Abstract
Murine typhus is a rickettsiosis caused by Rickettsia typhi, whose transmission is carried out by rat fleas in urban settlements as classically known, but it also has been related to cat fleas in a sub-urban alternative cycle that has been suggested by recent reports. These studies remarks that in addition to rats, other animals like cats, opossums and dogs could be implied in the transmission of Rickettsia typhi as infected fleas obtained from serologically positive animals have been detected in samples from endemic areas. In Mexico, the higher number of murine typhus cases have been detected in the Yucatan peninsula, which includes a great southeastern region of Mexico that shows ecologic characteristics similar to the sub-urban alternative cycle recently described in Texas and California at the United States. To find out which are the particular ecologic characteristics of murine typhus transmission in this region, we analyzed blood and Rhipicephalus sanguineus ticks obtained from domestic dogs by molecular approaches, demonstrating that both samples were infected by Rickettsia typhi. Following this, we obtained isolates that were analyzed by genetic sequencing to corroborate this infection in 100% of the analyzed samples. This evidence suggests for the first time that ticks and dogs could be actively participating in the transmission of murine typhus, in a role that requires further studies for its precise description.
Keywords: Dogs, Murine typhus, Rhipicephalus sanguineus, Rickettsia typhi, Vector
Introduction
Murine typhus is a human rickettsiosis caused by Rickettsia typhi, that is characterized by fever, headache, arthralgia, hepatomegaly, neurological deficits and a mortality rate close to 4% (Civen and Ngo, 2008). This disease has been typically concentrated in urban environments, in which the rat flea Xenopsylla cheopis and rats (Rattus rattus, Rattus norvegicus) are implied in its transmission. In addition, recent studies have demonstrated that in sub-urban areas with no rat presence, murine typhus transmission is related to a link between cats (Felis catus), dogs (Canis familiaris), opossums (Didelphis virginiana) and the flea Ctenocephalides felis that requires further research for a clearer description (Civen and Ngo, 2008; Blanton et al., 2016).
The highest number of human murine typhus reports in Mexico are from the Yucatan peninsula, in which the characteristics of peridomiciliary areas, weather and ecology, create suitable environments for the transmission of this illness however, the dynamics of its transmission remain unclear (Zavala-Castro et al., 2009, 2014; Dzul-Rosado et al., 2013a; Labruna, et al., 2011b). On this last subject, recent molecular evidence supports the existence of rickettsemia caused by Rickettsia typhi in dogs and rats from rural and suburban communities from Yucatan, but there are no data regarding a potential arthropod vector, the possible existence of a sylvatic cycle and, if this is the case, the possible connection with the urban and sub-urban cycles (Peniche-Lara et al., 2015; Martinez-Ortiz et al., 2016). Considering that previous studies found that Rhipicephalus sanguineus is the most prevalent tick among dogs in our region (Rodriguez-Vivas et al., 2016), and the close contact that people have with these animals, we analyzed ticks infesting domestic dogs from a rural community that has shown cases of murine typhus in Mexico, in order to find out the possible role of ticks in the transmission pathway of this disease.
Materials and Methods
Dog blood and tick DNA extraction
Ticks and blood were collected from 10 infested dogs from Teabo (20.4001° N, 89.2830° W) during the month of April from 2015 (dry season), which is a rural community in Mexico where human cases of rickettsiosis have been diagnosed. Population of Teabo includes 6,205 people distributed among 1,380 households which are occupying an area of 261, 87 Km2. This town is a sub humid zone surrounded by mid-elevation semi-deciduous jungle. The average annual temperature is 26.3 °C and the average annual rainfall is 65.7 millimeters (rainy season occurs from June to October). This community was selected because there have been human cases of rickettsiosis, people has a very close contact to dogs due to its different daily activities, its accessibility to the research center, and the good disposition of the people for working with the team.
Dog blood obtained by venous puncture in the forearm, was processed with the DNeasy Blood & Tissue Kit (Qiagen, Germantown MD, USA) following manufacturer directions and stored until its use. In the other hand, ticks that were identified as Rhipicephalus sanguineus adult males by taxonomical keys (Alekseev et al., 2001; Guzmán-Cornejo and Robbins, 2010), were employed for DNA extraction, using the ammonium hydroxide technique as described elsewhere (Guy and Stanek, 1991).
