Pachydermoperiostosis (PDP) or primary hypertrophic osteoathropathy (PHO) (also known as Touraine-Solente-Golé syndrome; OMIM 167100) is a rare genetic disease involving formation of periosteal new bone, clubbing of the digits, enlargement of joints, arthralgias and hypertrophic skin changes, especially on the face, forehead, and scalp. PDP may also display additional symptoms such as seborrhea, hyperhidrosis and hypertrophic gastritis (1–3).
PDP is a chronic condition that, while not life-threatening, decreases the patient’s quality of life. Among other clinical manifestations, the thickened skin of the upper third of the face gives a leonine appearance to the face (4). We report here 3 cases of PDP that were treated by injections of botulinum toxin type A (BTX-A). To the best of our knowledge, this is only the third report describing the use of BTX-A for treatment of PDP, and the first in which a patient (Case 1) received a course of several injections.
CASE REPORTS
The clinical features of the three patients are summarized in Table SI1. All patients signed informed consent and granted permission to publish these images and information about their cases. Our treatment strategy was designed to achieve a visible improvement in the facial appearance with BTX-A (Botox, Allergan INC., Ireland) (Fig. S11). A 4-point facial wrinkle scale (FWS) (5) (0 = none, 1 = mild, 2 = moderate, and 3 = severe) was used to assess wrinkle severity in relaxation at baseline, week 4 and week 16.
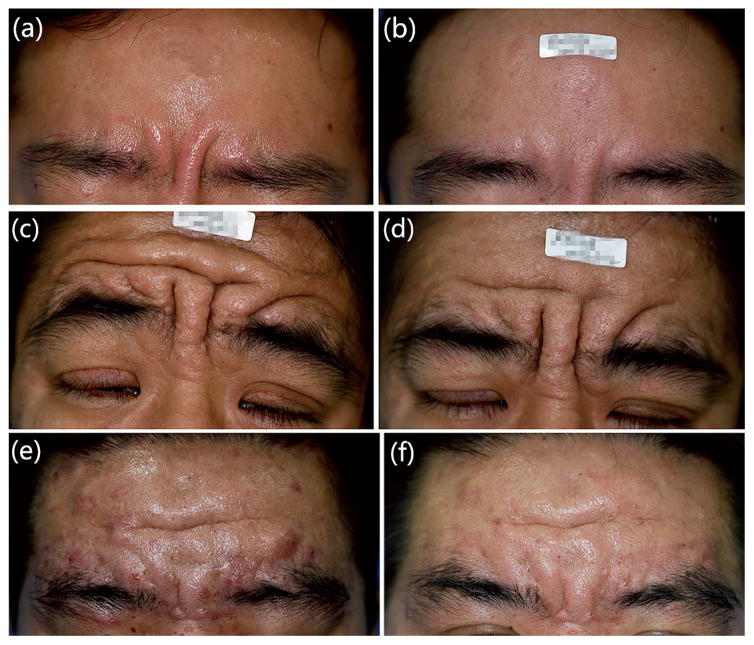
The facial examination of patient 1 revealed oily, thickened skin withsignificant furrows. BTX-A was injected into the relevant muscles. The patient was followed up at 2, 4, 8 and 16 weeks after the injection. The patient was classified as a responder at weeks 4 with good improvement (Fig. 1a, b). It was noted that the improvement started within the first week after treatment, and culminated between weeks 4 and 8. He underwent another 3 sets of treatments with the same dose injected at 16-week intervals; the results continued to be promising at each follow-up. There were no adverse events reported throughout the entire course of injections.
Fig. 1. Patients before and after treatment.
(a) Significant glabellar furrows at presentation in patient 1. (b) 4 weeks after treatment with BTX-A. (c) Significantly thickened skin on the eyelids and forehead with furrowing at presentation in patient 2. (d) 1 week after treatment with BTX-A, showing exacerbation of the eyelid ptosis that caused opening the eyes. (e) Facial enlargement with deep folds in the skin of the forehead and glabellar region and acne vulgaris on the forehead in patient 3. (f) 4, weeks after treatment with BTX-A.
