SUMMARY
In the last 2 decades, renewed attention to neglected tropical diseases (NTDs) has spurred the development of antiparasitic agents, especially in light of emerging drug resistance. The need for new drugs has required in vitro screening methods using parasite culture. Furthermore, clinical laboratories sought to correlate in vitro susceptibility methods with treatment outcomes, most notably with malaria. Parasites with their various life cycles present greater complexity than bacteria, for which standardized susceptibility methods exist. This review catalogs the state-of-the-art methodologies used to evaluate the effects of drugs on key human parasites from the point of view of drug discovery as well as the need for laboratory methods that correlate with clinical outcomes.
KEYWORDS: parasites, resistance, susceptibility tests
INTRODUCTION
The methods employed to test the activity of either established or novel medicines against parasites remain largely within the research domain. However, the development of public-private partnership consortia to align biological and parasitological testing platforms against the various parasite stages has started to pay off by strengthening and rationalizing the drug discovery and development pipeline. To give an example, with malaria now, there is a renewed effort to identify drugs that target liver stages and gametocytes in efforts not only to treat patients with acute infection but also to kill dormant liver stages (hypnozoites) and prevent transmission (gametocytes). Therefore, different assays have emerged in the research arena to address these pressing needs to identify compounds that serve as chemical starting points for drug development. Ultimately, from a clinical point of view, interpretive breakpoints (drug concentrations above which infectious agents are killed or growth is inhibited in patients) would be of immense use for treating patients. Here again, difficulty arises because parasites are difficult to culture from human specimens (so-called ex vivo testing), and in some cases, polyclonal infections may occur; thus, testing may select for certain strains and not others present in that human infection. Further confounding attempts to standardize testing is the fact that certain drugs act at a particular stage of the parasite life cycle, thus affecting the results of the assay. Standardized methods are also essential to drug discovery efforts so that promising leads do not fall by the wayside in preclinical testing and that “false-positive” leads do not progress too far in the development pipeline.
This review attempts to conduct an impartial environmental scan of the laboratory testing methods available to perform susceptibility testing against medically important parasites and to provide a critical appraisal from a clinical perspective (Table 1). Organisms covered in this review include Plasmodium, Leishmania, Trypanosoma, enteric protozoa (Giardia, Entamoeba, and Cryptosporidium), Schistosoma, and filariae. In the realm of bacteria and viruses, organizations like the Clinical and Laboratory Standards Institute (CLSI) (http://clsi.org/) in North America and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) (http://www.eucast.org/) in Europe deal directly with the standardization of phenotypic methods and also the interpretation of clinical breakpoints based on in vitro testing methods. While the CLSI and EUCAST do not always agree on breakpoints for certain bug-drug combinations, the decision of clinical breakpoints is based on evidence in the literature. This includes primarily pharmacokinetic and pharmacodynamic data from clinical trials, supported by in vitro growth and resistance data based on specified testing methods. In certain instances, there is a paucity of clinical evidence to guide the interpretation of in vitro data, and here epidemiological cutoffs (ECOFFs) are used to give a sense of the wild-type distribution of inhibitory concentrations in the population. The EUCAST also guides clinical interpretation of breakpoints for regulatory agencies such as the European Medicines Agency, whereas in the United States, it is not uncommon to have the Food and Drug Administration espouse different breakpoints to the CLSI (http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ucm275763.htm). However, for patient management, most clinical laboratories that test patient samples adhere to CLSI guidelines on testing methods for bacteria, viruses, and fungi and participate in rigorous quality assurance programs such as the College of American Pathology (CAP) in the United States (http://www.cap.org/).
TABLE 1.
Summary of susceptibility testing assays of medically important human parasites
| Parasite | Parasite stages | Type(s) of assaya | Measure(s) | Reference(s) |
|---|---|---|---|---|
| Plasmodium | Liver stages (sporozoites, schizonts, hypnozoites) | High-content imaging | P. berghei/P. yoelii liver schizont development | 161 |
| Bioluminescence imaging | P. berghei/P. yoelii liver schizont development | 27, 162 | ||
| Immunofluorescence | Development of P. chabaudi liver small (hypnozoites) and large (schizonts) forms | 33 | ||
| Immunofluorescence | P. falciparum/P. vivax sporozoite invasion and development | 28, 29 | ||
| Asexual blood stages (rings, trophozoites, schizonts) | Microscopic | P. falciparum/P. vivax schizont maturation | 163 | |
| P. falciparum ring-stage survival | 164 | |||
| Isotopic | P. falciparum intraerythrocytic growth | 4 | ||
| P. falciparum intraerythrocytic growth | 5 | |||
| Colorimetric | P. falciparum intraerythrocytic growth | 7 | ||
| ELISA | P. falciparum intraerythrocytic growth | 6 | ||
| Fluorescent DNA dye based | P. falciparum intraerythrocytic growth | 9, 165, 166 | ||
| Transmission stages (gametocytes, gametes, zygotes, ookinetes, oocysts, sporozoites) | ATP bioluminescence | P. falciparum gametocyte viability | 167 | |
| Flow cytometry | P. falciparum gametocyte viability | 168 | ||
| Microscopic-immunofluorescence | P. falciparum male (exflagellation) and female (fluorescence, shape, size) gamete formation | 23 | ||
| Microscopic | P. falciparum presence/no. of oocysts | 169 | ||
| Leishmania | Amastigotes, promastigotes | Macrophage infection model | Increase in no. of amastigotes | 40, 41 |
| Colorimetric | Promastigote viability | 39 | ||
| Flow cytometry | No. of parasites | 42 | ||
| Trypanosoma cruzi | Epimastigotes, trypomastigotes, amastigotes | Murine models of Chagas disease | Parasite burden measured using PCR, immunofluorescence, bioluminescence | 43, 44, 46, 47, 56 |
| Trypanosoma brucei | Trypomastigotes, epimastigotes | PCR-RFLP with SfaNI | Detection of mutated parasite transporter and drug-resistant parasites | 69 |
| Giardia lamblia | Trophozoites, cysts | Parasite culture | No. of parasites | 78 |
| Colorimetric | Color change due to parasite growth | 76 | ||
| Imaging | Parasite viability | 78 | ||
| Bioluminescence | Luminescence signal | 80 | ||
| Entamoeba histolytica | Trophozoites, cysts | Bioluminescence | ATP-dependent luciferase bioluminescence signal | 86 |
| Cryptosporidium | Oocysts, gametocytes | Microscopy, qRT-PCR | Parasite invasion and growth | 100, 102–105 |
| Schistosoma | Eggs, miracidiae, cercariae, schistosomulae, adult worms | Real-time parasite mobility | Worm viability and motility | 100, 101 |
| Isothermal microcalorimetry | Motility | 102 | ||
| Image-based high-content screening | Enzymatic and metabolic activity | 103 | ||
| Fluorescence | Viability and cytotoxicity | 104, 105 | ||
| Luminescence | Viability | 106 | ||
| Filaria | Microfilariae, L3 larvae, adult worms | Microscopy | Motility | 132–135 |
| Colorimetry | Viability | 154, 155 | ||
| Worm fecundity | Microfilaria release | 134, 138 | ||
| Embryogenesis | No. of embryos and stage | 134, 153, 157 | ||
| Trypan blue exclusion | Viability | 151, 152 | ||
| Third larval stage (L3) to fourth (L4) | Molting | 137, 141 | ||
| Histology and electron microscopy | Anatomical changes | 142, 143 | ||
| WormAssay (software) | Motility scoring | 156 |
qRT-PCR, quantitative reverse transcription-PCR; ELISA, enzyme-linked immunosorbent assay; PCR-RFLP, PCR-restriction fragment length polymorphism.
In this vein, nascent groups such as the Worldwide Antimalarial Resistance Network (http://www.wwarn.org/) have made a similar attempt to provide standardization of testing methods for malaria, quality control of reagents used, interpretation of data (graphing 50% inhibitory concentration [IC50] data), and epidemiologically relevant updates on molecular markers of resistance emerging throughout the endemic world (1). From a microbiological and clinical perspective, the science and medicine of antimalarial susceptibility testing lag far behind those for bacteria, fungi, and viruses. This review attempts to catalog the current state of affairs of testing methods, clinical validation studies, and molecular data available for susceptibility testing of parasites. Additionally, this review considers further developments, driven in large part by the stimulus to the new antimalarial clinical trial funding space, that may allow clinical interpretation of laboratory testing to guide patient management.
MALARIA
Malaria is one of the major causes of death and illness by an infectious disease. The World Health Organization (WHO) estimates that 3.2 billion people are at risk of acquiring malaria, with 214 million cases occurring worldwide, resulting in 438,000 deaths. Ninety percent of the deaths occurred in the WHO African region. Plasmodium falciparum and Plasmodium vivax are the main etiological agents. One area of concern is the rise in the incidence of artemisinin-resistant P. falciparum infection in Southeast Asian countries, with the consequent risk of further spread to sub-Saharan Africa (2).
Plasmodium falciparum Susceptibility Testing Assays
Trager and Jensen were the first to culture the intraerythrocytic stage of P. falciparum in human erythrocytes supplemented with human serum and other nutrients essential for parasite survival (3). The advent of this culture method permitted the laboratory investigation of this stage of the parasite life cycle. Soon after, in 1979, a susceptibility testing method was developed by Desjardins et al. using radioactive hypoxanthine incorporation into nucleic acids as the assay readout in the presence or absence of drugs (4). Others then developed a method using ethanolamine incorporation into membrane lipids (5). With both approaches, the presence of radioactivity was directly related to parasitemia, and thus, microscopy-based counting of Giemsa-stained parasites was not required. This enabled testing to be done in a microwell format with serial dilutions of the drug. Typically, parasites are exposed for a full 48-h life cycle in standard assays using unsynchronized cultures. More recently, enzyme-linked immunosorbent assays (ELISAs) relying on antibodies to detect malaria antigens have been used. These antigens include histidine-rich protein 2 (HRP2) (6) and lactate dehydrogenase (LDH) (7). Both proteins are expressed proportionally to the amount of viable parasites left after drug treatment (Fig. 1). Unlike LDH, HRP2 is secreted by the parasite and very stable. A fortunate consequence of intraerythrocytic survival is that the majority of DNA present in infected red blood cells is parasite derived. Thus, others developed an assay relying on a nucleic acid stain (SYBR green) to determine parasite levels (8, 9). This stain can be applied to flow cytometry-based susceptibility tests that monitor the drug effect by gating the red blood cell fraction after incubation with the drug. Taken together, these assays enable researchers to establish the IC50 or IC90, at which one-half or 90% of the parasites are killed by the drug being tested, respectively. However, Wein et al. showed that these tests are not all equal and in fact may be influenced by the mechanism of action of the drug (10). For example, when pyrimethamine was tested by the LDH and SYBR green methods, the parasites appeared resistant to the drug, whereas by radiolabeled hypoxanthine release and HRP2 methods, the parasites were sensitive, with IC50s below 10 nM. It has been proposed that hemoglobin may affect fluorescence-based assays relying on SYBR green (11). These experiments were conducted with exposure to the parasites to one full life cycle of 48 h. When the investigators repeated the assays with 72 h of exposure, all test methods displayed similar results (susceptible at IC50s of less than 10 nM). The question arises as to whether DNA replication, which is a function of the metabolic activity of the parasite, needs more than 48 h to reflect true parasite survival after drug exposure. The mechanism of action of the compound may also be stage specific, such as in the ring stage (e.g., artemisinins [12, 13]) or in the mature stages (e.g., mefloquine [10]), and thus, a 48- or 72-h exposure may mask the effects of the drug.
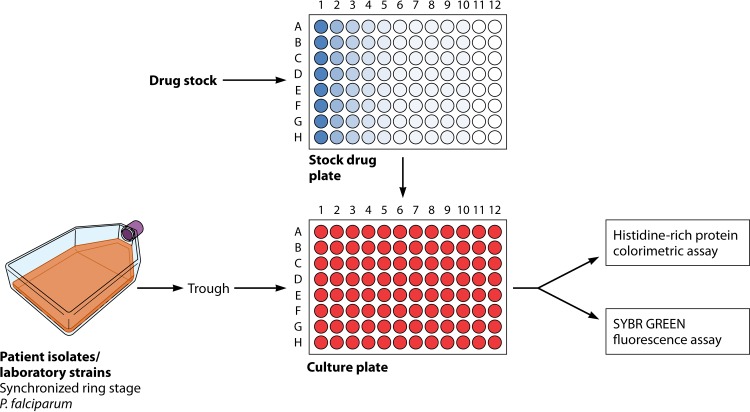
FIG 1.
Typical in vitro susceptibility testing workflow for Plasmodium falciparum. Synchronization of ring-stage intraerythrocytic parasites is required, followed by the addition of drugs to a microtiter plate. There are assays for culture-adapted laboratory strains as well as direct testing from patient blood. Parasitemia can be assessed in several ways, including by an ELISA or fluorescence via flow cytometry. There are several modifications of this basic format, including the RSA.
In the context of antimicrobial susceptibility for bacteria or viruses, there are no obvious analogies to find. In general, testing methods for viruses or bacteria may change by antimicrobial, not because of the mechanism of action per se but because drugs may interact with certain media or incubation conditions specific to the organism. Typically, bacterial testing is done by broth microdilution or by use of strips impregnated with an antibiotic. Bacterial proliferation is usually exponential, and thus, retardation of growth is easy to identify either on agar plates or in broth by the use of turbidity as a readout. Certain antibiotics are cidal, causing permanent effects on viability, whereas others may be static, implying that if the drug is removed, the microbe may recover. On the other hand, malaria may be similar in that dormant but not dead forms of the parasite have been noted by several investigators upon drug exposure, most notably with the artemisinins. Dormancy may in fact be analogous to the static effect of certain antibiotics on clinically relevant bacteria.
The discordance between in vitro testing results (IC50) and clinical treatment outcomes confounded researchers and prompted clinicians to use an in vivo surrogate. Here parasitemia is monitored on an hourly and then a daily basis to determine if there is a delay in parasite clearance. This led to the development of drug-specific assays such as the “ring-stage survival assay” (RSA) for artemisinin (14). The RSA is the first assay to correlate strongly with antimalarial clinical treatment outcomes when conducted on isolates derived from patients who failed artemisinin therapy (14, 15). An interesting feature of the RSA is that the parasites have to be very tightly synchronous in the phase of growth, and drug exposure is focused on one part of the life cycle stages (very early rings).