For this, ticks were disinfected with a mixture of 0.15M Iodine-70% ethanol for 15 minutes and washed with sterile water to remove iodine. Half of each tick was macerated and resuspended in 100 ul of 0.7M ammonium hydroxide to free the DNA, and after being cooled, the tubes were heated for 20 minutes at 90°C to evaporate the ammonia.
The resulting DNA was used for PCR or stored at -70°C until its use; the remaining half body was conserved for future isolation. We also inspected the dogs for fleas but none was found.
PCR and RFLPs reactions set up
The amplification of gltA was used as a first approach to identify infected ticks and dogs with the primers Rpcs877fw (5’-GGGGGCCTGCTCACGGCGG-3’) and Rpcs1258rv (5’-ATTGCAAAAAGTACAGTGAACA-3’) using Platinum Taq (Invitrogen) as described previously (Regnery et al., 1991; Dzul-Rosado et al., 2013b). For the follow up and identification of positive controls we amplified 17kDa with the primers:
17kDa1Fw (5’-GCTCTTGCAACTTCTATGTT-3’) and 17kDa1Rv (5’-CATTGTTCGTCAGGTTGGCG-3’); and OmpB with a nested reaction using the primers: rOmpBfw (5’- GCTTAGAATCAACTGATACAG-3’) and rOmpBrv (5’-GCTTTATAACCAGCTAAACCACC-3’) for the first round, followed by a second one with primers: rOmpBTGIfw (5’-AAGATCCTTCTGATGTTGCAACA-3’) and rOmpBTHIrv (5’-GGTTTGGCCCATATACCATAAG-3’).
PCR products were analyzed in BrEt stained gels of polyacrylamide (Webb et al., 1990). As a first and fast approach to identify the agents that could be infecting ticks prior to start the isolation steps, RFLP analysis were performed as described in a previous work (Zavala-Castro et al., 2009), using 1.2 UI of AluI and 50ng of the 17kDa PCR product overnight; and then the bands were analyzed expecting 200bp and 250bp fragments (Zavala-Castro et al., 2009). RFLP analysis were also useful to fast track the isolation experiments prior the corroboration of the isolates through DNA sequencing.
Rickettsia typhi isolation from Rhipicephalus sanguineus
As reported previously (Dzul-Rosado et al., 2013b), a total of 50,000 Vero cells were grown in 6 separated wells of a 24 well cell culture plate (Corning Inc, Corning NY USA) with Dulbecco’s modified eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) (Caisson Labs, Smithfield UT, USA), with 5% CO2, 37°C for 48 hours or until 95% confluence was achieved. The remaining half body from positive male ticks was macerated and resuspended in microtubes containing 600ul of brain heart infusion (BHI).
DMEM medium was carefully removed, and wells were refilled with 300 ul of each tick suspension; then the plate was covered with parafilm and centrifugued at 700g, 22°C, for 60 minutes. Finally, the supernatant was removed and wells were refilled with 1ml of DMEM supplemented with 4% FCS, 100 U of penicillin, 100 µg of streptomycin and 250 ng of amphotericin B (Sigma-Aldrich, St Louis MO, USA); then the plates were incubated at 33°C with 5% CO2. From this point, the medium was changed every 3 days using DMEM without antibiotics and supplemented with 5% of FCS and the supernatants were screened using Gimenez stain to verify the status of the infection on days 9 and 15 (Gimenez, 1964).
On the day 15, cells from positive wells were scrapped and transferred into 5 ml of DMEM to culture flasks (25cm2) that was changed every 3 days. Cultures were scrapped for DNA extraction using the kit DNeasy Blood and Tissue on day 15 according manufacturer (Qiagen, Germantown MD, USA). Species identification was performed by the amplification of 17kDa, gltA and OmpB, followed by RFLP analysis for 17kDa as it has been described. Finally, three PCR amplicons of 17kDa and OmpB from every positive well were fully sequenced by Sanger at the Biotechnology Institute (UNAM, Cuernavaca Morelos, Mexico), and compared with other sequences in the GenBank.
