Abstract
Chronic orofacial pain is a symptom associated with a wide range of neuropathic, neurovascular, idiopathic, and myofascial conditions that affect a significant proportion of the population. While the collective impact of the subset of the orofacial pain disorders involving neurogenic and idiopathic mechanisms is substantial, some of these are relatively uncommon. Hence, patients with these disorders can be vulnerable to misdiagnosis, sometimes for years, increasing the symptom burden and delaying effective treatment. This manuscript first reviews the decision tree to be followed in diagnosing any neuropathic pain condition, as well as the levels of evidence needed to make a diagnosis with each of several levels of confidence: definite, probable, or possible. It then examines the clinical literature related to the idiopathic and neurogenic conditions that can occasion chronic orofacial pain, including burning mouth syndrome, trigeminal neuralgia, glossopharyngeal neuralgia, post-herpetic neuralgia, and atypical odontalgia. Temporomandibular disorders also are examined as are other headache conditions, even though they are not neurologic conditions, because they are common and can mimic symptoms of the latter disorders. For each of these conditions, the paper reviews literature regarding incidence and prevalence, physiologic and other contributing factors, diagnostic signs and symptoms, and empirical evidence regarding treatments. Finally, in order to improve the quality and accuracy of clinical diagnosis, as well as the efficiency with which effective treatment is initiated and delivered, criteria are offered that can be instrumental in making a differential diagnosis.
Keywords: Orofacial pain disorders, Burning mouth syndrome, Neuropathic pain syndromes
Introduction
Chronic orofacial pain (COFP) disorders, collectively, affect a large proportion of the population [1]. They can involve dysfunction in multiple systems: musculoskeletal, vascular, neurovascular, neuropathic, idiopathic, and psychogenic [2]. Further, they can present singly or in combination. Even when a COFP disorder is associated with a single cause (e.g., neuropathic), it can present with a range of clinical features. Hence, it is common for COFP patients to consult multiple providers before an accurate diagnosis is made, including primary care providers, dentists, physical therapists, and mental health professionals. While several COFP diagnoses are relatively common (e.g., migraine, tension-type headache, and temporomandibular disorders), other COFP syndromes are less prevalent and are often misdiagnosed (Table 1).
Table 1.
Classification of neurologic, idiopathic, and musculoskeletal orofacial pain [3].
| Musculoskeletal |
| Temporomandibular disorders |
| Tension-type headache |
| Cervical headache |
| Vascular* |
| Giant cell arteritis |
| Carotid artery dissection |
| Neurovascular |
| Migraine |
| Cluster headache |
| Neuropathic-Episodic |
| Trigeminal neuralgia |
| Glossopharyngeal neuralgia |
| Neuropathic-Continuous |
| Herpetic neuralgia* |
| Postherpetic neuralgia |
| Eagle’s syndrome |
| Idiopathic |
| Burning mouth syndrome |
| Atypical odontalgia |
These conditions are not included in this manuscript as they are not considered COFP
This delays the initiation of effective treatment for years in patients with some of the less common conditions [4]. COFP syndromes may arise from a variety of underlying pathophysiologic causes and are typically categorized as musculoskeletal, vascular, neurovascular, psychogenic, neuropathic (episodic or continuous), and idiopathic [3].
Burning Mouth Syndrome (BMS) is one of the less common neuropathic/idiopathic COFP disorders. While most neuropathic and/or idiopathic COFP conditions are relatively uncommon, collectively, they are reasonably prevalent in the general population, affecting between 3.3% and 8.2% of that group [5]. To a large degree, the broad range reflected in such epidemiologic studies reflects the lack of specific clinical signs and symptoms that can be used to reach a definitive diagnosis for neuropathic/idiopathic COFP. Not only does the lack of definitive diagnostic criteria complicate epidemiologic studies, diagnosis and treatment are complicated by the fact that many of these COFP disorders, as diagnoses of exclusion, often suffer from misdiagnosis or delayed diagnosis (Table 2).
Table 2.
Burning mouth and other neuropathic/idiopathic COFP disorders: Differential diagnosis.
| Site | Pain severity pattern | Pain descriptors | Aggravating factors | Possible H and P findings |
|---|---|---|---|---|
| Burning Mouth Syndrome | ||||
| Labial mucosa (frequently tip of tongue), lips, buccal mucosa, palate, pharynx, floor of mouth. | Continuous. Increasing severity during the day. | Mild to moderate severity. Burning, stinging, scalding, and numb. Usually bilateral. | Hot or spicy food. Stress? | Primary BMS: normal oral mucosa, dysgeusia, dry mouth. Secondary BMS: diabetes, vitamin deficiency, conditions with reduced salivation, selected medications. |
| Trigeminal Neuralgia | ||||
| Along distribution of 2nd and 3rd divisions of trigeminal nerve | Paroxysmal. Attacks last seconds to minutes. Many (up to 30) attack daily. | Moderate to high severity. Usually unilateral. Stabbing, shooting, electrical lightning. | Chewing, light touch, talking, brushing teeth, cold. Sometimes spontaneous. Residual dull pain in affected area. | Hypesthesia, autonomic symptoms. |
| Glossopharyngeal Neuralgia | ||||
| Deep in throat, ear, and pharynx. | Paroxysmal. Attacks last seconds to minutes. | Moderate to high severity. Stabbing, sharp. | Swallowing, chewing, talking, coughing, and yawning. Residual aching pain. | Hypesthesia, syncope (rarely). |
| Post-herpetic Neuralgia | ||||
| Site of zoster rash. Usually follows dermatomal distribution. | Constant to intermittent. | Moderate to high severity. Burning, throbbing, aching, shooting, and itching. | Light touch. | History of zoster infection. Skin changes. Hyperesthesia, hypoesthesia, allodynia. |
| Atypical Odontalgia | ||||
| Tooth pain or pain at the site of a tooth extraction. More often in molars and in maxilla. | Continuous or almost continuous. Usually localized to tooth at onset. Can spread to wider area of face or neck. | Moderate severity. Deep, poorly localized. Dull, aching. Occasional sharp pain. | Mechanical stimulation or pressure to site of pain. | Normal radiographic and laboratory tests. Often follows endodontic treatment or tooth extraction. Hyperalgesia at facial area near oral symptoms. |
| Temporomandibular Disorder | ||||
| TMJ, masticatory muscles, ear. May include pain radiating to head and neck. | Usually intermittent. Can progress to continuous with exacerbations. | Mild to high Aching, sharp. severity. | Prolonged chewing. Bruxing. | Clicking at TMJ, reduced/deviated jaw opening, tenderness on palpation of masticatory musculature, bruxing, abnormal wearing of teeth, headache. |
This manuscript focuses primary attention on one idiopathic/neuropathic disorder, Burning Mouth Syndrome (BMS). It reviews the clinical features of BMS, putative mechanisms underlying its presentation, psychological factors often associated (BMS often has been misdiagnosed as “psychogenic”), and a review of empirical data relevant to treatment. In addition, the manuscript reviews the clinical characteristics of other neuropathic COFP disorders. Because most cases of COFP, including BMS, lack the level of medical evidence required for a definitive diagnosis, a practitioner needs to know both the characteristics of a given condition (i.e., the hallmarks of BMS) and those of other neuropathic/idiopathic conditions in sufficient detail so as to develop an appropriate differential. Before focusing specifically on BMS and other associated disorders, however, we first review a grading system that has proved to be useful in making clinical diagnoses across a broad range of neuropathic disorders.
Diagnosing Neuropathic Pain Disorders
Neuropathic pain is defined as “pain initiated or caused by a primary lesion or dysfunction in the nervous system” [6]. Historically, neuropathic pain has been under-recognized in primary care settings [7]. In an effort to facilitate diagnosis and the initiation of appropriate treatment, the Special Interest Group on Neuropathic Pain (NeuSIG), under the auspices of the International Association for the Study of Pain, developed a grading system to facilitate its diagnosis [5,8].
Figure 1 describes a decision tree for determining whether the medical evidence associated with a patient’s symptoms support the diagnosis of a neuropathic disorder as “definite,” “probable,” or “possible.”
Figure 1.
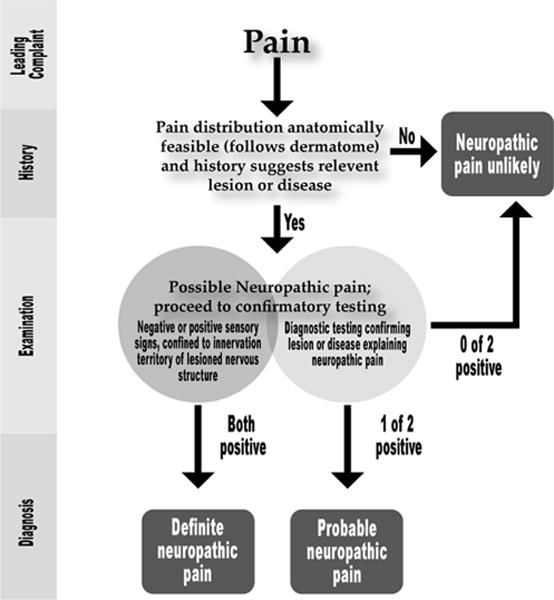
Diagnostic guidelines for neuropathic pain [8].
In order to reach a definite diagnosis, a practitioner must find confirmatory evidence on diagnostic testing or make a disease diagnosis consistent with the neurologic dysfunction. As is evident in the following pages, such evidence often is lacking in neuropathic COFP disorders.
Thus, the differential diagnosis of these conditions typically rests on a thorough history, a careful physical examination, and an understanding of the clinical features with which the disorders may present (Figure 1) [9].
Burning Mouth Syndrome
Clinical features
Burning Mouth Syndrome (BMS), or glossodynia, is a chronic pain disorder characterized by continuous, burning sensations that fluctuate in intensity and often are associated with taste alterations (dysgeusia) and dry mouth (xerostomia) [10]. These symptoms most often affect the anterior two-thirds of the tongue and other oropharyngeal structures. Estimates as to its prevalence vary widely with some studies reporting prevalence as low as 0.7% of the adult population, while others have estimated prevalence to be as high as 15% [11,12]. The wide variability evident in these studies likely reflects two elements that complicate its diagnosis:
There is no clear understanding of the neurophysiologic mechanisms underlying the symptoms, so that BMS lacks a confirmatory diagnostic test; and
BMS symptoms can be secondary to other medical (e.g., nutritional deficit) or oral/dental conditions (e.g., an ill-fitting bite splint or an inflammatory reaction to an oral appliance) that must be ruled out as causes. In the latter cases, BMS pain is considered “secondary,” while BMS pain that is not secondary to other medical or oral/dental conditions is considered “primary.” Many surveys do not distinguish between primary and secondary BMS.
Prevalence
While there is a lack of agreement as to its prevalence, other features are more consistent. For example, BMS is most commonly found in older adults (average age of onset is ~ 60 years) and is rare in patients younger than 40 [13]. Further, it is as much as five times more frequent in women than men. Because it is so frequently found in peri- or postmenopausal women, there is a growing conviction that its onset may be related to hormonal factors [14,15]. Similarly, there is growing belief that the pain and other symptoms of BMS are neuropathic in nature, largely owing to the typical descriptions [14,16]. While BMS typically presents as pain distributed symmetrically in the oropharyngeal area, that pattern can vary. It also tends to follow a typical diurnal pattern, such that patients often complain of pain that increases throughout the day. Most patients also report that pain is exacerbated by hot or spicy foods and is eased by cold foods.
Pathogenesis
The exact pathogenesis of BMS is in large part poorly understood. Psychogenic contributors likely play a role in the presentation of symptoms, as does hormonal fluctuations. No studies offer conclusive evidence of an identifiable etiology, however, symptoms of BMS have been reported in higher incidence in persons with significantly heightened perception of taste, increased number of fungiform papillae, and potentially the presence of the TAS2R38 gene [14]. Given the bilateral nature of the symptoms, it can be reasonably hypothesized that BMS represents a type of central pain.
