Highlights
-
•
At high hCG levels in molar pregnancies, a “hook effect” can cause an artificially negative value.
-
•
Delay in diagnosis of a molar pregnancy due to the “hook effect” can lead to severe complications.
-
•
Suspicion of a molar pregnancy should be communicated so a diluted sample is used to quantify hCG.
1. Introduction
Complete molar pregnancy is estimated to occur once in every 1000–1500 pregnancies. It most commonly presents with vaginal bleeding between 6 and 16 weeks gestation in approximately 80–90% of cases. The other classic clinical signs and symptoms, such as uterine enlargement greater than expected for gestation dates, hyperemesis, and medical complications, such as pregnancy induced hypertension, clinical hyperthyroidism and respiratory insufficiency, occur less frequently in recent years. This has been a result of earlier diagnosis from increased availability of ultrasonography and accurate tests for measurement of human chorionic gonadotropin (hCG) (Lurain, 2010, Berkowitz et al., 2014, Soto-Wright et al., 1995).
Molar pregnancies are more commonly associated with markedly elevated hCG levels, with approximately 50% of patients having hCG levels > 100,000 mIU/mL (Lurain, 2010, Berkowitz et al., 2014). At serum levels of hCG above 500,000 mIU/mL, a “hook effect” can occur, resulting in an artificially low or negative value when using the current commercially available immunometric hCG assays (Yeung and Cheung, 2014, Mori and Lurain, 2015). We report a case of a complete mole and normal fetus twin gestation where the diagnosis was delayed by a negative pregnancy test due to the “hook effect,” resulting in life-threatening complications.
2. Case
A 34-year-old gravida 3 para 1011 initially presented to another medical center with 4 weeks of palpitations, diaphoresis, difficulty breathing, worsening lower extremity edema, and persistent nausea and vomiting. She had a negative urine pregnancy test, her LMP was unknown due to irregular menses. She was diagnosed with thyroid storm that required hospitalization for further management. She was treated with methimazole 10 mg daily and propranolol 60 mg QID with stabilization of her condition and discharged for outpatient follow up.
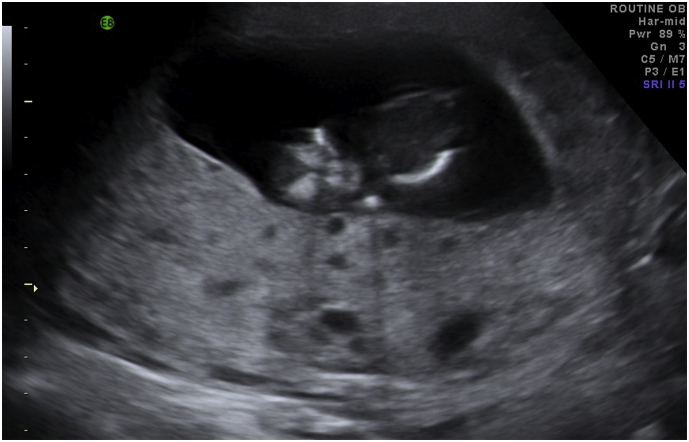
At presentation to our emergency department six days later, she continued to report worsening symptoms with irregular bleeding, nausea, and vomiting. Her vital signs were significant for a blood pressure of 190/110, heart rate of 105, and oxygen saturation of 98% on room air. Physical exam was notable for tachycardia with no murmur, 3 + bilateral lower extremity edema to her low back, and an enlarged irregular-shaped abdomen with a tender abdominal-pelvic mass. Labs included a negative urine pregnancy test, urinalysis > 300 mg/dL protein, uric acid 7.2 md/dL, LDH 221 U/L, Hgb 11.0 g/dL, Hct 33.9%, WBC 8.1 K/UL, platelets 288 K/UL, TSH 0.16 uIU/mL, and free T4 1.8 ng/dL. A bedside abdominal ultrasound showed a 13-week fetus with a large placenta and gynecology was consulted. On pelvic exam, there was scant blood in the vaginal vault and the uterus was enlarged to 28 weeks gestational size. A serum hCG after proper dilution was > 900,000 mIU/mL. A formal sonogram showed a nonviable fetus at 13 weeks, a large amount of amniotic fluid, and a disproportionately large hydropic placenta with diffuse cystic interfaces (Fig. 1).
Fig. 1.
Ultrasound of 13 week fetus and complete mole.
With a presumed twin mole-nonviable fetus pregnancy, she underwent suction evacuation with appropriate thyroid and beta-receptor blockade. Prior to the procedure blood products and uterotonics were available. An arterial line and a triple lumen central venous catheter were placed. The cervix was injected with 5000 units of vasopressin in 20 cm3 of 1% lidocaine. Under ultrasound guidance, the cervix was dilated to allow insertion of a 14 mm curved cannula and an intravenous pitocin infusion was started at 17 U/h. Four minutes after suction was applied in the uterus her oxygen saturation dropped to 87% and she became hypotensive with no arterial line waveform, although carotid pulses were palpable. Vital signs normalized with bag ventilation, epinephrine, calcium gluconate, intravenous fluids, and sodium bicarbonate. The suction curettage was completed to evacuate all the intra-uterine contents.
