Abstract
Background
Surgical removal of one or both eyes has been the most common way to treat children with retinoblastoma worldwide for more than 100 years. Ophthalmic artery chemosurgery (OAC) was introduced 10 years ago and it has been used as an alternative to enucleation for eyes with advanced retinoblastoma. The purpose of this report is to analyze our 9-year experience treating advanced retinoblastoma eyes with OAC.
Materials & Methods
Single-arm retrospective study from a single center of 226 eyes with eyes of retinoblastoma patients with advanced intraocular disease defined as both Reese-Ellsworth (RE) “Va” or ”Vb” and International Classification Retinoblastoma (ICRb) group “D” or ”E” (COG Classification). Ocular survival, patient survival, second cancers and electroretinography (ERG) were assessed.
Results
Ocular survival at five years for these advanced eyes was 70.2% (95% confidence interval, 57.3%–79.8%). When eyes were divided into groups either by RE classification or ICRb, no significant differences in ocular survival were seen. Ocular survival was significantly better in naïve compared to non-naïve eyes (80.2% vs 58.4%, p=0.041). The ERG distribution was very similar before and after OAC treatment for the patient population that did not receive intravitreal chemotherapy. Three patients (1.5%) have developed metastatic retinoblastoma (previously reported) and were successfully treated (no deaths).
Conclusion
Using OAC for advanced eyes (the majority of such eyes have been enucleated in the past) enables 70% 5-year ocular survival. Treated eyes have a similar ERG distribution before and after treatment. No patient has died of metastatic retinoblastoma.
Keywords: Electroretinography Melphalan, intrarterial chemotherapy, ophthalmic artery chemosurgery, retinoblastoma
INTRODUCTION
In high income countries the majority (>70%) of children present with advanced intraocular disease and almost all of them were enucleated primarily until 30 years ago.1 As clinicians became more experienced in the use of external beam irradiation some of these eyes were treated primarily with radiation, Sixty percent of such advanced eyes were primarily enucleated and of the remaining 40% of eyes only half were salvaged with radiation.2 Thus even with modern radiation techniques only 20% of advanced Rb eyes (Group V) could be salvaged. While this represented a meaningful step for the retinoblastoma patient (compared to salvaging no eyes), the subsequent development of second cancers ultimately led to less use of this approach.
Clinicians worldwide began using systemic chemotherapy instead of external beam irradiation for intraocular retinoblastoma about 25 years ago. Success rates vary widely and published success rates for “D” eyes managed initially with systemic chemotherapy vary between 0% and 83%3,4 with most in the 25–30% range.4–6 “E” eyes, the most advanced class of retinoblastoma eyes, have generally not been treated in the past but have mostly been enucleated.6–8 Direct comparison of salvage rates described in literature is difficult since different ICRb versions are used for the classification of the eyes. Also, primary enucleation rates are often not published and making it difficult to estimate the actual saved eyes.
In the 1950s delivery of local chemotherapy via the carotid artery was pioneered9 in order to lower the subsequent dose of EBRT. Later, enhanced techniques were developed which ensured more localized delivery to the ophthalmic artery.10 In New York we have developed direct ophthalmic artery infusion, also called ophthalmic artery chemosurgery (OAC), a technique in which chemotherapy is locally delivered to the eye by a micro-catheter that is advanced from the femoral artery into the orifice of the ophthalmic artery. OAC has now been performed more than 3,000 times worldwide since introduced in 2006.11 OAC has been used successfully for unilateral and bilateral disease, as primary (naïve) and salvage therapy, for eyes with vitreous and/or sub-retinal seeding, for eyes with extensive retinal detachment and for both advanced and less advanced intraocular disease.12–15 With time, the expanded the use of OAC included eyes with less advanced disease, and treated children as young as three months of age.14–17 Side effects, advantages and disadvantages of OAC have been described in various reviews 18–20.
In our initial report of 9 OAC cases, all 9 eyes were advanced and scheduled for enucleation prior to enrolling in our Institutional Review Board (IRB)-approved clinical trial. Each eye was classified as Reese-Ellsworth Group V.11 Our hope at that time was that OAC might be as effective as systemic chemotherapy but less toxic and that by eliminating systemic chemotherapy we could eliminate the short term and long-term side effects of systemic chemotherapy without sacrificing efficacy. We quickly learned that OAC could not only replace systemic chemotherapy (and eliminate those systemic side effects including the need for a vascular port) but, because of the higher drug concentration attained in the eye, it could salvage the majority of eyes that we would previously have enucleated.1
Seventy five percent of children with retinoblastoma in the US have disease that cannot be cured with focal treatments alone and thus require external beam radiation, systemic chemotherapy, or enucleation. Most of these have either RE “Va” or “Vb” eyes and ICRb “D” or “E” eyes. Unfortunately such eyes are usually not saved (whether naïve or recurrent) when managed with primary radiation or systemic chemotherapy alone.21 At the same time advanced intraocular retinoblastoma eyes that previously were almost always enucleated worldwide are now being treated.22 After 9 years of experience we decided to retrospectively review these cases and report on patient survival, ocular survival, second cancers and also ERG results before and after treatment for the sub-set of eyes classified as advanced by both classification schemes.
