections has rapidly increased since FDA approval of anti-VEGF injections in 2004, as noted above. A study utilizing a Medicare database reported that intravitreal injections increased from 83,000 in 2004 to 2.4 million in 2012 (47). Most injections involve use of anti-VEGF agents, given primarily for neovascular age-related macular degeneration (AMD) but also given for diabetic retinopathy and other indications. The risk of endophthalmitis is approximately 0.05% per injection (48), and since anti-VEGF injections are repeated at regular intervals (usually monthly) for AMD, the risk is cumulative. Other types of intravitreal injections, such as corticosteroids for inflammatory eye conditions, are also being used with increasing frequency. A retrospective study based on a U.S. medical claims database found a higher rate of endophthalmitis after corticosteroid than after anti-VEGF injections (0.13% versus 0.02%) (49), but this study has been questioned because it did not compare only culture-positive cases (culture results were not available). Sterile inflammation mimicking endophthalmitis may occur with higher frequency after corticosteroid than after anti-VEGF injections.
Patients with postinjection endophthalmitis typically present within 5 days after the injection, and the most common symptom is decreased vision. A British review of 47 patients with postinjection endophthalmitis found that 96% had decreased vision, 73% had eye pain/photophobia, and 49% had eye redness (50). The microbiology of postinjection endophthalmitis includes coagulase-negative staphylococci (65%), viridans streptococci (30%), S. aureus (0 to 5%), and others (0 to 4%) (48, 50, 51). This is similar to the microbiology of postcataract endophthalmitis except that the incidence of viridans streptococci is over 3-fold higher than in postcataract endophthalmitis, and this may be due to the fact that intravitreal injections are usually performed in the office rather than the operating room and without the use of masks. Oral flora, including viridans streptococci, may be aerosolized by speaking, and using masks or observing a strict “no talking” policy during injections decreases the rate of streptococcal endophthalmitis. A study of 25 centers in France that used masks for injections (some performed in operating rooms) reported a very low (0.007%) incidence of endophthalmitis, with streptococci causing only 4% of cases (52). A center in the United States decreased the postinjection endophthalmitis rate 2-fold (from 0.02% to 0.01%) and endophthalmitis due to oral pathogens 7-fold (from 0.015% to 0.002%) by instituting a no-talking policy during office-based injections (53).
Rarely, a postinjection endophthalmitis case may be part of an outbreak due to use of contaminated solutions. The onset of symptoms is usually rapid in bacterial cases but may be delayed in fungal cases. All 12 patients who developed streptococcal endophthalmitis after injection of contaminated anti-VEGF solutions presented 1 to 6 days postinjection, while 14 patients who developed Bipolaris hawaiiensis endophthalmitis following injection of contaminated triamcinolone developed symptoms a median of 83 days later (54, 55). Outcomes in outbreaks are typically poor: an outbreak of Escherichia coli and Citrobacter endophthalmitis related to a counterfeit anti-VEGF solution left 14% of eyes blind (no light perception) (56).
Treatment of postinjection endophthalmitis is the same as for postoperative endophthalmitis, described above, and visual outcomes depend partly on the pathogen: streptococci are particularly virulent in the eye. A review of 197 cases reported in 43 publications found that very poor outcomes (≤20/400 vision) were seen in 13% of coagulase-negative staphylococcal cases and 31% of culture-negative cases but in 94% of streptococcal cases (48).
POSTTRAUMATIC ENDOPHTHALMITIS
Penetrating eye trauma (open globe injury) occurs in 2 to 3.8/100,000 population in the United States. Posttraumatic endophthalmitis occurs in 0.9 to 18% of adults and 5 to 54% of children with such injuries (57, 58). Risk factors include delay in treatment of the eye trauma, rural setting of the injury, presence of an intraocular foreign body, lens capsule disruption, and a lacerating injury rather than blunt trauma with globe rupture. Endophthalmitis symptoms include decreased vision and eye pain. On examination, a hypopyon and intraocular inflammation are usually seen, and an eye wound with purulent drainage may sometimes be seen. Specific findings may suggest the pathogen. Bacillus infections are fulminant and sometimes associated with a ring corneal abscess, while Clostridium endophthalmitis cases may have gas bubbles in the anterior chamber and a green-brown hypopyon (59). Major causes of posttraumatic endophthalmitis include coagulase-negative staphylococci, Bacillus species, streptococci, Gram-negative bacilli (e.g., Pseudomonas and Klebsiella), and fungi. Treatment includes removal of any retained intraocular foreign object, intravitreal antibiotics, vitrectomy in most cases, and often adjunctive topical and systemic antibiotics. Tetanus vaccine is indicated after open globe injuries if the patient's last vaccination was ≥5 years earlier. Final visual acuity is variable and depends to a great extent on the pathogen, but one multicenter study found that 41% of all cases achieved 20/40 or better, while 47% were left with minimal (light perception only) or no vision (60).
BLEB-RELATED ENDOPHTHALMITIS
Bleb-related endophthalmitis usually occurs suddenly but months to years following glaucoma surgery in which a “bleb” is created. A filtering bleb is a defect in the sclera covered by conjunctiva that improves aqueous resorption into the systemic circulation. However, following this surgery, only a thin barrier of conjunctiva separates the aqueous from the outside world in the location of the bleb, and this carries an ongoing risk of developing endophthalmitis. A large study from Japan reported an endophthalmitis risk of 1% over 5 years (61), and other studies have reported higher rates. Blebs that are leaking (aqueous) increase the endophthalmitis risk by nearly 5-fold (61). An infection of the bleb, or blebitis, often precedes endophthalmitis. Blebitis should be promptly treated to prevent progression. Streptococci, primarily viridans streptococci but also S. pneumoniae, are the pathogens in over one-third of bleb-related endophthalmitis cases; other major pathogens include S. aureus, Haemophilus influenzae, and enterococci. Treatment of endophthalmitis is with intracameral and/or intravitreal antibiotic injections, vitrectomy in severe cases, plus topical antibiotics in most cases; systemic antibiotics such as quinolones are also sometimes given as adjunctive therapy. Visual outcome is poor for cases due to pathogens that are virulent in the eye, such as enterococci and any type of streptococci.
