Abstract
Prior work in amputees and partial limb immobilization have shown improved neural and behavioral outcomes in using their residual limb with prosthesis when undergoing observation-based training with a prosthesis-using actor compared to an intact limb. It was posited that these improvements are due to an alignment of user with the actor. It may be affected by visual angles that allow emphasis of critical joint actions which may promote behavioral changes. The purpose of this study was to examine how viewing perspective of observation-based training effects prosthesis adaptation in naïve device users. Twenty non-amputated prosthesis users (NAPUs) learned how to use an upper extremity prosthetic device while viewing a training video from either a sagittal or coronal perspective. These views were chosen as they place visual emphasis on different aspects of task performance to the device. We found that perspective of actions has a significant role in adaptation of the residual limb while using upper limb prostheses. Perspectives that demonstrate elbow adaptations to prosthesis usage may enhance the functional motor outcomes of action observation therapy. This work has potential implications on how prosthetic device operation is conveyed to persons adapting to prostheses through action observation based therapy.
Keywords: Motor control, biomechanics, upper extremity, prosthesis, action observation
1.0 Introduction
Prior studies have utilized methods of partial immobilization to understand methods of neural plasticity and behavioral adaptations (Hamzei et al., 2006;Wittenberg and Schaechter, 2009;Bassolino et al., 2012;Toussaint and Meugnot, 2013). However, partial limb immobilization can afford a unique opportunity to evaluate behavioral outcomes analogous to parital limb loss. Such studies can focus on how the residual limb functions with a prosthetic device. Several prior studies have utilized partial limb immobilization to model prosthesis use; with direct relevance to residual limb control following limb amputation (Yuen et al., 1994;Bouwsema et al., 2008;Carey et al., 2008;Cusack et al., 2014).
For persons with limb loss (“amputees”), the use of artificial limbs (prostheses) becomes a vital part of their lives. Unfortunately, ~33% of upper limb amputees reject prostheses, and ~75% use the devices as a non-functional aesthetic (Datta et al., 2004). Skilled movement is a tremendous challenge to persons with upper extremity limb loss, even when using prostheses (Schultz et al., 2007). A significant challenge is seen in learning how to modify control patterns of the residual limb with loss of degrees of freedom (Metzger et al., 2012). Yet, the present standard of rehabilitative training involves mimicking of a therapist (normally a person with intact limbs) for “shoulder, elbow, and terminal device control” (Smurr et al., 2008).
We have condidered the role of action observation in learning how to use prostheses. The investigation of action observation rehabilitation methods have increased in recent years (Buccino, 2014) and involve observance of motor skill demonstrations, followed by performance of the observed skills. It is generally regarded that since action observation drives similar parietofrontal and motor circuits as actual action (Gazzola and Keysers, 2009), observation will cause increased activity to promote motor function (Strafella and Paus, 2000;De Maeght and Prinz, 2004;Gangitano et al., 2004;Ferrari et al., 2005;Liepert et al., 2011).
Our prior studies suggest a specific type of action observation-based treatment may be optimal. In low-functional users of prostheses, greater engagement of parietofrontal areas is seen if amputees observe other prosthesis users perform actions, compared to when observing intact limbs perform the same tasks (Cusack et al., 2012). This work also revealed differences in muscle activation onset timing in the residual limb, driven by the type of limb they observe. This lead us to hypothesize that appropriate action observation may be beneficial in learning how to use the residual limb for tasks involving a prosthesis where the type of limb seen (prosthetic versus intact) can significantly effect behavioral adaptations. In a behavioral follow-up study, we evaluated the biomechanics of naïve prosthesis users when imitating actions of prosthesis users (matched limb training) versus persons with intact limbs (mismatched limb training) (Cusack et al., 2014). Results showed that matched limb imitation resulted in an angular displacement at the elbow that emulated the prosthesis user demonstration. Contrastingly, the mismatched limb-training group showed elbow angular displacements that matched the intact limb actor. Further, participants in the matched limb group appear to adapt to a novel shoulder-elbow coupling strategy which is similar to the prosthesis user actor, while the mismatched limb group continued using shoulder-elbow coupling of the intact actor. Together, these results suggest that when prosthesis users are faced with the impossible task of imitating the movements of an intact hand, they perform action with decreased parietofrontal activity, greater variability and poorer technique in the residual limb which will influence prosthesis success.
It is also known that perspective of action may impact the action observation system and subsequent behavioral outcomes (Saxe et al., 2006;Kelly and Wheaton, 2013). It is possible that varying the perspective may substantially affect action observation training. Our prior behavioral study (Cusack et al., 2014) demonstrated large angular displacement changes at the elbow (flexion/extension) during action observation. In that study, action demonstrations were only observed in the sagittal plane. It is possible that the effects seen could be due to the fact that the sagittal view emphasizes elbow extension as an adaptation to the prosthesis that the participants identify (Figure 1A). However, a more coronal view (Figure 1B) could help participants visualize other joint actions (namely shoulder ab/adduction), which may influence their motor adaptation to the prosthesis. As well, the effects could be due to stimulus-response compatibility (SRC; as described in (Vankov and Kokinov, 2013;Cross and Iacoboni, 2014)), where the state of the observer better matches that of the observed. We can evaluate the specificity of the effects by considering a paradigm where we compare the motor performance effects based on the viewing angle presented to the observer. We trained non-amputee prosthesis users (NAPUs) on how to use a body-powered prosthesis with matched limb training using two different viewing perspectives. One group saw a sagittal view, which emphasizes elbow adaptations to the device. The second group saw a coronal view that emphasizes shoulder adaptations to the device. We hypothesized that NAPUs would show decreases in motor variability in the elbow and shoulder joints after observing demonstrations in the sagittal and coronal perspective, respectively.