Results
Blood samples were obtained from 10 domestic dogs that roam freely in the town and act as companions to their owners in activities that take place in the surrounding jungle like agriculture or hunt. No tick co-infestation was observed. Additionally, we obtained a random number of Rhipicephalus sanguineus ticks from these dogs. DNA obtained from both kind of samples was subjected to conventional PCR and RFLP analysis, obtaining a 100% of ticks and dogs infected with Rickettsia typhi (Data not shown).
Six adult ticks positive to Rickettsia typhi were randomly selected for isolation, using a 24-well culture plate technique previously established by our group for the infection of Vero cells (Dzul-Rosado et al., 2013b). The infected monolayers were followed up by Gimenez stain and PCR/RFLP analysis (Fig. 1 and Fig. 2); after 3 passages, amplicons of OmpB and 17kDa obtained from the scrapping of the positive wells were fully sequenced in the Biomedical Institute of the National Autonomous University of Mexico (IBT-UNAM), and compared with sequences reported at the GenBank (NCBI). Amplicons showed 100% of identity with the OmpB (RT0699) and 17kDa genes (RT0821) of Rickettsia typhi str. Wilmington (Access Number AE017197.1).
Fig. 1.
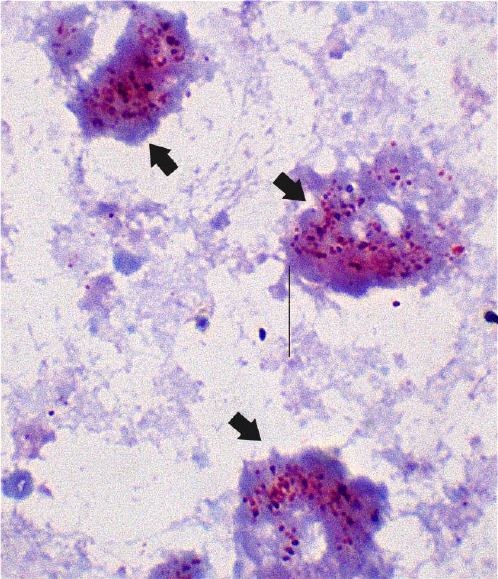
Gimenez stain of isolates. Representative micrograph showing the follow up of isolates by Gimenez stain. Arrows shows infected Vero cells.
Fig. 2.

PCR and RFLP analysis of isolates. Representative acrylamide gel electrophoresis showing how every isolate obtained from infected ticks was subjected to PCR amplification of OmpB and 17kDa (A), using previously characterized positive controls. PCR products were obtained according to expected;17kDa amplicon was subjected to further analysis by AluI RFLPs (B), obtaining fragments according to the molecular pattern expected for R. typhi.
Discussion
The importance of rickettsiosis for the public health in Mexico has begun to be showed by several epidemiologic studies but the ecologic characteristics of its transmission have not been deeply explored. It is classically known that Rickettsia typhi transmission to humans requires fleas (Xenopsylla cheopis) that acquire the bacteria from rats and mice in urban settlements, being the main route of infection worldwide. However, it is also recognized than some sub-urban regions like southern states of America, the cat flea (Ctenocephalides felis) and opossums are highly effective for the transmission and maintenance of murine typhus (Civen and Ngo, 2008). This last alternate cycle was described during epidemic outbreaks of murine typhus, in communities from Texas and California that were not in touch with the classic transmission, however, data supported the infection of fleas by Rickettsia typhi but no rickettsemia in opossums, only anti-Rickettsia typhi antibodies. (Blanton et al., 2016; Maina et al., 2016). Other studies have demonstrated that dogs can play an important role in the transmission of murine typhus, as high antibody titers against Rickettsia typhi and rickettsemia have been demonstrated by IFA and PCR/sequencing in dog blood samples from different world regions including Yucatan; however, its potential as reservoirs remains to be assessed (Nogueras et al., 2013; Martinez-Ortiz et al., 2016). Those studies have not described the participation of an arthropod as a possible vector which, considering the classic cycle could be a flea. Here, we show molecular and cellular evidence that the most abundant ticks in dogs of southeastern Mexico (Rhipicephalus sanguineus) could have an important