Main Text
Targeting therapeutically relevant biomolecules to protected sites of the body, such as the brain, is a major challenge in the design of molecular therapies. Exosomes recently emerged as promising vehicles for targeted drug delivery, offering advantages such as good biocompatibility, low immunogenicity, targeting specificity, and the ability to overcome barriers.1 While, to date, most strategies for linking biomolecules to exosomes have dealt with RNA or small molecules, a specific approach for the active and selective packaging of proteins has so far been lacking. In this issue of Molecular Therapy, Sterzenbach et al.2 present an elegant strategy for specific sorting of protein cargo to exosomes by engineering an exosome address signal to the protein of interest. The authors further demonstrate that, upon administration via the nasal route, exosomes carry the engineered protein across the blood-brain barrier and target its biological activity to neural cells. The study opens up new perspectives for therapeutic targeting of biologically active proteins to the brain as well as other tissues.
Exosomes belong to a group of naturally secreted extracellular vesicles (EVs) mediating short- and long-distance intercellular communication delivering different kinds of cargoes to recipient cells.3, 4 Exosomes originate in the endosomal system of donor cells, where they are formed at specific sites of the limiting membrane by budding into the lumen of multivesicular bodies (MVBs). These subsequently release the internal vesicles into the extracellular space. Like viruses, exosomes interact with specific target cells and elicit phenotypic responses upon internalization, yet exosomes do not self-replicate. To date, it is well accepted that exosomes, together with other EVs, represent a body-wide messenger system acting through the circulation and other body fluids,5 identifying them as attractive tools for diagnostic and therapeutic purposes.6, 7 Current therapeutic approaches either take advantage of unmodified EVs carrying intrinsic beneficial properties, such as stem cell-derived EVs facilitating tissue regeneration and dendritic cell-derived EVs used for immune therapy, or aim at loading EVs with curative cargo. Both strategies share the prospects of cell-free biological therapeutics exhibiting minimal side effects. Engineering of EVs requires efficient cargo loading techniques.8 Exogenous addition of hydrophilic biomolecules, such as small RNAs or proteins, is frequently achieved by electroporation or detergent treatment of isolated EVs, essentially resulting in partial disruption of the EV-membrane and, hence, a potential adverse impact on the functionality of the carrier vehicle. In contrast, endogenous loading employs ectopic overexpression of the therapeutic cargo (mostly small non-coding RNAs) in donor cells, resulting in the release of modified EVs into the culture supernatant. Overall, the prevailing loading techniques facilitate non-specific association of the cargo with EVs and do not make use of a mechanism enabling cargo selection.
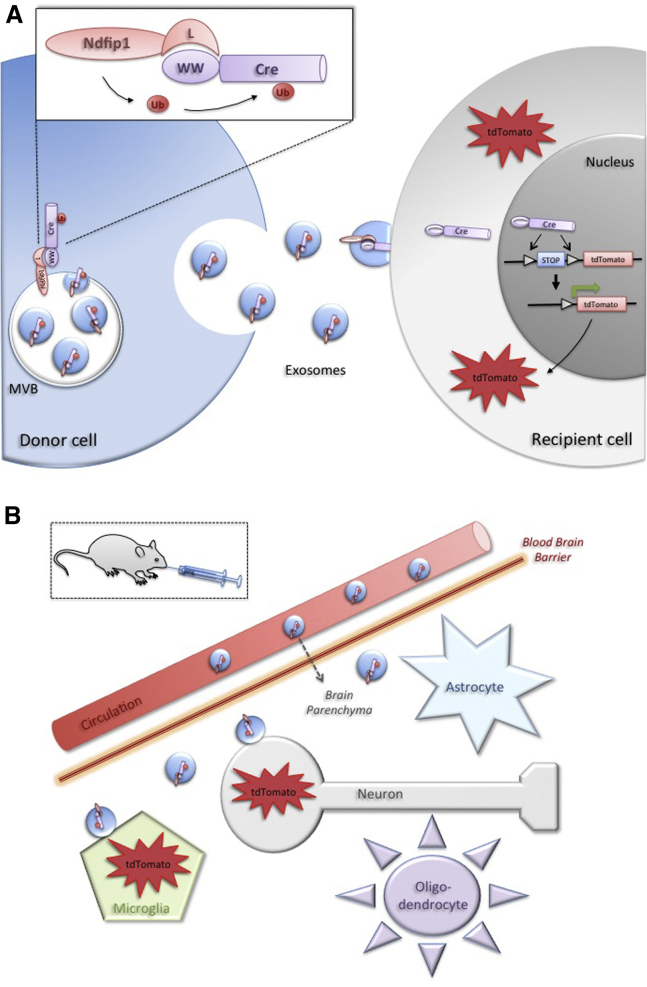
Although the EV biogenesis machinery is not fully understood, cargo sorting during exosome formation is known to involve ESCRT (endosomal sorting complex required for transport) proteins acting at the MVB-limiting membrane.9 At least part of the exosomal cargo is recruited by interaction with ESCRT-associated proteins mediating ubiquitination of cargo molecules. It was previously shown that L-domain-containing proteins recruit ESCRT-associated proteins as well as ubiquitin ligases to the MVB membrane regulating export via exosomes.10, 11 In the present study, Sterzenbach et al.2 utilize this mode of cargo selection by engineering WW domains to Cre recombinase serving as a reporter protein (Figure 1A). WW domains interact with the L-domain motifs of the adaptor protein Ndfip1, mediating recruitment to exosomes. The authors provide a careful step-by-step validation of the cargo selection mechanism and functionality of the exosome-associated WW-Cre reporter protein. WW-tagged Cre directly interacts with Ndfip1, promoting monoubiquitination and exosomal packing of WW-Cre, while untagged Cre is absent from exosomes. Furthermore, exosomal export of WW-Cre requires the presence of Ndfip1 in the donor cells. Thus, WW-domains serve as exosome address signals, which, upon addition to a protein of interest, direct this protein to exosomes in an Ndfip1-dependent manner.