Figure 1.
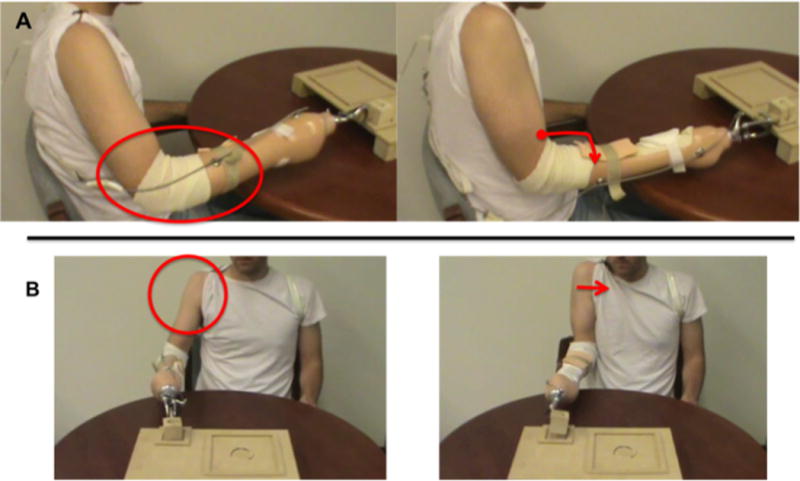
Perspectives captured from screenshots of the videos used in this study (circles and arrows were not a part of the video). The sagittal view (A) with elbow extension highlighted, and coronal view (B) with shoulder adduction highlighted.
2. Materials and Methods
2.1 Subjects
Twenty (20) healthy participants with intact limbs were recruited for this study. Of the 20 participants, individuals were randomly placed into one of two groups based on video perspective, coronal (n=10, 9 male, 24.6 +/− 2.7 years) and sagittal (n=10, 9 male, 28.5 +/− 2.5 years). All subjects were right-handed according to the Edinburgh handedness inventory (Oldfield, 1971). Signed informed consent was obtained based on experimental approvals given by the Georgia Tech Institutional Review Board.
2.2 Experimental Setup
The experimental setup mirrored our previous study (Cusack et al., 2014). The right arm of each subject was fitted with three twin-axis goniometers and one single-axis torsiometer (models SG110/150 and Q110/Q150, respectively, Biometrics Ltd, Newport, UK) that were connected to an 8-channel MyoSystem data collection system (model 1400L, Noraxon, Scottsdale, AZ, USA). Data was sampled with 1 kHz frequency and 12 bit resolution. There were three physiological degrees of freedom in the arm that were of interest in this study: elbow flexion/extension (EFE), shoulder abduction/adduction (SAA) and horizontal shoulder flexion/extension (SFE). Prior studies have verified that wrist flexion and forearm rotation were eliminated with the prosthesis (Cusack et al., 2014). Sensors were applied using guidelines provided by the manufacturer’s user manual and previous studies in upper extremity kinematics (Chao et al., 1980;Anglin and Wyss, 2000;Hansson et al., 2004;Wise et al., 2004;Magermans et al., 2005;Biometrics, 2010). This technique has been demonstrated as a successful method for studying upper limb joint motion and coordination during simulated activities of daily living (ADLs) (Chao et al., 1980; O’Neill et al., 1992).
For EFE, the distal endblock of the goniometer was placed along the medial midline of the forearm, with long axes of the endblock and radius aligned. The proximal endblock was placed along the medial midline of the upper arm, with the long axes of the endblock and humerus aligned. The endblocks were positioned such that the center of the goniometer was directly over the medial epicondyle of the humerus. For SAA and SFE, the distal endblock of the goniometer was placed along the lateral midline of the upper arm, with the long axes of the endblock and humerus aligned. The proximal endblock was placed over the acromion, in line with the distal endblock and perpendicular to the midaxillary line of the thorax. The endblocks were positioned such that the center of the goniometer was directly over the greater tubercle of the humerus. To monitor the absence of forearm rotation, a torsiometer was placed along the ventral midline of the forearm aligned with the long axis of the radius, with distal and proximal endblocks on the distal and proximal ends of the forearm, respectively. The distal endblock was placed approximately 1 cm from the ulnar and radial styloids. We were able to confirm that forearm rotation was absent in our participants.
Once the sensors were in place, each subject was fitted with a specially adapted prosthesis that accommodates an intact subject’s entire forearm and hand (Figure 2A). The prosthesis consists of an acrylic laminated socket that fits over the forearm with a hook terminal device. This design mimics the design of a transradial prosthesis; with the distal portion expanded to allow room for the hand and fingers. Subjects wore a coth sleeve over their hand and forearm. Foam padding was inserted to secure the hand and forearm within the socket and adjustable straps were applied to immobilize the wrist in all planes.
Figure 2.
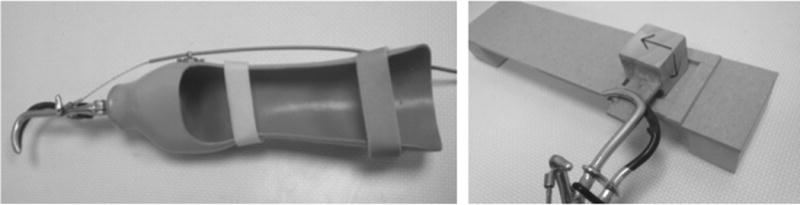
Prosthesis (left) and motor task board (right) used in this study (based on Cusack, et al, 2014, and relevant text).