Psychological factors
While psychological disorders are no longer seen by most as causing BMS, they frequently are associated with the disorder [13,17]. Research has found a high incidence of depression, anxiety, and fear of cancer in patients with BMS. In fact, between 30% and 50% of BMS patients have been found to experience symptoms of depression and 30% to 60% have been found to report symptoms consistent with an anxiety disorder [18,19]. Certainly, these conditions can co-exist.
While the frequency of these comorbidities seems high, it is important to note that the data reflect the presence of depressive or anxiety symptoms that may not meet the criteria for a formal psychiatric diagnosis, such as a Major Depressive Disorder. In this respect, BMS is similar to other chronic pain conditions where high rates of sub-syndromal psychological disorders also have been found. For example, epidemiologic studies based on health claims databases show diagnoses of depression in 13% of patients with chronic low back pain, while symptom-based (i.e., sub-syndromal) studies of clinic patients report depressive symptoms in almost 75% of patients with chronic pain [20,21]. Inasmuch as the symptom profile described above is consistent for BMS patients and patients experiencing other chronic pain conditions (e.g., low back pain, fibromyalgia), these co-morbidities cannot be considered unique to BMS [21,22]
More unique to BMS compared to other orofacial pain syndromes is the frequency with which patients fear that their symptoms reflect an underlying malignancy. Multiple studies have reported that a high percentage of BMS patients describe a fear of cancer, ranging from 20% to 47% [23,24]. While any explanation of this unusual finding is speculative, it likely reflects several factors. First, as BMS is a diagnosis of exclusion, the lag between symptom onset and diagnosis can be protracted. Research has shown that on average, patients with BMS may await a diagnosis for almost 7 years [3]. Hence, patients often experience an extended period of uncertainty regarding potential causes of their symptoms, during which time anxiety and catastrophic cognitions may develop. A second factor that likely contributes to cancer phobia involves BMS demographics: patients average 60 years of age at the time of BMS symptom onset. Cancer, as a disease associated with aging, is a much more prominent concern for the latter age group than for other chronic pain conditions, where onset commonly occurs at a younger age.
Treatment considerations
While treatment for BMS patients lacks evidence-based or consensus guidelines, the first step invariably involves ruling out other conditions that might be responsible for the symptom profile. Ill-fitting bite splints and selected acrylic appliances can result in inflammation and discomfort, while correcting the design and/or composition of the appliance can yield symptom relief [10]. In addition, metabolic disorders (e.g., diabetes) and nutritional deficiencies (e.g., Vitamin B12 deficiency) may represent other conditions that can occasion BMS symptoms [14]. Finally, BMS symptoms can be associated with adverse effects of medications. For example, select anti-depressant medications are frequently associated with side effects such as dry mouth, a frequent part of the BMS profile. In these cases, the replacement of a problematic medication with another that has a different side effect profile can be helpful in reducing secondary BMS symptoms.
For patients with primary BMS, the practitioner may want to adopt a “stepped care” model [25]. In this model, treatments are staged so that front-line, often less expensive treatments are initiated first with many patients responding to initial therapy. Of course, front-line treatments for primary BMS typically are dental or medical in nature. The following section outlines medical therapies with demonstrated effectiveness for BMS.
Pharmacological therapies
Several pharmacological agents have been studied as possible treatments for BMS although typically the evidence is from small, poorly designed studies, many of which lack blinding, randomization, and/or comparison to a placebo (an important consideration inasmuch as a demonstrated placebo effect exists in treating this pain syndrome). When combined with the subjectivity of pain assessment over a relatively short duration of treatment, these design considerations weaken some of the findings. Overall, this translates into low quality evidence to support efficacy and safety [26]. For this reason, non-pharmacological approaches to the management of this condition should be considered before initiating drug therapy.
As there is no standardized, clinically validated pain assessment tool specific to BMS, no consensus exists on how to effectively rate BMS pain. Therefore, some inconsistencies in results among trials may be secondary to how pain scores are characterized and interpreted. In addition, most studies did not assess the impact of treatment on quality of life (QOL). Notwithstanding these concerns, given the burden of pain and impact on QOL in those suffering from BMS, pharmacotherapeutic options may be reasonable once other etiologies are considered and other non-pharmacologic treatment options have failed. Unfortunately, many drug therapy studies have failed to show a substantial clinical benefit versus placebo for the treatment of BMS pain. When drug therapy is appropriate, co-occurring conditions are an important consideration in tailoring treatment to other patient-specific factors and needs.
Clonazepam
It has been proposed that GABA-A receptors may change in density or ligand concentration, resulting in the pain associated with BMS. As clonazepam modulates GABA-A receptors and has a strong effect on the brain’s serotonergic system, it has been widely studied as a treatment for BMS, both systemically and topically. It is thought that clonazepam is the preferred benzodiazepine for treating BMS, partially due to its unique pharmacokinetic profile [27].
Observational and interventional studies of clonazepam have evaluated topical administration of the drug (e.g., 0.5 mg clonazepam dissolved in the mouth for three minutes then spit out with remaining saliva up to four times daily), as well as once daily, low-dose systemic use [27–36]. With topical administration, clonazepam’s action has a quick effect in reducing BMS symptoms (within 10 minutes) and is association with minimal adverse effects. Unfortunately, the duration of action of analgesia and alleviation of other BMS symptoms is short (3 hours to 5 hours) [15].
One randomized, placebo-controlled study that assessed topical clonazepam found superior pain relief as compared to placebo over six months of treatment [32]. At one month, 23/33 showed a 50% reduction in symptoms vs. 4/33 in the placebo group (p<0.05). Similar findings were reported at six months: 23/33 on clonazepam experienced a 50% reduction in symptoms from baseline vs. 2/33 taking placebo. Although further studies would be beneficial to confirm these findings, topical clonazepam is a viable treatment in patients unresponsive to other treatments not associated with abuse-liability.
The efficacy of systemic clonazepam is generally conflicting among widely variable studies. Some studies didn’t demonstrate added clinical value over placebo, while other studies showed significant improvement in symptoms related to pain intensity and overall improvement.
Antidepressants
Efficacy regarding the use of antidepressants for the treatment of BMS is also conflicting, with drug-related side effects of greater concern. Trazodone, an antidepressant with multiple actions involving the serotonergic system, has been studied for pain due to BMS. One randomized, double-blind, placebo-controlled study assessing the use of trazodone over 8 weeks showed no benefit vs placebo in pain intensity [37]. Moreover, use of trazodone was associated with adverse effects that included dizziness and drowsiness. Though small studies assessing the use of sertraline, paroxetine, and moclobemide have demonstrated favorable efficacy results, confidence in those results is limited by the lack of placebo comparisons and blinding [38,39]. Aside from the methodological considerations associated with the latter studies, dose-dependent adverse effects and drug interactions limit these drugs’ usefulness. However, general provider comfort in dosing and monitoring of these agents may make them reasonable options for the patient experiencing pain due to BMS.
Milnacipran is a serotonin and norepinephrine reuptake inhibitor that is approved in the U.S. to treat fibromyalgia, rather than depression, even though it shares common pathways with the latter antidepressant drugs [40]. Three open-label, non-controlled studies have assessed the use of milnacipran for treatment of BMS [41–43]. As with the antidepressant literature, results were conflicting, and their interpretation complicated by such methodological issues as small sample sizes, possible bias, and a lack of comparators. Other confounds included the use of other drugs and the presence of other co-occurring conditions, all of which raise further questions about the quality of the evidence. Hence, the data provide little support for its use.
Vitamin B
Vitamin B has been studied for the treatment of BMS, but has also been used to treat other causes of burning mouth symptoms, such as vitamin deficiency. Different types of Vitamin B have been studied, including B1, B2, and B6. In one open-label, non-randomized study of 55 patients with BMS (with and without vitamin deficiency), 86% (24/28) of vitamin-deficient participants were asymptomatic at three months; however, only 7% (2/27) of non-deficient participants exhibited improvement [44]. In addition, A double-blind, non-randomized study of 16 patients found no benefit of Vitamin B (Vitamin B1, B2, and B6) VS placebo after four months of treatment [45]. Unfortunately, the study did not control for vitamin deficiency status. Based on available evidence, treatment with Vitamin B should be considered only if the patients are established to be vitamin deficient.
Gabapentin
One double-blind study assessed the use of gabapentin and ALA, alone and in combination, for the treatment of BMS pain. A total of 120 participants were randomized to one of four groups: gabapentin 300 mg daily, ALA 600 mg daily, the combination of both, or placebo [46]. Greatest efficacy (noted as positive changes or full recovery) was found with combination therapy (14/20 patients) relative to ALA (11/20 patients), gabapentin (10/20 patients), and placebo (9/60 patients). Adverse events were mild with no reported discontinuations. While these results provide some support for the combined treatment, the added benefits of the combined treatment were modest. Further, dose dependent adverse effects (e.g., somnolence, dizziness) might limit the use of gabapentin. Sub-therapeutic dosing of gabapentin compared to studies assessing its utility in other chronic pain syndromes may have contributed to the modest effect size.
Capsaicin
Topical capsaicin works by desensitizing nociceptors and depleting stores of the neuropeptide, Substance P, thereby reducing neuropathic inflammation and potentially leading to a reduction in the oral burning sensation characteristic of BMS. A study utilizing oral capsaicin was shown to have statistically significant improvement in visual analog score (VAS) ratings, but with the complication of serious gastric pain in 32% of the participants by the end of 4 weeks [47]. This is not surprising considering the typical adverse effect of burning when initiating this therapy for topical use. While other capsaicin studies also have shown reductions in VAS scores for BMS patients, formulations varied among studies, making interpretation difficult [48,49]. Hence, capsaicin may be an effective agent in reducing symptoms of BMS but its use is limited by poor patient tolerability and a lack of information regarding the optimal dose.
H2-Receptor antagonist (Lafutidine)
Lafutidine is an H2-receptor antagonist that has a unique mechanism related to the treatment of BMS as compared to other drugs within the class. Lafutidine is thought to activate mucosal defensive mechanisms in the gastrointestinal tract by sensitizing capsaicin-sensitive afferent neurons. One randomized, controlled study has demonstrated benefit for lafutidine 10 mg twice daily in treating 74 patients with BMS [50]. The control group received other H2RAs twice daily, and both arms rinsed with an anti-inflammatory solution (azulene) four times daily. After 12 weeks of treatment, those treated with lafutidine reported lower pain intensity relative to comparators. Although the difference was not statistically significant, complete symptom resolution was observed in 29% of those treated with lafutidine vs. 13% in the control arm. Minor adverse effects not warranting discontinuation were noted with lafutidine, including mild nausea and abdominal distention. Unfortunately, lafutidine is not available in the United States and the observable differences seen in treatment groups may preclude assuming the utility of this agent is a class-effect of HSRAs in general.
Complementary alternative medicine
Various complementary alternative medicines (CAM) have been studied for treatment of BMS. Several compounds have been shown not to differ in efficacy from placebo. Generally, the alternative medicine studies lack strong evidence of clinical efficacy (e.g., comparisons to placebo or other controls) and/or are limited by unknown safety concerns. For these reasons, utilization of these as bona fide treatment options for BMS is not recommended. That said, a reasonable amount of CAM research on BMS has been conducted.
Alpha Lipoic acid
Although the mechanism of BMS is not well understood, as noted above, some believe a neuropathic component may be involved. Alpha lipoic acid (ALA) has been used to help treat diabetic neuropathies and is believed to have a neuroprotective effect as an antioxidant and free radical scavenger. It also increases levels of glutathione and lowers blood glucose levels although the clinical significance of these pharmacologic actions are unknown [51]. Accordingly, ALA has been studied as a treatment for BMS.
