Abstract
Eight proteins potentially involved in cholesterol efflux [ABCA1, ABCG1, CYP27A1, phospholipid transfer protein (PLTP), scavenger receptor type BI (SR-BI), caveolin-1, cholesteryl ester transfer protein, and apolipoprotein A-I (apoA-I)] were overexpressed alone or in combination in RAW 264.7 macrophages. When apoA-I was used as an acceptor, overexpression of the combination of ABCA1, CYP27A1, PLTP, and SR-BI (Combination I) enhanced the efflux by 4.3-fold. It was established that the stimulation of efflux was due to increased abundance of ABCA1 and increased apoA-I binding to non-ABCA1 sites on macrophages. This combination caused only a small increase of the efflux to isolated HDL. When HDL was used as an acceptor, overexpression of caveolin-1 or a combination of caveolin-1 and SR-BI (Combination II) was the most active, doubling the efflux to HDL, without affecting the efflux to apoA-I. When tested in the in vivo mouse model of cholesterol efflux, overexpression of ABCA1 and Combination I elevated cholesterol export from macrophages to plasma, liver, and feces, whereas overexpression of caveolin-1 or Combination II did not have an effect. We conclude that pathways of cholesterol efflux using apoA-I as an acceptor make a predominant contribution to cholesterol export from macrophages in vivo.
Supplementary key words: reverse cholesterol transport, atherosclerosis, lipoproteins, lipids, ABC transporters
Cholesterol efflux is the first and most likely the rate-limiting step of reverse cholesterol transport (RCT). RCT is a pathway removing excess cholesterol from extrahepatic cells, most importantly vascular cells, thus protecting against development of atherosclerosis. Several pathways of cholesterol efflux have been described. One is a diffusion pathway, which does not depend on any specific cellular protein. Several specific pathways involve a number of cellular proteins. First are the ABC transporters ABCA1 (1) and ABCG1 (2), mediating cholesterol efflux to lipid-free apolipoprotein A-I (apoA-I) and mature HDL, respectively. Scavenger receptor type BI (SR-BI) is another cellular protein involved in cholesterol efflux for which HDL is an acceptor (3). We have recently demonstrated that CYP27A1 (4) and caveolin-1 (5) are also involved in cholesterol efflux. A number of other proteins have been demonstrated to contribute to cholesterol efflux, including intracellular apoA-I (6), phospholipid transfer protein (PLTP) (7), and cholesteryl ester transfer protein (CETP) (8); the mechanisms of their involvement in cholesterol efflux are mostly unknown.
Also unknown is how the different pathways and their components interact with each other, whether they are synergistic, competing, or redundant, and what the relative contribution of each pathway is to the overall export of cholesterol from cells. It has been suggested that ABCA1 and ABCG1 work in sequence and in synergy, leading to the formation of cholesterol-rich HDL (9–11), whereas SR-BI competes with ABCA1 and ABCG1 for cholesterol destined for efflux (12, 13); interactions between other components of the cholesterol efflux pathway have not been investigated. Easily transfectable cell lines were used in the majority of the studies; it is, however, unclear whether these observations accurately describe cholesterol efflux pathways in cells of the vessel wall, most importantly, macrophages. There are considerable differences in the pathways of cholesterol efflux and their regulation in different cells and tissues (14). Various efflux pathways use different cholesterol acceptors, and the contribution of these acceptors, and consequently of the cellular proteins interacting with these acceptors, to overall cholesterol export is unclear. The majority of apoA-I in plasma is present in the lipidated form, and yet inactivation of ABCA1, which uses lipid-free apoA-I as acceptor, results in severe atherosclerosis (15, 16), whereas inactivation of ABCG1, which uses lipidated apoA-I, has a small and inconsistent effect on atherosclerosis (17–19).
In this study, we took advantage of a new method of high-efficiency transfection of macrophages (20) to selectively enhance various pathways of cholesterol efflux and to investigate the contributions of the pathways using apoA-I versus those using HDL as acceptors to overall cholesterol export from macrophages in vitro and in vivo.
MATERIALS AND METHODS
Cells and plasmids
RAW 264.7 mouse macrophage cells were maintained and transfected as described previously (20). THP-1 cells were maintained in RPMI supplemented with 10% FBS. Cells were differentiated into macrophage-like cells by incubation in RPMI supplemented with 10% FBS, 100 nM phorbol 12-myristate 13-acetate (PMA) and 100 nM vitamin D3 for 48 h. Differentiated cells were transfected using Metafectene (Biontex, Munich, Germany) and manufacturer’s protocol and cultured in RPMI supplemented with 10% FBS, 100 nM PMA, 100 nM vitamin D3, and 1 μM 5-azacytidine. Plasmids containing human ABCA1 and human ABCG1-GFP were a kind gift from Dr. A. Remaley, plasmid containing ABCG1-myc was a gift from Dr. W. Jessup. Plasmid containing mouse SR-BI was a gift from Drs. Z. Chen and A. Bocharov, and plasmid containing human PLTP was a gift from Dr. C. Ehnholm. Genes for human apoA-I, caveolin-1, and CYP27A1 have been described previously (4, 5, 21). All genes were cloned into pcDNA3.1 plasmid. pCMV-β-Gal plasmid was used for mock transfections and to monitor efficiency of transfection.
Lipoproteins
HDL was isolated from human plasma (pooled plasma supplied by the Red Cross) by sequential centrifugation. ApoA-I was isolated from HDL as described previously (22). LDL was purified from human plasma by sequential centrifugation and acetylated as described by Basu et al. (23). ApoB-deficient plasma was obtained from normolipidemic plasma by precipitating the apoB-containing lipoproteins with 0.9 g/l dextran sulfate/45 mM MgCl2.
Immunoblotting
Cells were treated as indicated, washed, and harvested. Proteins were separated on SDS-PAGE, followed by immunoblotting. In brief, PolyVinyliDiene Fluoride membranes were blocked with 2.5% skim milk solution, washed, and incubated for 1 h at room temperature with the antibodies against ABCA1 [monoclonal, NDF4C2 (24)], ABCG1 (polyclonal, Abcam ab52617), PLTP (polyclonal, Abcam ab7735), SR-BI (polyclonal, Abcam ab369), caveolin-1 (polyclonal, BD Transduction Laboratories), or myc (monoclonal, clone 9E10) and for 1 h with anti-rabbit or anti-mouse IgG secondary antibodies or biotin-anti-mouse IgM and streptavidin-HRP conjugate. Bands were visualized by SuperSignal West Pico Chemiluminescent Substrate kit (Pierce), and the relative intensities of the bands were quantitated by densitometry. The abundance of β-actin was used as loading control (monoclonal antibody from Sigma).
Cholesterol efflux
Cellular cholesterol was labeled by incubating cells in serum-containing medium with [1α,2α(n)-3H]cholesterol (Amersham, final radioactivity 0.5 MBq/ml) for 48 h in a CO2 incubator. Cells were then washed and incubated for 18 h at 37°C in serum-free medium, and washed and incubated for another 3 h at 37°C in serum-free medium containing 30 μg/ml of lipid-free apoA-I or HDL, 2% human apoB-depleted plasma, or 5 mM methyl-β-cyclodextrin. The medium was collected, centrifuged for 15 min at 4°C at 10,000 g, and aliquots of supernatant were counted in a β-counter. Cells were harvested, and cell-associated radioactivity was counted. Cholesterol efflux was expressed as the proportion of [3H]cholesterol transferred from cells to medium. Cholesterol mass was measured using the colorimetric technique.
