Abstract
Background
Severe anaphylactic symptoms can occur during oral food challenges (OFCs). Thus, high-risk patients (e.g., patients with a history of anaphylaxis or high antigen-specific immunoglobulin E [IgE] levels) must carefully undergo OFCs in hospitals. We attempted to identify the risk factors for severe symptoms during OFC testing among high-risk patients.
Methods
We retrospectively evaluated patients' characteristics and severe symptoms that were experienced during a double-blind placebo-controlled food challenge test performed before the patients underwent oral immunotherapy between June 2008 and June 2012. Patients were ≥5 years old and had an anaphylactic history or antigen-specific IgE (>30 kUA/L). Severe symptoms were defined using the grading of the Japanese Anaphylaxis Guidelines, which are modified from the European Academy of Allergology and Clinical Immunology Guidelines.
Results
We evaluated 393 cases with positive test results, including 98 cases with severe symptoms. The most frequent severe symptoms were respiratory (77%), gastrointestinal (28%), cardiovascular (27%), and neurological (13%) symptoms. Multivariate analysis revealed that the significant factors for a severe reaction were a history of anaphylaxis to the causative food (adjusted odds ratio [OR]: 2.147, p = 0.003), older age (per 1 year increase, adjusted OR: 1.102, p = 0.044), and an egg OFC (adjusted OR: 0.433, p = 0.003).
Conclusions
The risk factors for a severe reaction to OFCs were a history of an anaphylactic reaction and older age. An egg OFC was associated with low risk of severe symptoms during OFC. Therefore, OFCs for patients with these risk factors should only be performed under specialist supervision with access to rapid treatment and full resuscitation equipment.
Keywords: Anaphylaxis, Children, Food hypersensitivity, Oral food challenge, Pediatric patients
Introduction
Oral food challenges (OFCs) are performed to diagnose and confirm food allergies, and to evaluate the tolerance of certain foods [1, 2]. In this context, the OFC test is the gold standard for diagnosing and confirming acquired tolerance to food allergies [3]. However, the development of anaphylaxis during OFCs is a threat to the patient's health [3, 4]. For high-risk patients who have a history of anaphylaxis or high antigen-specific immunoglobulin E (IgE) levels that are known to be associated with persistent food allergy, OFC tests should only be performed in hospitals [5, 6]. Therefore, these patients are often considered unfit to undergo OFC testing [2, 7, 8], and only a few reports have described OFC testing among these patients [9]. OFCs have an associated risk of severe reactions at all dose levels [10]. So it is important to determine the risk factors for a severe reaction during OFC testing [4]. This study aimed to clarify the symptoms that were induced during OFC testing among patients at high risk of a severe reaction as well as identify the risk factors that were associated with these severe symptoms.
Materials and Methods
Study Design
This retrospective chart review evaluated data from a double-blind placebo-controlled food challenge test conducted on high-risk patients (UMIN000011683) [11]; the patients underwent OFC testing to determine their initial threshold before oral immunotherapy, and their OFC-provoked symptoms and symptom severities were recorded. Data regarding the patients' characteristics and treatment methods were also recorded.
Ethical Considerations
According to the Declaration of Helsinki, the study design and the risks of OFC-provoked symptoms were fully explained to the patients and their guardians (both orally and in writing). Clinical data were deidentified to ensure patient confidentiality, and the study design was approved by the institutional review board of the Sagamihara National Hospital.
Eligibility Criteria
Between June 2008 and June 2012, the study enrolled patients who were ≥5 years old and had an anaphylactic history [8, 9, 12] or high levels of egg-, milk-, wheat-, or peanut-specific IgE (>30 kUA/L). These markers provide a >95% positive predictive value for milk, egg, and peanut allergies [13, 14] and an 80% positive predictive value for wheat allergy [15]. Any drugs that could affect the OFC results were discontinued ≥96 h before the OFC. We also excluded patients with no reaction to the OFCs, as our aim was to clarify the risk factors for a severe reaction among patients who responded to the OFCs. All OFC tests were implemented when the patients' symptoms of bronchial asthma, atopic dermatitis, and other related conditions were controlled [16]. We defined anaphylaxis according to the World Allergy Organization definition [17]. We subsequently excluded cases with negative OFC results.
Food Challenge Tests
The OFC tests used a double-blind method, and the challenge foods were prepared using pumpkin cake with cocoa or strawberry puree (Table 1, detailed methods). The OFCs were performed according to the European Academy of Allergology and Clinical Immunology (EAACI) guidelines [18] and the 2014 Japanese Guidelines for Food Allergy [16]. All foods for the OFCs were prepared by our institution's nutrition management staff.
Table 1.
Recipes for OFCs
| Food | Amount of protein | Ingredients |
|---|---|---|
| Milk | 1,700 mg | cow's-milk powder (equivalent to 50 mL of milk; Kewpie, Tokyo, Japan) was mixed with 120 mL of strawberry puree |
| Egg | 3,100 mg | half of a large whole egg was mixed with pumpkin cake, heated in a microwave to a core temperature of 91.9° C (1,000 W, 1.5 min), and masked with cocoa |
| Wheat | 1,300 mg | 16 g of soft flour was mixed with pumpkin cake, heated in a microwave to a core temperature of 89.4° C (1,000 W, 1.5 min), and masked with cocoa |
| Peanut | 795 mg | peanut flour powder was mixed with 120 mL of strawberry puree |
| Placebo (milk or peanut) | 0 mg | 4 g of sucrose powder was mixed with 120 mL of strawberry puree |
| Placebo (egg or wheat) | 0 mg | 40 g of pumpkin cake was masked with cocoa |
The total amounts of milk, egg, wheat, and peanut protein used were 1,700, 3,100, 1,300, and 795 mg, respectively. The samples for the milk and peanut OFCs were fractioned into doses of 5, 10, 20, 30, and 55 mL (for a total dose of 120 mL) [19]. The samples for the egg and wheat OFCs were fractioned into doses of 1/16, 1/16, 1/8, 1/4, and 1/2. Each OFC was administered over the course of 2 h, with 30 min between each fractional dose (online suppl. Table S1; see www.karger.com/doi/10.1159/000458724 for all online suppl. material).