role in the life cycle of Rickettsia typhi. To our knowledge extent, this is the first report of Rhipicephalus sanguineus ticks infected with Rickettsia typhi, which alongside with the demonstration of rickettsemia from the same species in their dogs hosts, highlights that vectorial competence and animal transmission studies are needed to a better understanding of the life cycle of Rickettsia typhi in our region, where several studies have demonstrated a close contact among ticks and humans in communities that have had positive cases of rickettsiosis (Martinez-Ortiz et al., 2016; Rodriguez-Vivas et al., 2016). These studies should include tests on the capacity of the vector to become infected from an animal source, the trans-ovarian transmission of the pathogen and the capabilities of nymphs to infect healthy animals, as it has been done in recent studies (Labruna et al., 2011a; Levin et al., 2017). Murine typhus is a public health problem in our region that, for its control, needs a deeper understanding of its ecologic characteristics. Here we show the first molecular and cellular evidence of Rickettsia typhi infecting Rhipicephalus sanguineus ticks from dogs that are in close contact with humans. Although additional experiments would be necessary to a better understanding of this phenomena, we could suggest that ticks, and not only fleas, could be participating in the transmission of this disease.
Conflict of interest
The authors declare that there is no conflict of interests.
Acknowledgements
This work was supported by a grant of the Mexican National Council for Science and Technology (Conacyt FOSIS 261885) to Dra. Karla Dzul-Rosado. The authors would also express their gratitude for the people of Teabo, Yucatan and to the people that supported us for data acquisition.
References
- Alekseev A.N, Dubinina H.V, Van De Pol I, Schouls L.M. Identification of Ehrlichia spp, Borrelia burgdorferi in Ixodes ticks in the Baltic regions of Russia. J. Clin. Microbiol. 2001;39:2237–2242. doi: 10.1128/JCM.39.6.2237-2242.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanton L.S, Idowu B.M, Tatsch T.N, Henderson J.M, Bouyer D.H, Walker D.H. Opossums and Cat Fleas:New Insights in the Ecology of Murine Typhus in Galveston, Texas. Am. J. Trop. Med. Hyg. 2016;95:457–461. doi: 10.4269/ajtmh.16-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civen R, Ngo V. Murine typhus:an unrecognized suburban vectorborne disease. Clin. Infect. Dis. 2008;46:913–8. doi: 10.1086/527443. [DOI] [PubMed] [Google Scholar]
- Dzul-Rosado K, Gonzalez-Martinez P, Peniche-Lara G, Zavala-Velazquez J, Zavala-Castro J. Murine typhus in humans, Yucatan, Mexico'. Emerg. Infect. Dis. 2013a;19:1021–1022. doi: 10.3201/eid1906.121400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzul-Rosado K, Peniche-Lara G, Tello-Martin R, Zavala-Velazquez J, Pacheco Rde C, Labruna M.B, Sanchez E.C, Zavala-Castro J. Rickettsia rickettsii isolation from naturally infected Amblyomma parvum ticks by centrifugation in a 24-well culture plate technique. Open Vet. J. 2013b;3:101–105. [PMC free article] [PubMed] [Google Scholar]
- Gimenez D.F. Staining rickettsiae in yolk-sac cultures. Stain. Technol. 1964;39:135–140. doi: 10.3109/10520296409061219. [DOI] [PubMed] [Google Scholar]
- Guy E.C, Stanek G. Detection of Borrelia burgdorferi in patients with Lyme disease by the polymerase chain reaction. J. Clin. Pathol. 1991;44:610–611. doi: 10.1136/jcp.44.7.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán-Cornejo C, Robbins R.G. The genus Ixodes (Acari:Ixodidae) in Mexico:adult identification keys, diagnoses, hosts, and distribution. Revista mexicana de biodiversidad. 2010;81:289–298. [Google Scholar]