2.3 Experimental task
Subjects placed their elbow in a fixed location and rested their arm on a table within a fixed perimeter. A workspace board (Figure 2B) was then positioned on the table in front of the subject such that the midline of the board was aligned with the vertical midline of the torso. The distance between the subject and the board was chosen such that the center of the workspace aligned with a fixed marker on the terminal device.
Subjects learned to use the prosthesis by observing a training video and then imitated a motor task. The task objective was to cyclically flip a wooden block 90° within a target area on a functional task board, as previously described (Cusack et al., 2014). The task involves: 1) placement of the wooden cube within the square target area, 2) lifting the cube, rotating 90° clockwise, placement back into target area, and 3) lifting the cube, rotating 90° counterclockwise, placement back into target area. This task was chosen as it mimicks using a key, a task that would normally involve forearm pronation/supination, which is eliminated by the prosthesis. No active prehension was required during the task (as we were interested in residual limb control), and the block was fixed in the terminal device. The block (4.5 cm on each side) must be confined within the square target area (5.5 cm on each side) in both position 1 and position 2. Thus, it is comprised of two principal motions: a rotation that permits the flipping of the block and a translation to control the end effector such that the block does not leave the workspace board target area. This motor task mimics that of turning a key in a lock. The wooden block was secured within the functional terminal device using tape. The functional task board was positioned directly in front of the subject so that the target area would be parallel to the wooden block. Subjects were not allowed to practice the motor task prior to the experiment.
Subjects first viewed a 30-second video demonstration of a prosthesis user wearing the same style of device and completing the motor task of cyclically flipping the wooden block within the functional task board. The videos were filmed from 2 perspectives, sagittal and coronal (screenshots in Figure 1). In Figure 1A, the sagittal perspective (which was used in our previous study, (Cusack et al., 2014)) emphasizes behavior in the elbow joint. In the coronal perspective (Figure 1B) the shoulder is the joint segment that is emphasized. In both videos, the actor maintained a consistent pace by following a metronome. Each video was muted as the participants watched the videos, as to not influence timing strategy of the action. Subjects were instructed to sit quietly in a resting position and to only focus on the video while it was playing.
After the presentation of each video, subjects were instructed to “imitate the movement seen in the video as quickly and as accurately as possible” for a total of ten continuous repetitions. Subjects were not directed how to move their arm segments or joints beyond these instructions. In order for the movements to be as natural as possible, no attempt was made to control the speed or pace of their movement repetitions. This pairing of observation followed by imitation was repeated 20 times; thus totaling approximately 10 minutes of focused action observation and 200 movements over 10 minutes of action imitation.
2.4 Data analysis
Kinematics from the three degrees of freedom of interest were obtained using ELGON and all further processing was performed using custom MATLAB software (version R2012a, The MathWorks Inc., Natick, MA, USA). All data were lowpass filtered at 6 Hz with a fourth-order Butterworth filter and then manually inspected on a subject level to identify the beginning and ending of each of the individual movements. Data were visually inspected on a trial-by-trial basis to identify the beginning and end of each individual movement by locating the maximum shoulder adduction between movement cycles and placing an event marker there to indicate the beginning of each movement cycle. These time points correspond to the transition between clockwise and counterclockwise rotations in the block rotation task. All displacements in this study are relative to this reference position. This varies from our previous study, which used the peak shoulder abduction to mark the beginning of each movement cycle (Cusack et al., 2014). This methodological change was made as it yielded more consistent individual movement identifications. A consequence of this change is a 180 deg shift in all angular displacement profiles relative to those presented in the prior study. The durations of each movement were computed, as decreased movement duration over consecutive trials has previously been shown to be an accurate measure for quantifying motor adaptation (Flament et al., 1999;Kempf et al., 2001;Smith et al., 2006).
Our prior study demonstrated that when NAPUs saw video demonstrations of other prosthesis users, adaptations were most prevalent in shoulder and elbow movement patterns that matched the video actor, along with decreases in motor variability (Cusack et al., 2014). Thus, we were particularly interested in analysis of angluar displacement and coefficient of variation in the elbow and shoulder based on the viewing perspective presented. Angular displacement data were time normalized to percentage of the movement cycle. Data from each of the 10 repetitions were averaged together into a representative movement for that particular trial.
The coefficient of variation (CV) for each of the 20 movement groups was calculated according to CV(%) = σ(%)/μ(%); where σ(%) and μ(%) are the angular displacement standard deviation and mean as functions of percent movement cycle, respectively. This process was repeated on a subject level for each of the 20 groups of movements in the recording session. Additionally, for each of these 20 representative movements, the average CV was computed over the length of the entire movement cycle.
2.5 Statistical design
Time series kinematic data and CV were divided and averaged into 8 contiguous time windows, each representing 12.5% of the complete movement cycle. A test of normality, the Shapiro-Wilks test, was performed indicating that the data were statistically normal. A 2-way ANOVA was performed with video (sagittal/coronal) and time window (1–8) as factors separately for shoulder (SAA, SFE) and elbow (EFE) displacement and CV. Subsequent post hoc t-tests were performed, with significance set at p<0.05 with Bonferroni correction. Time of movement was statistically assessed using a one-way ANOVA with video (sagittal/coronal) as the factor. All statistical tests were conducted using SPSS Statistics software (IBM, Armonk, NY, USA).
