Abstract
Enterochromaffin cells were the first endocrine cells of the gastrointestinal tract to be chemically distinguished, almost 150 years ago. It is now known that the chromaffin reaction of these cells was due to their content of the reactive aromatic amine, 5-hydroxytryptamine (5-HT, also known as serotonin). They have commonly been thought to be a special class of gut endocrine cells (enteroendocrine cells) that are distinct from the enteroendocrine cells that contain peptide hormones. The study by Martin et al in the current issue of this journal reveals that the patterns of expression of nutrient receptors and transporters differ considerably between chromaffin cells of the mouse duodenum and colon. However, even within regions chromaffin cells differ; in the duodenum there are chromaffin cells that contain both secretin and 5-HT, cholecystokinin and 5-HT, and all three of secretin, cholecystokinin and 5-HT. Moreover, the ratios of these different cell types differ substantially between species. And, in terms of function, 5-HT has many roles, including in appetite, motility, fluid secretion, release of digestive enzymes and bone metabolism. The paper thus emphasizes the need to define the many different classes of enterochromaffin cells and relate this to their roles.
Keywords: serotonin, 5-hydroxytryptamine, enteroendocrine cells, gut hormones
5-HT is at the same time one of the most studied and yet the most mysterious of the gut hormones. It is synthesized by a subtype of gastrointestinal enteroendocrine cells (EEC) that are referred to as enterochromaffin (EC) cells, which produce about 95% of the body’s 5-HT and are the most numerous of the gut endocrine cells.1 With endocrine cells in the islets of the pancreas, EEC form the gastro-entero-pancreatic (GEP) group of embryologically-related endocrine cells. EC cells are found throughout the gastrointestinal tract, from the stomach to the rectum. They were first recognized by the binding of chrome salts by Heidenhain in 1870 and they were named by Ciaccio et al in 1907.2 They have historically been considered different from peptide hormone containing EEC, 3–5 even though 25 years ago colocalisation of 5-HT with peptide hormones in EEC was reported.6, 7 The separation of EC cells from other EEC reached its extreme in a recent review in the authoritative journal, Cell, in which 5-HT is not even listed as a hormone of gastrointestinal endocrine cells.8 This is clearly wrong. In fact, 5-HT is contained in EEC, released into the circulation, has a range of important physiological roles, and may contribute to disease states.5, 9
HETEROGENEITY OF 5-HT ROLES AND CELL TYPES
Amongst the many roles attributed to 5-HT that is released from EC cells (Table 1) are initiation and/or enhancement of propulsive events in the small intestine and colon, enhancement of water and electrolyte secretion, exacerbation of inflammatory reactions, induction of nausea and vomiting in response to ingested toxins, regulation of obesity, control of pancreatic enzyme secretion, inhibition of bone formation, modulation of appetite, inhibition of gastric emptying and control of mucosal growth.5, 9 5-HT release is controlled by luminal chemicals, mechanical stimulation of the mucosa, neurotransmitters, other gut hormones and by inflammatory mediators.
Table 1.