The significance of this is that researchers applied the drug at the stage of the life cycle where the drug is purported to act (14). This raises the question of whether all susceptibility testing should be stage specific in such a way. Unfortunately, the mechanism of action and stage specificity of all antimalarials are not known. Furthermore, genetic mutations were identified in a protein (kelch 13), which presaged the resistance phenotype in both cultured laboratory strains and patient isolates (16). While there are no clinical breakpoints, clinicians began to use in vivo data such as day 3-positive parasitemia or delayed parasite clearance in patients as an indicator of resistance (16, 17). The assay readout is therefore relative to parasites not exposed to the drug. Researchers have suggested that parasite survival of >1%, when exposed to 700 nM dihydroartemisinin for 6 h at the ring stage, correlates with in vivo treatment failure (14). The association is arbitrary but seems to hold true, especially in Cambodia, where artemisinin resistance is on the rise. One cannot rule out that the RSA is a measure of dormancy of the ring stage in response to artemisinins and thus a propensity for clinical treatment failure in patients who cannot clear the dormant stages.
A further confounding issue is the testing of parasites either after in vitro culture adaptation or directly from the patient (so-called ex vivo testing). The former approach requires a long-term culture system to be set up and for the isolate to adapt to the artificial system. The risk here is that culture may select for a certain genotype when an infection is often polygenic (so-called multiplicity of infection), especially in regions of hyperendemicity such as Africa (18). Selection may occur for the fitter clone or genotype, which may not reflect the situation in the patient. Direct testing of patient blood via the ex vivo method may also be problematic in the situation where there are multiple clones. In general, ex vivo testing is more difficult to conduct in resource-limited settings and is time sensitive.
Transmission-Blocking Drug Tests
An important stage in the life cycle of malaria in humans is the production of gametocytes, which are taken up in a blood meal by anopheline mosquitoes. Therefore, drugs that are able to kill the gametocyte stage may prevent transmission. Culturing of gametocytes at the exclusion of asexual stages requires special culture conditions. In 1986, Ponnudurai et al. were able to demonstrate that culture of gametocytes alone was possible (19). The addition of N-acetylglucosamine to the culture medium likely blocks the entry of merozoites into uninfected red blood cells, which ultimately depletes the asexual stages that require this invasion step. Other methods, such as the addition of heparin and depletion of nutrients in parasite-oversaturated medium, can result in gametocyte differentiation. Gametocytogenesis, whose triggers are poorly understood, occurs at a low rate of 0.2 to 1% from sexually committed merozoites (20, 21). Mature gametocytes form over a period of 12 to 14 days and have five distinct stages (stages I to V), with the latter two stages being insensitive to many current antimalarials (22, 23). Mature gametocytes are relatively quiescent from a metabolic perspective and remain so until gamete formation is triggered upon mosquito feeding and the subsequent change of the milieu. It is the most mature gametocyte stage (the so-called stage V gametocyte) that is responsible for transmission once taken up in a mosquito blood meal.
Therefore, researchers have developed assays to factor in transmission-blocking properties in drug discovery programs. A sex bias ratio has been cited in the literature, whereby more female than male gametocytes (female-over-male gametocyte ratio of up to 4:1) are produced, and concerns that drugs may affect each sex differentially have been raised, although it is worth noting that both male and female gametocytes are required in a mosquito blood meal for propagation (22). This emphasized the need for assays with sex-specific readouts to account for differences in both drug sensitivity and thresholds of detection. The process by which male gametocytes emerge from the red blood cell is called exflagellation. Certain groups have used the percentage or rate of exflagellation as a measure of drug activity, whereas others may use metabolic readouts or reporter-driven transgenic parasites. The relative clinical merits of these assays are not well established, specifically whether they translate into better patient outcomes. The theoretical benefit of preventing gametocytogenesis is clear from a transmission-blocking perspective and, at the population level, may help promote disease control and eradication. Ultimately, the value of transmission-blocking activity will have to be assessed from clinical studies and correlated back to these in vitro assays to determine if there is an association between gametocytocidal concentrations in vitro, drug exposure at the patient level (or cohort of patients), and benefit at the population level.
Antihypnozoite Drug Tests
In addition to blocking transmission, another major pursuit in the drug development arena has been to target the liver stages for the use of new antimalarials for both prophylaxis and treatment. Currently, atovaquone and primaquine (and related 8-aminoquinolines) are the only approved drugs that retain activity against the liver stage of malaria. The 8-aminoquinolines but not atovaquone are able to kill the hypnozoite stage of P. vivax and Plasmodium ovale. The approved drug here is primaquine, which requires 14 days of treatment and carries a risk of hemolysis in patients with severe glucose-6-phosphate dehydrogenase (G6PD) deficiency (24, 25). Other drugs in this class in clinical development include tafenoquine, which is completing phase III studies and kills hypnozoites effectively after a single dose, but there are concerns about G6PD deficiency, and an accompanying diagnostic test may be required to rule out this potential toxicity (26). More drugs with activity against liver-stage parasites through new modes of action and lacking G6PD liability are needed. Recently, this gap in the portfolio of new antimalarials encouraged the malaria community to develop assays to measure drug activity at this specific stage of the life cycle.
Significant progress has been made in developing stable in vitro human hepatocyte assays using specific spatial control in three-dimensional arrays or with the specifically selected HC04 cell line (27–29). Certain groups have used the P. berghei liver-stage assay in murine models to good effect (30). Others took advantage of the fact that the primate malaria agent Plasmodium cynomolgi forms liver-stage hypnozoites, thus potentially making it a useful surrogate for P. vivax or P. ovale. However, early attempts relied on in vivo inoculation of rhesus macaques (31). An in vitro liver-stage model has remained elusive because primary hepatocytes dedifferentiate after 1 week of culture, making them refractory to hypnozoite formation, and also, infection of hepatocytes by sporozoites results in host cell demise. Recent laboratory studies were able to show the persistence of hypnozoite stages of P. cynomolgi in Macaca fascicularis hepatocytes up to 40 days under modified growth conditions (32, 33). Mikolajczak and colleagues successfully used a humanized mouse model to accommodate P. vivax infection, which shows promise (34). Persistence beyond 8 to 15 days in hepatocytes is essential for the study of the hypnozoite stage, as it forms only after this point. From a translational perspective, there are no direct diagnostic tests for the identification of latent malaria in the liver stage to enroll patients in a radical-cure trial. Detection of P. vivax relapse, as opposed to reinfection, is also difficult to assess in areas of endemicity, where both phenomena may occur.
KINETOPLASTIDS
Leishmania
Leishmania is a digenetic parasite that alternates between a flagellated promastigote form in the gastrointestinal tract of female phlebotomine sandflies and an intracellular amastigote form in mammalian host macrophages. Macrophages engulf promastigotes into the phagosome, where differentiation to the amastigote form occurs. Fibroblasts and dendritic cells can also serve as host cells for amastigotes in an infected patient. Disease manifestations of leishmania infection vary by species type and the geographic location where these species reside. Immune responses by the host may also affect clinical manifestations, which range from cutaneous disease to mucosal and even visceral (organ) infection.
Antimonial drugs and miltefosine treatment failure were reported for both visceral and cutaneous leishmaniases (35–38). Assessment of susceptibilities of different clinical strains of Leishmania to drugs has been limited by a lack of consensus on testing methodology, especially as it relates to the different life cycle stages. The intracellular amastigote stage is the target of treatment for leishmaniasis. Drug susceptibility testing against amastigotes is considered the “gold standard” for antimonial drugs (Fig. 2). Susceptibility testing against promastigotes shows results different from those for amastigotes (39). However, several factors influence the testing of clinical strains against amastigotes, making it a low-throughput assay (40). Traditional Leishmania drug susceptibility testing relies on the determination of the 50% effective dose (ED50) using either mouse peritoneal macrophages or other macrophage cell lines. These tests are conducted under diverse conditions with different concentrations of drugs, drug exposure times, and readout specifications. Since these variables affect the final results (41), drug susceptibility assay outcomes have not always been uniform. Fernández et al. (40) found that the determination of the reduction of the parasite load at a single predefined drug concentration at 34°C provided a better method for susceptibility assessment. However, this measurement depended on microscopic reading. Combining this approach with the detection of parasite RNA may provide a method to be routinely used to monitor clinical strains of Leishmania (40).
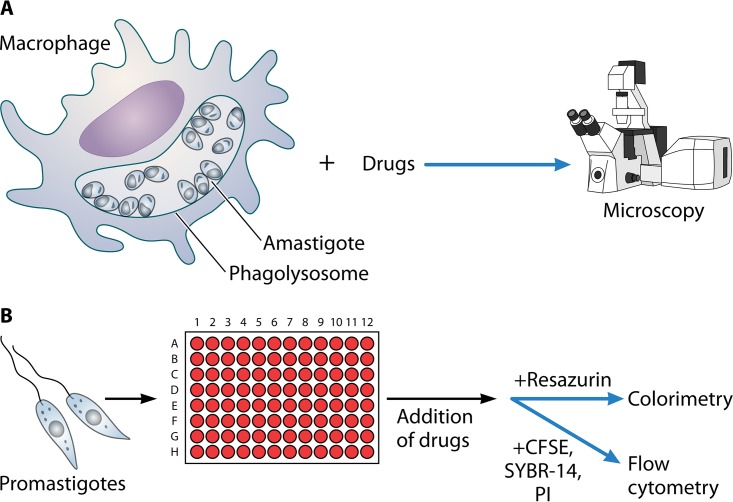
FIG 2.
Leishmania susceptibility testing can be performed by using either amastigotes within macrophages (A) or promastigotes (B). Endpoints can include microscopy or measurement of promastigote survival using colorimetric or fluorescent readouts.
A simplified colorimetric resazurin assay was developed for measuring Leishmania donovani susceptibility to miltefosine using promastigotes. Miltefosine susceptibility for clinical isolates correlated strongly by using the assay with intracellular amastigotes and the resazurin-based assay for promastigotes (39). However, further study is necessary to examine the usefulness of this assay as a surveillance tool for susceptibility testing with other drugs in clinical isolates.
Flow cytometry has been used to assess viability and cellular changes in Leishmania and is a promising tool for examining the sensitivity and resistance of Leishmania promastigotes to different drugs. The effect of the drug on the proliferation of promastigotes at different time intervals was determined by using 5 (and 6)-carboxyfluorescein diacetate succinimidyl ester (CFSE). The combination of SYBR-14 and propidium iodide (PI) stains was an efficient way to concurrently visualize both live and dead promastigotes of Leishmania. This staining method has the advantage of being rapid, without requiring extra processing before staining. Since promastigotes are easy to cultivate in vitro, this assay was performed with promastigotes to measure the activity of drugs on this parasitic form (42). Future research should be directed toward understanding the applicability of similar techniques in clinical isolates and the amastigote stage of the parasite and increasing the throughput of testing modalities to screen a large number of compounds.
Trypanosoma
Chagas disease.
Chagas disease, caused by an intracellular protozoan parasite, Trypanosoma cruzi, is the leading form of infectious heart disease in Central and South America. There are distinct stages of the clinical forms of Chagas disease (43). The acute phase remains asymptomatic in several cases, but this can be severe, typically characterized by high parasitemia fever and lymphadenopathy, causing death in up to 5% of diagnosed cases. Chronic infections result cardiomyopathy in 20 to 30% of cases and digestive megasyndromes in about 10% of cases (44, 45). Treatment is indicated for patients in the acute phase and in the early chronic phase, immunosuppressed patients with reactivation of infection, and children with congenital infection. Treatment of patients in the acute phase and in the indeterminate or asymptomatic period relies on nifurtimox and benznidazole. Variation in drug susceptibilities of T. cruzi was observed in a study by Filardi and Brener (46), and about 30% of the parasite populations led to infections that were resistant to both or either drug. A simple drug susceptibility assay can provide a routine, reproducible surveillance tool for measuring the worldwide development of drug resistance. Drug susceptibility testing in T. cruzi is particularly challenging because most Chagas disease patients are diagnosed only in the chronic stage or when tests are performed during blood donation or surgery (47). Drug susceptibility of T. cruzi has been evaluated in animal models of acute and chronic Chagas disease. Information on the effect of drugs on the dissemination of parasites in specific tissues after infection required the sacrifice of animals and enumeration of the parasite burden in organs and whole animals. Techniques such as PCR amplification or in situ hybridization of parasite-specific genes (48–51), parasite antigen-specific immunofluorescence (52–54), and quantification of amastigotes or trypomastigotes present in tissue sections or blood (55–58) have been cumbersome and inconsistent and suffered from significant limitations. There are no definitive cure criteria available for Chagas disease, and in the absence of a predictive treatment model, several diagnostic methods are frequently used, including fresh blood cultures and examination, parasite-specific gene PCR from blood and tissue DNA, and serological assays. Tissue PCR is arguably the most sensitive method and can identify several noncured mice (59). The inconsistency of PCR-based methodologies was due to the lack of knowledge of the locations of parasite persistence. This makes real-time assessment of the parasite burden difficult (47). In a recent clinical trial study with a new drug, posaconazole, identification of T. cruzi DNA was used in a PCR assay. Instead of using it as a marker of cure, those researchers used it to measure treatment failure. A negative PCR result, therefore, may indicate the absence of circulating DNA in fresh blood drawn for testing (60). A new, more sensitive, bioluminescence-based mouse model has been developed to monitor the progress of T. cruzi infection and to interrogate the activities of different drugs. This imaging method allowed the quantification of the parasite burden in specific tissue and minimized bias in tissue sampling (61) (Fig. 3). This shows the limitation of tissue PCR, especially the misclassification of treatment failures as cures, because of the localized distribution of parasites. An immunosuppressive drug, cyclophosphamide, has been used to increase the sensitivity of detection of parasites after drug treatment (62–64). Integrating bioluminescence imaging and posttreatment immunosuppression methods may provide a maximally sensitive way to estimate the true dynamics of T. cruzi reactivation and cure (65). Whether these methods can be utilized in human clinical specimens remains to be seen.
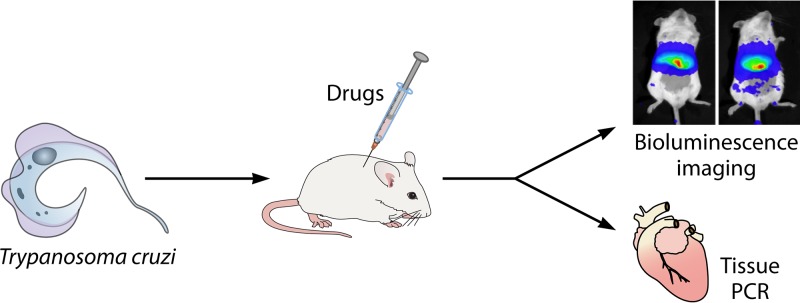
FIG 3.
Drug susceptibility in T. cruzi evaluated in animal models of acute and chronic Chagas disease. The mouse model for Trypanosoma cruzi depicted here is used for testing the preclinical efficacy of candidate drugs, followed by evaluation of tissue by molecular or imaging methods.