3.0 RESULTS
3.1 Angular displacement
SAA
ANOVA demonstrated a main effect of time window (F(7,3685.3)=380.2, p<0.001) but no main effect of video (F(1,0.221)=.023, p=0.880). A video x time window interaction effect was seen (F(7,65.5)=6.7, p<0.001). In the early part of the movement cycle, the coronal perspective showed a significantly higher displacement than the sagittal (Figure 3A, time windows 2–3, p<0.001). At the end of the movement cycle, this pattern had reversed where sagittal showed a higher displacement than coronal (time windows 6–7, p<0.001).
Figure 3.
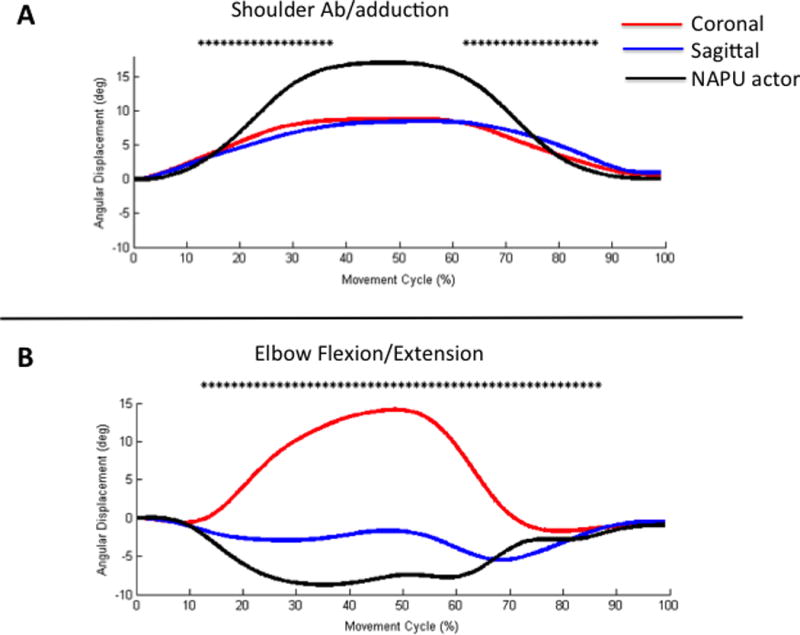
(A) Shoulder abduction/adduction and (B) elbow flexion/extension angular displacement effects over the duration of the movement cycle (%). Asterisks indicate time windows where there was a significant difference (p<0.05) between perspective groups. The NAPU actor trace is provided for visual comparison purposes.
SFE
ANOVA revealed a main effect of video (F(1,229.8)=33.3, p<0.001), time window (F(7,983.1)=142.5, p<0.001). No interaction effect was seen (F(7,11.9)=1.7, p=0.09). Post-hoc evaluation of the main effect of video shows a significantly larger angular displacement for the sagittal view compared to the coronal view (p<0.001).
EFE
ANOVA results indicate a main effect of video (F(1, 41289)=1245.5, p<0.001), time window (F(7,3776.5)=113.9, p<0.001), and an interaction effect (F(7,4286.7)=129.3, p<0.001). The two experimental groups show a different angular displacement (Figure 3B). The sagittal group shows a relative extension, which aligns with the prosthesis actor, while the coronal group shows a relative flexion.
3.2 Coefficient of Variation
SAA
ANOVA revealed a main effect of video (F(1,.004)=45.6, p<0.001), time window (F(7,0.001)=5.4, p<0.001), but no interaction effect (F(7,9.5×10−5)=1.2, p=.38). Post hoc analysis revealed that coronal group showed a significantly higher CV compared to the sagittal group (p<0.001, Figure 4A).
Figure 4.
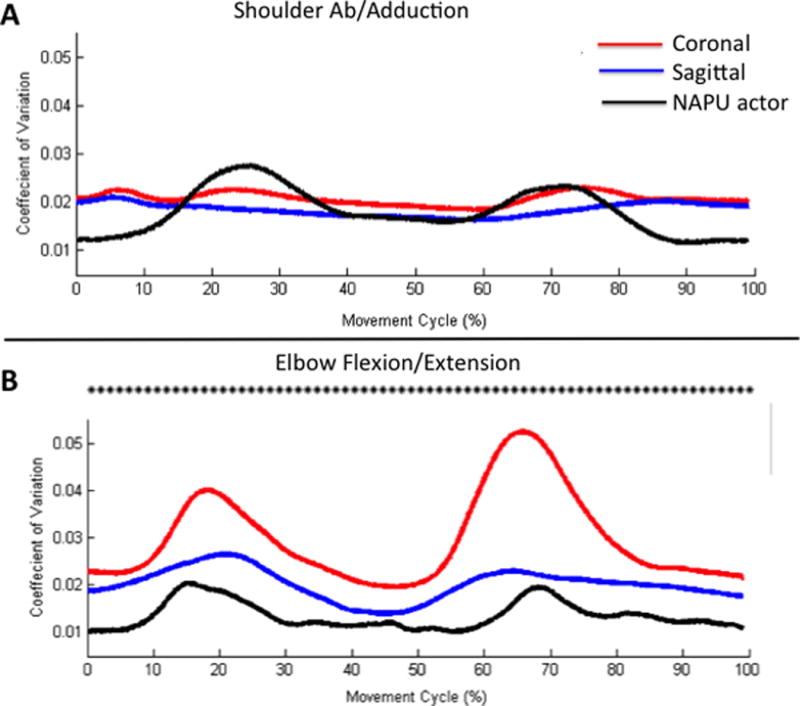
(A) Shoulder abduction/adduction and (B) elbow flexion/extension CV effects. Asterisks indicate time windows where there was a significant difference (p<0.05) between perspective groups. The NAPU actor trace is provided for visual comparison purposes.