KERATITIS-RELATED ENDOPHTHALMITIS
Keratitis means corneal infection, and most cases are treated with topical antibiotics. Progression to endophthalmitis is uncommon and usually occurs by extension of the infection through the cornea into the aqueous. A majority of cases of keratitis-related endophthalmitis are due to molds. A series from Florida of nearly 10,000 keratitis cases found that only 0.5% progressed to endophthalmitis; fungal keratitis (keratomycosis) was a significant risk factor for such progression (62). Over half the endophthalmitis cases in that series were due to molds (53%), while Gram-positive bacteria (27%) and Gram-negative bacilli (20%) comprised the remaining cases. A series from New Jersey reported a lower rate of fungal endophthalmitis (17%), but this reflects the much lower incidence of keratomycosis in colder, less humid climates (63). The risk of keratomycosis progressing to endophthalmitis varies, but one center reported that 6% of Fusarium keratitis cases seen during an outbreak developed endophthalmitis (64).
In cases of keratomycosis-related endophthalmitis, Fusarium and Aspergillus are the most common etiologies (65). Keratomycosis may be difficult to diagnose, as cultures of corneal scrapings may be falsely negative. The appearance of the corneal infiltrate often suggests the diagnosis. Unlike bacteria, molds often produce corneal infiltrates with fuzzy or feathery borders and satellite lesions (Fig. 5). Noninvasive techniques such as optical coherence tomography can support the diagnosis of keratomycosis. The finding of frond-like projections extending from the back of the cornea into the aqueous, or of thick clumped material in the aqueous, is concerning for keratomycosis-related endophthalmitis. Treatment of keratitis-related endophthalmitis includes intracameral and/or intravitreal antibiotics, topical antibiotics, adjunctive systemic antifungal antibiotics for keratomycosis-related cases, and vitrectomy as needed. Corneal transplant to debulk the infection may be required, particularly in cases due to molds.
FIG 5.
Endophthalmitis resulting from extension of Alternaria keratitis. Note the irregular borders of the corneal infiltrate and satellite lesions, both typical of mold keratitis.
ENDOGENOUS ENDOPHTHALMITIS
Endogenous endophthalmitis, which results from bacteremic or fungemic seeding of the eye, is rare. Only 5 to 15% of all endophthalmitis cases are endogenous (66). The choroid is usually seeded first since it is highly vascular, and as a consequence the intraocular infection usually starts in the posterior segment. Bacterial cases typically present acutely and fungal cases subacutely. Only half of the patients with endogenous endophthalmitis in one series had symptoms of an underlying infection on presentation, and only 75% had positive blood cultures (67). Infections commonly associated with endogenous endophthalmitis include liver abscess, endocarditis, and urinary tract infection (67, 68). Blood cultures are often positive in these cases but may be negative in cases due to transient bacteremia or fungemia, such as those related to i.v. drug use (IVDU), an indwelling central venous catheter, or an outpatient gastrointestinal procedure such as endoscopy or colonoscopy. The ophthalmologist must have a high index of suspicion in these cases, as misdiagnosis (usually as uveitis) results in delay in therapy. The risk of a hospitalized patient developing endophthalmitis from bacteremia or fungemia appears to be low overall, with one U.S. study reporting an overall rate of 0.05% although a higher rate (0.4%) following fungemia than following bacteremia (0.04%) (69). A higher endophthalmitis rate may also be seen in patients with K. pneumoniae bacteremia associated with liver abscess, as seen in East Asian centers.
Endogenous Bacterial Endophthalmitis
Patients with endogenous bacterial endophthalmitis (EBE) usually present acutely, and 90% complain of decreased vision, 50% have eye pain (50%), 35% have a hypopyon, and 33% have vitritis (70). Systemic symptoms may be absent, and one-half to two-thirds of patients present first to an ophthalmologist (26, 67). Fever (37% of cases) and flu-like symptoms (20%) were the most common systemic complaints in one series (70). One-quarter to one-third of cases may have a delayed diagnosis; a 3-day delay in diagnosis has been estimated (70). The pathogen is diagnosed by positive blood or intraocular cultures, and common pathogens are S. aureus, streptococci (including viridans streptococci, S. pneumoniae, and group A and B streptococci), and Gram-negative bacilli such as Escherichia coli and K. pneumoniae. In East Asian nations, many EBE cases are due to K. pneumoniae serotypes K1 and K2 and are associated with liver abscesses.
Treatment includes systemic therapy for the underlying infection and intravitreal antibiotics for the endophthalmitis. Vitrectomy is often indicated as well, due to the virulence of the pathogens and the severe endophthalmitis these produce. Prognosis is poor in the majority of cases. In a series of 75 EBE patients (89 eyes) treated between 2001 and 2012, only 41% of eyes recovered 20/200 vision or better, while 19% of eyes were enucleated or eviscerated (70). Vitrectomy was associated with a better visual prognosis and a lower rate of evisceration or enucleation.
Endogenous Fungal Endophthalmitis
Candida is the most common cause of endogenous fungal endophthalmitis (EFE). The initial manifestation is usually chorioretinitis, manifested as fluffy white chorioretinal lesions. This may be clinically silent, with symptoms developing only after development of significant vitritis. Of note, Candida chorioretinitis is counted as a type of endophthalmitis in some studies, while others distinguish chorioretinitis from endophthalmitis, meaning cases with significant vitritis; the latter convention will be followed here.