Positive Criteria for Oral Food Challenges and Severity of Symptoms
Positive responses to the OFCs were determined based on the presence of induced symptoms (Table 2), according to the grading in the Japanese anaphylaxis guidelines [20], which was modified from the symptom grading for anaphylactic reactions in the EAACI guidelines [18]. Objective symptoms, including a range of skin, respiratory tract, and gastrointestinal symptoms (vomiting and diarrhea), were considered positive criteria. When mild symptoms, such as abdominal pain and oral discomfort, were provoked, we observed for 30 min so as to not worsen symptoms, continued the OFC, and encouraged patients to consume additional food.
Table 2.
Symptom grading
| 1 (Mild) | 2 (Moderate) | 3 (Severe) | |
|---|---|---|---|
| Skin | Localized urticaria, exanthema, wheal, pruritus | Generalized urticaria, exanthema, wheal, pruritus | – |
| Swollen eyelid or lip | Swollen face | – | |
| Gastrointestinal tract | Pruritus of the throat or oral cavity | Throat pain | – |
| Mild abdominal pain | Moderate abdominal pain | Cramps | |
| Nausea, emesis, diarrhea | Recurrent emesis, diarrhea | Continuous emesis, loss of bowel control | |
| Respiratory tract | Intermittent cough, nasal congestion, sneezing, rhinorrhea | Repetitive cough | Persistent cough, hoarseness, “barking” cough |
| – | Chest tightness, wheezing detectable via auscultation | Audible wheezing, dyspnea, cyanosis, saturation <92%, swallowing or speaking difficulties, throat tightness, respiratory arrest | |
| Cardiovascular | – | Pale face, mild hypotension, tachycardia (increase >15 beats/min) | Hypotension, dysrhythmia, severe bradycardia, cardiac arrest |
| Neurological | Change in activity level, tiredness | Light-headedness, feeling of “pending doom,” somnolence, headache | Confusion, loss of consciousness, incontinence |
The severity score was based on the organ system that was most affected by the symptoms [21]. Hypotension was defined as a systolic blood pressure <70 mm Hg (age: 1 month to 1 year), <70 mm Hg + (2 × age) (age: 1–10 years), and <90 mm Hg (age: >11 years). Mild hypotension was defined as a systolic blood pressure of <80 mm Hg (age: 1 month to 1 year), <80 mm Hg + (2 × age) (age: 1–10 years), and <100 mm Hg (age: >11 years). Wheezing detectable via auscultation was defined as mild wheezing that was audible only through a stethoscope. Audible wheezing was defined as wheezing audible without a stethoscope. This definition was modified using the anaphylactic symptom grading of the EAACI guidelines [18].
This grading system in the Japanese anaphylaxis guidelines was also used for severity assessment [21]. Symptoms were graded by 2 physicians, and severe symptoms were classified as grade 3 (Table 2). Where necessary, the physicians selected appropriate treatment measures, which included fluid resuscitation, oxygenation, intravenous or oral antihistamines, intravenous steroids, inhaled β2 agonists or adrenaline, and intramuscular adrenaline.
Antigen-Specific IgE
Antigen-specific IgE titers and ovomucoid-specific titers were retrospectively reviewed within 3 months before the OFC (ImmunoCAP™, Thermo Fisher Scientific/Phadia, Uppsala, Sweden).
Statistical Analysis
All data were reported as number (%) or median (interquartile range [IQR]). Intergroup comparisons were performed using the Mann-Whitney U test or the Fisher exact test (with the Bonferroni correction if necessary), and p values <0.05 were considered statistically significant. To determine the risk factors for severe reaction or multiple doses of adrenaline, we first performed the Mann-Whitney U test or the Fisher exact test. Univariate analysis was performed using significant factors from these tests, and stepwise regression analyses were then performed using the statistically significant variables from the univariate analysis to obtain adjusted odd ratios (ORs). Univariate and multivariate analyses were analyzed by logistic regression. All analyses were performed using SPSS software (v20.0; SPSS Inc., Chicago, IL, USA).
Results
Patient Characteristics
This study evaluated 393 cases with positive test results, i.e. milk: 164 cases, egg: 152 cases, wheat: 50 cases, and peanut: 27 cases (Table 3). A total of 334 patients (median age, 8.3 years) underwent 393 OFCs (59 patients underwent OFCs for 2 different antigens). There was a history of an anaphylactic reaction to the causative food for 217 patients (55%). Of the 393 patients, 217 (55%) had antigen-specific IgE levels >30 kUA/L. Forty-one patients (10%) had a history of anaphylactic reaction and high levels of antigen-specific IgE. In 39 cases, placebo OFCs were not conducted because patients had had severe reactions to active OFCs and refused placebo OFCs. Placebo OFCs were conducted in 354 cases.