An examination of patient 2 disclosed that the eyelids were considerably thickened and enlarged; the palpebral apertures were reduced to 5 mm bilaterally. His visual acuity was impaired while other ocular examinations were within normal limits. He the forehead and glabellar region hadsignificant with furrowing. Injections of BTX-A were administered. One week after the injection, the patient reported an exacerbation of the eyelid ptosis that caused difficulty in opening his eyes (Fig. 1c, d). An excellent response in his forehead was noted. Upon visual examination, he had significant ptosis with a margin to reflex distance of 1 mm in the right eye and 2 mm in the left. The levator palpebrae superioris muscle function was poor. The patient rejected any medication, and he reported that the eyelid ptosis was the same as before treatment by week 6.
Patient 3 presented with facial enlargement with deep folds in the skin of the forehead and glabellar region. Several papules, pustules and nodules on his forehead were noted and diagnosed as acne vulgaris. The injection sites and doses in the glabellar region were the same as case 1; 5 intradermal injections along the forehead were given using 2 U each. He tolerated the procedure well and the results were noted to be satisfactory at weeks 2, 4, and 16 (Fig. 1e, f). Surprisingly his acne improved without any additional medication.
DISCUSSION
A genetic defect in either HPGD or recently solute carrier organic anion transporter family member 2A1 (SLCO2A1) gene is responsible for PDP. Impaired metabolism of PGE2 is considered as pathogenia in PDP (2, 6). The diagnosis of PDP is straightforward when all 3 characteristics are present. Mechanical ptosis is seen in PDP resulting from thickening of both eyelids (7). Histological findings in pachydermia frequently include dermal mucin deposition, elastic fiber degeneration, dermal fibrosis and adnexal hyperplasia (8).
There is no specific therapy for pachydermia in PDP. Plastic surgery has been indicated in some literature reviews (9, 10). The current study suggests that BTX-A may enhance the cosmetic appearance of cutaneous manifestations of PDP. We speculate that the reason that BTX-A contributes to the aesthetic improvement could be the inhibitory effects that BTX-A exerts on muscles. As the injected muscles relax, the surrounding tissue might relax as well. In addition, injection of BTX-A into the dermal–subdermal layer has been anecdotally reported to improve skin texture and turgor (11). Since the skin texture is better, the wrinkles will be improved and afterwards become shallower.
Patient 1 exhibited an excellent response using the same dose of BTX-A injected over 4 sessions, and although further mild improvement after subsequent injections was not able to be detected by the scoring tool, a more enhanced cosmetic outcome was observed after repeated treatments. However, the depth of the original glabellar folds was less pronounced than that of the other two patients. It remains to be seen how much efficacy there is for BTX-A injections in patients with severe facial cutaneous manifestations, because the mechanism of action is still uncertain.
Patient 2 reported an exacerbation of eyelid ptosis that caused some difficulty in normal vision after the injection. Exacerbated eyelid ptosis has been reported after BTX-A injection in a previous study, and suggested due to blepharitis secondary to isotretinoin therapy (4). However, in our opinion, it is more likely that the eyelid ptosis (both in our case and in the previous study) was an adverse effect of the injections. The relaxation of the frontalis muscle after BTX-A injection will cause both brow and eyelid ptosis, even though the weakening of the muscular depressors of the brow (orbicularis oculi, procerus, and corrugator supercilii muscles) had been achieved. Therefore, with regard to safety, we suggest that clinicians should begin with only a small dose of BTX-A (5 to 7 injection sites with 1–2 U each) injected into the forehead for patients with PDP.
Patients with severe PDP have sebaceous gland hyperplasia (12), the efficacy of BTX-A as a treatment for reducing sebum production may explain why the acne of patient 3 was alleviated after BTX-A injection (13). The sebum reducing effects of botulinum toxin will probably cause lower activity of sebaceous glands (14), and the thickness of the facial pachydermia will appear to be thinner afterwards. The effects of botulinum toxin in reducing wrinkles in thinner skin will be greater than that in thicker skin according to the clinical experiences of the authors.
The off-label use of BTX-A is gaining remarkable interest all over the world; all of the 3 patients in our report refused surgery and underwent BTX-A as an alternative therapy. As the typical course of PDP is self-limiting, the treatment has been mainly focused on cosmetic improvement, therefore BTX-A injection could be administered to some patients. It remains to be confirmed the optimum technique for injection of BTX-A, and the optimum doses and treatment repetition regimen in PDP patients.
