SUMMARY
Since the reclassification of the genus Bartonella in 1993, the number of species has grown from 1 to 45 currently designated members. Likewise, the association of different Bartonella species with human disease continues to grow, as does the range of clinical presentations associated with these bacteria. Among these, blood-culture-negative endocarditis stands out as a common, often undiagnosed, clinical presentation of infection with several different Bartonella species. The limitations of laboratory tests resulting in this underdiagnosis of Bartonella endocarditis are discussed. The varied clinical picture of Bartonella infection and a review of clinical aspects of endocarditis caused by Bartonella are presented. We also summarize the current knowledge of the molecular basis of Bartonella pathogenesis, focusing on surface adhesins in the two Bartonella species that most commonly cause endocarditis, B. henselae and B. quintana. We discuss evidence that surface adhesins are important factors for autoaggregation and biofilm formation by Bartonella species. Finally, we propose that biofilm formation is a critical step in the formation of vegetative masses during Bartonella-mediated endocarditis and represents a potential reservoir for persistence by these bacteria.
KEYWORDS: Bartonella, blood-culture-negative endocarditis, emerging infections, trimeric autotransporter adhesins, biofilm
INTRODUCTION
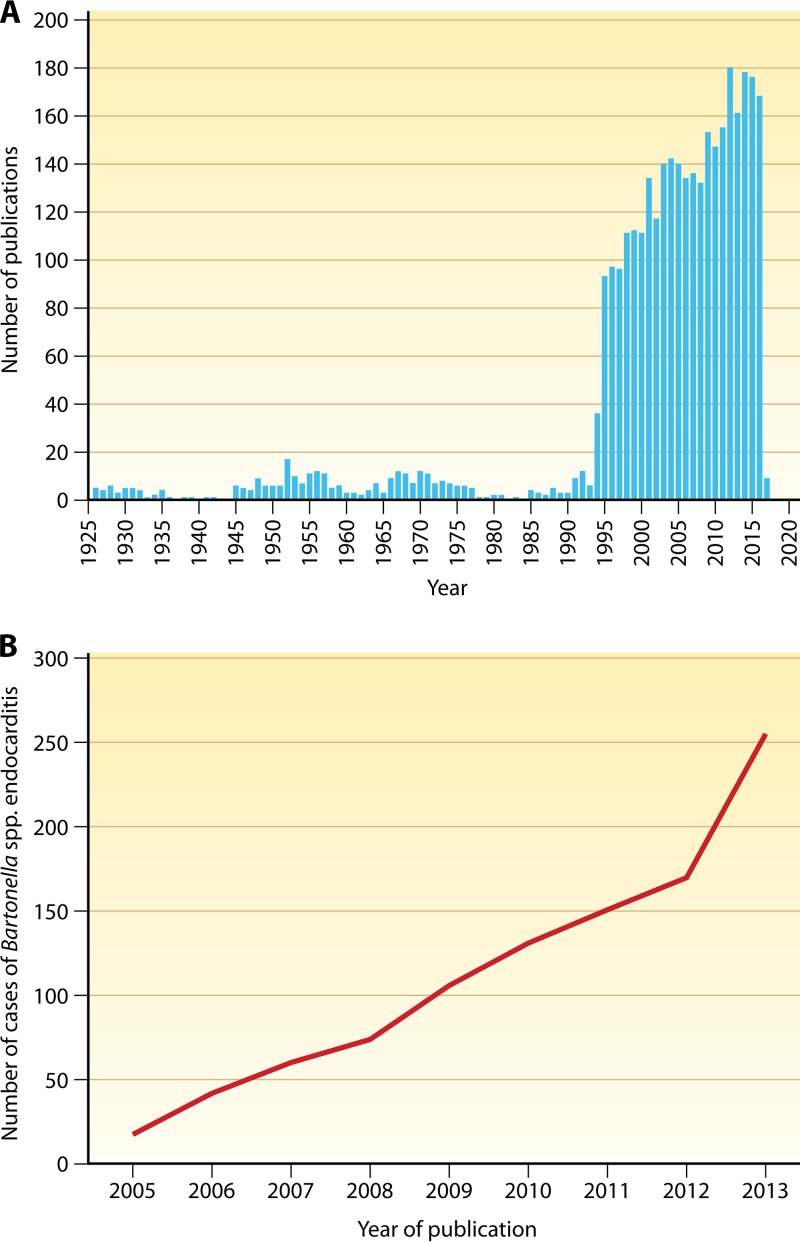
Just over a century ago, an emerging disease plagued almost a million frontline troops during World War I, rendering them unfit for duty for months at a time. The disease became known as “trench fever,” and it was subsequently shown to be caused by the louse-borne bacterium now known as Bartonella quintana (1). Interestingly, at that time many soldiers affected by trench fever were also reported to have cardiac involvement, and a complication called “disordered action of the heart” was described (2, 3). Diseases caused by bacteria in the current genus Bartonella, which once plagued the soldiers of World War I as trench fever, remained somewhat obscure until appearing as opportunistic infections in AIDS patients and homeless patients in urban areas in the early 1990s. Now characterized as reemerging, bacteria in the genus Bartonella are fastidious, Gram-negative, facultative intracellular pathogens with a unique intraerythrocytic lifestyle. Bartonellae usually exist in two specific habitats: the gut of the obligately bloodsucking arthropod vector, where they are exposed to toxic concentrations of heme, and the bloodstream of the mammalian host with deprivation of access to heme and iron (4). The ability of these bacteria to be transmitted by bloodsucking arthropods facilitates survival and dispersion while avoiding the host immune system. Over the past 20 years, there has been a rapid increase in the number of Bartonella species, with 45 species now designated and with some species containing more than one subspecies (Table 1). New species and subspecies are constantly being proposed, as evidenced by the description of Bartonella vinsonii subsp. yucatanensis as a distinct new taxon (5). Additionally, Bartonella isolates and candidate species from a wide range of animal reservoirs have been described but not yet assigned new species designations and will undoubtedly further expand this growing genus of bacteria. Bartonellae are zoonotic bacteria transmitted from host to host by a diverse range of hematogenous arthropod vectors, including fleas, lice, ticks, and sandflies (6). The association of Bartonella species with new vectors such as sheep keds has been recently reported (7). Likewise, the association of Bartonella species with vertebrate host reservoirs, including cats, rodents, and humans, has long been established, but a steadily expanding range of new animal reservoirs has been reported, including marine mammals (8), terrestrial herbivores such as camels (9), and wild carnivores, including lions, bears, and foxes (10). The emergence of Bartonella in a wide range of hosts and environments and the association of these bacteria with disease are mirrored by a steady increase in the number of articles about Bartonella which have been published in the last 2 decades compared to earlier time periods (Fig. 1A).
TABLE 1.
Currently designated Bartonella species, their hosts, and associated human disease
| Species | Host(s) | Human disease association |
|---|---|---|
| B. acomydis | Golden spiny mouse (Acomys russatus) (398) | |
| B. alsatica | Rabbits (39) | Endocarditis (40) |
| B. ancashensis | Human patient (41) | Verruga peruana (41, 399) |
| B. apis | Honeybee symbiont (400) | |
| B. australis | Kangaroos (58) | |
| B. bacilliformis | Human (26, 401) | Oroya fever, verruga peruana, Carrion's disease (26) |
| B. birtlesii | Mice (402) | |
| B. bovis | Dairy cattle (403) | |
| B. callosciuri | Plantain squirrel (398) | |
| B. capreoli | Deer (403) | |
| B. chomelii | French cattle (404) | |
| B. clarridgeiae | Cat (187) | Lymphadenopathy, fever, papule, CSD (44, 187) |
| B. coopersplainsensis | Rat (58) | |
| B. doshiae | Voles (405) | |
| B. dromedarii | Camels (406) | |
| B. elizabethae | Rats (24) | Endocarditis, neuroretinitis (18, 407) |
| B. florenciae | Shrew, mouse (408) | |
| B. fuyuanensis | Field mouse (409) | |
| B. grahamii | Rodents, voles (405) | Neuroretinitis, CSD (51, 53) |
| B. heixiaziensis | Vole (409) | |
| B. henselae | Cat (31, 140) | CSD, endocarditis, bacillary angiomatosis, bacteremia (140) |
| B. jaculi | Greater Egyptian jerboa (398) | |
| B. japonica | Mice (410) | |
| B. koehlerae | Cat (411) | Endocarditis (19) |
| B. koehlerae subsp. bothieri | Bobcat (412) | |
| B. koehlerae subsp. boulouisii | Mountain lion (412) | |
| B. mayotimonensis | Bats (55) | Endocarditis (20) |
| B. melophagi | Sheep (413) | |
| B. naantaliensis | Bats (55) | |
| B. peromysci | Mouse (405) | |
| B. pachyuromydis | Fat-tail gerbil (398) | |
| B. phoceensis | Rat (414) | |
| B. queenslandensis | Rats (58) | |
| B. quintana | Human (415) | Trench fever, endocarditis, bacteremia, bacillary angiomatosis |
| B. rattaustraliani | Rats (416) | |
| B. rattimassiliensis | Rats (414) | |
| B. rochalimae | Foxes, raccoons, coyotes (57, 417) | Bacteremia, splenomegaly (57) |
| B. silvatica | Mice (410) | |
| B. schoenbuchensis | Deer (418) | |
| B. senegalensis | Tick (419) | |
| B. talpae | Moles (405) | |
| B. tamiae | Rodents, humans (58) | Fever (58, 59) |
| B. taylorii | Rats (405) | |
| B. tribocorum | Rats (420) | |
| B. vinsonii subsp. arupensis | Mice (65) | Endocarditis (21) |
| B. vinsonii subsp. berkhoffii | Dog, coyotes (181, 421) | Endocarditis (23) |
| B. vinsonii subsp. vinsonii | Voles (24) | |
| B. vinsonii subsp. yucatanensis | Rodents (5) | |
| B. weissii | Cat (181) | |
| B. washoensis | Dog (422) |
FIG 1.
(A) Number of publications on Bartonella in PubMed. Source: https://www.ncbi.nlm.nih.gov/pubmed/?term=bartonella. (B) Increase in reported Bartonella endocarditis cases. (Adapted from reference 177 with permission.)
The role of Bartonella species in causing endocarditis was first reported in 1993 when B. quintana was identified in a patient with HIV infection (11). Soon thereafter, B. quintana was also isolated from several homeless patients with chronic alcoholism, some of whom were immunocompetent and had been diagnosed as having blood-culture-negative endocarditis (BCNE) (12–14). In those cases, specialized isolation techniques were used to isolate B. quintana from the patient's blood and/or PCR was used to confirm the etiology. That same year, Bartonella henselae was also shown to be responsible for a case of “culture-negative” endocarditis (15) and also in a second immunocompetent patient with endocarditis who owned a cat from which he most likely acquired the bacterium (16). Since that time, the number of cases of endocarditis and blood-culture-negative endocarditis that have been attributed to B. quintana and B. henselae has steadily increased (Fig. 1B). While these two species represent the vast majority of endocarditis cases attributed to Bartonella species, several other species, including B. alsatica (17), B. elizabethae (18), B. koehlerae (19), B. mayotimonensis (20), and B. vinsonii subsp. arupensis and berkhoffii (21–23), have been associated with endocarditis in humans. In this review, we summarize the current knowledge of the human-pathogenic Bartonella species, focusing on the two species, B. henselae and B. quintana, which are most commonly associated with endocarditis.
TAXONOMY OF THE GENUS BARTONELLA
Taxonomic History
Despite the recent rapid expansion of the genus Bartonella, B. bacilliformis was the only recognized species in the genus until 1993 (24). B. bacilliformis is the agent of the biphasic Carrion's disease, which includes the acute hemolytic anemia phase known as Oroya fever and the chronic phase known as verruga peruana (see reference 25 for a recent review). The skin lesions in patients with verruga peruana are unique in that they are highly vascularized nodules with evidence of angiogenesis (26). B. bacilliformis is restricted to certain regions in the Andes Mountains because of the distribution of the sandfly vector (25). Despite the unique pathology observed in patients with Carrion's disease, the study of B. bacilliformis was limited until the last 25 years, perhaps due to its vector-restricted geographic distribution. Similarly, the agent of trench fever was first known as Rickettsia quintana due to its cell association and difficulty in culturing, similar to the rickettsiae (1). In 1965, Rickettsia quintana was grown in axenic medium in the absence of host cells (27, 28), and it was subsequently reclassified as Rochalimaea quintana.
Interest in these bacteria increased greatly in the early 1990s when Rochalimaea quintana and a new species, Rochalimaea henselae, were first described in HIV-infected patients and subsequently in immunocompetent patients (29–33). Both species were established as etiologic agents of bacillary angiomatosis, which also exhibits angiogenic lesions similar to verruga peruana (33). Furthermore, Rochalimaea henselae was recognized as the primary etiologic agent of cat scratch disease (CSD) (34, 35). DNA relatedness studies and rRNA gene analysis showed that B. bacilliformis and members of the genus Rochalimaea were closely related, and so these two genera were merged, establishing the current genus Bartonella, and the family Bartonellaceae was removed from the order Rickettsiales (24).
Current Status
The genus Bartonella contains aerobic or microaerophilic, fastidious, Gram-negative bacilli belonging to the alpha-2 subgroup of the class Proteobacteria. Out of the 45 Bartonella species listed in Table 1 which infect animals, 13 have been implicated in human diseases. In addition to the currently recognized species, numerous subspecies exist, as do isolates from animal reservoirs that have not yet been fully characterized and named (candidate species). Thus, the genus is expanding rapidly in real time and very likely includes more distinct species than those listed in Table 1. It should also be recognized that not all of the species listed in Table 1 have been validated, but they are included here for the sake of completeness and because they have become established in the literature. Currently, there are 35 Bartonella species/subspecies with standing in nomenclature (http://www.bacterio.net/bartonella.html). Several recent studies/reviews have described the phylogenetic relationships among Bartonella species, strains, and isolates using different gene loci, but these are not addressed in this review (25, 36–38).
Bartonella Species Known To Infect Humans
Human infections caused by several different Bartonella species have been reported, and the list of potential human pathogens in the genus continues to grow. However, currently the vast majority of infections in humans are attributed most probably to B. bacilliformis, B. henselae, or B. quintana. The association of other Bartonella species with human disease relies on substantial information in the literature for some species and very limited information or even single case reports for other species. Isolation of the Bartonella species from diseased tissues is described in some reports, while in others, serology or molecular diagnostics supports the etiologic role. Accordingly, the strength of the association of each Bartonella species with human disease must be considered variable. Regardless, it has been proposed that any Bartonella species found in animals may be capable of infecting humans (20). Bartonella species that have been associated with human disease include the following.
B. alsatica.
B. alsatica was initially isolated from the blood of wild rabbits (39). B. alsatica was isolated from a patient diagnosed with BCNE who also had a preexisting valve lesion (40). A subsequent report implicated B. alsatica in a second case of BCNE in a patient who was a rabbit breeder (17), suggesting that rabbits may serve as the reservoir for transmission of B. alsatica to humans.
B. ancashensis.
B. ancashensis is a recently identified new species of Bartonella that was isolated from two patients with verruga peruana in the Ancash region of Peru (41). Initially, these patients were thought to be infected with B. bacilliformis, until molecular analysis indicated that their isolates were a distinct new species. This finding raises a question: do other Bartonella species infect patients in areas of South America where only B. bacilliformis is thought to be endemic?
B. bacilliformis.
Acute and chronic symptoms of B. bacilliformis infection are known as Oroya fever and verruga peruana, respectively, and are collectively referred to as Carrion's disease. Oroya fever is an acute life-threatening hemolytic anemia that is geographically limited to the high Andes, may result in death for more than 80% of infected patients in the absence of antibiotic treatment, and is increasing at an alarming rate in the pediatric population (42). The chronic form of B. bacilliformis infection results in angiogenic lesions on the skin called verruga peruana. The diverse clinical presentation of Carrion's disease suggests adaptation by B. bacilliformis to facilitate immune evasion in the human host to maintain the reservoir state for vector transmission (43).
B. clarridgeiae.
At least three Bartonella species, B. henselae, B. clarridgeiae, and B. koehlerae, are associated with cats. B. clarridgeiae was isolated from two different immunocompromised patients reported to have CSD (44, 187). Symptoms reported include severe headache, fever, lymphadenopathy, chills, sweating, and malaise (44). There is also evidence of coexistence of B. henselae and B. clarridgeiae in populations of cats and their fleas (46). Evidence of B. henselae and B. clarridgeiae DNA has also been reported in saliva of cats and dogs, and it has been suggested that B. clarridgeiae is a minor cause of CSD (47).
B. elizabethae.
B. elizabethae has been reported to cause human illness, and strains of this bacterium have been isolated from small mammals in Asia (48). It was originally isolated by Daly et al. (18) from a patient with endocarditis, and human serologic evidence of B. elizabethae infection has been reported in Thailand (49). Clinical characteristics may include headache, lethargy, muscle pain, conjunctival suffusion, and anemia. Almost 70% of patients with evidence of B. elizabethae infection also recorded exposure to rats, while the rest had cat exposure (50).
B. grahamii.
The first human isolate of B. grahamii was from an immunodeficiency virus-negative patient presenting as a case of neuroretinitis, proving that B. grahamii is pathogenic to humans (51). It has been reported as one of the most prevalent species in rodents (52) and has been reported as being a causative agent of CSD-like illness in an immunocompromised patient (53).
B. henselae.
First isolated in 1992 from a febrile patient infected with HIV (31), B. henselae commonly infects domestic and feral cats (Felis catus) causing long-term bacteremia. B. henselae is the primary etiologic agent of CSD and is the second most common Bartonella species causing endocarditis. B. henselae also causes bacteremia and bacillary angiomatosis.
B. koehlerae.
B. koehlerae was detected in heart valve tissue resected from a patient with BCNE (19). In an additional case, the patient reported depression and anxiety, headaches, joint stiffness, and hallucinations as a result of persistent infection with B. koehlerae that resolved following antibiotic treatment (54).
B. mayotimonensis.
B. mayotimonensis was isolated from the aortic valve tissue of a patient from the United States with infective endocarditis (20). The patient lived on a farm in Iowa and reported owning a cat prior to his illness and possible exposure to mouse fecal droppings. The authors of that study suggest the possibility that any Bartonella species can cause human infection and BCNE (20). A subsequent report isolated B. mayotimonensis from the blood of bats and detected Bartonella species in their ectoparasites (55).
B. quintana.
B. quintana is the cause of louse-borne trench fever; the bacterium is also recognized as the causative agent of bacteremia, bacillary angiomatosis, chronic lymphadenopathy, and endocarditis. It is one of the two species of Bartonella with a human reservoir. A recent review identified B. quintana as the most frequent cause of vector-borne infections in homeless and marginalized populations in the United States and Europe (56). B. quintana is the most common Bartonella species causing endocarditis.
B. rochalimae.
B. rochalimae has been reported to be a cause of bacteremia, fever, and splenomegaly in a patient who traveled to Peru (57).
B. tamiae.
Three isolates of B. tamiae were recovered from patients in Thailand who had fever (58, 59). B. tamiae DNA has since been detected in chigger mites and ticks, suggesting that these may serve as possible vectors for the transmission of this Bartonella species (60).
B. vinsonii.
B. vinsonii has been isolated from patients with endocarditis, arthritis, neurological disease, and vasoproliferative neoplasia (61, 62). Both B. vinsonii subsp. arupensis (21) and B. vinsonii subsp. berkhoffii (22, 23) have been associated with endocarditis. Vector transmission of B. vinsonii subsp. berkhoffii is suspected among dogs and wild canines (63), but cats have also been implicated as possible reservoirs (64). B. vinsonii subsp. arupensis is carried by rodents (65).
MICROBIOLOGY OF THE GENUS BARTONELLA
Growth Properties
Bartonella species are Gram-negative pleomorphic rods that stain poorly with the Gram stain but better with the Gimenez stain (66). Bartonella species are fastidious, relatively slow-growing bacteria with a requirement for heme. This growth requirement is met by growth supplements, including hemoglobin, erythrocytes, or hemin added to agar bases such as heart infusion agar, Columbia agar, brucella agar, or Trypticase soy agar. Additional supplements such as IsoVitaleX are used by some laboratories. Bartonella species grow best at 35 to 37°C with 5% supplemental CO2, with the exception of B. bacilliformis, which grows best at 28°C in the absence of supplemental CO2. Liquid medium has more recently been shown to support the growth of Bartonella species and has proven useful in both clinical and research laboratories (67, 68). Unique protein profiles, fatty acid composition, enzymatic activities, restriction fragment length polymorphisms, and PCR with and without DNA sequencing are all techniques used to identify Bartonella to the species level and are presented in depth in other reviews (66, 69).
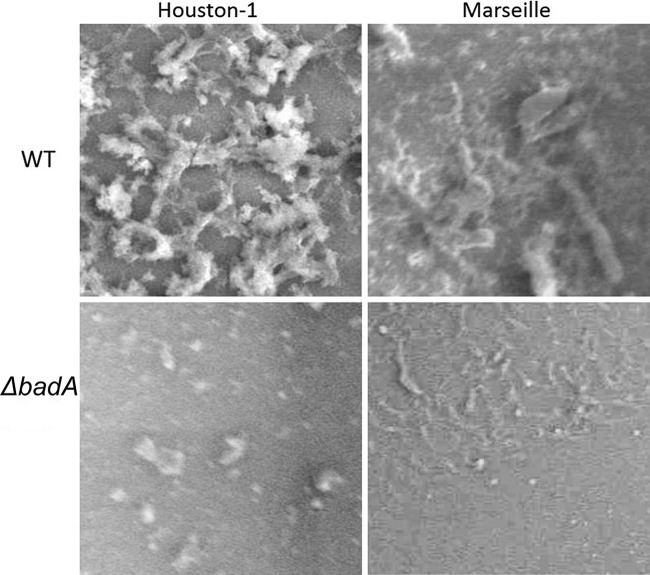
Some Bartonella species (B. bacilliformis, B. clarridgeiae, and B. rochalimae) possess flagella. All Bartonella species, except B. bacilliformis, are thought to have a VirB/VirD4 type IV secretion system (T4SS), and most are thought to have a surface-localized Trw T4SS. It has been hypothesized that the presence and functions of flagella and the Trw T4SS are mutually exclusive (70). All Bartonella species possess surface appendages that were initially described as type IV pili (71) but later shown to be comprised of trimeric autotransporter adhesins (TAAs) (72). The size of the appendages and the molecular mass of native TAAs have been shown to vary with species and are thought to be over 1 million Da for the protein trimer of the Brp TAA homologue in B. vinsonii (73). Expression of TAA genes is also highly variable among different species, within strains of the same species (72), and even under different conditions (74). The expression of TAA genes correlates with autoaggregation and has also been recently shown to play an important role in biofilm formation by B. henselae (75). It is well known that these autoaggregative properties are more apparent in recent low-passage-number isolates. The initial report of the isolation of B. henselae Houston-1 noted adherent colony morphology of the primary isolate (Fig. 2), which was lost upon serial subculture, resulting in more rapidly growing bacteria (31, 76). Spontaneous mutants lacking expression of the TAA gene in B. henselae have been reported (77) (see Pathogenesis of Bartonella Species), but it is not clear if this conversion from the highly autoaggregative phenotype to the nonaggregative phenotype occurs in nature or if it is only a result of laboratory growth and passage.
FIG 2.
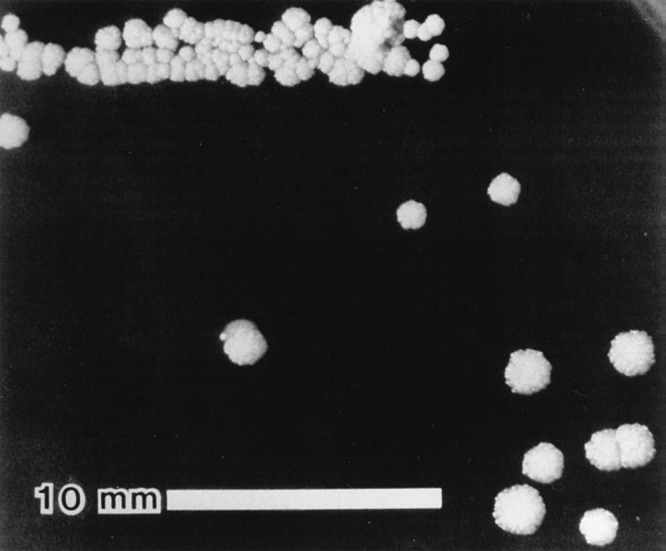
Colony morphology of low-passage-number Houston-1 type strain of B. henselae (ATCC 49882). A highly adherent colony phenotype was observed in this isolate which has subsequently been attributed to expression of badA. (Reproduced from reference 31.)
Genetics and Genome Organization
Bartonella species possess a single circular chromosome that varies in size from 1.45 Mbp for B. bacilliformis to 2.64 Mbp for B. tribocorum (36, 66). Based on the Bartonella genomes sequenced to date, the genome size loosely correlates with host specificity, with rodent-associated species having larger genomes and the human-specific species B. bacilliformis having the smallest. Rodent-associated Bartonella species show more evidence of horizontal gene transfer and gene duplication than do the human-specific Bartonella species. Of particular relevance to this review, it appears that the genomes of the rodent-associated species harbor more host adaptability factor genes such as the T4SSs as well as TAA, transporter, and adhesin genes than do the human-restricted species (78).
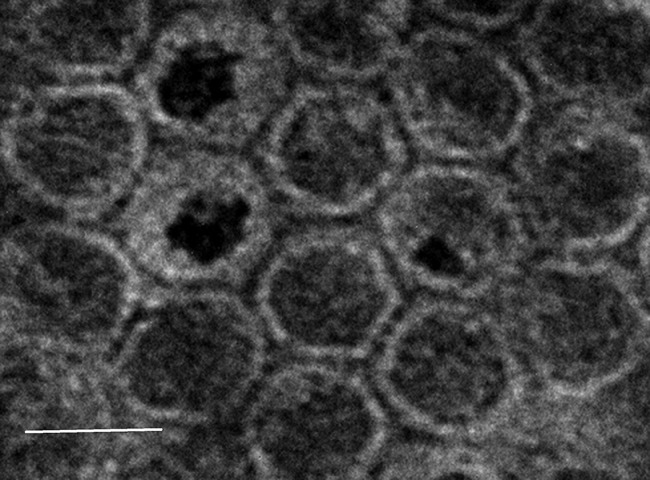
Some Bartonella species, including B. grahamii and B. tribocorum, possess plasmids (36, 78). The functions of plasmid-borne genes in these species are not well characterized but include putative small regulatory RNAs (75). Additional episomal DNA elements are the linear DNA fragments of a uniform 14-kb size that were observed in the cells of B. henselae (79). These linear fragments of DNA were shown to be a result of random packaging of genomic DNA into 40- to 50-nm icosahedral bacteriophage-like particles (BLPs) (Fig. 3) (79). A similar BLP was previously observed in B. bacilliformis and was described as a 40-nm icosahedral particle with a 16-nm tail (80). Subsequently, the B. bacilliformis BLP was shown to consist of specific proteins but nonselectively packaged genomic DNA (81). Initial experiments to demonstrate gene transfer by the BLPs of both B. henselae and B. bacilliformis were not successful (79, 81). The genes directing synthesis of components of the Bartonella bacteriophage-like particles have not definitively been identified; however, integrated in most of the Bartonella genomes sequenced to date are genes annotated as phage-related genes or prophage genes (36, 37, 78, 82–84). The BLP has also been described in B. grahamii, and it has been proposed as a gene transfer agent with successful in vitro particle-mediated transfer of genes being reported (37, 78). It has been further proposed that Bartonella BLPs have properties that are intermediate between those of gene transfer agents and transducing bacteriophages (85). Regardless, the function of these novel particles in packaging and exporting genomic DNA provides evidence that they play an active role in horizontal gene transfer and the evolution of Bartonella species.