Table 3.
Clinical and demographic characteristics of the patients who underwent the OFCs
| Milk (n = 164) | Egg (n = 152) | Wheat (n = 50) | Peanut (n = 27) | |
|---|---|---|---|---|
| Male sex | 113 (69) | 105 (69) | 31 (62) | 20 (74) |
| Age, years | 8.2 (6.9–10.2) | 8.6 (7.0–10.7) | 8.1 (6.7–10.9) | 9.1 (7.5–12.3) |
| History of immediate reaction to causative food | 149 (91) | 139 (91) | 48 (96) | 24 (89) |
| History of anaphylaxis to causative food | 109 (67) | 61 (40) | 36 (72) | 11 (41) |
| Atopic dermatitis, current | 98 (60) | 81 (53) | 22 (44) | 9 (33) |
| Bronchial asthma, current | 99 (60) | 84 (55) | 25 (50) | 7 (26) |
| Allergic rhinitis, current | 67 (40) | 67 (44) | 23 (46) | 17 (63) |
| Total IgE, IU/mL | 1,450 (699–2,670) | 1,238 (584–838) | 931 (589–2,483) | 688 (358–1,590) |
| Antigen-specific IgE, kUA/L | 41.0 (17.0–87.5) | 29.0 (12.0–78.3)a 24.7 (9.6–52.7)b |
77.5 (22.8–100) | 27.0 (7.0–60.0) |
Values are expressed as n (%) or median (IQR). IgE, immunoglobulin E.
The value for egg-white.
The value for ovomucoid.
OFC-Provoked Symptoms
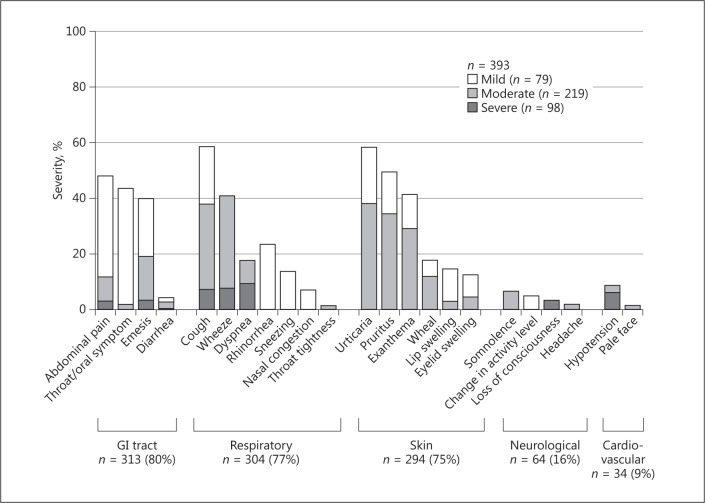
Figure 1 displays the frequencies of all OFC-provoked symptoms. The most frequent symptoms were gastrointestinal, followed by respiratory, skin, neurological, and cardiovascular. The most frequent gastrointestinal symptoms were oral mucosal, followed by abdominal pain. The most frequent respiratory symptoms were coughing, followed by wheezing. The most frequent skin symptoms were urticaria, followed by pruritus. The most frequent neurological symptom was somnolence. The threshold volumes and detailed symptoms for each food are shown in online supplementary Tables 2 and 3. The distribution of positive food challenges for each dose step is displayed in online supplementary Figure 1.
Fig. 1.
Symptoms induced by oral food challenges. Severity of symptoms was defined according to the Anaphylaxis Guidelines of Japan (Table 2). GI, gastrointestinal.
Symptom Severity
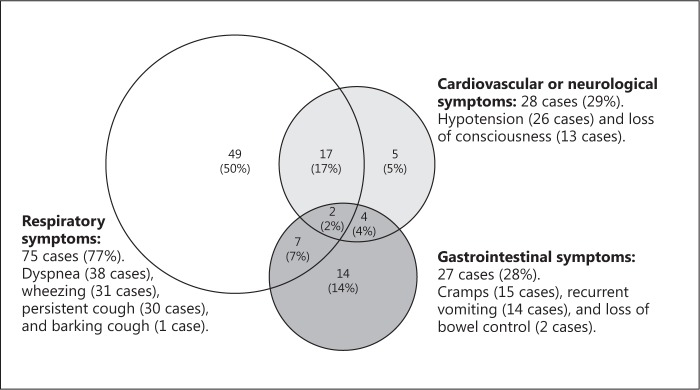
The symptom severity was mild in 79 cases (20%), moderate in 216 (55%), and severe in 98 (25%). Anaphylaxis was observed in 190 cases (48%). Severe symptoms (grade 3) were observed in 33% (54/164) of milk OFCs, 15% (22/152) of egg OFCs, 32% (54/164) of wheat OFCs, and 22% (6/27) of peanut OFCs. Details of these severe symptoms are displayed in Figure 2. All 28 cases of cardiovascular symptoms involved decreased blood pressure, and all 13 cases of neurological symptoms involved a loss of consciousness. Among the 13 neurological cases, 11 patients had decreased blood pressure, 1 had mildly decreased blood pressure, and 1 had no cardiovascular symptoms (Fig. 2). Two cases involved respiratory, cardiovascular, and abdominal symptoms; both patients experienced hypoxemia, a loss of consciousness, and decreased blood pressure, and they received 2 doses of adrenaline. The distribution of clinical reaction severity by dose steps is displayed in online supplementary Figure 1. Severe symptoms (grade 3) were less frequent after the fifth dose than after the first (p = 0.005) or second to fourth doses (p < 0.001).