Human African trypanosomiasis.
“Sleeping sickness,” or human African trypanosomiasis (HAT), causes morbidity and mortality in sub-Saharan Africa. Two protozoan parasites of the genus Trypanosoma, Trypanosoma brucei gambiense and Trypanosoma brucei rhodesiense, are responsible for HAT, and both parasites are transmitted by tsetse flies. According to the World Health Organization, in 2014, 3,796 actual cases were reported, with <15,000 estimated cases of HAT. The first stage of the disease is treated with pentamidine, whereas the second neurological stage can be treated with melarsoprol, eflornithine, or nifurtimox-eflornithine combination therapy (NECT). A promising compound in the pipeline at the Drugs for Neglected Diseases Initiative (DNDi) (Geneva, Switzerland) is fexinidazole. Fexinidazole is an oral agent used primarily for stage 2 of HAT but potentially stage 1 in children.
There has been an increasing incidence of melarsoprol treatment failure because of drug resistance. For example, 20 to 30% treatment failure rates were reported in Uganda, the Democratic Republic of Congo, and the Sudan (66). This rise in drug resistance underscores the need for the development of drug susceptibility testing against HAT. Fortunately, in 2009, the WHO Essential Medicines List (EML) added NECT to treat the neurological stage of HAT caused by T. brucei gambiense. NECT is efficacious, relatively safe, and easy to administer (67).
In a previous study to analyze the melarsoprol treatment failure rate among late-stage HAT patients in Aura, northern Uganda, a glandular puncture and a lumbar puncture were performed, and the number of trypanosomes was counted in cerebrospinal fluid (CSF) or lymph node fluid (68). The invasive nature and some false-positive diagnoses associated with testing emphasize the need for the development of new techniques accessible for routine use.
To determine the susceptibility of T. brucei gambiense in northwestern Uganda to drugs, PCR and SfaNI restriction digestion of the adenosine transporter gene were employed (69). It should be mentioned that out of 11 mutations described by Mäser et al. (70), SfaNI restriction fragment length polymorphism (RFLP) analysis examined only 1. It is also possible that other factors, such as ABC transporters, may play a role in reduced drug susceptibility in African trypanosomes (71). Moreover, T. brucei gambiense parasites isolated from patients adapt poorly to culture conditions, and the parasites underwent infection in rodent and adaptation in culture medium before they were ready for propagation. It is unclear if the adapted parasites retain characteristics similar to those of parasites originally isolated from patients and if rodent infection and culture conditions imposed selection pressures on the parasites before susceptibility to drugs could be ascertained (69).
Recently, genome-scale RNA interference (RNAi) screening linked two closely related aquaglyceroporins, AQP2 and AQP3, to melarsoprol-pentamidine cross-resistance (MPXR) (72), suggesting that aquaglyceroporins have an important role in influencing susceptibility to these drugs. In a follow-up paper, Baker et al. (73) showed that the loss of function of one of these proteins, AQP2, renders MPXR in a laboratory-selected MPXR strain. It will be important to understand the status of AQP2 in drug-resistant clinical isolates and to check if there is a relation between the clinical outcome and the status of AQP2 (73, 74).
STOOL PROTOZOA
Diarrheal infections are considered one of the top four contributors to the global burden of disease. Intestinal parasites are leading causes of morbidity and mortality associated with diarrheal diseases in both the developed and developing worlds. Infections by two anaerobic protozoan parasites, Entamoeba histolytica and Giardia lamblia, result in more than 300 million cases annually. In the absence of vaccines or prophylactic drugs, treatment of amebiasis and giardiasis depends on nitroimidazoles, with metronidazole being the most commonly used drug worldwide. Despite the efficacy of nitroimidazole drugs, treatment failures in giardiasis happen in up to 20% of cases (75). The clinical resistance of G. lamblia to metronidazole is evident, and cross-resistance to commonly used antigiardial drugs is a major concern (76). The potential resistance of E. histolytica to metronidazole is also an increasing concern, as in vitro, E. histolytica trophozoites adapt to therapeutically relevant levels of metronidazole (77). Global surveillance of drug resistance in these organisms requires a simple, reproducible drug susceptibility testing method for these anaerobic protozoa.
Giardia
Traditionally, susceptibility testing in Giardia has been performed with closed tubes, which requires more compounds and depends on tedious and labor-intensive manual counting of parasites, with few replicates available. The development of an assay in multiwell microtiter plates has been hindered due to the sensitivity of Giardia to oxygen. Several methods have been developed to form a low-oxygen intestinal atmosphere. These methods include a 24-h assay using 2-ml cultures in 24-well plates in a CO2 atmosphere; 96-well plates incubated at 35°C for 48 h in the presence of 3% O2, 4% CO2, and 93% N2; 96-well plates flushed with nitrogen; 96-well airtight plates incubated for 72 h; and 384-well plates under 5% CO2 in air for 24 h. However, all these methods resulted in unequal growth of parasites in different wells of the microtiter plates. To overcome these challenges, several changes of culture conditions were made by Gut et al. (78) and led to better growth of G. lamblia. Those researchers removed nonadherent and dead G. lamblia parasites from cultures and limited the presence of O2 to less than 1% in special incubation bags or 3% O2–5% CO2 without the use of incubation bags.
Giardia drug susceptibility testing was performed by microscopy, which included parasite counting and evaluation of motility, morphology, or adherence. Radioactive incorporation assays have also been used, but these assays require associated equipment and radioactive waste disposal. The accessibility and higher cost of radioactive compounds make these assays less user friendly. Both fluorescence-based assays using SYBR green and colorimetric tests with formazan dyes {e.g., MTT [3-(4,5 dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] assays} and synthetic substrates of purine salvage pathway enzymes (79) were used, but the assays required an ELISA reader and multiple washes, which affected signal intensity and reproducibility.
Recently, Gut et al. (78) developed an image-based high-throughput screen using 4′,6-diamidino-2-phenylindole (DAPI) nuclear stain and successfully identified 12 compounds that inhibited the growth of G. lamblia at low-micromolar or submicromolar concentrations. The disadvantages of this assay included the requirement for expensive automated cell imaging equipment and the storage of large electronic files.
A bioluminescence-based high-throughput screen was also developed to identify compounds that kill G. lamblia trophozoites (80). This assay monitors the viability of the trophozoites based on the ATP content of G. lamblia trophozoites.
The metabolic activity of trophozoites is inhibited due to the effect of a compound, and the corresponding ATP-dependent luciferase bioluminescence signal is also diminished (Fig. 4). These organisms may remain viable and resume growth after the completion of treatment. Moreover, the morphological changes of trophozoites due to treatment with compounds cannot be detected by this method. Therefore, a follow-up microscopic study and proliferation assay would be a reliable procedure for examining the effect of compounds on G. lamblia trophozoite viability. A target-based screen was developed by using Giardia carbamate kinase, and this luminescence-based enzyme assay can be a useful tool for drug screening (81). Recently, a microfluidic device was developed to culture G. lamblia, and two currently used drugs were tested with an on-chip drug susceptibility assay using an imaging method (82). This assay holds the possibility of using the microfluidic platform for future high-throughput drug susceptibility testing.
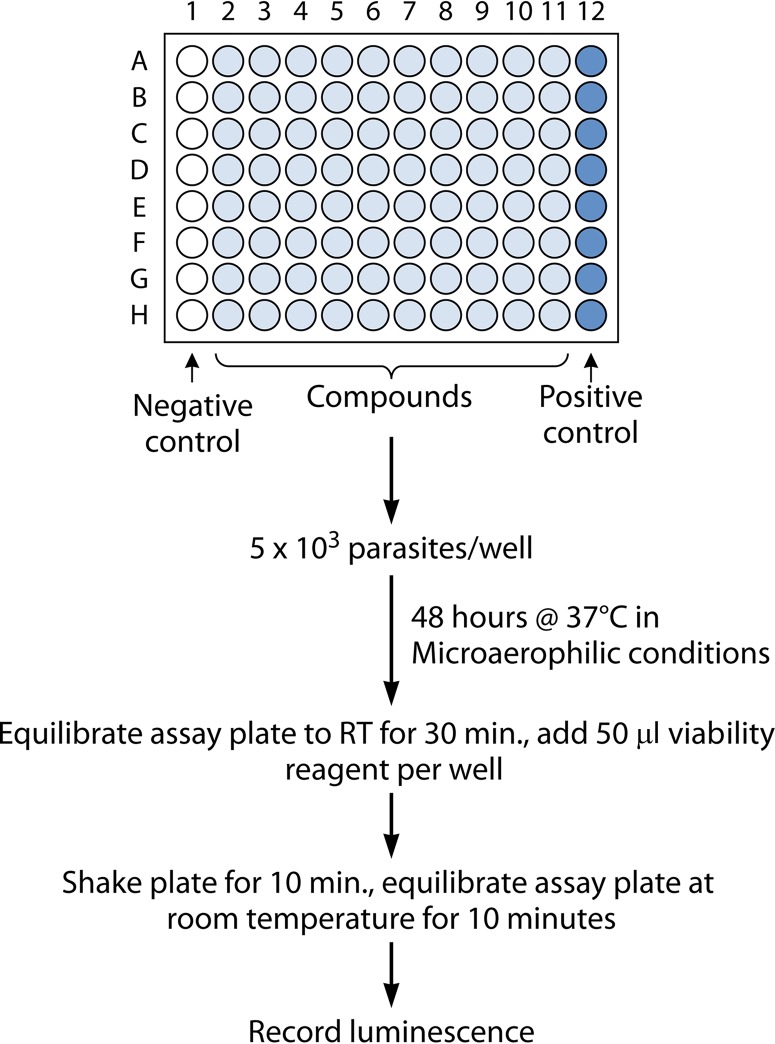
FIG 4.
In vitro susceptibility testing workflow using a luminescence assay of cultured Giardia or Entamoeba parasites under microaerophilic conditions in a 96-well-microtiter-plate format. RT, room temperature.
Entamoeba histolytica
Drug susceptibility testing in E. histolytica has been challenged by the absence of competent screening methods, with available assays being exhaustive, depending mostly on microscopy (83), radioisotopes (84), and a complex staining method (85). Like Giardia, E. histolytica is an anaerobe and cannot survive in an oxygen-rich environment present in the wells of microtiter plates, and this posed a challenge for developing a testing method with microtiter plates, and above all, there was no rapid readout assay available. Debnath et al. (86) developed a microtiter plate-based screen that could be performed under anaerobic conditions, imitating the ameba's natural growth environment. This robotic-driven assay used the CellTiter-Glo luminescent cell viability assay (Promega) technology that measures ATP bioluminescence and represents a speedy, sensitive, and labor-saving assay to identify potent compounds against E. histolytica (86) (Fig. 4). The assay was developed by using an inoculum of E. histolytica in the logarithmic phase of growth, which led to confluence but not excessive growth. This high-throughput screen is performed with 96-well and 384-well microtiter plates with 50,000 parasites/ml and 30,000 parasites/ml, respectively (87). Similarly to Giardia, the effect of compounds on trophozoite morphology cannot be determined by this assay. Combining a microscopic study with this method would provide a better testing method.
Cryptosporidium
Cryptosporidium species are sporozoans that infect small intestinal epithelial cells, within which they replicate asexually for several rounds prior to differentiation into male and female gametocytes, fertilization, and the production of oocysts. The oocysts sporulate rapidly, thus becoming infectious and enabling both spread into the environment and autoinfection characterized by additional rounds of asexual replication within the same host. Cryptosporidiosis was discovered in the 1970s but first came to attention as a major human pathogen in immunocompromised patients, especially those with AIDS, in whom it is a prominent cause of chronic diarrhea (88, 89). However, young children suffer the preponderance of morbidity due to cryptosporidiosis. The recently reported Global Enterics Multicenter Study (GEMS), a large molecular epidemiological study of life-threatening childhood diarrhea conducted at sites in Africa and the Indian subcontinent, highlighted the importance of Cryptosporidium, which ranked second to rotavirus as an etiological agent among children <1 year of age (90). In the MAL-ED study, which investigated causes of less severe diarrhea, cryptosporidium ranked lower on the list of causative agents (91). Nitazoxanide, which is effective in immunocompetent adults, is the only drug known to have any efficacy. Unfortunately, nitazoxanide is not effective in immunocompromised people and is unreliable for the treatment of malnourished infants with cryptosporidiosis (92–94).
In the absence of reliably efficacious drugs for the treatment of human cryptosporidiosis, correlations of the results of in vitro drug susceptibility testing and clinical efficacy are not possible. Furthermore, it is not yet clear if Cryptosporidium spp. will become resistant to drugs under drug pressure, as drugs have not been used in large-enough groups of humans or livestock, and standardized methods to evaluate drug sensitivity have yet to be developed. Nevertheless, a number of investigators working on drug development have optimized methods to measure in vitro drug susceptibility. All of the approaches to date rely upon inducing oocyst excystation by simulating entry into the small intestine (95–98). The freed sporozoites then invade mammalian tissue culture cells, within which the asexual forms of the parasite replicate for several rounds. The efficiency of host cell infection is highly dependent on the cell type used, with the human colonic carcinoma cell line HCT-8 being among the most efficient (98, 99). This method does not enable continual parasite culture, but replication and expansion of the asexual forms can be quantified by using a number of methods. Cai et al. (100) developed a quantitative real-time reverse transcription-PCR method to quantify parasite mRNA levels, using the abundance of host mRNA for normalization. This method takes advantage of the short half-life of mRNA following parasite death and thus provides a reliable means of measuring parasite elimination. However, this method is limited by its relatively low throughput and high cost. By using commercially available reagents, this assay was made amenable to high-throughput screening (101). Gut and Nelson first noted that Vicia villosa lectin specifically labels Cryptosporidium parasites, providing a simple method for microscopy-based assays (102). Bessoff et al. (103, 104) subsequently used this approach to develop a high-content imaging assay that has proven useful to screen small-molecule libraries. Recently, Vinayak et al. (105) succeeded at modifying the genome of Cryptosporidium parvum using CRISPR-Cas9 technology, which lays the groundwork for simpler screening assays using parasite lines modified to express reporter genes (105). Several groups are using luciferase-tagged parasites to measure the expansion of C. parvum. Another method of note is the use of multiplexed recombinase polymerase amplification (RPA) to detect Cryptosporidium, which may have useful applications for susceptibility testing (106). Each of these systems provides an in vitro method to assess the effects of drugs and drug-like compounds on Cryptosporidium invasion of and growth within host cells. At present, however, there are no methods to assess drug effects on the sexual forms of Cryptosporidium parasites and on Cryptosporidium transmission. Developing such methods is an active area of investigation.