FIG 3.
Transmission electron micrograph of the bacteriophage-like particles of B. henselae stained with uranyl acetate. White bar, 50 nm.
LABORATORY DIAGNOSIS OF INFECTIONS CAUSED BY BARTONELLA
Isolation and Culture
B. henselae and most likely the other newly recognized Bartonella species associated with human disease avoided detection by routine blood culture methods for many years before it was appreciated that primary isolation requires specific growth medium and extended incubation times. Endocarditis has traditionally been diagnosed based on a positive blood culture. However, blood culture methods have sensitivity as low as 20% for diagnosing Bartonella endocarditis, while tissue culture of surgically excised valves has a similarly low sensitivity of 30% (86). In general, direct plating of blood or tissue homogenates is preferable, and several different agar base formulas, including heart infusion, Trypticase soy, brucella agar, and Columbia agar supplemented with 5% rabbit blood or 5% hemoglobin, have been used successfully for primary isolation (66). For primary isolation, extended incubation times of up to 21 days may be required (33). Plates for primary isolation are incubated in 5% CO2 at 35 to 37°C, except for the isolation of B. bacilliformis, which prefers ambient CO2 and temperatures of 26 to 28°C. It has been reported that the lysis centrifugation method for sample preparation increases isolate recovery (29). Successful isolation of Bartonella species from automated blood culture systems and liquid culture media has also been reported (67, 87), as has isolation using cell culture systems (33).
Special Stains
Histopathology of valve tissue stained with hematoxylin-eosin typically reveals marked inflammation, fibrosis, and calcification compared to endocarditis not caused by Bartonella (88). Warthin-Starry silver staining has been a frequently employed method for the detection of Bartonella species and reveals small, dark-staining bacteria in the fibrotic areas of the affected valve in over 75% of Bartonella endocarditis cases (89), but this stain is not specific for Bartonella species. Other special stains include Giemsa and Gimenez stains, which can be used on valvular tissue for initial diagnosis, but these techniques are also not specific for Bartonella species (66). Acridine orange has also been used to nonspecifically detect Bartonella species in culture (90). Immunohistochemical staining of affected tissues has employed both monoclonal and polyclonal antibodies with various degrees of success and affords higher specificity than Warthin-Starry staining (91). Regardless, due to the low yield of blood culture and lack of specificity of specialized stains, current diagnosis of Bartonella endocarditis relies heavily on serology and/or molecular testing of blood or valvular tissue specimens.
Serology
Serology has played a critical role in diagnosis of Bartonella infections and, in fact, was crucial in the establishment of B. henselae as the etiologic agent of CSD (34). That initial serologic assay used B. henselae cocultivated with Vero cells as an antigen in an indirect fluorescent antibody assay (IFA) to test for IgM and/or IgG antibodies in the patient's serum. A positive titer was considered >16 for IgM and >64 for IgG, with a 4-fold rise in titer for IgG between acute-phase serum and convalescent-phase serum samples (collected at least 2 weeks apart) preferable for definitive diagnosis (34, 92). Further reports of Bartonella species cultivated in the absence of host cells or prepared using other approaches resulted in wide variations in both specificity and sensitivity (93, 94). It is very likely that the highly autoaggregative nature of most Bartonella species, subsequently attributed to expression of the TAAs on the surface of the bacterium, contributed to this variation, since the TAAs are antigenic and recognized by serum from patients infected with Bartonella species (77, 95). Additionally, the variable expression of the TAA genes in different B. henselae isolates may have further contributed to this problem (72). The IFA is not regarded as species specific, and there is considerable cross-reactivity between B. henselae and B. quintana and perhaps other Bartonella species as well (96). A similar IFA was used to study 22 cases of BCNE, and a positive predictive value of close to 90% was reported, but very high antibody titers (>1,600) were found in these patients, perhaps due to their subacute or chronic infection with Bartonella species (97).
Serologic assays employing enzyme-linked-immunosorbent-assay (ELISA)-based approaches have also been used for several years, but similar problems have been reported for these assays as well, including cross-reactivity and lack of specificity (98). One approach to serologic diagnosis to reduce cross-reactivity is to substitute specific antigens for whole bacterial antigens. An example is the use of the VirB5 17-kDa antigen recombinant protein expressed in Escherichia coli. In that case, the 17-kDa recombinant protein was examined in Western blot assays and shown to have reactivity with human sera from patients with CSD, very similar to the IFA (99). The recombinant 17-kDa antigen has subsequently been adapted to an ELISA-based assay to detect IgG antibody against B. henselae (100) and for an IgM capture assay where high sensitivity and specificity were reported (101). Another approach to serologic diagnosis is to employ subcellular fractions as an antigen in an ELISA to detect IgG for the diagnosis of CSD (102). Cross-adsorption and Western immunoblotting techniques also have been reported with high specificity and sensitivity in detecting Bartonella endocarditis; the cross-adsorption technique was used to overcome false positivity from cross-reactivity with other bacterial species, especially with Chlamydia species, which could also be a causative agent of endocarditis (86, 103–106).
Molecular Tools for Detection of Bartonella
PCR is not only one of the mainstays for diagnosing Bartonella infections, but it has played a critical role in fulfilling molecular Koch's postulates to associate Bartonella with new disease syndromes. The detection of Bartonella 16S rRNA gene sequences in the lesions of patients with bacillary angiomatosis provided the first link of Bartonella with this condition (32). Similarly, the detection of B. henselae 16S rRNA gene sequences in skin test antigens used to diagnose CSD helped resolve a longstanding mystery about the etiology of CSD (35). PCR alone or coupled with restriction fragment digestion to detect polymorphisms, enrichment broth culture, or DNA sequencing has all been used to identify Bartonella isolates (see reference 89 for a recent review). Many different primer pairs and techniques have been described to detect Bartonella DNA in clinical specimens by PCR (see reference 89 for a recent review). When specifically applied to Bartonella endocarditis, amplification of Bartonella DNA from valvular tissue by PCR has been shown in multiple case series to have higher sensitivity and specificity, ranging from 72 to 98% (86, 97, 107, 108). PCR testing can also be performed on whole blood, plasma, or serum samples, with studies reporting sensitivity of 58% and specificity of 100% (109). For a recent review of laboratory diagnostic procedures for Bartonella species, see the work of Gutierrez et al. (69).
EPIDEMIOLOGY OF BARTONELLA INFECTIONS
Natural Reservoirs and Arthropod Vectors
Bartonella species have been isolated or detected in a wide range of animal species, including terrestrial animals, rodents, bats, and marine animals, such as beluga whales and sea turtles (8, 110). In most cases, the presence of Bartonella species in the blood of these infected animal reservoirs does not result in serious disease. Thus, this vast range of infected animals serves as a ubiquitous reservoir for potential zoonotic infection. Only two species of Bartonella, B. bacilliformis and B. quintana, are known to infect humans as their reservoir host. These two species, together with B. henselae, cause the vast majority of human disease attributed to Bartonella species (111).
There are several examples illustrating how different Bartonella species have evolved with their mammalian hosts. This would include B. henselae with cats, B. vinsonii subsp. berkhoffii with dogs, B. bovis in cows, B. melophagi in sheep, and B. australis with kangaroos in Australia (112). However, the list of rodent-adapted Bartonella species is growing exponentially, as exemplified by species that have evolved with multiple types of squirrels: ground squirrels (B. washoensis), gray squirrels (“Candidatus Bartonella durdenii”), flying squirrels (“Candidatus Bartonella volans”), and even groundhogs (“Candidatus Bartonella monaxi”) (113, 114). Most recently, bats have been identified as the reservoirs of diverse and novel species of Bartonella. In addition to infected bats in eastern Africa (Kenya) and Guatemala, Peru has a similar overall prevalence (24.1%) but presents a greater variety of prevalence by species: for example, more than half of the population of common vampire bats in Peru is infected (113). The high prevalence is most likely due to the relatively long lifespan of bats, an average of 10 to 20 years. There is a theoretical possibility, yet to be confirmed, that transmission of Bartonella species, in addition to transmission via ectoparasites, may occur via direct bat bite, as is the case with rabies transmission. Because some bat species (especially Carollia and Glossophaga bats) share roosts with other species, there is a potential for both intra- and interspecies transmission of infections. Bats are frequent hosts to a wide variety of ectoparasites such as fleas, bat flies, soft ticks, and mites (115). Studies from Egypt and the United States have shown that arthropods have a role as vectors of Bartonella species to other wildlife, with humans being incidental hosts (116, 117).
Bartonella species have been labeled as emerging pathogens, and yet they have been detected in the dental pulp of humans dating back to antiquity (118). Bartonella was first recognized as an agent of endocarditis in 1993 (11). Transmission of Bartonella species may be via arthropod vectors or direct inoculation, depending on which species is involved. In the case of B. bacilliformis, disease incidence follows geographic boundaries that are limited by the distribution of its vector, the sandfly (Lutzomyia verrucarum), to 1 to 3 km of altitude in the Andes Mountains in Peru. Its presence in Ecuador and Colombia supports an argument for yet another vector or mode of transmission (119). While L. verrucarum is its most important arthropod vector, other phlebotomine sandflies—L. maranonensis and L. robusta—may serve as vectors in areas devoid of L. verrucarum (120). Humans are the only established reservoir of B. bacilliformis, and several reports examining nonhuman reservoirs, including plants (121), rodents (122, 123), and domesticated animals (124), were inconclusive. Infection with B. bacilliformis is biphasic, with both an acute hemolytic anemia (Oroya fever) and a chronic form with vascular proliferative lesions (verruga peruana) (125).
B. henselae is endemic worldwide, and transmission to humans has been linked to cats by both serology and epidemiologic studies (34, 92, 98, 126). Healthy cats bacteremic with B. henselae are associated with bacillary angiomatosis and CSD in their human contacts. The major vector of transmission between cats is the cat flea (Ctenocephalides felis) (127, 128), with about 50% of cats bearing signs of previous or current infection (129). Cat-to-cat transmission of the organism by the cat flea, with no direct contact transmission, has been demonstrated (130). B. henselae has been experimentally detected in oral swabs from infected cats (131, 132) and is able to replicate in the gut of the cat flea, is shed in the feces, and is also shown to live in flea feces for up to 3 days postexposure (133–135). Possible mechanisms of cat-to-cat transmission include flea bite and ingestion of fleas and flea feces (136). Of note, asymptomatic but bacteremic cats are more likely to harbor fleas, therefore leading to persistence in the flea vector. Bartonella species has been detected in other types of fleas, as well as ixodid and Dermacentor ticks (109, 137, 138), but no definitive transmission studies have shown that these vectors transmit B. henselae (139). No flea-to-human transmission has been identified as of yet (127, 131, 140). Rather, transmission of B. henselae occurs indirectly, primarily by contaminated flea feces that are inoculated by a cat scratch (141) and rarely through a cat bite (131). Bartonella clarridgeiae and B. koehlerae are widespread in cats but are uncommon causes of illness in humans (142–144). B. elizabethae has been isolated in humans, while its DNA has been amplified from the blood of dogs (145). The majority of CSD cases occur in children aged 5 to 9 years and those living in the southern United States. The estimates show 22,000 diagnoses of CSD each year in the United States with about 2,000 hospitalizations (146). The incidence of CSD varies by season, with most cases occurring during the fall and early-winter months, September through January. Some attribute the seasonal prevalence to the breeding patterns of cats, peak time of domestic cat adoptions, and the temporal presence of fleas on cats, which spread the bacteria among the cat population (146).
Unlike B. bacilliformis, B. quintana (trench fever) has worldwide distribution often associated with war zones and poor sanitation predisposing to infestation with the human body louse (Pediculus humanus), the only known vector for transmission (147). The disease presents with fever, rash, bone pain, and splenomegaly lasting for about 4 to 5 days, thus its name of quintan or 5-day fever. On rare occasions, the symptoms persist or recur as multiple paroxysms. World War I brought this relatively rare disease to light, since about 1 million troops were thought to have been infected. Now, the disease is seen mostly in alcoholic and homeless populations and has been dubbed “urban trench fever” (148, 149). The 1990s and the HIV epidemic brought the resurgence of the disease presenting with fever and bacteremia with and without endocarditis (107). Although the human body louse P. humanus is the main vector for its transmission, B. quintana has been detected in cat fleas, monkey fleas, and cat dental pulp, suggesting potential methods of transmission other than infestation and bite by the body louse (150, 151). B. quintana is transmitted by lice through its feces, and its mode of transmission is well researched (152). Much like B. henselae, it is also found to replicate in the gut of the vector (134). In a state of prolonged bacteremia, it is found in the erythrocytes (153), and nonhemolytic intracellular colonization of erythrocytes preserves the pathogen for efficient transmission by lice while protecting it from the host immune response and decreasing antimicrobial efficacy (153). It is also proposed that B. quintana could present a risk in blood transfusion, since undetected bacteria could be present in erythrocytes of blood donors (153). Recently, Bartonella species were detected in 3.2% of asymptomatic blood donors from Brazil (154). In its classic form of causing endocarditis, B. quintana multiplies in the louse intestine and is excreted in the louse's feces and deposited on human skin. Entry across the skin occurs when a pruritic area of the skin is scratched and abraded (155).
Epidemiology of Bartonella Endocarditis
B. henselae, when combined with B. quintana, accounts for over 90% of Bartonella endocarditis cases (107). Bartonella species in general have wide geographic distribution, possibly due to the geographic specificity of their respective hosts and vectors (156–162). For example, DNA of several species of Bartonella was isolated from bat flies and bats in Africa, Asia, Europe, and both North and South America (163). B. henselae and B. quintana, the two species most commonly associated with endocarditis, are also known to occur worldwide (58, 164–166). While the majority of cases of Bartonella endocarditis reported are from Europe and the Americas, cases have also been reported from Asia, East and West Africa, and Australia, suggesting worldwide distribution of Bartonella endocarditis (167–171). There is a clear preponderance of the male gender in the reported Bartonella endocarditis cases, with males accounting for 60 to 85% of cases (97, 107, 172). It is not clear whether there is a biologic basis for this difference other than the difference in demographic factors (such as homelessness or alcohol abuse) that exists between the genders. B. quintana endocarditis was originally reported in a patient with HIV; however, subsequent reports have shown that B. quintana endocarditis occurs in people without known immunodeficiency (11, 13). Some of the most frequently recurring epidemiologic associations with B. quintana endocarditis were homelessness, alcoholism, and exposure to body lice. These epidemiologic associations are likely to be interrelated and probably are surrogate markers for low socioeconomic status rather than each factor being individually associated with the risk of B. quintana endocarditis. However, interestingly the majority of these patients with B. quintana endocarditis did not have previously known valvular diseases, which would be expected in people of low socioeconomic status. B. henselae endocarditis accounted for about 25% of all Bartonella endocarditis and usually occurred in people who had previous valve diseases and had a history of exposure to cats or cat fleas (107, 173, 174). B. henselae also has been implicated as a coinfecting agent in a patient with other bacterial etiologies such as staphylococcal endocarditis (173). Even though this was a single case report, the possibility that the virulence and pathogenic features of Bartonella, such as endothelial proliferation, may prime the valve for subsequent infection by other, more commonly encountered bacteria such as staphylococci is an area for future investigation. Patients with Bartonella endocarditis generally tend to have a lower average age than patients with other types of bacterial endocarditis, with observed geographic variability in age possibly reflecting regional differences in demographics and socioeconomic status (175–177).
BARTONELLA AND DISEASE
Disease Syndromes Associated with Bartonella Infection
B. quintana was identified in human dental tissue dating as far back as 4,000 years, and relics of the Inca empire depict features of the disease verruca peruana, which is now known to be caused by B. bacilliforms (118, 178). However, until only a few decades ago only one other human disease; namely, trench fever, was attributed to bacteria in the current genus Bartonella. Since the early 1990s, several species and subspecies of Bartonella have been characterized, and the spectrum of natural reservoirs, vectors, and human diseases caused by Bartonella species has significantly expanded (179–181). Below is a brief description of some of the major human diseases caused by Bartonella species. Even though these diseases may be distinct from endocarditis in some ways, it is increasingly evident now that many of them are accompanied by intraerythrocytic (bacteremic) phases which may lead to endocarditis (182).
Carrion's disease.
Carrion's disease (bartonellosis) is caused by B. bacilliforms and transmitted by sandflies of the species L. verrucarum. It is endemic in higher-altitude areas of Peru, Colombia, and Ecuador, but sporadic cases have been reported in people returning from visits to areas of endemicity (57). The classic manifestation of Carrion's disease is described as having two phases, the first an acute febrile illness (sometimes known as Oroya fever) characterized by fever, generalized lymphadenopathy, myalgia, headache, jaundice, and severe hemolytic anemia with fatality rates of as high as 90%. Meningeal and cerebral involvement can occur in up to 20% of patients with Carrion's disease and manifests as delirium, paralysis, and seizures. A subsequent chronic and cutaneous eruptive phase of the disease is characterized by development of verrucous dermal eruptions that result from proliferation of vascular endothelial cells (183, 184).
Trench fever.
Trench fever is so named because it classically occurred in the trenches of World War I among various European armies' troops but is also linked with a more recent epidemic that has been reported among homeless people in impoverished urban areas of several countries (148, 185). Trench fever is caused by B. quintana and is transmitted person to person by the body louse, P. humanus. It is characterized by a sudden onset of high-grade fever, retro-orbital headache, myalgia, and bone pain, especially over the pretibial area. It is classically described as a 5-day relapsing fever, hence its other name, febris quintana, from the Latin for five. The disease can take a chronic course and last several weeks, during which bacteremia is common and cardiac involvement may complicate the course with insidious onset of endocarditis (186).
CSD.
Cat scratch disease (CSD) is caused primarily by B. henselae and is transmitted by the scratch or, less likely, by the bite or lick of cats (150). Other Bartonella species have been implicated in CSD-like disease in individual case reports (44, 53, 187). Even though more than 50% of domestic cats may be carriers of Bartonella as shown in some studies, they usually do not show signs or symptoms of infection (140, 188, 189). B. henselae has also been isolated in fleas recovered from infected cats (140). The classic description of CSD involves a scratch by a cat followed by local inflammation 10 to 14 days later and significant enlargement of regional lymph nodes. Systemic symptoms such as fever and malaise typically develop and could last for several weeks. Most cases run a benign course and resolve spontaneously, although serious complications such as meningitis, osteomyelitis, encephalitis, and endocarditis are known to occur (190, 191). Oculoglandular syndrome (also known as Parinaud's oculoglandular syndrome) is an ocular manifestation of cat scratch disease of granulomatous conjunctivitis with pre- and postauricular lymphadenopathy; a recent case report also highlights an expanding spectrum of ocular involvement by CSD presenting as an optic nerve granuloma (192, 193). Transmission between cats is mainly by the cat flea, although other arthropods, mainly ticks of the genus Ixodes, have been proposed as possible vectors (194).
BA.
Bacillary angiomatosis (BA) is a proliferative disease of vascular epithelia manifesting as a solitary or multiple papulonodular cutaneous lesions. The etiologic agents are both B. henselae and B. quintana (32, 33, 195). BA was originally described in patients with HIV and other immunocompromised status such as organ transplant recipients on immunosuppressive therapy; however, it has since been described in immunocompetent hosts (196). The cutaneous lesions are highly vascular, bruising or bleeding easily, due to the underlying effect of these species of Bartonella to cause abnormal vascular endothelial cell proliferation and neovascularization (183, 197). The skin lesions could be superficial or deep in the subdermal structures, at times even involving the bones. Regional lymphadenopathy and involvement of mucous membranes of the mouth, conjunctivae, and the gastrointestinal tract, including the perianal area, have also been described. Visceral involvement is also known to occur involving the liver, spleen, lymph nodes, and the bone marrow, with other reports documenting isolated visceral involvement in the absence of cutaneous lesions (198, 199).
Peliosis hepatis.
Peliosis hepatis is characterized by multiple vascular, hemorrhagic parenchymatous and cystic lesions of the liver ranging from a few millimeters to 3 cm in size. Peliosis was originally described in a case report associated with tuberculosis in 1916 (200). Several subsequent reports have shown association with many other pathological conditions, including multiple infectious and noninfectious diseases such as neoplastic processes and exposure to toxins and anabolic steroids (201, 202). In more recent years, peliosis has been distinctly associated with HIV infection (203, 204). Histologically, the lesions of peliosis show dilated capillaries, vascular hyperplasia, and inflammatory cells much as in the case of BA (30, 198, 205). B. henselae is the species most often associated with peliosis hepatis, and affected patients usually present with abdominal pain, fever, and weight loss. Hepatomegaly is usually present, and some patients may have concomitant cutaneous lesions of BA (206).
Bartonella-related mimics of autoimmune disease.
Juvenile arthritis and myositis associated with high serum titers for B. henselae that rise and fall with disease activity have been reported in children (207, 208). Although causal association has not been proven, increased rates of seropositivity for B. henselae have been described in patients with leukocytoclastic vasculitis and Henoch-Schönlein purpura (209, 423) as well as in a case of Coombs-positive autoimmune hemolytic anemia (210). Cases of uveitis associated with HLA-B27 seropositivity have also been described in B. henselae-infected patients presumed to have ocular involvement by Bartonella (211). It should be noted that the association of Bartonella species with autoimmune disorders has been largely limited to serology-based testing and could be compromised by cross-reacting antibodies.
Bartonella as a risk factor for atherosclerosis.
Several infectious agents, including Chlamydophila pneumoniae, Helicobacter pylori, cytomegalovirus, and periodontal pathogens, have been reported to contribute to atherosclerotic vascular disease (see reference 212 for a review). Antimicrobial activity of statins has been reported, and it has been suggested that this activity may, at least in part, be responsible for the reduction in cardiovascular mortality associated with the use of these drugs (213). However, antibiotic treatments have largely failed to significantly reduce cardiovascular mortality (214). B. henselae has been shown to infect human CD34+ hematopoietic progenitor cells, and it was proposed that these cells may serve as the primary niche of infections (215). A subsequent report showed that endothelial progenitor cells were infected with B. henselae, resulting in damage counteracted by nitric oxide as demonstrated by the administration of l-arginine (216). It has been proposed that B. henselae infection of endothelial progenitor cells could reduce both the number of these cells and their functionality (216). In so doing, the natural repair role for endothelial progenitor cells would be diminished, thereby indirectly contributing to the growth of atherosclerotic plaque (217). Further study is needed to support the role of the interaction of Bartonella with both endothelial progenitor cells and endothelial cells. The association of Bartonella species with the onset and progression of atherosclerosis remains speculative at this point.
Myocarditis.
Myocarditis associated with both B. henselae and B. quintana infection has been reported (218–220). While myocarditis is not a common clinical presentation of Bartonella infection, it warrants mention for the severe disease course reported to manifest in one specific affected group. During the period of 1979 to 1992, 16 sudden, unexplained cardiac deaths were reported in elite Swedish orienteers; orienteering is a popular activity in Sweden with extreme physical demands, extended outdoor exposure, and interaction with nature (221). Of the 16 fatal cases, myocarditis was the most common diagnosis; heart tissue from five orienteers was tested by PCR, and Bartonella species was detected in four (221). Additionally, four of the five patients' sera were tested and shown to have antibodies to Bartonella species (221). A subsequent retrospective study on 1,136 sera from orienteers showed that 31% had antibodies to Bartonella compared to 6.8% in time-matched healthy blood donor control sera (222). The authors of that study concluded that antibodies to Bartonella species in Swedish orienteers may be indicative of risk factors associated with the development of myocarditis and sudden unexpected cardiac death in these athletes who were previously in good health (221, 222).
Bacteremia.
Invasion of erythrocytes and prolonged intraerythrocytic presence in their respective reservoir hosts (intraerythrocytic bacteremia) are one of the hallmarks of inoculation of a reservoir host by Bartonella species (223, 224). Bartonella species have been shown to employ several molecular mechanisms as a basis for invasion of erythrocytes and evasion of the immune responses that would otherwise typically trigger symptoms in bacteremic hosts (225–228). Such asymptomatic bacteremia has been reported in various nonmammalian and mammalian hosts, including humans (8, 110, 229, 230). Asymptomatic bacteremia has been shown to persist from a few weeks to several months in studies conducted in healthy human volunteers (231). In humans, a small proportion of the erythrocytes, usually no more than 1%, is infected by B. quintana, and such low-level bacteremia can persist for months to years with no or only subclinical symptoms (153, 224, 232). In a rat model of infection with B. tribocorum, B-cell-deficient rats had a more prolonged bacteremia than immunocompetent rats (232, 233). It is plausible that low-level bacteremia may precede the pathogenesis and eventual clinical development of endocarditis in humans; however, this has not been epidemiologically or experimentally confirmed (234).
BCNE.
Blood-culture negative endocarditis (BCNE) is generally defined as endocarditis where the microbial etiology cannot be established after at least three different blood samples in a standard blood culture system fail to grow an organism after at least 5 days of incubation (235). The incidence of reported BCNE varies dramatically, ranging from 2.5% to 76% of all infective endocarditis cases (236–238). One of the reasons for such broad variability of the incidence of BCNE is the geographic variation in the reported rates of culture-negative endocarditis, probably reflecting the differences in availability of testing alternatives. For instance, reports from South Africa, Algeria, and Pakistan put the rates of culture negativity at about 50% of all endocarditis cases (238–240), whereas case series from Japan, France, and the United Kingdom report BCNE rates of 12 to 20% (235, 241, 242). In addition to geographic variations, earlier reports may have overestimated the incidence (of culture negativity) partly because of the limited testing capability in the past, even in developed nations, with ever-expanding contemporary options for serologic and molecular testing. It is now estimated that BCNE accounts for around 5% of all endocarditis cases (236, 241, 243–245). There are various reasons why a blood culture may be negative in the face of endocarditis (hence presumed bacteremia). The main reasons for culture negativity are pretreatment with antibiotics prior to the blood culture; nonbacterial etiologies for the endocarditis (such as fungal etiology, for instance); right-sided endocarditis, which may not be as bacteremic in systemic circulation; and the presence of implanted devices such as pacemakers or implantable defibrillators (246–248). The microbial etiologic agent of the endocarditis being fastidious and thus not easily grown on standard culture medium is one of the most important reasons why blood culture may be negative in the case of endocarditis (86, 177, 249). Bartonella is also thought to account for 3 to 4% of all cases of endocarditis (97, 177). While B. quintana and B. henselae account for the majority of cases of endocarditis, several other species of Bartonella have been shown to cause endocarditis, as shown in Table 1 (18, 86, 250, 251).
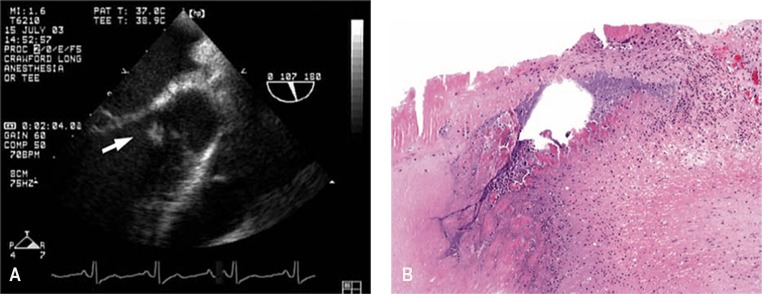
The majority of patients with Bartonella endocarditis have clinical presentations similar to other cases of subacute bacterial endocarditis. Nonspecific symptoms such as fever, fatigue, and weight loss predominate in the clinical picture. In one series of 348 cases of BCNE from France, Bartonella species accounted for 28% of the cases (86). Almost all of the patients had fever as a presenting symptom, whereas about 50 to 70% had symptoms of heart failure such as exertional dyspnea and about 50% had insidious weight loss (86, 252–254). Physical examination findings typically include cardiac murmur, and the aortic valve either in isolation or with another valve is the most frequently affected valve, including in the pediatric age group (255–258). In a detailed report, a 33-year-old man with a known bicuspid aortic valve was shown to have BCNE confirmed by echocardiography that was shown to be caused by B. henselae (258) (Fig. 4).