Cholesterol trafficking
The abundance of cholesterol in cholesterol-rich domains and trafficking of cholesterol to these domains were assessed as described previously (25). In brief, cells were labeled with [14C] cholesterol (final radioactivity 0.5 MBq/ml) for 48 h at 37°C, and washed and labeled with [3H]acetate (final radioactivity 5 MBq/ml) for 3 h at 15°C. Cells were then warmed to 37°C for 20 min, cooled to 4°C, washed, and treated with cholesterol oxidase (1 U/ml) for 3 h at 4°C. Cellular lipids were isolated and separated on TLC as described (25).
ApoA-I binding
To measure apoA-I binding specifically to ABCA1, a cross-linking assay was used as described by Wang et al. (26). Briefly, transfected cells were incubated with apoA-I (50 μg/ml) for 1 h at 37°C and washed. Dithiobis(succinimidyl)propionate (DSP, Pierce) was added to the final concentration of 250 μM and incubated for 1 h at room temperature. Cells were then lysed with RIPA buffer and incubated with polyclonal anti-ABCA1 antibody (Novus) overnight at 4°C. Complex of ABCA1 and antibodies was immunoprecipitated with Protein G Sepharose by coincubation for 4 h at 4°C. Immunoprecipitated and unbound fractions were diluted with loading buffer containing 5% 2-mercaptoethanol, boiled for 5 min, and subjected to 12% SDS-PAGE and Western blot; apoA-I was detected using monoclonal anti-apoA-I antibody AI-4.1.
In vivo studies
Cholesterol efflux in vivo was measured as described by Wang et al. (10) with modifications. Briefly, RAW 264.7 macrophage cells were transiently transfected with indicated plasmids as described above. Cells were simultaneously radiolabeled and loaded with cholesterol by incubation for 48 h with 1.1 GBq/ml [3H]cholesterol and acetylated LDL (50 μg/ml) for 48 h. Cells were washed, incubated for 24 h in serum-free medium, harvested, and resuspended in 0.15 M sterile saline at a concentration of 107 cells/ml. Cells were injected intraperitoneally into male C57BL/6 mice (2 × 106 cells containing 5 × 106 dpm per mouse). Each group consisted of six animals. After 24 h, mice were euthanized, and blood, liver, and feces were collected. Aliquots of plasma were counted, and cholesterol from liver and feces was extracted according to Folch, Lees, and Sloane-Stanley (27) Animal experimentation was approved by the Alfred Medical Research and Education Precinct Animal Ethics Committee; experiments were conducted in compliance with the “Principles of laboratory animal care” (NIH publication No 85-23) and Australian laws.
Statistical analysis
All experiments were reproduced two to four times, and representative experiments are shown. Unless indicated otherwise, experimental groups consisted of quadruplicates; means ± SEM are presented. The Student’s t-test was used to determine statistical significance of the differences.
RESULTS
Overexpression of various proteins and cholesterol efflux to apoA-I or HDL in vitro
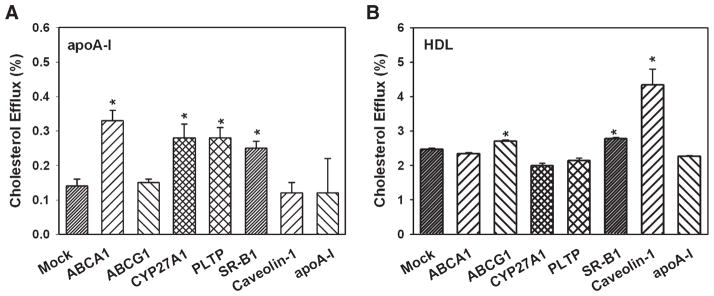
Eight proteins previously implicated in their involvement in cholesterol efflux were overexpressed in mouse macrophages RAW 264.7 using a previously described protocol (20), which provided an 80% efficiency of transfection and uniform expression of the heterologous gene in the entire cellular population. Unless indicated, cells were not loaded with cholesterol, because loading would have upregulated several cholesterol efflux pathways, including ABC transporters. When lipid-free apoA-I was used as an acceptor, overexpression of ABCA1 elevated cholesterol efflux by 2.4-fold, whereas overexpression of CYP27A1, PLTP, and SR-BI elevated cholesterol efflux by about 2-fold (Fig. 1A). Overexpression of ABCG1, caveolin-1, and apoA-I did not affect cholesterol efflux to apoA-I (Fig. 1A); as we demonstrated previously, overexpression of CETP also did not affect cholesterol efflux (28). When HDL was used as an acceptor, overexpression of caveolin-1 increased cholesterol efflux by 1.8-fold; small but statistically significant increases of cholesterol efflux were also observed after overexpression of ABCG1 and SR-BI (Fig. 1B). Overexpression of ABCA1 did not affect cholesterol efflux to HDL. A moderate effect of ABCA1 overexpression on the efflux to HDL observed by Okuhira et al. (29) was attributed to the efflux to lipid-free apoA-I dissociated from HDL. The amount of lipid-free apoA-I in fresh preparations of HDL used in this study was undetectable by native PAGE and Western blot; if a small amount was still present, this amount was insufficient to contribute to the overall efflux to HDL.
Fig. 1.
Overexpression of various genes and cholesterol efflux from macrophages. RAW 264.7 cells were transiently transfected with the indicated genes. Cells were labeled with [3H]cholesterol, and cholesterol efflux to lipid-free apolipoprotein A-I (apoA-I) (A) or HDL (B) (final concentration of both, 30 μg/ml) was assessed as described in Materials and Methods. Cholesterol efflux was expressed as a proportion of [3H]cholesterol transferred from cells to medium. Means ± SEM of quadruplicate determinations are shown. * P < 0.05 versus mock-transfected cells.
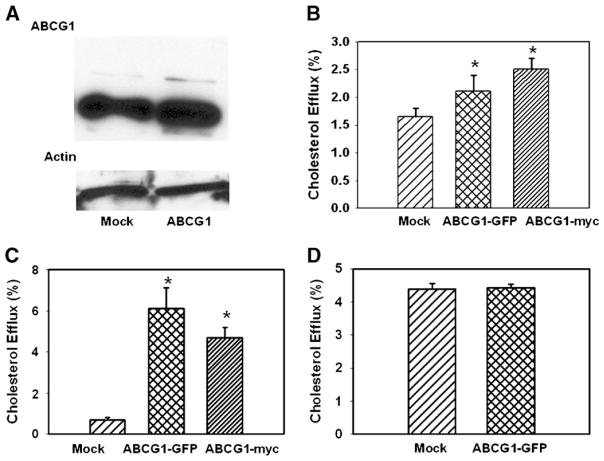
The small effect of ABCG1 overexpression on cholesterol efflux to HDL was unexpected and inconsistent with the results of studies overexpressing ABCG1 in other cell types (30), but was not dramatically different from the only study overexpressing ABCG1 in murine macrophages (10). One reason for this result could be the fact that in the present study, we used ABCG1-GFP conjugate, which may have properties different from those of ABCG1. We therefore tested another construct, ABCG1-myc. ABCG1-myc also caused only a small stimulation of cholesterol efflux from RAW 264.7 macrophages (not shown).
Another reason for the modest effect of ABCG1 overexpression in macrophages could be a high level of ABCG1 in RAW 264.7 cells (as reflected by the high level of efflux to HDL from nonactivated RAW 264.7 cells), reducing possible effects of its overexpression. Indeed, a relatively high level of ABCG1 was found in nonactivated RAW 264.7 cells (Fig. 2A), and although transfection increased the ABCG1 level by 70% (6.1 vs. 3.6 relative units, Fig. 2A), the concentration of endogenous ABCG1 may already have been approaching a functionally saturating level. To further test this possibility, we examined the overexpression of ABCG1 plasmids in three other cell types. Two human cell lines, HeLa cells, a cell model lacking ABC transporters, and THP-1 human macrophages, known to express low levels of ABC transporters unless activated with LXR agonist, were tested. Compared with RAW 264.7 cells, cholesterol efflux to HDL from mock-transfected HeLa and THP-1 cells was 1.6-fold and 3.6-fold lower, respectively. Overexpression of both ABCG1-myc and ABCG1-GFP elevated cholesterol efflux by approximately 1.5-fold and 7.5-fold in HeLa and THP-1 cells, respectively. (Fig. 2B, C). The third cell line tested was mouse 3T3 fibroblasts. Cholesterol efflux to HDL from these cells was 1.8-fold higher compared with RAW 264.7 cells, and overexpression of ABCG1 had no effect on cholesterol efflux to HDL (Fig. 2D). Thus, it appears that overexpression of ABCG1 stimulated cholesterol efflux to HDL from cells with a low basal level of the efflux, but not from cells with a high basal level of cholesterol efflux, pointing to a possibility that the ABCG1 level in the latter was functionally saturated. However, we cannot exclude a possibility of species-specific differences, inasmuch as overexpression of ABCG1 stimulated cholesterol efflux from human cells but not murine cells.