Fig. 2.
Severe symptoms in 98 cases. Severe symptoms were defined according to the Anaphylaxis Guidelines of Japan (Table 2). Patients could experience multiple symptoms in the same category.
Treatment for OFC-Provoked Symptoms
A total of 304 patients (77%) experienced OFC-provoked symptoms that required treatment. Treatment included antihistamines (272 cases, 69%), inhaled β2 agonists (215 cases, 55%), drip infusion (168 cases, 43%), steroids (144 cases, 37%), intramuscular adrenaline (90 cases, 23%), and adrenaline inhalation (4 cases, 13%) (online suppl. Table 4). All symptoms improved within 8 h after treatment. All patients with severe symptoms underwent treatment, which included antihistamines (94%), inhaled β2 agonists (85%), steroids (76%), and adrenaline (92%). All 8 patients with severe symptoms who did not receive adrenaline had respiratory symptoms. Six patients experienced repeated cough that was improved by a nebulized β2 agonist (n = 6), and 2 patients with a barking cough improved after receiving nebulized adrenaline (n = 2). We therefore did not need to administer an adrenaline injection. Only patients with severe symptoms received adrenaline. Multiple doses of adrenaline were administered to 20 patients, with 2 receiving 4 doses and 18 receiving 2 doses. Among the 89 cases without any treatment, 63 experienced mild symptoms and thus did not require any treatment. Among the 26 patients who experienced moderate symptoms and did not require treatment, 20 presented with vomiting and then became asymptomatic, and the 6 with repeated cough improved spontaneously.
Risk Factors for Severe Symptoms
Our univariate analysis revealed 3 potential significant factors for severe symptoms. A history of anaphylaxis to the causative food (OR: 2.517, 95% confidence interval [CI]: 1.536–4.126, p < 0.001) and age (per 1 year increase) (OR: 1.102, 95% CI: 1.004–1.210, p = 0.041) were significant risk factors for the development of severe symptoms. An egg OFC (OR: 0.367, 95% CI: 0.217–0.622, p < 0.001)was associated with low risk of severe symptoms during OFC. Our regression analysis with stepwise selection (Table 4) confirmed that a history of anaphylaxis to the causative food, age, and an egg OFC were significant factors. The multivariate analysis also confirmed that a history of anaphylaxis to the causative food (OR: 2.147, 95% CI: 1.289–3.573, p = 0.003) and age (per 1 year increase) (OR: 1.102, 95% CI: 1.003–1.211, p = 0.044) were significant risk factors for the development of severe symptoms. An egg OFC (OR: 0.433, 95% CI: 0.252–0.745, p = 0.003) was associated with low risk of severe symptoms during OFC. Comparison of an egg OFC and OFCs other than egg are detailed in online supplementary Table 5.
Table 4.
Multivariate analysis of factors related to severe reactions in 393 patients
| Crude OR (95% CI) | p value | Adjusted OR (95% CI) | p value | |
|---|---|---|---|---|
| Hen's egg OFC | 0.367 (0.217–0.622) | <0.001 | 0.433 (0.252–0.745) | 0.003 |
| Age (per 1 year increase) | 1.102 (l.004–1.210) | 0.041 | 1.102 (1.003–1.211) | 0.044 |
| History of anaphylaxis to causative food | 2.517 (1.536–4.126) | <0.001 | 2.147 (1.289–3.573) | 0.003 |
Multivariate logistic regression analysis was performed using stepwise regression for the variables in Table 3. Only the above 3 categories were statistically significant. The adjusted OR was calculated using the statistically significant predictors of a severe reaction: OFC to hen's egg, age, and a history of anaphylaxis to causative food. A history of anaphylaxis to the causative food and older age were significant risk factors for the development of severe symptoms. An egg OFC was associated with low risk of severe symptoms during OFC. OR, odds ratio; CI, confidence interval; OFC, oral food challenge.
Risk Factors for Multiple Doses of Adrenaline
We analyzed data from 90 patients who received intramuscular adrenaline. Twenty patients received multiple doses of adrenaline. The second dose was administered 45 min (IQR: 30–66.5 min) after the first. Of 70 patients for whom the time of steroid administration was recorded, 45 (64%) received steroids prior to the first adrenaline injection and 17 (24%) within 15 min after the injection. Our univariate analysis revealed 3 potential significant factors for multiple doses of adrenaline, i.e. the number of anaphylactic events to causative food (per 1 episode increase) (OR: 2.517, 95% CI: 1.536–4.126, p < 0.001), the use of oxygen (OR: 2.722, 95% CI: 1.606–4.611, p < 0.001), and the use of steroids (OR: 0.306, 95% CI: 0.106–0.880, p = 0.028). Our regression analysis with stepwise selection using significant variables from the univariate analysis confirmed that the number of anaphylactic events to causative food, oxygen use, and steroid administration were independent significant factors. The multivariate analysis using these 3 variants confirmed that the number of anaphylactic events to causative food (OR: 1.504, 95% CI: 1.124–2.012, p = 0.006), the use of oxygen (OR: 1.547, 95% CI: 1.348–3.973, p = 0.001), and the use of steroids (OR: 0.114, 95% CI: 0.026–0.500, p = 0.004) were significant factors for multiple doses of adrenaline (Table 5).
Table 5.