SCHISTOSOMA
Schistosomiasis is an important neglected tropical disease and causes 3.3 million disability-adjusted life years (DALYs) (107). For over 30 years, praziquantel has been the only drug available to treat and control this disease. There are several advantages of praziquantel, such as good safety, tolerability, efficacy, and low cost, although the latter may no longer be the case in the United States. However, its limitations include its inability to protect individuals from reinfection and its inactivity against the schistosomula, preadult, and juvenile-adult stages of the worm (108, 109). Moreover, the emergence of praziquantel-resistant strains of Schistosoma is looming, since praziquantel is estimated to cover 235 million people by 2018 (110, 111). There are variations among individual Schistosoma mansoni isolates in susceptibility to praziquantel in mice. Protocols have been developed to quantify the praziquantel ED50s of S. mansoni isolates to monitor the susceptibility of parasite isolates obtained from areas of endemicity to drugs. This will help to determine if variation in susceptibility to praziquantel in S. mansoni is a natural phenomenon or due to the effect of mass treatment (112). It has been noted that mass drug administration can reduce the efficacy of drugs like praziquantel (113).
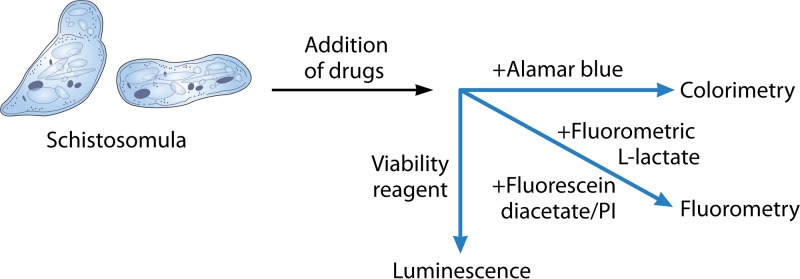
Previously, drug susceptibility testing was performed with adult worms after incubating them with different drugs for 72 h, and parasite viability was monitored by using a microscope (114, 115). Viability testing and determination of worm motility by microscopic readout are slow, laborious, and subjective. Recently, a range of automated technologies has emerged, and different readouts are available. These methods include the impedance-based real-time measurement of parasite mobility (116, 117), isothermal microcalorimetry (118), image-based high-content screening (119), and fluorescence-based (120, 121) and luminescence-based (122) assays (Fig. 5).
FIG 5.
Schistosoma in vitro testing relies on the use of culture-adapted schistosomula-stage parasites followed by the addition of drugs. Readouts include luminescence, colorimetry, and fluorometry.
Dye-based assays have several advantages, including being cheap, simple, and able to be read by an automated plate reader (123). The alamarBlue viability assay (resazurin), the fluorescein diacetate-propidium iodide bioassay, and a fluorometric l-lactate assay have been used as fluorescence assays. alamarBlue was able to discriminate only live and dead newly transformed schistosomulae (NTS) after a week of incubation with reference drugs but not at earlier time points, and this assay could not measure the dose-response of drugs (124). The fluorescein diacetate-propidium iodide fluorescence-based bioassay can detect viability and differentiate live and dead S. mansoni adults and NTS. With this method, fluorescein diacetate stains live NTS, and propidium iodide stains dead NTS (120). Practical issues, such as a low signal to discriminate between live and dead NTS, the requirement for a high number of NTS, the involvement of a rinsing step, and the potential to calculate the dose-response for all standard drugs, are major concerns for this assay (123). The fluorescence-based lactate assay was used to measure the viability of NTS and adult S. mansoni worms, and dose-dependent effects could be obtained for some but not all standard drugs (121). The limitation of this assay is that it requires a specific number of worms, and the supernatant needs to be removed to obtain high and consistent signals (123). The search for a simple, cheap, and specific dye without the requirement for additional equipment or analysis is still continuing.
Recently, a luminescence assay using CellTiter-Glo has been conducted on S. mansoni NTS and adult worms (122). This assay required a multidrop sorter to distribute a precise number of worms into each well. Panic et al. (123) tested and compared 11 fluorescence or luminescence viability and cytotoxicity marker assays and dyes to standardize assay conditions and to examine if they correlated with S. mansoni NTS viability. This study demonstrated that it was not easy to develop a simple, inexpensive, “just-add” colorimetric marker-based drug screening assay for the larval stage of S. mansoni. Those authors, however, confirmed that CellTiter-Glo could be used for differentiating live and dead NTS in a test with a single drug concentration and potentially also in dose-response studies (123). An important computational tool developed recently is the quantal dose-response calculator (QDREC), which can calculate the dose-response characteristics for helminths and possibly other parasites (125).
FILARIAE
Filariae are tissue-invasive roundworms transmitted by arthropods. Pathogenic human filariae include Wuchereria bancrofti, Brugia malayi, and Brugia timori, which cause lymphatic filariasis, and Onchocerca volvulus, the cause of river blindness. Other filarial pathogens of humans include Loa loa, the cause of African eyeworm, and several species of Mansonella. Filarial parasites cause substantial morbidity worldwide. Lymphatic filariasis infects over 67 million people and causes hydrocele in 20 million and lymphedema in 17 million people (126). Onchocerca volvulus infects 16 million to 37 million people and is a leading cause of skin disease and blindness (127, 128).
Filariae pass through several life cycle stages in the human host, and susceptibility of one stage to a medication does not guarantee susceptibility of the other stages. For example, ivermectin has potent activity against microfilarial stages but does not readily kill adult filarial worms (129). Thus, in vitro drug testing is often performed on several stages of the worm, including microfilariae, adult worms, and third-larval-stage (L3) larvae.
The infective form of the parasite that is transmitted from the vector to humans is the L3 stage. Once in their host, L3 larvae migrate over a period of a few days to their preferred niche in the human host. These sites include the lymphatic vessels for W. bancrofti and Brugia species, subcutaneous tissues and skin nodules for O. volvulus, subcutaneous tissues for L. loa, and the peritoneal cavity for Mansonella perstans (130). Over a period of several weeks to months, depending on the species of filarial nematode (reviewed in reference 131), L3 larvae molt twice and become mature adult worms, which mate and release microfilariae. Microfilariae are L1-stage larvae, which are acquired by the arthropod vector. Microfilariae of most species circulate in the bloodstream, although those of O. volvulus and Mansonella streptocerca reside in the skin (130). L3 larvae, adult worms, and microfilariae have all been used for in vitro susceptibility testing.
Because the Brugia malayi life cycle can be maintained in small mammals, this species is the one used most often for in vitro drug screening studies (132–143). Other filariae used for in vitro drug testing include Brugia pahangi (153), O. volvulus and other Onchocerca species (141, 144), L. loa (145), Litomosoides sigmodontis and Acanthocheilonema viteae (filarial nematodes of rodents) (146–148), and Setaria species (filarial nematodes of sheep, cattle, and other ruminant mammals) (149–152). Even though it is the most common filarial pathogen of humans, Wuchereria bancrofti is rarely used in screening assays because it cannot be maintained in animal models (147).
Traditionally, the primary endpoint used to determine filarial susceptibility during in vitro testing has been changes in motility as determined by observation by microscopy (132). For most studies evaluating the effects of potential antifilarial agents, microfilariae are observed on a regular basis for a period of 1 to 7 days, and adult filarial worms are observed for a period of days to weeks (132–136, 138, 140–144, 148, 153). While worms can be identified as simply motile or nonmotile (136, 139, 140, 142, 145), in many studies, scoring systems have been applied to characterize the degree of motility observed at different time points (132–135, 138, 143, 144, 148, 151–153).
A complementary approach to determine the viability of microfilariae is MTT-formazan colorimetry (154, 155). In this assay, microfilariae are incubated for 1 to 4 h with 0.5 mg/ml of the tetrazolium salt MTT. Viable filarial worms take up the colorless salt and reduce it to formazan, which is blue. Filariae can then be transferred manually into microtiter wells containing dimethyl sulfoxide (DMSO). Next, usually after 1 h of gentle agitation, absorbance is measured to obtain a semiquantitative measurement of filarial viability (155). This approach has been used successfully to evaluate compounds for activity against adult worms, L3 larvae, and microfilariae (132, 134, 135, 140, 144, 147–149, 151, 153).
When monitoring adult worm cultures, some studies also evaluate effects of potential antifilarial agents on worm fecundity by measuring the number of microfilariae released by adult female worms on a daily basis (134, 138, 146, 148, 153). Adult worms can also be dissected at the study endpoint to enable microscopic assessment of effects on embryogenesis with regard to both numbers of embryos and stages of embryo development (134, 153, 157).
Other tests that are done for drug susceptibility studies include trypan blue exclusion with microfilariae (151, 152), assessment of L3 to L4 molting (137, 141), and histology and electron microscopy to detect anatomical changes (134, 141–143, 147).
A major drawback of most filaria susceptibility assays is that they are highly labor-intensive, greatly limiting their potential use in high-throughput screening systems. To overcome this, Marcellino and colleagues developed a software program (WormAssay) that can be used with a video camera to measure movements of adult filarial worms in 24-well culture plates (156). Visualization and motility scoring can be automated and integrated with a robotic plate manipulation system (156). Recently, this system was used successfully to identify auranofin as having macrofilaricidal activity (141). An adaptation of the WormAssay software has been made, which enables its use for screening microfilaria and L3 stages using microscopy and multiple worms per well (158).
Another limitation of current screening assays is that in vitro culture puts substantial physiological stress on filariae. Microfilariae fail to develop substantially in vitro, and adult worms neither mate nor produce new oocytes in vitro (131). However, it is noteworthy that microfilariae do not develop substantially in the mammalian host either. Indeed, a recent transcriptomics study showed that adult B. malayi female worms exhibit marked changes in gene expression upon transfer into cell culture medium (159). Coculture with insect or mammalian cell lines often improves microfilaria and adult worm survival in vitro but does not overcome these developmental blocks (131).
Thus, the development of in vitro systems that better model the usual environment of filarial worms is an ongoing research goal. To date, the most advanced in vitro system developed is likely that of Kassis and colleagues (160). Using a motorized microscope and a polydimethylsiloxane microchannel lined with dermal lymphatic endothelial cells and human dermal fibroblasts, those researchers developed a highly advanced in vitro imaging platform that can continuously measure adult filarial worm speed, thrashing, and migration patterns (160). While it has not yet been used for drug screening, it has the ability to evaluate subtle changes in worm activity and behavior.
Biographies

Abebe Genetu Bayih completed his Ph.D. and postdoctoral training in Leishmania immunology and malaria drug development at the University of Calgary, Canada. He then returned to take up the post of Head, Department of Medical Parasitology, University of Gondar, Ethiopia, where he currently resides.

Anjan Debnath, Ph.D., is an Assistant Adjunct Professor at the Skaggs School of Pharmacy and Pharmaceutical Sciences at the University of California—San Diego (UCSD) and the Amoebozoa Core Director at the Center for Discovery and Innovation in Parasitic Diseases (http://www.cdipd.org/) at UCSD. Dr. Debnath received his M.Sc. (Zoology) from the University of Calcutta, India, and completed his Ph.D. work in Parasitology at the National Institute of Cholera and Enteric Diseases, Kolkata, India. He subsequently did his postdoctoral research with Dr. James H. McKerrow at the University of California—San Francisco. His research focuses on studying intestinal parasites and free-living amebae. His research interests include drug discovery for parasitic diseases and molecular mechanisms of pathogenesis.

Edward Mitre, M.D., is an Associate Professor in the Department of Microbiology and Immunology at the Uniformed Services University in Bethesda, MD. His laboratory (https://www.usuhs.edu/faculty/mitre) studies the biology of filariae and the immunological mechanisms by which helminths can protect against autoimmune diseases and allergy. Dr. Mitre obtained his medical degree from the Johns Hopkins School of Medicine in 1995 and completed his internal medicine residency at New York University. He then did an infectious diseases fellowship at the National Institutes of Health, followed by postdoctoral research work in helminth immunology as well as clinical training in tropical medicine at the Laboratory of Parasitic Diseases at the NIH. In addition to his laboratory research and teaching responsibilities at the university, Dr. Mitre regularly attends on internal medicine and infectious diseases consultation services at the Walter Reed National Military Medical Center.

Christopher D. Huston, M.D., is an Associate Professor of Medicine and Microbiology and Molecular Genetics at the University of Vermont College of Medicine, where he has been on faculty since 2003. He received an M.D. from Cornell University Medical College and trained in infectious diseases at the University of Virginia. He became interested in drug discovery in 2009 after caring for an immunocompromised patient with cryptosporidiosis for whom existing treatment options were inadequate. He has developed numerous in vitro and in vivo models that are widely used by the Cryptosporidium drug development field and serves in an advisory role on anti-Cryptosporidium drug development for numerous nongovernmental organizations.

Benoît Laleu received a Ph.D. in Chemistry from the University of Geneva in 2006 and then did a postdoctoral stay at the University of Toronto. He worked during 7 years as a medicinal chemist for GenKyoTex, a Swiss biotech company. Since 2015, he has been working for the Medicines for Malaria Venture (MMV) as a Research Scientist in the Drug Discovery Department. He is currently leading the Pathogen Box project at MMV, which is an open-source initiative to catalyze drug discovery for neglected diseases.

Didier Leroy, Ph.D., leads the biology department at MMV as well as drug discovery activities in the context of individual projects, miniportfolios of pharmaceutical companies, and pharmacology platforms. Dr. Leroy, a molecular pharmacologist/biologist, joined the MMV Drug Discovery Team in 2009 from Merck-Serono International, where he was managing a team of 10 collaborators and several projects in the Lead Discovery Department. He has broad experience in drug discovery and disease biology (infectious diseases, cancer, inflammation, and neurological disorders). He has worked in various phases of a project: target characterization, proteomics, lead identification and optimization, molecular/cellular and in vivo pharmacology, druggability evaluation, and cellular biology/biochemistry. Dr Leroy has a Ph.D. in Molecular Biology from the University J. Fourier of Grenoble in France and has more than 50 published scientific papers in various fields from enzymology and structural biology to parasitology.

Benjamin Blasco completed his Ph.D. under the guidance of Prof. Stewart Cole at the Swiss Federal Institute of Technology in Lausanne (EPFL), with virulence regulators of Mycobacterium tuberculosis as a research topic. As part of his postdoctoral training, he designed and developed biological assays to screen for inhibitors of mycobacterial virulence. In September 2014, he joined the MMV Drug Discovery team to support the validation and the deployment of in vitro and in vivo assays to test new compounds. He is also involved in several projects aiming at predicting the risk of resistance for antimalarials in development.