SFE
ANOVA revealed a main effect of video (F(1,0.01)=23.2, p<0.001), time window (F(7,0.001) = 6.6, p<0.001), but no interaction effect (F(7,8.9×10−5)=1.9, p=0.06). Post-hoc evaluation of the main effect of video shows significantly less CV for coronal view compared to the sagittal view (p<0.001).
EFE
ANOVA revealed a main effect of video (F(1, 0.075)=320.8, p<0.001), time window (F(7,0.012)=53.1, p<0.001), and an interaction effect (F(7, 0.005) = 21.3, p<0.001). Figure 4B illustrates the effect, where the coronal group showed a higher CV than the sagittal group at all time windows (p<0.001). Further the sagittal group showed a CV that was more similar to the actor.
3.3 Movement time
Statistical analysis was performed to determine if video presentation influenced movement time. One-way ANOVA revealed a significant effect of video (F(1,10075)=40.8, p<0.001), where participants performed faster in the sagittal (12.8s ± 4.4) than coronal groups (16.9s ±5.5).
4. Discussion
The purpose of this study was to assess whether varying perspective of action can affect the behavioral adaptations seen in the residual limb after partial limb immobilization (modeled with a prosthesis) (Cusack et al., 2012;Cusack et al., 2014). Using a model of action observation therapy, separate groups of naïve prosthesis users learned how to use devices to perform a task by watching a proficient prosthesis user from a coronal view (emphasizing shoulder behavior) or sagittal view (emphasizing elbow behavior). The hypothesis was that perspective of the observed actor may affect the joint adaptations to the prosthesis, where persons in the sagittal group would show angular displacement and CV adaptations primarily at the elbow, while the coronal group would show such adaptations primarily at the shoulder. Results generally support this hypothesis, notably at the elbow. At the elbow, the sagittal group showed angular displacement patterns that better matched to those of the actor, with decreased CV compared to the coronal group. At the shoulder (notably SAA), the sagittal group showed a lower CV compared to the coronal view, but the coronal perspective showed higher angular displacement than the sagittal perspective at the beginning of the movement, which switched by the end of the movement. While there were no interaction effects in SFE, the coronal view showed decreased CV and less angular displacement compared to the sagittal view. In summary, this work suggests that the effects seen in matched limb training are not driven solely by compatability of actor and observer, but that beign able to observe specific adaptations in a matched limb is most optimal. Further, this work potentially highlights a strong role in elbow adaptations to transradial prosthesis usage for this type of task.
This work is not without its limitations. We evaluated single session changes in motor control based on two perspectives. There are infinite possibilities of visual perspective, but we chose two which clearly (but not exclusively) emphasize shoulder and elbow movements, which were target outcomes. Based on these findings, we can better develop future studies in amputees focusing on long-term outcomes to action observation prosthesis training in a longitudinal design. As well, we focused on a single task, which was intentionally selected as it forced users to adapt to lost forearm rotation which is an essential adaptation in transradial amputees. Future studies will evaluate more tasks, transferrance across tasks, and further characterize outcomes at varying levels of upper limb loss in amputees. Given that we fixed the block to ther terminal device, it will also be vital to consider how prehension can affect these results, as active prehension for body-powered prostheses can involve shoulder flexion/extension, depending on the nature of the device. Motion capture can provide better flexibility in monitoring more behavioral changes. Related to this, while we noted that there are few changes to shoulder actions, we were only able to record shoulder ab/adduction and flexion/extension due to limitations of channels for recording. Future studies will also evaluate other actions (shoulder elevation, trunk movments) to determine how the adaptaions can affect these movements. As well, identifying how other types of control (myoelectric) and varying levels of limb loss (parital hand through shoulder disarticulation) will be most vital to future studies.
Joint specificity in residual limb motor adaptations
In our prior work, we demonstrated that elbow displacement showed the largest adaptation (Cusack et al., 2014). Similarly here, the elbow was the joint that showed the largest change in angular displacement profile, and also a significant reduction in CV when actions were observed in the sagittal plane. The sagittal view uniquely afforded the opportunity for participants to potentially observe the elbow motor adaptation to the task. In the present study, we also note that with sagittal views the NAPU group yielded elbow displacement results similar to the prosthesis user actor, whereas the coronal view group shows elbow displacement profiles that are more similar to the intact actor (as in Figure 2 of (Cusack et al., 2014)).
There is potential relevance to amputees in these observations. The elbow is the closest joint to the prosthesis in transradial limb loss. This and our previous study suggest the importance of elbow adaptations to performing skillful movement using prostheses. Previous investigation has also demonstrated the importance of intact elbow in transradial amputees to obtain normal control strategies during reaching (Metzger et al., 2010). In skillful motor tasks, transradial amputees wearing prostheses demonstrate significantly increased elbow range of movement compared to sound limbs, suggesting that elbow adaptations are a vital compensatory accommodation (Carey et al., 2008). Given that the sagittal view emphasized elbow adaptations, and behavioral changes (CV, displacement) along with increased movement speed, we suggest that the ability to emphasize the elbow behavior substantially benefitted these participants compared to emphasizing shoulder behavior. Our ongoing studies are seeking to validate whether long-term (days, weeks) adaptations are improved using sagittal versus coronal perspectives for functional rehabilitation in amputees that are learning prosthesis usage.