Univariate and multivariate analyses of factors related to multiple doses of adrenaline
| Crude OR (95% CI) | p value | Adjusted OR (95% CI) | p value | |
|---|---|---|---|---|
| Number of anaphylactic events to causative food (per 1 episode increase) | 2.517 (1.536–4.126) | <0.001 | 1.504 (1.124–2.012) | 0.006 |
| Use of oxygen | 2.722 (1.606–4.611) | <0.001 | 1.547 (1.348–3.973) | 0.001 |
| Use of steroids | 0.306 (0.106–0.880) | 0.028 | 0.114 (0.026–0.500) | 0.004 |
We analyzed 90 patients who received intramuscular adrenaline; 20 were administered multiple doses. Multivariate logistic regression analysis was performed using stepwise regression for the significant variables from the univariate analysis. Only the above 3 categories were statistically significant. The adjusted OR was calculated using the statistically significant predictors of multiple doses of adrenaline. OR, odds ratio; CI, confidence interval; OFC, oral food challenge.
Placebo OFC
Among 354 cases who received placebo OFC, 16 patients (5%) reacted. Of these 16, 13 had mild symptoms, including 5 with mild abdominal pain, 4 with limited local skin symptoms, 3 with mild respiratory symptoms, and 2 with oral discomfort. In 3 patients, moderate symptoms were observed, i.e. skin symptoms in an immediate whole-body reaction to strawberry puree for masking (first episode of immediate reaction to strawberry), wheezing because of worsening asthma, and vomiting twice because the patient disliked the challenge food. OFCs were stopped for these 3 patients. Nebulized β2 agonist was administered for the wheezing, which disappeared immediately.
Discussion
Patients' Background
In this study, OFCs were performed in cases where the induction of severe symptoms was expected [22]. Approximately 92% of these cases had a history of immediate symptoms, 55% had a history of anaphylaxis, and most cases had high antigen-specific IgE titers. Although these patients would not typically undergo OFCs and would typically be directed to food elimination, the OFCs were performed for threshold estimation before oral immunotherapy. Anaphylaxis was observed in 190 cases (48%). This process induced severe reactions in 98 cases (25%). Ninety of these patients (23%) were treated using adrenaline, and 20 (5%) were treated using multiple doses of adrenaline.
Only a few reports have detailed organ-specific symptoms for severe reactions [4, 23, 24], and food allergy symptoms have typically been divided into general gastrointestinal, respiratory tract, skin, nervous system, and cardiovascular symptoms. However, organ-specific information might be useful, as it would allow us to prepare more effectively for when symptoms emerge. In this study, we found that urticaria was the most common skin symptom, confirming the finding of a previous study [23]. The most frequent respiratory symptom was coughing, although a barking cough was only observed in only 2 cases. In contrast, Sampson [25] reported a barking cough only. Abdominal pain was another frequent symptom (also not reported by Sampson [25]). Common symptoms like coughing and abdominal pain were added to the EAACI grading [18]; these should be considered during future testing.
Treatment for OFC-Provoked Symptoms
Adrenaline is used in cases of shock, although it is also recommended during the preshock stage [26]. Adrenaline is indicated for severe symptoms at our institution, and it was administered in 90 cases (23%) when patients developed severe symptoms after the OFCs. In contrast, Perry et al. [4] and Järvinen et al. [27] reported that intramuscular adrenaline was only administered in approximately 11% of the cases with positive challenge results. Perry et al. [4] selected patients who were largely referred from primary and secondary care settings for the evaluation and management of food allergies. Järvinen et al. [27] selected subjects for OFCs based on the expectation that a child would have a <50% likelihood of a positive challenge on the basis of the food-specific IgE level. Therefore, their inclusion criteria were either nonselective or selected low-risk patients. There was a high incidence of history of anaphylaxis in patients selected for our study, and the participants were older than in previous studies. Our higher administration rate of adrenaline appears to indicate that our patient population had a higher risk of developing severe symptoms.
Severe Symptoms
The most frequent severe symptoms were respiratory, gastrointestinal, cardiovascular, and neurological, respectively. A previous report regarding cases of serious anaphylaxis [22] found that respiratory and cardiovascular symptoms were the most common and abdominal symptoms less common. In contrast, we found that abdominal (gastrointestinal) symptoms were the second-most common. However, the previous study included a relatively small proportion of patients with food allergies (32%) and a median age of 36 years, compared to our mainly pediatric patients with food allergies. Therefore, despite the discrepancy regarding the frequency of abdominal symptoms, our findings appear to indicate that respiratory, abdominal, and cardiovascular symptoms are common in cases of pediatric food allergies. Our severity classification consisted of 3 grades, not 5 [25], each grade included a wide range of clinical signs and symptoms. For example, grade 3 included loss of consciousness, cardiac arrest, an oxygen saturation <92%, and audible wheezing. The EAACI scale also has similar limitations [22].
Dose Regimen and Guidelines
Caution is required in the performance of any food challenge, and clinical guidelines must be followed. The food challenges in this study were carried out between June 2008 and June 2012. We were therefore unable to follow the PRACTALL guidelines, which were published in 2012. We should take into account that the target doses in our study were lower than those recommended by PRACTALL, and that patients who did not react to OFCs in this study might react to larger doses. OFCs have an associated risk of severe reactions at all dose levels [10]. Although symptoms after the fifth dose seemed to be significantly milder than those after other doses, symptoms after the first dose and second to fourth doses were very similar, with no significant differences in severity. Severe reactions occurred at all dose levels in our study. This finding is compatible with that of a previous study in which 7 accumulated doses were used [11].