Evidence regarding the efficacy of ALA for treatment of BMS is conflicting, complicated in part by differing definitions of efficacy among studies. Several randomized, blinded, controlled trials have demonstrated positive efficacy of ALA at improving pain [46,51]. Further, several studies have shown adverse effects to be mild and primarily involving gastrointestinal complaints [46,52]. Other studies, however, have shown no benefit of ALA as compared to placebo [52–54]. Although more studies are needed to clarify the efficacy of ALA in the treatment of BMS, overall evidence suggests that ALA may be somewhat effective for treatment of BMS. Moreover, ALA is relatively safe, with few side effects or drug/disease interactions. ALA is inexpensive and available without a prescription, although lack of consistency in commercially available products may complicate accurate dosing.
Lycopene
Lycopene was examined for treatment of BMS in a double-blind, placebo controlled study [55]. Sixty BMS patients were randomized to either lycopene-enriched olive oil 300 ppm or placebo (water and dye) for 12 weeks. Lycopene was administered orally as 1.5 ml of spray three times daily. Results showed improvement in both groups in VAS pain scores and other BMS symptoms, and no adverse events were reported. No statistically significant differences, however, were found between groups. Similarly, a randomized, double-blind study of Hypericum perforatum extract 300 mg (n=21) vs. placebo (n=22) three times daily resulted in no statistical differences in VAS scores between groups at 12 weeks [56].
Catuama is an herbal product composed of four medicinal plant extracts: Paullinia cupana (guarana), Trichilia catigua (catuaba), Zingiber officinalis (ginger) and Ptychopetalum olacoides (muirapuama). Catuama twice daily was compared to placebo in a randomized, double-blind study in 72 patients with BMS [57]. Statistically significant reductions in pain severity ratings favoring the catuama-treated group were noted at 8 weeks and 12 weeks. Six participants (3 in each arm) withdrew due to exacerbation of symptoms. Adverse effects also were noted for catuama, including somnolence, weight gain and insomnia. While the long-term safety of this product is unknown, abrupt discontinuation of one of its components, guarana, can result in symptoms of withdrawal because of its caffeine content [58].
Other agents
Other agents, such as olanzapine, topiramate and pramipexole, have only been reported descriptively in the literature [59–62]. In one case report, topiramate was proposed to induce BMS. Other therapies such as sucralose and hormone replacement have been studied for secondary causes of burning mouth pain versus BMS itself [59].
Psychological therapies
Patients not responsive to dental or pharmacologic treatments may require multi-disciplinary care that includes psychotherapy. In addition, patients demonstrating symptom reduction but not total or adequate resolution may benefit from psychological intervention to supplement medical/dental treatment. Although cognitive behavioral therapies have been recommended as part of a multidisciplinary intervention for BMS patients, the empirical literature regarding the effectiveness of psychological interventions is limited to studies of group therapies [63,64]. For example, in an early study, BMS patients were randomized into a cognitive therapy group that met weekly for 12 weeks to 15 weeks or into an attention control group [65]. The treatment group demonstrated a statistically significant decrease in reported pain severity following treatment. That benefit was maintained at six-month follow-up. Similar results were obtained in a more recent study of a three-month course of group psychotherapy against treatment as usual [66]. A significantly higher percentage of the group therapy participants reported symptomatic improvement than did patients who participated in standard care (70% Vs. 40%). Finally, one study examined the effects of group therapy (two psychoanalytic sessions per week for two months) crossed with medical treatment (ALA) or placebo. Results at two months showed that all active treatments yielded symptom improvement relative to placebo, although the greatest improvement occurred in the ALA and group therapy condition [67].
While the above findings suggest benefit from group interventions, the mechanisms responsible for symptom improvement are not clear, especially as each of the above interventions differed in the content of the intervention from the others. Indeed, the issue is highlighted further by results derived from a very brief group intervention [68]. The latter intervention, described as group cognitive behavioral therapy, involved two 20-minute presentations (by a neurologist and a dentist) to small groups of BMS patients. The presentations provided basic information regarding BMS, pain physiology and stress, as well as brief training in relaxation; this was followed by an hour of open discussion among group participants. The session then was repeated at an interval of six months. Despite the brevity of the intervention, significant reductions in pain intensity and anxiety are described relative to symptoms reported by patients who received treatment as usual.
The results described above suggest that psychological interventions, even some that are quite basic, can be useful adjuncts to the treatment of BMS. These results also raise questions about how intensive such interventions should be, especially as benefits have been found for both brief and more extended interventions. As with the medical treatment modalities, the best approach could involve “stepped care”-a set of interventions that are scaffolded, such that less intensive/costly interventions are delivered initially (e.g., interventions with an educational focus) and are followed by more intensive interventions only for patients that do not benefit from the previous level of care [69]. With such a model, psychological distress that accrues over the extended course of the search for diagnosis and treatment would likely be responsive to a low-level intervention, while patients who bring pre-morbid psychosocial vulnerabilities to the clinic would likely require more intensive care [3].
Other Neuropathic/Idiopathic COFP Disorders
Trigeminal Neuralgia
Trigeminal neuralgia (TN) is an orofacial pain condition that is characterized by paroxysms of sharp, stabbing, and electric, shock-like pain that typically follows either the 2nd branch or the 3rd branch of the trigeminal nerve unilaterally to the cheek or chin. Usually episodes last several seconds, but can last for several minutes in duration. Patients often describe periods in which painful episodes are frequent for several weeks followed by pain-free intervals lasting months or years. The pain generally is of high severity and frequently triggered by stimulation of “trigger zones” that is typically of a light mechanical nature (e.g., brushing, talking, and shaving). Provocation can also be associated with other stimuli, such as bright lights or loud sounds.
Two categories of TN have been identified. Symptomatic TN is characterized by an identifiable structural lesion, such as nerve demyelination. Classic TN, while technically considered to be idiopathic, is thought to be related to vascular compression of the trigeminal nerve root at the root entry zone. This can result in focal demyelination and hyperexcitability of the afferent fiber. Hyperexcitability of the trigeminal brainstem complex also is suspected in TN, perhaps, as a consequence of repetitive “ignition” of the injured sensory nerve (i.e., central sensitization). If so, this could account for the effectiveness of pharmacotherapies that target the central nervous system (CNS).
TN is a relatively uncommon condition, with estimates of incidence that vary from a low of 4.3 new cases per 100,000 annually to rates as high as 27 per 100,000 [70,71]. Rates also vary significantly with age, with very few young adults experiencing TN and a much higher incidence in adults over 60 years of age, reaching rates as high as 80 per 100,000 annually among adults over 70 [71,72]. It is more frequently found in women than men, with recent studies estimating the relative rates at 2.3 to 1 [73].
Differential diagnosis of TN is generally clinical in nature although ruling out metabolic and endocrinologic etiologies is important. Imaging may be pursued in an effort to further classify the syndrome and rule out structural causes that may be alleviated by surgical correction. Although TN is almost always unilateral in nature, following the respective dermatome(s) of the nerve branches affected, bilateral distribution of symptoms has been described. Differentiating between TN and other facial neuralgias may be difficult, at best. Suspicion of active herpes zoster infection in and around the ophthalmologic orbit (herpes zoster ophthalmicus) should be specifically ruled out as a possible cause of symptoms and high suspicion of this disorder resulting in referral to a specialist [74].
Psychological factors in TN generally are viewed as “downstream” phenomena associated with the prolonged experience of severe, lancinating pain. Consistent with that view, research has shown that, relative to patients with lower pain severity, those with more severe TN pain describe more disability and more impaired health status [75]. Further, in a study that compared patients with chronic, atypical facial pain against those with both atypical facial pain and episodic TN, those with both atypical pain and TN reported significantly higher levels of pain severity, pain-related disability, anxiety, and depression [76].
Many non-pharmacologic, pharmacologic, complementary, and surgical interventions have been extensively studied for the treatment of pain due to TN and are beyond the scope of this manuscript [77]. In general, antidepressants and anticonvulsants are the mainstay of pharmacologic therapy with perhaps carbamazepine, gabapentin, pregabalin, tricyclic antidepressants, and duloxetine being the most widely studied [78]. Given the neurogenic nature of TN, it is not surprising that therapies that have shown efficacy for other neuropathic pain syndromes also show promise for TN. As with most chronic pain syndromes, multi-disciplinary treatment involving cognitive behavioral therapy also has shown promise.
Glossopharyngeal neuralgia
Glossopharyngeal neuralgia (GPN) symptoms are similar to those of TN: attacks of pain of a sharp, stabbing, shooting, or lancinating nature that typically is unilateral (although 25% of patients may have bilateral symptoms) and that typically lasts less than a minute. In addition to pain, some patients also experience some throat discomfort owing to the innervation of glossopharyngeal (cranial IX) nerve [71]. Following an attack, it is common for patients to experience a series of episodes of GPN-related discomfort at a frequency of 5 hour to 12 per hour; clusters of such attacks can occur for weeks or months [71,79]. Subsequent to a GPN cluster, however, many patients experience a long symptom-free period with up to 75% experiencing complete remission [80]. This type of symptom clustering makes systematic investigation of treatment modalities incredibly difficult.
There are two forms of GPN, secondary to the differences in the pain distribution associated with each form. Pharyngeal GPN usually is located in the pharynx, tonsil, soft palate, or posterior one-third of the tongue, with radiation to the ear and/or mandible possible. Tympanic GPN pain, however, is generally restricted solely to the ear for unclear reasons. Regardless of the form, GPN triggers generally are located in the tonsillar region and posterior pharynx, so that attacks can be activated by swallowing, chewing, talking, coughing, or yawning [79]. In the majority of cases, a thorough examination of the patient is unrevealing of pathology, other than the identification of trigger points [81].
GPN is an uncommon condition, with an incidence estimated around 0.7 cases/100,000 per year, substantially lower than that of TN. It occurs predominantly in adults, typically over the age of 50, and is twice as common in women as men [71]. Secondary to its rarity, it is difficult to diagnose, with the differential diagnosis most frequently between TN and GPN, although the referred mandibular pain also can be confused with temporomandibular disorders, a much more common group of pain syndromes [82].
No studies were found that address psychological assessment of patients with GPN, nor were studies found that addressed GPN-specific treatment. That said, given the similarities described above between TN and GPN, many of the same recommendations are likely to apply. In particular, it is likely that GPN patients who experience severe symptoms, especially those who experience GPN symptoms on a recurrent basis, are likely to demonstrate QOL effects similar to those of TN patients [75]. Those patients may benefit from complementary psychiatric (i.e., anti-depressant) or psychological treatment [83].
Similar to BMS, GPN lacks clear evidence-based or consensus diagnostic or treatment guidelines. Additionally, the lack of predictability in presence of symptoms makes designing well-controlled studies difficult. Several treatment modalities have been investigated and treatment options for GPN typically follow that of a plan for TN. As with the other COFP syndromes discussed already, a multi-disciplinary approach to treatment, including psychologic modalities, should be made available to patients.
Post-herpetic neuralgia
Acute herpes zoster eruptions are associated with reactivation of latent varicella virus infections, often occurring decades after the initial infection. Acute zoster infections are common in the general population, with the lifetime risk as high as 20% and increasing with age [84]. The eruptions typically follow a dermatomal pattern and occur in the thoracic region in up to 50% of patients, in the trigeminal region in 5% to 28% of cases, and in cervical nerves in approximately 20% of those diagnosed [85–88]. Approximately 10% of patients with acute herpes zoster will experience postherpetic neuralgia (PHN), defined as pain of at least moderate severity (>3/10 on a 0 to 10 scale) that persists for more than three (3) months after the herpes zoster rash has healed [84,89]. When PHN is defined differently (e.g., at a lower severity) and an older age group is the study target, the incidence of PHN can be as high as 73% [90].
Factors that predict the pain severity in acute herpes zoster have been well studied with several factors appearing as consistent predictors: older age, acute pain severity, and the presence of a prodrome [91]. In turn, several factors have been found to predict PHN pain severity: older age, acute pain severity, and previous or most recent rash severity [85,92,93]. In addition, functional limitations prior to an initial infection have also been found to predict PHN pain severity [85]. PHN pain has been described variously as deep, aching, burning, stabbing, itching, and electrical. It can be the product of several different mechanisms:
allodynia (an ordinarily non-painful stimulus is perceived as painful);
hyperalgesia (a somewhat painful stimulus is perceived as more painful than expected); and
dysesthesia (abnormal sensations are experienced in the absence of stimulation). Of these, the pain of allodynia is often described as most debilitating by PHN patients [94,95].