Action observation therapy in residual limb motor control
Our pervious work suggested that the activation of the left parietofrontal system can be affected by the type of limb seen (Cusack et al., 2012). In this work, amputees with prostheses showed left parietofrontal activation when watching other prosthesis users. However, when the amputees watched persons with intact limbs perform the same tasks, left parietofrontal activation was decreased while right parieto-occipital activation was increased. We proposed that there was vulnerability to the action observation model in amputees due to the altered kinematics of prostheses compared to intact limbs. Such differences are not only modified biomechanics (such as range of movement as may occur in stroke) but involve missing degrees of freedom that are not functionally replaced by prostheses (Lake, 1997;Biddiss and Chau, 2007;Carey et al., 2008). Our behavioral studies demonstrate that naïve prosthesis users imitating actions of intact limbs perform movements with greater variability and poorer technique compared to imitation of prosthetic limbs (Cusack et al., 2012).
It is also proposed that perspective can significantly affect action-observation-mediated motor planning. During a motor imagery task, investigators have shown evidence that visual perspective can interfere with action planning mechanisms (Conson et al., 2012). Other studies have suggested that perspective-action interference may arise from premotor, rather than parietal cortex (Oosterhof et al., 2012). The significance of interference in premotor areas may highlight impacts on cortical motor planning mechanisms (Buccino et al., 2001;Rushworth et al., 2003;Jastorff et al., 2010;Caggiano et al., 2011), which are vital for skillful motor control (Murata et al., 1997;Rushworth et al., 2003;Wheaton et al., 2005;Jastorff et al., 2010). Viewing touch stimuli in egocentric versus allocentric perspectives can also significantly affect modulation of sensorimotor cortex (Schaefer et al., 2009). However, modeling has revealed perspective-invariant representation of actions in the parietofrontal activation (Oh et al., 2012). It has also been shown that perspective of seen actions may only mildly affect facilitation of primary motor cortex in observers (Alaerts et al., 2009).
In the case of prosthesis use, we propose that perspective matters greatly to facilitate the demonstration of essential motor adaptations that must be made with the prosthesis by the residual limb. This may be distinct from perspective in a sound limb, in which it may be easier to infer joint specific outcomes of an action based on shared biomechanics (de Vignemont and Haggard, 2008;Sadeghipour and Kopp, 2011). Studies have suggested that in cases where there is greater positional disparity between the actor and the imitator (assessed by rotating an avatar around its longitudinal axis, viewed from an overhead perspective), trajectory and velocity can be negatively affected (Krause and Kobow, 2013).
Based on Figure 1, it is apparent that perspective illustrates very different joint-level behaviors in prosthesis users. As in this and prior work (Cusack et al., 2014), participants were never told to focus on a particular joint or aspect of movement. Despite this, clear behavioral effects are seen. When degrees of freedom of the limb are eliminated due to limb loss, adaptation to a prosthesis may be optimized by a clear demonstration of how to adapt the residuum to perform the task. It is possible that the effects seen in the sagittal perspective reflect an “implicit imitation”, where prosthesis users are better able to adapt to the demands of the task without the need of specific instructions (Bisio et al., 2010). However, implicit imitation was not clearly seen at the shoulder with coronal perspective. As we did not see similar effects between the sagittal and coronal perspective video groups, this would suggest that the effects are beyond stimulus-response compatibility (SRC; as described in (Vankov and Kokinov, 2013;Cross and Iacoboni, 2014)), where the state of the observer better matches that of the observed. In this study, the motoric limitations of both the actor and the participant’s limbs are similarly mitigating a role of simple compatibility.
Conclusion
This work demonstrates that perspective of actions may have a significant role in adapting the residual limb following immobalization. Specifically, perspectives that demonstrate elbow adaptations to prosthesis usage may enhance functional motor outcomes to action observation therapies in transradial prosthesis usage. Future studies will involve focusing on rehabilitative outcomes to action observation prosthesis training across more tasks, evaluate transferrance across tasks, and further characterize outcomes at varying levels of upper limb loss.
References
- Alaerts K, Heremans E, Swinnen SP, Wenderoth N. How are observed actions mapped to the observer’s motor system? Influence of posture and perspective. Neuropsychologia. 2009;47:415–422. doi: 10.1016/j.neuropsychologia.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Anglin C, Wyss UP. Review of arm motion analyses. Proc Inst Mech Eng H. 2000;214:541–555. doi: 10.1243/0954411001535570. [DOI] [PubMed] [Google Scholar]
- Bassolino M, Bove M, Jacono M, Fadiga L, Pozzo T. Functional effect of short-term immobilization: kinematic changes and recovery on reaching-to-grasp. Neuroscience. 2012;215:127–134. doi: 10.1016/j.neuroscience.2012.04.019. [DOI] [PubMed] [Google Scholar]
