Abstract
Background and aims: Cardiovascular disease (CVD) is the leading cause of death in the Middle East. We sought to study the prevalence and coexistence of 6 cardiovascular risk factors (RFs) among patients who underwent percutaneous coronary intervention (PCI), and to evaluate the impact of age and gender on the presence of multiple RFs. Methods and results. In this prospective, multicenter study, 2426 consecutive patients were enrolled. Mean age was 59.0 ± 10.1 years and 500 (20.6%) were women. Acute coronary syndrome and stable coronary disease were the indications for PCI in 77.1% and 22.9%, respectively. Hypertension was present in 62.3%, diabetes in 53.8%, hypercholesterolemia in 48.8%, smoking in 43.5%, family history of premature CVD 39.4% and obesity in 28.8%. Only 3.8% did not have any of these RFs. Presence of ⩾3 and ⩾4 RFS was observed in 57.4% and 29.5% of patients, respectively. Presence of ⩾3 RFs was more common in women than men (69.0% vs. 54.5%, p < 0.0001), and among patients 41–65 years of age than older or younger patients (60.1% vs. 52.0% vs. 48.3%, respectively, p = 0.017). Conclusions: Cardiovascular RFs are highly prevalent in this PCI Middle Eastern population undergoing PCI. More than half and more than one-fourth of the patients had at least 3 or 4 RFs; respectively. More women than men and more middle aged patients than older or younger patients had significantly higher rates of presence of multiple RFs.
Keywords: Cardiovascular disease, Risk factors, Middle Eastern patients, Percutaneous coronary intervention
Abbreviations
- ACS
acute coronary syndrome
- BMI
body mass index
- CAD
coronary artery disease
- CVD
cardiovascular disease
- DM
diabetes mellitus
- JoPCR1
The first Jordanian PCI Registry
- HbA1c
glycosylated hemoglobin
- HTN
hypertension
- NSTEACS
non-ST-segment elevation ACS
- PCI
percutaneous coronary intervention
- RF
risk factor
- SD
standard deviation
- STEMI
ST-segment elevation myocardial infarction
1. Introduction
Cardiovascular disease (CVD) is the leading cause of death worldwide and in the Middle East [1], [2], [3]. By 2020, the mortality burden from noncommunicable diseases, mostly attributable to CVD, is expected to occur mainly in low- and middle-income countries [4]. International [5], [6] and regional [7], [8], [9], [10] studies have identified a set of common CVD risk factors (RFs) that are found to be highly prevalent in Middle Eastern communities, including hypertension (HTN), diabetes mellitus (DM), hypercholesterolemia, obesity, cigarette smoking, physical inactivity, family history of premature CVD, and poor diet.
The vast majority (⩾90%) of Middle Eastern patients diagnosed to have coronary artery disease (CAD) have at least one of the four classical RFs, i.e., HTN, DM, cigarette smoking, and hypercholesterolemia, and the coexistence of two or more RFs [11], [12] or three or more RFs [5], [8], [10] is present in the majority of these patients. The presence of multiple cardiovascular RFs negatively impacts patient prognosis by potentiating the development of atherosclerosis [13] and increasing the intensity, diversity, and cost of long-term medical care. Prevalence of RFs has not been studied in an exclusive percutaneous coronary intervention (PCI) population in this region. The studies that addressed the prevalence of RFs involved patients admitted with acute coronary syndrome (ACS) and treated conservatively or those who underwent coronary angiography and subsequent PCI or coronary surgical revascularization [7], [8], [9], patients diagnosed to have stable coronary disease who did not have coronary angiography [8], patients who sustained cerebrovascular events [6], or individuals assessed in an outpatient clinic setup [5], [10].
Data from the recently completed first Jordanian PCI Registry were analyzed to determine the prevalence of six cardiovascular RFs among patients who underwent PCI for ACS or stable coronary disease.
2. Methods
2.1. Study population
The first Jordanian PCI Registry is a multicenter, prospective study that enrolled consecutive patients who underwent PCI in 12 tertiary care centers in Jordan (January 2013–February 2014) and were then followed up for 1 year after the index hospitalization. Patients signed an informed consent form prior to entry to the study. The two major indications for PCI were ACS, including ST-segment elevation myocardial infarction and non-ST-segment elevation ACS, or stable coronary disease, including chronic stable angina and silent ischemia. The prevalence rates of the following six RFs were assessed in every patient during the index hospital stay: HTN, DM, cigarette smoking, hypercholesterolemia, obesity, and family history of premature CVD.