FIG 4.
(A) Transesophageal echocardiogram from a patient with BCNE caused by B. henselae. Bicuspid aortic valve with left coronary leaflet almost entirely replaced by a large vegetation (arrow). (B) Giemsa stain of the patient in panel A showing extensive fibrosis and coccobacilli on the aortic valve that were confirmed to be B. henselae. (Both panels reproduced from reference 258 with permission from Elsevier.)
While the majority of the valves affected were native valves, prosthetic valve involvement has been reported, and prosthetic valve involvement by Bartonella seems to take a more aggressive course with valve perforation and rapid development to heart failure (12, 259, 260). Additional physical exam findings include splenomegaly, which has been reported in up to 40% of Bartonella endocarditis cases in one series; thromboembolic phenomena; digital clubbing; and hepatomegaly (86, 175, 177). The most commonly observed laboratory abnormalities include elevated inflammatory markers such as erythrocyte sedimentation rate (76 to 83%), anemia (55 to 68%), thrombocytopenia (33 to 50%), elevated liver enzymes (20%), evidence of renal failure (40 to 50%), leukocytosis, and positive rheumatoid factor (86, 175, 177). Earlier reports showed Bartonella endocarditis with significant mortality rates of 7 to 30%; however, more recent studies report mortality rates in the lower range, probably signifying improved diagnostic and therapeutic measures, including improved surgical techniques (255, 261, 262).
Currently, there is a lack of criteria for the diagnosis of endocarditis caused by Bartonella species (177). The use of traditional blood culture methods is hampered by the low rate of culture positivity and the need for prolonged incubation time, use of specialized media, and special growth conditions. Despite measures to optimize the yield, blood cultures are likely to remain negative, with some estimates showing that up to 75% of Bartonella endocarditis cases could be culture negative (90, 97, 263). Therefore, diagnostic criteria that rely on blood culture positivity, such as the Duke criteria, are likely to miss a significant proportion of Bartonella endocarditis cases. Moreover, due to the indolent nature of Bartonella infections, the other major Duke criterion of echocardiographic evidence of vegetation may not be as readily apparent as it is for the other types of endocarditis. Thus, the utility of the Duke criteria to diagnose Bartonella endocarditis using blood culture and echocardiographic evidence as major criteria has been in question (264). Serologic testing for IgG antibodies to either B. henselae or B. quintana using microimmunofluorescence techniques has been used for the diagnosis of Bartonella endocarditis in several studies. A Bartonella IgG titer of ≥1:800 is recommended as the cutoff for a positive test, offering high sensitivity, specificity, and positive predictive value (177, 265, 266). Likewise, PCR testing of blood, plasma, or serum samples taken from confirmed cases of Bartonella endocarditis was shown to have a sensitivity of about 58% and specificity of 100% (267). The use of serology and PCR testing has been proposed and eventually added as major Duke criteria for Coxiella burnetii infection, which is epidemiologically and demographically closely related to Bartonella endocarditis (268, 269). Similarly, incorporating a positive Bartonella serology or PCR test as a major Duke criterion for the diagnosis of Bartonella endocarditis has been proposed (177, 241, 264, 265, 270–273). Thus, available evidence advocates including B. henselae or B. quintana IgG serology at ≥1:800 or a positive PCR as a major Duke criterion for the diagnosis of Bartonella endocarditis.
(i) B. quintana endocarditis.
B. quintana accounts for about three-fourths of Bartonella endocarditis cases. After transmission by the human body louse P. humanus, bacterial entry across the skin occurs and adherence to and infection of erythrocytes and endothelial cells ensue. The resulting bacteremia can persist for prolonged periods, at times lasting for years. B. quintana has evolved with variably expressed outer membrane proteins (Vomps) that are believed to be essential for infectivity as well as evasion of detection by the host immune system (74, 274). B. quintana has also been shown to induce intracellular signals that lead to decreased apoptosis and increased proliferation of vascular endothelial cells (see Pathogenesis of Bartonella Species), attributes believed to enhance its capacity to cause chronic infection and intracellular aggregation in endothelial cells, including valvular endothelium (275). Vascular and valvular endothelial cells are also targets of B. quintana colonization and production of cytokines and mitogenic factors, leading to endothelial proliferation and cytoskeletal rearrangement (228, 276). Thus, B. quintana employs several strategies to evade host immunity and cause a prolonged and asymptomatic bacteremia that culminates in the development of endocarditis. These pathogenic mechanisms of B. quintana underlie the insidious clinical presentation and subtle variations in clinical findings of endocarditis caused by B. quintana.
(ii) B. henselae endocarditis.
B. henselae accounts for about one-fourth of Bartonella endocarditis cases. The majority, but not all, of B. henselae endocarditis cases have a history of contact or interaction with a cat. B. henselae shows tropism for endothelial cells and replicates and persists in the endothelium similarly to B. quintana. Pathogenic mechanisms include Bartonella adhesin A (BadA) and the TAA, which is expressed in B. henselae as well as in B. quintana (70, 277, 278).
(iii) Treatment considerations for Bartonella endocarditis.
By virtue of their pathogenic and virulence mechanisms, namely, intraerythrocytic propagation and ability to persist in a primary niche, Bartonella species are in general endowed with a substantial capacity to evade the host immune system and to resist antimicrobial agents (156, 181, 224, 279, 280). Earlier studies have reported widespread in vitro susceptibility of several Bartonella species to various classes of antibiotics, including penicillins, beta-lactams, macrolides, and aminoglycosides (280–282). However, subsequent studies and clinical experience have shown that treatment failures of Bartonella infections are a significant problem despite seemingly low MICs suggesting susceptibility (282–284). Moreover, several of the antimicrobial classes tested against Bartonella species exhibit only bacteriostatic properties, with the exception of aminoglycosides such as gentamicin (283, 285, 286). The issue of host defense evasion, potential biofilm formation, and hence resistance to antimicrobials is even more acutely important in cases of Bartonella endocarditis where cultures are likely to be negative and diagnosis delayed. Based on experience from the past 2 decades in the treatment of Bartonella endocarditis, multiple reports and recommendations advocate the use of at least two antibiotics, one of them being an aminoglycoside (172, 287, 288). The recommended duration of therapy is generally for a minimum of 4 weeks in native valve disease and a minimum of 6 weeks in prosthetic valve endocarditis. Aminoglycosides are recommended at least for the first 2 weeks of therapy, and the duration of combined aminoglycoside use and the total duration of therapy correlate with a beneficial clinical outcome (98). Thus, current recommendations for treatment of Bartonella endocarditis stress the use of an aminoglycoside at least for the first 2 weeks of therapy in conjunction with a two-drug regimen, the second drug being a beta-lactam, a macrolide, or a tetracycline depending on the specific concomitant clinical considerations (289, 290). The combination of gentamicin and doxycycline has been suggested in a recent report (291).
PATHOGENESIS OF BARTONELLA
Human Infection
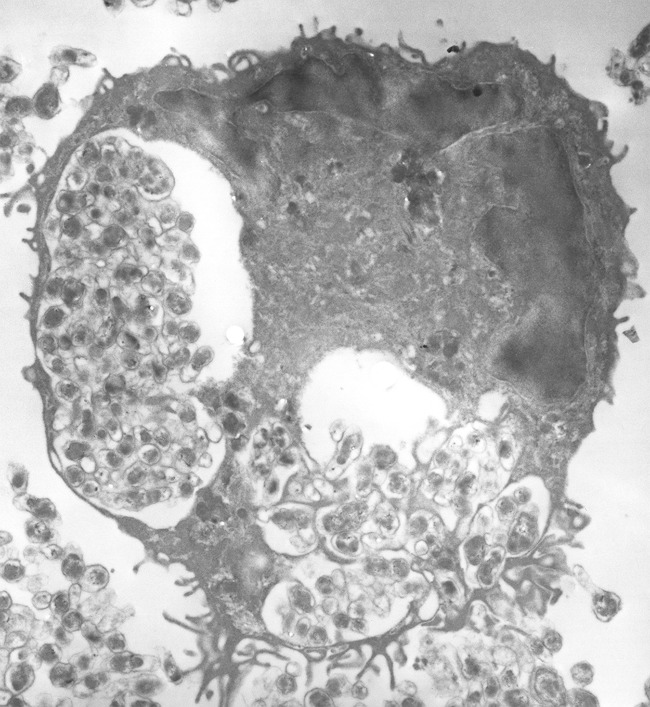
Pathogen-associated molecular patterns (PAMPs) are molecules associated with specific pathogen groups that are recognized by cells of the innate immune system. To detect invading pathogens such as bacteria and viruses, the immune system is equipped with pattern recognition receptors that are specialized in their recognition, including the Toll-like receptor (TLR). Following intravenous or intradermal inoculation with Bartonella, the bacteria evade the host innate immune system. This has been attributed to the inability of the TLR to identify the lipopolysaccharide on the outer membrane of the bacteria as a result of the reduced endotoxic activity of the lipopolysaccharide of Bartonella (292). Moreover, B. quintana employs strategies to dampen the host inflammatory response through overproduction of the anti-inflammatory interleukin-10 (IL-10) and antagonizing proinflammatory factors such as Toll-like receptor 4 (293, 294). B. bacilliformis, which possesses flagella, structures that are recognized by the TLR, is also known to evade the innate immune system because of a primary amino acid sequence change in the flagellin which promotes its evasion (295). After innate immune system evasion, the bacteria are cleared from the circulating bloodstream for their primary niche, most likely the endothelium, where they grow and seed back to reinfect the blood, causing bacteremic relapses in cats, mouse models, and rhesus macaques (74, 127, 224, 296). Bartonella is known to infect a range of host cells, but the endothelial cell is thought to be the primary niche location (296). This hypothesis stems from the evidence shown in the verruga peruana of B. bacilliformis characterized by tumors from endothelial cell proliferation (297) and studied in vitro (183, 298). B. quintana and B. henselae have also been shown to invade endothelial cells (299, 300). B. henselae has been shown to invade human endothelial cells in vitro by both a VirB/VirD4 T4SS-dependent mechanism resulting in intracellular invasion of large aggregates of bacteria termed invasomes (Fig. 5) and a VirB/VirD4 T4SS-independent mechanism (275, 277). While B. henselae has been shown to invade human endothelial cells in vitro by these two different mechanisms, the role of intracellular growth in facilitating human disease is not clear (301, 302).
FIG 5.
Transmission electron micrograph of a B. henselae invasome after internalization into an endothelial cell. Magnification, ×12,000.
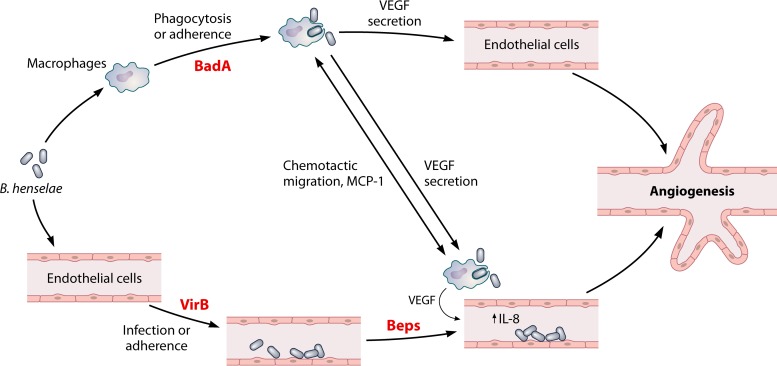
Regardless of the proposed role of endothelial cells in serving as the primary niche of infection by Bartonella species, it is clear that the interaction with the endothelium results in a host response that is unique to these bacteria. This unique angiogenic host response results from infection by each of the three major human pathogens in the genus Bartonella, B. bacilliformis, B. henselae, or B. quintana. Angiogenesis caused by B. henselae is induced by production of proangiogenic factors such as vascular endothelial growth factor (VEGF), by promoting endothelial cell proliferation, and by suppressing apoptosis of vascular endothelial cells (303–306). VEGF is also a potent mitogen and tumor angiogenesis stimulator, and during B. henselae infection, production of VEGF is shown to be increased in microvascular endothelial cells (300). The production of VEGF by B. henselae has been shown to be regulated by the TAA-dependent activation of hypoxia-inducible factor 1 (Hif-1) (77, 307). B. henselae has also been shown to reprogram human endothelial progenitor cells (308). B. bacilliformis infection shows evidence of endothelial cell proliferation in its vascular tumor (Peruvian warts) evident during the chronic phase, and in vitro models have shown that it stimulates endothelial cells and also induces angiogenesis (183). The production of VEGF during B. henselae infection has been shown to require BadA and occurs through a hypoxia-inducible factor 1-dependent mechanism (77). A similar role has been proposed for the Vomps of B. quintana (309). In contrast, the inhibition of apoptosis has been shown to be a result of delivery of the Bartonella effector proteins (Beps), namely, BepA, by the VirB/VirD4 T4SS of B. henselae (310). Thus, in the case of B. henselae, the coordinated efforts of BadA and the VirB/VirD4 T4SS are thought to be required for the angiogenic host response induced by this bacterium (311). A paracrine angiogenic loop model has been proposed to explain this unique host response in B. henselae (Fig. 6) (312). However, it should be noted that B. bacilliformis is able to induce dramatic angiogenic lesions in infected patients, but this species has no known VirB T4SS encoded in its genome. Thus, the role of the VirB/VirD4 T4SS appears to be dispensable for at least some Bartonella species to induce angiogenesis. Alternatively, it is possible that B. bacilliformis has as-yet-undefined factors that function in a capacity similar to that of the Beps to augment the role of the TAAs in eliciting a proangiogenic host response. Mitogenic activity associated with the B. bacilliformis GroEL chaperone for human umbilical vein endothelial cells has been demonstrated, suggesting a possible role for this protein in Bartonella-induced angiogenesis as well (313).
FIG 6.
Paracrine angiogenic loop model for B. henselae. The role of BadA, VirB, and the cognate effectors (Beps) in inducing the angiogenic host response that is unique to Bartonella species is shown. (Adapted from reference 312.)
Virulence Factors
Every bacterium carries virulence factors specifically adapted to its needs for invasion, colonization, replication, and survival in the host cell, and Bartonella species are no exception. With the exception of virulence-associated surface-exposed proteins, most bacterial virulence factors are delivered either to the extracellular environment or directly into host cells (314). The virulence factors described to date for Bartonella species fall into these two categories. While many virulence factors for these bacteria have been described, infection by Bartonella species is facilitated by the presence of two major virulence factors, the TAAs and the T4SSs.
The TAA family of proteins.
The TAAs of Gram-negative bacteria are a family of proteins that are considered type V secretion systems. While there is considerable variability in TAA size, sequence conservation, and the number of gene copies in the genomes of different Bartonella species, all Bartonella species appear to have at least one TAA gene (315). The TAA family of proteins is found in many other Gram-negative bacteria (316). The TAA protein family is termed BadA in B. henselae (77), Vomps in B. quintana (74), and Bartonella repeat proteins (Brps) in B. vinsonii (73). The role of BadA and the Vomps in virulence in the genus Bartonella is the best-studied facet of these TAAs.
(i) BadA.
BadA has been ascribed several functions, the first of which is adherence to host extracellular matrix proteins or target endothelial cells. BadA is a monomer of 328 kDa that forms filaments that are about 240 nm long on the surface of B. henselae (315). Other bacteria possess TAAs with similar characterized adherence functions such as Yersinia (YadA) (317) or Neisseria meningitidis (NadA) (318). TAAs form extracellular filaments composed of head and stalk domains assembled on a C-terminal membrane anchor, forming a “lollipop-like” structural architecture (315, 319, 320). During assembly, the TAA is secreted into the periplasm while the membrane anchor builds a homotrimeric 12-stranded beta-barrel in the outer membrane. This trimerization is required to maintain the stability and adhesive property of the protein (319). The trimer barrel forms a pore which transports the head and stalk domains to the cell surface, and the C-terminal part of the stalk clamps the pore (321). Previous experiments with mutant strains show that while the BadA head is responsible for adherence to the extracellular matrix and autoagglutination (AAG), the stalk is required for fibronectin adherence (322). BadA prevents bacteria from being phagocytized and also stimulates endothelial cell proliferation by inducing a proangiogenic host cell response through activation of Hif-1, a crucial transcription factor for angiogenic cytokine secretion (77, 323, 324). A ΔbadA mutant of B. henselae was shown to exhibit reduced replication and a weakened proangiogenic host response in a zebrafish embryo model (325).
Even within B. henselae, the size of the BadA protein is variable, and it is thought that much of this variation is due to the different size of the repeating neck/stalk regions (72). Likewise, the amount of surface-localized BadA is also highly variable, ranging from no detectable BadA in the Berlin-1 and ATCC 49793 strains to very high expression in the Marseille strain (72). Regulation of the badA gene has been attributed to the BatR/S two-component regulatory system (326) and the general stress response system (327). More recent studies have shown that a family of nine unannotated and highly transcribed RNAs designated Bartonella regulatory transcript (Brt1 to Brt9) found upstream of a putative transcriptional regulator protein are important in the regulation of badA (75).
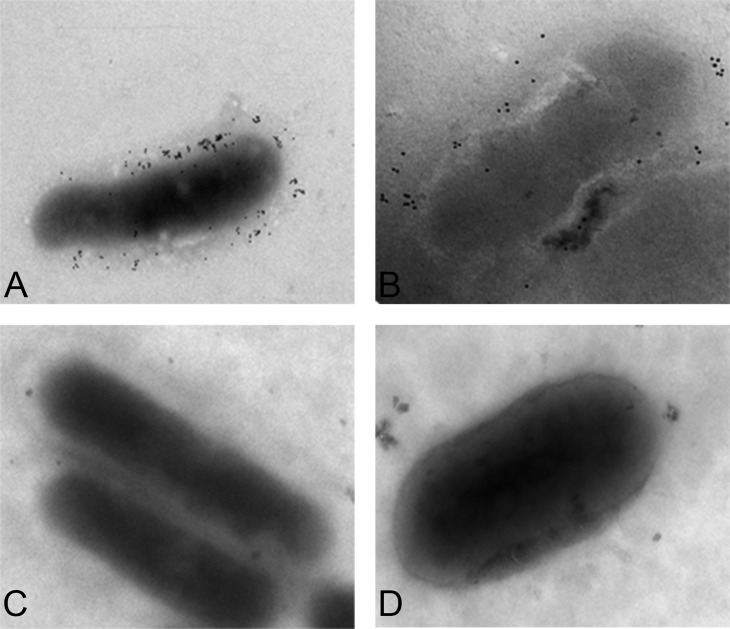
Considerable confusion exists in the literature with regard to the genome sequence (accession no. BX897699) of the badA locus for the Houston-1 type strain of B. henselae (ATCC 48892), which reports a 1-bp deletion in the anchor of the badA gene (BH01510) (82). This results in the BadA anchor region being annotated as a separate 67-amino-acid-protein-encoding gene (BH01520) which partly overlaps the 3′ end of BH01510 (77). Riess et al. subsequently reported that the B. henselae Houston-1 strain exhibits surface-localized BadA and does not contain this mutation in the membrane anchor region of BadA (72). Independent sequence analysis of low-passage-number B. henselae Houston-1 in our laboratory shows BH01510 and BH01520 as a single merged open reading frame (unpublished data). Furthermore, immunoelectron microscopy shows a surface-localized BadA, indicating a functional membrane anchor domain (Fig. 7), suggesting that the genome sequence reported by Alsmark et al. (82) may reflect a higher-passage-number variant with a laboratory-derived mutation in the badA gene. It should be noted that other strains or laboratory-derived variants have also been shown to be defective for either badA expression or BadA surface localization (72).
FIG 7.
Expression and surface localization of BadA in B. henselae. Houston-1 (A) and Marseille (B) strains were reacted with rabbit anti-BadA antibody, followed by goat anti-rabbit IgG conjugated to 10-nm colloidal gold particles. Cells were washed, suspended in phosphate-buffered saline, transferred onto a copper-coated grid, air dried, and imaged using a JEOL JEM 1400 microscope. Surface localization of BadA can be seen in both the Houston-1 and Marseille strains but not the isogenic badA deletion mutants (Houston-1 ΔbadA mutant [C] and Marseille ΔbadA mutant [D]). The markerless, nonpolar in-frame Houston-1 deletion mutant was constructed as previously described (325). The Marseille deletion mutant was constructed by the same approach (unpublished data). Rabbit anti-BadA antibody was raised to the stalk region of the BadA protein (77) and was generously provided by Volkhard Kempf.
(ii) Vomps.
Vomps are a multigene family of TAA proteins found in B. quintana that have a similar modular structure (head-neck-membrane anchor) as BadA of B. henselae (74, 328). There are four Vomps (A to D), which, like BadA, function as adhesins mediating host cell adhesion and autoaggregation in B. quintana (74). Expression of the vomp genes varies in the host and is thought to suppress the host immune response, favoring adaptive interaction (74). The Vomps are closely related to the afimbrial adhesin YadA, a TAA of Yersinia enterocolitica. The surface-expressed Vomps contain conserved structural features of YadA, including collagen-binding motifs (74, 329). VompC confers the ability to bind collagen IV, and VompA is necessary and sufficient for autoaggregation (74). In vivo, Vomp genes are differentially expressed, and gene deletions were shown to occur during prolonged bloodstream infection (74).
VirB/VirD4 T4SS.
Bacterial T4SSs are present in many Gram-negative bacteria, including Helicobacter pylori, Coxiella burnetii, Agrobacterium tumefaciens, and Legionella pneumophila (224, 330–332). In B. henselae, the VirB/VirD4 T4SS is the best-characterized T4SS among the Bartonella species (see reference 223 for a review). The B. henselae VirB/VirD4 T4SS is comprised of a multiprotein system (VirB2 to VirB11) which translocates Beps to target cells through a contact-dependent process that is thought to require a pilus-like surface-protruding filament that is believed to consist of VirB2 and VirB5 (303, 333–336).
Postadherence, host cell signaling interruption is mediated via the translocation of seven unique Bartonella effector proteins (BepA to -G) (337), all encoded immediately downstream of the VirB/VirD4 T4SS on the genome, by using the B. henselae VirB/VirD4 T4SS for delivery. VirB T4SSs consist of a substrate translocation channel which spans the two membranes of Gram-negative bacteria and a surface filament which extends from the bacterial envelope and establishes contact with target cells. This translocation channel extends to the host cell membrane and facilitates translocation of substrate. Bacteria gain entry into human endothelial cells either as a single bacterium using a VirB/VirD4 T4SS-independent zipper-like mechanism (302) or through an invasome-mediated uptake requiring the VirB/VirD4 T4SS (Fig. 5) (275). The VirB/VirD4 T4SS delivers BepG for invasome-mediated uptake (338) and/or BepC and BepF to extensively rearrange the actin cytoskeleton (339). This rearrangement produces bacterial aggregation and ultimately engulfment by host cell membranes and entry into endothelial cells via the invasome (275). The presence of the bacterium causes a proinflammatory response activating NF-κB, and cytokines that promote inflammation such as tumor necrosis factor alpha (TNF-α) are released. Activation of NF-κB induces the secretion of interleukin-8 and the expression of intercellular adhesion molecule 1 (ICAM-1) and E-selectin.
This VirB/VirD4 T4SS is also crucial for the inhibition of endothelial cell apoptosis (340) and intraerythrocyte infection; a mutant defective in virB4 and virD4 was not bacteremic in a model system (332). On delivery into the endothelial cell, BepA also localizes to the plasma membrane to induce production of second messenger cyclic AMP conveying an antiapoptotic property to the cells (341). Bartonella infection is known to suppress early and late events in apoptosis, namely, caspase activation and DNA fragmentation, respectively (304). The translocation of the Beps into endothelial cells, together with the induction of VEGF production, which is dependent on the presence of BadA on the surface of B. henselae, is thought to work together to induce the angiogenic host response (Fig. 6). The interplay of BadA and the VirB/VirD4 T4SS has been studied, and it was shown that BadA can affect effector translocation (311). The contributions of each of these two virulence factors in inducing angiogenesis are not yet entirely clear, and study has been hampered by the lack of a practical animal model.
Trw T4SS.
Asides from VirB/VirD4, a second type of T4SS, Trw, has been identified as a molecular determinant of host-specific erythrocyte infection (342). It is a set of virulence genes that were laterally acquired, and the gene products are effective in promoting prolonged bacteremia through erythrocyte adhesion and infection (342–344). Trw does not translocate any known effectors but produces multiple variant pili involved in the attachment and invasion of host erythrocytes (345). The presence of Trw has been shown to correlate with loss of flagella, which represents a major pathogenicity factor for erythrocyte invasion by B. bacilliformis and probably other flagellated Bartonella (70). Variable expression of some Trw protein genes appears to correlate with the presence or absence of other Trw proteins. The Trw T4SS has several components: of more importance are the adherent components, TrwL and TrwJ, which are surface-exposed components that mediate host specificity (342). TrwL and TrwJ have been referred to as anchoring and pilus proteins, respectively (346). In in vitro adhesion and invasion assays, the TrwJ and TrwL transposon mutants showed reduced adhesive properties and lacked the ability to invade the erythrocyte (342).
Hbps.
Heme is essential for Bartonella survival. In the gut of their arthropod vector, Bartonella species are exposed to toxic concentrations of heme, which is otherwise confined to the bloodstream of the mammalian host. Thus, one of the roles of the multigene family of hemin binding proteins (Hbps) is to facilitate survival in the vector, as is the case for B. henselae in the cat flea (347). It is thought that Bartonella uses HbpA for iron acquisition and to bind hemin required for bacterial growth (348). Five different Hbp genes have been identified in both B. henselae (349) and B. quintana (350). The Hbps also serve as adhesins and have been shown to bind fibronectin and facilitate entry into endothelial cells (351). HbpC, in contrast to HbpA, is sensitive to environmental changes such as those in temperature and hemin and is overexpressed in the arthropod gut. HbpC is hypothesized to bind hemin to prevent access to the bacterial cell and counter the resultant heme toxicity (4). Deletion of the hbpC gene also affected the ability of B. henselae to infect the cat host. HbpA and HbpC were also observed on the outer membrane vesicles produced by B. henselae, and constitutive expression of HbpC increased the amount of hemin associated with the vesicle. It should be noted that Pap31, originally described as a protein associated with the BLP of B. henselae (352), is homologous to the multigene Hbp family of Bartonella, Omp31 of Brucella, Agrobacterium tumefaciens Omp25, and opacity proteins of Neisseria species (350). Pap31 is also thought to be involved in heme acquisition and virulence and is also said to bind fibronectin and heparin, possibly on the same domain (349, 350, 353).
Fhas.
The filamentous hemagglutinins (Fhas) are a family of type V secretion system proteins encoded in the genome of some Bartonella species, including B. henselae, but not all species. Fhas are known to be a virulence factor in other Gram-negative bacteria like Bordetella pertussis (354, 355) and Pasteurella multocida (356) by facilitating adhesion. Nine copies of full-length and truncated fhaB genes (fhaB1 to -9) are found on the B. henselae genome with each gene copy being immediately downstream of or in close proximity to the hemolysin activator gene (hecA); hemolysin activator is a separately encoded protein that is thought to serve as a transporter domain for FhaB (315). While the Fhas represent additional potential pathogenicity factors, it is not clear which of the Fha gene loci are expressed in B. henselae. Proteomics studies have shown the presence of antibodies in cat sera that are reactive with the FhaB2 protein of B. henselae, providing indirect evidence that at least one of the FhaB loci is expressed in vivo (357). It has been proposed that the Fha proteins may play a supporting role for adherence when BadA is not expressed in B. henselae (315). Regardless, the function of the Fha proteins in Bartonella adherence and virulence remains unknown.