- Biddiss EA, Chau TT. Upper limb prosthesis use and abandonment: a survey of the last 25 years. Prosthet Orthot Int. 2007;31:236–257. doi: 10.1080/03093640600994581. [DOI] [PubMed] [Google Scholar]
- Biometrics L. Goniometer and torsiometer operating manual 2010 [Google Scholar]
- Bisio A, Stucchi N, Jacono M, Fadiga L, Pozzo T. Automatic versus voluntary motor imitation: effect of visual context and stimulus velocity. PLoS One. 2010;5:e13506. doi: 10.1371/journal.pone.0013506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwsema H, Van Der Sluis CK, Bongers RM. The role of order of practice in learning to handle an upper-limb prosthesis. Arch Phys Med Rehabil. 2008;89:1759–1764. doi: 10.1016/j.apmr.2007.12.046. [DOI] [PubMed] [Google Scholar]
- Buccino G. Action observation treatment: a novel tool in neurorehabilitation. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130185. doi: 10.1098/rstb.2013.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, Seitz RJ, Zilles K, Rizzolatti G, Freund HJ. Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study. Eur J Neurosci. 2001;13:400–404. [PubMed] [Google Scholar]
- Caggiano V, Fogassi L, Rizzolatti G, Pomper JK, Thier P, Giese MA, Casile A. View-based encoding of actions in mirror neurons of area f5 in macaque premotor cortex. Current biology : CB. 2011;21:144–148. doi: 10.1016/j.cub.2010.12.022. [DOI] [PubMed] [Google Scholar]
- Carey SL, Jason Highsmith M, Maitland ME, Dubey RV. Compensatory movements of transradial prosthesis users during common tasks. Clin Biomech (Bristol, Avon) 2008;23:1128–1135. doi: 10.1016/j.clinbiomech.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Chao EY, An KN, Askew LJ, Morrey BF. Electrogoniometer for the measurement of human elbow joint rotation. J Biomech Eng. 1980;102:301–310. doi: 10.1115/1.3138227. [DOI] [PubMed] [Google Scholar]
- Conson M, Mazzarella E, Donnarumma C, Trojano L. Judging hand laterality from my or your point of view: interactions between motor imagery and visual perspective. Neurosci Lett. 2012;530:35–40. doi: 10.1016/j.neulet.2012.09.051. [DOI] [PubMed] [Google Scholar]
- Cross KA, Iacoboni M. To imitate or not: Avoiding imitation involves preparatory inhibition of motor resonance. Neuroimage. 2014;91:228–236. doi: 10.1016/j.neuroimage.2014.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusack W, Cope M, Nathanson S, Pirouz N, Kistenberg R, Wheaton LA. Neural activation differences in amputees during imitation of intact versus amputee movements. Front Human Neurosci. 2012:1–13. doi: 10.3389/fnhum.2012.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusack WF, Patterson R, Thach S, Kistenberg RS, Wheaton LA. Motor performance benefits of matched limb imitation in prosthesis users. Exp Brain Res. 2014;232:2143–2154. doi: 10.1007/s00221-014-3904-2. [DOI] [PubMed] [Google Scholar]
- Datta D, Selvarajah K, Davey N. Functional outcome of patients with proximal upper limb deficiency–acquired and congenital. Clin Rehabil. 2004;18:172–177. doi: 10.1191/0269215504cr716oa. [DOI] [PubMed] [Google Scholar]
- De Maeght S, Prinz W. Action induction through action observation. Psychol Res. 2004;68:97–114. doi: 10.1007/s00426-003-0148-3. [DOI] [PubMed] [Google Scholar]
- De Vignemont F, Haggard P. Action observation and execution: what is shared? Soc Neurosci. 2008;3:421–433. doi: 10.1080/17470910802045109. [DOI] [PubMed] [Google Scholar]
- Ferrari PF, Rozzi S, Fogassi L. Mirror neurons responding to observation of actions made with tools in monkey ventral premotor cortex. J Cogn Neurosci. 2005;17:212–226. doi: 10.1162/0898929053124910. [DOI] [PubMed] [Google Scholar]
- Flament D, Shapiro MB, Kempf T, Corcos DM. Time course and temporal order of changes in movement kinematics during learning of fast and accurate elbow flexions. Exp Brain Res. 1999;129:441–450. doi: 10.1007/s002210050911. [DOI] [PubMed] [Google Scholar]
- Gangitano M, Mottaghy FM, Pascual-Leone A. Modulation of premotor mirror neuron activity during observation of unpredictable grasping movements. Eur J Neurosci. 2004;20:2193–2202. doi: 10.1111/j.1460-9568.2004.03655.x. [DOI] [PubMed] [Google Scholar]
- Gazzola V, Keysers C. The observation and execution of actions share motor and somatosensory voxels in all tested subjects: single-subject analyses of unsmoothed fMRI data. Cereb Cortex. 2009;19:1239–1255. doi: 10.1093/cercor/bhn181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamzei F, Liepert J, Dettmers C, Weiller C, Rijntjes M. Two different reorganization patterns after rehabilitative therapy: an exploratory study with fMRI and TMS. Neuroimage. 2006;31:710–720. doi: 10.1016/j.neuroimage.2005.12.035. [DOI] [PubMed] [Google Scholar]
- Hansson GA, Balogh I, Ohlsson K, Skerfving S. Measurements of wrist and forearm positions and movements: effect of, and compensation for, goniometer crosstalk. J Electromyogr Kinesiol. 2004;14:355–367. doi: 10.1016/j.jelekin.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Jastorff J, Begliomini C, Fabbri-Destro M, Rizzolatti G, Orban GA. Coding observed motor acts: different organizational principles in the parietal and premotor cortex of humans. J Neurophysiol. 2010;104:128–140. doi: 10.1152/jn.00254.2010. [DOI] [PubMed] [Google Scholar]
- Kelly RL, Wheaton LA. Differential mechanisms of action understanding in left and right handed subjects: the role of perspective and handedness. Front Psychol. 2013;4:957–965. doi: 10.3389/fpsyg.2013.00957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempf T, Corcos DM, Flament D. Time course and temporal order of changes in movement kinematics during motor learning: effect of joint and instruction. Exp Brain Res. 2001;136:295–302. doi: 10.1007/s002210000584. [DOI] [PubMed] [Google Scholar]