Fig. 2.
The effect of overexpression of ABCG1 on ABCG1 abundance in RAW 264.7 cells (A) and cholesterol efflux to HDL from HeLa cells (B), THP-1 macroophages (C), and 3T3 fibroblasts (D). A: RAW 264.7 cells were transiently transfected with ABCG1-GFP and the abundance of ABCG1 was assessed by Western blot. B–D: HeLa cells (B), THP-1 human macrophages (C), or 3T3 murine fibroblasts (D) were transfected as indicated and labeled with [3H]cholesterol, and cholesterol efflux to HDL (final concentration 30 μg/ml) was assessed as described in Materials and Methods. Cholesterol efflux was expressed as a proportion of [3H]cholesterol transferred from cells to medium. Means ± SEM of quadruplicate determinations are shown. * P < 0.05 versus mock-transfected cells.
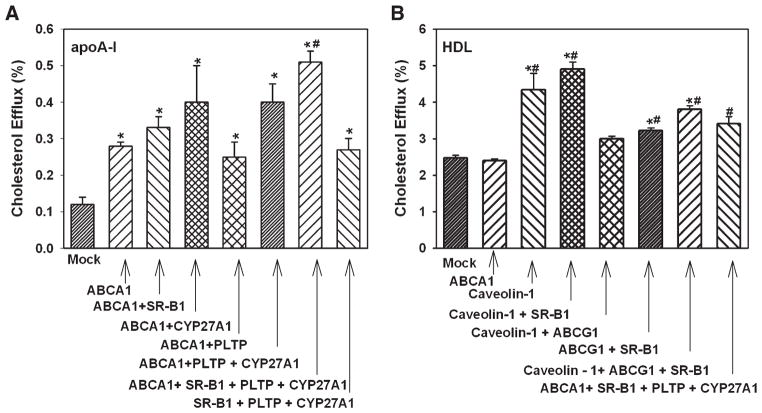
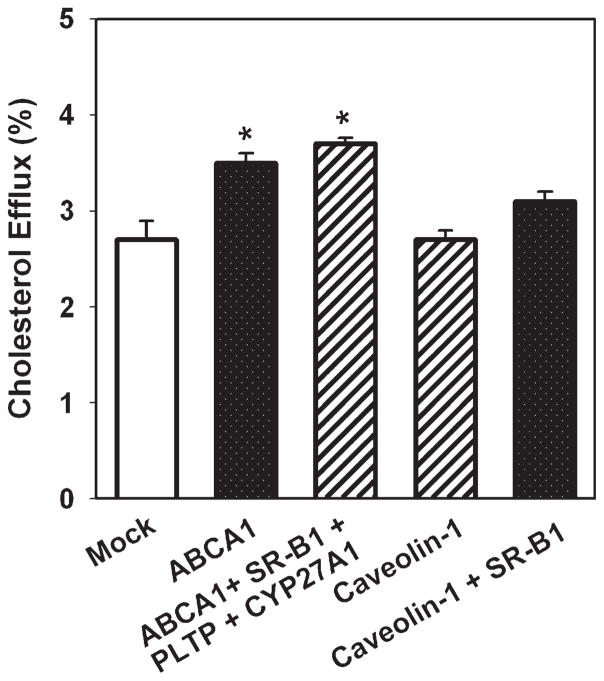
To test the effect of overexpression of combinations of the proteins, the following approach was used. The cells were cotransfected with the one of two proteins that had the biggest effect on cholesterol efflux, i.e., ABCA1 or CYP27A1 for the efflux to apoA-I and caveolin-1 or SR-BI for the efflux to HDL, and one of the remaining seven proteins. The combination of two proteins that caused the biggest effect was then cotransfected with one of the remaining six proteins, and so on. The maximum number of proteins we were able to coexpress was four; coexpression of the fifth protein caused a sharp reduction of cholesterol efflux independently of what the combination was.
The results of the analysis of the effects of overexpression of combinations of proteins are presented in Fig. 3. When apoA-I was used as an acceptor, overexpression of SR-BI or CYP27A1 together with ABCA1 added to the stimulation of cholesterol efflux, whereas addition of PLTP did not (Fig. 3A). The combination of proteins that caused the biggest stimulation of cholesterol efflux was ABCA1 + SR-BI + PLTP + CYP27A1; this combination caused a 4.3-fold stimulation of cholesterol efflux (Fig. 3A). The combination of SR-BI + PLTP + CYP27A1 without ABCA1 was less effective, causing a 2-fold increase of cholesterol efflux. When HDL was used as an acceptor, overexpression of SR-BI together with caveolin-1 slightly increased cholesterol efflux; the difference with overexpression of caveolin-1 alone, however, was not statistically significant (Fig. 3B). All other combinations only minimally affected cholesterol efflux to HDL. Importantly, overexpression of a combination of ABCA1 + SR-BI + PLTP + CYP27A1 that caused a considerable elevation of cholesterol efflux to apoA-I caused only a small (30%) elevation of cholesterol efflux to HDL (Fig. 3B).
Fig. 3.
Overexpression of a combination of genes and cholesterol efflux from macrophages. RAW 264.7 cells were transiently transfected with the indicated genes. Cells were labeled with [3H]cholesterol, and cholesterol efflux to lipid-free apoA-I (A) or HDL (B) (final concentration of both, 30 μg/ml) was assessed as described in Materials and Methods. Cholesterol efflux was expressed as a proportion of [3H] cholesterol transferred from cells to medium. Means ± SEM of quadruplicate determinations are shown. * P < 0.05 versus mock-transfected cells. # P < 0.01 versus ABCA1.
Although the efflux of labeled cholesterol to lipid-free apoA-I reflects the movement of cholesterol mass, the efflux to HDL is accompanied by cholesterol exchange between cells and HDL, therefore not necessarily reflecting the movement of cholesterol mass. When total cholesterol mass was measured in the cells and the medium, incubation with HDL resulted in 4% of cellular cholesterol moved to HDL after 2 h. However, there was no statistically significant difference between mock-transfected cells and cells transfected with ABCA1, ABCA1 + SR-BI + PLTP + CYP27A1, caveolin-1 or caveolin-1 + SR-BI (not shown).