Brice Campo, Ph.D., is a Director of Drug Discovery at MMV. He leads drug discovery activities in the context of individual projects and pharmacology platforms. He joined MMV in November 2011, bringing with him more than 13 years of experience in drug discovery, gained primarily with the Genomic Institute of the Novartis Foundation (GNF) and Addex Pharmaceuticals SA. He has broad experience in molecular pharmacology and drug discovery in several disease areas such as neuroscience, metabolic disease, and inflammation and has successfully led and contributed to teams at all stages of drug discovery. Dr. Campo holds a Ph.D. in Neuroscience and Immunology from the University of Sheffield (UK) and has published a significant number of scientific papers and patent applications.

Timothy N. C. Wells, Ph.D., is the Chief Scientific Officer at the Medicines for Malaria Venture, a role he has held since 2007. He received a Ph.D. in Chemistry from the Imperial College London and an Sc.D. from Cambridge in Biology. He is a fellow of the UK Royal Society of Chemistry and the Academy of Medical Sciences. Previously, he was head of Research for the Swiss biotechnology company Serono. He became interested in drug discovery for neglected diseases after starting a collaboration with the WHO back in 2003.

Paul A. Willis, Ph.D., is a Medicinal Chemist and a Director of Drug Discovery at the Medicines for Malaria Venture. He was previously a team leader and project leader at AstraZeneca, working on cardiovascular, respiratory, and anti-inflammatory drug discovery projects.

Peter Sjö, Ph.D., is Senior Director, Medicinal Chemistry, at AstraZeneca R&D, Gothenburg, Sweden. He received a Ph.D. in Organic Chemistry focused on the design and stereoselective synthesis of novel muscarinic antagonists in 1993 from the University of Oslo, Norway. After completion of his Ph.D. studies, he moved into industrial drug discovery research at Astra Draco in Lund, Sweden, in 1993. His current research interest is focused on the discovery and development of drugs aimed at the treatment of respiratory diseases.

Wesley C. Van Voorhis, M.D., Ph.D., attended MIT as an undergraduate degree and Cornell Medical College and Rockefeller University (RU) for his M.D./Ph.D. At RU, he was the first to discover and characterize human dendritic cells (antigen-presenting accessory cells). His Ph.D. advisor, Dr. Ralph Steinman, was awarded the 2011 Nobel Prize for Medicine for Dr. Steinman's discovery of dendritic cells. Dr. Van Voorhis worked in Brazil, studying leprosy, and resolved to work on problems of global health importance. He trained in Internal Medicine at the University of California—San Francisco, and infectious diseases at the University of Washington (UW). He practices medicine, teaches, and leads the Division of Allergy and Infectious Diseases (>80 faculty and >250 staff) at UW. He is the Director of CERID, the Center for Emerging and Re-Emerging Infectious Diseases at UW. For the past 25 years, he has worked on preclinical drug development for malaria, trypanosomes, leishmania, and cryptosporidium.

Dylan R. Pillai, M.D., Ph.D., is an Associate Professor at the University of Calgary, Medical Microbiologist at Calgary Laboratory Services, and Infectious Diseases physician in the Department of Medicine. He completed his M.D. and Ph.D. at the University of Toronto and postgraduate training at Stanford and the University of California—San Francisco. His research interests focus on describing the clinical epidemiology and understanding the mechanisms underlying antimicrobial resistance in human pathogens such as P. falciparum, S. pneumoniae, and C. difficile. His group conducts translational research with the aim of developing new treatment and diagnostic strategies in resource-limited settings.
REFERENCES
- 1.Woodrow CJ, Dahlström S, Cooksey R, Flegg JA, Le Nagard H, Mentré F, Murillo C, Ménard D, Nosten F, Sriprawat K, Musset L, Quashie NB, Lim P, Fairhurst RM, Nsobya SL, Sinou V, Noedl H, Pradines B, Johnson JD, Guerin PJ, Sibley CH, Le Bras J. 2013. High-throughput analysis of antimalarial susceptibility data by the WorldWide Antimalarial Resistance Network (WWARN) in vitro analysis and reporting tool. Antimicrob Agents Chemother 57:3121–3130. doi: 10.1128/AAC.02350-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. 2015. World malaria report 2015. WHO, Geneva, Switzerland. [Google Scholar]
- 3.Trager W, Jensen JB. 1976. Human malaria parasites in continuous culture. Science 193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 4.Desjardins RE, Canfield CJ, Haynes JD, Chulay JD. 1979. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob Agents Chemother 16:710–718. doi: 10.1128/AAC.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elabbadi N, Ancelin ML, Vial HJ. 1992. Use of radioactive ethanolamine incorporation into phospholipids to assess in vitro antimalarial activity by the semiautomated microdilution technique. Antimicrob Agents Chemother 36:50–55. doi: 10.1128/AAC.36.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noedl H, Wernsdorfer WH, Miller RS, Wongsrichanalai C. 2002. Histidine-rich protein II: a novel approach to malaria drug sensitivity testing. Antimicrob Agents Chemother 46:1658–1664. doi: 10.1128/AAC.46.6.1658-1664.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Druilhe P, Moreno A, Blanc C, Brasseur PH, Jacquier P. 2001. A colorimetric in vitro drug sensitivity assay for Plasmodium falciparum based on a highly sensitive double-site lactate dehydrogenase antigen-capture enzyme-linked immunosorbent assay. Am J Trop Med Hyg 64:233–241. [DOI] [PubMed] [Google Scholar]
- 8.Bennett TN, Paguio M, Gligorijevic B, Seudieu C, Kosar AD, Davidson E, Roepe PD. 2004. Novel, rapid, and inexpensive cell-based quantification of antimalarial drug efficacy. Antimicrob Agents Chemother 48:1807–1810. doi: 10.1128/AAC.48.5.1807-1810.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smilkstein M, Sriwilaijaroen N, Kelly JX, Wilairat P, Riscoe M. 2004. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob Agents Chemother 48:1803–1806. doi: 10.1128/AAC.48.5.1803-1806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wein S, Maynadier M, Tran Van Ba C, Cerdan R, Peyrottes S, Fraisse L, Vial H. 2010. Reliability of antimalarial sensitivity tests depends on drug mechanisms of action. J Clin Microbiol 48:1651–1660. doi: 10.1128/JCM.02250-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheruiyot AC, Auschwitz JM, Lee PJ, Yeda RA, Okello CO, Leed SE, Talwar M, Murthy T, Gaona HW, Hickman MR, Akala HM, Kamau E, Johnson JD. 2016. Assessment of the Worldwide Antimalarial Resistance Network standardized procedure for in vitro malaria drug sensitivity testing using SYBR green assay for field samples with various initial parasitemia levels. Antimicrob Agents Chemother 60:2417–2424. doi: 10.1128/AAC.00527-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skinner TS, Manning LS, Johnston WA, Davis TM. 1996. In vitro stage-specific sensitivity of Plasmodium falciparum to quinine and artemisinin drugs. Int J Parasitol 26:519–525. doi: 10.1016/0020-7519(96)89380-5. [DOI] [PubMed] [Google Scholar]
- 13.Klonis N, Xie SC, McCaw JM, Crespo-Ortiz MP, Zaloumis SG, Simpson JA, Tilley L. 2013. Altered temporal response of malaria parasites determines differential sensitivity to artemisinin. Proc Natl Acad Sci U S A 110:5157–5162. doi: 10.1073/pnas.1217452110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Witkowski B, Amaratunga C, Khim N, Sreng S, Chim P, Kim S, Lim P, Mao S, Sopha C, Sam B, Anderson JM, Duong S, Chuor CM, Taylor WRJ, Suon S, Mercereau-Puijalon O, Fairhurst RM, Menard D. 2013. Novel phenotypic assays for the detection of artemisinin-resistant Plasmodium falciparum malaria in Cambodia: in-vitro and ex-vivo drug-response studies. Lancet Infect Dis 13:1043–1049. doi: 10.1016/S1473-3099(13)70252-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Z, Wang Y, Cabrera M, Zhang Y, Gupta B, Wu Y, Kemirembe K, Hu Y, Liang X, Brashear A, Shrestha S, Li X, Miao J, Sun X, Yang Z, Cui L. 2015. Artemisinin resistance at the China-Myanmar border and association with mutations in the K13 propeller gene. Antimicrob Agents Chemother 59:6952–6959. doi: 10.1128/AAC.01255-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois A-C, Khim N, Kim S, Duru V, Bouchier C, Ma L, Lim P, Leang R, Duong S, Sreng S, Suon S, Chuor CM, Bout DM, Ménard S, Rogers WO, Genton B, Fandeur T, Miotto O, Ringwald P, Le Bras J, Berry A, Barale J-C, Fairhurst RM, Benoit-Vical F, Mercereau-Puijalon O, Ménard D. 2014. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 505:50–55. doi: 10.1038/nature12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nyunt MH, Hlaing T, Oo HW, Tin-Oo L-LK, Phway HP, Wang B, Zaw NN, Han SS, Tun T, San KK, Kyaw MP, Han E-T. 2015. Molecular assessment of artemisinin resistance markers, polymorphisms in the k13 propeller, and a multidrug-resistance gene in the eastern and western border areas of Myanmar. Clin Infect Dis 60:1208–1215. doi: 10.1093/cid/ciu1160. [DOI] [PubMed] [Google Scholar]
- 18.Chen K, Sun L, Lin Y, Fan Q, Zhao Z, Hao M, Feng G, Wu Y, Cui L, Yang Z. 2014. Competition between Plasmodium falciparum strains in clinical infections during in vitro culture adaptation. Infect Genet Evol 24:105–110. doi: 10.1016/j.meegid.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ponnudurai T, Lensen AH, Meis JF, Meuwissen JH. 1986. Synchronization of Plasmodium falciparum gametocytes using an automated suspension culture system. Parasitology 93(Part 2):263–274. doi: 10.1017/S003118200005143X. [DOI] [PubMed] [Google Scholar]
- 20.Sinden RE. 1983. Sexual development of malarial parasites. Adv Parasitol 22:153–216. doi: 10.1016/S0065-308X(08)60462-5. [DOI] [PubMed] [Google Scholar]
- 21.Talman AM, Domarle O, McKenzie FE, Ariey F, Robert V. 2004. Gametocytogenesis: the puberty of Plasmodium falciparum. Malar J 3:24. doi: 10.1186/1475-2875-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delves MJ, Ruecker A, Straschil U, Lelièvre J, Marques S, López-Barragán MJ, Herreros E, Sinden RE. 2013. Male and female Plasmodium falciparum mature gametocytes show different responses to antimalarial drugs. Antimicrob Agents Chemother 57:3268–3274. doi: 10.1128/AAC.00325-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruecker A, Mathias DK, Straschil U, Churcher TS, Dinglasan RR, Leroy D, Sinden RE, Delves MJ. 2014. A male and female gametocyte functional viability assay to identify biologically relevant malaria transmission-blocking drugs. Antimicrob Agents Chemother 58:7292–7302. doi: 10.1128/AAC.03666-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baird JK, Hoffman SL. 2004. Primaquine therapy for malaria. Clin Infect Dis 39:1336–1345. doi: 10.1086/424663. [DOI] [PubMed] [Google Scholar]
- 25.WHO. 2015. Guidelines for the treatment of malaria, 3rd ed World Health Organization, Geneva, Switzerland. [Google Scholar]
- 26.Llanos-Cuentas A, Lacerda MV, Rueangweerayut R, Krudsood S, Gupta SK, Kochar SK, Arthur P, Chuenchom N, Möhrle JJ, Duparc S, Ugwuegbulam C, Kleim J-P, Carter N, Green JA, Kellam L. 2014. Tafenoquine plus chloroquine for the treatment and relapse prevention of Plasmodium vivax malaria (DETECTIVE): a multicentre, double-blind, randomised, phase 2b dose-selection study. Lancet 383:1049–1058. doi: 10.1016/S0140-6736(13)62568-4. [DOI] [PubMed] [Google Scholar]
- 27.Lacrue AN, Sáenz FE, Cross RM, Udenze KO, Monastyrskyi A, Stein S, Mutka TS, Manetsch R, Kyle DE. 2013. 4(1H)-Quinolones with liver stage activity against Plasmodium berghei. Antimicrob Agents Chemother 57:417–424. doi: 10.1128/AAC.00793-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.March S, Ng S, Velmurugan S, Galstian A, Shan J, Logan DJ, Carpenter AE, Thomas D, Sim BKL, Mota MM, Hoffman SL, Bhatia SN. 2013. A microscale human liver platform that supports the hepatic stages of Plasmodium falciparum and vivax. Cell Host Microbe 14:104–115. doi: 10.1016/j.chom.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sattabongkot J, Yimamnuaychoke N, Leelaudomlipi S, Rasameesoraj M, Jenwithisuk R, Coleman RE, Udomsangpetch R, Cui L, Brewer TG. 2006. Establishment of a human hepatocyte line that supports in vitro development of the exo-erythrocytic stages of the malaria parasites Plasmodium falciparum and P. vivax. Am J Trop Med Hyg 74:708–715. [PubMed] [Google Scholar]
- 30.Baragaña B, Hallyburton I, Lee MCS, Norcross NR, Grimaldi R, Otto TD, Proto WR, Blagborough AM, Meister S, Wirjanata G, Ruecker A, Upton LM, Abraham TS, Almeida MJ, Pradhan A, Porzelle A, Martínez MS, Bolscher JM, Woodland A, Norval S, Zuccotto F, Thomas J, Simeons F, Stojanovski L, Osuna-Cabello M, Brock PM, Churcher TS, Sala KA, Zakutansky SE, Jiménez-Díaz MB, Sanz LM, Riley J, Basak R, Campbell M, Avery VM, Sauerwein RW, Dechering KJ, Noviyanti R, Campo B, Frearson JA, Angulo-Barturen I, Ferrer-Bazaga S, Gamo FJ, Wyatt PG, Leroy D, Siegl P, Delves MJ, Kyle DE, Wittlin S, Marfurt J, et al. 2015. A novel multiple-stage antimalarial agent that inhibits protein synthesis. Nature 522:315–320. doi: 10.1038/nature14451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corcoran KD, Hansukjariya P, Sattabongkot J, Ngampochjana M, Edstein MD, Smith CD, Shanks GD, Milhous WK. 1993. Causal prophylactic and radical curative activity of WR182393 (a guanylhydrazone) against Plasmodium cynomolgi in Macaca mulatta. Am J Trop Med Hyg 49:473–477. [DOI] [PubMed] [Google Scholar]