Risk Factors for Severe Reactions
Severe anaphylactic reactions are associated with older age, preexisting lung disease, and drug-induced anaphylaxis [22]. Our multivariate analysis revealed that a history of anaphylaxis to the causative food and older age are risk factors for a severe reaction. Egg OFCs were associated with low prevalence of a severe reaction. In addition, we observed an association between older age and severe reactions, which was compatible with the findings of a previous study [22]. Although we only evaluated patients who were ≥5 years of age, older age is also a risk factor for severe reactions.
Interestingly, an egg OFC was associated with reduced risk of a severe reaction, which may be due to the known tendency of milk, wheat, and peanut OFCs to aggravate the induced symptoms [4, 9, 15, 23, 28]. Previous studies have also described mild symptoms after egg OFCs [29, 30]. This may be because egg induces fewer respiratory and cardiovascular symptoms than other foods. In addition, the egg OFC was reported to be associated with more gastrointestinal symptoms [31]. In our study, among the 36 patients who were excluded due to negative OFC findings, 25 had previously had an egg allergy. This result is also compatible with our finding that an egg allergy was not a risk factor. It is important to prepare for the emergence of severe symptoms in all cases. Furthermore, although it is clear that a history of anaphylaxis to the causative food is a risk factor for a severe reaction [17, 18, 32], the severity of the reaction warrants more careful attention to this factor when performing OFC testing.
Multiple Doses of Adrenaline
Risk factors for multiple doses of adrenaline included a history of anaphylaxis and the use of oxygen, whereas the use of steroids was associated with a reduced risk of multiple doses of adrenaline. Oxygen was used to treat severe respiratory symptoms; this is compatible with it being a risk factor for multiple doses of adrenaline. Generally, high-risk patients tend to experience repeated anaphylaxis, and so this is also compatible. Surprisingly, the use of steroids was significantly associated with the reduced administration of multiple doses of adrenaline, whereas other treatments, like antihistamine, did not reduce the need for additional adrenaline administration. Steroid use may prevent deterioration after administration of adrenaline. The role of steroids to prevent biphasic anaphylaxis needs further investigation [33]. In the Cochrane Reviews, the effect of steroids on anaphylaxis was not evaluated because of lack of a randomized study [34]. In our study, the median time for administration of the second dose of adrenaline was 45 min after the first dose. Although the onset of the peak of effect of steroids is at 4 h after administration, a significant effect emerges after 1 h [33]. However, our study was a retrospective study, and further randomized prospective studies are needed that will examine the protective effects of steroids to prevent multiple administrations of adrenaline.
Limitations
Our study has several limitations. First, our severity classification had 3 levels, as does the EAACI scale. Grade 3 covered the full spectrum of severity, from severe symptoms that would improve with some treatment [35] to near-fatal symptoms [36]. Among severe symptoms, distinguishing between extremely severe and severe symptoms requires comparing detailed symptoms. Second, we only evaluated patients who were ≥5 years of age at a single center, and our findings may not extrapolate to this age group or be representative of the population at other centers. It would be more informative to include a broader sample of patients undergoing OFCs, including open OFCs, to increase the sample size and thereby reduce bias. Third, this study's retrospective design precludes any conclusions regarding the causality of the relationships that we observed. Therefore, prospective multicenter studies are needed to validate our findings.
Conclusion
Among patients who had a high risk of severe symptoms, the most frequent severe symptoms were respiratory, gastrointestinal, cardiovascular, and neurological after undergoing OFCs. The risk factors for a severe reaction were a history of anaphylactic reaction to the causative food and a patient's age. The egg OFC was associated with a reduced risk of severe reactions. Although it is important to prepare for the emergence of severe symptoms in all cases, when OFCs are performed in patients with these risk factors, we should carefully observe emerging symptoms to make sure that they do not become severe symptoms. Such challenges should therefore only be performed under specialist supervision with access to rapid treatment and full resuscitation equipment. Prospective multicenter studies in other patient populations are needed to confirm our findings.
Statement of Ethics
All study participants or their guardians provided informed consent, and the study design was approved by the appropriate ethics review board.
Disclosure Statement
Noriyuki Yangida, Sakura Sato, Tomoyuki Asaumi, and Kiyotake Ogura have no conflicts of interest to declare. Motohiro Ebisawa received lecture fees from Pfizer and Siemens.
Acknowledgements
We thank all of the pediatricians, dietitians, and nurses who participated in patient recruitment and data collection at Sagamihara National Hospital. This study was supported by Health and Labour Sciences Research Grants for Research on Allergic Disease and Immunology from the Ministry of Health, Labour, and Welfare of Japan for study design, data collection, analysis, and interpretation of data (grant No. 201414009A). We would like to thank Editage (www.editage.jp) for English language editing.