2.1. Study measures
All RFs were defined according to standard definitions [14]. Patients were considered to have HTN if they had either systolic blood pressure elevated above 140 mmHg and/or diastolic blood pressure above 90 mmHg on several occasions during hospital stay, were diagnosed to have HTN, or were prescribed anti-HTN medications by a treating physician. DM was defined according to the standard criteria set by the American Diabetes Association, i.e., fasting serum glucose ⩾126 mg/dL, 2-hour glucose level ⩾200 mg/dL, or glycosylated hemoglobin value ⩾6.5%. DM was also diagnosed in patients who had unequivocal hyperglycemia and classical symptoms of DM (polyuria, polydipsia, and unexplained weight loss) and casual plasma glucose ⩾200 mg/dL, and those with a prior diagnosis of DM or who were prescribed antidiabetic medications by a treating physician [15], [16]. Body mass index (BMI) was calculated according to the standard formula [body weight (kg)/height (m2)]. Weight and height were measured as early as possible after hospital admission and when the clinical situation of each patient was stable enough to allow taking these measurements. Patients who were cigarette smokers at enrollment were considered current smokers. Patients who never smoked and past smokers who quit at least 1 month prior to enrollment were considered nonsmokers. Patients were considered to have hypercholesterolemia if they had a past diagnosis by a treating physician, were prescribed lipid-lowering agents, or were found to have serum total cholesterol >240 mg/dL during the index admission. Obesity was defined as BMI of ⩾30 kg/m2. A family history of premature CVD was defined as myocardial infarction, coronary revascularization, or sudden death before 55 years of age in father or any other male first-degree relative, or before 65 years of age in mother or any other female first-degree relative. This study was approved by the Institutional Review Board in each of the participating hospitals.
Statistical analysis
IBM SPSS Statistics 20 (IBM Corp., Armonk, NY, USA, 2011) was used for data entry and analysis. Data were described using mean values ± standard deviation for continuous variables. Frequencies and percentages were used to describe categorical variables. The differences in percentages of RFs among men and women, and among the three prespecified age groups were analyzed using chi-square test. A p value of <0.05 was considered statistically significant.
Results
A total of 2426 patients were enrolled consecutively in the study. Their mean age (±standard deviation) was 59.0 ± 10.1 years, and 500 (20.6%) of them were women. There were 114 patients (4.7%) younger than 41 years of age, 1679 (69.2%) were 41–65 years of age, and 633 (26.1%) were older than 65 years of age. PCI was indicated for ACS in 1870 patients (77.1%) and for stable coronary disease in 556 patients (22.9%). Patients who had ACS were diagnosed to have either ST-segment elevation myocardial infarction (725; 38.8%) or non-ST-segment elevation ACS (1145; 61.2%).
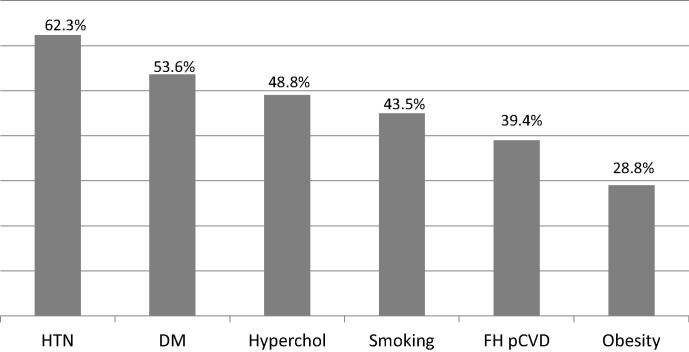
Table 1 shows the baseline clinical profiles, coronary angiographic features, and PCI procedure details in the whole group, and Fig. 1 depicts the overall prevalence of the six prespecified RFs. The presence of HTN was confirmed in all patients based on a prior diagnosis of this RF rather than on blood pressure measurements during admission. The majority of diabetic (90%) and hypercholesterolemic (95%) patients had these diagnoses prior to admission, and the other patients (10% of diabetic and 5% of hypercholesterolemic patients) had the diagnoses made based on the results of blood tests during admission. Only 3.8% of patients did not have any of these RFs. A past history of myocardial infarction and that of PCI were present in 11% and 24% of patients, respectively. Nearly 60% of patients had single-vessel CAD and 70% single-vessel PCI.
Table 1.