She was admitted to the intensive care unit intubated. A transesophageal echocardiogram revealed global reduced cardiac function. A nitroprusside drip was started for blood pressure control, initially at 1 mcg/kg/min and titrated to 2.5 mcg/kg/min. She received multiple blood transfusions because labs suggested a coagulopathy; Hgb downtrended from 11.0 to 7.4 g/dL, platelets nadired at 59 K/UL, and fibrinogen fell to 132 mg/dL. Once stabilized, she was transferred out of intensive care two days after her procedure. She was discharged home the following day on carvedilol 50 mg BID and methimazole 5 mg daily for 3 weeks. At her follow-up visit, carvedilol and methimazole were discontinued and she was started on oral contraceptive pills. Bi-weekly hCG levels downtrended to 1 mIU/mL at 15 weeks postoperatively and she continued to do well. Pathology on the uterine contents showed a 12–13 week fetus and a complete hydatidiform mole.
3. Discussion
With the increasing availability of rapid, accurate tests for the detection of hCG to diagnose pregnancy and the use of early ultrasound examination, molar pregnancy is now typically discovered in the first trimester before the classic clinical signs and symptoms develop (Lurain, 2010, Berkowitz et al., 2014). Comparison of clinical presentation of complete hydatidiform moles from 1988–1993 to 1965–1975 at the New England Trophoblastic Disease Center revealed a decrease in vaginal bleeding (97% to 84%), hyperemesis (26% to 8%), uterine size greater than dates (51% to 28%), anemia (54% to 5%), preeclampsia (27% to 1.3%), hyperthyroidism (7% to 0), and respiratory distress (2% to 0). This was the result of earlier first trimester diagnosis, which increased from 47% to 84% between the study periods (Soto-Wright et al., 1995). Delay in diagnosis of a molar pregnancy in our patient as a result of a falsely negative pregnancy test due to the “hook effect” led to life threatening complications.
The “hook effect” resulting in a false negative pregnancy test can occur with high hCG levels typically associated with advanced molar pregnancies (Yeung and Cheung, 2014, Mori and Lurain, 2015). All current commercially available hCG tests are two-site noncompetitive immunometric assays, also known as “sandwich” assays. When hCG is present, it is immobilized or captured by the capture antibody and then labeled by a tracer or signal antibody. As a result, hCG molecules link up both immobilized (capture) and tracer (signal) antibody to form a sandwich (immobilized-hCG-tracer antibody). After washing away excess material, the amount of now immobilized label is measured and is directly proportional to the amount of hCG joining the sandwiches together. The hCG level in the sample is then obtained by comparing the amount of tracer signal in the sample to a standard hCG concentration curve (Fig. 2A) (Griffey et al., 2013).
Fig. 2.
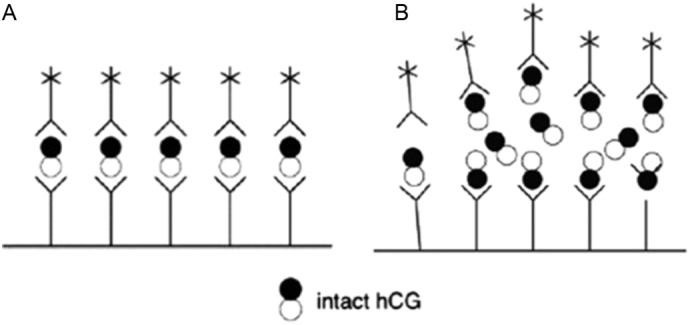
Sandwich assay schematic demonstrating a normal positive hCG test (A) where intact hCG molecule is recognized by both fixed and free-floating antibodies with a reporter label and a false negative hCG test due to the hook-effect (B) where excess hCG saturates both fixed and free-floating antibodies preventing sandwich formation (Griffey et al., 2013).
At very high serum levels of hCG, usually above 500,000 mIU/mL, both the capture and tracer antibodies used in the immunometric assays become simultaneously saturated preventing binding of the two to create a sandwich. Since the non-sandwiched tracer antibodies are washed away with the excess material, the hCG test will be negative (Fig. 2B) (Griffey et al., 2013). The sensitivity of most hCG assays are set to the normal pregnancy hCG range at 8 to 11 weeks of about 25,000 to 250,000 mIU/mL. If a diagnosis of gestational trophoblastic disease associated with a higher hCG level is suspected, this should be communicated to the laboratory so that the hCG assay will be performed on a diluted sample.