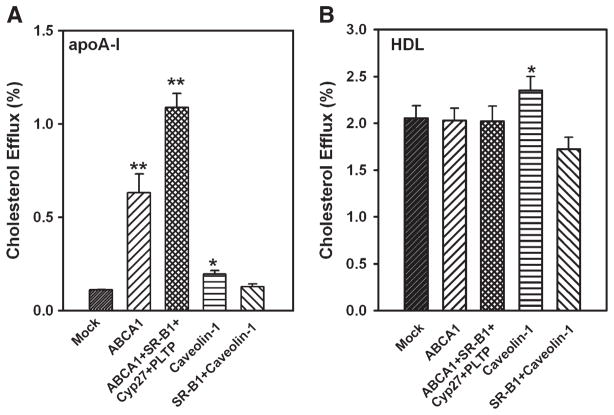
To further characterize the effect of overexpression of combinations of proteins on cholesterol efflux, the above experiments were repeated with cholesterol-loaded cells. Transfected cells were loaded with cholesterol by incubation with acetylated LDL (50 μg/ml) for 24 h. When apoA-I was used as an acceptor, transfection with ABCA1 and with ABCA1 + SR-BI + PLTP + CYP27A1 elevated cholesterol efflux by 6- and 11-fold, respectively (Fig. 4A). Transfection of cells with caveolin-1 marginally increased cholesterol efflux (P = 0.04), whereas transfection with a combination of caveolin-1 + SR-BI had no statistically significant effect (Fig. 4A). When HDL was used as an acceptor, only transfection with caveolin-1 increased cholesterol efflux (Fig. 4B). The effect of caveolin-1 was attenuated in cholesterol-loaded cells, suggesting that the contribution of this pathway to cholesterol efflux to HDL may have changed after cholesterol loading, i.e., due to upregulation of ABC transporters.
Fig. 4.
Overexpression of a combination of genes and cholesterol efflux from cholesterol-loaded macrophages. RAW 264.7 cells were transiently transfected with the indicated genes. Cells were loaded with cholesterol by incubation with acetylated LDL (50 μg/ml) for 24 h and simultaneously labeled with [3H]cholesterol. Cholesterol efflux to lipid-free apoA-I (A) or HDL (B) (final concentration of both, 30 μg/ml) was assessed as described in Materials and Methods. Cholesterol efflux was expressed as a proportion of [3H]cholesterol transferred from cells to medium. Means ± SEM of quadruplicate determinations are shown. * P < 0.05, ** P < 0.001 (versus mock-transfected cells).
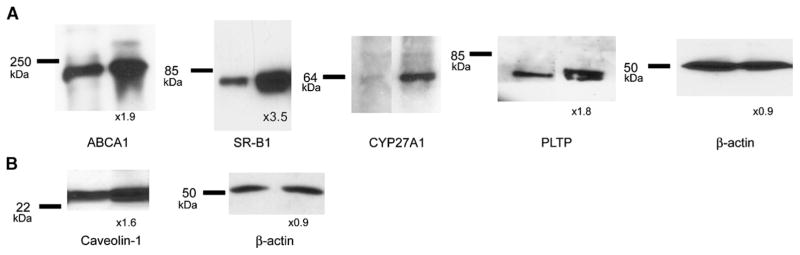
To assess the increment of abundance of each protein after overexpression of the combination of genes, cells were cotransfected with ABCA1 + SR-BI + PLTP + CYP27A1 or transfected with caveolin-1 alone, and expression of each protein was assessed by Western blotting and compared with its expression in mock-transfected cells (Fig. 5). The abundances of ABCA1, SR-BI, and PLTP were increased by 1.9-, 3.5-, and 1.8-fold, respectively (Fig. 5A). These values represent a combination of abundances of endogenous and heterologous proteins, and although it cannot be ruled out that expression of endogenous proteins was affected, it is more likely that most of the increment was due to heterologous expression. The exception is the abundance of endogenous ABCA1, which could be affected by CYP27A1 and PLTP; this issue is examined below. Overexpression of CYP27A1 was assayed using anti-myc antibody, preventing a comparison with the basal expression of CYP27A. Overexpression of caveolin-1 resulted in a 1.6-fold increase of its abundance (Fig. 5B). Transfection of cells did not affect abundance of β-actin (Fig. 5A, B).
Fig. 5.
The effect of overexpression of a combination of genes on their abundance. RAW 264.7 cells were transiently transfected with ABCA1 + scavenger receptor type BI (SR-BI) + phospholipid transfer protein (PLTP) + CYP27A1 (A) or caveolin-1 (B). The abundance of individual proteins was assessed by Western blot and quantitated by densitometry as described in Materials and Methods. Numbers under the blots indicate increase of abundance of the indicated protein (fold, relative to mock-transfected cells).
Cholesterol efflux to plasma in vitro
Testing the effects of overexpression of various proteins on cholesterol efflux resulted in establishing two distinct cellular models. One model, RAW 264.7 cells cotransfected with ABCA1 or ABCA1 + SR-BI + PLTP + CYP27A1, had elevated efflux to lipid-free apoA-I, but not to HDL. Another model, RAW 264.7 cells transfected with caveolin-1 or caveolin-1 + SR-BI had elevated efflux to HDL, but not to lipid-free apoA-I. These models presented an opportunity to compare the contribution of these two acceptors to cholesterol export in vitro and in vivo. To test the efflux in vitro, we removed apoB-containing lipoproteins from plasma by precipitation with dextran-sulfate, thus minimizing nonspecific (diffusional) efflux. The plasma was used at a concentration of 2%, the concentration within the linear part of the dose-dependent curve (not shown). When apoB-depleted human plasma was used as an acceptor, overexpression of ABCA1 or ABCA1 + SR-BI + PLTP + CYP27A1 stimulated cholesterol efflux by 30% and 40%, respectively, whereas overexpression of caveolin-1 or caveolin-1 + SR-BI failed to affect cholesterol efflux (Fig. 6). The latter finding was unexpected, inasmuch as lipidated HDL represents the majority of plasma apoA-I; this is consistent, however, with findings showing that plasma HDL level is not always a major determinant of plasma cholesterol efflux capacity (31, 32). We hypothesize that the increase of cholesterol efflux was mainly due to the efflux to plasma lipid-free apoA-I. These data indicate that cholesterol efflux from macrophages may be enhanced by manipulating cellular pathways responsible for the efflux to apoA-I.
Fig. 6.
Overexpression of various genes and cholesterol efflux to human plasma. RAW 264.7 cells were transiently transfected with the indicated genes. Cells were labeled with [3H]cholesterol, and cholesterol efflux to 2% apoB-depleted human plasma was assessed as described in Materials and Methods. Cholesterol efflux was expressed as a proportion of [3H]cholesterol transferred from cells to medium. Means ± SEM of quadruplicate determinations are shown. * P < 0.05 versus mock-transfected cells.
Cholesterol efflux in vivo
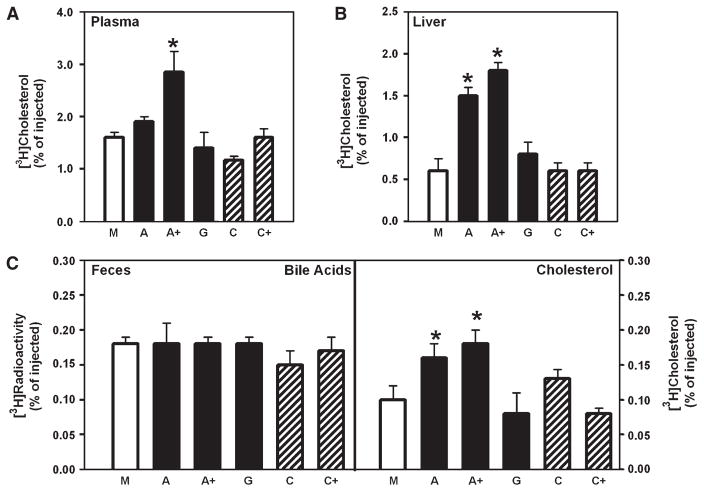
To further test the effect of stimulation of cholesterol efflux to apoA-I or HDL on cholesterol export from macrophages, the in vivo model described by Wang et al. (10) was used. Labeled cholesterol-loaded macrophages overexpressing indicated proteins were implanted into the peritoneal cavity of mice; the appearance of labeled cholesterol in plasma, liver, and feces was followed after 24 h. Overexpression of various genes or their combinations had no effect on cholesterol loading of cells, inasmuch as the cellular cholesterol content and the specific activity of cellular [3H]cholesterol were not affected by transfections. Overexpression of ABCA1 (“A”) only slightly increased the export of labeled cholesterol to plasma, but increased the amount of labeled cholesterol in the liver and feces by 2.5- and 1.6-fold, respectively (Fig. 7A–C). This finding is consistent with that of Calpe-Berdiel et al. (33) and Wang et al. (10), who demonstrated impaired cholesterol transport from ABCA1-deficient macrophages in vivo. Overexpression of ABCA1 + SR-BI + PLTP + CYP27A1 (“A+”) increased the export of cholesterol to plasma, liver, and feces by 1.8-, 3.0-, and 1.8-fold, respectively (Fig. 7A–C). Overexpression of ABCG1 (“G”), caveolin-1 (“C”), or caveolin-1 + SR-BI (“C+”) did not affect cholesterol export from macrophages to plasma, liver, or feces. Appearance of labeled cholesterol in the bile acid fraction of feces was not affected by any of the transfections (Fig. 7C, left panel).