Demographic, clinical, and angiographic features of the study patients.
| Feature | % |
|---|---|
| Age (y), mean ± SD | 59.0 ± 10.1 |
| Females | 20.6 |
| Hypertension | 62.3 |
| Diabetes mellitus | 53.6 |
| Hypercholesterolemia | 48.8 |
| Current smoker | 43.5 |
| Mean BMI (kg/m2) | 28.5 ± 4.5 |
| <25 | 22.6 |
| 25–29.9 | 48.6 |
| ⩾30 | 28.8 |
| Chronic kidney disease | 50 (2.7) |
| Past myocardial infarction | 10.8 |
| Past stroke | 2.1 |
| Peripheral arterial disease | 0.9 |
| Prior PCI | 24.3 |
| Prior coronary artery bypass surgery | 3.5 |
| ST-segment deviation | 48.6 |
| Elevated serum cardiac biomarkers | 40.0 |
| LVEF < 45% | 12.5 |
| Heart failure on admission | 11.1 |
| Diagnosis | |
| ACS | 77.1 |
| STEMI | 29.9 |
| NSTEACS | 47.2 |
| Stable coronary syndrome: | 22.9 |
| Chronic stable angina | 20.6 |
| Silent ischemia | 2.3 |
| Number of diseased coronary arteries | |
| 1 | 58.4 |
| 2 | 29.6 |
| ⩾3 | 12.0 |
| Treated coronary arteries with PCI | |
| 1 coronary artery | 71.4 |
| 2 coronary arteries | 23.4 |
| ⩾3 coronary arteries | 5.6 |
| PCI of left main coronary artery | 1.2 |
| PCI of saphenous vein graft | 1.0 |
ACS = acute coronary syndrome; BMI = body mass index; LVEF = left ventricular ejection fraction; NSTEACS = non-ST-segment elevation acute coronary syndrome; PCI = percutaneous coronary intervention; SD = standard deviation; STEMI = ST-segment myocardial infarction.
Figure 1.
Overall prevalence of the six cardiovascular risk factors. DM = diabetes mellitus; FH pCVD = family history of premature cardiovascular disease; HTN = hypertension; hyperchol = hypercholesterolemia.
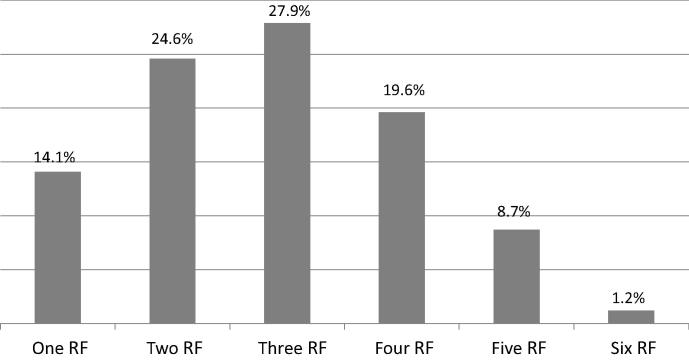
The prevalence of RFs is shown in Fig. 2. Two or more RFs were observed in 82.0% of patients. At least three RFs were present in 57.4% of patients and at least four RFs were present in 29.5%. Table 2 demonstrates the coexistence of each of the five RFs (smoking, DM, HTN, obesity, and hypercholesterolemia) with the other RFs. The presence of at least three RFs was observed in 54.8% of obese patients, 51.0% of those with hypercholesterolemia, 44.9% of those with DM, 43.0% of those with HTN, and 38.6% of smokers. The RF most likely to coexist with at least four RFs was obesity (25.6%), followed by hypercholesterolemia (19.2%).
Figure 2.
Proportions of patients who have one or more of the six risk factors (hypertension, diabetes mellitus, hypercholesterolemia, obesity, cigarette smoking, and family history of premature cardiovascular disease).
Table 2.
Number of risk factors (0–5) coexisting with hypertension, diabetes mellitus, smoking, hypercholesterolemia, and obesity.