Roles and effects of 5-HT derived from enteroendocrine cells
| Role / actions of 5-HT derived from enteroendocrine cells | Comments |
|---|---|
| Supplying 5-HT to platelets | Platelets lack machinery to synthesise 5-HT |
| Activation of propulsive reflexes in intestine and colon | Stimulant action discovered in the 1950s, but 5-HT’s role still debated |
| Slowing of gastric emptying | Effect mediated through a vago-vagal reflex |
| Enhancement of intestinal inflammation | Increasing 5-HT availability by inhibiting uptake increases inflammation |
| Increase of ionic movement across the mucosal epithelium | Measured by increase in short circuit current. Equivalent to movement of anions (Cl−, HCO3−) into the lumen. High levels of 5-HT can cause diarrhea. |
| Mucosal vasodilation | Activated by mechanical stimulation of the epithelium causing 5-HT release from EC. |
| Reduction in bone mass | 5-HT inhibits osteoblast proliferation |
| Liver regeneration | Inhibition of TPH1 compromises liver regeneration |
| Stimulation of pancreatic enzyme secretion | Acts synergistically with CCK |
| Modulation of visceral pain | Intraluminal 5-HT reduces pain, but depleting EC cells also reduces pain |
| Appetite | Increases in 5-HT levels and 5-HT mimetics decrease food intake, and antagonists of 5-HT receptors increase feeding |
| Nausea and vomiting | Triggered by toxins in the stomach and upper intestine. May require high levels of 5-HT |
In this issue of Neurogastroenterology and Motility, Martin et al.10 reveal remarkable differences in expression levels for nutrient receptor and transporter genes in highly enriched isolates of EC cells from mouse duodenum and colon. Examples are mRNA for the glucose transporter, GLUT2, being enriched more than 400 fold in EC of the duodenum compared to those from colon, and the long chain fatty acid receptor, FFAR4, being enriched 45 fold in the colon compared to duodenum. The short chain fatty acid receptor, FFAR2, was also enriched in colonic EC, but FFARs 1 (medium and long chain fatty acid receptor) and 3 (propionate- and butyrate-preferring short chain fatty acid receptor) were expressed at greater levels in duodenal EC.
5-HT is not exclusive to a class of EEC that is distinct from those EEC that produce peptide hormones (Fig. 1). Evidence for this conclusion dates back at least to the 1990s when Roth and Gordon7 reported that 5-HT was present in about 80% of secretin cells in the mouse duodenum (see Fig. 1). Co-expression of 5-HT in most secretin cells in mouse was confirmed using a transgenic reporter for secretin.11 In bovine, cat and guinea-pig, all secretin cells of the duodenum and jejunum contained 5-HT. In human duodenum about 70% of secretin cells were immunoreactive for 5-HT, whereas in pig very few secretin cells contained 5-HT,6 which we have confirmed. Ablation of secretin-expressing cells of the mouse small intestine significantly reduced the production of 5-HT, CCK, glucagon, PYY, GIP, and somatostatin, indicating a close developmental relationship between cells that produce these hormones.12
Figure 1.
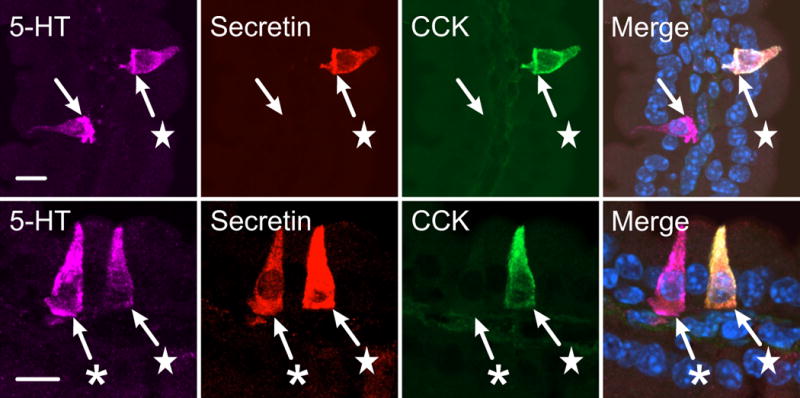
Colocalisation of 5-HT with secretin and CCK in enteroendocrine cells of the mouse duodenum. Examples of 5-HT cells that do not contain either of these peptide hormones (unlabeled arrows), 5-HT plus secretin (✱) and 5-HT plus both CCK and secretin (★) are illustrated. In the merged image cell nuclei are stained by DAPI (blue). Scale bars: 10 μm.