Other Bartonella adhesins and outer membrane proteins.
Surface labeling of B. henselae demonstrated several surface-localized proteins, a subset of which were shown to bind human endothelial cells (358). One of those, Omp43, functions as an adhesin during B. henselae interaction with endothelial cells (359), binds fibronectin (360), and bears homology to the Omp2 family of porins from Brucella spp. Omp43 was shown to be immunogenic in cats, with sera from most infected cats being reactive to this protein (361). Another putative adhesin of B. henselae is Omp89, an immunogenic surface protein containing a zinc metalloprotease domain that has been shown to bind fibronectin (360). Other membrane-associated proteins, GroEL, HtrA, and Omp89, are also thought to be involved in protein folding or degradation (360–362). In B. bacilliformis, several outer membrane proteins between 11.2 and 100 kDa have been identified (363, 364). Six of these have been shown to mediate bacterial interactions with erythrocytes (363). A 43-kDa immunogenic lipoprotein, which is a homologue of LppB proteins of Haemophilus spp. that are associated with virulence, has been reported (365). B. bacilliformis also possess a flagellin, a 42-kDa protein subunit of the flagella which has been shown to be resistant to protease or trypsin treatment and provides the bacteria with motility (43, 366).
Invasins.
Invasion-associated locus A and B genes (ialAB) of B. bacilliformis were first described by Mitchell and Minnick (226). These genes are necessary for both the invasive phenotype and its erythrocyte parasitism (367). In B. bacilliformis, IalB is localized to the inner membrane, but in B. henselae, it is localized on the outer membrane (226, 361, 367). IalB has been implicated in invasion of erythrocytes in both B. bacilliformis and B. henselae. In B. bacilliformis, IalB is produced in increasing amounts as the temperature drops below 37°C (368), but it has also been noted that other environmental changes like those in iron availability and pH have an effect on expression (367). In B. henselae, IalB and Omp43 elicit an antibody response that varies between cats (226, 361, 367). IalB has also been shown to be required for intraerythrocytic bacteremia (367). In B. birtlesii, IalB was localized to the outer membrane and shown to be required for invasion of erythrocytes but not adherence (342). In B. henselae, IalB is thought to facilitate entry into erythrocytes, and recombinant IalB protein has been reported to be immunogenic and proposed as a possible diagnostic tool for an immunoassay (369). Genome analysis of B. elizabethae and B. grahamii also shows the presence of an ialB locus (370, 371).
Growth in Biofilms
A biofilm may be defined as a cluster of bacterial cells embedded in a matrix, which is more tolerant of most antimicrobials and host defenses than are free-floating single cells (372). Surface protein adhesins facilitate substrate adherence and autoagglutination (AAG). Adherence has been described as a two-phase process: phase 1, in which the bacterial cells first behave as dormant colloidal particles making reversible contact with surfaces according to their physiochemical properties, and a subsequent phase in which biologically produced adhesins anchor the cells more irreversibly to the surface (373). AAG activity is a known signal for host cell interaction and virulence in several Gram-negative bacteria, and outer membrane proteins of these bacteria have been demonstrated to be autoagglutinins (374). Once autoagglutinated, bacteria communicate with one another using chemical signal molecules, a process called quorum sensing, which allows bacteria to monitor the environment and to change behavior depending on conditions in the community. Quorum sensing is critical for biofilm formation (375). Biofilms are formed when bacteria irreversibly adhere to a surface, communicate, and excrete a protective matrix. Production of an extracellular polymeric substance (EPS) matrix, reduced growth rates, and alternating regulation of specific genes distinguish biofilm sessile aggregates from their planktonic counterparts (376). Biofilms are characterized by their stability, chronic bacterial infections, and increased resistance to antibiotics. Biofilm EPS consists of proteins, DNA, polysaccharides, and excreted cellular components (377). It ensures that the bacteria are protected from host immune responses such as macrophage engulfment, antibiotics, or host immune defenses while sequestering valuable enzymes and nutrients, exchanging genetic information, and recycling lysed cell components (378).
Despite the well-known role of both BadA of B. henselae and the Vomps of B. quintana in facilitating autoaggregation and adherence, which are critical initial steps in biofilm formation, very little information about the growth of Bartonella species in biofilms and the role that the TAAs play in the process has been reported. In the case of B. henselae, growth in biofilms was first reported by Kyme et al. (76). In that report, the autoaggregative nature was described as phase variation, which has subsequently been shown to be attributable to the presence of BadA on the surface of B. henselae (72). Studies show that BadA accumulates as a dense surface layer of long hair-like structures (∼240 nm) and that the absence of BadA prevents AAG (379). The role of TAAs in biofilm formation has been proposed or experimentally demonstrated for a wide range of Gram-negative bacteria, including Acinetobacter baumannii (380), Burkholderia species (381–383), uropathogenic Escherichia coli (UPEC) (384), enterohemorrhagic E. coli (EHEC) (385), and Salmonella enterica (386). Not surprisingly, we have shown that BadA plays an important role in biofilm formation by B. henselae, as a badA deletion mutant has an impaired ability to form biofilms (Fig. 8) (75). When our laboratory constructed nonpolar deletion mutants of the badA gene in both the Houston-1 type strain and the Marseille strain of B. henselae, they were shown to have a reduced ability to form biofilms. Scanning electron micrographs show that the ΔbadA strains have reduced adherence and biomass accumulation, whereas the wild-type parental strains for both Houston-1 and Marseille have the ability to form well-structured biofilms (unpublished data) (Fig. 8).
FIG 8.
Biofilm formation by B. henselae. Scanning electron microscopic images of the adherent cells for both the Houston-1 and Marseille wild-type (WT) strains compared to reduced adherence, autoaggregation, and biofilm formation for the isogenic mutants in which the badA gene is deleted (ΔbadA). Bacterial cells (105) were inoculated onto a coverslip in a six-well plate and grown for 24 h in Schneider's liquid medium at 37°C and 5% CO2. Cells were fixed with 2% paraformaldehyde plus 2% glutaraldehyde and 0.15% alcian blue (to preserve the polysaccharide moieties found in the EPS of biofilms [396, 397]) in 0.2 M sodium cacodylate buffer, pH 7.2. Samples were washed, postfixed for 90 min in 1% OsO4, and dehydrated. Samples were air dried overnight; the coverslip was mounted on adhesive carbon film, coated for 20 s with Au/Pd (60:40) at 16.40 g/cm and 25 mA, and examined using a JEOL JSM6490LV microscope operated at 3-kV low vacuum; and secondary images were collected as JPEG files. The ΔbadA mutants were the same strains as those described in the legend to Fig. 7.
The autoaggregative nature of low-passage-number Bartonella species grown under laboratory conditions is well known, and large aggregates of bacteria have been observed in histopathological specimens from patients with BA (387), verruga peruana (388), and CSD (389). The ability of B. henselae (75, 76) and perhaps other Bartonella species to aggregate and form biofilms suggests that these bacterial communities are an integral component of the vegetative masses reported for endocarditis caused by Bartonella. Molecular studies and histopathology of heart valves from cases of Bartonella endocarditis demonstrate the presence of these bacteria on the surface of damaged valves (97, 177). A damaged heart valve could serve as the substrate on which Bartonella biofilms form and establish infections that are resistant to antibiotic treatment, as is the case for BCNE.
It is also tempting to speculate on a possible role of Bartonella biofilms in colonization of their arthropod vectors. Biofilm formation by Yersinia pestis in the proventriculus of the rat flea vector has been shown to promote transmission (390). Y. pestis apparently evolved from non-vector-borne Yersinia species for rat flea transmission by three loss-of-function mutations that enhanced biofilm formation in the flea foregut (391). It may also be possible that biofilm formation plays a limited role in transmission of Y. pestis by the cat flea (392). Five different Bartonella species have been shown to persist in the cat flea vector (393). B. henselae replicates in the flea gut and is excreted in the flea feces (128, 135). It has been proposed that B. henselae forms a biofilm in the flea gut and possibly on the flea feces to persist before transmission on the claws of cats (394). It has also been hypothesized that B. quintana may also form a biofilm on louse feces (133). Thus, it is possible that the biofilm formation in the arthropod vectors of Bartonella species and/or their feces enhances transmission of these bacteria, much as it does for Y. pestis.
Bartonella species share the ability to infect erythrocytes in their natural hosts, and it has been speculated that a primary niche exists to allow persistence of these bacteria in the host and to seed the bloodstream for subsequent transmission by the arthropod vector. The preponderance of evidence supports the role of the endothelium in serving as this primary niche (296), but it has also been suggested that endothelial progenitor cells may also function as a niche reservoir (215–217, 308). Intracellular survival in endothelial cells would afford an opportunity for immune evasion and may explain the stealthy nature of these pathogens (223). However, in humans bartonellae are not widely reported inside endothelial cells, suggesting that this may be a laboratory-generated phenomenon of unclear clinical relevance (203, 301, 387, 388). Similarly, an immunocompromised mouse model (SCID/BEIGE) using Bartonella taylorii was developed and shown to establish a chronic infection with the bacteria localized as extracellular aggregates embedded within the collagen matrix (395). An alternate explanation is that Bartonella species form stable biofilms (rather than carrying out intracellular invasion of endothelial cells), which allow immune evasion and persistence in their hosts. Extracellular attachment of bartonellae to endothelial cells or extracellular matrix proteins may provide the substrate for the formation of a focus that becomes a structured biofilm for seeding of the bloodstream and persistence in the face of both antimicrobial treatment and the host immune response.
CONCLUSIONS
The genus Bartonella is a rapidly expanding group of ubiquitous bacteria that are found in a diverse array of animal reservoirs. Three species of Bartonella, B. bacilliformis, B. henselae, and B. quintana, are responsible for the vast majority of human disease caused by this group of bacteria. In particular, B. henselae and B. quintana are frequent causes of BCNE that often go undiagnosed due to technical challenges in diagnosing infections caused by Bartonella species. Despite this challenge, the number of reported cases of Bartonella endocarditis is rapidly rising, and it represents a significant cause of infective endocarditis that remains understudied. The role of the TAAs (BadA and Vomps) and the T4SSs of Bartonella in the interaction of this bacterium with the vascular endothelium has been well studied, and they certainly play critical roles in the establishment of endocarditis. Furthermore, the ability of Bartonella to form biofilms is likely to be a crucial step in infection of heart valves and therefore in causing endocarditis. Additionally, the role that biofilms play in colonization and transmission in the arthropod vectors responsible for transmission of Bartonella species and persistence in both the natural and incidental hosts should also be considered.
ACKNOWLEDGMENTS
We thank Volkhard Kempf for kindly providing the BadA-specific antibody used for the immunoelectron microscopy. We thank Gael Nicolas, Amada Garces, and Truitt Sutton for assistance with electron microscopy. We also thank Richard Birtles for helpful discussion and suggestions regarding the taxonomy and nomenclature of Bartonella species.
U.O. is supported by award HRD1400837 of the NSF Florida-Georgia Louis Stokes Alliance for Minority Participation Bridge to the Doctorate Project to USF. B.A. is supported by NIH grant 1R21AI102560.
Biographies

Udoka Okaro (M.S.) is a doctoral student in the Department of Molecular Medicine at the University of South Florida (USF) Morsani College of Medicine (MCOM), working under the mentorship of Burt Anderson. She received her undergraduate degree in biochemistry at Enugu State University of Science and Technology, Nigeria, and a master's degree in biotechnology at USF MCOM. She began her scientific career at the State House Clinic in Nigeria, where she worked as a laboratory technician for four years, and then the USF Byrd Alzheimer's Institute, where she worked as a graduate assistant for a year before admission into the Ph.D. program at USF MCOM. Her research interests are currently focused on regulatory systems which control biofilm formation in bacteria.

Anteneh Addisu (M.D., Ph.D.) is currently a fellow in the Department of Infectious Diseases and International Medicine at USF MCOM. He obtained his medical degree from Addis Ababa University in Ethiopia and a Ph.D. from the Department of Molecular Pharmacology and Physiology at USF MCOM. His research interests encompass translational research in general, focusing on how cytoskeletal and molecular motor components of human and bacterial cells interact in health and disease and how they can be therapeutically targeted.

Beata Casanas (D.O.) is an Associate Professor and Fellowship Program Director at USF MCOM in the Division of Infectious Diseases and International Medicine. She received her medical degree from the Nova Southeastern University College of Osteopathic Medicine and subsequently completed her Internal Medicine and Infectious Disease training at USF MCOM. She currently serves as Executive Medical Director of the Hillsborough County Health Department overseeing the Tuberculosis, HIV and Sexually Transmitted Infections clinics, and she is heavily involved in various projects concerning HIV and HIV-related diseases. She has always had a special interest in cardiovascular infections, especially endocarditis.

Burt Anderson (Ph.D.) is a Professor in the Department of Molecular Medicine at USF MCOM. He obtained his Ph.D. from Georgia State University in Molecular Genetics. He started his career at the Centers for Disease Control and Prevention, Viral and Rickettsial Zoonoses Branch, where he was part of the research team that described Bartonella (Rochalimaea) henselae, Ehrlichia chaffeensis, and Ehrlichia ewingii as distinct newly recognized species of bacteria associated with zoonotic diseases. He has studied the molecular biology of Bartonella species for over 25 years. Currently, his research interests focus on regulation of virulence and biofilm formation by bacteria in the genus Bartonella.
REFERENCES
- 1.Anstead GM. 2016. The centenary of the discovery of trench fever, an emerging infectious disease of World War 1. Lancet Infect Dis 16:e164–e172. doi: 10.1016/S1473-3099(16)30003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray LM. 1918. The common factor in disordered action of the heart. Br Med J 2:650–652. doi: 10.1136/bmj.2.3024.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Venning JA. 1919. The etiology of disordered action of the heart: a report on 7,803 cases. Br Med J 2:337–339. doi: 10.1136/bmj.2.3063.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roden JA, Wells DH, Chomel BB, Kasten RW, Koehler JE. 2012. Hemin binding protein C is found in outer membrane vesicles and protects Bartonella henselae against toxic concentrations of hemin. Infect Immun 80:929–942. doi: 10.1128/IAI.05769-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schulte Fischedick FB, Stuckey MJ, Aguilar-Setien A, Moreno-Sandoval H, Galvez-Romero G, Salas-Rojas M, Arechiga-Ceballos N, Overgaauw PA, Kasten RW, Chomel BB. 2016. Identification of Bartonella species isolated from rodents from Yucatan, Mexico, and isolation of Bartonella vinsonii subsp. yucatanensis subsp nov. Vector Borne Zoonotic Dis 16:636–642. doi: 10.1089/vbz.2016.1981. [DOI] [PubMed] [Google Scholar]
- 6.Hertig MP. 1942. Phlebotomus and Carrion's disease. Am J Trop Med 22(Suppl):1–80. [Google Scholar]
- 7.Rudolf I, Betasova L, Bischof V, Venclikova K, Blazejova H, Mendel J, Hubalek Z, Kosoy M. 2016. Molecular survey of arthropod-borne pathogens in sheep keds (Melophagus ovinus), Central Europe. Parasitol Res 115:3679–3682. doi: 10.1007/s00436-016-5175-2. [DOI] [PubMed] [Google Scholar]
- 8.Maggi RG, Raverty SA, Lester SJ, Huff DG, Haulena M, Ford SL, Nielsen O, Robinson JH, Breitschwerdt EB. 2008. Bartonella henselae in captive and hunter-harvested beluga (Delphinapterus leucas). J Wildl Dis 44:871–877. doi: 10.7589/0090-3558-44.4.871. [DOI] [PubMed] [Google Scholar]
- 9.Ereqat S, Nasereddin A, Vayssier-Taussat M, Abdelkader A, Al-Jawabreh A, Zaid T, Azmi K, Abdeen Z. 2016. Molecular evidence of Bartonella species in ixodid ticks and domestic animals in Palestine. Front Microbiol 7:1217. doi: 10.3389/fmicb.2016.01217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bai Y, Gilbert A, Fox K, Osikowicz L, Kosoy M. 2016. Bartonella rochalimae and B. vinsonii subsp. berkhoffii in wild carnivores from Colorado, USA. J Wildl Dis 52:844–849. doi: 10.7589/2016-01-015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spach DH, Callis KP, Paauw DS, Houze YB, Schoenknecht FD, Welch DF, Rosen H, Brenner DJ. 1993. Endocarditis caused by Rochalimaea quintana in a patient infected with human immunodeficiency virus. J Clin Microbiol 31:692–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spach DH, Kanter AS, Daniels NA, Nowowiejski DJ, Larson AM, Schmidt RA, Swaminathan B, Brenner DJ. 1995. Bartonella (Rochalimaea) species as a cause of apparent “culture-negative” endocarditis. Clin Infect Dis 20:1044–1047. doi: 10.1093/clinids/20.4.1044. [DOI] [PubMed] [Google Scholar]
- 13.Drancourt M, Mainardi JL, Brouqui P, Vandenesch F, Carta A, Lehnert F, Etienne J, Goldstein F, Acar J, Raoult D. 1995. Bartonella (Rochalimaea) quintana endocarditis in three homeless men. N Engl J Med 332:419–423. doi: 10.1056/NEJM199502163320702. [DOI] [PubMed] [Google Scholar]
- 14.Spach DH, Kanter AS, Dougherty MJ, Larson AM, Coyle MB, Brenner DJ, Swaminathan B, Matar GM, Welch DF, Root RK, Stamm WE. 1995. Bartonella (Rochalimaea) quintana bacteremia in inner-city patients with chronic alcoholism. N Engl J Med 332:424–428. doi: 10.1056/NEJM199502163320703. [DOI] [PubMed] [Google Scholar]
- 15.Hadfield TL, Warren R, Kass M, Brun E, Levy C. 1993. Endocarditis caused by Rochalimaea henselae. Hum Pathol 24:1140–1141. doi: 10.1016/0046-8177(93)90196-N. [DOI] [PubMed] [Google Scholar]
- 16.Holmes AH, Greenough TC, Balady GJ, Regnery RL, Anderson BE, O'Keane JC, Fonger JD, McCrone EL. 1995. Bartonella henselae endocarditis in an immunocompetent adult. Clin Infect Dis 21:1004–1007. doi: 10.1093/clinids/21.4.1004. [DOI] [PubMed] [Google Scholar]
- 17.Jeanclaude D, Godmer P, Leveiller D, Pouedras P, Fournier PE, Raoult D, Rolain JM. 2009. Bartonella alsatica endocarditis in a French patient in close contact with rabbits. Clin Microbiol Infect 15(Suppl 2):S110–S111. doi: 10.1111/j.1469-0691.2008.02187.x. [DOI] [PubMed] [Google Scholar]
- 18.Daly JS, Worthington MG, Brenner DJ, Moss CW, Hollis DG, Weyant RS, Steigerwalt AG, Weaver RE, Daneshvar MI, O'Connor SP. 1993. Rochalimaea elizabethae sp. nov. isolated from a patient with endocarditis. J Clin Microbiol 31:872–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Avidor B, Graidy M, Efrat G, Leibowitz C, Shapira G, Schattner A, Zimhony O, Giladi M. 2004. Bartonella koehlerae, a new cat-associated agent of culture-negative human endocarditis. J Clin Microbiol 42:3462–3468. doi: 10.1128/JCM.42.8.3462-3468.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin EY, Tsigrelis C, Baddour LM, Lepidi H, Rolain JM, Patel R, Raoult D. 2010. Candidatus Bartonella mayotimonensis and endocarditis. Emerg Infect Dis 16:500–503. doi: 10.3201/eid1603.081673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fenollar F, Sire S, Raoult D. 2005. Bartonella vinsonii subsp. arupensis as an agent of blood culture-negative endocarditis in a human. J Clin Microbiol 43:945–947. doi: 10.1128/JCM.43.2.945-947.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olarte L, Ampofo K, Thorell EA, Sanderson S, Doby E, Pavia AT, Rosado H, Raoult D, Socolovschi C, Hersh AL. 2012. Bartonella vinsonii endocarditis in an adolescent with congenital heart disease. Pediatr Infect Dis J 31:531–534. doi: 10.1097/INF.0b013e31824ba95a. [DOI] [PubMed] [Google Scholar]
- 23.Roux V, Eykyn SJ, Wyllie S, Raoult D. 2000. Bartonella vinsonii subsp. berkhoffii as an agent of afebrile blood culture-negative endocarditis in a human. J Clin Microbiol 38:1698–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brenner DJ, O'Connor SP, Winkler HH, Steigerwalt AG. 1993. Proposals to unify the genera Bartonella and Rochalimaea, with descriptions of Bartonella quintana comb. nov., Bartonella vinsonii comb. nov., Bartonella henselae comb. nov., and Bartonella elizabethae comb. nov., and to remove the family Bartonellaceae from the order Rickettsiales. Int J Syst Bacteriol 43:777–786. doi: 10.1099/00207713-43-4-777. [DOI] [PubMed] [Google Scholar]
- 25.Minnick MF, Anderson BE, Lima A, Battisti JM, Lawyer PG, Birtles RJ. 2014. Oroya fever and verruga peruana: bartonelloses unique to South America. PLoS Negl Trop Dis 8:e2919. doi: 10.1371/journal.pntd.0002919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noguchi H, Battistini TS. 1926. Etiology of Oroya fever: I. Cultivation of Bartonella bacilliformis. J Exp Med 43:851–864. doi: 10.1084/jem.43.6.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ito S, Vinson JW. 1965. Fine structure of Rickettsia quintana cultivated in vitro and in the louse. J Bacteriol 89:481–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vinson JW. 1966. In vitro cultivation of the rickettsial agent of trench fever. Bull World Health Organ 35:155–164. [PMC free article] [PubMed] [Google Scholar]
- 29.Slater LN, Welch DF, Hensel D, Coody DW. 1990. A newly recognized fastidious gram-negative pathogen as a cause of fever and bacteremia. N Engl J Med 323:1587–1593. doi: 10.1056/NEJM199012063232303. [DOI] [PubMed] [Google Scholar]
- 30.Welch DF, Pickett DA, Slater LN, Steigerwalt AG, Brenner DJ. 1992. Rochalimaea henselae sp. nov., a cause of septicemia, bacillary angiomatosis, and parenchymal bacillary peliosis. J Clin Microbiol 30:275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Regnery RL, Anderson BE, Clarridge JE III, Rodriquez-Barradas M, Jones DC, Carr JH. 1992. Characterization of a novel Rochalimaea species, R. henselae sp. nov., isolated from blood of a febrile, human immunodeficiency virus-positive patient. J Clin Microbiol 30:265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Relman DA, Loutit JS, Schmidt TM, Falkow S, Tompkins LS. 1990. The agent of bacillary angiomatosis. An approach to the identification of uncultured pathogens. N Engl J Med 323:1573–1580. [DOI] [PubMed] [Google Scholar]
- 33.Koehler JE, Quinn FD, Berger TG, LeBoit PE, Tappero JW. 1992. Isolation of Rochalimaea species from cutaneous and osseous lesions of bacillary angiomatosis. N Engl J Med 327:1625–1631. doi: 10.1056/NEJM199212033272303. [DOI] [PubMed] [Google Scholar]
- 34.Regnery RL, Olson JG, Perkins BA, Bibb W. 1992. Serological response to “Rochalimaea henselae” antigen in suspected cat-scratch disease. Lancet 339:1443–1445. doi: 10.1016/0140-6736(92)92032-B. [DOI] [PubMed] [Google Scholar]
- 35.Anderson B, Kelly C, Threlkel R, Edwards K. 1993. Detection of Rochalimaea henselae in cat-scratch disease skin test antigens. J Infect Dis 168:1034–1036. doi: 10.1093/infdis/168.4.1034. [DOI] [PubMed] [Google Scholar]
- 36.Engel P, Salzburger W, Liesch M, Chang CC, Maruyama S, Lanz C, Calteau A, Lajus A, Medigue C, Schuster SC, Dehio C. 2011. Parallel evolution of a type IV secretion system in radiating lineages of the host-restricted bacterial pathogen Bartonella. PLoS Genet 7:e1001296. doi: 10.1371/journal.pgen.1001296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guy L, Nystedt B, Toft C, Zaremba-Niedzwiedzka K, Berglund EC, Granberg F, Naslund K, Eriksson AS, Andersson SG. 2013. A gene transfer agent and a dynamic repertoire of secretion systems hold the keys to the explosive radiation of the emerging pathogen Bartonella. PLoS Genet 9:e1003393. doi: 10.1371/journal.pgen.1003393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu Q, Kosoy M, Olival KJ, Dittmar K. 2014. Horizontal transfers and gene losses in the phospholipid pathway of bartonella reveal clues about early ecological niches. Genome Biol Evol 6:2156–2169. doi: 10.1093/gbe/evu169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heller R, Kubina M, Mariet P, Riegel P, Delacour G, Dehio C, Lamarque F, Kasten R, Boulouis HJ, Monteil H, Chomel B, Piemont Y. 1999. Bartonella alsatica sp. nov., a new Bartonella species isolated from the blood of wild rabbits. Int J Syst Bacteriol 49:283–288. doi: 10.1099/00207713-49-1-283. [DOI] [PubMed] [Google Scholar]