The pathophysiology of PHN is complex, involving a combination of peripheral and central mechanisms. Peripheral factors involve injury to nerves secondary to inflammation and the immune response to the varicella infection; the damage can involve both nociceptive (i.e., C-polymodal and αδ) and non-nociceptive fibers (i.e., αβ) [96]. In addition to the peripheral inputs, central sensitization also is believed to contribute to PHN pain, secondary to the deafferentation associated with peripheral nerve damage.
The differential diagnosis of PHN is based on a sound physical examination, and perhaps more importantly, a thorough history of symptoms and past medical problems. Pain and dysesthesia may precede the appearance of rash in an acute zoster infection, which may make differentiation from PHN and the appropriate treatment approach less clear.
A substantial literature documents an association between the severity of PHN pain and a patient’s QOL: higher levels of pain severity are associated with depression and anxiety in high percentages of PHN patients [97–100]. Moreover, PHN pain is likely to cause such emotional responses: patients with acute herpes zoster whose pain resolves (typically after approximately one month) do not demonstrate the levels of emotional distress exhibited by patients whose PHN pain persists [94]. PHN pain, however, can impact other QOL domains, as well, including disability/role functioning and sleep [97,99,100]. There also is some evidence that patients who cope poorly with the pain of acute infection may be at greater risk of developing PHN: patients who catastrophized at higher levels at baseline were likely to report higher levels of pain severity when assessed 8 weeks later and may have been more vulnerable to developing depression over the course of the study [101].
Much research has focused on the early implementation of pharmacologic modalities during an acute zoster outbreak (e.g., corticosteroids, anti-virals, and anticonvulsants) to reduce the later appearance of PHN symptoms [102,103]. Numerous non-pharmacologic and pharmacologic treatments have been studied for the treatment of pain associated with PHN. These treatment options generally follow similar strategies to that of other common neuropathic pain disorders [104]. Of those investigated various antidepressants, namely tricyclic antidepressants (e.g., amitriptyline or nortriptyline) and anticonvulsants. Topical lidocaine is FDA approved for PHN, although studies supporting its use are small with modest treatment effect [105,106].
Atypical Odontalgia
Atypical odontalgia (AO) involves “a severe throbbing in the tooth without major pathology” [15,107]. Because AO is idiopathic, its diagnosis can be challenging and typically is a diagnosis of exclusion. It is common, however, for AO to begin following a dental procedure, such as a tooth extraction or pulp extirpation [81] It is most often found in women in their mid-forties, although it can affect adults of any age [107] While the mechanisms of AO remain unclear, it is increasingly thought that it is occasioned by several events or risk factors that include local inflammation, infection, mechanical irritation, and minor nerve injury.
No evidence-based diagnostic guidelines exist for AO which makes the systematic evaluation of treatment modalities difficult. Unfortunately, therapies typically effective for other orofacial pain syndromes have been largely disappointing in studies of AO [108].
Eagle’s syndrome
Eagle’s syndrome is characterized by a constellation of features that may include recurrent throat pain, foreign body sensations in the pharynx, dysphagia, ear pain, and neck pain [109]. These symptoms can be exacerbated by head rotation, swallowing, chewing, and speaking. Rarely, Eagle’s syndrome can be associated with “yawning headache” [110]. The symptoms are related to elongation of the styloid process, normally 2.5 cm to 3 cm in length, to a length exceeding 3 cm. At this length, a range of neurovascular structures near the tip of the styloid process can be stimulated. Interestingly, elongated styloid processes are not uncommon: it is estimated that 4% of the population will exhibit elongation, although it is symptomatic in only a small percentage of that group [111]. In the latter group, it is thought that ossification and/or scar tissue formation may be responsible for symptom onset, sometimes following tonsillectomy [112].
Two forms of the syndrome have been identified: classic type and carotid artery type. The classic type is associated with stimulation of the trigeminal, facial, glossopharyngeal, and vagus nerves, while the carotid artery type is linked to styloid irritation of the carotid nerve plexus [113]. Symptoms associated with Eagle’s syndrome can be similar to those of GPN, although those of the former syndrome tend to be more dull and persistent, while those of the latter can be more sharp and episodic. Eagle’s syndrome symptoms also have been misdiagnosed as TN and as a temporomandibular disorder [109,112].
For patients with moderate symptoms, medical management is advocated, although no clear preference has emerged for optimal pharmacologic benefit. A range of medications has been tried that include non-steroidal anti-inflammatory medications, anticonvulsants, and anti-depressants, all of which have yielded satisfactory results [114]. Similarly, transpharyngeal infiltration with steroids or analgesics has proved effective. Physical therapy approaches also have been tried, including manual therapy and exercise, although with mixed results [115].
For patients with severe symptoms, surgical interventions have proved effective. Two approaches are used, an extra- and an intra-oral [112]. There is some disagreement as to which is preferred as both have demonstrated good success [109,112]. While the intra-oral approach is somewhat less invasive, complications such as poor visualization of the surgical field, injury of local structures, and infection have caused the current preference toward extra-oral approaches secondary to better visualization and a reduced risk of infection [116].
Headache Disorders
As noted previously, headache disorders, whether of musculoskeletal (e.g., tension-type) or neurovascular (e.g., migraine) origin, differ in their clinical presentations from the disorders of primary interest in this manuscript and, consequently, are not a major focus. That said, headache disorders are common and are considered by some to be another form of COFP [2]. Further, in some cases, headache disorders can mimic neuropathic/idiopathic COFP symptoms (e.g., some temporomandibular disorders [TMD]) and, more commonly, can complicate treatment as co-existing conditions. For these reasons, headache disorders warrant mention here. More attention is paid to TMD than to other headache conditions (migraine, cluster, tension type) as its symptoms can mimic neuropathic/idiopathic disorders in some patients.
Temporomandibular disorders
Unlike BMS, TMD is a positive diagnosis (i.e., it can be diagnosed when an identifiable set of physical observations and symptoms are found), rather than a diagnosis of exclusion. Characteristic TMD symptoms include pain on palpation of the masticatory musculature, pain on jaw opening, restricted jaw opening, and clicking or crepitus on jaw opening. Patients commonly report pain that is more widespread than just the masticatory musculature and are likely to report headaches and pain elsewhere, even low back pain [117]. Patients with TMD also demonstrate a number of parafunctional oral behaviors that can support clinical examination findings [118]. In fact, patients that exhibit high levels of parafunctional activity are 17 times more likely to have a TMD disorder than patients with low levels of such activity [117]. Finally, the medical history reported by TMD patients also can support a diagnosis: they are more likely than non-TMD patients to describe an external injury to the jaw, a jaw injury due to yawning, or a jaw injury associated with prolonged opening.
Also unlike BMS, TMD is quite prevalent. The 2002 National Health Interview Survey suggests that approximately 4.6% of U.S. adults (6.3% for women; 1.8% for men) reported symptoms consistent with TMD [119]. Moreover, it has been estimated that as much as 75% of the population has at least one of three TMD signs: joint noise, deviation on opening, or episodic locking [120]. As a consequence of its prevalence, TMD has been the target of a large project, the Orofacial Pain: Prospective Evaluation and Risk Assessment (OPPERA) study [121]. Because this project followed a large sample of asymptomatic individuals to the point where a substantial number experienced a first instance of TMD, prospective OPPERA studies have examined medical and psychological factors associated with the onset of the condition, providing predictive data not available for the idiopathic/neuropathic disorders considered previously.
The strongest predictors of TMD onset, based on the OPPERA study, are psychological. Increased risk of TMD onset was associated with higher baseline levels of reported somatic symptoms, psychosocial stress, and affective distress [121,122]. Further analysis of the data suggested that a general predisposing factor underlay the above predictors: global psychological and somatic symptom levels appear to represent a risk factor associated with the development of TMD symptoms. Inasmuch as the latter findings also overlap with risk factors associated with the likelihood that TMD symptoms will become chronic, TMD appears to represent a condition with a strong biopsychosocial profile, a profile that suggests the potential importance of psychological interventions integrated into a multidisciplinary treatment plan [123].
The diagnosis of TMD has been outlined in multiple guidelines [124,125]. As with other COFP disorders, a comprehensive history and physical examination is paramount. Non-pharmacologic and pharmacologic treatments have been extensively studied. In general, patient education is the cornerstone of any treatment plan for TMD. This includes trigger avoidance, disorder pathogenesis, positioning, and parafunctional habit discontinuation [126]. Splinting and various physical therapy approaches also have been investigated with variable evidence for efficacy [127]. Pharmacologic approaches are generally limited by the lack of well-controlled studies assessing effectiveness of a given agent in the chronic setting. Acute treatment may include nonsteroidal anti-inflammatory drugs (NSAIDs), skeletal muscle relaxants (SMRs), or short term night-time benzodiazepines [128]. The tricyclic antidepressant, amitriptyline, may be useful in the symptomatic treatment of TMD, although large randomized, placebo-controlled studies are not available to support that recommendation [129].
Tension-type headache
Tension-type headache (TTH) is one of the three most prevalent primary headache disorders (others are migraine and cluster headache), which together represent the most common reason that patients seek neurologic consultation [79]. Of the three, TTH is the most common, estimated to affect up to 46% of adults annually worldwide [130]. It is somewhat more common among women than men, by a ratio of approximately 5:4 [130]. There are three forms of TTH:
Infrequent episodic, <1 day/month;
Frequent episodic, >1 day/month but <15 days/month; and
Chronic, >15 days/month.
Typically, patients with TTH present with complaints of mild to moderate pain (described as “tightness” or “pressure”) in a band-like pattern extending bilaterally from the cervical to the frontalis region. On physical examination, patients will demonstrate no neurologic findings, although palpation generally (but not always) reveals pericranial and/or cervical muscle tenderness. TTH patients also demonstrate trigger points, hyper-irritable nodes with bands of skeletal muscle fibers that can produce radiating pain, often in a pericranial distribution, when palpated [131]. Many patients with TTH (88%) also present with co-existing neck pain with associated myofascial tenderness in the cervical region [132].
The two most common TTH precipitants include stress/mental tension and fatigue/poor sleep, although precipitants also can include weather changes, poor health, long work hours, and frequent traveling [133]. These precipitants and recent research suggest that myofascial sensitivity is likely to be the most common pathway to episodic tension-type headache [134]. With chronic TTH, central sensitization mechanisms are likely to be implicated [135]. Aside from central sensitization, there also is a link between psychological distress and TTH, especially chronic TTH. Among people with chronic TTH seen at specialty clinics, rates of both mood (~ 30%) and anxiety (~ 48%) disorders are significantly higher than rates found in the general population, and chronic TTH patients with psychiatric comorbidities also exhibit higher levels of disability [136].
Multiple treatment approaches can benefit patients with episodic TTH, including pharmacotherapy, physiotherapy, psychological therapies, and other therapies as needed (e.g., nutrition and dietetics), alone and in combination. The choice of treatments depends upon the factors contributing to the headache. Generally, preventive pharmacotherapy has been the first line of treatment for episodic TTH, except when a more aggressive approach is needed to interrupt a headache cycle. Physical therapy for TTH is also considered a first-line approach, especially in patients that demonstrate trigger points and sensitivity to palpation of pericranial and cervical musculature or postural deviations [137]. Inasmuch as lifestyle/behavioral factors can precipitate and maintain episodes of TTH, it is not surprising that a range of psychological interventions has been employed in TTH treatment, including relaxation training, biofeedback (BFB) training, and cognitive behavioral therapy, all of which have demonstrated good treatment effectiveness [138].
Migraine
There are two recognized forms of migraine: migraine with aura (classic) and migraine without aura (common). Common migraine is approximately twice as prevalent as classic migraine. Each form of migraine also can be characterized by its frequency: episodic (EM) or chronic (CM). Diagnostic criteria for CM include three criteria:
15 or more headache days/month;
Of those headache days, at least 8 must meet criteria for migraine with or without aura or respond to migraine-specific treatment; and
headache duration must be at least 3 months [79].