| Risk factor | No. of coexisting risk factors |
|||||
|---|---|---|---|---|---|---|
| 0 (%) | 1 (%) | 2 (%) | 3 (%) | 4 (%) | 5 (%) | |
| Smoking | 12.3 | 22.9 | 26.2 | 22.4 | 13.4 | 2.8 |
| Diabetes | 4.9 | 18.2 | 32.0 | 28.3 | 14.4 | 2.2 |
| Hypertension | 4.4 | 20.1 | 32.5 | 27.5 | 13.6 | 1.9 |
| Obesity | 3.9 | 13.9 | 27.5 | 29.2 | 21.5 | 4.1 |
| Hypercholesterolemia | 1.6 | 13.8 | 33.6 | 31.8 | 16.7 | 2.5 |
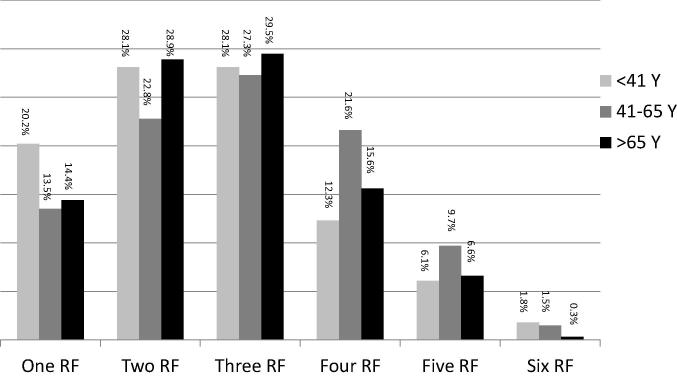
The prevalence of RFs was evaluated according to age (Fig. 3). The presence of two or more RFs was observed in more patients aged 41–65 years of age compared with younger and older patients. The presence of three or more RFs was observed in 60.1% of patients in the middle age group, compared with 52.0% of older and 48.3% of younger patients (p = 0.017). Furthermore, the presence of four or more RFs was observed in 32.8% of patients in the middle age group, compared with 22.5% of older and 20.2% of younger patients (p = 0.007).
Figure 3.
Proportions of patients in three age groups (younger than 41 years, 41–65 years, and older than 65 years of age) who have one or more of the six risk factors (hypertension, diabetes mellitus, hypercholesterolemia, obesity, cigarette smoking, and family history of premature cardiovascular disease). RF = risk factor.
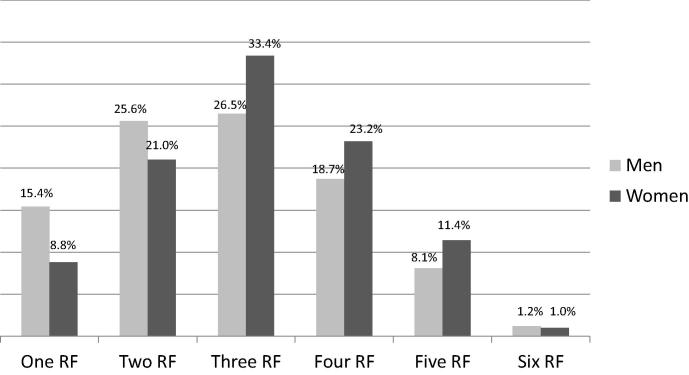
The prevalence of RFs in 500 women was compared with that in 1926 men (Fig. 4). Absence of any RF was observed in more men than women (4.5% vs. 1.2%, p < 0.001). Women were more likely than men to have at least three RFs (69.0% vs. 54.5%) or at least four RFs than men (35.6% vs. 28.0%, p < 0.001).
Figure 4.
Proportions of men and women who have one or more of the six risk factors (hypertension, diabetes mellitus, hypercholesterolemia, obesity, cigarette smoking, and family history of premature cardiovascular disease). RF = risk factor.
Discussion
The main findings of this study are as follows: (1) HTN, DM, and hypercholesterolemia were the most prevalent RFs, and each was present in nearly half of this Middle Eastern contemporary PCI population; (2) coexistence of two or more cardiovascular RFs was the rule, not the exception, where 82% have two or more RFs, 55% three or more RFs, and 30% four or more RFs; and (3) more women than men, and patients 41–65 years of age compared with younger and older age groups had a higher prevalence of two or more RFs. The 12 participating centers perform about 80% of all PCI procedures in the country. These centers cover most of the local healthcare sectors including public, university, and nonteaching private and teaching private hospitals. Thus, the studied group of PCI patients fairly represents the whole PCI patient population.
Conventional cardiovascular RFs are broadly classified into two categories: modifiable and nonmodifiable factors [3], [5]. Modifiable RFs can reduce the CVD risk if treated and controlled, and nonmodifiable RFs are not amenable to adjustment or control, e.g., age, sex, family history of CVD, and ethnicity. Other than the five modifiable RFs studied in this registry (HTN, DM, cigarette smoking, hypercholesterolemia, and obesity), the list includes sedentary lifestyle, bad nutritional habits, low socioeconomic class, alcohol consumption, low educational level, urbanization, poor housing quality, and pollution [3], [5], [17], [18], [19]. Several studies have demonstrated that these modifiable RFs are responsible for a large number of premature CV deaths worldwide and in the low- and middle-income countries [20], [21].