Colocalisation of 5-HT and CCK has also been found in EEC of the mouse proximal small intestine13 and more recently, the colocalisation of 5-HT and CCK has been mapped in the mouse small and large intestine.14 There was colocalisation in the duodenum, jejunum, ileum and cecum, but not in the colon. Overlap ranged from about 15% of 5-HT cells containing CCK in the duodenum, to 50% in the cecum. In addition, 5-HT is co-expressed with PYY in EEC of the human colon and anal canal15, 16 and with GLP-1 cells in the human anal canal.17
Although the different roles of 5-HT released from gastrointestinal EEC is consistent with there being different subtypes of 5-HT producing EEC, the various physiological roles of 5-HT have not yet been ascribed to EC with different patterns of receptor, transporter or co-hormone expression and different locations in the gut. To determine the relationships of expression patterns and physiological roles is an important challenge for the future.
The paper of Martin et al in the current issue reveals free fatty acid receptor (FFAR) expression by duodenal EEC that contain 5-HT.10 It is feasible that duodenal EC that express FFAR are the 5-HT/CCK cells discussed above. Infusion of nutrients into the upper small intestine causes a vagus nerve-dependent increase in pancreatic enzyme release that has both CCK mediated and 5-HT mediated components.18, 19 In conscious rats, intraluminal application of rodent chow evoked increases in pancreatic enzyme secretion that were reduced by 54% when CCK receptors were blocked and 92% when both CCK and 5-HT receptors were blocked.19 Thus, CCK and 5-HT may be released from the same EEC in response to fats.
5-HT and CCK released by activation of FFARs may also act synergistically to increase mixing activity during digestion. Mixing activity that was induced by luminal fat was mimicked by increasing 5-HT availability (by inhibiting 5-HT uptake) and by CCK.20 Moreover, intraluminal decanoic acid induced mixing movements that were reduced by both 5-HT and CCK receptor antagonists. 5-HT and CCK possibly also act together in causing satiety. CCK is well established as a gastrointestinal hormone that is released by nutrients and stimulates vagal afferent endings in the gut to induce satiety.21 5-HT, 5-HT receptor stimulants and increased availability of 5-HT (caused by inhibiting its metabolism) all decrease food intake, and antagonists of 5-HT receptors increase feeding.22, 23
The 5-HT plus secretin EEC of the upper small intestine may express a different range of receptors. 5-HT mimics the effect of secretin to release bicarbonate; low doses of 5-HT (20–200 nmol/kg/h) infused into the vasculature of the rat duodenum24 or direct application of 5-HT to the isolated duodenum from mice increases bicarbonate secretion.25 Thus, synergistic effects of 5-HT and secretin released from the same cells, which presumably express acid receptors, are predicted.
In the human colon about 25% of 5-HT cells also contain PYY,16, 17 although in the mouse very few colonic 5-HT cells contain PYY.26 This points to one of the major puzzles revealed by in-depth studies of EEC, the differences between species in their distribution patterns.6, 27
INVOLVEMENT OF EC CELLS IN DISEASE
Celiac Disease (CD)
In CD the number of 5-HT-containing EEC per mm of duodenal tissue is about double or more than double control values, despite there being villous atrophy.28, 29 In a study of newly diagnosed CD patients and healthy controls consuming a gluten containing meal (~6–8 g), 5-HT levels in plasma over the first 3 post-prandial hours were significantly higher in CD compared to controls.28 Peak plasma 5-HT levels correlated significantly with dyspeptic symptoms, with levels comparable to that seen in carcinoid syndrome and cisplatin-induced emesis. Furthermore, in patients with persistently active (refractory) CD, gene expression of tryptophan hydroxylase-1 (Tph1), the rate-limiting enzyme in 5-HT synthesis, is significantly increased in duodenal samples.29 Infusion of 5-HT causes symptoms similar to those caused by a gluten challenge: abdominal cramps, nausea and diarrhea. However, it is not known which subtypes of EC cells in the proximal small intestine respond to gluten challenge.