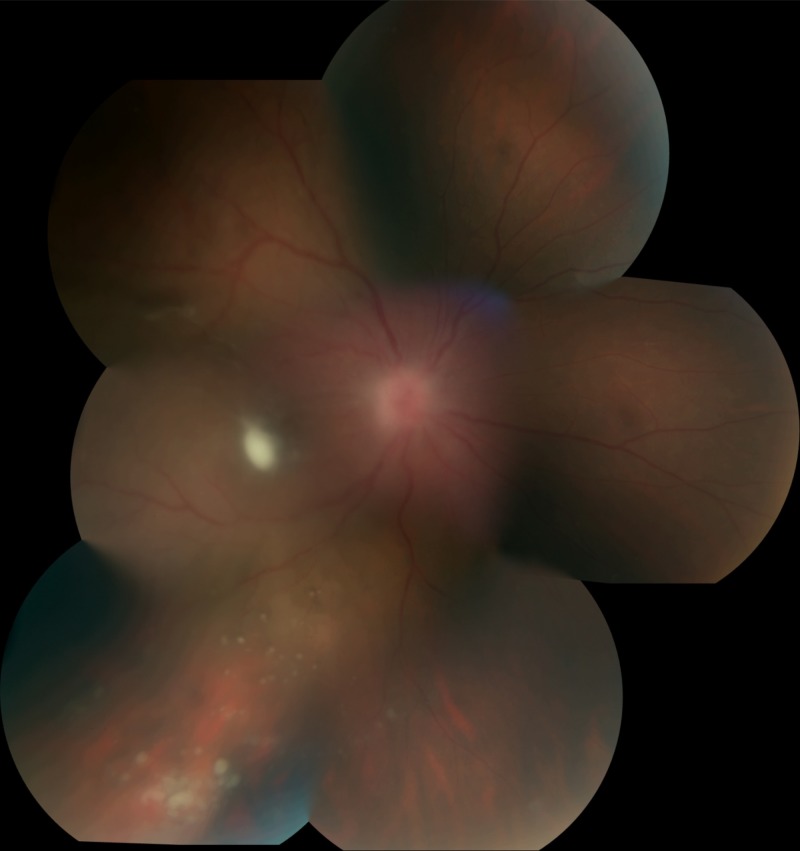
In patients with candidemia, the incidence of chorioretinitis is much higher than that of endophthalmitis; a prospective trial of 370 patients with candidemia found that 11% had chorioretinitis while only 1.6% had endophthalmitis (71). Because ocular candidiasis may be clinically silent until late in the infection and because many candidemic patients are too ill to relay visual symptoms, all patients with candidemia should have a funduscopic examination. Risk factors for Candida chorioretinitis and endophthalmitis reflect those for candidemia: central venous catheters, total parenteral nutrition, broad-spectrum antibiotics, recent abdominal surgery, neutropenia, and glucocorticoid therapy in inpatients and IVDU and recent central venous catheters, including peripherally inserted central catheters (PICC lines), in outpatients. Eye examination typically reveals fluffy white chorioretinal lesions, and there may be overlying vitritis or extension into the vitreous (Fig. 6). Fluff balls suspended in the vitreous may appear as a “string of pearls.” Diagnosis is by blood or vitreous cultures. The diagnosis of EFE is based on eye findings rather than vitreous cultures in most cases of documented candidemia. In outpatients, blood cultures may be negative because candidemia was transient and occurred days or weeks earlier; culture of intraocular fluids is required in these cases, including vitrectomy if initial aspirate cultures are negative. Candida albicans is the predominant species of EFE, but all Candida species have been described.
FIG 6.
Endogenous Candida albicans endophthalmitis following illicit injection drug use. A vitreous aspirate was culture negative, so a vitrectomy was performed, and this yielded the diagnosis. Note white lesions overlying the retina, typical of endogenous fungal endophthalmitis.
Treatment of candidemia has been discussed in recent Infectious Disease Society of America guidelines, which also discuss treatment of Candida chorioretinitis and endophthalmitis (72). Treatment with systemic agents is usually adequate for cases of chorioretinitis that do not have macula-threatening lesions. Macula-threatening chorioretinitis and cases of endophthalmitis require intravitreal antifungal injections (amphotericin or voriconazole) in addition to systemic therapy. Endophthalmitis cases with significant vitritis usually require vitrectomy as well. For systemic agents, fluconazole is recommended for fluconazole-susceptible Candida, voriconazole for fluconazole-resistant but voriconazole-susceptible isolates, and liposomal amphotericin, with or without 5-flucytosine, for azole-resistant strains. Fluconazole or voriconazole is preferred to amphotericin for susceptible isolates because azoles are less toxic and produce higher levels in the vitreous. Voriconazole, for example, achieves vitreous levels that are approximately 40% of serum levels even in uninflamed eyes. Systemic echinocandins do not reach adequate concentrations in the vitreous to treat endophthalmitis. Echinocandins may achieve reasonable levels in the choroid, but their role in treating chorioretinitis alone is unknown because there are few data in humans. One study measured micafungin levels in a patient given i.v. micafungin prior to a scheduled enucleation and found reasonable levels in the choroid (34% of plasma levels) but very poor levels in the retina or vitreous (7% and 0.9% of plasma levels, respectively) (73).
Endogenous mold endophthalmitis is rare and seen primarily in immunocompromised patients, such as patients with hematologic malignancies or transplant recipients, or patients with IVDU. Aspergillus and Fusarium are the major pathogens, although Scedosporium and other fungi are also seen. Therapy includes systemic antifungal therapy plus intravitreal amphotericin or voriconazole and usually vitrectomy.
VISUAL OUTCOMES AND MICROBIOLOGY
Some loss of vision is common after endophthalmitis, but the final visual acuity usually cannot be determined for weeks to months later. Unless the eye has no light perception, vision may improve once the acute inflammation has resolved. Any vision is worth saving, so every effort should be made to save even light perception vision.
Visual outcomes in endophthalmitis are related to a number of factors, including presenting visual acuity and the promptness of appropriate therapy, but the pathogen involved is a major factor in nearly all cases. In postcataract endophthalmitis, the EVS found that a “good” final visual acuity of 20/100 or better occurred in approximately 80% of culture-negative or coagulase-negative staphylococcal cases but in only 50% due to S. aureus, 30% due to streptococci (of any type), and 56% due to Gram-negative bacilli (86). Considering outcomes by pathogen regardless of endophthalmitis category, a group in Florida reported that very poor visual acuity (20/400 or worse) occurred in 75% of cases due to streptococci (with no difference in outcomes between viridans streptococci, S. pneumoniae, and beta-hemolytic streptococci), 93% of enterococcal cases, 64% of Bacillus cases, 69% of H. influenzae cases, 70% of Serratia cases, and 92% of Pseudomonas cases (74–79). These cases were seen over a 10-year period at a tertiary eye hospital where the most severe endophthalmitis cases are likely to be referred. However, there is hope even in endophthalmitis cases due to bacteria associated with a poor visual prognosis. Bacillus produces a fulminant endophthalmitis, but 18% of cases at that eye hospital had a final acuity of 20/60 or better (76).