The finding that over 90% of patients with CAD had at least one RF in the majority of these patients concurs with other investigators’ findings [8], [10], [13], [18], [22], [23]. Studies have shown that multiple RFs are more prevalent among lower social class households, singles, and people who are economically inactive, and less prevalent among homeowners and older age groups [22]. Coexistence of RFs varies across populations studied, that is, populations in different geographic regions in the world, urban versus rural populations, different ethnic groups within the same country, individuals who do not have overt CVD compared with those who have symptomatic CVD, men versus women, and old versus young individuals [24], [25]. An outpatient clinic-based study in 14 Middle Eastern and African nations showed that the presence of three or more RFs was observed in more than half (53%) of the overall cohort [10]. This rate ranged from 33% in low-income countries to 57% in high-income countries. In a local study of 1534 individuals who had symptomatic CAD, two RFs were present in 28% and three in 25% of the patients, and the presence of three or less RFs was observed in 41% of patients [8].
The rate of coexistence of RF was shown by several studies to suddenly drop, after a threshold of three RFs, to a fairly low level [26]. This study has demonstrated that the coexistence of RFs dropped suddenly to a single digit percentage (<10%) at a threshold of four RFs, reflecting a worse risk profile among the study individuals, all of whom had undergone PCI.
The study also showed that multiple RF coexistence varies according to age and gender. The presence of three or more RFs was observed in six of 10 middle-aged patients, compared with about five of 10 of younger and older age groups. RF coexistence dropped to a single-digit percentage after four RFs in the three age groups. The observation of a decreasing prevalence of RFs among the older age group compared with those aged 41–65 years is due to the fact that age per se steadily increases the absolute baseline risk of CAD independent of the conventional RFs; therefore, patients without RFs tend to present at a much later age once their baseline absolute risk increases sufficiently to cause a significant prevalence of CAD. Furthermore, the decreasing prevalence of RFs in the older patients reflects an inherent survivor bias because patients with conventional RFs die at a much younger age [27].
Recent evidence from the ongoing sex-specific research has demonstrated that although men and women share similar RFs for CAD, certain RFs, such as cigarette smoking, DM, depression, and other psychosocial RFs, tend to exert a worse health impact in women [28]. It has been suggested that nine potentially modifiable RFs (smoking, HTN, DM, waist-to-hip ratio, dietary patterns, physical activity, alcohol consumption, plasma apolipoproteins, and psychosocial factors) account for 96% of the population-attributable risk of myocardial infarction in women [5], [21]. Overall, the prevalence of RFs is greater in women than in men, and women are more likely to have a higher number of cardiovascular RFs [23], [27]. The current study showed that about seven of 10 women had three or more RFs, and four of 10 had four or more RFs. Furthermore, the sudden drop in the number of RFs to a single digit observed in men after the coexistence of four RFs occurred in women after five RFs.
Among enrolled patients who had HTN, the most prevalent other RF seems to be hypercholesterolemia [28], [29]. This study showed that among patients with HTN, 62% also had DM and 60% had hypercholesterolemia. Moreover, among patients with DM, 72%, 57%, and 36% had HTN, hypercholesterolemia, and smoking, respectively. The presence of other RFs among diabetic patients predicts higher mortality and necessitates aggressive medical therapy [30], [31]. The two most common RFs coexisting with hypercholesterolemia in this study were HTN (77%) and DM (63%). Similarly, obesity coexisted with HTN in 69% and with DM in 57% of patients.
Contributory causes to the high prevalence of coexistence of multiple RFs include growing population with demographic shifts, an altered age profile, low education levels, lifestyle changes due to urbanization, dietary habits, lack of adoption of regular physical activity in a large proportion of the population, ineffective antitobacco campaigns, and rise of prevalence rates of DM, metabolic syndrome, and obesity [30], [32], [33], [34]. Patients with multiple RFs are at increased risk of having coronary artery calcification, and premature mortality and morbidity [13], [35]. The population-attributable risk for these RFs was higher in the Middle East than in other regions of the world, highlighting the substantial potential benefits expected from aggressive preventive measures. Primary prevention strategies contingent on early detection and adequate control of conventional RFs are critical to combat the global burden of CVD.
A few limitations in our study warrant discussion. Inherent to similar observational registries, this study is subject to selection bias, collection of nonrandomized data, and missing or incomplete information [36]. Some of the cardiovascular RFs may be subject to information and/or recall bias since the data were self-reported. Participation was voluntary and the enrolment of consecutive patients was encouraged, but this was not verified, as it is the case with other registries. ACS patients who died before or shortly after admission and those who did not undergo angiography were not represented in this study. The mere presence of a particular RF in an individual patient with CVD does not guarantee that it plays a causal role in the pathogenesis of atherosclerosis due to the coexistence of nontraditional RFs and genetic causes at the individual level. Other than high levels of total cholesterol, levels of high- or low-density lipoproteins were not considered in the RF analysis. Inclusion of high-density lipoprotein cholesterol, a highly prevalent finding in Middle Eastern men and women [8], would diminish the number of patients who do not have any of the conventional RFs. BMI, rather than the waist circumference, was used to define obesity. Although BMI is easy to calculate and commonly used in registries, it is a crude and flawed anthropometric biomarker that lacks the discriminating power in differentiating between lean body mass and fat mass. Despite these limitations, this study is unique in that it evaluated the prevalence and coexistence of six RFs in a relatively large cohort who underwent PCI in the Middle East, a region that is not well presented in cardiovascular interventional studies and registries.