Irritable Bowel Syndrome (IBS)
There is substantial evidence to link 5-HT with IBS.9, 30 Increased plasma 5-HT levels are reported in diarrhea-predominant IBS (IBS-D) and reduced levels in constipation-predominant IBS (IBS-C). Alterations in mucosal levels of 5-HT and decreased expression and activity of the serotonin uptake transporter (SERT) in IBS has also been reported.9 5-HT may also increase visceral hypersensitivity in IBS.31 5-HT3 receptor antagonists and TPH1 inhibitors have been shown to significantly improve symptoms in IBS-D patients.32, 33 Again, which subpopulations of EC cells contribute to the symptoms of IBS is unknown.
UNDERSTANDING THE HETEROGENEITY
Gene expression studies and immunohistochemical investigations indicate different aspects of the heterogeneity of 5-HT containing EEC. In the duodenum, the total population of EC cells expresses greater levels of GLUT2, FFAR1 and FFAR3 than the colon population. But in the duodenum there are several EC cell sub-populations, including EC cells that also contain secretin, EC cells that also contain CCK, and EC cells with both or neither of these peptide hormones (Fig. 1). These subgroups are likely to have uneven distributions of GLUT2, FFAR1 and FFAR3. For example, CCK cells are sensitive to FFAs, whereas the major physiological stimulus for secretin secretion is reduced pH. Thus, to gain a fuller understanding of the heterogeneity it will be necessary to define and characterise EC both by region and by subtypes within regions, and relate this characterisation to the functions of the different types of EC. It will also be necessary to define between-species differences, and which subtypes of EC contribute to disease processes. Big challenges await us.
Key Points.
Cells that contain 5-HT (serotonin) are the most common enteroendocrine cells in the gastrointestinal tract
They are commonly referred to as enterochromaffin cells and are classified as a group separate from the enteroendocrine cells that store and secrete peptide hormones.
In this Mini-Review, evidence for considerable heterogeneity of 5-HT and their overlap, through colocalisation, with peptide hormone cells is discussed.
Acknowledgments
FUNDING
Work on gastrointestinal endocrine cells is funded in part by NIH grant (ID #1OT2OD023847).
Footnotes
CONFLICTS OF INTEREST
No competing interests are declared
AUTHOR CONTRIBUTIONS
SD, LJF, JF, BC and JBF wrote the mini-review together
References
- 1.Sjölund K, Sandén G, Håkanson R, Sundler F. Endocrine cells in the human intestine: an immunocytochemical study. Gastroenterology. 1983;85:1120–1130. [PubMed] [Google Scholar]
- 2.de Herder WW, Rehfeld JF, Mark Kidd M, Irvin M, Modlin IM. A short history of neuroendocrine tumours and their peptide hormones. Best Practice and Research Clinical Endocrinology and Metabolism. 2016;30:3–17. doi: 10.1016/j.beem.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Vialli M, Erspamer V. Celluli enterocromaffini e cellule basigranulose acidofile nei vertebrati. Z Zellforsch. 1933;19:743–773. [Google Scholar]
- 4.Erspamer V, Asero B. Identification of enteramine, the specific hormone of the enterochromaffin cell system, as 5-hydroxytryptamine. Nature. 1952;169:800–801. doi: 10.1038/169800b0. [DOI] [PubMed] [Google Scholar]
- 5.Gershon MD. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr Op Endoc Diab Obes. 2013;20:14–21. doi: 10.1097/MED.0b013e32835bc703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cetin Y. Secretin-cells of the mammalian intestine contain serotonin. Histochemistry. 1990;93:601–606. doi: 10.1007/BF00272202. [DOI] [PubMed] [Google Scholar]