- 32.Dembele L, Gego A, Zeeman A-M, Franetich J-F, Silvie O, Rametti A, Le Grand R, Dereuddre-Bosquet N, Sauerwein R, van Gemert G-J, Vaillant J-C, Thomas AW, Snounou G, Kocken CHM, Mazier D. 2011. Towards an in vitro model of Plasmodium hypnozoites suitable for drug discovery. PLoS One 6:e18162. doi: 10.1371/journal.pone.0018162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dembélé L, Franetich J-F, Lorthiois A, Gego A, Zeeman A-M, Kocken CHM, Le Grand R, Dereuddre-Bosquet N, van Gemert G-J, Sauerwein R, Vaillant J-C, Hannoun L, Fuchter MJ, Diagana TT, Malmquist NA, Scherf A, Snounou G, Mazier D. 2014. Persistence and activation of malaria hypnozoites in long-term primary hepatocyte cultures. Nat Med 20:307–312. doi: 10.1038/nm.3461. [DOI] [PubMed] [Google Scholar]
- 34.Mikolajczak SA, Vaughan AM, Kangwanrangsan N, Roobsoong W, Fishbaugher M, Yimamnuaychok N, Rezakhani N, Lakshmanan V, Singh N, Kaushansky A, Camargo N, Baldwin M, Lindner SE, Adams JH, Sattabongkot J, Prachumsri J, Kappe SHI. 2015. Plasmodium vivax liver stage development and hypnozoite persistence in human liver-chimeric mice. Cell Host Microbe 17:526–535. doi: 10.1016/j.chom.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lira R, Sundar S, Makharia A, Kenney R, Gam A, Saraiva E, Sacks D. 1999. Evidence that the high incidence of treatment failures in Indian kala-azar is due to the emergence of antimony-resistant strains of Leishmania donovani. J Infect Dis 180:564–567. doi: 10.1086/314896. [DOI] [PubMed] [Google Scholar]
- 36.Rojas R, Valderrama L, Valderrama M, Varona MX, Ouellette M, Saravia NG. 2006. Resistance to antimony and treatment failure in human Leishmania (Viannia) infection. J Infect Dis 193:1375–1383. doi: 10.1086/503371. [DOI] [PubMed] [Google Scholar]
- 37.Bhandari V, Kulshrestha A, Deep DK, Stark O, Prajapati VK, Ramesh V, Sundar S, Schonian G, Dujardin JC, Salotra P. 2012. Drug susceptibility in Leishmania isolates following miltefosine treatment in cases of visceral leishmaniasis and post kala-azar dermal leishmaniasis. PLoS Negl Trop Dis 6:e1657. doi: 10.1371/journal.pntd.0001657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Obonaga R, Fernández OL, Valderrama L, Rubiano LC, Castro MDM, Barrera MC, Gomez MA, Gore Saravia N. 2014. Treatment failure and miltefosine susceptibility in dermal leishmaniasis caused by Leishmania subgenus Viannia species. Antimicrob Agents Chemother 58:144–152. doi: 10.1128/AAC.01023-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kulshrestha A, Bhandari V, Mukhopadhyay R, Ramesh V, Sundar S, Maes L, Dujardin JC, Roy S, Salotra P. 2013. Validation of a simple resazurin-based promastigote assay for the routine monitoring of miltefosine susceptibility in clinical isolates of Leishmania donovani. Parasitol Res 112:825–828. doi: 10.1007/s00436-012-3212-3. [DOI] [PubMed] [Google Scholar]
- 40.Fernández O, Diaz-Toro Y, Valderrama L, Ovalle C, Valderrama M, Castillo H, Perez M, Saravia NG. 2012. Novel approach to in vitro drug susceptibility assessment of clinical strains of Leishmania spp. J Clin Microbiol 50:2207–2211. doi: 10.1128/JCM.00216-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seifert K, Escobar P, Croft SL. 2010. In vitro activity of anti-leishmanial drugs against Leishmania donovani is host cell dependent. J Antimicrob Chemother 65:508–511. doi: 10.1093/jac/dkp500. [DOI] [PubMed] [Google Scholar]
- 42.Kamau SW, Nunez R, Grimm F. 2001. Flow cytometry analysis of the effect of allopurinol and the dinitroaniline compound (Chloralin) on the viability and proliferation of Leishmania infantum promastigotes. BMC Pharmacol 1:1. doi: 10.1186/1471-2210-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rassi A, Rassi A, Marin-Neto JA. 2010. Chagas disease. Lancet 375:1388–1402. doi: 10.1016/S0140-6736(10)60061-X. [DOI] [PubMed] [Google Scholar]
- 44.Köberle F. 1970. The causation and importance of nervous lesions in American trypanosomiasis. Bull World Health Organ 42:739–743. [PMC free article] [PubMed] [Google Scholar]
- 45.Arantes RME, Marche HHF, Bahia MT, Cunha FQ, Rossi MA, Silva JS. 2004. Interferon-gamma-induced nitric oxide causes intrinsic intestinal denervation in Trypanosoma cruzi-infected mice. Am J Pathol 164:1361–1368. doi: 10.1016/S0002-9440(10)63222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Filardi LS, Brener Z. 1987. Susceptibility and natural resistance of Trypanosoma cruzi strains to drugs used clinically in Chagas disease. Trans R Soc Trop Med Hyg 81:755–759. doi: 10.1016/0035-9203(87)90020-4. [DOI] [PubMed] [Google Scholar]
- 47.Francisco AF, Lewis MD, Jayawardhana S, Taylor MC, Chatelain E, Kelly JM. 2015. Reply to Urbina and McKerrow, “Drug Susceptibility of Genetically Engineered Trypanosoma cruzi Strains and Sterile Cure in Animal Models as a Criterion for Potential Clinical Efficacy of Anti-T. cruzi Drugs.” Antimicrob Agents Chemother 59:7925. doi: 10.1128/AAC.02022-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lane JE, Olivares-Villagomez D, Vnencak-Jones CL, McCurley TL, Carter CE. 1997. Detection of Trypanosoma cruzi with the polymerase chain reaction and in situ hybridization in infected murine cardiac tissue. Am J Trop Med Hyg 56:588–595. [DOI] [PubMed] [Google Scholar]
- 49.Lane JE, Ribeiro-Rodrigues R, Olivares-Villagómez D, Vnencak-Jones CL, McCurley TL, Carter CE. 2003. Detection of Trypanosoma cruzi DNA within murine cardiac tissue sections by in situ polymerase chain reaction. Mem Inst Oswaldo Cruz 98:373–376. doi: 10.1590/S0074-02762003000300013. [DOI] [PubMed] [Google Scholar]
- 50.Zhang L, Tarleton RL. 1999. Parasite persistence correlates with disease severity and localization in chronic Chagas' disease. J Infect Dis 180:480–486. doi: 10.1086/314889. [DOI] [PubMed] [Google Scholar]
- 51.James MJ, Yabsley MJ, Pung OJ, Grijalva MJ. 2002. Amplification of Trypanosoma cruzi-specific DNA sequences in formalin-fixed raccoon tissues using polymerase chain reaction. J Parasitol 88:989–993. doi: 10.2307/3285543. [DOI] [PubMed] [Google Scholar]
- 52.Ben Younès-Chennoufi A, Hontebeyrie-Joskowicz M, Tricottet V, Eisen H, Reynes M, Said G. 1988. Persistence of Trypanosoma cruzi antigens in the inflammatory lesions of chronically infected mice. Trans R Soc Trop Med Hyg 82:77–83. doi: 10.1016/0035-9203(88)90269-6. [DOI] [PubMed] [Google Scholar]
- 53.Chandler FW, Watts JC. 1988. Immunofluorescence as an adjunct to the histopathologic diagnosis of Chagas' disease. J Clin Microbiol 26:567–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taniwaki NN, da Silva CV, da Silva S, Mortara RA. 2007. Distribution of Trypanosoma cruzi stage-specific epitopes in cardiac muscle of Calomys callosus, BALB/c mice, and cultured cells infected with different infective forms. Acta Trop 103:14–25. doi: 10.1016/j.actatropica.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 55.Nunes MP, Coutinho SG, Louis JA, Souza WJ. 1990. Trypanosoma cruzi: quantification in tissues of experimentally infected mice by limiting dilution analysis. Exp Parasitol 70:186–192. doi: 10.1016/0014-4894(90)90099-X. [DOI] [PubMed] [Google Scholar]
- 56.Mortatti RC, Fonseca LS, Coelho J, Oliveira A, Moreno M. 1992. Follow-up of patent and subpatent parasitemias and development of muscular lesions in mice inoculated with very small numbers of Trypanosoma cruzi. Exp Parasitol 75:233–239. doi: 10.1016/0014-4894(92)90183-B. [DOI] [PubMed] [Google Scholar]
- 57.Russo M, Starobinas N, Marcondes MC, Minoprio P, Honteyberie-Joskowicz M. 1996. The influence of T cell subsets on Trypanosoma cruzi multiplication in different organs. Immunol Lett 49:163–168. doi: 10.1016/0165-2478(96)02498-4. [DOI] [PubMed] [Google Scholar]
- 58.Pinto PL, Takami R, Nunes EV, Guilherme CS, Oliveira OC, Gama-Rodrigues J, Okumura M. 1999. Life cycle of Trypanosoma cruzi (Y strain) in mice. Rev Hosp Clin 54:141–146. [DOI] [PubMed] [Google Scholar]
- 59.Martins HR, Figueiredo LM, Valamiel-Silva JCO, Carneiro CM, Machado-Coelho GLL, Vitelli-Avelar DM, Bahia MT, Martins-Filho OA, Macedo AM, Lana M. 2008. Persistence of PCR-positive tissue in benznidazole-treated mice with negative blood parasitological and serological tests in dual infections with Trypanosoma cruzi stocks from different genotypes. J Antimicrob Chemother 61:1319–1327. doi: 10.1093/jac/dkn092. [DOI] [PubMed] [Google Scholar]
- 60.Molina I, Gómez i Prat J, Salvador F, Treviño B, Sulleiro E, Serre N, Pou D, Roure S, Cabezos J, Valerio L, Blanco-Grau A, Sánchez-Montalvá A, Vidal X, Pahissa A. 2014. Randomized trial of posaconazole and benznidazole for chronic Chagas' disease. N Engl J Med 370:1899–1908. doi: 10.1056/NEJMoa1313122. [DOI] [PubMed] [Google Scholar]
- 61.Lewis MD, Fortes Francisco A, Taylor MC, Burrell-Saward H, McLatchie AP, Miles MA, Kelly JM. 2014. Bioluminescence imaging of chronic Trypanosoma cruzi infections reveals tissue-specific parasite dynamics and heart disease in the absence of locally persistent infection. Cell Microbiol 16:1285–1300. doi: 10.1111/cmi.12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bustamante JM, Bixby LM, Tarleton RL. 2008. Drug-induced cure drives conversion to a stable and protective CD8+ T central memory response in chronic Chagas disease. Nat Med 14:542–550. doi: 10.1038/nm1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cencig S, Coltel N, Truyens C, Carlier Y. 2012. Evaluation of benznidazole treatment combined with nifurtimox, posaconazole or AmBisome in mice infected with Trypanosoma cruzi strains. Int J Antimicrob Agents 40:527–532. doi: 10.1016/j.ijantimicag.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 64.Bustamante JM, Craft JM, Crowe BD, Ketchie SA, Tarleton RL. 2014. New, combined, and reduced dosing treatment protocols cure Trypanosoma cruzi infection in mice. J Infect Dis 209:150–162. doi: 10.1093/infdis/jit420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lewis MD, Francisco AF, Taylor MC, Kelly JM. 2015. A new experimental model for assessing drug efficacy against Trypanosoma cruzi infection based on highly sensitive in vivo imaging. J Biomol Screen 20:36–43. doi: 10.1177/1087057114552623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pépin J, Milord F. 1994. The treatment of human African trypanosomiasis. Adv Parasitol 33:1–47. doi: 10.1016/S0065-308X(08)60410-8. [DOI] [PubMed] [Google Scholar]
- 67.Yun O, Priotto G, Tong J, Flevaud L, Chappuis F. 2010. NECT is next: implementing the new drug combination therapy for Trypanosoma brucei gambiense sleeping sickness. PLoS Negl Trop Dis 4:e720. doi: 10.1371/journal.pntd.0000720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Legros D, Evans S, Maiso F, Enyaru JC, Mbulamberi D. 1999. Risk factors for treatment failure after melarsoprol for Trypanosoma brucei gambiense trypanosomiasis in Uganda. Trans R Soc Trop Med Hyg 93:439–442. doi: 10.1016/S0035-9203(99)90151-7. [DOI] [PubMed] [Google Scholar]
- 69.Matovu E, Geiser F, Schneider V, Mäser P, Enyaru JC, Kaminsky R, Gallati S, Seebeck T. 2001. Genetic variants of the TbAT1 adenosine transporter from African trypanosomes in relapse infections following melarsoprol therapy. Mol Biochem Parasitol 117:73–81. doi: 10.1016/S0166-6851(01)00332-2. [DOI] [PubMed] [Google Scholar]
- 70.Mäser P, Sütterlin C, Kralli A, Kaminsky R. 1999. A nucleoside transporter from Trypanosoma brucei involved in drug resistance. Science 285:242–244. doi: 10.1126/science.285.5425.242. [DOI] [PubMed] [Google Scholar]
- 71.Mäser P, Kaminsky R. 1998. Identification of three ABC transporter genes in Trypanosoma brucei spp. Parasitol Res 84:106–111. [DOI] [PubMed] [Google Scholar]
- 72.Alsford S, duBois K, Horn D, Field MC. 2012. Epigenetic mechanisms, nuclear architecture and the control of gene expression in trypanosomes. Expert Rev Mol Med 14:e13. doi: 10.1017/erm.2012.7. [DOI] [PubMed] [Google Scholar]
- 73.Baker N, Glover L, Munday JC, Aguinaga Andrés D, Barrett MP, de Koning HP, Horn D. 2012. Aquaglyceroporin 2 controls susceptibility to melarsoprol and pentamidine in African trypanosomes. Proc Natl Acad Sci U S A 109:10996–11001. doi: 10.1073/pnas.1202885109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Horn D, Duraisingh MT. 2014. Antiparasitic chemotherapy: from genomes to mechanisms. Annu Rev Pharmacol Toxicol 54:71–94. doi: 10.1146/annurev-pharmtox-011613-135915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Upcroft P, Upcroft JA. 2001. Drug targets and mechanisms of resistance in the anaerobic protozoa. Clin Microbiol Rev 14:150–164. doi: 10.1128/CMR.14.1.150-164.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wright JM, Dunn LA, Upcroft P, Upcroft JA. 2003. Efficacy of antigiardial drugs. Expert Opin Drug Saf 2:529–541. doi: 10.1517/14740338.2.6.529. [DOI] [PubMed] [Google Scholar]
- 77.Wassmann C, Hellberg A, Tannich E, Bruchhaus I. 1999. Metronidazole resistance in the protozoan parasite Entamoeba histolytica is associated with increased expression of iron-containing superoxide dismutase and peroxiredoxin and decreased expression of ferredoxin 1 and flavin reductase. J Biol Chem 274:26051–26056. doi: 10.1074/jbc.274.37.26051. [DOI] [PubMed] [Google Scholar]