- Labruna M.B, Ogrzewalska M, Soares J.F, Martins T.F, Soares H.S, Moraes-Filho J, Nieri-Bastos F.A, Almeida A.P, Pinter A. Experimental infection of Amblyomma aureolatum ticks with Rickettsia rickettsii. Emerg. Infect. Dis. 2011a;17:829–834. doi: 10.3201/eid1705.101524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labruna M.B, Mattar S.V, Nava S, Bermudez S, Venzal J.M, Dolz G, Katia A, Romero L, de Sousa R, Oteo J, Zavala-Castro J. Rickettsioses in Latin America, Caribbean, Spain and Portugal. Revista MVZ Córdoba. 2011b;16:2435–2457. [Google Scholar]
- Levin M.L, Zemtsova G.E, Killmaster L.F, Snellgrove A, Schumacher L.B.M. Vector competence of Amblyomma americanum (Acari:Ixodidae) for Rickettsia rickettsii. Ticks Tick Borne Dis. 2017;8:615–622. doi: 10.1016/j.ttbdis.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maina A.N, Fogarty C, Krueger L, Macaluso K.R, Odhiambo A, Nguyen K, Farris C.M, Luce-Fedrow A, Bennett S, Jiang J, Sun S, Cummings R.F, Richards A.L. Rickettsial Infections among Ctenocephalides felis and Host Animals during a Flea-Borne Rickettsioses Outbreak in Orange County, California. PLoS One. 2016;11:e0160604. doi: 10.1371/journal.pone.0160604. http://doi.org/10.1371/journal.pone.0160604 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Ortiz D, Torres-Castro M, Koyoc-Cardena E, Lopez K, Panti-May A, Rodriguez-Vivas I, Puc A, Dzul K, Zavala-Castro J, Medina-Barreiro A, Chable-Santos J, Manrique-Saide P. Molecular evidence of Rickettsia typhi infection in dogs from a rural community in Yucatan, Mexico. Biomedica. 2016;36:45–50. doi: 10.7705/biomedica.v36i2.2913. [DOI] [PubMed] [Google Scholar]
- Nogueras M.M, Pons I, Pla J, Ortuno A, Miret J, Sanfeliu I, Segura F. The role of dogs in the eco-epidemiology of Rickettsia typhi, etiological agent of Murine typhus. Vet. Microbiol. 2013;163:97–102. doi: 10.1016/j.vetmic.2012.11.043. [DOI] [PubMed] [Google Scholar]
- Peniche-Lara G, Dzul-Rosado K, Perez-Osorio C, Zavala-Castro J. Rickettsia typhi in rodents and R. felis in fleas in Yucatan as a possible causal agent of undefined febrile cases. Rev. Inst. Med. Trop. Sao Paulo. 2015;57:129–132. doi: 10.1590/S0036-46652015000200005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnery R.L, Spruill C.L, Plikaytis B.D. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J. Bacteriol. 1991;173:1576–1589. doi: 10.1128/jb.173.5.1576-1589.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Vivas R.I, Apanaskevich D.A, Ojeda-Chi M.M, Trinidad-Martinez I, Reyes-Novelo E, Esteve-Gassent M.D, Perez de Leon A.A. Ticks collected from humans, domestic animals, and wildlife in Yucatan, Mexico. Vet. Parasitol. 2016;215:106–113. doi: 10.1016/j.vetpar.2015.11.010. [DOI] [PubMed] [Google Scholar]
- Webb L, Carl M, Malloy D.C, Dasch G.A, Azad A.F. Detection of murine typhus infection in fleas by using the polymerase chain reaction. J. Clin. Microbiol. 1990;28:530–534. doi: 10.1128/jcm.28.3.530-534.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala-Castro J.E, Zavala-Velazquez J.E, Sulu Uicab J.E. Murine typhus in child, Yucatan, Mexico. Emerg. Infect. Dis. 2009;15:972–974. doi: 10.3201/eid1506.081367. http://doi.org/10.3201/eid1506.081367 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala-Castro J.E, Dzul-Rosado K.R, Peniche-Lara G, Tello-Martín R, Zavala-Velázquez J.E. Isolation of Rickettsia typhi from Human, Mexico. Emerg. Infect. Dis. 2014;20:1411–1412. doi: 10.3201/eid2008.130095. [DOI] [PMC free article] [PubMed] [Google Scholar]