- 7.Roth KA, Gordon JI. Spatial differentiation of the intestinal epithelium: Analysis of enteroendocrine cells containing immunoreactive serotonin, secretin, and substance P in normal and transgenic mice. Proc Natl Acad Sci USA. 1990;87:6408–6412. doi: 10.1073/pnas.87.16.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coate KC, Kliewer SA, Mangelsdorf DJ. SnapShot: Hormones of the gastrointestinal tract. Cell. 2014;159:1478–1479. doi: 10.1016/j.cell.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 9.Mawe GM, Hoffman JM. Serotonin signalling in the gut—functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2013;10:473–486. doi: 10.1038/nrgastro.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin AM, Lumsden AL, Young RL, Jessup CF, Spencer NJ, Keating DJ. The nutrient-sensing repertoires of mouse enterochromaffin cells differ between duodenum and colon. Neurogastroenterol Motil. 2017 doi: 10.1111/nmo.13046. [DOI] [PubMed] [Google Scholar]
- 11.Lopez MJ, Upchurch BH, Rindi G, Leiter AB. Studies in transgenic mice reveal potential relationships between secretin-producing cells and other endocrine cell types. The Journal of Biological Chemistry. 1995;270:885–891. doi: 10.1074/jbc.270.2.885. [DOI] [PubMed] [Google Scholar]
- 12.Rindi G, Ratineau C, Ronco A, Candusso ME, Tsai M-J, Leiter AB. Targeted ablation of secretin-producing cells in transgenic mice reveals a common differentiation pathway with multiple enteroendocrine cell lineages in the small intestine. Development. 1999;126:4149–4156. doi: 10.1242/dev.126.18.4149. [DOI] [PubMed] [Google Scholar]
- 13.Cho H-J, Callaghan B, Bron R, Bravo DM, Furness JB. Identification of enteroendocrine cells that express TRPA1 channels in the mouse intestine. Cell Tissue Res. 2014;356:77–82. doi: 10.1007/s00441-013-1780-x. [DOI] [PubMed] [Google Scholar]
- 14.Fakhry J, Wang J, Martins P, Fothergill LJ, Hunne B, Prieur P, Shulkes A, Rehfeld JF, Callaghan B, Furness JB. Distribution and characterisation of CCK containing enteroendocrine cells of the mouse small and large intestine. Cell Tissue Res. doi: 10.1007/s00441-017-2612-1. (In press, Accepted 20 March 2017) [DOI] [PubMed] [Google Scholar]
- 15.Lukinius AIC, Ericsson JLE, Lundqvist MK, Wilander EMO. Ultrastructural localization of serotonin and polypeptide YY (PYY) in endocrine cells of the human rectum. The Journal of Histochemistry and Cytochemistry. 1986;34:719–726. doi: 10.1177/34.6.3517149. [DOI] [PubMed] [Google Scholar]
- 16.Martins P, Fakhry J, Chaves de Oliveira E, et al. Analysis of eneteroendocrine cell populations in the human colon. Cell Tissue Res. 2017;367:361–368. doi: 10.1007/s00441-016-2530-7. [DOI] [PubMed] [Google Scholar]
- 17.Hörsch D, Fink T, Göke B, Arnold R, Büchler M, Weihe E. Distribution and chemical phenotypes of neuroendocrine cells in the human anal canal. Regul Pept. 1994;54:527–542. doi: 10.1016/0167-0115(94)90550-9. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Owyang C. Pancreatic secretion evoked by cholecystokinin and non-cholecystokinin-dependent duodenal stimuli via vagal afferent fibes in the rat. J Physiol (Lond) 1996;494:773–782. doi: 10.1113/jphysiol.1996.sp021531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Hao Y, Zhu J, Owyang C. Serotonin released from intestinal enterochromaffin cells mediates luminal non-cholecystokinin-stimulated pancreatic secretion in rats. Gastroenterology. 2000;118:1197–1207. doi: 10.1016/s0016-5085(00)70373-8. [DOI] [PubMed] [Google Scholar]