- 78.Gut J, Ang KKH, Legac J, Arkin MR, Rosenthal PJ, McKerrow JH. 2011. An image-based assay for high throughput screening of Giardia lamblia. J Microbiol Methods 84:398–405. doi: 10.1016/j.mimet.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 79.Kang EW, Clinch K, Furneaux RH, Harvey JE, Schofield PJ, Gero AM. 1998. A novel and simple colorimetric method for screening Giardia intestinalis and anti-giardial activity in vitro. Parasitology 117(Part 3):229–234. doi: 10.1017/S0031182098002959. [DOI] [PubMed] [Google Scholar]
- 80.Chen CZ, Kulakova L, Southall N, Marugan JJ, Galkin A, Austin CP, Herzberg O, Zheng W. 2011. High-throughput Giardia lamblia viability assay using bioluminescent ATP content measurements. Antimicrob Agents Chemother 55:667–675. doi: 10.1128/AAC.00618-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen CZ, Southall N, Galkin A, Lim K, Marugan JJ, Kulakova L, Shinn P, van Leer D, Zheng W, Herzberg O. 2012. A homogenous luminescence assay reveals novel inhibitors for Giardia lamblia carbamate kinase. Curr Chem Genomics 6:93–102. doi: 10.2174/1875397301206010093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zheng G-X, Zhang X-M, Yang Y-S, Zeng S-R, Wei J-F, Wang Y-H, Li Y-J. 2014. An integrated microfluidic device for culturing and screening of Giardia lamblia. Exp Parasitol 137:1–7. doi: 10.1016/j.exppara.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 83.Seifert K, Duchêne M, Wernsdorfer WH, Kollaritsch H, Scheiner O, Wiedermann G, Hottkowitz T, Eibl H. 2001. Effects of miltefosine and other alkylphosphocholines on human intestinal parasite Entamoeba histolytica. Antimicrob Agents Chemother 45:1505–1510. doi: 10.1128/AAC.45.5.1505-1510.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ghosh S, Chan JMW, Lea CR, Meints GA, Lewis JC, Tovian ZS, Flessner RM, Loftus TC, Bruchhaus I, Kendrick H, Croft SL, Kemp RG, Kobayashi S, Nozaki T, Oldfield E. 2004. Effects of bisphosphonates on the growth of Entamoeba histolytica and Plasmodium species in vitro and in vivo. J Med Chem 47:175–187. doi: 10.1021/jm030084x. [DOI] [PubMed] [Google Scholar]
- 85.Singh S, Athar F, Azam A. 2005. Synthesis, spectral studies and in vitro assessment for antiamoebic activity of new cyclooctadiene ruthenium(II) complexes with 5-nitrothiophene-2-carboxaldehyde thiosemicarbazones. Bioorg Med Chem Lett 15:5424–5428. doi: 10.1016/j.bmcl.2005.08.103. [DOI] [PubMed] [Google Scholar]
- 86.Debnath A, Parsonage D, Andrade RM, He C, Cobo ER, Hirata K, Chen S, García-Rivera G, Orozco E, Martínez MB, Gunatilleke SS, Barrios AM, Arkin MR, Poole LB, McKerrow JH, Reed SL. 2012. A high-throughput drug screen for Entamoeba histolytica identifies a new lead and target. Nat Med 18:956–960. doi: 10.1038/nm.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Upcroft JA, Upcroft P. 2001. Drug susceptibility testing of anaerobic protozoa. Antimicrob Agents Chemother 45:1810–1814. doi: 10.1128/AAC.45.6.1810-1814.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Malebranche R, Arnoux E, Guérin JM, Pierre GD, Laroche AC, Péan-Guichard C, Elie R, Morisset PH, Spira T, Mandeville R. 1983. Acquired immunodeficiency syndrome with severe gastrointestinal manifestations in Haiti. Lancet ii:873–878. doi: 10.1016/S0140-6736(83)90868-1. [DOI] [PubMed] [Google Scholar]
- 89.Navin TR, Weber R, Vugia DJ, Rimland D, Roberts JM, Addiss DG, Visvesvara GS, Wahlquist SP, Hogan SE, Gallagher LE, Juranek DD, Schwartz DA, Wilcox CM, Stewart JM, Thompson SE, Bryan RT. 1999. Declining CD4+ T-lymphocyte counts are associated with increased risk of enteric parasitosis and chronic diarrhea: results of a 3-year longitudinal study. J Acquir Immune Defic Syndr Hum Retrovirol 20:154–159. doi: 10.1097/00042560-199902010-00007. [DOI] [PubMed] [Google Scholar]
- 90.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acácio S, Biswas K, O'Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. 2013. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 91.Platts-Mills JA, Babji S, Bodhidatta L, Gratz J, Haque R, Havt A, McCormick BJ, McGrath M, Olortegui MP, Samie A, Shakoor S, Mondal D, Lima IF, Hariraju D, Rayamajhi BB, Qureshi S, Kabir F, Yori PP, Mufamadi B, Amour C, Carreon JD, Richard SA, Lang D, Bessong P, Mduma E, Ahmed T, Lima AA, Mason CJ, Zaidi AK, Bhutta ZA, Kosek M, Guerrant RL, Gottlieb M, Miller M, Kang G, Houpt ER, MAL-ED Network Investigators. 2015. Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED). Lancet Glob Health 3:e564–e575. doi: 10.1016/S2214-109X(15)00151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Amadi B, Mwiya M, Musuku J, Watuka A, Sianongo S, Ayoub A, Kelly P. 2002. Effect of nitazoxanide on morbidity and mortality in Zambian children with cryptosporidiosis: a randomised controlled trial. Lancet 360:1375–1380. doi: 10.1016/S0140-6736(02)11401-2. [DOI] [PubMed] [Google Scholar]
- 93.Diaz E, Mondragon J, Ramirez E, Bernal R. 2003. Epidemiology and control of intestinal parasites with nitazoxanide in children in Mexico. Am J Trop Med Hyg 68:384–385. [PubMed] [Google Scholar]
- 94.Rossignol JF, Hidalgo H, Feregrino M, Higuera F, Gomez WH, Romero JL, Padierna J, Geyne A, Ayers MS. 1998. A double-‘blind' placebo-controlled study of nitazoxanide in the treatment of cryptosporidial diarrhoea in AIDS patients in Mexico. Trans R Soc Trop Med Hyg 92:663–666. doi: 10.1016/S0035-9203(98)90804-5. [DOI] [PubMed] [Google Scholar]
- 95.Arrowood MJ. 2002. In vitro cultivation of Cryptosporidium species. Clin Microbiol Rev 15:390–400. doi: 10.1128/CMR.15.3.390-400.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gold D, Stein B, Tzipori S. 2001. The utilization of sodium taurocholate in excystation of Cryptosporidium parvum and infection of tissue culture. J Parasitol 87:997–1000. doi: 10.2307/3285221. [DOI] [PubMed] [Google Scholar]
- 97.Flanigan T, Marshall R, Redman D, Kaetzel C, Ungar B. 1991. In vitro screening of therapeutic agents against Cryptosporidium: hyperimmune cow colostrum is highly inhibitory. J Protozool 38:225S–227S. [PubMed] [Google Scholar]
- 98.Upton SJ, Tilley M, Nesterenko MV, Brillhart DB. 1994. A simple and reliable method of producing in vitro infections of Cryptosporidium parvum (Apicomplexa). FEMS Microbiol Lett 118:45–49. doi: 10.1111/j.1574-6968.1994.tb06801.x. [DOI] [PubMed] [Google Scholar]
- 99.Upton SJ, Tilley M, Brillhart DB. 1995. Effects of select medium supplements on in vitro development of Cryptosporidium parvum in HCT-8 cells. J Clin Microbiol 33:371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cai X, Woods KM, Upton SJ, Zhu G. 2005. Application of quantitative real-time reverse transcription-PCR in assessing drug efficacy against the intracellular pathogen Cryptosporidium parvum in vitro. Antimicrob Agents Chemother 49:4437–4442. doi: 10.1128/AAC.49.11.4437-4442.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang H, Zhu G. 2015. Quantitative RT-PCR assay for high-throughput screening (HTS) of drugs against the growth of Cryptosporidium parvum in vitro. Front Microbiol 6:991. doi: 10.3389/fmicb.2015.00991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gut J, Nelson RG. 1999. Cryptosporidium parvum: synchronized excystation in vitro and evaluation of sporozoite infectivity with a new lectin-based assay. J Eukaryot Microbiol 46:56S–57S. [PubMed] [Google Scholar]
- 103.Bessoff K, Sateriale A, Lee KK, Huston CD. 2013. Drug repurposing screen reveals FDA-approved inhibitors of human HMG-CoA reductase and isoprenoid synthesis that block Cryptosporidium parvum growth. Antimicrob Agents Chemother 57:1804–1814. doi: 10.1128/AAC.02460-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bessoff K, Spangenberg T, Foderaro JE, Jumani RS, Ward GE, Huston CD. 2014. Identification of Cryptosporidium parvum active chemical series by repurposing the open access malaria box. Antimicrob Agents Chemother 58:2731–2739. doi: 10.1128/AAC.02641-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vinayak S, Pawlowic MC, Sateriale A, Brooks CF, Studstill CJ, Bar-Peled Y, Cipriano MJ, Striepen B. 2015. Genetic modification of the diarrhoeal pathogen Cryptosporidium parvum. Nature 523:477–480. doi: 10.1038/nature14651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Crannell Z, Castellanos-Gonzalez A, Nair G, Mejia R, White AC, Richards-Kortum R. 2016. Multiplexed recombinase polymerase amplification assay to detect intestinal protozoa. Anal Chem 88:1610–1616. doi: 10.1021/acs.analchem.5b03267. [DOI] [PubMed] [Google Scholar]
- 107.Murray CJL, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basáñez M-G, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabé E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, et al. 2012. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 108.Holtfreter MC, Loebermann M, Klammt S, Sombetzki M, Bodammer P, Riebold D, Kinzelbach R, Reisinger EC. 2011. Schistosoma mansoni: schistosomicidal effect of mefloquine and primaquine in vitro. Exp Parasitol 127:270–276. doi: 10.1016/j.exppara.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 109.Bertão HG, da Silva RAR, Padilha RJR, de Azevedo Albuquerque MCP, Rádis-Baptista G. 2012. Ultrastructural analysis of miltefosine-induced surface membrane damage in adult Schistosoma mansoni BH strain worms. Parasitol Res 110:2465–2473. doi: 10.1007/s00436-011-2786-5. [DOI] [PubMed] [Google Scholar]
- 110.Cupit PM, Cunningham C. 2015. What is the mechanism of action of praziquantel and how might resistance strike? Future Med Chem 7:701–705. doi: 10.4155/fmc.15.11. [DOI] [PubMed] [Google Scholar]
- 111.Secor WE, Montgomery SP. 2015. Something old, something new: is praziquantel enough for schistosomiasis control? Future Med Chem 7:681–684. doi: 10.4155/fmc.15.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cioli D, Botros SS, Wheatcroft-Francklow K, Mbaye A, Southgate V, Tchuenté L-AT, Pica-Mattoccia L, Troiani AR, El-Din SHS, Sabra A-NA, Albin J, Engels D, Doenhoff MJ. 2004. Determination of ED50 values for praziquantel in praziquantel-resistant and -susceptible Schistosoma mansoni isolates. Int J Parasitol 34:979–987. doi: 10.1016/j.ijpara.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 113.Crellen T, Walker M, Lamberton PH, Kabatereine NB, Tukahebwa EM, Cotton JA, Webster JP. 2016. Reduced efficacy of praziquantel against Schistosoma mansoni is associated with multiple rounds of mass drug administration. Clin Infect Dis 63:1151–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ramirez B, Bickle Q, Yousif F, Fakorede F, Mouries M-A, Nwaka S. 2007. Schistosomes: challenges in compound screening. Expert Opin Drug Discov 2:S53–S61. doi: 10.1517/17460441.2.S1.S53. [DOI] [PubMed] [Google Scholar]
- 115.Abdulla M-H, Ruelas DS, Wolff B, Snedecor J, Lim K-C, Xu F, Renslo AR, Williams J, McKerrow JH, Caffrey CR. 2009. Drug discovery for schistosomiasis: hit and lead compounds identified in a library of known drugs by medium-throughput phenotypic screening. PLoS Negl Trop Dis 3:e478. doi: 10.1371/journal.pntd.0000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Smout MJ, Kotze AC, McCarthy JS, Loukas A. 2010. A novel high throughput assay for anthelmintic drug screening and resistance diagnosis by real-time monitoring of parasite motility. PLoS Negl Trop Dis 4:e885. doi: 10.1371/journal.pntd.0000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rinaldi G, Loukas A, Brindley PJ, Irelan JT, Smout MJ. 2015. Viability of developmental stages of Schistosoma mansoni quantified with xCELLigence worm real-time motility assay (xWORM). Int J Parasitol Drugs Drug Resist 5:141–148. doi: 10.1016/j.ijpddr.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Manneck T, Braissant O, Haggenmüller Y, Keiser J. 2011. Isothermal microcalorimetry to study drugs against Schistosoma mansoni. J Clin Microbiol 49:1217–1225. doi: 10.1128/JCM.02382-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Paveley RA, Mansour NR, Hallyburton I, Bleicher LS, Benn AE, Mikic I, Guidi A, Gilbert IH, Hopkins AL, Bickle QD. 2012. Whole organism high-content screening by label-free, image-based Bayesian classification for parasitic diseases. PLoS Negl Trop Dis 6:e1762. doi: 10.1371/journal.pntd.0001762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Peak E, Chalmers IW, Hoffmann KF. 2010. Development and validation of a quantitative, high-throughput, fluorescent-based bioassay to detect schistosoma viability. PLoS Negl Trop Dis 4:e759. doi: 10.1371/journal.pntd.0000759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Howe S, Zöphel D, Subbaraman H, Unger C, Held J, Engleitner T, Hoffmann WH, Kreidenweiss A. 2015. Lactate as a novel quantitative measure of viability in Schistosoma mansoni drug sensitivity assays. Antimicrob Agents Chemother 59:1193–1199. doi: 10.1128/AAC.03809-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lalli C, Guidi A, Gennari N, Altamura S, Bresciani A, Ruberti G. 2015. Development and validation of a luminescence-based, medium-throughput assay for drug screening in Schistosoma mansoni. PLoS Negl Trop Dis 9:e0003484. doi: 10.1371/journal.pntd.0003484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Panic G, Flores D, Ingram-Sieber K, Keiser J. 2015. Fluorescence/luminescence-based markers for the assessment of Schistosoma mansoni schistosomula drug assays. Parasit Vectors 8:624. doi: 10.1186/s13071-015-1233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mansour NR, Bickle QD. 2010. Comparison of microscopy and Alamar blue reduction in a larval based assay for schistosome drug screening. PLoS Negl Trop Dis 4:e795. doi: 10.1371/journal.pntd.0000795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Asarnow D, Rojo-Arreola L, Suzuki BM, Caffrey CR, Singh R. 2015. The QDREC Web server: determining dose-response characteristics of complex macroparasites in phenotypic drug screens. Bioinformatics 31:1515–1518. doi: 10.1093/bioinformatics/btu831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ramaiah KD, Ottesen EA. 2014. Progress and impact of 13 years of the global programme to eliminate lymphatic filariasis on reducing the burden of filarial disease. PLoS Negl Trop Dis 8:e3319. doi: 10.1371/journal.pntd.0003319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Global Burden of Disease Study 2013 Collaborators. 2015. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 386:743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Taylor MJ, Hoerauf A, Bockarie M. 2010. Lymphatic filariasis and onchocerciasis. Lancet 376:1175–1185. doi: 10.1016/S0140-6736(10)60586-7. [DOI] [PubMed] [Google Scholar]