- 40.Raoult D, Roblot F, Rolain JM, Besnier JM, Loulergue J, Bastides F, Choutet P. 2006. First isolation of Bartonella alsatica from a valve of a patient with endocarditis. J Clin Microbiol 44:278–279. doi: 10.1128/JCM.44.1.278-279.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mullins KE, Hang J, Jiang J, Leguia M, Kasper MR, Ventosilla P, Maguina C, Jarman RG, Blazes D, Richards AL. 2015. Description of Bartonella ancashensis sp. nov., isolated from the blood of two patients with verruga peruana. Int J Syst Evol Microbiol 65:3339–3343. doi: 10.1099/ijsem.0.000416. [DOI] [PubMed] [Google Scholar]
- 42.Huarcaya E, Maguiña C, Torres R, Rupay J, Fuentes L. 2004. Bartonellosis (Carrion's disease) in the pediatric population of Peru: an overview and update. Braz J Infect Dis 8:331–339. doi: 10.1590/S1413-86702004000500001. [DOI] [PubMed] [Google Scholar]
- 43.Henriquez-Camacho C, Ventosilla P, Minnick MF, Ruiz J, Maguiña C. 2015. Proteins of Bartonella bacilliformis: candidates for vaccine development. Int J Pept 2015:702784. doi: 10.1155/2015/702784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Margileth AM, Baehren DF. 1998. Chest-wall abscess due to cat-scratch disease (CSD) in an adult with antibodies to Bartonella clarridgeiae: case report and review of the thoracopulmonary manifestations of CSD. Clin Infect Dis 27:353–357. doi: 10.1086/514671. [DOI] [PubMed] [Google Scholar]
- 45.Reference deleted. [Google Scholar]
- 46.Bai Y, Rizzo MF, Alvarez D, Moran D, Peruski LF, Kosoy M. 2015. Coexistence of Bartonella henselae and B. clarridgeiae in populations of cats and their fleas in Guatemala. J Vector Ecol 40:327–332. doi: 10.1111/jvec.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim YS, Seo KW, Lee JH, Choi EW, Lee HW, Hwang CY, Shin NS, Youn HJ, Youn HY. 2009. Prevalence of Bartonella henselae and Bartonella clarridgeiae in cats and dogs in Korea. J Vet Sci 10:85–87. doi: 10.4142/jvs.2009.10.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Colton L, Kabeya H, Kosoy M. 2012. Experimental infection of three laboratory mouse stocks with a shrew origin Bartonella elizabethae strain: an evaluation of bacterial host switching potential. Infect Ecol Epidemiol 2. doi: 10.3402/iee.v2i0.17132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bhengsri S, Baggett HC, Peruski LF, Morway C, Bai Y, Fisk TL, Sitdhirasdr A, Maloney SA, Dowell SF, Kosoy M. 2011. Bartonella seroprevalence in rural Thailand. Southeast Asian J Trop Med Public Health 42:687–692. [PubMed] [Google Scholar]
- 50.Kosoy M, Bai Y, Sheff K, Morway C, Baggett H, Maloney SA, Boonmar S, Bhengsri S, Dowell SF, Sitdhirasdr A, Lerdthusnee K, Richardson J, Peruski LF. 2010. Identification of Bartonella infections in febrile human patients from Thailand and their potential animal reservoirs. Am J Trop Med Hyg 82:1140–1145. doi: 10.4269/ajtmh.2010.09-0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kerkhoff FT, Bergmans AM, van Der Zee A, Rothova A. 1999. Demonstration of Bartonella grahamii DNA in ocular fluids of a patient with neuroretinitis. J Clin Microbiol 37:4034–4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Birtles RJ, Laycock G, Kenny MJ, Shaw SE, Day MJ. 2002. Prevalence of Bartonella species causing bacteraemia in domesticated and companion animals in the United Kingdom. Vet Rec 151:225–229. doi: 10.1136/vr.151.8.225. [DOI] [PubMed] [Google Scholar]
- 53.Oksi J, Rantala S, Kilpinen S, Silvennoinen R, Vornanen M, Veikkolainen V, Eerola E, Pulliainen AT. 2013. Cat scratch disease caused by Bartonella grahamii in an immunocompromised patient. J Clin Microbiol 51:2781–2784. doi: 10.1128/JCM.00910-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Breitschwerdt EB, Mascarelli PE, Schweickert LA, Maggi RG, Hegarty BC, Bradley JM, Woods CW. 2011. Hallucinations, sensory neuropathy, and peripheral visual deficits in a young woman infected with Bartonella koehlerae. J Clin Microbiol 49:3415–3417. doi: 10.1128/JCM.00833-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Veikkolainen V, Vesterinen EJ, Lilley TM, Pulliainen AT. 2014. Bats as reservoir hosts of human bacterial pathogen, Bartonella mayotimonensis. Emerg Infect Dis 20:960–967. doi: 10.3201/eid2006.130956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leibler JH, Zakhour CM, Gadhoke P, Gaeta JM. 2016. Zoonotic and vector-borne infections among urban homeless and marginalized people in the United States and Europe, 1990–2014. Vector Borne Zoonotic Dis 16:435–444. doi: 10.1089/vbz.2015.1863. [DOI] [PubMed] [Google Scholar]
- 57.Eremeeva ME, Gerns HL, Lydy SL, Goo JS, Ryan ET, Mathew SS, Ferraro MJ, Holden JM, Nicholson WL, Dasch GA, Koehler JE. 2007. Bacteremia, fever, and splenomegaly caused by a newly recognized Bartonella species. N Engl J Med 356:2381–2387. doi: 10.1056/NEJMoa065987. [DOI] [PubMed] [Google Scholar]
- 58.Saisongkorh W, Rolain JM, Suputtamongkol Y, Raoult D. 2009. Emerging Bartonella in humans and animals in Asia and Australia. J Med Assoc Thai 92:707–731. [PubMed] [Google Scholar]
- 59.Kosoy M, Morway C, Sheff KW, Bai Y, Colborn J, Chalcraft L, Dowell SF, Peruski LF, Maloney SA, Baggett H, Sutthirattana S, Sidhirat A, Maruyama S, Kabeya H, Chomel BB, Kasten R, Popov V, Robinson J, Kruglov A, Petersen LR. 2008. Bartonella tamiae sp. nov., a newly recognized pathogen isolated from three human patients from Thailand. J Clin Microbiol 46:772–775. doi: 10.1128/JCM.02120-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kabeya H, Colborn JM, Bai Y, Lerdthusnee K, Richardson JH, Maruyama S, Kosoy MY. 2010. Detection of Bartonella tamiae DNA in ectoparasites from rodents in Thailand and their sequence similarity with bacterial cultures from Thai patients. Vector Borne Zoonotic Dis 10:429–434. doi: 10.1089/vbz.2009.0124. [DOI] [PubMed] [Google Scholar]
- 61.Breitschwerdt EB, Maggi RG, Duncan AW, Nicholson WL, Hegarty BC, Woods CW. 2007. Bartonella species in blood of immunocompetent persons with animal and arthropod contact. Emerg Infect Dis 13:938–941. doi: 10.3201/eid1306.061337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Breitschwerdt EB, Maggi RG, Nicholson WL, Cherry NA, Woods CW. 2008. Bartonella sp. bacteremia in patients with neurological and neurocognitive dysfunction. J Clin Microbiol 46:2856–2861. doi: 10.1128/JCM.00832-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Breitschwerdt EB, Maggi RG, Lantos PM, Woods CW, Hegarty BC, Bradley JM. 2010. Bartonella vinsonii subsp. berkhoffii and Bartonella henselae bacteremia in a father and daughter with neurological disease. Parasit Vectors 3:29. doi: 10.1186/1756-3305-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Varanat M, Travis A, Lee W, Maggi RG, Bissett SA, Linder KE, Breitschwerdt EB. 2009. Recurrent osteomyelitis in a cat due to infection with Bartonella vinsonii subsp. berkhoffii genotype II. J Vet Intern Med 23:1273–1277. doi: 10.1111/j.1939-1676.2009.0372.x. [DOI] [PubMed] [Google Scholar]
- 65.Welch DF, Carroll KC, Hofmeister EK, Persing DH, Robison DA, Steigerwalt AG, Brenner DJ. 1999. Isolation of a new subspecies, Bartonella vinsonii subsp. arupensis, from a cattle rancher: identity with isolates found in conjunction with Borrelia burgdorferi and Babesia microti among naturally infected mice. J Clin Microbiol 37:2598–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Minnick MF, Anderson B. 2015. Bartonella, p 1911–1939. In Tang Y-W, Liu D, Poxton I, Schwartzman J, Sussman M, Williams H, Versteeg-Buschman L (ed), Molecular medical microbiology, 2nd ed Elsevier, New York, NY. [Google Scholar]
- 67.Maggi RG, Duncan AW, Breitschwerdt EB. 2005. Novel chemically modified liquid medium that will support the growth of seven Bartonella species. J Clin Microbiol 43:2651–2655. doi: 10.1128/JCM.43.6.2651-2655.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Riess T, Dietrich F, Schmidt KV, Kaiser PO, Schwarz H, Schafer A, Kempf VA. 2008. Analysis of a novel insect cell culture medium-based growth medium for Bartonella species. Appl Environ Microbiol 74:5224–5227. doi: 10.1128/AEM.00621-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gutierrez R, Vayssier-Taussat M, Buffet JP, Harrus S. 2017. Guidelines for the isolation, molecular detection, and characterization of Bartonella species. Vector Borne Zoonotic Dis 17:42–50. doi: 10.1089/vbz.2016.1956. [DOI] [PubMed] [Google Scholar]
- 70.Dehio C. 2008. Infection-associated type IV secretion systems of Bartonella and their diverse roles in host cell interaction. Cell Microbiol 10:1591–1598. doi: 10.1111/j.1462-5822.2008.01171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Batterman HJ, Peek JA, Loutit JS, Falkow S, Tompkins LS. 1995. Bartonella henselae and Bartonella quintana adherence to and entry into cultured human epithelial cells. Infect Immun 63:4553–4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Riess T, Raddatz G, Linke D, Schafer A, Kempf VA. 2007. Analysis of Bartonella adhesin A expression reveals differences between various B. henselae strains. Infect Immun 75:35–43. doi: 10.1128/IAI.00963-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gilmore RD Jr, Bellville TM, Sviat SL, Frace M. 2005. The Bartonella vinsonii subsp. arupensis immunodominant surface antigen BrpA gene, encoding a 382-kilodalton protein composed of repetitive sequences, is a member of a multigene family conserved among Bartonella species. Infect Immun 73:3128–3136. doi: 10.1128/IAI.73.5.3128-3136.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang P, Chomel BB, Schau MK, Goo JS, Droz S, Kelminson KL, George SS, Lerche NW, Koehler JE. 2004. A family of variably expressed outer-membrane proteins (Vomp) mediates adhesion and autoaggregation in Bartonella quintana. Proc Natl Acad Sci U S A 101:13630–13635. doi: 10.1073/pnas.0405284101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tu N, Carroll RK, Weiss A, Shaw LN, Nicolas G, Thomas S, Lima A, Okaro U, Anderson B. 2017. A family of genus-specific RNAs in tandem with DNA-binding proteins control expression of the badA major virulence factor gene in Bartonella henselae. Microbiologyopen 6(2). doi: 10.1002/mbo3.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kyme P, Dillon B, Iredell J. 2003. Phase variation in Bartonella henselae. Microbiology 149:621–629. doi: 10.1099/mic.0.26014-0. [DOI] [PubMed] [Google Scholar]
- 77.Riess T, Andersson SG, Lupas A, Schaller M, Schafer A, Kyme P, Martin J, Walzlein JH, Ehehalt U, Lindroos H, Schirle M, Nordheim A, Autenrieth IB, Kempf VA. 2004. Bartonella adhesin a mediates a proangiogenic host cell response. J Exp Med 200:1267–1278. doi: 10.1084/jem.20040500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Berglund EC, Frank AC, Calteau A, Vinnere Pettersson O, Granberg F, Eriksson AS, Naslund K, Holmberg M, Lindroos H, Andersson SG. 2009. Run-off replication of host-adaptability genes is associated with gene transfer agents in the genome of mouse-infecting Bartonella grahamii. PLoS Genet 5:e1000546. doi: 10.1371/journal.pgen.1000546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Anderson B, Goldsmith C, Johnson A, Padmalayam I, Baumstark B. 1994. Bacteriophage-like particle of Rochalimaea henselae. Mol Microbiol 13:67–73. doi: 10.1111/j.1365-2958.1994.tb00402.x. [DOI] [PubMed] [Google Scholar]
- 80.Umemori E, Sasaki Y, Amano K, Amano Y. 1992. A phage in Bartonella bacilliformis. Microbiol Immunol 36:731–736. doi: 10.1111/j.1348-0421.1992.tb02075.x. [DOI] [PubMed] [Google Scholar]
- 81.Barbian KD, Minnick MF. 2000. A bacteriophage-like particle from Bartonella bacilliformis. Microbiology 146:599–609. doi: 10.1099/00221287-146-3-599. [DOI] [PubMed] [Google Scholar]
- 82.Alsmark CM, Frank AC, Karlberg EO, Legault BA, Ardell DH, Canback B, Eriksson AS, Naslund AK, Handley SA, Huvet M, La Scola B, Holmberg M, Andersson SG. 2004. The louse-borne human pathogen Bartonella quintana is a genomic derivative of the zoonotic agent Bartonella henselae. Proc Natl Acad Sci U S A 101:9716–9721. doi: 10.1073/pnas.0305659101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Saenz HL, Engel P, Stoeckli MC, Lanz C, Raddatz G, Vayssier-Taussat M, Birtles R, Schuster SC, Dehio C. 2007. Genomic analysis of Bartonella identifies type IV secretion systems as host adaptability factors. Nat Genet 39:1469–1476. doi: 10.1038/ng.2007.38. [DOI] [PubMed] [Google Scholar]
- 84.Li H, Tong Y, Huang Y, Bai J, Yang H, Liu W, Cao W. 2012. Complete genome sequence of Bartonella quintana, a bacterium isolated from rhesus macaques. J Bacteriol 194:6347. doi: 10.1128/JB.01602-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lang AS, Zhaxybayeva O, Beatty JT. 2012. Gene transfer agents: phage-like elements of genetic exchange. Nat Rev Microbiol 10:472–482. doi: 10.1038/nrmicro2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Houpikian P, Raoult D. 2005. Blood culture-negative endocarditis in a reference center: etiologic diagnosis of 348 cases. Medicine (Baltimore) 84:162–173. doi: 10.1097/01.md.0000165658.82869.17. [DOI] [PubMed] [Google Scholar]
- 87.Probert W, Louie JK, Tucker JR, Longoria R, Hogue R, Moler S, Graves M, Palmer HJ, Cassady J, Fritz CL. 2009. Meningitis due to a “Bartonella washoensis”-like human pathogen. J Clin Microbiol 47:2332–2335. doi: 10.1128/JCM.00511-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lepidi H, Fournier PE, Raoult D. 2000. Quantitative analysis of valvular lesions during Bartonella endocarditis. Am J Clin Pathol 114:880–889. doi: 10.1309/R0KQ-823A-BTC7-MUUJ. [DOI] [PubMed] [Google Scholar]
- 89.Litwin CM, Anderson B, Tsolis R, Rasley A. 2016. The Bartonellaceae, Brucellaceae, and Francisellaceae, p 473–481. In Detrick B, Schmitz JL, Hamilton RG (ed), Manual of molecular and clinical laboratory immunology, 8th ed ASM Press, Washington, DC. [Google Scholar]
- 90.Larson AM, Dougherty MJ, Nowowiejski DJ, Welch DF, Matar GM, Swaminathan B, Coyle MB. 1994. Detection of Bartonella (Rochalimaea) quintana by routine acridine orange staining of broth blood cultures. J Clin Microbiol 32:1492–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Caponetti GC, Pantanowitz L, Marconi S, Havens JM, Lamps LW, Otis CN. 2009. Evaluation of immunohistochemistry in identifying Bartonella henselae in cat-scratch disease. Am J Clin Pathol 131:250–256. doi: 10.1309/AJCPMNULMO9GPLYU. [DOI] [PubMed] [Google Scholar]
- 92.Zangwill KM, Hamilton DH, Perkins BA, Regnery RL, Plikaytis BD, Hadler JL, Cartter ML, Wenger JD. 1993. Cat scratch disease in Connecticut. epidemiology, risk factors, and evaluation of a new diagnostic test. N Engl J Med 329:8–13. doi: 10.1056/NEJM199307013290102. [DOI] [PubMed] [Google Scholar]
- 93.Bergmans AM, Peeters MF, Schellekens JF, Vos MC, Sabbe LJ, Ossewaarde JM, Verbakel H, Hooft HJ, Schouls LM. 1997. Pitfalls and fallacies of cat scratch disease serology: evaluation of Bartonella henselae-based indirect fluorescence assay and enzyme-linked immunoassay. J Clin Microbiol 35:1931–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vermeulen MJ, Verbakel H, Notermans DW, Reimerink JH, Peeters MF. 2010. Evaluation of sensitivity, specificity and cross-reactivity in Bartonella henselae serology. J Med Microbiol 59:743–745. doi: 10.1099/jmm.0.015248-0. [DOI] [PubMed] [Google Scholar]
- 95.Wagner CL, Riess T, Linke D, Eberhardt C, Schafer A, Reutter S, Maggi RG, Kempf VA. 2008. Use of Bartonella adhesin A (BadA) immunoblotting in the serodiagnosis of Bartonella henselae infections. Int J Med Microbiol 298:579–590. doi: 10.1016/j.ijmm.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 96.Dalton MJ, Robinson LE, Cooper J, Regnery RL, Olson JG, Childs JE. 1995. Use of Bartonella antigens for serologic diagnosis of cat-scratch disease at a national referral center. Arch Intern Med 155:1670–1676. [PubMed] [Google Scholar]
- 97.Raoult D, Fournier PE, Drancourt M, Marrie TJ, Etienne J, Cosserat J, Cacoub P, Poinsignon Y, Leclercq P, Sefton AM. 1996. Diagnosis of 22 new cases of Bartonella endocarditis. Ann Intern Med 125:646–652. doi: 10.7326/0003-4819-125-8-199610150-00004. [DOI] [PubMed] [Google Scholar]
- 98.Barka NE, Hadfield T, Patnaik M, Schwartzman WA, Peter JB. 1993. EIA for detection of Rochalimaea henselae-reactive IgG, IgM, and IgA antibodies in patients with suspected cat-scratch disease. J Infect Dis 167:1503–1504. doi: 10.1093/infdis/167.6.1503. [DOI] [PubMed] [Google Scholar]
- 99.Anderson B, Lu E, Jones D, Regnery R. 1995. Characterization of a 17-kilodalton antigen of Bartonella henselae reactive with sera from patients with cat scratch disease. J Clin Microbiol 33:2358–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Loa CC, Mordechai E, Tilton RC, Adelson ME. 2006. Production of recombinant Bartonella henselae 17-kDa protein for antibody-capture enzyme-linked immunosorbent assay. Diagn Microbiol Infect Dis 55:1–7. doi: 10.1016/j.diagmicrobio.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 101.Hoey JG, Valois-Cruz F, Goldenberg H, Voskoboynik Y, Pfiffner J, Tilton RC, Mordechai E, Adelson ME. 2009. Development of an immunoglobulin M capture-based enzyme-linked immunosorbent assay for diagnosis of acute infections with Bartonella henselae. Clin Vaccine Immunol 16:282–284. doi: 10.1128/CVI.00305-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tsuruoka K, Tsuneoka H, Kawano M, Yanagihara M, Nojima J, Tanaka T, Yamamoto M, Ichihara K. 2012. Evaluation of IgG ELISA using N-lauroyl-sarcosine-soluble proteins of Bartonella henselae for highly specific serodiagnosis of cat scratch disease. Diagn Microbiol Infect Dis 74:230–235. doi: 10.1016/j.diagmicrobio.2012.06.028. [DOI] [PubMed] [Google Scholar]
- 103.Houpikian P, Raoult D. 2003. Western immunoblotting for Bartonella endocarditis. Clin Diagn Lab Immunol 10:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Houpikian P, Raoult D. 2003. Diagnostic methods. Current best practices and guidelines for identification of difficult-to-culture pathogens in infective endocarditis. Cardiol Clin 21:207–217. [DOI] [PubMed] [Google Scholar]
- 105.Liang Z, Raoult D. 2000. Species-specific monoclonal antibodies for rapid identification of Bartonella quintana. Clin Diagn Lab Immunol 7:21–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liang Z, Raoult D. 2000. Differentiation of Bartonella species by a microimmunofluorescence assay, sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and Western immunoblotting. Clin Diagn Lab Immunol 7:617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fournier PE, Lelievre H, Eykyn SJ, Mainardi JL, Marrie TJ, Bruneel F, Roure C, Nash J, Clave D, James E, Benoit-Lemercier C, Deforges L, Tissot-Dupont H, Raoult D. 2001. Epidemiologic and clinical characteristics of Bartonella quintana and Bartonella henselae endocarditis: a study of 48 patients. Medicine (Baltimore) 80:245–251. doi: 10.1097/00005792-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 108.Bosshard PP, Kronenberg A, Zbinden R, Ruef C, Bottger EC, Altwegg M. 2003. Etiologic diagnosis of infective endocarditis by broad-range polymerase chain reaction: a 3-year experience. Clin Infect Dis 37:167–172. doi: 10.1086/375592. [DOI] [PubMed] [Google Scholar]
- 109.Sanogo YO, Zeaiter Z, Caruso G, Merola F, Shpynov S, Brouqui P, Raoult D. 2003. Bartonella henselae in Ixodes ricinus ticks (Acari: Ixodida) removed from humans, Belluno province, Italy. Emerg Infect Dis 9:329–332. doi: 10.3201/eid0903.020133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Valentine KH, Harms CA, Cadenas MB, Birkenheuer AJ, Marr HS, Braun-McNeill J, Maggi RG, Breitschwerdt EB. 2007. Bartonella DNA in loggerhead sea turtles. Emerg Infect Dis 13:949–950. doi: 10.3201/eid1306.061551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Anderson BE, Neuman MA. 1997. Bartonella spp. as emerging human pathogens. Clin Microbiol Rev 10:203–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chomel BB, Boulouis HJ, Maruyama S, Breitschwerdt EB. 2006. Bartonella spp. in pets and effect on human health. Emerg Infect Dis 12:389–394. doi: 10.3201/eid1203.050931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bai Y, Recuenco S, Gilbert AT, Osikowicz LM, Gomez J, Rupprecht C, Kosoy MY. 2012. Prevalence and diversity of Bartonella spp. in bats in Peru. Am J Trop Med Hyg 87:518–523. doi: 10.4269/ajtmh.2012.12-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Muhldorfer K. 2013. Bats and bacterial pathogens: a review. Zoonoses Public Health 60:93–103. doi: 10.1111/j.1863-2378.2012.01536.x. [DOI] [PubMed] [Google Scholar]
- 115.Autino AG, Claps GL, Barquez RM, Diaz MM. 2011. Ectoparasitic insects (Diptera: Streblidae and Siphonaptera: Ischnopsyllidae) of bats from Iquitos and surrounding areas (Loreto, Peru). Mem Inst Oswaldo Cruz 106:917–925. doi: 10.1590/S0074-02762011000800004. [DOI] [PubMed] [Google Scholar]
- 116.Reeves WK, Rogers TE, Durden LA, Dasch GA. 2007. Association of Bartonella with the fleas (Siphonaptera) of rodents and bats using molecular techniques. J Vector Ecol 32:118–122. [DOI] [PubMed] [Google Scholar]
- 117.Reeves WK, Streicker DG, Loftis AD, Dasch GA. 2006. Serologic survey of Eptesicus fuscus from Georgia, USA, for Rickettsia and Borrelia and laboratory transmission of a Rickettsia by bat ticks. J Vector Ecol 31:386–389. [DOI] [PubMed] [Google Scholar]
- 118.Drancourt M, Tran-Hung L, Courtin J, Lumley H, Raoult D. 2005. Bartonella quintana in a 4000-year-old human tooth. J Infect Dis 191:607–611. doi: 10.1086/427041. [DOI] [PubMed] [Google Scholar]
- 119.Chamberlin J, Laughlin LW, Romero S, Solorzano N, Gordon S, Andre RG, Pachas P, Friedman H, Ponce C, Watts D. 2002. Epidemiology of endemic Bartonella bacilliformis: a prospective cohort study in a Peruvian mountain valley community. J Infect Dis 186:983–990. doi: 10.1086/344054. [DOI] [PubMed] [Google Scholar]
- 120.Caceres AG, Galati EA, Le Pont F, Velasquez C. 1997. Possible role of Lutzomyia maranonensis and Lutzomyia robusta (Diptera: Psychodidae) as vectors of human bartonellosis in three provinces of region nor Oriental del Maranon, Peru. Rev Inst Med Trop Sao Paulo 39:51–52. doi: 10.1590/S0036-46651997000100011. [DOI] [PubMed] [Google Scholar]
- 121.Herrer A. 1953. Carrion's disease. I. Studies on plants claimed to be reservoirs of Bartonella bacilliformis. Am J Trop Med Hyg 2:637–643. [PubMed] [Google Scholar]
- 122.Cooper P, Guderian R, Orellana P, Sandoval C, Olalla H, Valdez M, Calvopina M, Guevara A, Griffin G. 1997. An outbreak of bartonellosis in Zamora Chinchipe province in Ecuador. Trans R Soc Trop Med Hyg 91:544–546. doi: 10.1016/S0035-9203(97)90019-5. [DOI] [PubMed] [Google Scholar]
- 123.Cooper P, Guderian R, Paredes W, Daniels R, Perera D, Espinel M, Valdez M, Griffin G. 1996. Bartonellosis in Zamora Chinchipe province in Ecuador. Trans R Soc Trop Med Hyg 90:241–243. doi: 10.1016/S0035-9203(96)90229-1. [DOI] [PubMed] [Google Scholar]
- 124.Birtles RJ, Canales J, Ventosilla P, Alvarez E, Guerra H, Llanos-Cuentas A, Raoult D, Doshi N, Harrison TG. 1999. Survey of Bartonella species infecting intradomicillary animals in the Huayllacallan Valley, Ancash, Peru, a region endemic for human bartonellosis. Am J Trop Med Hyg 60:799–805. [DOI] [PubMed] [Google Scholar]
- 125.Blazes DL, Mullins K, Smoak BL, Jiang J, Canal E, Solorzano N, Hall E, Meza R, Maguina C, Myers T, Richards AL, Laughlin L. 2013. Novel Bartonella agent as cause of verruga peruana. Emerg Infect Dis 19:1111–1114. doi: 10.3201/eid1907.121718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tappero JW, Mohle-Boetani J, Koehler JE, Swaminathan B, Berger TG, LeBoit PE, Smith LL, Wenger JD, Pinner RW, Kemper CA, Reingold AL. 1993. The epidemiology of bacillary angiomatosis and bacillary peliosis. JAMA 269:770–775. [PubMed] [Google Scholar]
- 127.Chomel BB, Kasten RW, Floyd-Hawkins K, Chi B, Yamamoto K, Roberts-Wilson J, Gurfield AN, Abbott RC, Pedersen NC, Koehler JE. 1996. Experimental transmission of Bartonella henselae by the cat flea. J Clin Microbiol 34:1952–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Higgins JA, Radulovic S, Jaworski DC, Azad AF. 1996. Acquisition of the cat scratch disease agent Bartonella henselae by cat fleas (Siphonaptera: Pulicidae). J Med Entomol 33:490–495. doi: 10.1093/jmedent/33.3.490. [DOI] [PubMed] [Google Scholar]
- 129.Karem KL, Paddock CD, Regnery RL. 2000. Bartonella henselae, B. quintana, and B. bacilliformis: historical pathogens of emerging significance. Microbes Infect 2:1193–1205. doi: 10.1016/S1286-4579(00)01273-9. [DOI] [PubMed] [Google Scholar]
- 130.Chomel BB. 2000. Cat-scratch disease. Rev Sci Tech 19:136–150. [DOI] [PubMed] [Google Scholar]
- 131.Demers DM, Bass JW, Vincent JM, Person DA, Noyes DK, Staege CM, Samlaska CP, Lockwood NH, Regnery RL, Anderson BE. 1995. Cat-scratch disease in Hawaii: etiology and seroepidemiology. J Pediatr 127:23–26. doi: 10.1016/S0022-3476(95)70251-2. [DOI] [PubMed] [Google Scholar]