Fig. 7.
Overexpression of various genes and cholesterol efflux in vivo. RAW 264.7 macrophages were transfected with mock (M), ABCA1 (A), ABCA1 + SR-BI + PLTP+ CYP27A1 (A+), ABCG1 (G), caveolin-1 (C), or caveolin-1+ SR-BI (C+), loaded with cholesterol, labeled with [3H]cholesterol, and injected intraperitoneally into mice. The appearance of label in plasma (A), liver (B), and feces (C) was assessed after 24 h as described in Materials and Methods. Means ± SEM (n = 6) are shown; * P < 0.01 versus mock-transfected.
Mechanisms of cholesterol efflux stimulation
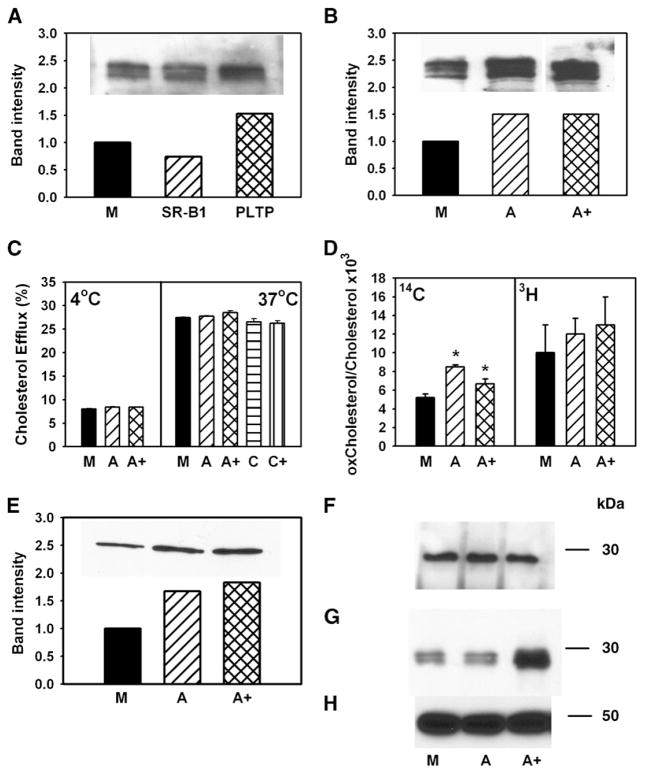
The effect of overexpression of ABCA1 on cholesterol efflux was expected, but the mechanism of how SR-BI, PLTP, and CYP27A1 further stimulated the efflux was unclear. The first possibility we considered was that SR-BI, PLTP, and CYP27A1 increased the abundance of ABCA1. We have previously demonstrated that overexpression of CYP27A1 increases the abundance of ABCA1 in RAW 246.7 macrophages (20). We further found that transfection of RAW 246.7 cells with PLTP also increased the abundance of ABCA1 in the cells (Fig. 8A), a finding consistent with that of Oram et al. (7). In contrast, overexpression of SR-BI, if anything, decreased the abundance of ABCA1, a finding consistent with the observations of Chen et al. (12). However, if the abundance of ABCA1 was compared in the cells transfected with ABCA1 alone or with the combination ABCA1 + SR-BI + PLTP + CYP27A1, the abundance of ABCA1 was similar in single transfections and in transfections with a combination of genes (Fig. 8B). Thus, although enhancement of ABCA1 abundance explains stimulation of cholesterol efflux by PLTP and CYP27A1 alone (Fig. 1A) and in combination with each other (Fig. 3), it does not explain further stimulation of cholesterol efflux by the ABCA1 + SR-BI + PLTP + CYP27A1 combination.
Fig. 8.
Possible mechanisms of stimulation of cholesterol efflux. A, B: RAW 264.7 cells were transiently transfected with mock (M), SR-BI, PLTP, ABCA1 (A), or ABCA1 + SR-BI + PLTP+ CYP27A1 (A+). The abundance of ABCA1 in cells was assessed by Western blotting. C: RAW 264.7 cells were transiently transfected with mock (M), ABCA1 (A), ABCA1 + SR-BI + PLTP+ CYP27A1 (A+), caveolin-1 (C), or caveolin-1 + SR-BI (C+). Cells were labeled with [3H]cholesterol, and cholesterol efflux to methyl-β-cyclodextrin (final concentration 5 mM) at 4°C (left) or 37°C (right) was assessed as described in Materials and Methods. Cholesterol efflux was expressed as a proportion of [3H]cholesterol transferred from cells to medium. Means ± SEM of quadruplicate determinations are shown. D: RAW 264.7 cells were transiently transfected with mock (M), ABCA1 (A), or ABCA1 + SR-BI + PLTP + CYP27A1 (A+). Cells were labeled with [14C]cholesterol for 48 h at 37°C, washed, and labeled with [3H]acetate for 3 h at 15°C. Cells were then warmed to 37°C for 20 min, cooled to 4°C, washed, and treated with cholesterol oxidase (final concentration 1 U/ml) for 3 h at 4°C. Cellular lipids were isolated and separated on TLC as described in Materials and Methods. The ratio of oxidized cholesterol to cholesterol is shown. * P < 0.01 (versus mock-transfected cells). E: RAW 264.7 cells were transiently transfected with mock (M), ABCA1 (A), or ABCA1 + SR-BI + PLTP+ CYP27A1 (A+). The abundance of ABCA1 on cell surface was assessed as accessibility of ABCA1 to biotinilation as described in Materials and Methods. F, G: RAW 264.7 cells were transiently transfected with mock (M), ABCA1 (A), or ABCA1 + SR-BI + PLTP+ CYP27A1 (A+). ApoA-I was added to the final concentration of 50 μg/ml, incubated for 1 h at 37°C, and washed out. Dithiobis(succinimidyl)propionate was added to the final concentration of 250 μM and incubated for 1 h at room temperature. Cells were then lysed, and ABCA1 was immunoprecipitated with polyclonal anti-ABCA1 antibodies. Precipitated (F) and unbound (G) fractions were diluted with loading buffer containing 5% 2-mercaptoethanol, boiled for 5 min, and subjected to SDS-PAGE and Western blot using monoclonal antibody against apoA-I (F, G) or β-actin (H).
We next investigated whether the difference in cholesterol efflux was due to increased amount of cholesterol on the plasma membrane or enhanced trafficking of cholesterol to the plasma membrane. Labeled cells were incubated for 1 h with 5 mM methyl-β-cyclodextrin at either 4°C or 37°C. Rapid nonspecific removal of cholesterol by cyclodextrin at 4°C, when cholesterol trafficking is blocked, was used to assess the amount of cholesterol on the plasma membrane (34). At 37°C, cholesterol efflux to cyclodextrin is a reflection of the amount of both preexisting cholesterol and cholesterol delivered to the plasma membrane during the 1 h incubation (34). Overexpression of neither ABCA1 nor ABCA1 + SR-BI + PLTP + CYP27A1 had any effect on the efflux to cyclodextrin at 4°C and 37°C (Fig. 8C), indicating that neither the amount of cholesterol located on the plasma membrane nor its delivery to the plasma membrane was affected by the transfections. Overexpression of caveolin-1 or the caveolin-1 + SR-BI combination also did not affect cholesterol efflux to cyclodextrin at 37°C (Fig. 8C). Thus, the observed effects on cholesterol efflux to both lipid-free apoA-I and HDL were specific.