Figure 1.
Addressing Proteins to Exosomes for Targeted Delivery
(A) Engineering WW domains to a protein of interest, such as Cre recombinase, results in Ndfip-mediated ubiquitination and sorting to exosomes. Exosomes deliver functional Cre to target cells mediating recombination and expression of a reporter gene as a read out of protein activity. (B) Engineered exosomes enter the brain parenchyma after nasal administration (most likely by passing the blood-brain barrier from the circulation) and deliver Cre activity preferentially to neurons and some microglia.
An important question is whether such engineered exosomes are able to deliver the biological activity of WW-tagged cargo. Cre/loxP-mediated recombination of reporter genes in target cells provides a sensitive readout to address this issue and has been employed by previous studies to monitor exosomal transfer of Cre,12, 13, 14 which was largely ascribed to the delivery of Cre mRNA. Indeed, the present study shows that WW-Cre-engineered exosomes initiate reporter gene expression in target cells, demonstrating that WW-Cre taken up by recipient cells is functional. It is important to note, that reporter gene recombination in recipient cells was only detected when exosome donor cells were co-expressing Ndfip1 and WW-Cre and was not observed in the absence of Ndfip1 or upon expression of untagged Cre. This observation finally confirms that reporter gene recombination is due to Ndfip1-dependent exosomal delivery of WW-Cre protein and not Cre mRNA, which would occur independent of Ndfip1 and lead to the observation of recombination events in control situations, where Ndfip1 or the WW-tag of Cre were omitted. The complete absence of Cre mRNA leakiness into engineered exosomes may come as a surprise. However, this could be explained by exosome donor cell specifications, such as the exosome biogenesis machinery or the level of Cre overexpression. Intriguingly, the biological activity of WW-Cre was lost upon storage of exosomes before the assay in frozen or unfrozen condition, even though WW-Cre protein remained physically intact. Most likely, storage compromises the ability of exosomes to interact with target cells, perhaps by aggregation or proteolytic damage of surface receptors. However, with regard to therapeutic considerations, it will be important to determine conditions allowing a defined shelf-life of engineered exosomes.
Finally, the study addresses the suitability of WW-Cre-engineered exosomes for potential therapeutic applications in vivo by monitoring cargo delivery to the brain of reporter mice. Nasal administration of WW-Cre-engineered exosomes derived from glioblastoma LN18 cells successfully accomplished entry into the brain parenchyma, targeting Cre-activity primarily to neurons and a few microglia across different brain regions (Figure 1B). Thus, protein-engineered exosomes can pass the blood-brain barrier and target the biological activity of the cargo to a specific subset of neural target cells. How exosomes gain entry to the brain and the determinants of specific targeting are questions that need to be addressed in the future.
Sterzenbach et al.2 provide a proof of principle that addition of a sorting motif facilitates targeting of proteins to exosomes, which can be subsequently utilized as shuttles, directing their biological activity to distant target cells. Compared to previous work promoting exosome-based therapeutics, two aspects of this study are unique. First, it provides a solution for endogenous loading of proteins in exosomes as therapeutic cargo. Second, it provides a sensitive readout of the biological activity delivered by exosomes on a single cell level, ultimately relevant for the efficacy of putative therapeutic applications. Indeed, the percentage of target cells exhibiting reporter gene recombination reported in this study appears relatively low, suggesting that optimization of the targeting efficiency will be required for therapeutic application. This could be achieved either at the level of the donor cell by increasing the efficiency of sorting cargo to exosomes or by improving exosome-target cell interaction, hence targeting efficiency. The sorting efficiency may be influenced by competing sorting signals present in the cargo protein favoring localization to other subcellular compartments. In fact, WW-Cre also contains a nuclear sorting motif, which most likely hampers its association with exosomes and may contribute to lower delivery rates as compared to proteins containing no additional sorting information. To increase targeting efficiency of engineered exosomes, the addition of surface epitopes mediating interaction with specific target cell receptors, such as the RGV-peptide for selective targeting of acetylcholine receptor-positive neurons, has been successfully applied.15 However, there are still significant gaps in our knowledge of surface molecules that define exosome tropism and contribute to target cell selectivity. The WW-Cre reporter system described here may actually be adopted as a basic tool for studying exosome-target cell interactions, revealing fundamental insight into donor-target cell networks, which, in turn, will be instructive for the design of exosome-based therapeutics. Similarly, to the field of recombinant viruses, the development of specific cellular expression systems specialized for packaging of exosomes is envisioned.