- 132.Lappin MR, Hawley J. 2009. Presence of Bartonella species and Rickettsia species DNA in the blood, oral cavity, skin and claw beds of cats in the United States. Vet Dermatol 20:509–514. doi: 10.1111/j.1365-3164.2009.00800.x. [DOI] [PubMed] [Google Scholar]
- 133.Seki N, Kasai S, Saito N, Komagata O, Mihara M, Sasaki T, Tomita T, Sasaki T, Kobayashi M. 2007. Quantitative analysis of proliferation and excretion of Bartonella quintana in body lice, Pediculus humanus L. Am J Trop Med Hyg 77:562–566. [PubMed] [Google Scholar]
- 134.Fournier PE, Minnick MF, Lepidi H, Salvo E, Raoult D. 2001. Experimental model of human body louse infection using green fluorescent protein-expressing Bartonella quintana. Infect Immun 69:1876–1879. doi: 10.1128/IAI.69.3.1876-1879.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Finkelstein JL, Brown TP, O'Reilly KL, Wedincamp J Jr, Foil LD. 2002. Studies on the growth of Bartonella henselae in the cat flea (Siphonaptera: Pulicidae). J Med Entomol 39:915–919. doi: 10.1603/0022-2585-39.6.915. [DOI] [PubMed] [Google Scholar]
- 136.Foil L, Andress E, Freeland RL, Roy AF, Rutledge R, Triche PC, O'Reilly KL. 1998. Experimental infection of domestic cats with Bartonella henselae by inoculation of Ctenocephalides felis (Siphonaptera: Pulicidae) feces. J Med Entomol 35:625–628. doi: 10.1093/jmedent/35.5.625. [DOI] [PubMed] [Google Scholar]
- 137.Chang CC, Hayashidani H, Pusterla N, Kasten RW, Madigan JE, Chomel BB. 2002. Investigation of Bartonella infection in ixodid ticks from California. Comp Immunol Microbiol Infect Dis 25:229–236. doi: 10.1016/S0147-9571(02)00012-7. [DOI] [PubMed] [Google Scholar]
- 138.Stevenson HL, Bai Y, Kosoy MY, Montenieri JA, Lowell JL, Chu MC, Gage KL. 2003. Detection of novel Bartonella strains and Yersinia pestis in prairie dogs and their fleas (Siphonaptera: Ceratophyllidae and Pulicidae) using multiplex polymerase chain reaction. J Med Entomol 40:329–337. doi: 10.1603/0022-2585-40.3.329. [DOI] [PubMed] [Google Scholar]
- 139.Telford SR III, Wormser GP. 2010. Bartonella spp. transmission by ticks not established. Emerg Infect Dis 16:379–384. doi: 10.3201/eid1603.090443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Koehler JE, Glaser CA, Tappero JW. 1994. Rochalimaea henselae infection. A new zoonosis with the domestic cat as reservoir. JAMA 271:531–535. [DOI] [PubMed] [Google Scholar]
- 141.Chomel BB, Boulouis HJ, Breitschwerdt EB, Kasten RW, Vayssier-Taussat M, Birtles RJ, Koehler JE, Dehio C. 2009. Ecological fitness and strategies of adaptation of Bartonella species to their hosts and vectors. Vet Res 40:29. doi: 10.1051/vetres/2009011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Clarridge JE III, Raich TJ, Pirwani D, Simon B, Tsai L, Rodriguez-Barradas MC, Regnery R, Zollo A, Jones DC, Rambo C. 1995. Strategy to detect and identify Bartonella species in routine clinical laboratory yields Bartonella henselae from human immunodeficiency virus-positive patient and unique Bartonella strain from his cat. J Clin Microbiol 33:2107–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Heller R, Artois M, Xemar V, De Briel D, Gehin H, Jaulhac B, Monteil H, Piemont Y. 1997. Prevalence of Bartonella henselae and Bartonella clarridgeiae in stray cats. J Clin Microbiol 35:1327–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gurfield AN, Boulouis HJ, Chomel BB, Heller R, Kasten RW, Yamamoto K, Piemont Y. 1997. Coinfection with Bartonella clarridgeiae and Bartonella henselae and with different Bartonella henselae strains in domestic cats. J Clin Microbiol 35:2120–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mexas AM, Hancock SI, Breitschwerdt EB. 2002. Bartonella henselae and Bartonella elizabethae as potential canine pathogens. J Clin Microbiol 40:4670–4674. doi: 10.1128/JCM.40.12.4670-4674.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jackson LA, Perkins BA, Wenger JD. 1993. Cat scratch disease in the United States: an analysis of three national databases. Am J Public Health 83:1707–1711. doi: 10.2105/AJPH.83.12.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sasaki T, Poudel SK, Isawa H, Hayashi T, Seki N, Tomita T, Sawabe K, Kobayashi M. 2006. First molecular evidence of Bartonella quintana in Pediculus humanus capitis (Phthiraptera: Pediculidae), collected from Nepalese children. J Med Entomol 43:110–112. [DOI] [PubMed] [Google Scholar]
- 148.Brouqui P, Lascola B, Roux V, Raoult D. 1999. Chronic Bartonella quintana bacteremia in homeless patients. N Engl J Med 340:184–189. doi: 10.1056/NEJM199901213400303. [DOI] [PubMed] [Google Scholar]
- 149.Jackson LA, Spach DH, Kippen DA, Sugg NK, Regnery RL, Sayers MH, Stamm WE. 1996. Seroprevalence to Bartonella quintana among patients at a community clinic in downtown Seattle. J Infect Dis 173:1023–1026. doi: 10.1093/infdis/173.4.1023. [DOI] [PubMed] [Google Scholar]
- 150.Rolain JM, Franc M, Davoust B, Raoult D. 2003. Molecular detection of Bartonella quintana, B. koehlerae, B. henselae, B. clarridgeiae, Rickettsia felis, and Wolbachia pipientis in cat fleas, France. Emerg Infect Dis 9:338–342. doi: 10.3201/eid0903.020278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.La VD, Tran-Hung L, Aboudharam G, Raoult D, Drancourt M. 2005. Bartonella quintana in domestic cat. Emerg Infect Dis 11:1287–1289. doi: 10.3201/eid1108.050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Byam W, Lloyd L. 1920. Trench fever: its epidemiology and endemiology. Proc R Soc Med 13:1–27. [PMC free article] [PubMed] [Google Scholar]
- 153.Rolain JM, Foucault C, Guieu R, La Scola B, Brouqui P, Raoult D. 2002. Bartonella quintana in human erythrocytes. Lancet 360:226–228. doi: 10.1016/S0140-6736(02)09462-X. [DOI] [PubMed] [Google Scholar]
- 154.Diniz PP, Velho PE, Pitassi LH, Drummond MR, Lania BG, Barjas-Castro ML, Sowy S, Breitschwerdt EB, Scorpio DG. 2016. Risk factors for Bartonella species infection in blood donors from Southeast Brazil. PLoS Negl Trop Dis 10:e0004509. doi: 10.1371/journal.pntd.0004509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Raoult D, Roux V. 1999. The body louse as a vector of reemerging human diseases. Clin Infect Dis 29:888–911. doi: 10.1086/520454. [DOI] [PubMed] [Google Scholar]
- 156.Jacomo V, Kelly PJ, Raoult D. 2002. Natural history of Bartonella infections (an exception to Koch's postulate). Clin Diagn Lab Immunol 9:8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chmielewski T, Podsiadly E, Tylewska-Wierzbanowska S. 2007. Presence of Bartonella spp. in various human populations. Pol J Microbiol 56:33–38. [PubMed] [Google Scholar]
- 158.Sun J, Fu G, Lin J, Song X, Lu L, Liu Q. 2010. Seroprevalence of Bartonella in Eastern China and analysis of risk factors. BMC Infect Dis 10:121. doi: 10.1186/1471-2334-10-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sackal C, Laudisoit A, Kosoy M, Massung R, Eremeeva ME, Karpathy SE, Van Wyk K, Gabitzsch E, Zeidner NS. 2008. Bartonella spp. and Rickettsia felis in fleas, Democratic Republic of Congo. Emerg Infect Dis 14:1972–1974. doi: 10.3201/eid1412.080610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tea A, Alexiou-Daniel S, Arvanitidou M, Diza E, Antoniadis A. 2003. Occurrence of Bartonella henselae and Bartonella quintana in a healthy Greek population. Am J Trop Med Hyg 68:554–556. [DOI] [PubMed] [Google Scholar]
- 161.Brenner EC, Chomel BB, Singhasivanon OU, Namekata DY, Kasten RW, Kass PH, Cortes-Vecino JA, Gennari SM, Rajapakse RP, Huong LT, Dubey JP. 2013. Bartonella infection in urban and rural dogs from the tropics: Brazil, Colombia, Sri Lanka and Vietnam. Epidemiol Infect 141:54–61. doi: 10.1017/S0950268812000519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.De Sousa R, Edouard-Fournier P, Santos-Silva M, Amaro F, Bacellar F, Raoult D. 2006. Molecular detection of Rickettsia felis, Rickettsia typhi and two genotypes closely related to Bartonella elizabethae. Am J Trop Med Hyg 75:727–731. [PubMed] [Google Scholar]
- 163.Morse SF, Olival KJ, Kosoy M, Billeter S, Patterson BD, Dick CW, Dittmar K. 2012. Global distribution and genetic diversity of Bartonella in bat flies (Hippoboscoidea, Streblidae, Nycteribiidae). Infect Genet Evol 12:1717–1723. doi: 10.1016/j.meegid.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 164.Sangare AK, Boutellis A, Drali R, Socolovschi C, Barker SC, Diatta G, Rogier C, Olive MM, Doumbo OK, Raoult D. 2014. Detection of Bartonella quintana in African body and head lice. Am J Trop Med Hyg 91:294–301. doi: 10.4269/ajtmh.13-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Drali R, Sangare AK, Boutellis A, Angelakis E, Veracx A, Socolovschi C, Brouqui P, Raoult D. 2014. Bartonella quintana in body lice from scalp hair of homeless persons, France. Emerg Infect Dis 20:907–908. doi: 10.3201/eid2005.131242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Marie JL, Fournier PE, Rolain JM, Briolant S, Davoust B, Raoult D. 2006. Molecular detection of Bartonella quintana, B. elizabethae, B. koehlerae, B. doshiae, B. taylorii, and Rickettsia felis in rodent fleas collected in Kabul, Afghanistan. Am J Trop Med Hyg 74:436–439. [PubMed] [Google Scholar]
- 167.Woolley MW, Gordon DL, Wetherall BL. 2007. Analysis of the first Australian strains of Bartonella quintana reveals unique genotypes. J Clin Microbiol 45:2040–2043. doi: 10.1128/JCM.00175-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Goldstein LH, Saliba WR, Elias M, Zlotnik A, Raz R, Giladi M. 2005. Bartonella quintana endocarditis in east Africa. Eur J Intern Med 16:518–519. doi: 10.1016/j.ejim.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 169.Rolain JM, Bourry O, Davoust B, Raoult D. 2005. Bartonella quintana and Rickettsia felis in Gabon. Emerg Infect Dis 11:1742–1744. doi: 10.3201/eid1111.050861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lim MH, Chung DR, Kim WS, Park KS, Ki CS, Lee NY, Kim SM. 2012. First case of Bartonella quintana endocarditis in Korea. J Korean Med Sci 27:1433–1435. doi: 10.3346/jkms.2012.27.11.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Znazen A, Rolain JM, Hammami N, Kammoun S, Hammami A, Raoult D. 2005. High prevalence of Bartonella quintana endocarditis in Sfax, Tunisia. Am J Trop Med Hyg 72:503–507. [PubMed] [Google Scholar]
- 172.Raoult D, Fournier PE, Vandenesch F, Mainardi JL, Eykyn SJ, Nash J, James E, Benoit-Lemercier C, Marrie TJ. 2003. Outcome and treatment of Bartonella endocarditis. Arch Intern Med 163:226–230. doi: 10.1001/archinte.163.2.226. [DOI] [PubMed] [Google Scholar]
- 173.Barbier F, Fournier PE, Dauge MC, Gallien S, Raoult D, Andremont A, Ruimy R. 2009. Bartonella quintana coinfection in Staphylococcus aureus endocarditis: usefulness of screening in high-risk patients? Clin Infect Dis 48:1332–1333. doi: 10.1086/597826. [DOI] [PubMed] [Google Scholar]
- 174.Tattevin P, Watt G, Revest M, Arvieux C, Fournier PE. 2015. Update on blood culture-negative endocarditis. Med Mal Infect 45:1–8. doi: 10.1016/j.medmal.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 175.Werner M, Fournier PE, Andersson R, Hogevik H, Raoult D. 2003. Bartonella and Coxiella antibodies in 334 prospectively studied episodes of infective endocarditis in Sweden. Scand J Infect Dis 35:724–727. doi: 10.1080/00365540310015980. [DOI] [PubMed] [Google Scholar]
- 176.Kurland S, Enghoff E, Landelius J, Nystrom SO, Hambraeus A, Friman G. 1999. A 10-year retrospective study of infective endocarditis at a university hospital with special regard to the timing of surgical evaluation in S. viridans endocarditis. Scand J Infect Dis 31:87–91. doi: 10.1080/00365549950161952. [DOI] [PubMed] [Google Scholar]
- 177.Edouard S, Nabet C, Lepidi H, Fournier PE, Raoult D. 2015. Bartonella, a common cause of endocarditis: a report on 106 cases and review. J Clin Microbiol 53:824–829. doi: 10.1128/JCM.02827-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Schultz MG. 1968. A history of bartonellosis (Carrion's disease). Am J Trop Med Hyg 17:503–515. [DOI] [PubMed] [Google Scholar]
- 179.Houpikian P, Raoult D. 2001. Molecular phylogeny of the genus Bartonella: what is the current knowledge? FEMS Microbiol Lett 200:1–7. doi: 10.1111/j.1574-6968.2001.tb10684.x. [DOI] [PubMed] [Google Scholar]
- 180.Kosoy M, Hayman DT, Chan KS. 2012. Bartonella bacteria in nature: where does population variability end and a species start? Infect Genet Evol 12:894–904. doi: 10.1016/j.meegid.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 181.Breitschwerdt EB, Kordick DL. 2000. Bartonella infection in animals: carriership, reservoir potential, pathogenicity, and zoonotic potential for human infection. Clin Microbiol Rev 13:428–438. doi: 10.1128/CMR.13.3.428-438.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gouriet F, Lepidi H, Habib G, Collart F, Raoult D. 2007. From cat scratch disease to endocarditis, the possible natural history of Bartonella henselae infection. BMC Infect Dis 7:30. doi: 10.1186/1471-2334-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Garcia FU, Wojta J, Broadley KN, Davidson JM, Hoover RL. 1990. Bartonella bacilliformis stimulates endothelial cells in vitro and is angiogenic in vivo. Am J Pathol 136:1125–1135. [PMC free article] [PubMed] [Google Scholar]
- 184.Alexander B. 1995. A review of bartonellosis in Ecuador and Colombia. Am J Trop Med Hyg 52:354–359. [DOI] [PubMed] [Google Scholar]
- 185.Ohl ME, Spach DH. 2000. Bartonella quintana and urban trench fever. Clin Infect Dis 31:131–135. doi: 10.1086/313890. [DOI] [PubMed] [Google Scholar]
- 186.Maurin M, Raoult D. 1996. Bartonella (Rochalimaea) quintana infections. Clin Microbiol Rev 9:273–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kordick DL, Hilyard EJ, Hadfield TL, Wilson KH, Steigerwalt AG, Brenner DJ, Breitschwerdt EB. 1997. Bartonella clarridgeiae, a newly recognized zoonotic pathogen causing inoculation papules, fever, and lymphadenopathy (cat scratch disease). J Clin Microbiol 35:1813–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chomel BB, Abbott RC, Kasten RW, Floyd-Hawkins KA, Kass PH, Glaser CA, Pedersen NC, Koehler JE. 1995. Bartonella henselae prevalence in domestic cats in California: risk factors and association between bacteremia and antibody titers. J Clin Microbiol 33:2445–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bergmans AM, de Jong CM, van Amerongen G, Schot CS, Schouls LM. 1997. Prevalence of Bartonella species in domestic cats in The Netherlands. J Clin Microbiol 35:2256–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Florin TA, Zaoutis TE, Zaoutis LB. 2008. Beyond cat scratch disease: widening spectrum of Bartonella henselae infection. Pediatrics 121:e1413–e1425. doi: 10.1542/peds.2007-1897. [DOI] [PubMed] [Google Scholar]
- 191.Nelson CA, Saha S, Mead PS. 2016. Cat-scratch disease in the United States, 2005–2013. Emerg Infect Dis 22:1741–1746. doi: 10.3201/eid2210.160115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Carithers HA. 1978. Oculoglandular disease of Parinaud. A manifestation of cat-scratch disease. Am J Dis Child 132:1195–1200. doi: 10.1001/archpedi.1978.02120370043011. [DOI] [PubMed] [Google Scholar]
- 193.Aziz HA, Plesec TP, Sabella C, Udayasankar UK, Singh AD. 2016. Cat scratch disease: expanded spectrum. Ocul Oncol Pathol 2:246–250. doi: 10.1159/000447063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hercik K, Hasova V, Janecek J, Branny P. 2007. Molecular evidence of Bartonella DNA in ixodid ticks in Czechia. Folia Microbiol (Praha) 52:503–509. doi: 10.1007/BF02932111. [DOI] [PubMed] [Google Scholar]
- 195.Spach DH. 1992. Bacillary angiomatosis. Int J Dermatol 31:19–24. doi: 10.1111/j.1365-4362.1992.tb03512.x. [DOI] [PubMed] [Google Scholar]
- 196.Cockerell CJ, Bergstresser PR, Myrie-Williams C, Tierno PM. 1990. Bacillary epithelioid angiomatosis occurring in an immunocompetent individual. Arch Dermatol 126:787–790. [PubMed] [Google Scholar]
- 197.Cockerell CJ, LeBoit PE. 1990. Bacillary angiomatosis: a newly characterized, pseudoneoplastic, infectious, cutaneous vascular disorder. J Am Acad Dermatol 22:501–512. doi: 10.1016/0190-9622(90)70071-O. [DOI] [PubMed] [Google Scholar]
- 198.Kemper CA, Lombard CM, Deresinski SC, Tompkins LS. 1990. Visceral bacillary epithelioid angiomatosis: possible manifestations of disseminated cat scratch disease in the immunocompromised host: a report of two cases. Am J Med 89:216–222. doi: 10.1016/0002-9343(90)90301-S. [DOI] [PubMed] [Google Scholar]
- 199.LeBoit PE. 1990. The expanding spectrum of a new disease, bacillary angiomatosis. Arch Dermatol 126:808–811. [PubMed] [Google Scholar]
- 200.Tsokos M, Erbersdobler A. 2005. Pathology of peliosis. Forensic Sci Int 149:25–33. doi: 10.1016/j.forsciint.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 201.Nadell J, Kosek J. 1977. Peliosis hepatis. Twelve cases associated with oral androgen therapy. Arch Pathol Lab Med 101:405–410. [PubMed] [Google Scholar]
- 202.McGiven AR. 1970. Peliosis hepatis: case report and review of pathogenesis. J Pathol 101:283–285. doi: 10.1002/path.1711010312. [DOI] [PubMed] [Google Scholar]
- 203.Perkocha LA, Geaghan SM, Yen TS, Nishimura SL, Chan SP, Garcia-Kennedy R, Honda G, Stoloff AC, Klein HZ, Goldman RL, Van Meter S, Ferrell L, LeBoit PE. 1990. Clinical and pathological features of bacillary peliosis hepatis in association with human immunodeficiency virus infection. N Engl J Med 323:1581–1586. doi: 10.1056/NEJM199012063232302. [DOI] [PubMed] [Google Scholar]
- 204.Czapar CA, Weldon-Linne CM, Moore DM, Rhone DP. 1986. Peliosis hepatis in the acquired immunodeficiency syndrome. Arch Pathol Lab Med 110:611–613. [PubMed] [Google Scholar]
- 205.Tappero JW, Koehler JE, Berger TG, Cockerell CJ, Lee TH, Busch MP, Stites DP, Mohle-Boetani J, Reingold AL, LeBoit PE. 1993. Bacillary angiomatosis and bacillary splenitis in immunocompetent adults. Ann Intern Med 118:363–365. doi: 10.7326/0003-4819-118-5-199303010-00007. [DOI] [PubMed] [Google Scholar]
- 206.Spach DH, Koehler JE. 1998. Bartonella-associated infections. Infect Dis Clin North Am 12:137–155. doi: 10.1016/S0891-5520(05)70414-1. [DOI] [PubMed] [Google Scholar]
- 207.Hayem F, Chacar S, Hayem G. 1996. Bartonella henselae infection mimicking systemic onset juvenile chronic arthritis in a 2 1/2-year-old girl. J Rheumatol 23:1263–1265. [PubMed] [Google Scholar]
- 208.Al-Matar MJ, Petty RE, Cabral DA, Tucker LB, Peyvandi B, Prendiville J, Forbes J, Cairns R, Rothstein R. 2002. Rheumatic manifestations of Bartonella infection in 2 children. J Rheumatol 29:184–186. [PubMed] [Google Scholar]
- 209.Robinson JL, Spady DW, Prasad E, McColl D, Artsob H. 2005. Bartonella seropositivity in children with Henoch-Schonlein purpura. BMC Infect Dis 5:21. doi: 10.1186/1471-2334-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Van Audenhove A, Verhoef G, Peetermans WE, Boogaerts M, Vandenberghe P. 2001. Autoimmune haemolytic anaemia triggered by Bartonella henselae infection: a case report. Br J Haematol 115:924–925. doi: 10.1046/j.1365-2141.2001.03165.x. [DOI] [PubMed] [Google Scholar]
- 211.Kerkhoff FT, Rothova A. 2000. Bartonella henselae associated uveitis and HLA-B27. Br J Ophthalmol 84:1125–1129. doi: 10.1136/bjo.84.10.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Chatzidimitriou D, Kirmizis D, Gavriilaki E, Chatzidimitriou M, Malisiovas N. 2012. Atherosclerosis and infection: is the jury still not in? Future Microbiol 7:1217–1230. doi: 10.2217/fmb.12.87. [DOI] [PubMed] [Google Scholar]
- 213.Kozarov E, Padro T, Badimon L. 2014. View of statins as antimicrobials in cardiovascular risk modification. Cardiovasc Res 102:362–374. doi: 10.1093/cvr/cvu058. [DOI] [PubMed] [Google Scholar]
- 214.Steinberg D. 2006. Thematic review series: the pathogenesis of atherosclerosis. An interpretive history of the cholesterol controversy, part V: the discovery of the statins and the end of the controversy. J Lipid Res 47:1339–1351. [DOI] [PubMed] [Google Scholar]
- 215.Mandle T, Einsele H, Schaller M, Neumann D, Vogel W, Autenrieth IB, Kempf VA. 2005. Infection of human CD34+ progenitor cells with Bartonella henselae results in intraerythrocytic presence of B. henselae. Blood 106:1215–1222. doi: 10.1182/blood-2004-12-4670. [DOI] [PubMed] [Google Scholar]
- 216.Salvatore P, Casamassimi A, Sommese L, Fiorito C, Ciccodicola A, Rossiello R, Avallone B, Grimaldi V, Costa V, Rienzo M, Colicchio R, Williams-Ignarro S, Pagliarulo C, Prudente ME, Abbondanza C, Lamberti F, Baroni A, Buommino E, Farzati B, Tufano MA, Ignarro LJ, Napoli C. 2008. Detrimental effects of Bartonella henselae are counteracted by l-arginine and nitric oxide in human endothelial progenitor cells. Proc Natl Acad Sci U S A 105:9427–9432. doi: 10.1073/pnas.0803602105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Salvatore P, Zullo A, Sommese L, Colicchio R, Picascia A, Schiano C, Mancini FP, Napoli C. 2015. Infections and cardiovascular disease: is Bartonella henselae contributing to this matter? J Med Microbiol 64:799–809. doi: 10.1099/jmm.0.000099. [DOI] [PubMed] [Google Scholar]
- 218.Meininger GR, Nadasdy T, Hruban RH, Bollinger RC, Baughman KL, Hare JM. 2001. Chronic active myocarditis following acute Bartonella henselae infection (cat scratch disease). Am J Surg Pathol 25:1211–1214. doi: 10.1097/00000478-200109000-00015. [DOI] [PubMed] [Google Scholar]
- 219.Montcriol A, Benard F, Fenollar F, Ribeiri A, Bonnet M, Collart F, Guidon C. 2009. Fatal myocarditis-associated Bartonella quintana endocarditis: a case report. J Med Case Rep 3:7325. doi: 10.4076/1752-1947-3-7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Pipili C, Katsogridakis K, Cholongitas E. 2008. Myocarditis due to Bartonella henselae. South Med J 101:1186. doi: 10.1097/SMJ.0b013e318182481c. [DOI] [PubMed] [Google Scholar]
- 221.Wesslen L, Ehrenborg C, Holmberg M, McGill S, Hjelm E, Lindquist O, Henriksen E, Rolf C, Larsson E, Friman G. 2001. Subacute bartonella infection in Swedish orienteers succumbing to sudden unexpected cardiac death or having malignant arrhythmias. Scand J Infect Dis 33:429–438. doi: 10.1080/00365540152029891. [DOI] [PubMed] [Google Scholar]
- 222.McGill S, Wesslen L, Hjelm E, Holmberg M, Rolf C, Friman G. 2001. Serological and epidemiological analysis of the prevalence of Bartonella spp. antibodies in Swedish elite orienteers 1992-93. Scand J Infect Dis 33:423–428. doi: 10.1080/00365540152029882. [DOI] [PubMed] [Google Scholar]
- 223.Harms A, Dehio C. 2012. Intruders below the radar: molecular pathogenesis of Bartonella spp. Clin Microbiol Rev 25:42–78. doi: 10.1128/CMR.05009-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Schulein R, Seubert A, Gille C, Lanz C, Hansmann Y, Piemont Y, Dehio C. 2001. Invasion and persistent intracellular colonization of erythrocytes. A unique parasitic strategy of the emerging pathogen Bartonella. J Exp Med 193:1077–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Cartwright JL, Britton P, Minnick MF, McLennan AG. 1999. The IalA invasion gene of Bartonella bacilliformis encodes a (de)nucleoside polyphosphate hydrolase of the MutT motif family and has homologs in other invasive bacteria. Biochem Biophys Res Commun 256:474–479. doi: 10.1006/bbrc.1999.0354. [DOI] [PubMed] [Google Scholar]
- 226.Mitchell SJ, Minnick MF. 1995. Characterization of a two-gene locus from Bartonella bacilliformis associated with the ability to invade human erythrocytes. Infect Immun 63:1552–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Conyers GB, Bessman MJ. 1999. The gene, ialA, associated with the invasion of human erythrocytes by Bartonella bacilliformis, designates a nudix hydrolase active on dinucleoside 5′-polyphosphates. J Biol Chem 274:1203–1206. doi: 10.1074/jbc.274.3.1203. [DOI] [PubMed] [Google Scholar]
- 228.Minnick MF, Battisti JM. 2009. Pestilence, persistence and pathogenicity: infection strategies of Bartonella. Future Microbiol 4:743–758. doi: 10.2217/fmb.09.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kosoy MY, Regnery RL, Kosaya OI, Jones DC, Marston EL, Childs JE. 1998. Isolation of Bartonella spp. from embryos and neonates of naturally infected rodents. J Wildl Dis 34:305–309. doi: 10.7589/0090-3558-34.2.305. [DOI] [PubMed] [Google Scholar]
- 230.Chang CC, Chomel BB, Kasten RW, Heller RM, Kocan KM, Ueno H, Yamamoto K, Bleich VC, Pierce BM, Gonzales BJ, Swift PK, Boyce WM, Jang SS, Boulouis HJ, Piemont Y. 2000. Bartonella spp. isolated from wild and domestic ruminants in North America. Emerg Infect Dis 6:306–311. doi: 10.3201/eid0603.000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Vinson JW, Varela G, Molina-Pasquel C. 1969. Trench fever. 3. Induction of clinical disease in volunteers inoculated with Rickettsia quintana propagated on blood agar. Am J Trop Med Hyg 18:713–722. [PubMed] [Google Scholar]
- 232.Kordick DL, Breitschwerdt EB. 1995. Intraerythrocytic presence of Bartonella henselae. J Clin Microbiol 33:1655–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Koesling J, Aebischer T, Falch C, Schulein R, Dehio C. 2001. Cutting edge: antibody-mediated cessation of hemotropic infection by the intraerythrocytic mouse pathogen Bartonella grahamii. J Immunol 167:11–14. doi: 10.4049/jimmunol.167.1.11. [DOI] [PubMed] [Google Scholar]
- 234.Chomel BB, Kasten RW, Sykes JE, Boulouis HJ, Breitschwerdt EB. 2003. Clinical impact of persistent Bartonella bacteremia in humans and animals. Ann N Y Acad Sci 990:267–278. doi: 10.1111/j.1749-6632.2003.tb07376.x. [DOI] [PubMed] [Google Scholar]