Each form of migraine is characterized by similar features:
Unilateral pain that typically has a pulsating quality,
Moderate to severe levels of pain severity,
Symptoms that are aggravated by routine physical activity, and
Nausea, photophobia, or phonophobia.
For both types of migraine, many patients (~ 60%) experience a prodrome that precedes headache onset, although the features of the prodrome vary greatly (e.g., hyper-/hypo-activity, depression, food cravings, sensitivity to smells, stiff muscles). In migraine with aura, patients also experience visual, sensory, or motor symptoms just prior to headache onset. The most common aura is visual, involving scotoma that surrounds a blind spot. Next most common is sensory, with one-sided symptoms of numbness or paresthesia that affect an upper or lower limb.
The prevalence of migraine in the U.S. is high, affecting approximately 14% of adults annually, of which approximately 15% have chronic migraine [139,140]. CM patients are much more likely to experience severe impact from migraine (interference with work, school or social activities, severe pain, fatigue, frustration, difficulty concentrating) relative to EM patients (72.9% vs. 42.3%). Further, rates of depression (25.2%) and anxiety (23.6%) are significantly greater for the CM group compared to the EM group (rates were 10.0% and 8.5%, respectively). In fact, the odds of experiencing a major depressive disorder (MDD), anxiety disorder (AD), or combined MDD and AD in the past year are roughly twice as high in migraineurs than in persons without migraine [141,142]. Interestingly, the reverse relationship also applies for the odds of a migraine disorder among people with psychiatric disorders: among people with MDD or AD, the odds of experiencing migraine are significantly higher than are those for people without a psychiatric disorder. The bidirectional relationship, of course, raises questions about whether the disorders share a common neurophysiology.
The physiologic mechanisms of migraine remain poorly understood. Current theories emphasize central nervous system activity:
Cortical spreading depression that activates the trigeminovascular system, triggering a wave of cortical, meningeal, and vascular events that produce a migraine attack, and/or
Central sensitization of trigeminal nociceptors, associated with inflammatory mediators, growth factors, and neuropeptides [143,144].
Given the lack of a clear understanding of migraine pathophysiology, it is not surprising that a range of pharmacological and multidisciplinary approaches have been used. As it is beyond the scope of this review to detail the various pharmacological therapies that have proved effective for acute treatment and optimal care, the interested reader is directed to Canadian practice guidelines developed recently [145]. Similarly, for information regarding effective multidisciplinary approaches to episodic and chronic migraine, the interested reader is directed to research that has examined the effects of medical and behavioral treatment, alone and in combination [146].
Cluster headache
Cluster headache (CH) is typified by recurrent episodes of unilateral pain, usually involving the orbital or periorbital region that is innervated by the ophthalmic division of the trigeminal nerve. Common descriptions of CH pain are sharp, piercing, burning, and pulsating. Onset tends to be rapid, with an attack increasing from discomfort to excruciating pain in a span of minutes, and the duration of excruciating pain lasting until the headache ends, often quite suddenly [147]. Aside from severe unilateral headache, over 80% of CH patients present with same-sided unilateral lacrimation, nasal congestion, and/or rhinorrhea, and over 40% describe photophobia or phonophobia [148]. Patients often demonstrate agitation and restlessness during an attack, such as pacing, rocking back and forth, or banging a head against a wall [149]. Cluster headaches are not common, as epidemiologic studies estimate a lifetime prevalence of 0.12%, and are more common in men than women with a ratio of 2.5:1 [150,151].
The term, cluster headache, derives from the clinical presentation of frequent headache attacks that cluster together into bouts. To qualify as CH, headaches should occur at least five times per bout; each bout can last several weeks. Often, patients experience far more than five headaches in a cluster [152]. Most patients present with one bout per year with a mean duration of about two months per bout [147]. Almost three out of every four patients report recurrent attacks during the night that awaken them from sleep [152].
CH is considered to be one of a group of trigeminal autonomic cephalalgias [79]. Both peripheral and central factors are thought to play a part. Peripheral involvement is associated with trigeminovascular innervation of the ophthalmic and middle cerebral arteries, both of which have demonstrated local vasodilatation during attacks [153]. Central factors are implicated by features that suggest that the attacks may be occasioned by serotonergic dysfunction and/or hypothalamic discharge [143,154].
Not surprisingly, rates of depression and anxiety disorders are high in CH patients: one study reported that 43.0% reported clinically significant levels of depression, and 75.7% reported clinically significant anxiety [155]. Unlike migraine, where a bidirectional relationship appears to exist between headache and psychological distress, the relationship in CH appears to go in only one direction-CH appears to occasion psychological distress [156,157].
Despite the unique characteristics of CH that should aid differential diagnosis when a careful history is taken, the data consistently show a substantial lag between the onset of CH symptoms and its accurate diagnosis that can exceed 5 years [148]. Common misdiagnoses included migraine, trigeminal neuralgia, and sinusitis [158]. For patients who are correctly diagnosed, all guidelines point to the importance of patient education in order to diminish the emotional anguish that can be associated with recurrent bouts [148,158]. Several trials have shown benefit from administration of high-dose, high-flow-rate, and normobaric oxygen therapies, and others show benefit from intranasal and subcutaneous sumatriptan and oral and intranasal zolmitriptan [159,160]. Sumatriptan in an oral form also has been used for long-term reduction of CH attacks, although verapamil has emerged as the drug of choice for preventive care [160].
Summary and Recommendations
While BMS and other idiopathic and neuropathic COFP disorders may not singly be common in the general population, collectively, their prevalence supports the need for a general understanding by the generalist health provider and a thorough understanding by the neurologist or oral health professional. These disorders should be considered especially carefully for older adults, as their prevalence escalates with advancing age. Further, several of the disorders described previously are diagnoses of exclusion, requiring a careful history and physical examination, secondary to the lack of definitive diagnostic tests that can be adduced to reach a high level of confidence (“probable” or “possible” are most likely). Because of the diverse symptom profiles that these disorders can present, patients with idiopathic/neuropathic COFP are likely to appear for treatment to a broad range of providers.
Acknowledgments
The preparation of this manuscript was supported by a grant from the National Institute on Drug Abuse to the Southern Illinois University – Edwardsville and Saint Louis University Center of Excellence in Pain Education (Contract No. HHSN271201500056C).
References
- 1.Setty S, David J. Classification and epidemiology of orofacial pain. In: Vadivelu N, Vadivelu A, Kay AD, editors. Orofacial Pain: A Clinician’s Guide. Swtizerland: Springer; 2014. pp. 15–24. [Google Scholar]
- 2.Balasubramaniam R, Klasser GD, Delcanho R. Separating oral burning from burning mouth syndrome: unravelling a diagnostic enigma. Aust Dent J. 2009;54:293–299. doi: 10.1111/j.1834-7819.2009.01153.x. [DOI] [PubMed] [Google Scholar]
- 3.Sarlani E, Balciunas BA, Grace EG. Orofacial pain-Part I: Assessment and management of musculoskeletal and neuropathic causes. AACN Clin Issues. 2005;16:333–346. doi: 10.1097/00044067-200507000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Bonathan CJ, Zakrzewska JM, Love J, Williams AC. Beliefs and distress about orofacial pain: patient journey through a specialist pain consultation. J Oral Facial Pain Headache. 2014;28:223–232. doi: 10.11607/ofph.1184. [DOI] [PubMed] [Google Scholar]
- 5.Haanpaa M, Attal N, Backonja M, Baron R, Bennett M, et al. NeuPSIG guidelines on neuropathic pain assessment. Pain. 2011;152:14–27. doi: 10.1016/j.pain.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 6.Descriptions of chronic pain syndromes and definitions of pain terms. International Association for the Study of Pain, Subcommittee on Taxonomy. 1986;3:S1–S226. [PubMed] [Google Scholar]
- 7.Mantyselka P, Kumpusalo E, Ahonen R, Kumpusalo A, Kauhanen J, et al. Pain as a reason to visit the doctor: a study in Finnish primary health care. Pain. 2001;89:175–180. doi: 10.1016/s0304-3959(00)00361-4. [DOI] [PubMed] [Google Scholar]
- 8.Treede RD, Jensen TS, Campbell JN, Cruccu G, Dostrovsky JO, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70:1630–1635. doi: 10.1212/01.wnl.0000282763.29778.59. [DOI] [PubMed] [Google Scholar]
- 9.Geber C, Baumgartner U, Schwab R, et al. Revised definition of neuropathic pain and its grading system: an open case series illustrating its use in clinical practice. Am J Med. 2009;122:S3–S12. doi: 10.1016/j.amjmed.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Lamey PJ. Burning mouth syndrome. Dermatol Clin. 1996;14:339–354. doi: 10.1016/s0733-8635(05)70361-2. [DOI] [PubMed] [Google Scholar]
- 11.Tammiala-Salonen T, Hiidenkari T, Parvinen T. Burning mouth in a finnish adult population. Community Dent Oral Epidemiol. 1993;21:67–71. doi: 10.1111/j.1600-0528.1993.tb00723.x. [DOI] [PubMed] [Google Scholar]
- 12.Lipton JA, Ship JA, Larach-Robinson D. Estimated prevalence and distribution of reported orofacial pain in the United States. J Am Dent Assoc. 1993;124:115–121. doi: 10.14219/jada.archive.1993.0200. [DOI] [PubMed] [Google Scholar]
- 13.Gurvits GE, Tan A. Burning mouth syndrome. World J Gastroenterol. 2013;19:665–672. doi: 10.3748/wjg.v19.i5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ducasse D, Courtet P, Olie E. Burning mouth syndrome: current clinical, physiopathologic, and therapeutic data. Reg Anesth Pain Med. 2013;38:380–390. doi: 10.1097/AAP.0b013e3182a1f0db. [DOI] [PubMed] [Google Scholar]
- 15.Woda A, Dao T, Gremeau-Richard C. Steroid dysregulation and stomatodynia (burning mouth syndrome) J Orofac Pain. 2009;23:202–210. [PubMed] [Google Scholar]
- 16.Jaaskelainen SK. Pathophysiology of primary burning mouth syndrome. Clin Neurophysiol. 2012;123:71–77. doi: 10.1016/j.clinph.2011.07.054. [DOI] [PubMed] [Google Scholar]