PREVENTION
There are almost no published randomized controlled trials evaluating the efficacy of various proposed measures to prevent endophthalmitis, so the optimal methods are largely unknown (80). Prophylaxis for eye surgery with topical povidone-iodine preoperatively and topical antibiotics postoperatively is routinely given, but these measures have not been evaluated by a randomized controlled trial. Intracameral antibiotics are being used with increasing frequency as prophylaxis for cataract surgery. A randomized controlled trial in Europe found that intracameral cefuroxime prophylaxis at the end of cataract surgery was associated with a postoperative endophthalmitis rate of 0.06%, versus 0.3% in control eyes (81). However, the study results were questioned in the United States because the control group's endophthalmitis rate was approximately 3-fold higher than that seen at most U.S. centers. Rare cases of anaphylaxis to prophylactic intracameral cefuroxime have been reported (82), and use of prophylactic intracameral vancomycin has been associated with a rare but devastating complication of hemorrhagic occlusive retinal vasculitis (83, 84). For decreasing the risk of postinjection endophthalmitis, many centers have adopted the use of masks or observing a strict no-talking policy during injections. For patients presenting with penetrating eye trauma, prompt surgical repair and 48 h of prophylactic broad-spectrum systemic antibiotics (e.g., i.v. vancomycin plus ceftazidime) have been associated with a very low rate of posttraumatic endophthalmitis (57). Patients who have filtering blebs for glaucoma should be treated for blebitis without delay in order to prevent this relatively minor infection from leading to bleb-related endophthalmitis. I also recommend that patients with filtering blebs receive pneumococcal vaccination (80, 85). Endogenous bacterial endophthalmitis cannot usually be anticipated or prevented, but some cases of endogenous Candida endophthalmitis can be prevented by screening all candidemic patients with funduscopic examinations.
CONCLUSION
Endophthalmitis is a severe eye infection that requires rapid diagnosis and treatment to save vision. Endophthalmitis may occur from pathogens introduced into the eye from an external source (exogenous endophthalmitis) or via the bloodstream (endogenous endophthalmitis). Most cases of exogenous endophthalmitis occur as a complication of cataract surgery, an intravitreal injection, or penetrating ocular trauma. Endogenous endophthalmitis may develop as a result of metastatic spread of an extraocular focus of infection, such as endocarditis or a liver abscess, or from transient bacteremia or fungemia related to illicit injection drug use or an indwelling central venous catheter. Nearly all patients present with decreased vision, and some also have eye pain. A hypopyon and intraocular inflammation are typical findings on eye examination. Diagnosis is clinical, supported by cultures of vitreous and/or aqueous or also by blood cultures in endogenous cases. Newer diagnostic techniques such as PCR offer the promise of rapid identification of intraocular pathogens, including in culture-negative cases. Prompt treatment with intravitreal antibiotics is essential; some cases also benefit from a surgical vitrectomy. The visual outcome depends partly on the pathogen. Cases due to coagulase-negative staphylococci usually recover good vision, while half or more of the eyes infected with streptococci (of any type), S. aureus, or Gram-negative bacilli are left with poor vision. Few randomized controlled trials have been performed to determine the most effective ways to prevent endophthalmitis, and more such trials are needed.
Biography

Marlene L. Durand, M.D., graduated from Stanford University and Harvard Medical School, completed her residency and infectious disease fellowship at Massachusetts General Hospital (MGH), and then joined the MGH staff. She remains a member of the MGH Infectious Disease staff and is also the Director of the Infectious Disease Service at Massachusetts Eye and Ear Infirmary. She is an Associate Professor of Medicine and an Associate Professor of Ophthalmology at Harvard Medical School. Her clinical work treating patients with eye or ears, nose, and throat (ENT) infections led to her research interest in identifying optimal ways to diagnose and treat these infections. She has enjoyed collaborating with her colleagues in infectious diseases, microbiology, ophthalmology, and otolaryngology on clinical research topics.
REFERENCES
- 1.Melo GB, Bispo PJM, Yu MCZ, Pignatari AC, Höfling-Lima AL. 2011. Microbial profile and antibiotic susceptibility of culture-positive bacterial endophthalmitis. Eye 25:382–388. doi: 10.1038/eye.2010.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta A, Orlans HO, Hornby SJ, Bowler ICJ. 2014. Microbiology and visual outcomes of culture-positive bacterial endophthalmitis in Oxford, UK. Graefes Arch Clin Exp Ophthalmol 252:1825–1830. doi: 10.1007/s00417-014-2658-7. [DOI] [PubMed] [Google Scholar]
- 3.Kessner R, Golan S, Barak A. 2014. Changes in the etiology of endophthalmitis from 2003 to 2010 in a large tertiary medical center. Eur J Ophthalmol 24:918–924. doi: 10.5301/ejo.5000473. [DOI] [PubMed] [Google Scholar]