- 235.Raoult D, Casalta JP, Richet H, Khan M, Bernit E, Rovery C, Branger S, Gouriet F, Imbert G, Bothello E, Collart F, Habib G. 2005. Contribution of systematic serological testing in diagnosis of infective endocarditis. J Clin Microbiol 43:5238–5242. doi: 10.1128/JCM.43.10.5238-5242.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Tunkel AR, Kaye D. 1992. Endocarditis with negative blood cultures. N Engl J Med 326:1215–1217. doi: 10.1056/NEJM199204303261809. [DOI] [PubMed] [Google Scholar]
- 237.Watanakunakorn C, Burkert T. 1993. Infective endocarditis at a large community teaching hospital, 1980–1990. A review of 210 episodes. Medicine (Baltimore) 72:90–102. doi: 10.1097/00005792-199303000-00003. [DOI] [PubMed] [Google Scholar]
- 238.Benslimani A, Fenollar F, Lepidi H, Raoult D. 2005. Bacterial zoonoses and infective endocarditis, Algeria. Emerg Infect Dis 11:216–224. doi: 10.3201/eid1102.040668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Koegelenberg CF, Doubell AF, Orth H, Reuter H. 2003. Infective endocarditis in the Western Cape Province of South Africa: a three-year prospective study. QJM 96:217–225. doi: 10.1093/qjmed/hcg028. [DOI] [PubMed] [Google Scholar]
- 240.Tariq M, Alam M, Munir G, Khan MA, Smego RA Jr. 2004. Infective endocarditis: a five-year experience at a tertiary care hospital in Pakistan. Int J Infect Dis 8:163–170. doi: 10.1016/j.ijid.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 241.Lamas CC, Eykyn SJ. 2003. Blood culture negative endocarditis: analysis of 63 cases presenting over 25 years. Heart 89:258–262. doi: 10.1136/heart.89.3.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Nakatani S, Mitsutake K, Hozumi T, Yoshikawa J, Akiyama M, Yoshida K, Ishizuka N, Nakamura K, Taniguchi Y, Yoshioka K, Kawazoe K, Akaishi M, Niwa K, Nakazawa M, Kitamura S, Miyatake K, Committee on Guideline for Prevention Management of Infective Endocarditis, Japanese Circulation Society. 2003. Current characteristics of infective endocarditis in Japan: an analysis of 848 cases in 2000 and 2001. Circ J 67:901–905. doi: 10.1253/circj.67.901. [DOI] [PubMed] [Google Scholar]
- 243.Cannady PB Jr, Sanford JP. 1976. Negative blood cultures in infective endocarditis: a review. South Med J 69:1420–1424. doi: 10.1097/00007611-197611000-00008. [DOI] [PubMed] [Google Scholar]
- 244.Von Reyn CF, Levy BS, Arbeit RD, Friedland G, Crumpacker CS. 1981. Infective endocarditis: an analysis based on strict case definitions. Ann Intern Med 94:505–518. doi: 10.7326/0003-4819-94-4-505. [DOI] [PubMed] [Google Scholar]
- 245.Van Scoy RE. 1982. Culture-negative endocarditis. Mayo Clin Proc 57:149–154. [PubMed] [Google Scholar]
- 246.Nadir E, Rubinstein E. 2004. Fungal endocarditis. Curr Infect Dis Rep 6:276–282. doi: 10.1007/s11908-004-0048-8. [DOI] [PubMed] [Google Scholar]
- 247.Pazin GJ, Saul S, Thompson ME. 1982. Blood culture positivity: suppression by outpatient antibiotic therapy in patients with bacterial endocarditis. Arch Intern Med 142:263–268. [PubMed] [Google Scholar]
- 248.Massoure PL, Reuter S, Lafitte S, Laborderie J, Bordachard P, Clementy J, Roudaut R. 2007. Pacemaker endocarditis: clinical features and management of 60 consecutive cases. Pacing Clin Electrophysiol 30:12–19. [DOI] [PubMed] [Google Scholar]
- 249.Subedi S, Jennings Z, Chen SC. 2017. Laboratory approach to the diagnosis of culture-negative infective endocarditis. Heart Lung Circ doi: 10.1016/j.hlc.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 250.Drancourt M, Birtles R, Chaumentin G, Vandenesch F, Etienne J, Raoult D. 1996. New serotype of Bartonella henselae in endocarditis and cat-scratch disease. Lancet 347:441–443. doi: 10.1016/S0140-6736(96)90012-4. [DOI] [PubMed] [Google Scholar]
- 251.Maurin M, Birtles R, Raoult D. 1997. Current knowledge of Bartonella species. Eur J Clin Microbiol Infect Dis 16:487–506. doi: 10.1007/BF01708232. [DOI] [PubMed] [Google Scholar]
- 252.Lamas C, Favacho A, Ramos RG, Santos MS, Ferravoli GI, Weksler C, Rozental T, Boia MN, Lemos ER. 2007. Bartonella native valve endocarditis: the first Brazilian case alive and well. Braz J Infect Dis 11:591–594. doi: 10.1590/S1413-86702007000600012. [DOI] [PubMed] [Google Scholar]
- 253.De La Rosa GR, Barnett BJ, Ericsson CD, Turk JB. 2001. Native valve endocarditis due to Bartonella henselae in a middle-aged human immunodeficiency virus-negative woman. J Clin Microbiol 39:3417–3419. doi: 10.1128/JCM.39.9.3417-3419.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Oteo JA, Castilla A, Arosey A, Blanco JR, Ibarra V, Morano LE. 2006. Endocarditis due to Bartonella spp. Three new clinical cases and Spanish literature review. Enferm Infecc Microbiol Clin 24:297–301. doi: 10.1157/13089663. [DOI] [PubMed] [Google Scholar]
- 255.Werner M, Andersson R, Olaison L, Hogevik H. 2003. A clinical study of culture-negative endocarditis. Medicine (Baltimore) 82:263–273. [DOI] [PubMed] [Google Scholar]
- 256.Dreier J, Vollmer T, Freytag CC, Baumer D, Korfer R, Kleesiek K. 2008. Culture-negative infectious endocarditis caused by Bartonella spp.: 2 case reports and a review of the literature. Diagn Microbiol Infect Dis 61:476–483. doi: 10.1016/j.diagmicrobio.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 257.Baorto E, Payne RM, Slater LN, Lopez F, Relman DA, Min KW, St Geme JW III. 1998. Culture-negative endocarditis caused by Bartonella henselae. J Pediatr 132:1051–1054. doi: 10.1016/S0022-3476(98)70410-X. [DOI] [PubMed] [Google Scholar]
- 258.Albrich WC, Kraft C, Fisk T, Albrecht H. 2004. A mechanic with a bad valve: blood-culture-negative endocarditis. Lancet Infect Dis 4:777–784. doi: 10.1016/S1473-3099(04)01226-5. [DOI] [PubMed] [Google Scholar]
- 259.Vento A, Patila T, Vaara M, Larinkari U, Sipponen J. 2008. Bartonella quintana and Bartonella pediococcus infection after aortic valve replacement. Heart Surg Forum 11:E94–E95. doi: 10.1532/HSF98.20071204. [DOI] [PubMed] [Google Scholar]
- 260.Klein JL, Nair SK, Harrison TG, Hunt I, Fry NK, Friedland JS. 2002. Prosthetic valve endocarditis caused by Bartonella quintana. Emerg Infect Dis 8:202–203. doi: 10.3201/eid0802.010206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Hoen B, Selton-Suty C, Lacassin F, Etienne J, Briancon S, Leport C, Canton P. 1995. Infective endocarditis in patients with negative blood cultures: analysis of 88 cases from a one-year nationwide survey in France. Clin Infect Dis 20:501–506. doi: 10.1093/clinids/20.3.501. [DOI] [PubMed] [Google Scholar]
- 262.Pesanti EL, Smith IM. 1979. Infective endocarditis with negative blood cultures. An analysis of 52 cases. Am J Med 66:43–50. [DOI] [PubMed] [Google Scholar]
- 263.La Scola B, Raoult D. 1999. Culture of Bartonella quintana and Bartonella henselae from human samples: a 5-year experience (1993 to 1998). J Clin Microbiol 37:1899–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Fournier PE, Thuny F, Richet H, Lepidi H, Casalta JP, Arzouni JP, Maurin M, Celard M, Mainardi JL, Caus T, Collart F, Habib G, Raoult D. 2010. Comprehensive diagnostic strategy for blood culture-negative endocarditis: a prospective study of 819 new cases. Clin Infect Dis 51:131–140. doi: 10.1086/653675. [DOI] [PubMed] [Google Scholar]
- 265.Fournier PE, Mainardi JL, Raoult D. 2002. Value of microimmunofluorescence for diagnosis and follow-up of Bartonella endocarditis. Clin Diagn Lab Immunol 9:795–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Rolain JM, Lecam C, Raoult D. 2003. Simplified serological diagnosis of endocarditis due to Coxiella burnetii and Bartonella. Clin Diagn Lab Immunol 10:1147–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Zeaiter Z, Fournier PE, Greub G, Raoult D. 2003. Diagnosis of Bartonella endocarditis by a real-time nested PCR assay using serum. J Clin Microbiol 41:919–925. doi: 10.1128/JCM.41.3.919-925.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Fournier PE, Casalta JP, Habib G, Messana T, Raoult D. 1996. Modification of the diagnostic criteria proposed by the Duke Endocarditis Service to permit improved diagnosis of Q fever endocarditis. Am J Med 100:629–633. doi: 10.1016/S0002-9343(96)00040-X. [DOI] [PubMed] [Google Scholar]
- 269.Wegdam-Blans MC, Kampschreur LM, Delsing CE, Bleeker-Rovers CP, Sprong T, van Kasteren ME, Notermans DW, Renders NH, Bijlmer HA, Lestrade PJ, Koopmans MP, Nabuurs-Franssen MH, Oosterheert JJ, Dutch Q Fever Consensus Group. 2012. Chronic Q fever: review of the literature and a proposal of new diagnostic criteria. J Infect 64:247–259. doi: 10.1016/j.jinf.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 270.Lamas CC, Eykyn SJ. 1997. Suggested modifications to the Duke criteria for the clinical diagnosis of native valve and prosthetic valve endocarditis: analysis of 118 pathologically proven cases. Clin Infect Dis 25:713–719. doi: 10.1086/513765. [DOI] [PubMed] [Google Scholar]
- 271.Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG Jr, Ryan T, Bashore T, Corey GR. 2000. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 30:633–638. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 272.Prendergast BD. 2004. Diagnostic criteria and problems in infective endocarditis. Heart 90:611–613. doi: 10.1136/hrt.2003.029850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Millar B, Moore J, Mallon P, Xu J, Crowe M, McClurg R, Raoult D, Earle J, Hone R, Murphy P. 2001. Molecular diagnosis of infective endocarditis—a new Duke's criterion. Scand J Infect Dis 33:673–680. doi: 10.1080/00365540110026764. [DOI] [PubMed] [Google Scholar]
- 274.MacKichan JK, Gerns HL, Chen YT, Zhang P, Koehler JE. 2008. A SacB mutagenesis strategy reveals that the Bartonella quintana variably expressed outer membrane proteins are required for bloodstream infection of the host. Infect Immun 76:788–795. doi: 10.1128/IAI.01174-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Dehio C, Meyer M, Berger J, Schwarz H, Lanz C. 1997. Interaction of Bartonella henselae with endothelial cells results in bacterial aggregation on the cell surface and the subsequent engulfment and internalisation of the bacterial aggregate by a unique structure, the invasome. J Cell Sci 110:2141–2154. [DOI] [PubMed] [Google Scholar]
- 276.Dehio C. 2003. Recent progress in understanding Bartonella-induced vascular proliferation. Curr Opin Microbiol 6:61–65. doi: 10.1016/S1369-5274(03)00006-7. [DOI] [PubMed] [Google Scholar]
- 277.Dehio C. 2001. Bartonella interactions with endothelial cells and erythrocytes. Trends Microbiol 9:279–285. doi: 10.1016/S0966-842X(01)02047-9. [DOI] [PubMed] [Google Scholar]
- 278.Seubert A, Schulein R, Dehio C. 2002. Bacterial persistence within erythrocytes: a unique pathogenic strategy of Bartonella spp. Int J Med Microbiol 291:555–560. doi: 10.1078/1438-4221-00167. [DOI] [PubMed] [Google Scholar]
- 279.Zbinden R, Hochli M, Nadal D. 1995. Intracellular location of Bartonella henselae cocultivated with Vero cells and used for an indirect fluorescent-antibody test. Clin Diagn Lab Immunol 2:693–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Rolain JM, Maurin M, Mallet MN, Parzy D, Raoult D. 2003. Culture and antibiotic susceptibility of Bartonella quintana in human erythrocytes. Antimicrob Agents Chemother 47:614–619. doi: 10.1128/AAC.47.2.614-619.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Maurin M, Gasquet S, Ducco C, Raoult D. 1995. MICs of 28 antibiotic compounds for 14 Bartonella (formerly Rochalimaea) isolates. Antimicrob Agents Chemother 39:2387–2391. doi: 10.1128/AAC.39.11.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Rolain JM, Brouqui P, Koehler JE, Maguina C, Dolan MJ, Raoult D. 2004. Recommendations for treatment of human infections caused by Bartonella species. Antimicrob Agents Chemother 48:1921–1933. doi: 10.1128/AAC.48.6.1921-1933.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Musso D, Drancourt M, Raoult D. 1995. Lack of bactericidal effect of antibiotics except aminoglycosides on Bartonella (Rochalimaea) henselae. J Antimicrob Chemother 36:101–108. doi: 10.1093/jac/36.1.101. [DOI] [PubMed] [Google Scholar]
- 284.Myers WF, Grossman DM, Wisseman CL Jr. 1984. Antibiotic susceptibility patterns in Rochalimaea quintana, the agent of trench fever. Antimicrob Agents Chemother 25:690–693. doi: 10.1128/AAC.25.6.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Ives TJ, Manzewitsch P, Regnery RL, Butts JD, Kebede M. 1997. In vitro susceptibilities of Bartonella henselae, B. quintana, B. elizabethae, Rickettsia rickettsii, R conorii, R akari, and R prowazekii to macrolide antibiotics as determined by immunofluorescent-antibody analysis of infected Vero cell monolayers. Antimicrob Agents Chemother 41:578–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Rolain JM, Maurin M, Raoult D. 2000. Bactericidal effect of antibiotics on Bartonella and Brucella spp.: clinical implications. J Antimicrob Chemother 46:811–814. doi: 10.1093/jac/46.5.811. [DOI] [PubMed] [Google Scholar]
- 287.Elliott TS, Foweraker J, Gould FK, Perry JD, Sandoe JA, Working Party of the British Society for Antimicrobial Chemotherapy. 2004. Guidelines for the antibiotic treatment of endocarditis in adults: report of the Working Party of the British Society for Antimicrobial Chemotherapy. J Antimicrob Chemother 54:971–981. doi: 10.1093/jac/dkh474. [DOI] [PubMed] [Google Scholar]
- 288.Gould FK, Denning DW, Elliott TS, Foweraker J, Perry JD, Prendergast BD, Sandoe JA, Spry MJ, Watkin RW, Working Party of the British Society for Antimicrobial Chemotherapy. 2012. Guidelines for the diagnosis and antibiotic treatment of endocarditis in adults: a report of the Working Party of the British Society for Antimicrobial Chemotherapy. J Antimicrob Chemother 67:269–289. doi: 10.1093/jac/dkr450. [DOI] [PubMed] [Google Scholar]
- 289.Murdoch DR, Corey GR, Hoen B, Miro JM, Fowler VG Jr, Bayer AS, Karchmer AW, Olaison L, Pappas PA, Moreillon P, Chambers ST, Chu VH, Falco V, Holland DJ, Jones P, Klein JL, Raymond NJ, Read KM, Tripodi MF, Utili R, Wang A, Woods CW, Cabell CH, International Collaboration on Endocarditis-Prospective Cohort Study Investigators. 2009. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med 169:463–473. doi: 10.1001/archinternmed.2008.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Pendle S, Ginn A, Iredell J. 2006. Antimicrobial susceptibility of Bartonella henselae using Etest methodology. J Antimicrob Chemother 57:761–763. doi: 10.1093/jac/dki485. [DOI] [PubMed] [Google Scholar]
- 291.Angelakis E, Raoult D. 2014. Pathogenicity and treatment of Bartonella infections. Int J Antimicrob Agents 44:16–25. doi: 10.1016/j.ijantimicag.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 292.Zahringer U, Lindner B, Knirel YA, van den Akker WM, Hiestand R, Heine H, Dehio C. 2004. Structure and biological activity of the short-chain lipopolysaccharide from Bartonella henselae ATCC 49882T. J Biol Chem 279:21046–21054. doi: 10.1074/jbc.M313370200. [DOI] [PubMed] [Google Scholar]
- 293.Popa C, Abdollahi-Roodsaz S, Joosten LAB, Takahashi N, Sprong T, Matera G, Liberto MC, Foca A, van Deuren M, Kullberg BJ, van den Berg WB, van der Meer JWM, Netea MG. 2007. Bartonella quintana lipopolysaccharide is a natural antagonist of Toll-like receptor 4. Infect Immun 75:4831–4837. doi: 10.1128/IAI.00237-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Matera G, Liberto MC, Quirino A, Barreca GS, Lamberti AG, Iannone M, Mancuso E, Palma E, Cufari FA, Rotiroti D, Foca A. 2003. Bartonella quintana lipopolysaccharide effects on leukocytes, CXC chemokines and apoptosis: a study on the human whole blood and a rat model. Int Immunopharmacol 3:853–864. doi: 10.1016/S1567-5769(03)00059-6. [DOI] [PubMed] [Google Scholar]
- 295.Andersen-Nissen E, Smith KD, Strobe KL, Barrett SLR, Cookson BT, Logan SM, Aderem A. 2005. Evasion of Toll-like receptor 5 by flagellated bacteria. Proc Natl Acad Sci U S A 102:9247–9252. doi: 10.1073/pnas.0502040102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Dehio C. 2005. Bartonella-host-cell interactions and vascular tumour formation. Nat Rev Microbiol 3:621–631. doi: 10.1038/nrmicro1209. [DOI] [PubMed] [Google Scholar]
- 297.Maguina C, Guerra H, Ventosilla P. 2009. Bartonellosis. Clin Dermatol 27:271–280. doi: 10.1016/j.clindermatol.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 298.Verma A, Davis GE, Ihler GM. 2000. Infection of human endothelial cells with Bartonella bacilliformis is dependent on Rho and results in activation of Rho. Infect Immun 68:5960–5969. doi: 10.1128/IAI.68.10.5960-5969.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Muller NF, Kaiser PO, Linke D, Schwarz H, Riess T, Schafer A, Eble JA, Kempf VA. 2011. Trimeric autotransporter adhesin-dependent adherence of Bartonella henselae, Bartonella quintana, and Yersinia enterocolitica to matrix components and endothelial cells under static and dynamic flow conditions. Infect Immun 79:2544–2553. doi: 10.1128/IAI.01309-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Berrich M, Kieda C, Grillon C, Monteil M, Lamerant N, Gavard J, Boulouis HJ, Haddad N. 2011. Differential effects of Bartonella henselae on human and feline macro- and micro-vascular endothelial cells. PLoS One 6e20204. doi: 10.1371/journal.pone.0020204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Monteil RA, Michiels JF, Hofman P, Saint-Paul MC, Hitzig C, Perrin C, Santini J. 1994. Histological and ultrastructural study of one case of oral bacillary angiomatosis in HIV disease and review of the literature. Eur J Cancer B Oral Oncol 30B:65–71. doi: 10.1016/0964-1955(94)90054-X. [DOI] [PubMed] [Google Scholar]
- 302.Kempf VA, Schaller M, Behrendt S, Volkmann B, Aepfelbacher M, Cakman I, Autenrieth IB. 2000. Interaction of Bartonella henselae with endothelial cells results in rapid bacterial rRNA synthesis and replication. Cell Microbiol 2:431–441. doi: 10.1046/j.1462-5822.2000.00072.x. [DOI] [PubMed] [Google Scholar]
- 303.Kempf VA, Volkmann B, Schaller M, Sander CA, Alitalo K, Riess T, Autenrieth IB. 2001. Evidence of a leading role for VEGF in Bartonella henselae-induced endothelial cell proliferations. Cell Microbiol 3:623–632. doi: 10.1046/j.1462-5822.2001.00144.x. [DOI] [PubMed] [Google Scholar]
- 304.Kirby JE, Nekorchuk DM. 2002. Bartonella-associated endothelial proliferation depends on inhibition of apoptosis. Proc Natl Acad Sci U S A 99:4656–4661. doi: 10.1073/pnas.072292699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Kirby JE. 2004. In vitro model of Bartonella henselae-induced angiogenesis. Infect Immun 72:7315–7317. doi: 10.1128/IAI.72.12.7315-7317.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Lax AJ, Thomas W. 2002. How bacteria could cause cancer: one step at a time. Trends Microbiol 10:293–299. doi: 10.1016/S0966-842X(02)02360-0. [DOI] [PubMed] [Google Scholar]
- 307.Kempf VA, Lebiedziejewski M, Alitalo K, Walzlein JH, Ehehalt U, Fiebig J, Huber S, Schutt B, Sander CA, Muller S, Grassl G, Yazdi AS, Brehm B, Autenrieth IB. 2005. Activation of hypoxia-inducible factor-1 in bacillary angiomatosis: evidence for a role of hypoxia-inducible factor-1 in bacterial infections. Circulation 111:1054–1062. doi: 10.1161/01.CIR.0000155608.07691.B7. [DOI] [PubMed] [Google Scholar]
- 308.O'Rourke F, Mandle T, Urbich C, Dimmeler S, Michaelis UR, Brandes RP, Flotenmeyer M, Doring C, Hansmann ML, Lauber K, Ballhorn W, Kempf VA. 2015. Reprogramming of myeloid angiogenic cells by Bartonella henselae leads to microenvironmental regulation of pathological angiogenesis. Cell Microbiol 17:1447–1463. doi: 10.1111/cmi.12447. [DOI] [PubMed] [Google Scholar]
- 309.Schulte B, Linke D, Klumpp S, Schaller M, Riess T, Autenrieth IB, Kempf VA. 2006. Bartonella quintana variably expressed outer membrane proteins mediate vascular endothelial growth factor secretion but not host cell adherence. Infect Immun 74:5003–5013. doi: 10.1128/IAI.00663-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Scheidegger F, Ellner Y, Guye P, Rhomberg TA, Weber H, Augustin HG, Dehio C. 2009. Distinct activities of Bartonella henselae type IV secretion effector proteins modulate capillary-like sprout formation. Cell Microbiol 11:1088–1101. doi: 10.1111/j.1462-5822.2009.01313.x. [DOI] [PubMed] [Google Scholar]
- 311.Lu YY, Franz B, Truttmann MC, Riess T, Gay-Fraret J, Faustmann M, Kempf VA, Dehio C. 2013. Bartonella henselae trimeric autotransporter adhesin BadA expression interferes with effector translocation by the VirB/D4 type IV secretion system. Cell Microbiol 15:759–778. doi: 10.1111/cmi.12070. [DOI] [PubMed] [Google Scholar]
- 312.Resto-Ruiz SI, Schmiederer M, Sweger D, Newton C, Klein TW, Friedman H, Anderson BE. 2002. Induction of a potential paracrine angiogenic loop between human THP-1 macrophages and human microvascular endothelial cells during Bartonella henselae infection. Infect Immun 70:4564–4570. doi: 10.1128/IAI.70.8.4564-4570.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Minnick MF, Smitherman LS, Samuels DS. 2003. Mitogenic effect of Bartonella bacilliformis on human vascular endothelial cells and involvement of GroEL. Infect Immun 71:6933–6942. doi: 10.1128/IAI.71.12.6933-6942.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Depluverez S, Devos S, Devreese B. 2016. The role of bacterial secretion systems in the virulence of Gram-negative airway pathogens associated with cystic fibrosis. Front Microbiol 7:1336. doi: 10.3389/fmicb.2016.01336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.O'Rourke F, Schmidgen T, Kaiser PO, Linke D, Kempf VA. 2011. Adhesins of Bartonella spp. Adv Exp Med Biol 715:51–70. doi: 10.1007/978-94-007-0940-9_4. [DOI] [PubMed] [Google Scholar]
- 316.Qin W, Wang L, Lei L. 2015. New findings on the function and potential applications of the trimeric autotransporter adhesin. Antonie Van Leeuwenhoek 108:1–14. doi: 10.1007/s10482-015-0477-4. [DOI] [PubMed] [Google Scholar]
- 317.El Tahir Y, Skurnik M. 2001. YadA, the multifaceted Yersinia adhesin. Int J Med Microbiol 291:209–218. doi: 10.1078/1438-4221-00119. [DOI] [PubMed] [Google Scholar]
- 318.Capecchi B, Adu-Bobie J, Di Marcello F, Ciucchi L, Masignani V, Taddei A, Rappuoli R, Pizza M, Arico B. 2005. Neisseria meningitidis NadA is a new invasin which promotes bacterial adhesion to and penetration into human epithelial cells. Mol Microbiol 55:687–698. doi: 10.1111/j.1365-2958.2004.04423.x. [DOI] [PubMed] [Google Scholar]
- 319.Cotter SE, Surana NK, Grass S, St Geme JW III. 2006. Trimeric autotransporters require trimerization of the passenger domain for stability and adhesive activity. J Bacteriol 188:5400–5407. doi: 10.1128/JB.00164-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Linke D, Riess T, Autenrieth IB, Lupas A, Kempf VA. 2006. Trimeric autotransporter adhesins: variable structure, common function. Trends Microbiol 14:264–270. doi: 10.1016/j.tim.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 321.Henderson IR, Navarro-Garcia F, Desvaux M, Fernandez RC, Ala'Aldeen D. 2004. Type V protein secretion pathway: the autotransporter story. Microbiol Mol Biol Rev 68:692–744. doi: 10.1128/MMBR.68.4.692-744.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Kaiser PO, Riess T, Wagner CL, Linke D, Lupas AN, Schwarz H, Raddatz G, Schafer A, Kempf VA. 2008. The head of Bartonella adhesin A is crucial for host cell interaction of Bartonella henselae. Cell Microbiol 10:2223–2234. doi: 10.1111/j.1462-5822.2008.01201.x. [DOI] [PubMed] [Google Scholar]
- 323.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. 1989. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 324.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. 2000. Vascular-specific growth factors and blood vessel formation. Nature 407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 325.Lima A, Cha BJ, Amin J, Smith LK, Anderson B. 2014. Zebrafish embryo model of Bartonella henselae infection. Zebrafish 11:434–446. doi: 10.1089/zeb.2014.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Quebatte M, Dehio M, Tropel D, Basler A, Toller I, Raddatz G, Engel P, Huser S, Schein H, Lindroos HL, Andersson SG, Dehio C. 2010. The BatR/BatS two-component regulatory system controls the adaptive response of Bartonella henselae during human endothelial cell infection. J Bacteriol 192:3352–3367. doi: 10.1128/JB.01676-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Tu N, Lima A, Bandeali Z, Anderson B. 2016. Characterization of the general stress response in Bartonella henselae. Microb Pathog 92:1–10. doi: 10.1016/j.micpath.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Boonjakuakul JK, Gerns HL, Chen YT, Hicks LD, Minnick MF, Dixon SE, Hall SC, Koehler JE. 2007. Proteomic and immunoblot analyses of Bartonella quintana total membrane proteins identify antigens recognized by sera from infected patients. Infect Immun 75:2548–2561. doi: 10.1128/IAI.01974-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Hoiczyk E, Roggenkamp A, Reichenbecher M, Lupas A, Heesemann J. 2000. Structure and sequence analysis of Yersinia YadA and Moraxella UspAs reveal a novel class of adhesins. EMBO J 19:5989–5999. doi: 10.1093/emboj/19.22.5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Backert S, Clyne M. 2011. Pathogenesis of Helicobacter pylori infection. Helicobacter 16(Suppl 1):S19–S25. doi: 10.1111/j.1523-5378.2011.00876.x. [DOI] [PubMed] [Google Scholar]
