Abstract
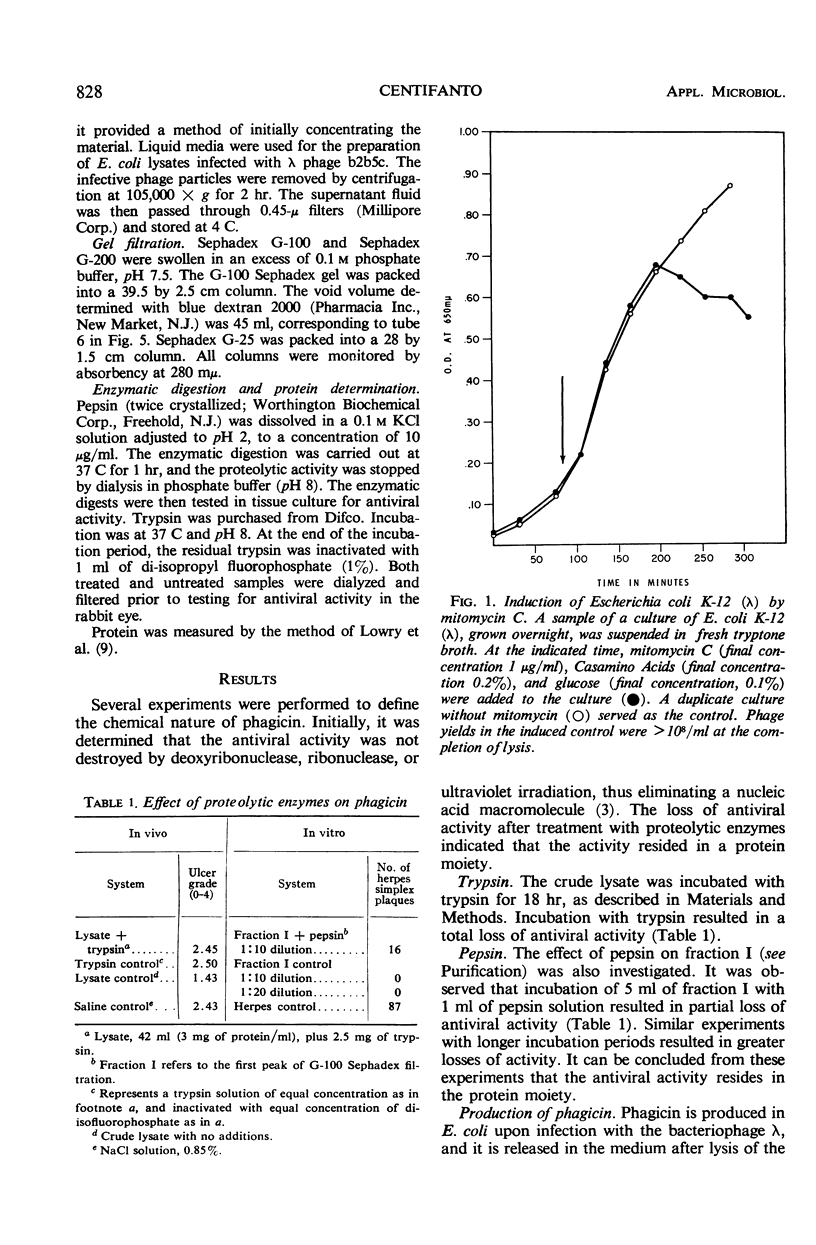
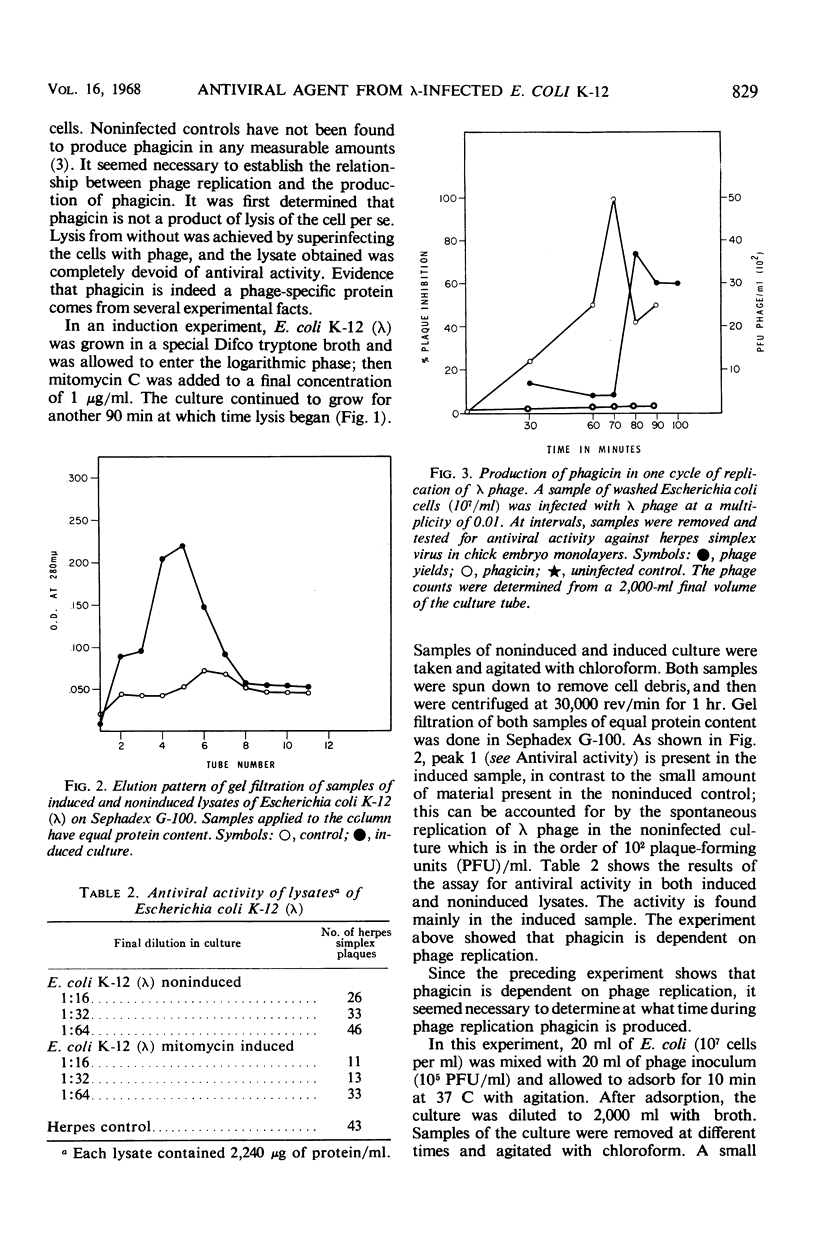
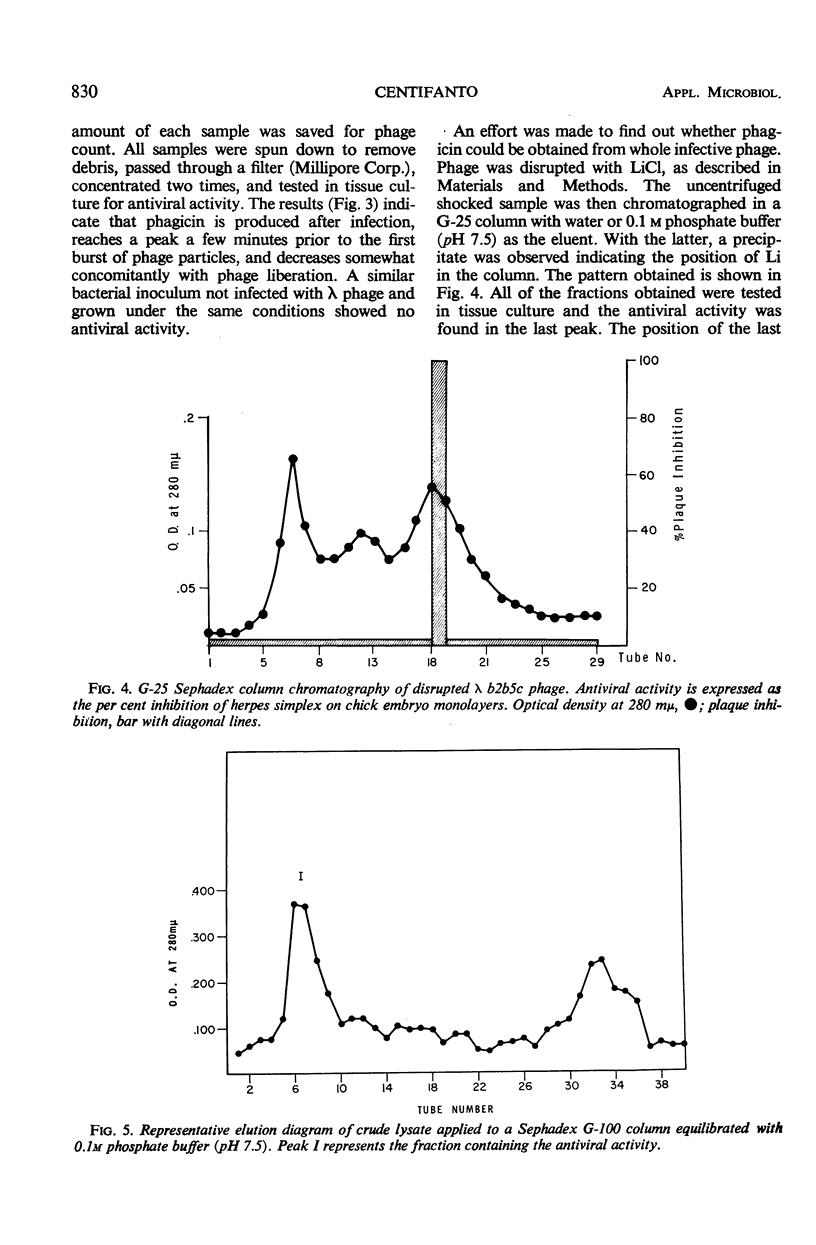
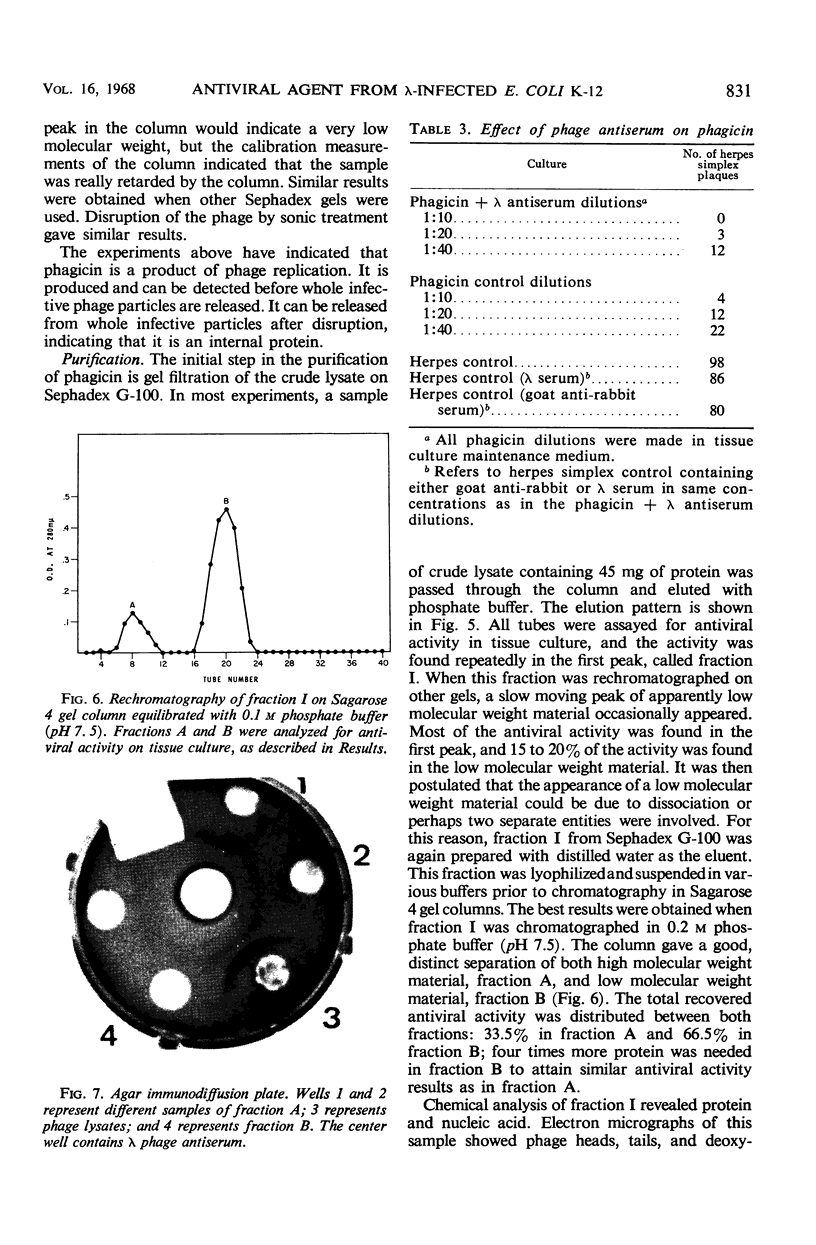
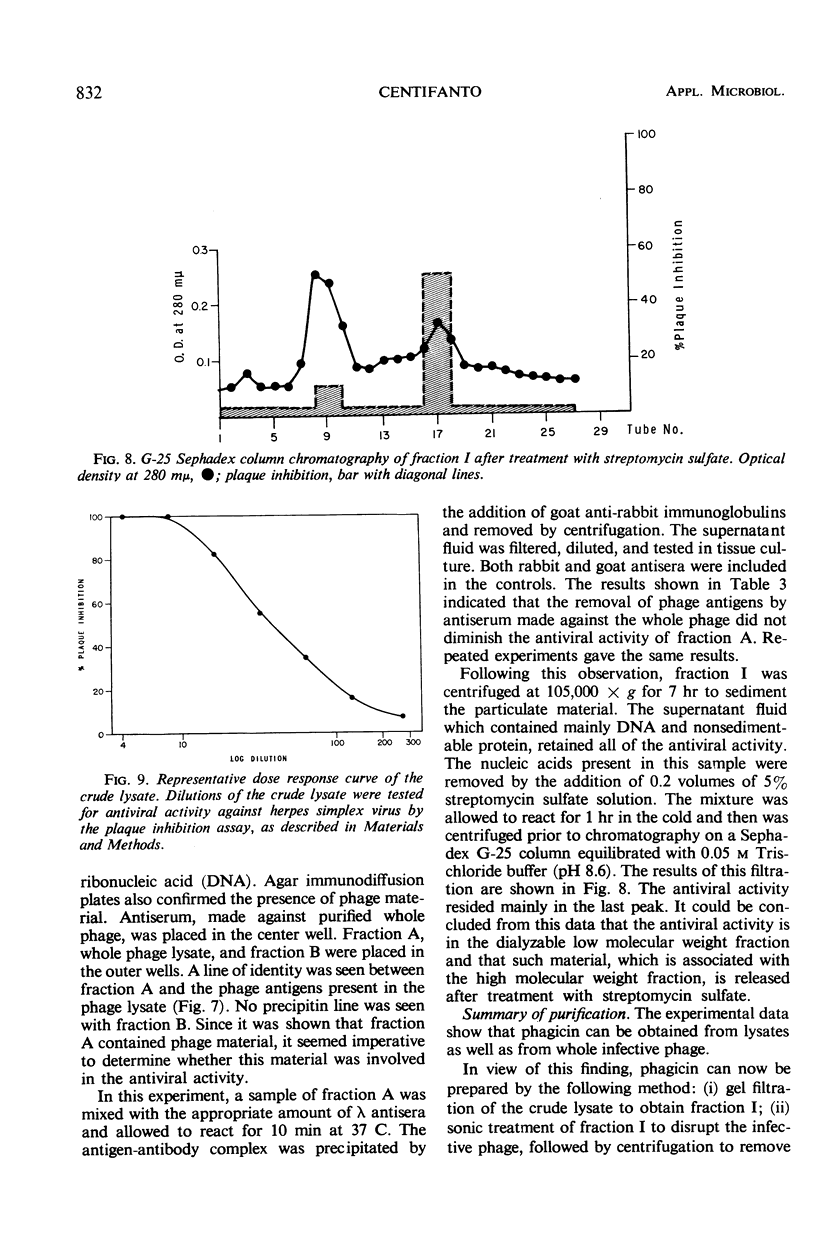
Phagicin, which is an antiviral agent active against deoxyribonucleic acid (DNA) viruses such as vaccinia and herpes simplex, has been identified as a phage internal protein. It was found in infected Escherichia coli lysates, but could also be obtained by disruption of the purified infective particles after incubation