- 4.Falavarjani KG, Nekoozadeh S, Modarres M, Parvaresh MM, Hashemi M, Soodi R, Alemzadeh SA. 2012. Isolates and antibiotic resistance of culture-proven endophthalmitis cases presented to a referral center in Tehran. Middle East Afr J Ophthalmol 19:361–363. doi: 10.4103/0974-9233.102740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moloney TP, Park J. 2014. Microbiological isolates and antibiotic sensitivities in culture-proven endophthalmitis: a 15-year review. Br J Ophthalmol 98:1492–1497. doi: 10.1136/bjophthalmol-2014-305030. [DOI] [PubMed] [Google Scholar]
- 6.Nam KY, Lee JE, Lee JE, Jeung WJ, Park JM, Park JM, Chung IY, Han YS, Yun IH, Kim HW, Byon IS. 2015. Clinical features of infectious endophthalmitis in South Korea: a five-year multicenter study. BMC Infect Dis 15:177–183. doi: 10.1186/s12879-015-0900-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma S, Padhi TR, Basu S, Kar S, Roy A, Das T. 2014. Endophthalmitis patients seen in a tertiary eye care centre in Odisha: a clinicomicrobiological analysis. Indian J Med Res 139:91–98. [PMC free article] [PubMed] [Google Scholar]
- 8.Gharamah AA, Moharram AM, Ismail MA, Al-Hussaini AK. 2012. Bacterial and fungal endophthalmitis in Upper Egypt: related species and risk factors. Asian Pac J Trop Biomed 2:655–659. doi: 10.1016/S2221-1691(12)60115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duan F, Wu K, Liao J, Zheng Y, Yuan Z, Tan J, Lin X. 2016. Causative microorganisms of infectious endophthalmitis: a 5-year retrospective study. J Ophthalmol doi: 10.1155/2016/6764192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simunovic MP, Rush RB, Hunyor AP, Chang AA. 2012. Endophthalmitis following intravitreal injection versus endophthalmitis following cataract surgery: clinical features, causative organisms and post-treatment outcomes. Br J Ophthalmol 96:862–866. doi: 10.1136/bjophthalmol-2011-301439. [DOI] [PubMed] [Google Scholar]
- 11.Valdez-Garcia JE, Climent A, Chavez-Mondragon E, Lozano-Ramirez JF. 2014. Anterior chamber bacterial contamination in cataract surgery. BMC Ophthalmol 14:57. doi: 10.1186/1471-2415-14-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Srinivasan R, Tiroumal S, Kanungo R, Natarajan MK. 2002. Microbial contamination of the anterior chamber during phacoemulsification. J Cataract Refract Surg 28:2173–2176. doi: 10.1016/S0886-3350(02)01493-1. [DOI] [PubMed] [Google Scholar]
- 13.Lundstrom M, Wejde G, Stenevi U, Thorbum W, Montan P. 2007. Endophthalmitis after cataract surgery: a nationwide prospective study evaluating incidence in relation to incision type and location. Ophthalmology 114:866–870. doi: 10.1016/j.ophtha.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 14.Fang CT, Lai SY, Yi WC, Hsueh PR, Liu KL, Chang SC. 2007. Klebsiella pneumoniae genotype K1: an emerging pathogen that causes septic ocular or central nervous system complications from pyogenic liver abscess. Clin Infect Dise 45:284–293. doi: 10.1086/519262. [DOI] [PubMed] [Google Scholar]
- 15.Wiskur BJ, Hunt JJ, Callegan MC. 2008. Hypermucoviscosity as a virulence factor in experimental Klebsiella pneumoniae endophthalmitis. Invest Ophthalmol Vis Sci 49:4931–4938. doi: 10.1167/iovs.08-2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin TL, Hsieh PF, Huang YT, Lee WC, Tsai YT, Su PA, Pan YJ, Hsu CR, Wu MC, Wang JT. 2014. Isolation of a bacteriophage and its depolymerase specific for K1 capsule of Klebsiella pneumoniae: implication in typing and treatment. J Infect Dis 210:1734–1744. doi: 10.1093/infdis/jiu332. [DOI] [PubMed] [Google Scholar]
- 17.Parkunan SM, Callegan MC. 2016. The pathogenesis of bacterial endophthalmitis, p 17–47. In Durand ML, Miller JW, Young LH (ed), Endophthalmitis. Springer International Publishing, Basel, Switzerland. [Google Scholar]
- 18.Callegan MC, Kane ST, Cochran DC, Ramadan RT, Chodosh J, McLean C, Stroman DW. 2006. Virulence factor profiles and antimicrobial susceptibilities of ocular Bacillus isolates. Cur Eye Res 31:693–702. doi: 10.1080/02713680600850963. [DOI] [PubMed] [Google Scholar]
- 19.Callegan MC, Kane ST, Cochran DC, Gilmore MS, Gominet M, Lereclus D. 2003. Relationship of plcR-regulated factors to Bacillus endophthalmitis virulence. Infect Immun 71:3116–3124. doi: 10.1128/IAI.71.6.3116-3124.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Callegan MC, Kane ST, Cochran DC, Novosad B, Gilmore MS, Gominet M, Lereclus D. 2005. Bacillus endophthalmitis: roles of bacterial toxins and motility during infection. Invest Ophthalmol Vis Sci 46:3233–3238. doi: 10.1167/iovs.05-0410. [DOI] [PubMed] [Google Scholar]
- 21.Parkunan SM, Randall CB, Astley RA, Furtado GC, Lira SA, Callegan MC. 10 June 2016. CXCL1, but not IL-6, significantly impacts intraocular inflammation during infection. J Leukoc Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ng EW, Costa JR, Samiy N, Ruoff KL, Connolly E, Cousins FV, D'Amico DJ. 2002. Contribution of pneumolysin and autolysin to the pathogenesis of experimental pneumococcal endophthalmitis. Retina 22:622–632. doi: 10.1097/00006982-200210000-00014. [DOI] [PubMed] [Google Scholar]
- 23.Sanders ME, Norcross EW, Robertson ZM, Moore QC 3rd, Fratkin J, Marquart ME. 2011. The Streptococcus pneumoniae capsule is required for full virulence in pneumococcal endophthalmitis. Invest Ophthalmol Vis Sci 52:865–872. doi: 10.1167/iovs.10-5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baillif S, Ecochard R, Casoli E, Freney J, Burillon C, Kodjikian L. 2008. Adherence and kinetics of biofilm formation of Staphylococcus epidermidis to different types of intraocular lenses under dynamic flow conditions. J Cataract Refract Surg 34:153–158. doi: 10.1016/j.jcrs.2007.07.058. [DOI] [PubMed] [Google Scholar]