References
- 1.National Asthma Education and Prevention Program Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma – summary report 2007. J Allergy Clin Immunol. 2007;120:S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 2.Nowak-Wegrzyn A, Assa'ad AH, Bahna SL, Bock SA, Sicherer SH, Teuber SS. Adverse Reactions to Food Committee of American Academy of Allergy, Asthma and Immunology: Work Group report: oral food challenge testing. J Allergy Clin Immunol. 2009;123:S365–S383. doi: 10.1016/j.jaci.2009.03.042. [DOI] [PubMed] [Google Scholar]
- 3.Muraro A, Werfel T, Hoffmann-Sommergruber K, Roberts G, Beyer K. EAACI food allergy and anaphylaxis guidelines: diagnosis and management of food allergy. Allergy. 2014;69:1008–1025. doi: 10.1111/all.12429. [DOI] [PubMed] [Google Scholar]
- 4.Perry TT, Matsui EC, Conover-Walker MK, Wood RA. Risk of oral food challenges. J Allergy Clin Immunol. 2004;114:1164–1168. doi: 10.1016/j.jaci.2004.07.063. [DOI] [PubMed] [Google Scholar]
- 5.Arik Yilmaz E, Cavkaytar O, Buyuktiryaki B, Sekerel BE, Soyer O, Sackesen C. Factors associated with the course of egg allergy in children. Ann Allergy Asthma Immunol. 2015;115:434–438. doi: 10.1016/j.anai.2015.08.012. e1. [DOI] [PubMed] [Google Scholar]
- 6.Tan JW, Campbell DE, Turner PJ, Kakakios A, Wong M, Mehr S, Joshi P. Baked egg food challenges – clinical utility of skin test to baked egg and ovomucoid in children with egg allergy. Clin Exp Allergy. 2013;43:1189–1195. doi: 10.1111/cea.12153. [DOI] [PubMed] [Google Scholar]
- 7.Burks AW, Jones SM, Boyce JA, Sicherer SH, Wood RA, Assa'ad A, Sampson HA. NIAID-sponsored 2010 guidelines for managing food allergy: applications in the pediatric population. Pediatrics. 2011;128:955–965. doi: 10.1542/peds.2011-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sampson HA, Gerth van Wijk R, Bindslev-Jensen C, Sicherer S, Teuber SS, Burks AW, Dubois AE, Beyer K, Eigenmann PA, Spergel JM, Werfel T, Chinchilli VM. Standardizing double-blind, placebo-controlled oral food challenges: American Academy of Allergy, Asthma and Immunology-European Academy of Allergy and Clinical Immunology PRACTALL consensus report. J Allergy Clin Immunol. 2012;130:1260–1274. doi: 10.1016/j.jaci.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 9.Cianferoni A, Khullar K, Saltzman R, Fiedler J, Garrett JP, Naimi DR, Spergel JM. Oral food challenge to wheat: a near-fatal anaphylaxis and review of 93 food challenges in children. World Allergy Organ J. 2013;6:14. doi: 10.1186/1939-4551-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rolinck-Werninghaus C, Niggemann B, Grabenhenrich L, Wahn U, Beyer K. Outcome of oral food challenges in children in relation to symptom-eliciting allergen dose and allergen-specific IgE. Allergy. 2012;67:951–957. doi: 10.1111/j.1398-9995.2012.02838.x. [DOI] [PubMed] [Google Scholar]
- 11.Sato S, Yanagida N, Ogura K, Imai T, Utsunomiya T, Iikura K, Goto M, Asaumi T, Okada Y, Koike Y, Syukuya A, Ebisawa M. Clinical studies in oral allergen-specific immunotherapy: differences among allergens. Int Arch Allergy Immunol. 2014;164:1–9. doi: 10.1159/000361025. [DOI] [PubMed] [Google Scholar]
- 12.Sampson HA, Aceves S, Bock SA, James J, Jones S, Joint Task Force on Practice Parameters Food allergy: a practice parameter update – 2014. J Allergy Clin Immunol. 2014;134:1016–1025. doi: 10.1016/j.jaci.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 13.Komata T, Soderstrom L, Borres MP, Tachimoto H, Ebisawa M. The predictive relationship of food-specific serum IgE concentrations to challenge outcomes for egg and milk varies by patient age. J Allergy Clin Immunol. 2007;119:1272–1274. doi: 10.1016/j.jaci.2007.01.038. [DOI] [PubMed] [Google Scholar]
- 14.Maloney JM, Rudengren M, Ahlstedt S, Bock SA, Sampson HA. The use of serum-specific IgE measurements for the diagnosis of peanut, tree nut, and seed allergy. J Allergy Clin Immunol. 2008;122:145–151. doi: 10.1016/j.jaci.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 15.Komata T, Soderstrom L, Borres MP, Tachimoto H, Ebisawa M. Usefulness of wheat and soybean specific IgE antibody titers for the diagnosis of food allergy. Allergol Int. 2009;58:599–603. doi: 10.2332/allergolint.09-OA-0096. [DOI] [PubMed] [Google Scholar]
- 16.Urisu A, Ebisawa M, Ito K, Aihara Y, Ito S, Mayumi M, Kohno Y, Kondo N, Committee for Japanese Pediatric Guideline for Food Allergy; Japanese Society of Pediatric Allergy and Clinical Immunology; Japanese Society of Allergology Japanese guideline for food allergy 2014. Allergol Int. 2014;63:399–419. doi: 10.2332/allergolint.14-RAI-0770. [DOI] [PubMed] [Google Scholar]
- 17.Simons FE, Ardusso LR, Dimov V, Ebisawa M, El-Gamal YM, Lockey RF, Sanchez-Borges M, Senna GE, Sheikh A, Thong BY, Worm M. World Allergy Organization Anaphylaxis Guidelines: 2013 update of the evidence base. Int Arch Allergy Immunol. 2013;162:193–204. doi: 10.1159/000354543. [DOI] [PubMed] [Google Scholar]