Supplementary Material
Acknowledgments
We thank Po-Han Huang for his valuable suggestions on this manuscript.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Castori M, Sinibaldi L, Mingarelli R, Lachman RS, Rimoin DL, Dallapiccola B. Pachydermoperiostosis: an update. Clin Genet. 2005;68:477–486. doi: 10.1111/j.1399-0004.2005.00533.x. [DOI] [PubMed] [Google Scholar]
- 2.Sasaki T, Niizeki H, Shimizu A, Shiohama A, Hirakiyama A, Okuyama T, et al. Identification of mutations in the prostaglandin transporter geneSLCO2A1 and its phenotype-genotype correlation in Japanese patients with pachydermoperiostosis. J Dermatol Sci. 2012;68:36–44. doi: 10.1016/j.jdermsci.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Supradeeptha C, Shandilya SM, Vikram Reddy K, Satyaprasad J. Pachydermoperiostosis-A case report of complete form and literature review. J Clin Orthop Trauma. 2014;5:27–32. doi: 10.1016/j.jcot.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghosn S, Uthman I, Dahdah M, Kibbi AG, Rubeiz N. Treatment of pachydermoperiostosis pachydermia with botulinum toxin type A. J Am Acad Dermatol. 2010;63:1036–1041. doi: 10.1016/j.jaad.2009.08.067. [DOI] [PubMed] [Google Scholar]
- 5.Carruthers A, Carruthers J, Cohen J. A prospective, double-blind, randomized, parallel-group, dose-ranging study of botulinum toxin type A in female subjects with horizontal forehead rhytides. Dermatol Surg. 2003;29:461–467. doi: 10.1046/j.1524-4725.2003.29114.x. [DOI] [PubMed] [Google Scholar]
- 6.Niizeki H, Shiohama A, Sasaki T, Seki A, Kabashima K, Otsuka A, et al. The novel SLCO2A1 heterozygous missense mutation p. E427K and nonsense mutation p. R603* in a female patient with pachydermoperiostosis with an atypical phenotype. Br J Dermatol. 2014;170:1187–1189. doi: 10.1111/bjd.12790. [DOI] [PubMed] [Google Scholar]
- 7.Ding J, Li B, Chen T, Hao L, Li D. Eyelid thickening and ptosis associated with pachydermoperiostosis: A case report and review of literature. Aesthetic Plast Surg. 2013;37:464–467. doi: 10.1007/s00266-013-0062-z. [DOI] [PubMed] [Google Scholar]
- 8.Tanese K, Niizeki H, Seki A, Otsuka A, Kabashima K, Kosaki K, et al. Pathological characterization of pachydermia in pachydermoperiostosis. J Dermatol. 2015;42:710–714. doi: 10.1111/1346-8138.12869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giancane G, Diggle CP, Legger EG, Tekstra J, Prakken B, Brenkman AB, et al. Primary hypertrophic osteoarthropathy: an update on patient features and treatment. J Rheumatol. 2015;42:2211–2214. doi: 10.3899/jrheum.150364. [DOI] [PubMed] [Google Scholar]
- 10.Bingol UA, Cinar C. Pachydermoperiostosis: aesthetic treatment of prematurely aging face with facelift and botulinum toxin A. J Craniofac Surg. 2014;25:e563–564. doi: 10.1097/SCS.0000000000001149. [DOI] [PubMed] [Google Scholar]
- 11.Chang SP, Tsai HH, Chen WY, Lee WR, Chen PL, Tsai TH. The wrinkles soothing effect on the middle and lower face by intradermal injection of botulinum toxin type A. Int J Dermatol. 2008;47:1287–1294. doi: 10.1111/j.1365-4632.2008.03895.x. [DOI] [PubMed] [Google Scholar]
- 12.Tanese K, Niizeki H, Seki A, Otsuka A, Kabashima K, Kosaki K, et al. Pathological characterization of pachydermia in pachydermoperiostosis. J Dermatol. 2015;42:710–714. doi: 10.1111/1346-8138.12869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah AR. Use of intradermal botulinum toxin to reduce sebum production and facial pore size. J Drugs Dermatol. 2008;7:847–850. [PubMed] [Google Scholar]
- 14.Li ZJ, Park SB, Sohn KC, Lee Y, Seo YJ, Kim CD, et al. Regulation of lipid production by acetylcholine signaling in human. doi: 10.1016/j.jdermsci.2013.06.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.