- 25.Mazoteras P, Quiles MG, Martins Bispo PJ, Höfling-Lima AL, Pignatari AC, Casaroli-Marano RP. 2016. Analysis of intraocular lens biofilms and fluids after long-term uncomplicated cataract surgery. Am J Ophthalmol 169:46–57. doi: 10.1016/j.ajo.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 26.Jackson TL, Eykyn SJ, Graham EM, Stanford MR. 2003. Endogenous bacterial endophthalmitis: a 17-year prospective series and review of 267 reported cases. Survey Ophthalmol 48:403–423. doi: 10.1016/S0039-6257(03)00054-7. [DOI] [PubMed] [Google Scholar]
- 27.Lalwani GA, Flynn HW Jr, Scott IU, Quinn CM, Berrocal AM, Davis JL, Murray TG, Smiddy WE, Miller D. 2008. Acute-onset endophthalmitis after clear corneal cataract surgery (1996-2005). Clinical features, causative organisms, and visual acuity outcomes. Ophthalmology 115:473–476. [DOI] [PubMed] [Google Scholar]
- 28.Taban M, Behrens A, Newcomb RL, Nobe MY, Saedi G, Sweet PM, McDonnell PJ. 2005. Acute endophthalmitis following cataract surgery: a systematic review of the literature. Arch Ophthalmol 123:613–620. doi: 10.1001/archopht.123.5.613. [DOI] [PubMed] [Google Scholar]
- 29.Chiquet C, Boisset S, Cornut P-L, Maurin M. 2016. The molecular diagnosis of endophthalmitis, p 77–97. In Durand ML, Miller JW, Young LH (ed), Endophthalmitis. Springer International Publishing, Basel, Switzerland. [Google Scholar]
- 30.Hykin PG, Tobal K, McIntye G, Matheson MM, Towler HM, Lightman SL. 1994. The diagnosis of delayed post-operative endophthalmitis by polymerase chain reaction of bacterial DNA in vitreous samples. J Med Microbiol 40:408–415. doi: 10.1099/00222615-40-6-408. [DOI] [PubMed] [Google Scholar]
- 31.Chiquet C, Cornut P-L, Benito Y, Thuret G, Maurin M, Lafontaine PO, Pechinot A, Palombi K, Lina G, Bron A, Denis P, Carricajo A, Creuzot C, Romanet JP, Vandenesch F, French Institutional Endophthalmitis Study Group . 2008. Eubacterial PCR for bacterial detection and identification in 100 acute postcataract surgery endophthalmitis. Invest Ophthalmol Vis Sci 49:1971–1978. doi: 10.1167/iovs.07-1377. [DOI] [PubMed] [Google Scholar]
- 32.Hong BK, Lee CS, Van Gelder RN, Garg SJ. 2015. Emerging techniques for pathogen discovery in endophthalmitis. Curr Opin Ophthalmol 26:221–225. doi: 10.1097/ICU.0000000000000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee AY, Akileswaran L, Tibbetts MD, Garg SJ, Van Gelder RN. 2015. Identification of torque teno virus in culture-negative endophthalmitis by representational deep DNA sequencing. Ophthalmology 122:524–530. doi: 10.1016/j.ophtha.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vasilyev EV, Trofimov DY, Tonevitsky AG, Ilinsky VV, Korostin DO, Rebrikov DV. 2009. Torque teno virus (TTV) distribution in healthy Russian population. Virology J 6:134. doi: 10.1186/1743-422X-6-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Endophthalmitis Vitrectomy Study Group. 1995. Results of the Endophthalmitis Vitrectomy Study: a randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Arch Ophthalmol 113:1479–1496. doi: 10.1001/archopht.1995.01100120009001. [DOI] [PubMed] [Google Scholar]
- 36.Bannerman TL, Rhoden DL, McAllister SK, Miller JM, Wilson LA. 1997. The source of coagulase-negative staphylococci in the Endophthalmitis Vitrectomy Study. A comparison of eyelid and intraocular isolates using pulsed-field gel electropheresis. Arch Ophthalmol 115:357–361. [DOI] [PubMed] [Google Scholar]
- 37.Leong JK, Shah R, McCluskey PJ, Benn RA, Taylor RF. 2002. Bacterial contamination of the anterior chamber during phacoemulsification cataract surgery. J Cataract Refract Surg 28:826–833. doi: 10.1016/S0886-3350(01)01160-9. [DOI] [PubMed] [Google Scholar]
- 38.Buchta V, Feuermannova A, Vasa M, Bašková L, Kutová R, Kubátová A, Vejsová M. 2014. Outbreak of fungal endophthalmitis due to Fusarium oxysporum following cataract surgery. Mycopathologia 177:115–121. doi: 10.1007/s11046-013-9721-5. [DOI] [PubMed] [Google Scholar]
- 39.Anand AR, Therese KL, Madhavan HN. 2000. Spectrum of aetiological agents of postoperative endophthalmitis and antibiotic susceptibility of bacterial isolates. Indian J Ophthalmol 48:123–128. [PubMed] [Google Scholar]
- 40.Sharma S, Sahu SK, Dhillon V, Das S, Rath S. 2015. Reevaluating intracameral cefuroxime as a prophylaxis against endophthalmitis after cataract surgery in India. J Cataract Refract Surg 41:393–399. doi: 10.1016/j.jcrs.2014.05.038. [DOI] [PubMed] [Google Scholar]
- 41.Doft BH, Kelsey SF, Wisniewski SR. 1998. Additional procedures after the initial vitrectomy or tap-biopsy in the Endophthalmitis Vitrectomy Study. Ophthalmology 105:707. doi: 10.1016/S0161-6420(98)94028-3. [DOI] [PubMed] [Google Scholar]
- 42.Shirodkar AR, Pathengay A, Flynn HW, Albini TA, Berrocal AM, Davis JL, Lalwani GA, Murray TG, Smiddy WE, Miller D. 2012. Delayed versus acute-onset endophthalmitis after cataract surgery. Am J Ophthalmol 153:391–398. doi: 10.1016/j.ajo.2011.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dave VP, Schwartz SG, Flynn HW Jr. 2014. Endophthalmitis following pars plana vitrectomy: a literature review of incidence, causative organisms, and treatment outcomes. Clin Ophthalmol 8:2183–2188. doi: 10.2147/OPTH.S71293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taban M, Behrens A, Newcomb RL, Nobe MY, McDonnell PJ. 2005. Incidence of acute endophthalmitis following penetrating keratoplasty: a systematic review. Arch Ophthalmol 123:605–609. doi: 10.1001/archopht.123.5.605. [DOI] [PubMed] [Google Scholar]