- 17.Bogetto F, Maina G, Ferro G, Carbone M, Gandolfo S. Psychiatric comorbidity in patients with burning mouth syndrome. Psychosom Med. 1998;60:378–385. doi: 10.1097/00006842-199805000-00028. [DOI] [PubMed] [Google Scholar]
- 18.Lamey PJ, Lewis MA. Oral medicine in practice: burning mouth syndrome. Br Dent J. 1989;167:197–200. doi: 10.1038/sj.bdj.4806969. [DOI] [PubMed] [Google Scholar]
- 19.Rojo L, Silvestre FJ, Bagan JV, De Vicente T. Psychiatric morbidity in burning mouth syndrome. Psychiatric interview versus depression and anxiety scales. Oral Surg Oral Med Oral Pathol. 1993;75:308–311. doi: 10.1016/0030-4220(93)90142-q. [DOI] [PubMed] [Google Scholar]
- 20.Gore M, Sadosky A, Stacey BR, Tai KS, Leslie D. The burden of chronic low back pain: clinical comorbidities, treatment patterns, and health care costs in usual care settings. Spine (Phila Pa 1976) 2012;37:E668–E677. doi: 10.1097/BRS.0b013e318241e5de. [DOI] [PubMed] [Google Scholar]
- 21.Salazar A, Duenas M, Mico JA, Ojeda B, Agüera-Ortiz L, et al. Undiagnosed mood disorders and sleep disturbances in primary care patients with chronic musculoskeletal pain. Pain Med. 2013;14:1416–1425. doi: 10.1111/pme.12165. [DOI] [PubMed] [Google Scholar]
- 22.Dersh J, Gatchel RJ, Mayer T, Polatin P, Temple OR. Prevalence of psychiatric disorders in patients with chronic disabling occupational spinal disorders. Spine (Phila Pa 1976) 2006;31:1156–1162. doi: 10.1097/01.brs.0000216441.83135.6f. [DOI] [PubMed] [Google Scholar]
- 23.de Souza FT, Teixeira AL, Amaral TM, dos Santos TP, Abreu MH, et al. Psychiatric disorders in burning mouth syndrome. J Psychosom Res. 2012;72:142–146. doi: 10.1016/j.jpsychores.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Lamey PJ, Lamb AB. Prospective study of aetiological factors in burning mouth syndrome. Br Med J (Clin Res Ed) 1988;296:1243–1246. doi: 10.1136/bmj.296.6631.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Von Korff M, Tiemens B. Individualized stepped care of chronic illness. West J Med. 2000;172:133–137. doi: 10.1136/ewjm.172.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zakrzewska JM, Forssell H, Glenny AM. Interventions for the treatment of burning mouth syndrome. Cochrane Database Syst Rev. 2005;1:CD002779. doi: 10.1002/14651858.CD002779.pub2. [DOI] [PubMed] [Google Scholar]
- 27.Cui Y, Xu H, Chen FM, Liu JL, Jiang L, et al. Efficacy evaluation of clonazepam for symptom remission in burning mouth syndrome: a meta-analysis. Oral Dis. 2016;22:503–511. doi: 10.1111/odi.12422. [DOI] [PubMed] [Google Scholar]
- 28.Ko JY, Kim MJ, Lee SG, Kho HS. Outcome predictors affecting the efficacy of clonazepam therapy for the management of burning mouth syndrome (BMS) Arch Gerontol Geriatr. 2012;55:755–761. doi: 10.1016/j.archger.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Grushka M, Epstein J, Mott A. An open-label, dose escalation pilot study of the effect of clonazepam in burning mouth syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;86:557–561. doi: 10.1016/s1079-2104(98)90345-6. [DOI] [PubMed] [Google Scholar]
- 30.de Castro LA, Ribeiro-Rotta RF. The effect of clonazepam mouthwash on the symptomatology of burning mouth syndrome: an open pilot study. Pain Med. 2014;15:2164–2165. doi: 10.1111/pme.12552. [DOI] [PubMed] [Google Scholar]
- 31.Heckmann SM, Kirchner E, Grushka M, Wichmann MG, Hummel T. A double-blind study on clonazepam in patients with burning mouth syndrome. Laryngoscope. 2012;122:813–816. doi: 10.1002/lary.22490. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez de Rivera Campillo E, Lopez-Lopez J, Chimenos-Kustner E. Response to topical clonazepam in patients with burning mouth syndrome: a clinical study. Bull Group Int Rech Sci Stomatol Odontol. 2010;49:19–29. [PubMed] [Google Scholar]
- 33.Amos K, Yeoh SC, Farah CS. Combined topical and systemic clonazepam therapy for the management of burning mouth syndrome: a retrospective pilot study. J Orofac Pain. 2011;25:125–130. [PubMed] [Google Scholar]
- 34.Jurisic Kvesic A, Zavoreo I, Basic Kes V, Vucicevic Boras V, Ciliga D, et al. The effectiveness of acupuncture versus clonazepam in patients with burning mouth syndrome. Acupunct Med. 2015;33:289–292. doi: 10.1136/acupmed-2015-010759. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez-de Rivera-Campillo E, Lopez-Lopez J. Evaluation of the response to treatment and clinical evolution in patients with burning mouth syndrome. Med Oral Patol Oral Cir Bucal. 2013;18:e403–e410. doi: 10.4317/medoral.18142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gremeau-Richard C, Woda A, Navez ML, Navel ML, Attan L, et al. Topical clonazepam in stomatodynia: a randomised placebo-controlled study. Pain. 2004;108:51–57. doi: 10.1016/j.pain.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 37.Tammiala-Salonen T, Forssell H. Trazodone in burning mouth pain: a placebo-controlled, double-blind study. J Orofac Pain. 1999;13:83–88. [PubMed] [Google Scholar]
- 38.Yamazaki Y, Hata H, Kitamori S, Onodera M, Kitagawa Y. An open-label, noncomparative, dose escalation pilot study of the effect of paroxetine in treatment of burning mouth syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:e6–e11. doi: 10.1016/j.tripleo.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 39.Maina G, Vitalucci A, Gandolfo S, Bogetto F. Comparative efficacy of SSRIs and amisulpride in burning mouth syndrome: a single-blind study. J Clin Psychiatry. 2002;63:38–43. doi: 10.4088/jcp.v63n0108. [DOI] [PubMed] [Google Scholar]
- 40.Milnacipran. U.S. National Library of Medicine. 2009. [Google Scholar]
- 41.Kato Y, Sato T, Katagiri A, Umezaki Y, Takenoshita M, et al. Milnacipran dose-effect study in patients with burning mouth syndrome. Clin Neuropharmacol. 2011;34:166–169. doi: 10.1097/WNF.0b013e318227f100. [DOI] [PubMed] [Google Scholar]
- 42.Sugimoto K. The dubious effect of milnacipran for the treatment of burning mouth syndrome. Clin Neuropharmacol. 2011;34:170–173. doi: 10.1097/WNF.0b013e31822511c4. [DOI] [PubMed] [Google Scholar]
- 43.Ito M, Kimura H, Yoshida K, Kimura Y, Ozaki N, et al. Effectiveness of milnacipran for the treatment of chronic pain in the orofacial region. Clin Neuropharmacol. 2010;33:79–83. doi: 10.1097/WNF.0b013e3181cb5793. [DOI] [PubMed] [Google Scholar]
- 44.Lamey PJ, Hammond A, Allam BF, McIntosh WB. Vitamin status of patients with burning mouth syndrome and the response to replacement therapy. Br Dent J. 1986;160:81–84. doi: 10.1038/sj.bdj.4805773. [DOI] [PubMed] [Google Scholar]
- 45.Hugoson A, Thorstensson B. Vitamin B status and response to replacement therapy in patients with burning mouth syndrome. Acta Odontol Scand. 1991;49:367–375. doi: 10.3109/00016359109005933. [DOI] [PubMed] [Google Scholar]
- 46.Lopez-D’alessandro E, Escovich L. Combination of alpha lipoic acid and gabapentin, its efficacy in the treatment of Burning Mouth Syndrome: a randomized, double-blind, placebo controlled trial. Med Oral Patol Oral Cir Bucal. 2011;16:e635–e640. doi: 10.4317/medoral.16942. [DOI] [PubMed] [Google Scholar]
- 47.Petruzzi M, Lauritano D, De Benedittis M, Baldoni M, Serpico R. Systemic capsaicin for burning mouth syndrome: short-term results of a pilot study. J Oral Pathol Med. 2004;33:111–114. doi: 10.1111/j.1600-0714.2004.0194n.x. [DOI] [PubMed] [Google Scholar]
- 48.Epstein JB, Marcoe JH. Topical application of capsaicin for treatment of oral neuropathic pain and trigeminal neuralgia. Oral Surg Oral Med Oral Pathol. 1994;77:135–140. doi: 10.1016/0030-4220(94)90275-5. [DOI] [PubMed] [Google Scholar]
- 49.Silvestre FJ, Silvestre-Rangil J, Tamarit-Santafe C, Bautista D. Application of a capsaicin rinse in the treatment of burning mouth syndrome. Med Oral Patol Oral Cir Bucal. 2012;17:e1–e4. doi: 10.4317/medoral.17219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Toida M, Kato K, Makita H, et al. Palliative effect of lafutidine on oral burning sensation. J Oral Pathol Med. 2009;38:262–268. doi: 10.1111/j.1600-0714.2008.00736.x. [DOI] [PubMed] [Google Scholar]
- 51.Femiano F, Gombos F, Scully C, Busciolano M, De Luca P. Burning mouth syndrome (BMS): controlled open trial of the efficacy of alpha-lipoic acid (thioctic acid) on symptomatology. Oral Dis. 2000;6:274–277. doi: 10.1111/j.1601-0825.2000.tb00138.x. [DOI] [PubMed] [Google Scholar]
- 52.Cavalcanti DR, da Silveira FR. Alpha lipoic acid in burning mouth syndrome–a randomized double-blind placebo-controlled trial. J Oral Pathol Med. 2009;38:254–261. doi: 10.1111/j.1600-0714.2008.00735.x. [DOI] [PubMed] [Google Scholar]
- 53.Carbone M, Pentenero M, Carrozzo M, Ippolito A, Gandolfo S. Lack of efficacy of alpha-lipoic acid in burning mouth syndrome: a double-blind, randomized, placebo-controlled study. Eur J Pain. 2009;13:492–496. doi: 10.1016/j.ejpain.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 54.Lopez-Jornet P, Camacho-Alonso F, Leon-Espinosa S. Efficacy of alpha lipoic acid in burning mouth syndrome: a randomized, placebo-treatment study. J Oral Rehabil. 2009;36:52–57. doi: 10.1111/j.1365-2842.2008.01914.x. [DOI] [PubMed] [Google Scholar]
- 55.Cano-Carrillo P, Pons-Fuster A, Lopez-Jornet P. Efficacy of lycopene-enriched virgin olive oil for treating burning mouth syndrome: a double-blind randomised. J Oral Rehabil. 2014;41:296–305. doi: 10.1111/joor.12147. [DOI] [PubMed] [Google Scholar]
- 56.Sardella A, Lodi G, Demarosi F, Tarozzi M, Canegallo L, et al. Hypericum perforatum extract in burning mouth syndrome: a randomized placebo-controlled study. J Oral Pathol Med. 2008;37:395–401. doi: 10.1111/j.1600-0714.2008.00663.x. [DOI] [PubMed] [Google Scholar]
- 57.Spanemberg JC, Cherubini K, de Figueiredo MA, Gomes AP, Campos MM, et al. Effect of an herbal compound for treatment of burning mouth syndrome: randomized, controlled, double-blind clinical trial. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113:373–377. doi: 10.1016/j.oooo.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 58.An evidence-based systematic review of guarana. Natural Medicines 2016 [Google Scholar]
- 59.Friedman DI. Topirimate-induced burning mouth syndrome. Headache. 2010;50:1383–1385. doi: 10.1111/j.1526-4610.2010.01720.x. [DOI] [PubMed] [Google Scholar]
- 60.Siniscalchi A, Gallelli L, Marigliano NM, Orlando P, De Sarro G. Use of topiramate for glossodynia. Pain Med. 2007;8:531–534. doi: 10.1111/j.1526-4637.2006.00152.x. [DOI] [PubMed] [Google Scholar]
- 61.Stuginski-Barbosa J, Rodrigues GG, Bigal ME, Speciali JG. Burning mouth syndrome responsive to pramipexol. J Headache Pain. 2008;9:43–45. doi: 10.1007/s10194-008-0003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ueda N, Kodama Y, Hori H, Umene W, Sugita A, et al. Two cases of burning mouth syndrome treated with olanzapine. Psychiatry Clin Neurosci. 2008;62:359–361. doi: 10.1111/j.1440-1819.2008.01806.x. [DOI] [PubMed] [Google Scholar]