To assess the amount and the rate of delivery of cholesterol to specifically exposed cholesterol-rich domains, we investigated the effect of transfections on the susceptibility of total and newly synthesized cholesterol to oxidation by cholesterol oxidase, a method we employed previously to investigate trafficking of cholesterol to cholesterol-rich domains (25). Cells were labeled with [14C]cholesterol for 48 h at 37°C to label the entire cellular cholesterol pool, and with [3H]acetate for 3 h at 15°C to label the intracellular newly synthesized cholesterol pool, followed by 20 min incubation at 37°C to allow some of the newly synthesized cholesterol to move to the plasma membrane. The total amount of cholesterol in the cholesterol-rich domains increased by 30–60% after both ABCA1 and ABCA1 + SR-BI + PLTP + CYP27A1 transfections (Fig. 8D), whereas the rate of transfer of cholesterol to cholesterol-rich domains was not statistically different in transfected versus mock-transfected cells. Thus, consistent with findings of Vaughan and Oram (35), transfection with ABCA1 increased the abundance of cholesterol in cholesterol-rich domains, but this was not the mechanism for the additional increase of cholesterol efflux after cotransfection with SR-BI + PLTP + CYP27A1.
To test whether overexpression of the combination of genes increased the abundance of cell-surface ABCA1, we assessed the accessibility of ABCA1 to biotinylation after transfection with ABCA1 alone and with ABCA1 + SR-BI + PLTP + CYP27A1. As expected, the abundance of ABCA1 on the cell surface was increased in both cases, but the difference between the two transfections was minimal (Fig. 8E). Further, we analyzed binding of apoA-I to cells, and more specifically, the amount of apoA-I bound to ABCA1. ApoA-I was incubated with cells, followed by cross-linking of bound apoA-I to cell surface proteins. ABCA1 was then precipitated with anti-ABCA1 antibody (cross-reacting with human and mouse ABCA1) (24), and the amount of apoA-I bound to ABCA1 (precipitated) and the amount bound to other proteins (supernatant) were assessed by Western blot. Transfections did not affect the amount of apoA-I bound to ABCA1 (Fig. 8F), consistent with our previous finding that apoA-I binding to ABCA1 and ABCA1-dependent cholesterol efflux may be dissociated (24). However, there was twice as much apoA-I in the supernatant fraction (Fig. 8G), indicating that there is an enhanced binding of apoA-I to cells after transfection with ABCA1 + SR-BI + PLTP + CYP27A1, but the binding site was not ABCA1. This fraction was then immunoprecipitated with anti-apoA-I antibodies and developed with anti-PLTP, anti-SR-BI, or anti-myc antibodies. None of the three proteins coprecipitated with apoA-I (data not shown). Thus, coexpression of SR-BI, PLTP, and CYP27A with ABCA1 may enhance cholesterol efflux by increasing binding of apoA-I to the cells. The exact nature of the binding sites and their composition are not clear; they may include binding to protein and/or lipid components of the plasma membrane, i.e., phospholipid-rich domains.
DISCUSSION
A number of cholesterol efflux pathways were described, but the relative contribution of each of them to cholesterol export from cells remains unclear. Inactivation of a specific efflux pathway in some cells results in significant impairment of cholesterol export and accumulation of cholesterol, whereas in other cells, the same inactivation has minimal effect on cholesterol efflux and cholesterol accumulation. For example, ABCA1 knockout in mouse macrophages, resulting in a severe impairment of cholesterol efflux, leads to an accumulation of cholesterol in these cells (36), but there is no accumulation of cholesterol in Tangier fibroblasts or endothelial cells lacking ABCA1 (11). Further, overexpression of CYP27A1 (4, 20), caveolin-1 (5), and ABCG1 (30) leads to a significant enhancement of cholesterol efflux in vitro, and yet knockout of these genes in vivo has only a modest effect on cholesterol accumulation in macrophages (19, 37, 38). These and other results indicate that different cells may use different pathways for cholesterol efflux to maintain cholesterol homeostasis and/or there is a considerable redundancy in the pathways of cholesterol export. Another possibility is that utilization of different pathways depends on prevailing metabolic circumstances and intracellular location of the excess cholesterol. Thus, cellular cholesterol pools in macrophages overloaded by exposure to LDL were more effectively reduced by the ABCG1-dependent pathway, whereas cellular pools in cells overloaded by exposure to acetylated LDL were reduced mainly by the ABCA1-dependent pathway (39).
The main finding of this study is that enhancing cholesterol efflux pathways that use apoA-I as acceptor leads to enhanced cholesterol export from macrophages in vivo, whereas enhancing pathways that use HDL as acceptor is ineffective. The fact that cholesterol efflux in vivo was enhanced coordinately with the increase of cholesterol efflux through pathways utilizing apoA-I, but not HDL, indicates that the former may have a predominant contribution to overall cholesterol export or at least to its upregulated component, which can be potentially targeted for treatment. This is consistent with findings that inactivation of macrophage ABCA1, the main element of the cholesterol efflux pathway using apoA-I, promotes atherosclerosis (36), whereas overexpression of ABCA1 protects against atherosclerosis (15). It is also consistent with the recent findings of Adorni et al. (40) that ABCA1-dependent efflux to human serum ex vivo is a predominant cholesterol efflux pathway. Inactivation or overexpression of key elements of the efflux pathway that uses HDL as primary acceptor, such as ABCG1, has been shown to have a limited effect on the development of atherosclerosis (19). It must be recognized, however, that the effects of overexpression and inactivation of proteins are not necessarily complementary. For example, overexpression of a protein may not affect the function if the step it is involved in is not rate-limiting, or if the protein is already at a functionally saturating concentration, as might be the case for ABCG1. Thus, our findings are indicative of possible approaches to enhance RCT rather than of possible causes of its impairment.
Another finding of this study is that CYP27A1, PLTP, and SR-BI supplement ABCA1-dependent cholesterol efflux to apoA-I. The exact mechanism of interaction between these proteins and the role of each individual protein remain unclear. Comparison of cholesterol efflux after various transfections clearly shows the requirement for ABCA1 in this combination, inasmuch as only a modest increase in cholesterol efflux was achieved when ABCA1 was omitted, and even that was probably due to the effect of overexpressed proteins on ABCA1 abundance. However, the abundance of ABCA1 in the cells and the abundance of cell-surface ABCA1 did not increase after coexpression of ABCA1 + CYP27A1 + PLTP + SR-BI over the amount found after overexpression of ABCA1 alone. The amount of cholesterol on the plasma membrane also did not change, and although ABCA1 increased the amount of cholesterol in cholesterol-rich domains, coexpression of three other genes did not further increase it. Neither cholesterol trafficking to the plasma membrane nor binding of apoA-I to ABCA1 was affected by the cotransfections. However, the total amount of apoA-I bound to cells transfected with ABCA1 + CYP27A1 + PLTP + SR-BI doubled, compared with mock- and ABCA1-transfected cells, which may explain the enhanced cholesterol efflux. The additional apoA-I binding sites were not on the four overexpressed proteins, and their nature remains unclear. It has been suggested recently that effective cholesterol efflux requires formation of phospholipid-rich curved membrane domains capable of high-affinity binding to apoA-I (41, 42). It is possible that overexpression of the combination of proteins stimulates the formation of such domains and enhances association of apoA-I with these domains or their protein and/or lipid constituents. The role of individual proteins, especially that of SR-BI, which was mainly implicated in cholesterol efflux to HDL, remains unclear, and the mechanism is not yet defined.