In conclusion, the high prevalence of the six cardiovascular RFs in this Middle Eastern PCI cohort is a viable concern for patients and for the community at large. The coexistence of multiple RFs provides a clear impression of the true burden of CVD risk in the population, emphasizing the urgent need to support the concept of multifaceted primary and secondary cardiovascular preventive medical and behavioral interventions.
Acknowledgments
We thank the following members of the first Jordanian PCI Registry Investigation Group for recruiting the patients: Abdelbasit Khatib, MD; Abdelfattah Al-Nadi, RN; Abeer Al Bashaireh, PharmD; Ahmad Abdulsattar, MD; Ahmad Harassis, MD, FACC; Aktham Hiari, MD, FACC; Ali Shakhatreh, MD; Amr Rasheed, MD; Ayed Al Hindi, MD; Azzam Jamil, MD; Bashar Al A’Amar, MD; Batool Haddad, PharmD; Delia Y. Omar, BSc Pharmacy, MSc Clinical Pharmacy; Enas Hijjih, PharmD; Hadi Abu-Hantash, MD, FACC; Hanan Abunimeh, PharmD; Haneen Kharabsheh, PharmD; Hasan Tayyim, PharmD; Hatem Tarawneh, MD, FACC; Husam Khader, RN; Husham Al-Janabi, MD; Hussein Al-Amrat, MD; Ghaida Melhem, PharmD; Ibrahim Abu Ata, MD, FACC; Ibrahim Jarrad, MD, FESC; Jamal Dabbas, MD; Kamel Touqan, MD; Laith Nassar, MD; Lewa Al Hazaymeh, MD; Lina Tashman, PharmD; Mahmoud Eswed, MD; Mazen Sudqi, MD; Medhat Bakri, MD; Mohammad Bakri, MD; Mohammed Mohialdeen, MD; Mohannad Momani, RN; Monther Hassan, MD; Nadeen Kufoof, PharmD; Najat Afaneh, PharmD; Nael Shobaki, MD; Nidal Hamad, MD, FACC; Nuha Abu-Diak, PharmD; Osama Baghal, MD, FACC; Osama Okkeh, MD; Qasem Al-Shamayleh, MD, FACC; Raed Awaysheh, MD; Ryad Jumaa, MD; Sahm Gharaibeh, MD; Saleh Eliamat, RN; Yousef Qossous, MD, FACC; Zakaria Qaqa, MD, FACC; Ziad Abu Taleb, MD. The study was supported by a nonrestricted grant from AstraZeneca.
Disclosure: Authors have nothing to disclose with regard to commercial support.
Footnotes
Peer review under responsibility of King Saud University.