- 331.Nagai H, Kubori T. 2011. Type IVB secretion systems of Legionella and other Gram-negative bacteria. Front Microbiol 2:136. doi: 10.3389/fmicb.2011.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Schulein R, Dehio C. 2002. The VirB/VirD4 type IV secretion system of Bartonella is essential for establishing intraerythrocytic infection. Mol Microbiol 46:1053–1067. doi: 10.1046/j.1365-2958.2002.03208.x. [DOI] [PubMed] [Google Scholar]
- 333.Christie PJ, Atmakuri K, Krishnamoorthy V, Jakubowski S, Cascales E. 2005. Biogenesis, architecture, and function of bacterial type IV secretion systems. Annu Rev Microbiol 59:451–485. doi: 10.1146/annurev.micro.58.030603.123630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Christie PJ, Whitaker N, González-Rivera C. 2014. Mechanism and structure of the bacterial type IV secretion systems. Biochim Biophys Acta 1843:1578–1591. doi: 10.1016/j.bbamcr.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Alvarez-Martinez CE, Christie PJ. 2009. Biological diversity of prokaryotic type IV secretion systems. Microbiol Mol Biol Rev 73:775–808. doi: 10.1128/MMBR.00023-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Cascales E, Christie PJ. 2003. The versatile bacterial type IV secretion systems. Nat Rev Microbiol 1:137–149. doi: 10.1038/nrmicro753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Schulein R, Guye P, Rhomberg TA, Schmid MC, Schroder G, Vergunst AC, Carena I, Dehio C. 2005. A bipartite signal mediates the transfer of type IV secretion substrates of Bartonella henselae into human cells. Proc Natl Acad Sci U S A 102:856–861. doi: 10.1073/pnas.0406796102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Rhomberg TA, Truttmann MC, Guye P, Ellner Y, Dehio C. 2009. A translocated protein of Bartonella henselae interferes with endocytic uptake of individual bacteria and triggers uptake of large bacterial aggregates via the invasome. Cell Microbiol 11:927–945. doi: 10.1111/j.1462-5822.2009.01302.x. [DOI] [PubMed] [Google Scholar]
- 339.Truttmann MC, Guye P, Dehio C. 2011. BID-F1 and BID-F2 domains of Bartonella henselae effector protein BepF trigger together with BepC the formation of invasome structures. PLoS One 6:e25106. doi: 10.1371/journal.pone.0025106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Schmid MC, Schulein R, Dehio M, Denecker G, Carena I, Dehio C. 2004. The VirB type IV secretion system of Bartonella henselae mediates invasion, proinflammatory activation and antiapoptotic protection of endothelial cells. Mol Microbiol 52:81–92. doi: 10.1111/j.1365-2958.2003.03964.x. [DOI] [PubMed] [Google Scholar]
- 341.Schmid MC, Scheidegger F, Dehio M, Balmelle-Devaux N, Schulein R, Guye P, Chennakesava CS, Biedermann B, Dehio C. 2006. A translocated bacterial protein protects vascular endothelial cells from apoptosis. PLoS Pathog 2:e115. doi: 10.1371/journal.ppat.0020115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Vayssier-Taussat M, Le Rhun D, Deng HK, Biville F, Cescau S, Danchin A, Marignac G, Lenaour E, Boulouis HJ, Mavris M, Arnaud L, Yang H, Wang J, Quebatte M, Engel P, Saenz H, Dehio C. 2010. The Trw type IV secretion system of Bartonella mediates host-specific adhesion to erythrocytes. PLoS Pathog 6:e1000946. doi: 10.1371/journal.ppat.1000946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Seubert A, Hiestand R, de la Cruz F, Dehio C. 2003. A bacterial conjugation machinery recruited for pathogenesis. Mol Microbiol 49:1253–1266. doi: 10.1046/j.1365-2958.2003.03650.x. [DOI] [PubMed] [Google Scholar]
- 344.Dehio C. 2004. Molecular and cellular basis of bartonella pathogenesis. Annu Rev Microbiol 58:365–390. doi: 10.1146/annurev.micro.58.030603.123700. [DOI] [PubMed] [Google Scholar]
- 345.Mavris M, Saenz H, Monteil M, Boulouis HJ, Dehio C, Vayssier-Taussat M. 2005. Characterization of genes involved in long-term bacteremia in mice by Bartonella birtlesii. Ann N Y Acad Sci 1063:312–314. doi: 10.1196/annals.1355.050. [DOI] [PubMed] [Google Scholar]
- 346.Nystedt B, Frank AC, Thollesson M, Andersson SG. 2008. Diversifying selection and concerted evolution of a type IV secretion system in Bartonella. Mol Biol Evol 25:287–300. doi: 10.1093/molbev/msm252. [DOI] [PubMed] [Google Scholar]
- 347.Liu M, Ferrandez Y, Bouhsira E, Monteil M, Franc M, Boulouis HJ, Biville F. 2012. Heme binding proteins of Bartonella henselae are required when undergoing oxidative stress during cell and flea invasion. PLoS One 7:e48408. doi: 10.1371/journal.pone.0048408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Carroll JA, Coleman SA, Smitherman LS, Minnick MF. 2000. Hemin-binding surface protein from Bartonella quintana. Infect Immun 68:6750–6757. doi: 10.1128/IAI.68.12.6750-6757.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Zimmermann R, Kempf VAJ, Schiltz E, Oberle K, Sander A. 2003. Hemin binding, functional expression, and complementation analysis of Pap 31 from Bartonella henselae. J Bacteriol 185:1739–1744. doi: 10.1128/JB.185.5.1739-1744.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Minnick MF, Sappington KN, Smitherman LS, Andersson SG, Karlberg O, Carroll JA. 2003. Five-member gene family of Bartonella quintana. Infect Immun 71:814–821. doi: 10.1128/IAI.71.2.814-821.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Stugard CE, Daskaleros PA, Payne SM. 1989. A 101-kilodalton heme-binding protein associated with Congo red binding and virulence of Shigella flexneri and enteroinvasive Escherichia coli strains. Infect Immun 57:3534–3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Bowers TJ, Sweger D, Jue D, Anderson B. 1998. Isolation, sequencing and expression of the gene encoding a major protein from the bacteriophage associated with Bartonella henselae. Gene 206:49–52. doi: 10.1016/S0378-1119(97)00580-5. [DOI] [PubMed] [Google Scholar]
- 353.Dabo SM, Confer AW, Anderson BE, Gupta S. 2006. Bartonella henselae Pap31, an extracellular matrix adhesin, binds the fibronectin repeat III13 module. Infect Immun 74:2513–2521. doi: 10.1128/IAI.74.5.2513-2521.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Zlamy M. 2016. Rediscovering pertussis. Front Pediatr 4:52. doi: 10.3389/fped.2016.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Arnal L, Longo G, Stupar P, Castez MF, Cattelan N, Salvarezza RC, Yantorno OM, Kasas S, Vela ME. 2015. Localization of adhesins on the surface of a pathogenic bacterial envelope through atomic force microscopy. Nanoscale 7:17563–17572. doi: 10.1039/C5NR04644K. [DOI] [PubMed] [Google Scholar]
- 356.Megroz M, Kleifeld O, Wright A, Powell D, Harrison P, Adler B, Harper M, Boyce JD. 2016. The RNA-binding chaperone Hfq is an important global regulator of gene expression in Pasteurella multocida and plays a crucial role in production of a number of virulence factors, including hyaluronic acid capsule. Infect Immun 84:1361–1370. doi: 10.1128/IAI.00122-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Vigil A, Ortega R, Jain A, Nakajima-Sasaki R, Tan X, Chomel BB, Kasten RW, Koehler JE, Felgner PL. 2010. Identification of the feline humoral immune response to Bartonella henselae infection by protein microarray. PLoS One 5:e11447. doi: 10.1371/journal.pone.0011447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Burgess AW, Anderson BE. 1998. Outer membrane proteins of Bartonella henselae and their interaction with human endothelial cells. Microb Pathog 25:157–164. doi: 10.1006/mpat.1998.0223. [DOI] [PubMed] [Google Scholar]
- 359.Burgess AW, Paquet JY, Letesson JJ, Anderson BE. 2000. Isolation, sequencing and expression of Bartonella henselae omp43 and predicted membrane topology of the deduced protein. Microb Pathog 29:73–80. doi: 10.1006/mpat.2000.0366. [DOI] [PubMed] [Google Scholar]
- 360.Dabo SM, Confer AW, Saliki JT, Anderson BE. 2006. Binding of Bartonella henselae to extracellular molecules: identification of potential adhesins. Microb Pathog 41:10–20. doi: 10.1016/j.micpath.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 361.Chenoweth MR, Greene CE, Krause DC, Gherardini FC. 2004. Predominant outer membrane antigens of Bartonella henselae. Infect Immun 72:3097–3105. doi: 10.1128/IAI.72.6.3097-3105.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Resto-Ruiz SI, Sweger D, Widen RH, Valkov N, Anderson BE. 2000. Transcriptional activation of the htrA (high-temperature requirement A) gene from Bartonella henselae. Infect Immun 68:5970–5978. doi: 10.1128/IAI.68.10.5970-5978.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Iwaki-Egawa S, Ihler GM. 1997. Comparison of the abilities of proteins from Bartonella bacilliformis and Bartonella henselae to deform red cell membranes and to bind to red cell ghost proteins. FEMS Microbiol Lett 157:207–217. doi: 10.1111/j.1574-6968.1997.tb12775.x. [DOI] [PubMed] [Google Scholar]
- 364.Minnick MF. 1994. Identification of outer membrane proteins of Bartonella bacilliformis. Infect Immun 62:2644–2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Padmalayam I, Kelly T, Baumstark B, Massung R. 2000. Molecular cloning, sequencing, expression, and characterization of an immunogenic 43-kilodalton lipoprotein of Bartonella bacilliformis that has homology to NlpD/LppB. Infect Immun 68:4972–4979. doi: 10.1128/IAI.68.9.4972-4979.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Scherer DC, DeBuron-Connors I, Minnick MF. 1993. Characterization of Bartonella bacilliformis flagella and effect of antiflagellin antibodies on invasion of human erythrocytes. Infect Immun 61:4962–4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Coleman SA, Minnick MF. 2001. Establishing a direct role for the Bartonella bacilliformis invasion-associated locus B (IalB) protein in human erythrocyte parasitism. Infect Immun 69:4373–4381. doi: 10.1128/IAI.69.7.4373-4381.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Coleman SA, Minnick MF. 2003. Differential expression of the invasion-associated locus B (ialB) gene of Bartonella bacilliformis in response to environmental cues. Microb Pathog 34:179–186. doi: 10.1016/S0882-4010(03)00005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Deng H, Pang Q, Xia H, Le Rhun D, Le Naour E, Yang C, Vayssier-Taussat M, Zhao B. 2016. Identification and functional analysis of invasion associated locus B (IalB) in Bartonella species. Microb Pathog 98:171–177. doi: 10.1016/j.micpath.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 370.Tay ST, Kho KL, Wee WY, Choo SW. 2016. Whole-genome sequence analysis and exploration of the zoonotic potential of a rat-borne Bartonella elizabethae. Acta Trop 155:25–33. doi: 10.1016/j.actatropica.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 371.Ko S, Kang JG, Kim HC, Klein TA, Choi KS, Song JW, Youn HY, Chae JS. 2016. Prevalence, isolation and molecular characterization of Bartonella species in Republic of Korea. Transbound Emerg Dis 63:56–67. doi: 10.1111/tbed.12217. [DOI] [PubMed] [Google Scholar]
- 372.Bjarnsholt T. 2013. The role of bacterial biofilms in chronic infections. APMIS Suppl 136:1–51. doi: 10.1111/apm.12099. [DOI] [PubMed] [Google Scholar]
- 373.Marshall K, Stout R, Mitchell R. 1971. Mechanism of the initial events in the sorption of marine bacteria to surfaces. Microbiology 68:337–348. [Google Scholar]
- 374.Misawa N, Blaser MJ. 2000. Detection and characterization of autoagglutination activity by Campylobacter jejuni. Infect Immun 68:6168–6175. doi: 10.1128/IAI.68.11.6168-6175.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Waters CM, Bassler BL. 2005. Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol 21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 376.Donlan RM. 2002. Biofilms: microbial life on surfaces. Emerg Infect Dis 8:881–890. doi: 10.3201/eid0809.020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Sutherland IW. 2001. The biofilm matrix—an immobilized but dynamic microbial environment. Trends Microbiol 9:222–227. doi: 10.1016/S0966-842X(01)02012-1. [DOI] [PubMed] [Google Scholar]
- 378.Okshevsky M, Meyer RL. 2015. The role of extracellular DNA in the establishment, maintenance and perpetuation of bacterial biofilms. Crit Rev Microbiol 41:341–352. doi: 10.3109/1040841X.2013.841639. [DOI] [PubMed] [Google Scholar]
- 379.Kaiser PO, Linke D, Schwarz H, Leo JC, Kempf VA. 2012. Analysis of the BadA stalk from Bartonella henselae reveals domain-specific and domain-overlapping functions in the host cell infection process. Cell Microbiol 14:198–209. doi: 10.1111/j.1462-5822.2011.01711.x. [DOI] [PubMed] [Google Scholar]
- 380.Bentancor LV, Camacho-Peiro A, Bozkurt-Guzel C, Pier GB, Maira-Litran T. 2012. Identification of Ata, a multifunctional trimeric autotransporter of Acinetobacter baumannii. J Bacteriol 194:3950–3960. doi: 10.1128/JB.06769-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Zimmerman SM, Michel F, Hogan RJ, Lafontaine ER. 2015. The autotransporter BpaB contributes to the virulence of Burkholderia mallei in an aerosol model of infection. PLoS One 10:e0126437. doi: 10.1371/journal.pone.0126437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Mil-Homens D, Fialho AM. 2011. Trimeric autotransporter adhesins in members of the Burkholderia cepacia complex: a multifunctional family of proteins implicated in virulence. Front Cell Infect Microbiol 1:13. doi: 10.3389/fcimb.2011.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Mil-Homens D, Leca MI, Fernandes F, Pinto SN, Fialho AM. 2014. Characterization of BCAM0224, a multifunctional trimeric autotransporter from the human pathogen Burkholderia cenocepacia. J Bacteriol 196:1968–1979. doi: 10.1128/JB.00061-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Valle J, Mabbett AN, Ulett GC, Toledo-Arana A, Wecker K, Totsika M, Schembri MA, Ghigo JM, Beloin C. 2008. UpaG, a new member of the trimeric autotransporter family of adhesins in uropathogenic Escherichia coli. J Bacteriol 190:4147–4161. doi: 10.1128/JB.00122-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Totsika M, Wells TJ, Beloin C, Valle J, Allsopp LP, King NP, Ghigo JM, Schembri MA. 2012. Molecular characterization of the EhaG and UpaG trimeric autotransporter proteins from pathogenic Escherichia coli. Appl Environ Microbiol 78:2179–2189. doi: 10.1128/AEM.06680-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Raghunathan D, Wells TJ, Morris FC, Shaw RK, Bobat S, Peters SE, Paterson GK, Jensen KT, Leyton DL, Blair JM, Browning DF, Pravin J, Flores-Langarica A, Hitchcock JR, Moraes CT, Piazza RM, Maskell DJ, Webber MA, May RC, MacLennan CA, Piddock LJ, Cunningham AF, Henderson IR. 2011. SadA, a trimeric autotransporter from Salmonella enterica serovar Typhimurium, can promote biofilm formation and provides limited protection against infection. Infect Immun 79:4342–4352. doi: 10.1128/IAI.05592-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.LeBoit PE, Berger TG, Egbert BM, Beckstead JH, Yen TS, Stoler MH. 1989. Bacillary angiomatosis. The histopathology and differential diagnosis of a pseudoneoplastic infection in patients with human immunodeficiency virus disease. Am J Surg Pathol 13:909–920. [PubMed] [Google Scholar]
- 388.Arias-Stella J, Lieberman PH, Erlandson RA, Arias-Stella J Jr. 1986. Histology, immunohistochemistry, and ultrastructure of the verruga in Carrion's disease. Am J Surg Pathol 10:595–610. doi: 10.1097/00000478-198609000-00002. [DOI] [PubMed] [Google Scholar]
- 389.Min KW, Reed JA, Welch DF, Slater LN. 1994. Morphologically variable bacilli of cat scratch disease are identified by immunocytochemical labeling with antibodies to Rochalimaea henselae. Am J Clin Pathol 101:607–610. doi: 10.1093/ajcp/101.5.607. [DOI] [PubMed] [Google Scholar]
- 390.Jarrett CO, Deak E, Isherwood KE, Oyston PC, Fischer ER, Whitney AR, Kobayashi SD, DeLeo FR, Hinnebusch BJ. 2004. Transmission of Yersinia pestis from an infectious biofilm in the flea vector. J Infect Dis 190:783–792. doi: 10.1086/422695. [DOI] [PubMed] [Google Scholar]
- 391.Sun YC, Jarrett CO, Bosio CF, Hinnebusch BJ. 2014. Retracing the evolutionary path that led to flea-borne transmission of Yersinia pestis. Cell Host Microbe 15:578–586. doi: 10.1016/j.chom.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Bland DM, Hinnebusch BJ. 2016. Feeding behavior modulates biofilm-mediated transmission of Yersinia pestis by the cat flea, Ctenocephalides felis. PLoS Negl Trop Dis 10:e0004413. doi: 10.1371/journal.pntd.0004413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Bouhsira E, Ferrandez Y, Liu M, Franc M, Boulouis HJ, Biville F. 2013. Ctenocephalides felis an in vitro potential vector for five Bartonella species. Comp Immunol Microbiol Infect Dis 36:105–111. doi: 10.1016/j.cimid.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 394.Bouhsira E, Franc M, Boulouis HJ, Jacquiet P, Raymond-Letron I, Lienard E. 2013. Assessment of persistence of Bartonella henselae in Ctenocephalides felis. Appl Environ Microbiol 79:7439–7444. doi: 10.1128/AEM.02598-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Chiaraviglio L, Duong S, Brown DA, Birtles RJ, Kirby JE. 2010. An immunocompromised murine model of chronic Bartonella infection. Am J Pathol 176:2753–2763. doi: 10.2353/ajpath.2010.090862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Erlandsen SL, Kristich CJ, Dunny GM, Wells CL. 2004. High-resolution visualization of the microbial glycocalyx with low-voltage scanning electron microscopy: dependence on cationic dyes. J Histochem Cytochem 52:1427–1435. doi: 10.1369/jhc.4A6428.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Fassel TA, Edmiston CE Jr. 1999. Bacterial biofilms: strategies for preparing glycocalyx for electron microscopy. Methods Enzymol 310:194–203. doi: 10.1016/S0076-6879(99)10017-X. [DOI] [PubMed] [Google Scholar]
- 398.Sato S, Kabeya H, Fujinaga Y, Inoue K, Une Y, Yoshikawa Y, Maruyama S. 2013. Bartonella jaculi sp. nov., Bartonella callosciuri sp. nov., Bartonella pachyuromydis sp. nov. and Bartonella acomydis sp. nov., isolated from wild Rodentia. Int J Syst Evol Microbiol 63:1734–1740. doi: 10.1099/ijs.0.041939-0. [DOI] [PubMed] [Google Scholar]
- 399.Mullins KE, Hang J, Jiang J, Leguia M, Kasper MR, Maguina C, Jarman RG, Blazes DL, Richards AL. 2013. Molecular typing of “Candidatus Bartonella ancashi,” a new human pathogen causing verruga peruana. J Clin Microbiol 51:3865–3868. doi: 10.1128/JCM.01226-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Kesnerova L, Moritz R, Engel P. 2016. Bartonella apis sp. nov., a honey bee gut symbiont of the class Alphaproteobacteria. Int J Syst Evol Microbiol 66:414–421. doi: 10.1099/ijsem.0.000736. [DOI] [PubMed] [Google Scholar]
- 401.Gray GC, Johnson AA, Thornton SA, Smith WA, Knobloch J, Kelley PW, Obregon Escudero L, Arones Huayda M, Wignall FS. 1990. An epidemic of Oroya fever in the Peruvian Andes. Am J Trop Med Hyg 42:215–221. [DOI] [PubMed] [Google Scholar]
- 402.Bermond D, Heller R, Barrat F, Delacour G, Dehio C, Alliot A, Monteil H, Chomel B, Boulouis HJ, Piemont Y. 2000. Bartonella birtlesii sp. nov., isolated from small mammals (Apodemus spp.). Int J Syst Evol Microbiol 50:1973–1979. [DOI] [PubMed] [Google Scholar]
- 403.Bermond D, Boulouis HJ, Heller R, Van Laere G, Monteil H, Chomel BB, Sander A, Dehio C, Piemont Y. 2002. Bartonella bovis Bermond et al. sp. nov. and Bartonella capreoli sp. nov., isolated from European ruminants. Int J Syst Evol Microbiol 52:383–390. doi: 10.1099/00207713-52-2-383. [DOI] [PubMed] [Google Scholar]
- 404.Maillard R, Riegel P, Barrat F, Bouillin C, Thibault D, Gandoin C, Halos L, Demanche C, Alliot A, Guillot J, Piémont Y, Boulouis HJ, Vayssier-Taussat M. 2004. Bartonella chomelii sp. nov., isolated from French domestic cattle (Bos taurus). Int J Syst Evol Microbiol 54:215–220. doi: 10.1099/ijs.0.02770-0. [DOI] [PubMed] [Google Scholar]
- 405.Birtles RJ, Harrison TG, Saunders NA, Molyneux DH. 1995. Proposals to unify the genera Grahamella and Bartonella, with descriptions of Bartonella talpae comb. nov., Bartonella peromysci comb. nov., and three new species, Bartonella grahamii sp. nov., Bartonella taylorii sp. nov., and Bartonella doshiae sp. nov. Int J Syst Bacteriol 45:1–8. doi: 10.1099/00207713-45-1-1. [DOI] [PubMed] [Google Scholar]
- 406.Rasis M, Rudoler N, Schwartz D, Giladi M. 2014. Bartonella dromedarii sp. nov. isolated from domesticated camels (Camelus dromedarius) in Israel. Vector Borne Zoonotic Dis 14:775–782. doi: 10.1089/vbz.2014.1663. [DOI] [PubMed] [Google Scholar]
- 407.O'Halloran HS, Draud K, Minix M, Rivard AK, Pearson PA. 1998. Leber's neuroretinitis in a patient with serologic evidence of Bartonella elizabethae. Retina 18:276–278. doi: 10.1097/00006982-199803000-00015. [DOI] [PubMed] [Google Scholar]
- 408.Mediannikov O, El Karkouri K, Robert C, Fournier P-E, Raoult D. 2013. Non-contiguous finished genome sequence and description of Bartonella florenciae sp. nov. Stand Genomic Sci 9:185–196. doi: 10.4056/sigs.4358060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Li DM, Hou Y, Song XP, Fu YQ, Li GC, Li M, Eremeeva ME, Wu HX, Pang B, Yue YJ, Huang Y, Lu L, Wang J, Liu QY. 2015. High prevalence and genetic heterogeneity of rodent-borne Bartonella species on Heixiazi Island, China. Appl Environ Microbiol 81:7981–7992. doi: 10.1128/AEM.02041-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Inoue K, Kabeya H, Shiratori H, Ueda K, Kosoy MY, Chomel BB, Boulouis HJ, Maruyama S. 2010. Bartonella japonica sp. nov. and Bartonella silvatica sp. nov., isolated from Apodemus mice. Int J Syst Evol Microbiol 60:759–763. doi: 10.1099/ijs.0.011528-0. [DOI] [PubMed] [Google Scholar]
- 411.Droz S, Chi B, Horn E, Steigerwalt AG, Whitney AM, Brenner DJ. 1999. Bartonella koehlerae sp. nov., isolated from cats. J Clin Microbiol 37:1117–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Chomel BB, Molia S, Kasten RW, Borgo GM, Stuckey MJ, Maruyama S, Chang CC, Haddad N, Koehler JE. 2016. Isolation of Bartonella henselae and two new Bartonella subspecies, Bartonella koehlerae subspecies boulouisii subsp. nov. and Bartonella koehlerae subspecies bothieri subsp. nov. from free-ranging Californian mountain lions and bobcats. PLoS One 11:e0148299. doi: 10.1371/journal.pone.0148299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Kosoy M, Bai Y, Enscore R, Rizzo MR, Bender S, Popov V, Albayrak L, Fofanov Y, Chomel B. 2016. Bartonella melophagi in blood of domestic sheep (Ovis aries) and sheep keds (Melophagus ovinus) from the southwestern US: cultures, genetic characterization, and ecological connections. Vet Microbiol 190:43–49. doi: 10.1016/j.vetmic.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 414.Gundi V, Davoust B, Khamis A, Boni M, Raoult D, La Scola B. 2004. Isolation of Bartonella rattimassiliensis sp. nov. and Bartonella phoceensis sp. nov. from European Rattus norvegicus. J Clin Microbiol 42:3816–3818. doi: 10.1128/JCM.42.8.3816-3818.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.McNee JW, Renshaw A, Brunt EH. 1916. Trench fever: a relapsing fever occurring with the British forces in France. Br Med J 12:225–234. doi: 10.1136/bmj.1.2876.225. [DOI] [Google Scholar]
- 416.Gundi VA, Taylor C, Raoult D, La Scola B. 2009. Bartonella rattaustraliani sp. nov., Bartonella queenslandensis sp. nov. and Bartonella coopersplainsensis sp. nov., identified in Australian rats. Int J Syst Evol Microbiol 59:2956–2961. doi: 10.1099/ijs.0.002865-0. [DOI] [PubMed] [Google Scholar]
- 417.Henn JB, Chomel BB, Boulouis H-J, Kasten RW, Murray WJ, Bar-Gal GK, King R, Courreau JF, Baneth G. 2009. Bartonella rochalimae in raccoons, coyotes, and red foxes. Emerg Infect Dis 15:1984–1987. doi: 10.3201/eid1512.081692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Dehio C, Lanz C, Pohl R, Behrens P, Bermond D, Piemont Y, Pelz K, Sander A. 2001. Bartonella schoenbuchii sp. nov., isolated from the blood of wild roe deer. Int J Syst Evol Microbiol 51:1557–1565. doi: 10.1099/00207713-51-4-1557. [DOI] [PubMed] [Google Scholar]
- 419.Mediannikov O, El Karkouri K, Diatta G, Robert C, Fournier PE, Raoult D. 2013. Non-contiguous finished genome sequence and description of Bartonella senegalensis sp. nov. Stand Genomic Sci 8:279–289. doi: 10.4056/sigs.3807472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Heller R, Riegel P, Hansmann Y, Delacour G, Bermond D, Dehio C, Lamarque F, Monteil H, Chomel B, Piemont Y. 1998. Bartonella tribocorum sp. nov., a new Bartonella species isolated from the blood of wild rats. Int J Syst Bacteriol 48:1333–1339. doi: 10.1099/00207713-48-4-1333. [DOI] [PubMed] [Google Scholar]
- 421.Chang CC, Kasten RW, Chomel BB, Simpson DC, Hew CM, Kordick DL, Heller R, Piemont Y, Breitschwerdt EB. 2000. Coyotes (Canis latrans) as the reservoir for a human pathogenic Bartonella sp.: molecular epidemiology of Bartonella vinsonii subsp. berkhoffii infection in coyotes from central coastal California. J Clin Microbiol 38:4193–4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Chomel BB, Wey AC, Kasten RW. 2003. Isolation of Bartonella washoensis from a dog with mitral valve endocarditis. J Clin Microbiol 41:5327–5332. doi: 10.1128/JCM.41.11.5327-5332.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Ayoub EM, McBride J, Schmiederer M, Anderson B. 2002. Role of Bartonella henselae in the etiology of Henoch-Schonlein purpura. Pediatr Infect Dis J 21:28–31. doi: 10.1097/00006454-200201000-00006. [DOI] [PubMed] [Google Scholar]