Exosomes have attracted the spotlight of therapeutic drug delivery. While a great deal of effort has been expended in investigating the delivery of potentially therapeutic RNAs, this study has generated a new possibility for attaching proteins to exosomes, which subsequently function as shuttles directing their activity to less accessible tissues, such as the brain. Much work remains as we await the development of specific cellular expression systems specialized for packaging of exosomes, as seen in the past for recombinant viruses.
References
- 1.EL Andaloussi S., Mäger I., Breakefield X.O., Wood M.J. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 2013;12:347–357. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 2.Sterzenbach U., Putz U., Low L.H., Silke J., Tan S.S., Howitt J. Engineered exosomes as vehicles for biologically active proteins. Mol. Ther. 2017;25:1269–1278. doi: 10.1016/j.ymthe.2017.03.030. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colombo M., Raposo G., Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 4.Maas S.L., Breakefield X.O., Weaver A.M. Extracellular Vesicles: Unique Intercellular Delivery Vehicles. Trends Cell Biol. 2017;27:172–188. doi: 10.1016/j.tcb.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yáñez-Mó M., Siljander P.R., Andreu Z., Zavec A.B., Borràs F.E., Buzas E.I., Buzas K., Casal E., Cappello F., Carvalho J. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fais S., O’Driscoll L., Borras F.E., Buzas E., Camussi G., Cappello F., Carvalho J., Cordeiro da Silva A., Del Portillo H., El Andaloussi S. Evidence-Based Clinical Use of Nanoscale Extracellular Vesicles in Nanomedicine. ACS Nano. 2016;10:3886–3899. doi: 10.1021/acsnano.5b08015. [DOI] [PubMed] [Google Scholar]
- 7.Lener T., Gimona M., Aigner L., Börger V., Buzas E., Camussi G., Chaput N., Chatterjee D., Court F.A., Del Portillo H.A. Applying extracellular vesicles based therapeutics in clinical trials - an ISEV position paper. J. Extracell. Vesicles. 2015;4:30087. doi: 10.3402/jev.v4.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sutaria D.S., Badawi M., Phelps M.A., Schmittgen T.D. Achieving the Promise of Therapeutic Extracellular Vesicles: The Devil is in Details of Therapeutic Loading. Pharm. Res. 2017;34:1053–1066. doi: 10.1007/s11095-017-2123-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schöneberg J., Lee I.H., Iwasa J.H., Hurley J.H. Reverse-topology membrane scission by the ESCRT proteins. Nat. Rev. Mol. Cell Biol. 2017;18:5–17. doi: 10.1038/nrm.2016.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baietti M.F., Zhang Z., Mortier E., Melchior A., Degeest G., Geeraerts A., Ivarsson Y., Depoortere F., Coomans C., Vermeiren E. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 2012;14:677–685. doi: 10.1038/ncb2502. [DOI] [PubMed] [Google Scholar]
- 11.Putz U., Howitt J., Lackovic J., Foot N., Kumar S., Silke J., Tan S.S. Nedd4 family-interacting protein 1 (Ndfip1) is required for the exosomal secretion of Nedd4 family proteins. J. Biol. Chem. 2008;283:32621–32627. doi: 10.1074/jbc.M804120200. [DOI] [PubMed] [Google Scholar]
- 12.Frühbeis C., Fröhlich D., Kuo W.P., Amphornrat J., Thilemann S., Saab A.S., Kirchhoff F., Möbius W., Goebbels S., Nave K.A. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol. 2013;11:e1001604. doi: 10.1371/journal.pbio.1001604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ridder K., Keller S., Dams M., Rupp A.K., Schlaudraff J., Del Turco D., Starmann J., Macas J., Karpova D., Devraj K. Extracellular vesicle-mediated transfer of genetic information between the hematopoietic system and the brain in response to inflammation. PLoS Biol. 2014;12:e1001874. doi: 10.1371/journal.pbio.1001874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zomer A., Maynard C., Verweij F.J., Kamermans A., Schäfer R., Beerling E., Schiffelers R.M., de Wit E., Berenguer J., Ellenbroek S.I. In Vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell. 2015;161:1046–1057. doi: 10.1016/j.cell.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alvarez-Erviti L., Seow Y., Yin H., Betts C., Lakhal S., Wood M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]