References
- 1.Lee M.S., Flammer A.J., Kim H.S., Hong J.Y., Li J., Lennon R.J. The prevalence of cardiovascular disease risk factors and the Framingham risk score in patients undergoing percutaneous intervention over the last 17 years by gender: time-trend analysis from the Mayo Clinic PCI Registry. J Prev Med Public Health. 2014;47:216–229. doi: 10.3961/jpmph.2014.47.4.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mendis S., Lindholm L.H., Mancia G., Whitworth J., Alderman M., Lim S. World Health Organization (WHO) and International Society of Hypertension (ISH) risk prediction charts: assessment of cardiovascular risk for prevention and control of cardiovascular disease in low and middle-income countries. J Hypertension. 2007;25:1578–1582. doi: 10.1097/HJH.0b013e3282861fd3. [DOI] [PubMed] [Google Scholar]
- 3.Yusuf S., Rangarajan S., Teo K., Islam S., Li W., Liu L. Cardiovascular risk and events in 17 low-, middle-, and high-income countries. N Engl J Med. 2014;371:818–827. doi: 10.1056/NEJMoa1311890. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization Cardiovascular Disease Factsheet 2015. 2015 <http://www.who.int/mediacentre/factsheets/fs317/en/> Available from: [Google Scholar]
- 5.Yusuf S., Hawken S., Ounpuu S., Dans T., Avezum A., Lanas F. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case–control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 6.O’Donnell M.J., Xavier D., Liu L., Zhang H., Chin S.L., Rao-Melacini P. Risk factors for ischemic and intracerebral hemorrhagic stroke in 22 countries (the INTERSTROKE study): a case–control study. Lancet. 2010;376:112–123. doi: 10.1016/S0140-6736(10)60834-3. [DOI] [PubMed] [Google Scholar]
- 7.Zubaid M., Rashed W.A., Almahmeed W., Al-Lawati J., Sulaiman K., Al-Motarreb A. Management and outcomes of Middle Eastern patients admitted with acute coronary syndromes in the Gulf Registry of Acute Coronary Events (Gulf RACE) Acta Cardiol. 2009;64:439–446. doi: 10.2143/AC.64.4.2041607. [DOI] [PubMed] [Google Scholar]
- 8.Hammoudeh A.J., Al-Tarawneh H., Elharassis A., Haddad J., Mahadeen Z., Badran N. Prevalence of conventional cardiovascular risk factors in Middle Eastern patients with coronary heart disease: the Jordan Hyperlipidemia and Related Targets Study (JoHARTS 2) Intern J Cardiol. 2006;110:179–183. doi: 10.1016/j.ijcard.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 9.AlHabib K.F., Hersi A., AlFaleh H., Kurdi M., Arafah M., Youssef M. The Saudi Project for Assessment of Coronary Events (SPACE) registry: design and results of a phase I pilot study. Can J Cardiol. 2009;25:e255–e258. doi: 10.1016/s0828-282x(09)70513-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alsheikh-Ali A.A., Omar M.I., Raal F.J., Rashed W., Hamoui O., Kane A. Cardiovascular risk factor burden in Africa and the Middle East: the Africa Middle East Cardiovascular Epidemiological (ACE) study. PLoS One. 2014;9:e102830. doi: 10.1371/journal.pone.0102830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao B, Zhang L, Wang H. The China National Survey of Chronic Kidney Disease Working Group. Clustering of major cardiovascular risk factors and the association with unhealthy lifestyles in the Chinese adult population. PLoS One 2013; 8: e66780. http://dx.doi.org/10.1371/journal.pone.0066780. [DOI] [PMC free article] [PubMed]
- 12.Balaji R., Logaraj M., John K.R. A study on clustering of cardiovascular risk factors among a rural adult population in Tamil Nadu. J Cardiovasc Dis Res. 2015;6:85–90. [Google Scholar]
- 13.Mamudu H.M., Paul T.K., Budof M. The effects of multiple coronary artery disease risk factors on subclinical atherosclerosis in a rural population in the United States. Prev Med. 2016;88:140–146. doi: 10.1016/j.ypmed.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Cannon C.P., Brindis R.G., Chaitman B.R., Cohen D.J., Cross J.T., Jr., Drozda J.P., Jr. 2013 ACCF/AHA key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes and coronary artery disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Acute Coronary Syndromes and Coronary Artery Disease Clinical Data Standards) J Am Coll Cardiol. 2013;61:992–1025. doi: 10.1016/j.jacc.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryden L., Standl E., Bartnik M., Van den Berghe G., Betteridge J., de Boer M.J. Guidelines on diabetes, pre-diabetes, and cardiovascular disease: executive summery. The Task Force on Diabetes and Cardiovascular Disease of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD) Eur Heart J. 2007;28:88–136. doi: 10.1093/eurheartj/ehl260. [DOI] [PubMed] [Google Scholar]
- 16.American Diabetes Association Standards of medical care in diabetes–2015. Diabetes Care. 2015;38:S1–S89. doi: 10.2337/dc14-2142. [DOI] [PubMed] [Google Scholar]