- 63.Miziara I, Chagury A, Vargas C, Freitas L, Mahmoud A. Therapeutic options in idiopathic burning mouth syndrome: literature review. Int Arch Otorhinolaryngol. 2015;19:86–89. doi: 10.1055/s-0034-1378138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Patton LL, Siegel MA, Benoliel R, De Laat A. Management of burning mouth syndrome: systematic review and management recommendations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103(Suppl):S39 e1–e13. doi: 10.1016/j.tripleo.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 65.Bergdahl J, Anneroth G, Perris H. Cognitive therapy in the treatment of patients with resistant burning mouth syndrome: a controlled study. J Oral Pathol Med. 1995;24:213–215. doi: 10.1111/j.1600-0714.1995.tb01169.x. [DOI] [PubMed] [Google Scholar]
- 66.Miziara ID, Filho BC, Oliveira R, Rodrigues dos Santos RM. Group psychotherapy: an additional approach to burning mouth syndrome. J Psychosom Res. 2009;67:443–448. doi: 10.1016/j.jpsychores.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 67.Femiano F, Gombos F, Scully C. Burning mouth syndrome: the efficacy of lipoic acid on subgroups. J Eur Acad Dermatol Venereol. 2004;18:676–678. doi: 10.1111/j.1468-3083.2004.01049.x. [DOI] [PubMed] [Google Scholar]
- 68.Komiyama O, Nishimura H, Makiyama Y, Iida T, Obara R, et al. Group cognitive-behavioral intervention for patients with burning mouth syndrome. J Oral Sci. 2013;55:17–22. doi: 10.2334/josnusd.55.17. [DOI] [PubMed] [Google Scholar]
- 69.Carlson CR. Psychological considerations for chronic orofacial pain. Oral Maxillofac Surg Clin North Am. 2008;20:185–195. doi: 10.1016/j.coms.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 70.Hall GC, Carroll D, Parry D, McQuay HJ. Epidemiology and treatment of neuropathic pain: the UK primary care perspective. Pain. 2006;122:156–162. doi: 10.1016/j.pain.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 71.Katusic S, Williams DB, Beard CM, Bergstralh E, Kurland LT. Incidence and clinical features of glossopharyngeal neuralgia, Rochester, Minnesota, 1945–1984. Neuroepidemiology. 1991;10:266–275. doi: 10.1159/000110283. [DOI] [PubMed] [Google Scholar]
- 72.Dieleman JP, Kerklaan J, Huygen FJ, Bouma PA, Sturkenboom MC. Incidence rates and treatment of neuropathic pain conditions in the general population. Pain. 2008;137:681–688. doi: 10.1016/j.pain.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 73.Koopman JS, Dieleman JP, Huygen FJ, de Mos M, Martin CG, et al. Incidence of facial pain in the general population. Pain. 2009;147:122–127. doi: 10.1016/j.pain.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 74.Johnson JL, Amzat R, Martin N. Herpes zoster ophthalmicus. Prim Care. 2015;42:285–303. doi: 10.1016/j.pop.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 75.Tolle T, Dukes E, Sadosky A. Patient burden of trigeminal neuralgia: results from a cross-sectional survey of health state impairment and treatment patterns in six European countries. Pain Pract. 2006;6:153–160. doi: 10.1111/j.1533-2500.2006.00079.x. [DOI] [PubMed] [Google Scholar]
- 76.Macianskyte D, Januzis G, Kubilius R, Adomaitiene V, Sciupokas A. Associations between chronic pain and depressive symptoms in patients with trigeminal neuralgia. Medicina (Kaunas) 2011;47:386–392. [PubMed] [Google Scholar]
- 77.Zakrzewska JM, Linskey ME. Trigeminal Neuralgia. Am Fam Physician. 2016;94:133–135. [PubMed] [Google Scholar]
- 78.Chole R, Patil R, Degwekar SS, Bhowate RR. Drug treatment of trigeminal neuralgia: a systematic review of the literature. J Oral Maxillofac Surg. 2007;65:40–45. doi: 10.1016/j.joms.2005.12.063. [DOI] [PubMed] [Google Scholar]
- 79.The International Classification of Headache Disorders, 3rd edition (beta version) Headache Classification Committee of the International Headache S; 2013. [DOI] [PubMed] [Google Scholar]
- 80.Rushton JG, Stevens JC, Miller RH. Glossopharyngeal (vagoglossopharyngeal) neuralgia: a study of 217 cases. Arch Neurol. 1981;38:201–205. doi: 10.1001/archneur.1981.00510040027002. [DOI] [PubMed] [Google Scholar]
- 81.Koratkar H, Parashar V, Koratkar S. A review of neuropathic pain conditions affecting teeth. Gen Dent. 2010;58:436–441. [PubMed] [Google Scholar]
- 82.Benoliel R, Eliav E. Neuropathic orofacial pain. Oral Maxillofac Surg Clin North Am. 2008;20:237–254. doi: 10.1016/j.coms.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 83.Myers CD, White BA, Heft MW. A review of complementary and alternative medicine use for treating chronic facial pain. J Am Dent Assoc. 2002;133:1189–1196. doi: 10.14219/jada.archive.2002.0360. [DOI] [PubMed] [Google Scholar]
- 84.Ragozzino MW, Melton LJ, Kurland LT, Chu CP, Perry HO. Population-based study of herpes zoster and its sequelae. Medicine (Baltimore) 1982;61:310–316. doi: 10.1097/00005792-198209000-00003. [DOI] [PubMed] [Google Scholar]
- 85.Drolet M, Brisson M, Schmader K, Levin M, Johnson R, et al. Predictors of postherpetic neuralgia among patients with herpes zoster: a prospective study. J Pain. 2010;11:1211–1221. doi: 10.1016/j.jpain.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 86.Goh CL, Khoo L. A retrospective study of the clinical presentation and outcome of herpes zoster in a tertiary dermatology outpatient referral clinic. Int J Dermatol. 1997;36:667–672. doi: 10.1046/j.1365-4362.1997.00241.x. [DOI] [PubMed] [Google Scholar]
- 87.Haanpaa M, Laippala P, Nurmikko T. Pain and somatosensory dysfunction in acute herpes zoster. Clin J Pain. 1999;15:78–84. doi: 10.1097/00002508-199906000-00003. [DOI] [PubMed] [Google Scholar]
- 88.Lewis MA, Sankar V, De Laat A, Benoliel R. Management of neuropathic orofacial pain. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007:S32 e1–e24. doi: 10.1016/j.tripleo.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 89.Schmader KE. Epidemiology and impact on quality of life of postherpetic neuralgia and painful diabetic neuropathy. Clin J Pain. 2002;18:350–354. doi: 10.1097/00002508-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 90.Weaver BA. The burden of herpes zoster and postherpetic neuralgia in the United States. J Am Osteopath Assoc. 2007;107:S2–S7. [PubMed] [Google Scholar]
- 91.Dworkin RH, Nagasako EM, Johnson RW, Griffin DR. Acute pain in herpes zoster: the famciclovir database project. Pain. 2001;94:113–119. doi: 10.1016/S0304-3959(01)00347-5. [DOI] [PubMed] [Google Scholar]
- 92.Dworkin RH, Boon RJ, Griffin DR, Phung D. Postherpetic neuralgia: impact of famciclovir, age, rash severity, and acute pain in herpes zoster patients. J Infect Dis. 1998;178:S76–S80. doi: 10.1086/514260. [DOI] [PubMed] [Google Scholar]
- 93.Opstelten W, Zuithoff NP, van Essen GA, van Loon AM, van Wijck AJ, et al. Predicting postherpetic neuralgia in elderly primary care patients with herpes zoster: prospective prognostic study. Pain. 2007;132:S52–S59. doi: 10.1016/j.pain.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 94.Drolet M, Brisson M, Schmader KE, Levin MJ, Jhonson R, et al. The impact of herpes zoster and postherpetic neuralgia on health-related quality of life: a prospective study. CMAJ. 2010;182:1731–1736. doi: 10.1503/cmaj.091711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hupp WS, Firriolo FJ. Cranial neuralgias. Dent Clin North Am. 2013;57:481–495. doi: 10.1016/j.cden.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 96.Truini A, Galeotti F, Haanpaa M, Zucchi R, Albanesi A, et al. Pathophysiology of pain in postherpetic neuralgia: a clinical and neurophysiological study. Pain. 2008;140:405–410. doi: 10.1016/j.pain.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 97.Daniel HC, Narewska J, Serpell M, Hoggart B, Johnson R, et al. Comparison of psychological and physical function in neuropathic pain and nociceptive pain: implications for cognitive behavioral pain management programs. Eur J Pain. 2008;12:731–741. doi: 10.1016/j.ejpain.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 98.Jensen MP, Chodroff MJ, Dworkin RH. The impact of neuropathic pain on health-related quality of life: review and implications. Neurology. 2007;68:1178–1182. doi: 10.1212/01.wnl.0000259085.61898.9e. [DOI] [PubMed] [Google Scholar]
- 99.Laurent B, Chavent M, Cragnolini T, Dahl AC, Pasquali S, et al. Epock: rapid analysis of protein pocket dynamics. Bioinformatics. 2015;31:1478–1480. doi: 10.1093/bioinformatics/btu822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.van Seventer R, Sadosky A, Lucero M, Dukes E. A cross-sectional survey of health state impairment and treatment patterns in patients with postherpetic neuralgia. Age Ageing. 2006;35:132–137. doi: 10.1093/ageing/afj048. [DOI] [PubMed] [Google Scholar]
- 101.Haythornthwaite JA, Clark MR, Pappagallo M, Raja SN. Pain coping strategies play a role in the persistence of pain in post-herpetic neuralgia. Pain. 2003;106:453–460. doi: 10.1016/j.pain.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 102.Dworkin RH, Perkins FM, Nagasako EM. Prospects for the prevention of postherpetic neuralgia in herpes zoster patients. Clin J Pain. 2002;16:S90–S100. doi: 10.1097/00002508-200006001-00016. [DOI] [PubMed] [Google Scholar]
- 103.Tenser RB, Dworkin RH. Herpes zoster and the prevention of postherpetic neuralgia: beyond antiviral therapy. Neurology. 2005;65:349–350. doi: 10.1212/01.wnl.0000173198.07267.8f. [DOI] [PubMed] [Google Scholar]
- 104.Finnerup NB, Attal N, Haroutounian S, Mcnicol E, Baron R, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14:162–173. doi: 10.1016/S1474-4422(14)70251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gimbel J, Linn R, Hale M, Nicholson B. Lidocaine patch treatment in patients with low back pain: results of an open-label, nonrandomized pilot study. Am J Ther. 2005;12:311–319. doi: 10.1097/01.mjt.0000164828.57392.ba. [DOI] [PubMed] [Google Scholar]
- 106.Hashmi JA, Baliki MN, Huang L, Parks EL, Chanda ML, et al. Lidocaine patch (5%) is no more potent than placebo in treating chronic back pain when tested in a randomised double blind placebo controlled brain imaging study. Mol Pain. 2012;8:29. doi: 10.1186/1744-8069-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Woda A, Pionchon P. A unified concept of idiopathic orofacial pain: pathophysiologic features. J Orofac Pain. 2000;14:196–212. [PubMed] [Google Scholar]
- 108.Abiko Y, Matsuoka H, Chiba I, Toyofuku A. Current evidence on atypical odontalgia: diagnosis and clinical management. Int J Dent. 2012;2012:518548. doi: 10.1155/2012/518548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mayrink G, Figueiredo EP, Sato FR, Moreira RW. Cervicofacial pain associated with Eagle’s syndrome misdiagnosed as trigeminal neuralgia. Oral Maxillofac Surg. 2012;16:207–210. doi: 10.1007/s10006-011-0276-7. [DOI] [PubMed] [Google Scholar]
- 110.Jacome DE. Primary yawning headache. Cephalalgia. 2001;21:697–699. doi: 10.1046/j.1468-2982.2001.00223.x. [DOI] [PubMed] [Google Scholar]