- 18.Vetander M, Helander D, Lindquist C, Hedlin G, Alfvén T, Ostblom E, Nilsson C, Lilja G, Wickman M. Classification of anaphylaxis and utility of the EAACI Taskforce position paper on anaphylaxis in children. Pediatr Allergy Immunol. 2011;22:369–373. doi: 10.1111/j.1399-3038.2010.01115.x. [DOI] [PubMed] [Google Scholar]
- 19.Komata T, Shukuya A, Imai T, Tachimoto H, Ebisawa M. Single-blind food challenge using dried food powder – 2nd report: milk (in Japanese) Arerugi. 2009;58:779–789. [PubMed] [Google Scholar]
- 20.Ebisawa M. JSA Anaphylaxis Guideline – importance of basic management and prevention (in Japanese) Arerugi. 2015;64:24–31. [PubMed] [Google Scholar]
- 21.Yanagida N, Imai T, Sato S, Ebisawa M. Do longer intervals between challenges reduce the risk of adverse reactions in oral wheat challenges? PLoS One. 2015;10:e0143717. doi: 10.1371/journal.pone.0143717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown SG, Stone SF, Fatovich DM, Burrows SA, Holdgate A, Celenza A, Coulson A, Hartnett L, Nagree Y, Cotterell C, Isbister GK. Anaphylaxis: clinical patterns, mediator release, and severity. J Allergy Clin Immunol. 2013;132:1141–1149. doi: 10.1016/j.jaci.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 23.Ahrens B, Niggemann B, Wahn U, Beyer K. Organ-specific symptoms during oral food challenge in children with food allergy. J Allergy Clin Immunol. 2012;130:549–551. doi: 10.1016/j.jaci.2012.05.045. [DOI] [PubMed] [Google Scholar]
- 24.Noone S, Ross J, Sampson HA, Wang J. Epinephrine use in positive oral food challenges performed as a screening test for food allergy therapy trials. J Allerg Clin Immunol Pract. 2015;3:424–428. doi: 10.1016/j.jaip.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sampson HA. Anaphylaxis and emergency treatment. Pediatrics. 2003;111:1601–1608. [PubMed] [Google Scholar]
- 26.Muraro A, Roberts G, Clark A, Eigenmann PA, Halken S, Lack G, Moneret-Vautrin A, Niggemann B, Rancé F, EAACI Task Force on Anaphylaxis in Children The management of anaphylaxis in childhood: position paper of the European Academy of Allergology and Clinical Immunology. Allergy. 2007;62:857–871. doi: 10.1111/j.1398-9995.2007.01421.x. [DOI] [PubMed] [Google Scholar]
- 27.Järvinen KM, Amalanayagam S, Shreffler WG, Noone S, Sicherer SH, Sampson HA, Nowak-Wegrzyn A. Epinephrine treatment is infrequent and biphasic reactions are rare in food-induced reactions during oral food challenges in children. J Allergy Clin Immunol. 2009;124:1267–1272. doi: 10.1016/j.jaci.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lifschitz C, Szajewska H. Cow's milk allergy: evidence-based diagnosis and management for the practitioner. Eur J Pediatr. 2015;174:141–150. doi: 10.1007/s00431-014-2422-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lieberman JA, Huang FR, Sampson HA, Nowak-Wegrzyn A. Outcomes of 100 consecutive open, baked-egg oral food challenges in the allergy office. J Allergy Clin Immunol. 2012;129:1682–1684. doi: 10.1016/j.jaci.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 30.Buelow BJ, Lee C, Zafra HT, Dasgupta M, Hoffmann RG, Vasudev M. Egg baked in product open oral food challenges are safe in selected egg-allergic patients. Allergy Rhinol (Providence) 2014;5:110–112. doi: 10.2500/ar.2014.5.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta M, Grossmann L, Spergel J, Cianferoni A. Egg food challenges are associated with more gastrointestinal reactions. Children. 2015;2:371–381. doi: 10.3390/children2030371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee S, Sadosty AT, Campbell RL. Update on biphasic anaphylaxis. Curr Opin Allergy Clin Immunol. 2016;16:346–351. doi: 10.1097/ACI.0000000000000279. [DOI] [PubMed] [Google Scholar]
- 33.Olnes MJ, Kotliarov Y, Biancotto A, Cheung F, Chen J, Shi R, Zhou H, Wang E, Tsang JS, Nussenblatt R, CHI Consortium Effects of systemically administered hydrocortisone on the human immunome. Sci Rep. 2016;6:23002. doi: 10.1038/srep23002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choo KJ, Simons FE, Sheikh A. Glucocorticoids for the treatment of anaphylaxis. Evid Based Child Health. 2013;8:1276–1294. doi: 10.1002/ebch.1925. [DOI] [PubMed] [Google Scholar]
- 35.Wainstein BK, Studdert J, Ziegler M, Ziegler JB. Prediction of anaphylaxis during peanut food challenge: usefulness of the peanut skin prick test (SPT) and specific IgE level. Pediatr Allergy Immunol. 2010;21:603–611. doi: 10.1111/j.1399-3038.2010.01063.x. [DOI] [PubMed] [Google Scholar]
- 36.Umasunthar T, Leonardi-Bee J, Hodes M, Turner PJ, Gore C, Habibi P, Warner JO, Boyle RJ. Incidence of fatal food anaphylaxis in people with food allergy: a systematic review and meta-analysis. Clin Exp Allergy. 2013;43:1333–1341. doi: 10.1111/cea.12211. [DOI] [PMC free article] [PubMed] [Google Scholar]