- 45.Chen JY, Jones MN, Srinivasan S, Neal TJ, Armitage WJ, Kaye SB, NHSBT Ocular Tissue Advisory Group and Contributing Ophthalmologists . 2015. OTAG audit study 18. Endophthalmitis after penetrating keratoplasty. Ophthalmology 122:25–30. [DOI] [PubMed] [Google Scholar]
- 46.Wilhelmus KR, Hassan SS. 2007. The prognostic role of donor corneoscleral rim cultures in corneal transplantation. Ophthalmology 114:440–445. doi: 10.1016/j.ophtha.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 47.Williams GA. 2014. IVT injections: health policy implications. Rev Ophthalmol 21:62–64. [Google Scholar]
- 48.Fileta JB, Scott IU, Flynn HW. 2014. Meta-analysis of infectious endophthalmitis after intravitreal injection of anti-vascular endothelial growth factor agents. Ophthalmic Surg Lasers Imaging Retina 45:143–149. doi: 10.3928/23258160-20140306-08. [DOI] [PubMed] [Google Scholar]
- 49.VanderBeek BL, Bonaffini SG, Ma L. 2015. The association between intravitreal steroids and post-injection endophthalmitis rates. Ophthalmology 122:2311–2315. doi: 10.1016/j.ophtha.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lyall DAM, Tey A, Foot B, Roxburgh ST, Virdi M, Robertson C, MacEwen CJ. 2012. Post-intravitreal anti-VEGF endophthalmitis in the United Kingdom: incidence, features, risk factors, and outcomes. Eye (Lond) 26:1517–1526. doi: 10.1038/eye.2012.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCannel CA. 2011. Meta-analysis of endophthalmitis after intravitreal injection of anti-vascular endothelial growth factor agents. Retina 31:654–661. doi: 10.1097/IAE.0b013e31820a67e4. [DOI] [PubMed] [Google Scholar]
- 52.Dossarps D, Bron AM, Koehrer P, Aho-Glélé LS, Creuzot-Garcher C, FRCR net (FRenCh Retina specialists net) . 2015. Endophthalmitis after intravitreal injections: incidence, presentation, management, and visual outcome. Am J Ophthalmol 160:17–25. doi: 10.1016/j.ajo.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 53.Garg SJ, Dollin M, Hsu J, Storey P, Vander JF. 2015. Effect of a strict “no-talking” policy during intravitreal injection on post-injection endophthalmitis. Ophthalmic Surg Lasers Imaging Retina 46:1028–1034. doi: 10.3928/23258160-20151027-07. [DOI] [PubMed] [Google Scholar]
- 54.Goldberg RA, Flynn HW Jr, Isom RF, Miller D, Gonzalez S. 2012. An outbreak of streptococcus endophthalmitis after intravitreal injection of bevacizumab. Am J Ophthalmol 153:204–208. doi: 10.1016/j.ajo.2011.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Small KW, Chan CK, Silva-Garcia R, Walsh TJ. 2014. Onset of an outbreak of Bipolaris hawaiiensis fungal endophthalmitis after intravitreal injections of triamcinolone. Ophthalmology 121:952–958. doi: 10.1016/j.ophtha.2013.10.040. [DOI] [PubMed] [Google Scholar]
- 56.Entezari M, Karimi S, Ahmadieh H, Mahmoudi AH, Parhizgar H, Yaseri M. 2016. A large outbreak of fulminant bacterial endophthalmitis after intravitreal injection of counterfeit bevacizumab. Graefes Arch Clin Exp Ophthalmol 254:1851–1856. doi: 10.1007/s00417-016-3426-7. [DOI] [PubMed] [Google Scholar]
- 57.Andreoli CM, Andreoli MT, Kloek CE, Ahuero AE, Vavvas D, Durand ML. 2009. Low rate of endophthalmitis in a large series of open globe injuries. Am J Ophthalmol 147:601–608. doi: 10.1016/j.ajo.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 58.Li XL, Zarbin MA, Bhagat N. 2015. Pediatric open globe injury: a review of the literature. J Emerg Trauma Shock 8:216–223. doi: 10.4103/0974-2700.166663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bhagat N, Li X, Zarbin MA. 2016. Post-traumatic endophthalmitis, p 151–170. In Durand ML, Miller JW, Young LH (ed), Endophthalmitis. Springer International, Basel, Switzerland. [Google Scholar]
- 60.Cornut PL, el Youssef B, Bron A, Thuret G, Gain P, Burillon C, Romanet JP, Vandenesch F, Maurin M, Creuzot-Garcher C, Chiquet C, French Institutional Endophthalmitis Study (FRIENDS) Group . 2013. A multicentre prospective study of post-traumatic endophthalmitis. Acta Ophthalmol 91:475–482. doi: 10.1111/j.1755-3768.2011.02349.x. [DOI] [PubMed] [Google Scholar]
- 61.Yamamoto T, Sawada A, Mayama C, Araie M, Ohkubo S, Sugiyama K, Kuwayama Y, Collaborative Bleb-Related Infection Incidence and Treatment Study Group . 2014. The 5 year incidence of bleb-related infection and its risk factors following filtering surgeries with adjunctive mitomycin C: collaborative bleb-related infection incidence and treatment study 2. Ophthalmology 121:1001–1006. doi: 10.1016/j.ophtha.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 62.Henry CR, Flynn HW Jr, Miller D, Forster RK, Alfonso EC. 2012. infectious keratitis progressing to endophthalmitis: a 15-year study of microbiology, associated factors, and clinical outcomes. Ophthalmology 119:2443–2449. doi: 10.1016/j.ophtha.2012.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Malihi M, Li X, Patel S, Eck T, Chu DS, Zarbin MA, Bhagat N. 14 July 2016. Infectious keratitis-associated endophthalmitis: a 14-year study. Retina. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 64.Rosenberg KD, Flynn HW Jr, Alfonso EC, Miller D. 2006. Jul-Aug Fusarium endophthalmitis following keratitis associated with contact lenses. Ophthalmic Surg Lasers Imaging 37:310–313. [DOI] [PubMed] [Google Scholar]