- 111.Kim SM, Seo MH, Myoung H, Choi JY, Kim YS, et al. Osteogenetic changes in elongated styloid processes of Eagle syndrome patients. J Craniomaxillofac Surg. 2014;42:661–667. doi: 10.1016/j.jcms.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 112.Costantinides F, Vidoni G, Bodin C, Di Lenarda R. Eagle’s syndrome: signs and symptoms. Cranio. 2013;31:56–60. doi: 10.1179/crn.2013.008. [DOI] [PubMed] [Google Scholar]
- 113.Kim E, Hansen K, Frizzi J. Eagle syndrome: case report and review of the literature. Ear Nose Throat J. 2008;87:631–633. [PubMed] [Google Scholar]
- 114.Othieno FA, Williams JR, Myers LL. A Patient With Cervicalgia. JAMA Otolaryngol Head Neck Surg. 2015;141:767–768. doi: 10.1001/jamaoto.2015.1225. [DOI] [PubMed] [Google Scholar]
- 115.Wong ML, Rossi MD, Groff W, Castro S, Powell J. Physical therapy management of a patient with Eagle syndrome. Physiother Theory Pract. 2011;27:319–327. doi: 10.3109/09593985.2010.498036. [DOI] [PubMed] [Google Scholar]
- 116.Ceylan A, Koybasioglu A, Celenk F, Yilmaz O, Uslu S. Surgical treatment of elongated styloid process: experience of 61 cases. Skull Base. 2008;18:289–295. doi: 10.1055/s-0028-1086057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ohrbach R, Fillingim RB, Mulkey F, Gonzalez Y, Gordon S, et al. Clinical findings and pain symptoms as potential risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case-control study. J Pain. 2011;12:27–45. doi: 10.1016/j.jpain.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Markiewicz MR, Ohrbach R, McCall WD., Jr Oral behaviors checklist: reliability of performance in targeted waking-state behaviors. J Orofac Pain. 2006;20:306–316. [PubMed] [Google Scholar]
- 119.Isong U, Gansky SA, Plesh O. Temporomandibular joint and muscle disorder-type pain in U.S. adults: the National Health Interview Survey. J Orofac Pain. 2008;22:317–322. [PMC free article] [PubMed] [Google Scholar]
- 120.Schiffman E, Fricton JR. TMJ and craniofacial pain: Diagnosis and management. St. Louis: Ishiaku Euro American; 1988. Epidemiology of TMJ and craniofacial pain. [Google Scholar]
- 121.Bair E, Brownstein NC, Ohrbach R, Greenspan JD, Dubner R, et al. Study protocol, sample characteristics, and loss to follow-up: the OPPERA prospective cohort study. J Pain. 2013;14:2–19. doi: 10.1016/j.jpain.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fillingim RB, Ohrbach R, Greenspan JD, Knott C, Diatchenko L, et al. Psychological factors associated with development of TMD: the OPPERA prospective cohort study. J Pain. 2013;14:75–90. doi: 10.1016/j.jpain.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fillingim RB, Ohrbach R, Greenspan JD, Knott C, Dubner R, et al. Potential psychosocial risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case-control study. J Pain. 2011;12:46–60. doi: 10.1016/j.jpain.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhu S, Wang D, Yin Q, Hu J. Treatment guidelines for temporomandibular joint ankylosis with secondary dentofacial deformities in adults. J Craniomaxillofac Surg. 2013;41:117–127. doi: 10.1016/j.jcms.2012.11.038. [DOI] [PubMed] [Google Scholar]
- 125.Guidelines for diagnosis and management of disorders involving the temporomandibular joint and related musculoskeletal structures. ASTJS. 2003;21:68–76. [PubMed] [Google Scholar]
- 126.Greene CS, American Association for Dental R Diagnosis and treatment of temporomandibular disorders: emergence of a new care guidelines statement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110:137–139. doi: 10.1016/j.tripleo.2010.04.032. [DOI] [PubMed] [Google Scholar]
- 127.Craane B, Dijkstra PU, Stappaerts K, De Laat A. Randomized controlled trial on physical therapy for TMJ closed lock. J Dent Res. 2012;91:364–369. doi: 10.1177/0022034512438275. [DOI] [PubMed] [Google Scholar]
- 128.List T, Axelsson S, Leijon G. Pharmacologic interventions in the treatment of temporomandibular disorders, atypical facial pain, and burning mouth syndrome. A qualitative systematic review. J Orofac Pain. 2003;17:301–310. [PubMed] [Google Scholar]
- 129.Rizzatti-Barbosa CM, Nogueira MT, de Andrade ED, Ambrosano GM, de Barbosa JR. Clinical evaluation of amitriptyline for the control of chronic pain caused by temporomandibular joint disorders. Cranio. 2003;21:221–225. doi: 10.1080/08869634.2003.11746254. [DOI] [PubMed] [Google Scholar]
- 130.Stovner LJ, Hagen K, Jensen R, Katsarava Z, Lipton R, et al. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia. 2007;27:193–210. doi: 10.1111/j.1468-2982.2007.01288.x. [DOI] [PubMed] [Google Scholar]
- 131.Abboud J, Marchand AA, Sorra K, Descarreaux M. Musculoskeletal physical outcome measures in individuals with tension-type headache: a scoping review. Cephalalgia. 2013;33:1319–1336. doi: 10.1177/0333102413492913. [DOI] [PubMed] [Google Scholar]
- 132.Ashina S, Bendtsen L, Lyngberg AC, Lipton RB, Hajiyeva N, et al. Prevalence of neck pain in migraine and tension-type headache: a population study. Cephalalgia. 2015;35:211–219. doi: 10.1177/0333102414535110. [DOI] [PubMed] [Google Scholar]
- 133.Sahler K. Epidemiology and cultural differences in tension-type headache. Curr Pain Headache Rep. 2012;16:525–532. doi: 10.1007/s11916-012-0296-5. [DOI] [PubMed] [Google Scholar]
- 134.Bendtsen L, Fernandez-de-la-Penas C. The role of muscles in tension-type headache. Curr Pain Headache Rep. 2011;15:451–458. doi: 10.1007/s11916-011-0216-0. [DOI] [PubMed] [Google Scholar]
- 135.Bendtsen L. Central sensitization in tension-type headache–possible pathophysiological mechanisms. Cephalalgia. 2000;20:486–508. doi: 10.1046/j.1468-2982.2000.00070.x. [DOI] [PubMed] [Google Scholar]
- 136.Heckman BD, Holroyd KA. Tension-type headache and psychiatric comorbidity. Curr Pain Headache Rep. 2006;10:439–447. doi: 10.1007/s11916-006-0075-2. [DOI] [PubMed] [Google Scholar]
- 137.Chaibi A, Russell MB. Manual therapies for primary chronic headaches: a systematic review of randomized controlled trials. J Headache Pain. 2014;15:67. doi: 10.1186/1129-2377-15-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Holroyd KA. Behavioral and psychologic aspects of the pathophysiology and management of tension-type headache. Curr Pain Headache Rep. 2002;6:401–407. doi: 10.1007/s11916-002-0083-9. [DOI] [PubMed] [Google Scholar]
- 139.Burch RC, Loder S, Loder E, Smitherman TA. The prevalence and burden of migraine and severe headache in the United States: updated statistics from government health surveillance studies. Headache. 2015;55:21–34. doi: 10.1111/head.12482. [DOI] [PubMed] [Google Scholar]
- 140.Starling AJ, Vargas BB. A narrative review of evidence-based preventive options for chronic migraine. Curr Pain Headache Rep. 2015;19:49. doi: 10.1007/s11916-015-0521-0. [DOI] [PubMed] [Google Scholar]
- 141.Jette N, Patten S, Williams J, Becker W, Wiebe S. Comorbidity of migraine and psychiatric disorders–a national population-based study. Headache. 2008;48:501–516. doi: 10.1111/j.1526-4610.2007.00993.x. [DOI] [PubMed] [Google Scholar]
- 142.McWilliams LA, Goodwin RD, Cox BJ. Depression and anxiety associated with three pain conditions: results from a nationally representative sample. Pain. 2004;111:77–83. doi: 10.1016/j.pain.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 143.Goadsby PJ. Pathophysiology of cluster headache: a trigeminal autonomic cephalgia. Lancet Neurol. 2002;1:251–257. doi: 10.1016/s1474-4422(02)00104-7. [DOI] [PubMed] [Google Scholar]
- 144.Silberstein SD. Migraine preventive treatment. Handb Clin Neurol. 2010;97:337–354. doi: 10.1016/S0072-9752(10)97027-9. [DOI] [PubMed] [Google Scholar]
- 145.Becker WJ, Findlay T, Moga C, Scott NA, Harstall C, et al. Guideline for primary care management of headache in adults. Can Fam Physician. 2015;61:670–679. [PMC free article] [PubMed] [Google Scholar]
- 146.Holroyd KA, Cottrell CK, O’Donnell FJ, Cordingley GE, Drew JB, et al. Effect of preventive (beta blocker) treatment, behavioural migraine management, or their combination on outcomes of optimised acute treatment in frequent migraine: randomised controlled trial. BMJ. 2010;341:4871. doi: 10.1136/bmj.c4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nesbitt AD, Goadsby PJ. Cluster headache. BMJ. 2012;344:2407. doi: 10.1136/bmj.e2407. [DOI] [PubMed] [Google Scholar]
- 148.Rozen TD, Fishman RS. Cluster headache in the United States of America: demographics, clinical characteristics, triggers, suicidality, and personal burden. Headache. 2012;52:99–113. doi: 10.1111/j.1526-4610.2011.02028.x. [DOI] [PubMed] [Google Scholar]
- 149.Schürks M, Kurth T, de Jesus J, Jonjic M, Rosskopf D, et al. Cluster headache: clinical presentation, lifestyle features, and medical treatment. Headache. 2006;46:1246–1254. doi: 10.1111/j.1526-4610.2006.00534.x. [DOI] [PubMed] [Google Scholar]
- 150.Bahra A, Goadsby PJ. Diagnostic delays and mis-management in cluster headache. Acta Neurol Scand. 2004;109:175–179. doi: 10.1046/j.1600-0404.2003.00237.x. [DOI] [PubMed] [Google Scholar]
- 151.Fischera M, Marziniak M, Gralow I, Evers S. The incidence and prevalence of cluster headache: a meta-analysis of population-based studies. Cephalalgia. 2008;28:614–618. doi: 10.1111/j.1468-2982.2008.01592.x. [DOI] [PubMed] [Google Scholar]
- 152.Bahra A, May A, Goadsby PJ. Cluster headache: a prospective clinical study with diagnostic implications. Neurology. 2002;58:354–361. doi: 10.1212/wnl.58.3.354. [DOI] [PubMed] [Google Scholar]
- 153.Waldenlind E, Ekbom K, Torhall J. MR-angiography during spontaneous attacks of cluster headache: a case report. Headache. 1993;33:291–295. doi: 10.1111/j.1526-4610.1993.hed3306291.x. [DOI] [PubMed] [Google Scholar]
- 154.Robbins MS. The psychiatric comorbidities of cluster headache. Curr Pain Headache Rep. 2013;17:313. doi: 10.1007/s11916-012-0313-8. [DOI] [PubMed] [Google Scholar]
- 155.Donnet A, Lanteri-Minet M, Guegan-Massardier E, Mick G, Fabre N, et al. Chronic cluster headache: a French clinical descriptive study. J Neurol Neurosurg Psychiatry. 2007;78:1354–1358. doi: 10.1136/jnnp.2006.112037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Breslau N, Schultz LR, Stewart WF, Lipton RB, Lucia VC, et al. Headache and major depression: is the association specific to migraine? Neurology. 2000;54:308–313. doi: 10.1212/wnl.54.2.308. [DOI] [PubMed] [Google Scholar]
- 157.Liang JF, Chen YT, Fuh JL, Li SY, Liu CJ, et al. Cluster headache is associated with an increased risk of depression: a nationwide population-based cohort study. Cephalalgia. 2013;33:182–189. doi: 10.1177/0333102412469738. [DOI] [PubMed] [Google Scholar]
- 158.Sanchez Del Rio M, Leira R, Pozo-Rosich P, Lainez JM, Alvarez R, et al. Errors in recognition and management are still frequent in patients with cluster headache. Eur Neurol. 2014;72:209–212. doi: 10.1159/000362517. [DOI] [PubMed] [Google Scholar]
- 159.Bennett MH, French C, Schnabel A, Wasiak J, Kranke P, et al. Normobaric and hyperbaric oxygen therapy for the treatment and prevention of migraine and cluster headache. Cochrane Database Syst Rev. 2015;12:CD005219. doi: 10.1002/14651858.CD005219.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Matharu M, Silver N. Cluster headache. BMJ Clin Evid. 2008 [PubMed] [Google Scholar]