Thyroid storm is now a rare but life-threatening complication of a complete molar pregnancy. Our patient exhibited some of the classic symptoms of clinical hyperthyroidism with diaphoresis, palpitations, dyspnea, nausea and vomiting, and edema indicative of cardiac failure. Although she was treated with both an antithyroid drug and beta blocker, she developed cardiovascular collapse during molar evacuation requiring blood pressure support and prolonged mechanical ventilation. Her condition rapidly improved over a few days after removing the precipitating event – the molar pregnancy.
The symptoms of hyperthyroidism associated with hydatidiform moles are caused by the high concentration of hCG acting on the thyroid TSH receptors and inducing release of thyroid hormones. Although the usual potency of hCG for TSH receptors is 4000 times less than TSH, the hCG produced by hydatidiform moles has more thyrotropic activity compared with hCG in normal pregnancy. This enhanced thyrotropic activity of hCG in conjunction with relatively high hCG levels, usually in excess of 100,000 mIU/mL, accounts for the clinical manifestations of hyperthyroidism observed in some patients with complete molar pregnancy (Walkington et al., 2011).
Twin gestations with a complete mole and coexistant normal fetus, such as occurred in our patient, are estimated to occur once in every 22,000 to 100,000 pregnancies and are associated with increased obstetrical complications, such as bleeding, hyperemesis, preeclampsia, and hyperthyroidism (Sebire et al., 2002). This is probably related to the diagnosis of twin mole/fetus pregnancies at later gestational ages with larger uteri and higher hCG levels than singleton complete moles. Also, patients with twin mole/fetus pregnancies who develop medical or obstetrical complications are more likely to experience fetal demise. Data are conflicting, however, whether twin pregnancies with complete mole and coexisting fetus are at increased risk of developing postmolar gestational trophoblastic neoplasia.
In summary, our patient exhibited several complications of molar pregnancy: twin complete mole/normal fetus pregnancy, life-threatening hyperthyroidism, and a false negative pregnancy test. Twin mole/fetus pregnancies are typically diagnosed at a later gestation resulting in more obstetrical complications, larger uteri, and high hCG levels. Delay in diagnosis due to a false negative pregnancy test related to the “hook effect” resulted in thyroid storm in our patient. A heightened awareness of the analytical limitations of hCG immunoassays and exercise of clinical judgment based on patient symptoms, physical examination, and other laboratory tests are essential to prevent misdiagnosis with subsequent mismanagement and improve patient outcomes.
Conflict of interest statement
None of the authors have any conflicts of interest related to the material presented in this case report.
Patient consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
References
- Berkowitz R.A., Goldstein D.P., Horowitz N.S. Management options of gestational trophoblastic disease. Curr. Obstet. Gynecol. Rep. 2014;3:76–83. doi: 10.1007/s13669-013-0071-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffey R.T., Trent C.J., Bavolek R.A., Keeperman J.B., Sampson C., Poirer R.F. "Hook-like effect" causes false-negative point-of-care urine pregnancy testing in emergency patients. J. Emerg. Med. 2013;44:155–160. doi: 10.1016/j.jemermed.2011.05.032. [DOI] [PubMed] [Google Scholar]
- Lurain J.R. Gestational trophoblastic disease I: epidemiology, pathology, clinical presentation and diagnosis of gestational trophoblastic disease, and management of hydatidiform mole. Am. J. Obstet. Gynecol. 2010;203:531–539. doi: 10.1016/j.ajog.2010.06.073. [DOI] [PubMed] [Google Scholar]
- Mori K.M., Lurain J.R. In: Human Chorionic Gonadotropin: Testing in Pregnancy and Gestational Trophoblastic Disease and Causes of Persistent Low Levels. Goff Barbara., editor. Wolters Kluwer; 2015. UpToDate. [Google Scholar]
- Sebire N.J., Foskett M., Pradinas F.J., Fisher R.A., Francis R.J., Short D., Newlands E.S., Seckl M.J. Outcome of twin pregnancies with complete hydatidiform mole and healthy co-twin. Lancet. 2002;359:2165–2166. doi: 10.1016/S0140-6736(02)09085-2. [DOI] [PubMed] [Google Scholar]
- Soto-Wright V., Bernstein M., Goldstein D.P., Berkowitz R.S. The changing clinical presentation of complete molar pregnancy. Obstet. Gynecol. 1995;86:775–779. doi: 10.1016/0029-7844(95)00268-V. [DOI] [PubMed] [Google Scholar]
- Walkington L., Webster J., Hancock B.W., Everard J., Coleman R.E. Hyperthyroidism and human chorionic gonadotropin production in gestational trophoblastic disease. Br. J. Cancer. 2011;104:1665–1669. doi: 10.1038/bjc.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung C.-W., Cheung A.N.Y. Negative pregnancy test in patients with trophoblastic diseases. Curr. Obstet. Gynecol. Rep. 2014;3:102–106. [Google Scholar]