- 17.Brook R.D., Rajagopalan S., Pope C.A., Brook J.R., Bhatnagar A., Diez-Roux A.V. Particulate matter air pollution and cardiovascular disease. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 18.Butt Z., Shahbaz U., Hashmi A.T., Naseem T., Khan M.M., Bukhari M.H. Frequency of conventional risk factors in patients with acute coronary syndrome in males and females. Ann King Edward Med Univ. 2010;16:55–58. [Google Scholar]
- 19.Bundhun P.K., Wu Z.J., Chen M.H. Impact of modifiable cardiovascular risk factors on mortality after percutaneous coronary intervention: a systematic review and meta-analysis of 100 studies. Medicine (Baltimore) 2015;94:e2313. doi: 10.1097/MD.0000000000002313. http://dx.doi:10.1097/MD.0000000000002313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alwan A. WHO Library Cataloguing-in-Publication Data; 2011. Global status report on noncommunicable diseases 2010. [Google Scholar]
- 21.Yusuf S., Reddy S., Ounpuu S., Anand S. Global burden of cardiovascular diseases. Part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104:2746–2753. doi: 10.1161/hc4601.099487. [DOI] [PubMed] [Google Scholar]
- 22.Poortinga W. The prevalence and clustering of four major lifestyle risk factors in an English adult population. Prev Med. 2007;44:124–128. doi: 10.1016/j.ypmed.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Selvarajah S., Haniff J., Kaur G., Hiong T.G., Cheong K.C., Lim C.M. Clustering of cardiovascular risk factors in a middle-income country: a call for urgency. Eur J Prev Cardiol. 2013;20:368–375. doi: 10.1177/2047487312437327. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Bots ML, Yang F, Sun J, He S, Hoes AW, et al. A comparison of the prevalence and clustering of major cardiovascular risk factors in the Netherlands and China. Eur J Prev Cardiol 2016; pii:2047487316648474. [DOI] [PubMed]
- 25.Daviglus M.L., Talavera G.A., Aviles-Santa M.L., Allison M., Cai J., Criqui M.H. Prevalence of major cardiovascular risk factors and cardiovascular diseases among Hispanic/Latino individuals of diverse backgrounds in the United States. JAMA. 2012;308:1775–1784. doi: 10.1001/jama.2012.14517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zaman M.M., Bhuiyan M.R., Karim N., Zaman M., Rahman M., Akanda A.W. Clustering of non-communicable diseases risk factors in Bangladeshi adults: an analysis of STEPS survey 2013. BMC Public Health. 2015;15:659. doi: 10.1186/s12889-015-1938-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khot U.N., Khot M.B., Bajzer C.T., Sapp S.K., Ohman E.M., Brener S.J. Prevalence of conventional risk factors in patients with coronary heart disease. JAMA. 2003;290:898–904. doi: 10.1001/jama.290.7.898. [DOI] [PubMed] [Google Scholar]
- 28.Mehta L.S., Beckie T.M., DeVon H.A., Grines C.L., Krumholz H.M., Johnson M.N. Acute myocardial infarction in women: A scientific statement from the American Heart Association. Circulation. 2016;133:916–947. doi: 10.1161/CIR.0000000000000351. Epub 2016 Jan 25. [DOI] [PubMed] [Google Scholar]
- 29.Stern R., Puoane T., Tsolekile L. An exploration into the determinants of noncommunicable diseases among rural-to-urban migrants in periurban South Africa. Prev Chronic Dis. 2010;7:A131. [PMC free article] [PubMed] [Google Scholar]
- 30.Zindah M., Belbeisi A., Walke H., Mokdad A.H. Obesity and diabetes in Jordan: findings from the behavioral risk factors surveillance system. Prev Chronic Dis. 2008;5 Available from: < http://www.cdc.gov/pcd/issues/2008/jan/06_0172.htm> [accessed 3.11.2016] [PMC free article] [PubMed] [Google Scholar]
- 31.Harati H., Hadaegh F., Saadat N., Azizi F. Population-based incidence of type 2 diabetes and its associated risk factors: results from a six-year cohort study in Iran. BMC Public Health. 2009;9:186. doi: 10.1186/1471-2458-9-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ajlouni K., Jaddou H., Batieha A. Obesity in Jordan. Int J Obes Relat Metab Disord. 1998;22:624–628. doi: 10.1038/sj.ijo.0800637. [DOI] [PubMed] [Google Scholar]
- 33.Esteghamati A., Khalilzadeh O., Ashraf H., Zandieh A., Morteza A., Rashidi A. Physical activity is correlated with serum leptin independent of obesity: results of the national surveillance of risk factors of noncommunicable diseases in Iran (SuRFSNCD-2007) Metabolism. 2010;59:1730–1735. doi: 10.1016/j.metabol.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization. WHO report on the global tobacco epidemic, 2011. Warning about the dangers of tobacco. Geneva, Switzerland: World Health Organization; 2011.
- 35.deRuiter W.K., Cairney J., Faulkner G. The period prevalence of risk behavior co-occurrence among Canadians. Prev Med. 2016;85:11–16. doi: 10.1016/j.ypmed.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 36.Lenzen M.J., Boersma E., Bertrand M.E., Maier W., Moris C., Piscione F. Management and outcome of patients with established coronary artery disease: the Euro Heart Survey on coronary revascularization. Eur Heart J. 2005;26:1169–1179. doi: 10.1093/eurheartj/ehi238. [DOI] [PubMed] [Google Scholar]