- 129.Fox LM. 2006. Ivermectin: uses and impact 20 years on. Curr Opin Infect Dis 19:588–593. doi: 10.1097/QCO.0b013e328010774c. [DOI] [PubMed] [Google Scholar]
- 130.Molyneux DH, Mitre E, Bockarie MJ, Kelly-Hope LA. 2014. Filaria zoogeography in Africa: ecology, competitive exclusion, and public health relevance. Trends Parasitol 30:163–169. doi: 10.1016/j.pt.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 131.Smyth JD. 1990. In vitro cultivation of parasitic helminths. CRC Press, Boca Raton, FL. [Google Scholar]
- 132.Rao UR, Salinas G, Mehta K, Klei TR. 2000. Identification and localization of glutathione S-transferase as a potential target enzyme in Brugia species. Parasitol Res 86:908–915. doi: 10.1007/s004360000255. [DOI] [PubMed] [Google Scholar]
- 133.Zaridah MZ, Idid SZ, Omar AW, Khozirah S. 2001. In vitro antifilarial effects of three plant species against adult worms of subperiodic Brugia malayi. J Ethnopharmacol 78:79–84. doi: 10.1016/S0378-8741(01)00286-0. [DOI] [PubMed] [Google Scholar]
- 134.Rao RU, Huang Y, Fischer K, Fischer PU, Weil GJ. 2009. Brugia malayi: effects of nitazoxanide and tizoxanide on adult worms and microfilariae of filarial nematodes. Exp Parasitol 121:38–45. doi: 10.1016/j.exppara.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 135.Lakshmi V, Joseph SK, Srivastava S, Verma SK, Sahoo MK, Dube V, Mishra SK, Murthy PK. 2010. Antifilarial activity in vitro and in vivo of some flavonoids tested against Brugia malayi. Acta Trop 116:127–133. doi: 10.1016/j.actatropica.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 136.Mahajan RS, Veerpathran A, Dakshinamoorthy G, Sharma RD, Goswami K, Reddy MV. 2010. Effect of certain antibiotics against filarial parasite Brugia malayi in vitro: possible role of oxidative stress. Indian J Clin Biochem 25:362–366. doi: 10.1007/s12291-010-0068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rao RU, Moussa H, Weil GJ. 2002. Brugia malayi: effects of antibacterial agents on larval viability and development in vitro. Exp Parasitol 101:77–81. doi: 10.1016/S0014-4894(02)00019-X. [DOI] [PubMed] [Google Scholar]
- 138.Li Z, Garner AL, Gloeckner C, Janda KD, Carlow CK. 2011. Targeting the Wolbachia cell division protein FtsZ as a new approach for antifilarial therapy. PLoS Negl Trop Dis 5:e1411. doi: 10.1371/journal.pntd.0001411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sashidhara KV, Kumar A, Rao KB, Kushwaha V, Saxena K, Murthy PK. 2012. In vitro and in vivo antifilarial activity evaluation of 3,6-epoxy [1,5]dioxocines: a new class of antifilarial agents. Bioorg Med Chem Lett 22:1527–1532. doi: 10.1016/j.bmcl.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 140.Kalani K, Kushwaha V, Sharma P, Verma R, Srivastava M, Khan F, Murthy PK, Srivastava SK. 2014. In vitro, in silico and in vivo studies of ursolic acid as an anti-filarial agent. PLoS One 9:e111244. doi: 10.1371/journal.pone.0111244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bulman CA, Bidlow CM, Lustigman S, Cho-Ngwa F, Williams D, Rascón AA, Tricoche N, Samje M, Bell A, Suzuki B, Lim KC, Supakorndej N, Supakorndej P, Wolfe AR, Knudsen GM, Chen S, Wilson C, Ang K-H, Arkin M, Gut J, Franklin C, Marcellino C, McKerrow JH, Debnath A, Sakanari JA. 2015. Repurposing auranofin as a lead candidate for treatment of lymphatic filariasis and onchocerciasis. PLoS Negl Trop Dis 9:e0003534. doi: 10.1371/journal.pntd.0003534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.O'Connell EM, Bennuru S, Steel C, Dolan MA, Nutman TB. 2015. Targeting filarial Abl-like kinases: orally available, Food and Drug Administration-approved tyrosine kinase inhibitors are microfilaricidal and macrofilaricidal. J Infect Dis 212:684–693. doi: 10.1093/infdis/jiv065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.O'Neill M, Geary JF, Agnew DW, Mackenzie CD, Geary TG. 2015. In vitro flubendazole-induced damage to vital tissues in adult females of the filarial nematode Brugia malayi. Int J Parasitol Drugs Drug Resist 5:135–140. doi: 10.1016/j.ijpddr.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Townson S, Tagboto S, McGarry HF, Egerton GL, Taylor MJ. 2006. Onchocerca parasites and Wolbachia endosymbionts: evaluation of a spectrum of antibiotic types for activity against Onchocerca gutturosa in vitro. Filaria J 5:4. doi: 10.1186/1475-2883-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mengome L-E, Akue JP, Souza A, Feuya Tchoua GR, Nsi Emvo E. 2010. In vitro activities of plant extracts on human Loa loa isolates and cytotoxicity for eukaryotic cells. Parasitol Res 107:643–650. doi: 10.1007/s00436-010-1910-2. [DOI] [PubMed] [Google Scholar]
- 146.Tiwari P, Tripathi LM, Raghu KG, Srivastava VML. 2004. Acanthocheilonema viteae: octopamine and its physiological role. Exp Parasitol 108:53–58. doi: 10.1016/j.exppara.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 147.Murthy PK, Joseph SK, Murthy PSR. 2011. Plant products in the treatment and control of filariasis and other helminth infections and assay systems for antifilarial/anthelmintic activity. Planta Med 77:647–661. doi: 10.1055/s-0030-1250452. [DOI] [PubMed] [Google Scholar]
- 148.Lentz CS, Halls V, Hannam JS, Niebel B, Strübing U, Mayer G, Hoerauf A, Famulok M, Pfarr KM. 2013. A selective inhibitor of heme biosynthesis in endosymbiotic bacteria elicits antifilarial activity in vitro. Chem Biol 20:177–187. doi: 10.1016/j.chembiol.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 149.Nisha M, Kalyanasundaram M, Paily KP, Abidha, Vanamail P, Balaraman K. 2007. In vitro screening of medicinal plant extracts for macrofilaricidal activity. Parasitol Res 100:575–579. doi: 10.1007/s00436-006-0294-9. [DOI] [PubMed] [Google Scholar]
- 150.Nayak A, Gayen P, Saini P, Maitra S, Sinha Babu SP. 2011. Albendazole induces apoptosis in adults and microfilariae of Setaria cervi. Exp Parasitol 128:236–242. doi: 10.1016/j.exppara.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 151.Mukherjee N, Saini P, Mukherjee S, Roy P, Sinha Babu SP. 2014. In vitro antifilarial activity of Azadirachta indica aqueous extract through reactive oxygen species enhancement. Asian Pac J Trop Med 7:841–848. doi: 10.1016/S1995-7645(14)60147-4. [DOI] [PubMed] [Google Scholar]
- 152.Zafar A, Ahmad I, Ahmad A, Ahmad M. 2016. Copper(II) oxide nanoparticles augment antifilarial activity of albendazole: in vitro synergistic apoptotic impact against filarial parasite Setaria cervi. Int J Pharm 501:49–64. doi: 10.1016/j.ijpharm.2016.01.059. [DOI] [PubMed] [Google Scholar]
- 153.Gunawardena NK, Fujimaki Y, Aoki Y, Mishima N, Ezaki T, Uni S, Kimura E. 2005. Differential effects of diethylcarbamazine, tetracycline and the combination on Brugia pahangi adult females in vitro. Parasitol Int 54:253–259. doi: 10.1016/j.parint.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 154.Comley JC, Rees MJ, Turner CH, Jenkins DC. 1989. Colorimetric quantitation of filarial viability. Int J Parasitol 19:77–83. doi: 10.1016/0020-7519(89)90024-6. [DOI] [PubMed] [Google Scholar]
- 155.Comley JC, Townson S, Rees MJ, Dobinson A. 1989. The further application of MTT-formazan colorimetry to studies on filarial worm viability. Trop Med Parasitol 40:311–316. [PubMed] [Google Scholar]
- 156.Marcellino C, Gut J, Lim KC, Singh R, McKerrow J, Sakanari J. 2012. WormAssay: a novel computer application for whole-plate motion-based screening of macroscopic parasites. PLoS Negl Trop Dis 6:e1494. doi: 10.1371/journal.pntd.0001494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Arumugam S, Wei J, Ward D, Abraham D, Lustigman S, Zhan B, Klei TR. 2014. Vaccination with a genetically modified Brugia malayi cysteine protease inhibitor-2 reduces adult parasite numbers and affects the fertility of female worms following a subcutaneous challenge of Mongolian gerbils (Meriones unguiculatus) with B. malayi infective larvae. Int J Parasitol 44:675–679. doi: 10.1016/j.ijpara.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Storey B, Marcellino C, Miller M, Maclean M, Mostafa E, Howell S, Sakanari J, Wolstenholme A, Kaplan R. 2014. Utilization of computer processed high definition video imaging for measuring motility of microscopic nematode stages on a quantitative scale: “the Worminator.” Int J Parasitol Drugs Drug Resist 4:233–243. doi: 10.1016/j.ijpddr.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ballesteros C, Tritten L, O'Neill M, Burkman E, Zaky WI, Xia J, Moorhead A, Williams SA, Geary TG. 2016. The effect of in vitro cultivation on the transcriptome of adult Brugia malayi. PLoS Negl Trop Dis 10:e0004311. doi: 10.1371/journal.pntd.0004311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kassis T, Skelton HM, Lu IM, Moorhead AR, Dixon JB. 2014. An integrated in vitro imaging platform for characterizing filarial parasite behavior within a multicellular microenvironment. PLoS Negl Trop Dis 8:e3305. doi: 10.1371/journal.pntd.0003305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Meister S, Plouffe DM, Kuhen KL, Bonamy GMC, Wu T, Barnes SW, Bopp SE, Borboa R, Bright AT, Che J, Cohen S, Dharia NV, Gagaring K, Gettayacamin M, Gordon P, Groessl T, Kato N, Lee MCS, McNamara CW, Fidock DA, Nagle A, Nam T, Richmond W, Roland J, Rottmann M, Zhou B, Froissard P, Glynne RJ, Mazier D, Sattabongkot J, Schultz PG, Tuntland T, Walker JR, Zhou Y, Chatterjee A, Diagana TT, Winzeler EA. 2011. Imaging of Plasmodium liver stages to drive next-generation antimalarial drug discovery. Science 334:1372–1377. doi: 10.1126/science.1211936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mwakingwe A, Ting L-M, Hochman S, Chen J, Sinnis P, Kim K. 2009. Noninvasive real-time monitoring of liver-stage development of bioluminescent Plasmodium parasites. J Infect Dis 200:1470–1478. doi: 10.1086/606115. [DOI] [PubMed] [Google Scholar]
- 163.Rieckmann KH, Campbell GH, Sax LJ, Mrema JE. 1978. Drug sensitivity of Plasmodium falciparum. An in-vitro microtechnique. Lancet i:22–23. doi: 10.1016/S0140-6736(78)90365-3. [DOI] [PubMed] [Google Scholar]
- 164.Witkowski B, Khim N, Chim P, Kim S, Ke S, Kloeung N, Chy S, Duong S, Leang R, Ringwald P, Dondorp AM, Tripura R, Benoit-Vical F, Berry A, Gorgette O, Ariey F, Barale J-C, Mercereau-Puijalon O, Menard D. 2013. Reduced artemisinin susceptibility of Plasmodium falciparum ring stages in western Cambodia. Antimicrob Agents Chemother 57:914–923. doi: 10.1128/AAC.01868-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Corbett Y, Herrera L, Gonzalez J, Cubilla L, Capson TL, Coley PD, Kursar TA, Romero LI, Ortega-Barria E. 2004. A novel DNA-based microfluorimetric method to evaluate antimalarial drug activity. Am J Trop Med Hyg 70:119–124. [PubMed] [Google Scholar]
- 166.Duffy S, Avery VM. 2012. Development and optimization of a novel 384-well anti-malarial imaging assay validated for high-throughput screening. Am J Trop Med Hyg 86:84–92. doi: 10.4269/ajtmh.2012.11-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lelièvre J, Almela MJ, Lozano S, Miguel C, Franco V, Leroy D, Herreros E. 2012. Activity of clinically relevant antimalarial drugs on Plasmodium falciparum mature gametocytes in an ATP bioluminescence “transmission blocking” assay. PLoS One 7:e35019. doi: 10.1371/journal.pone.0035019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chevalley S, Coste A, Lopez A, Pipy B, Valentin A. 2010. Flow cytometry for the evaluation of anti-plasmodial activity of drugs on Plasmodium falciparum gametocytes. Malar J 9:49. doi: 10.1186/1475-2875-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Churcher TS, Blagborough AM, Delves M, Ramakrishnan C, Kapulu MC, Williams AR, Biswas S, Da DF, Cohuet A, Sinden RE. 2012. Measuring the blockade of malaria transmission—an analysis of the Standard Membrane Feeding Assay. Int J Parasitol 42:1037–1044. doi: 10.1016/j.ijpara.2012.09.002. [DOI] [PubMed] [Google Scholar]