- Krause D, Kobow S. Effects of model orientation on the visuomotor imitation of arm movements: the role of mental rotation. Hum Mov Sci. 2013;32:314–327. doi: 10.1016/j.humov.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Lake C. Effects of Prosthetic Training on Upper-Extremity Prosthesis Use. J Pros Orthotics. 1997;9:3–12. [Google Scholar]
- Liepert J, Hassa T, Tuscher O, Schmidt R. Motor excitability during movement imagination and movement observation in psychogenic lower limb paresis. J Psychosom Res. 2011;70:59–65. doi: 10.1016/j.jpsychores.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Magermans DJ, Chadwick EK, Veeger HE, Van Der Helm FC. Requirements for upper extremity motions during activities of daily living. Clin Biomech (Bristol, Avon) 2005;20:591–599. doi: 10.1016/j.clinbiomech.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Metzger AJ, Dromerick AW, Holley RJ, Lum PS. Characterization of compensatory trunk movements during prosthetic upper limb reaching tasks. Arch Phys Med Rehabil. 2012;93:2029–2034. doi: 10.1016/j.apmr.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Metzger AJ, Dromerick AW, Schabowsky CN, Holley RJ, Monroe B, Lum PS. Feedforward control strategies of subjects with transradial amputation in planar reaching. J Rehabil Res Dev. 2010;47:201–211. doi: 10.1682/jrrd.2009.06.0075. [DOI] [PubMed] [Google Scholar]
- Murata A, Fadiga L, Fogassi L, Gallese V, Raos V, Rizzolatti G. Object representation in the ventral premotor cortex (area F5) of the monkey. J Neurophysiol. 1997;78:2226–2230. doi: 10.1152/jn.1997.78.4.2226. [DOI] [PubMed] [Google Scholar]
- O’neill OR, Morrey BF, Tanaka S, An KN. Compensatory motion in the upper extremity after elbow arthrodesis. Clin Orthop Relat Res. 1992:89–96. [PubMed] [Google Scholar]
- Oh H, Gentili RJ, Reggia JA, Contreras-Vidal JL. Modeling of visuospatial perspectives processing and modulation of the fronto-parietal network activity during action imitation. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:2551–2554. doi: 10.1109/EMBC.2012.6346484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Oosterhof NN, Tipper SP, Downing PE. Viewpoint (in)dependence of action representations: an MVPA study. J Cogn Neurosci. 2012;24:975–989. doi: 10.1162/jocn_a_00195. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Johansen-Berg H, Gobel SM, Devlin JT. The left parietal and premotor cortices: motor attention and selection. Neuroimage. 2003;20(Suppl 1):S89–100. doi: 10.1016/j.neuroimage.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Sadeghipour A, Kopp S. Embodied Gesture Processing: Motor-Based Integration of Perception and Action in Social Artificial Agents. Cognit Comput. 2011;3:419–435. doi: 10.1007/s12559-010-9082-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe R, Jamal N, Powell L. My body or yours? The effect of visual perspective on cortical body representations. Cereb Cortex. 2006;16:178–182. doi: 10.1093/cercor/bhi095. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Xu B, Flor H, Cohen LG. Effects of different viewing perspectives on somatosensory activations during observation of touch. Hum Brain Mapp. 2009;30:2722–2730. doi: 10.1002/hbm.20701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz AE, Baade SP, Kuiken TA. Expert opinions on success factors for upper-limb prostheses. J Rehabil Res Dev. 2007;44:483–489. doi: 10.1682/jrrd.2006.08.0087. [DOI] [PubMed] [Google Scholar]
- Smith MA, Ghazizadeh A, Shadmehr R. Interacting adaptive processes with different timescales underlie short-term motor learning. PLoS Biol. 2006;4:e179. doi: 10.1371/journal.pbio.0040179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smurr LM, Gulick K, Yancosek K, Ganz O. Managing the upper extremity amputee: a protocol for success. J Hand Ther. 2008;21:160–175. doi: 10.1197/j.jht.2007.09.006. quiz 176. [DOI] [PubMed] [Google Scholar]
- Strafella AP, Paus T. Modulation of cortical excitability during action observation: a transcranial magnetic stimulation study. Neuroreport. 2000;11:2289–2292. doi: 10.1097/00001756-200007140-00044. [DOI] [PubMed] [Google Scholar]
- Toussaint L, Meugnot A. Short-term limb immobilization affects cognitive motor processes. J Exp Psychol Learn Mem Cogn. 2013;39:623–632. doi: 10.1037/a0028942. [DOI] [PubMed] [Google Scholar]
- Vankov I, Kokinov B. The role of the motor system in conceptual processing: effects of object affordances beyond response interference. Acta Psychol (Amst) 2013;143:52–57. doi: 10.1016/j.actpsy.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Wheaton LA, Nolte G, Bohlhalter S, Fridman E, Hallett M. Synchronization of parietal and premotor areas during preparation and execution of praxis hand movements. Clin Neurophysiol. 2005;116:1382–1390. doi: 10.1016/j.clinph.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Wise MB, Uhl TL, Mattacola CG, Nitz AJ, Kibler WB. The effect of limb support on muscle activation during shoulder exercises. J Shoulder Elbow Surg. 2004;13:614–620. doi: 10.1016/j.jse.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Wittenberg GF, Schaechter JD. The neural basis of constraint-induced movement therapy. Curr Opin Neurol. 2009;22:582–588. doi: 10.1097/WCO.0b013e3283320229. [DOI] [PubMed] [Google Scholar]
- Yuen HK, Nelson DL, Peterson CQ, Dickinson A. Prosthesis training as a context for studying occupational forms and motoric adaptation. Am J Occup Ther. 1994;48:55–61. doi: 10.5014/ajot.48.1.55. [DOI] [PubMed] [Google Scholar]