In conclusion, we demonstrated that cholesterol efflux to apoA-I may make a predominant contribution to cholesterol export from macrophages. The key element of this pathway seems to be ABCA1, with CYP27A1, PLTP, and SR-BI playing a supplementary role.
Acknowledgments
This work was supported by a grant from the National Heart Foundation of Australia to D.S. and Z.K. (G 04M 1536), a grant from the Swiss National Foundation to G.E., and by a grant from the Perpetual Charitable Trust to D.S. D.S. is a Fellow of the National Health and Medical Research Council of Australia.
The authors acknowledge the technical assistance of Ms. S. Penfold and Ms. A. Gatt.
Abbreviations
- apo
apolipoprotein
- CETP
cholesteryl ester transfer protein
- PLTP
phospholipid transfer protein
- RCT
reverse cholesterol transport
- SR-BI
scavenger receptor type BI
References
- 1.Oram JF, Vaughan AM. ABCA1-mediated transport of cellular cholesterol and phospholipids to HDL apolipoproteins. Curr Opin Lipidol. 2000;11:253–260. doi: 10.1097/00041433-200006000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Kennedy MA, Barrera GC, Nakamura K, Baldan A, Tarr P, Fishbein MC, Frank J, Francone OL, Edwards PA. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 2005;1:121–131. doi: 10.1016/j.cmet.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Ji Y, Jian B, Wang N, Sun Y, Moya ML, Phillips MC, Rothblat GH, Swaney JB, Tall AR. Scavenger receptor BI promotes high density lipoprotein-mediated cellular cholesterol efflux. J Biol Chem. 1997;272:20982–20985. doi: 10.1074/jbc.272.34.20982. [DOI] [PubMed] [Google Scholar]
- 4.Escher G, Krozowski Z, Croft KD, Sviridov D. Expression of sterol 27-hydroxylase (CYP27A1) enhances cholesterol efflux. J Biol Chem. 2003;278:11015–11019. doi: 10.1074/jbc.M212780200. [DOI] [PubMed] [Google Scholar]
- 5.Fu Y, Hoang A, Escher G, Parton RG, Krozowski Z, Sviridov D. Expression of caveolin-1 enhances cholesterol efflux in hepatic cells. J Biol Chem. 2004;279:14140–14146. doi: 10.1074/jbc.M311061200. [DOI] [PubMed] [Google Scholar]
- 6.Major AS, Dove DE, Ishiguro H, Su YR, Brown AM, Liu L, Carter KJ, Linton MF, Fazio S. Increased cholesterol efflux in apolipoprotein AI (ApoAI)-producing macrophages as a mechanism for reduced atherosclerosis in ApoAI(−/−) Mice. Arterioscler Thromb Vasc Biol. 2001;21:1790–1795. doi: 10.1161/hq1101.097798. [DOI] [PubMed] [Google Scholar]
- 7.Oram JF, Wolfbauer G, Vaughan AM, Tang C, Albers JJ. Phospholipid transfer protein interacts with and stabilizes ATP-binding cassette transporter A1 and enhances cholesterol efflux from cells. J Biol Chem. 2003;278:52379–52385. doi: 10.1074/jbc.M310695200. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Z, Yamashita S, Hirano K, Nakagawa-Toyama Y, Matsuyama A, Nishida M, Sakai N, Fukasawa M, Arai H, Miyagawa J, et al. Expression of cholesteryl ester transfer protein in human atherosclerotic lesions and its implication in reverse cholesterol transport. Atherosclerosis. 2001;159:67–75. doi: 10.1016/s0021-9150(01)00490-7. [DOI] [PubMed] [Google Scholar]
- 9.Gelissen IC, Harris M, Rye KA, Quinn C, Brown AJ, Kockx M, Cartland S, Packianathan M, Kritharides L, Jessup W. ABCA1 and ABCG1 synergize to mediate cholesterol export to ApoA-I. Arterioscler Thromb Vasc Biol. 2006;26:534–540. doi: 10.1161/01.ATV.0000200082.58536.e1. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Collins HL, Ranalletta M, Fuki IV, Billheimer JT, Rothblat GH, Tall AR, Rader DJ. Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J Clin Invest. 2007;117:2216–2224. doi: 10.1172/JCI32057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fielding PE, Nagao K, Hakamata H, Chimini G, Fielding CJ. A two-step mechanism for free cholesterol and phospholipid efflux from human vascular cells to apolipoprotein A-1. Biochemistry. 2000;39:14113–14120. doi: 10.1021/bi0004192. [DOI] [PubMed] [Google Scholar]
- 12.Chen W, Silver DL, Smith JD, Tall AR. Scavenger receptor-BI inhibits ATP-binding cassette transporter 1-mediated cholesterol efflux in macrophages. J Biol Chem. 2000;275:30794–30800. doi: 10.1074/jbc.M004552200. [DOI] [PubMed] [Google Scholar]
- 13.Yvan-Charvet L, Pagler TA, Wang N, Senokuchi T, Brundert M, Li H, Rinninger F, Tall AR. SR-BI inhibits ABCG1-stimulated net cholesterol efflux from cells to plasma HDL. J Lipid Res. 2008;49:107–114. doi: 10.1194/jlr.M700200-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Wang M-D, Franklin V, Sundaram M, Kiss RS, Ho K, Gallant M, Marcel YL. Differential regulation of ATP binding cassette protein A1 expression and ApoA-I lipidation by Niemann-Pick Type C1 in murine hepatocytes and macrophages. J Biol Chem. 2007;282:22525–22533. doi: 10.1074/jbc.M700326200. [DOI] [PubMed] [Google Scholar]
- 15.Singaraja RR, Fievet C, Castro G, James ER, Hennuyer N, Clee SM, Bissada N, Choy JC, Fruchart JC, McManus BM, et al. Increased ABCA1 activity protects against atherosclerosis. J Clin Invest. 2002;110:35–42. doi: 10.1172/JCI15748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joyce CW, Amar MJA, Lambert G, Vaisman BL, Paigen B, Najib-Fruchart J, Hoyt RF, Jr, Neufeld ED, Remaley AT, Fredrickson DS, et al. The ATP binding cassette transporter A1 (ABCA1) modulates the development of aortic atherosclerosis in C57BL/6 and apoE-knockout mice. Proc Natl Acad Sci USA. 2002;99:407–412. doi: 10.1073/pnas.012587699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Out R, Hoekstra M, Meurs I, de Vos P, Kuiper J, Van Eck M, Van Berkel TJC. Total body ABCG1 expression protects against early atherosclerotic lesion development in mice. Arterioscler Thromb Vasc Biol. 2007;27:594–599. doi: 10.1161/01.ATV.0000257136.24308.0c. [DOI] [PubMed] [Google Scholar]
- 18.Basso F, Amar MJ, Wagner EM, Vaisman B, Paigen B, Santamarina-Fojo S, Remaley AT. Enhanced ABCG1 expression increases atherosclerosis in LDLr-KO mice on a western diet. Biochem Biophys Res Commun. 2006;351:398–404. doi: 10.1016/j.bbrc.2006.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Out R, Hoekstra M, Hildebrand RB, Kruit JK, Meurs I, Li Z, Kuipers F, Van Berkel TJC, Van Eck M. Macrophage ABCG1 deletion disrupts lipid homeostasis in alveolar macrophages and moderately influences atherosclerotic lesion development in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2006;26:2295–2300. doi: 10.1161/01.ATV.0000237629.29842.4c. [DOI] [PubMed] [Google Scholar]