with LiCl at 46 C for 15 min or by sonic treatment. After centrifugation at high speed, the antiviral activity was found in the DNA phase and could be separated by chromatography on Sephadex gels with 0.2 M phosphate buffer (pH 7.5) as the eluent. Phagicin present in lysates after removal of infective particles was nondialyzable and was bound to nucleic acids. It could be released during precipitation of nucleic acids by streptomycin sulfate, and in this form it could be easily dialyzed. The antiviral activity of phagicin was specific for herpes simplex and vaccinia viruses.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachrach U., Friedmann A. Purification and some possible functions of internal proteins from coliphage T2. Biochem Biophys Res Commun. 1967 Mar 9;26(5):596–601. doi: 10.1016/0006-291x(67)90107-6. [DOI] [PubMed] [Google Scholar]
- Dyson R. D. A procedure for the extraction of DNA and protein ghosts from bacteriophage lambda. Biochem Biophys Res Commun. 1966 Jan 4;22(1):106–111. doi: 10.1016/0006-291x(66)90610-3. [DOI] [PubMed] [Google Scholar]
- KAISER A. D., HOGNESS D. S. The transformation of Escherichia coli with deoxyribonucleic acid isolated from bacteriophage lambda-dg. J Mol Biol. 1960 Dec;2:392–415. doi: 10.1016/s0022-2836(60)80050-2. [DOI] [PubMed] [Google Scholar]
- KAUFMAN H. E., NESBURN A. B., MALONEY E. D. IDU therapy of herpes simplex. Arch Ophthalmol. 1962 May;67:583–591. doi: 10.1001/archopht.1962.00960020583012. [DOI] [PubMed] [Google Scholar]
- KORN D., WEISSBACH A. Thymineless induction in Escherichia coli K12 (lambda). Biochim Biophys Acta. 1962 Nov 26;61:775–790. doi: 10.1016/0926-6550(62)90060-9. [DOI] [PubMed] [Google Scholar]
- LEVINE L., BARLOW J. L., VAN VUNAKIS H. An internal protein in T2 and T4 bacteriophages. Virology. 1958 Dec;6(3):702–717. doi: 10.1016/0042-6822(58)90116-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Vilcek J., Freer J. H. Inhibition of Sindbis virus plaque formation by extracts of Escherichia coli. J Bacteriol. 1966 Dec;92(6):1716–1722. doi: 10.1128/jb.92.6.1716-1722.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]