- 65.Shen YC, Wang CY, Tsai HY, Lee HN. 2010. Intracameral voriconazole injection in the treatment of fungal endophthalmitis resulting from keratitis. Am J Ophthalmol 149:916–921. doi: 10.1016/j.ajo.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 66.Durand ML. 2016. Endophthalmitis: an overview, p 1–16. In Durand ML, Miller JW, Young LH (ed), Endophthalmitis. Springer International Publishing, Basel, Switzerland. [Google Scholar]
- 67.Okada AA, Johnson RP, Liles WC, D'Amico DJ, Baker AS. 1994. Endogenous bacterial endophthalmitis. Report of a ten-year retrospective study. Ophthalmology 101:832–838. [PubMed] [Google Scholar]
- 68.Cho H, Shin YU, Siegel NH, Yu HG, Sobrin L, Patel A, Durand ML, Miller JW, Husain D. 2016. Endogenous endophthalmitis in the American and Korean population: an 8-year retrospective study. Ocul Immunol Inflamm. 26:1–8. [DOI] [PubMed] [Google Scholar]
- 69.Vaziri K, Pershing S, Albini TA, Moshfeghi DM, Moshfeghi AA. 2015. Risk factors predictive of endogenous endophthalmitis among hospitalized patients with hematogenous infections in the United States. Am J Ophthalmol 159:498–504. doi: 10.1016/j.ajo.2014.11.032. [DOI] [PubMed] [Google Scholar]
- 70.Jackson TL, Paraskevopoulos T, Georgalas I. 2014. Systematic review of 342 cases of endogenous bacterial endophthalmitis. Surv Ophthalmol. 59:627–635. doi: 10.1016/j.survophthal.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 71.Oude Lashof AM, Rothova A, Sobel JD, Ruhnke M, Pappas PG, Viscoli C, Schlamm HT, Oborska IT, Rex JH, Kullberg BJ. 2011. Ocular manifestations of candidemia. Clin Infect Dis 53:262. doi: 10.1093/cid/cir355. [DOI] [PubMed] [Google Scholar]
- 72.Pappas PG, Kauffman CA, Andes DR, et al. 2016. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 62:e1–e50. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mochizuki K, Sawada A, Suemonri S, Kawakami H, Niwa Y, Kondo Y, Ohkusu K, Yamada N, Ogura S, Yaguchi T, Nishimura K, Kishino S. 2013. Intraocular penetration of intravenous micafungin in inflamed human eyes. Antimicrob Agents Chemother 57:4027–4030. doi: 10.1128/AAC.02300-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kuriyan AE, Weiss KD, Flynn HW Jr, Smiddy WE, Berrocal AM, Albini TA, Miller D. 2014. Endophthalmitis caused by streptococcal species: clinical settings, microbiology, management, and outcomes. Am J Ophthalmol. 157:774–780. doi: 10.1016/j.ajo.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kuriyan AE, Sridhar J, Flynn HW Jr, Smiddy WE, Albini TA, Berrocal AM, Forster RK, Belin PJ, Miller D. 2014. Endophthalmitis caused by Enterococcus faecalis: clinical features, antibiotic sensitivities, and outcomes. Am J Ophthalmol 158:1018–1023. doi: 10.1016/j.ajo.2014.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miller JJ, Scott IU, Flynn HW Jr, Smiddy WE, Murray TG, Berrocal A, Miller D. 2016. Endophthalmitis caused by Bacillus species. Am J Ophthalmol 145:883–888. [DOI] [PubMed] [Google Scholar]
- 77.Yoder DM, Scott IU, Flynn HW Jr, Miller D. 2004. Endophthalmitis caused by Haemophilus influenzae. Ophthalmology 111:2023–2026. doi: 10.1016/j.ophtha.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 78.Sridhar J, Kuriyan AE, Flynn HW Jr, Smiddy WE, Venincasa VD, Miller D. 2015. Endophthalmitis caused by Serratia marcescens: clinical features, antibiotic susceptibilities, and treatment outcomes. Retina 35:1095–1100. doi: 10.1097/IAE.0000000000000509. [DOI] [PubMed] [Google Scholar]
- 79.Sridhar J, Kuriyan AE, Flynn HW Jr, Miller D. 2015. Endophthalmitis caused by Pseudomonas aeruginosa: clinical features, antibiotic susceptibilities, and treatment outcomes. Retina 35:1101–1106. doi: 10.1097/IAE.0000000000000469. [DOI] [PubMed] [Google Scholar]
- 80.Durand ML. 2016. Preventing endophthalmitis, p 261–278. In Durand ML, Miller JW, Young LH (ed), Endophthalmitis. Springer International, Basel, Switzerland. [Google Scholar]
- 81.ECSRS Endophthalmitis Study Group. 2007. Prophylaxis of postoperative endophthalmitis following cataract surgery: results of the ESCRS multicenter study and identification of risk factors. J Cataract Refract Surg 33:978–988. doi: 10.1016/j.jcrs.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 82.Moisseiev E, Levinger E. 2013. Anaphylactic reaction following intracameral cefuroxime injection during cataract surgery. J Cataract Refract Surg 39:1432–1434. doi: 10.1016/j.jcrs.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 83.Nicholson LB, Kim BT, Jardón J, Townsend-Pico W, Santos C, Moshfeghi AA, Albini TA, Eliott D, Sobrin L. 2014. Severe bilateral ischemic retinal vasculitis following cataract surgery. Ophthalmic Surg Lasers Imaging Retina. 45:338–342. doi: 10.3928/23258160-20140605-01. [DOI] [PubMed] [Google Scholar]
- 84.Witkin AJ, Shah AR, Engstrom RE, Kron-Gray MM, Baumal CR, Johnson MW, Witkin DI, Leung J, Albini TA, Moshfeghi AA, Batlle IR, Sobrin L, Eliott D. 2015. Postoperative hemorrhagic occlusive retinal vasculitis: expanding the clinical spectrum and possible association with vancomycin. Ophthalmology 122:1438–1451. doi: 10.1016/j.ophtha.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 85.Durand ML. 2003. Eye infections, p 222–250. In Betts RF, Chapman SW, Penn RL (ed), Reese and Betts' a practical approach to infectious diseases. Lippincott, Williams and Wilkins, Philadelphia, PA. [Google Scholar]
- 86.Endophthalmitis Vitrectomy Study Group. 1996. Microbiologic factors and visual outcome in the Endophthalmitis Vitrectomy Study. Am J Ophthalmol 122:830–846. [DOI] [PubMed] [Google Scholar]