- 20.Escher G, Hoang A, Georges S, Tchoua U, El-Osta A, Krozowski Z, Sviridov D. Demethylation using the epigenetic modifier, 5-azacytidine, increases the efficiency of transient transfection of macrophages. J Lipid Res. 2005;46:356–365. doi: 10.1194/jlr.D400014-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Pyle L, Sviridov D, Fidge N. Characterization of the maturation of human pro-apolipoprotein A-I in an in vitro model. Biochemistry. 2001;40:3101–3108. doi: 10.1021/bi002025g. [DOI] [PubMed] [Google Scholar]
- 22.Sviridov D, Pyle L, Fidge N. Efflux of cellular cholesterol and phospholipid to apolipoprotein A-I mutants. J Biol Chem. 1996;271:33277–33283. doi: 10.1074/jbc.271.52.33277. [DOI] [PubMed] [Google Scholar]
- 23.Basu SK, Goldstein JL, Anderson GW, Brown MS. Degradation of cationized low density lipoprotein and regulation of cholesterol metabolism in homozygous familial hypercholesterolemia fibroblasts. Proc Natl Acad Sci USA. 1976;73:3178–3182. doi: 10.1073/pnas.73.9.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mukhamedova N, Fu Y, Bukrinsky M, Remaley AT, Sviridov D. The role of different regions of ATP-binding cassette transporter A1 in cholesterol efflux. Biochemistry. 2007;46:9388–9398. doi: 10.1021/bi700473t. [DOI] [PubMed] [Google Scholar]
- 25.Sviridov D, Fidge N, Beaumier-Gallon G, Fielding C. Apolipoprotein A-I stimulates the transport of intracellular cholesterol to cell-surface cholesterol-rich domains (caveolae) Biochem J. 2001;358:79–86. doi: 10.1042/0264-6021:3580079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang N, Silver DL, Costet P, Tall AR. Specific binding of ApoA-I, enhanced cholesterol efflux, and altered plasma membrane morphology in cells expressing ABC1. J Biol Chem. 2000;275:33053–33058. doi: 10.1074/jbc.M005438200. [DOI] [PubMed] [Google Scholar]
- 27.Folch J, Lees M, Sloane-Stanley GM. A simple method for isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 28.Tchoua U, D’Souza W, Mukhamedova N, Blum D, Niesor E, Mizrahi J, Maugeais C, Sviridov D. The effect of cholesteryl ester transfer protein overexpression and inhibition on reverse cholesterol transport. Cardiovasc Res. 2008;77:732–739. doi: 10.1093/cvr/cvm087. [DOI] [PubMed] [Google Scholar]
- 29.Okuhira K-i, Tsujita M, Yamauchi Y, Abe-Dohmae S, Kato K, Handa T, Yokoyama S. Potential involvement of dissociated apoA-I in the ABCA1-dependent cellular lipid release by HDL. J Lipid Res. 2004;45:645–652. doi: 10.1194/jlr.M300257-JLR200. [DOI] [PubMed] [Google Scholar]
- 30.Wang N, Lan D, Chen W, Matsuura F, Tall AR. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc Natl Acad Sci USA. 2004;101:9774–9779. doi: 10.1073/pnas.0403506101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sviridov D, Hoang A, Ooi E, Watts G, Barrett PHR, Nestel P. Indices of reverse cholesterol transport in subjects with metabolic syndrome after treatment with rosuvastatin. Atherosclerosis. 2008;197:732–739. doi: 10.1016/j.atherosclerosis.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Catalano G, Duchene E, Julia Z, Le Goff W, Bruckert E, Chapman MJ, Guerin M. Cellular SR-BI and ABCA1-mediated cholesterol efflux are gender-specific in healthy subjects. J Lipid Res. 2008;49:635–643. doi: 10.1194/jlr.M700510-JLR200. [DOI] [PubMed] [Google Scholar]
- 33.Calpe-Berdiel L, Rotllan N, Palomer X, Ribas V, Blanco-Vaca F, Escola-Gil JC. Direct evidence in vivo of impaired macrophage-specific reverse cholesterol transport in ATP-binding cassette transporter A1-deficient mice. Biochim Biophys Acta. 2005;1738:6–9. doi: 10.1016/j.bbalip.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 34.Nagao K, Takahashi K, Hanada K, Kioka N, Matsuo M, Ueda K. Enhanced ApoA-I-dependent cholesterol efflux by ABCA1 from sphingomyelin-deficient Chinese hamster ovary cells. J Biol Chem. 2007;282:14868–14874. doi: 10.1074/jbc.M611230200. [DOI] [PubMed] [Google Scholar]
- 35.Vaughan AM, Oram JF. ABCA1 redistributes membrane cholesterol independent of apolipoprotein interactions. J Lipid Res. 2003;44:1373–1380. doi: 10.1194/jlr.M300078-JLR200. [DOI] [PubMed] [Google Scholar]
- 36.Aiello RJ, Brees D, Bourassa PA, Royer L, Lindsey S, Coskran T, Haghpassand M, Francone OL. Increased atherosclerosis in hyperlipidemic mice with inactivation of ABCA1 in macrophages. Arterioscler Thromb Vasc Biol. 2002;22:630–637. doi: 10.1161/01.atv.0000014804.35824.da. [DOI] [PubMed] [Google Scholar]
- 37.Meir K, Kitsberg D, Alkalay I, Szafer F, Rosen H, Shpitzen S, Avi LB, Staels B, Fievet C, Meiner V, et al. Human sterol 27-hydroxylase (CYP27) overexpressor transgenic mouse model. Evidence against 27-hydroxycholesterol as a critical regulator of cholesterol homeostasis. J Biol Chem. 2002;277:34036–34041. doi: 10.1074/jbc.M201122200. [DOI] [PubMed] [Google Scholar]
- 38.Razani B, Combs TP, Wang XB, Frank PG, Park DS, Russell RG, Li M, Tang B, Jelicks LA, Scherer PE, et al. Caveolin-1-deficient mice are lean, resistant to diet-induced obesity, and show hypertriglyceridemia with adipocyte abnormalities. J Biol Chem. 2002;277:8635–8647. doi: 10.1074/jbc.M110970200. [DOI] [PubMed] [Google Scholar]
- 39.Wang MD, Kiss RS, Franklin V, McBride HM, Whitman SC, Marcel YL. Different cellular traffic of LDL-cholesterol and acetylated LDL-cholesterol leads to distinct reverse cholesterol transport pathways. J Lipid Res. 2007;48:633–645. doi: 10.1194/jlr.M600470-JLR200. [DOI] [PubMed] [Google Scholar]
- 40.Adorni MP, Zimetti F, Billheimer JT, Wang N, Rader DJ, Phillips MC, Rothblat GH. The roles of different pathways in the release of cholesterol from macrophages. J Lipid Res. 2007;48:2453–2462. doi: 10.1194/jlr.M700274-JLR200. [DOI] [PubMed] [Google Scholar]
- 41.Vedhachalam C, Duong PT, Nickel M, Nguyen D, Dhanasekaran P, Saito H, Rothblat GH, Lund-Katz S, Phillips MC. Mechanism of ATP-binding cassette transporter A1-mediated cellular lipid efflux to apolipoprotein A-I and formation of high density lipoprotein particles. J Biol Chem. 2007;282:25123–25130. doi: 10.1074/jbc.M704590200. [DOI] [PubMed] [Google Scholar]
- 42.Hassan HH, Denis M, Lee DYD, Iatan I, Nyholt D, Ruel I, Krimbou L, Genest J. Identification of an ABCA1-dependent phospholipid-rich plasma membrane apolipoprotein A-I binding site for nascent HDL formation: implications for current models of HDL biogenesis. J Lipid Res. 2007;48:2428–2442. doi: 10.1194/jlr.M700206-JLR200. [DOI] [PubMed] [Google Scholar]