- 20.Ellis M, Chambers JD, Gwynne RM, Bornstein JC. Serotonin and cholecystokinin mediate nutrient-induced segmentation in guinea pig small intestine. Am J Physiol Gastrointest Liver Physiol. 2013;304:G749–G761. doi: 10.1152/ajpgi.00358.2012. [DOI] [PubMed] [Google Scholar]
- 21.Morley JE. Appetite regulation by gut peptides. Annu Rev Nutr. 1990;10:383–395. doi: 10.1146/annurev.nu.10.070190.002123. [DOI] [PubMed] [Google Scholar]
- 22.Blundell JE. Serotonin manipulations and the structure of feeding behaviour. Appetite. 1986;7:39–56. doi: 10.1016/s0195-6663(86)80051-4. [DOI] [PubMed] [Google Scholar]
- 23.Cooper SJ, Dourish CT. Multiple cholecystokinin (CCK) receptors and CCK-monoamine interactions are instrumental in the control of feeding. Physiol Behav. 1990;48:849–857. doi: 10.1016/0031-9384(90)90239-z. [DOI] [PubMed] [Google Scholar]
- 24.Säfsten B, Sjöblom M, Flemström G. Serotonin increases protective duodenal bicarbonate secretion via enteric ganglia and a 5-HT4-dependent pathway. Scand J Gastroenterol. 2006;41:1279–1289. doi: 10.1080/00365520600641480. [DOI] [PubMed] [Google Scholar]
- 25.Tuo B-G, Isenberg JI. Effect of 5-hydroxytryptamine on duodenal mucosal bicarbonate secretion in mice. Gastroenterology. 2003;125:805–814. doi: 10.1016/s0016-5085(03)01045-x. [DOI] [PubMed] [Google Scholar]
- 26.Reynaud Y, Fakhry J, Fothergill L, et al. The chemical coding of 5-hydroxytryptamine containing enteroendocrine cells in the mouse gastrointestinal tract. Cell Tissue Res. 2016;364:489–497. doi: 10.1007/s00441-015-2349-7. [DOI] [PubMed] [Google Scholar]
- 27.Cho H-J, Kosari S, Hunne B, et al. Differences in hormone localisation patterns of K and L type enteroendocrine cells in the mouse and pig small intestine and colon. Cell Tissue Res. 2015;359:693–698. doi: 10.1007/s00441-014-2033-3. [DOI] [PubMed] [Google Scholar]
- 28.Coleman NS, Foley S, Dunlop SP, et al. Abnormalities of serotonin metabolism and their relation to symptoms in untreated celiac disease. Clin Gastroenterol Hepatol. 2006;4:874–881. doi: 10.1016/j.cgh.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 29.Di Sabatino A, Giuffrida P, Vanoli A, et al. Increase in neuroendocrine cells in the duodenal mucosa of patients with refractory celiac disease. Am J Gastroenterol. 2014;109:258–269. doi: 10.1038/ajg.2013.426. [DOI] [PubMed] [Google Scholar]
- 30.Dunlop SP, Coleman NS, Blackshaw E, et al. Abnormalities of 5-hydroxytryptamine metabolism in irritable bowel syndrome. Clin Gastroenterol Hepatol. 2005;3:349–357. doi: 10.1016/s1542-3565(04)00726-8. [DOI] [PubMed] [Google Scholar]
- 31.Park JH, Rhee P-L, Kim G, et al. Enteroendocrine cell counts correlate with visceral hypersensitivity in patients with diarrhoea-predominant irritable bowel syndrome. Neurogastroenterol Motil. 2006;18:539–546. doi: 10.1111/j.1365-2982.2006.00771.x. [DOI] [PubMed] [Google Scholar]
- 32.Andresen V, Montori VM, Keller J, West CP, Layer P, Camilleri M. Effects of 5-hydroxytryptamine (Serotonin) type 3 antagonists on symptom relief and constipation in nonconstipated irritable bowel syndrome: A systematic review and meta-analysis of randomized controlled trials. Clin Gastroenterol Hepatol. 2008;6:545–555. doi: 10.1016/j.cgh.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown PM, Drossman DA, Wood AJJ, et al. The tryptophan hydroxylase inhibitor LX1031 shows clinical benefit in patients with nonconstipating irritable bowel syndrome. Gastroenterology. 2011;141:507–516. doi: 10.1053/j.gastro.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009;60:355–366. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]