MATERIALS & METHODS
Subjects
This is a single center retrospective review of all patients with advanced retinoblastoma managed with OAC since we began using it in May 2006 until December 2014. Memorial Sloan Kettering Institutional Review Board approval was obtained for the retrospective review. Advanced retinoblastoma was defined as either Reese-Ellsworth Groups “Va” or “Vb” and ICRb Classification Groups “D” or “E” using the Children’s Oncology group (COG) version. This was done because some eyes with small tumors and localized vitreous seeding are classified as RE Vb but ICRb “C” and we do not consider those advanced. No patient who came to our institution after May 2006 with advanced intraocular retinoblastoma was excluded.
Ocular and patient survival
Statistical analysis was performed with Prism (GraphPad Software, Inc, La Jolla, CA). Kaplan-Meier survival data with the log-rank test were used to evaluate ocular survival (defined as survival of the eye; no enucleation), and the Mantel-Cox test was used to compare survival curves. In all cases, 95% confidence intervals were used and 5-year (60 months) ocular survival was reported.
The stratification between non-naïve and naïve eyes was defined as follows; non-naïve eyes did receive prior therapy with either systemic chemotherapy (including bridge systemic chemotherapy) and/or External beam radiation therapy. Naïve eyes had not had any of those therapies; however naïve eyes potentially were treated previously with local therapies (e.g. plaque brachytherapy, transpupillary thermotherapy (TT), periocular chemotherapy, cryotherapy, or intravitreal chemotherapy) or OAC at another hospital. Primarily enucleated eyes were defined as eyes that were enucleated fewer than 30 days after coming to Memorial Sloan Kettering. For the OAC treatments the following chemotherapeutics were used; melphalan, topotecan, carboplatin and methotrexate or a combinations of those. For bilateral patients we have previously described that patients need on average 3.48 OAC treatments per eye (range 1–8).23 Furthermore, patients that have received additional intravitreal therapy (n=43) have received median of 5 intravitreal injections (range 1–12).24 Metastasis and information on second cancers in these patients were tabulated.
Electroretinography
We used an adaptation of the International Society for Clinical Electrophysiology of Vision standard ERG protocol to obtain ERG recordings. More specifically, 30-Hz photopic flicker amplitude data were used, since we have previously reported that this is representative of the total ERG response. The initial ERG was compared to the most recent follow-up ERG. Based on statistical analysis of the ERGs of normal eyes that were recorded under anesthesia, 30-Hz response amplitude changes of >25 μV were considered clinically meaningful.25
Comparisons between ERG responses were considered to be the ‘same’ when ERG changes were no greater than a decrement or increment of 25 μV, ‘better’ with an increment of at least 25 μV or ‘worse’ with a decrement of at least 25μV. Moreover, consistent with our prior publications, ERG amplitudes were capped at 100 μV for analysis, as increases in ERG amplitude beyond this level were considered of no clinical significance, representing changes within the “excellent” category. ERG amplitudes were classified according to the following 6-point scale: less than 0.1 μV, undetectable; 0.1–25 μV, poor; 25.1–50 μV, fair; 50.1–75 μV, good; 75.1–100 μV very good; more than 100 μV, excellent.26
RESULTS
From the start of OAC treatment in May 2006 through December 2014, 331 eyes of 263 patients were treated. Two hundred twenty-six of these were advanced eyes (167 naïve) from 202 patients and are included in the present analysis. Patient demographics are given in Table 1.
Table 1.
Patient Demographics.
| Features at time of Ophthalmic artery chemosurgery | Number |
|---|---|
| Treated patients | 202 |
| Treated number of eyes | 226 |
| Mean Age (Mos) (n=202 patients) | 23 |
| Median (range) | 18 (0.3–195) |
| Mean follow-up (Mos) | 28 |
| Median (range) | 19 (0.5–101) |
| Sex in treated eyes | |
| Female | 121 (54%) |
| Male | 105 (46%) |
| Heredity in treated eyes | |
| Sporadic | 214 (95%) |
| Familial | 12 (5%) |
| Laterality in treated eyes | |
| Unilateral | 96 (42%) |
| Bilateral | 130 (58%) |
| Eyes undergoing treatment* | |
| Right | 110 (49%) |
| Left | 116 (51%) |
Both eyes treated with ophthalmic artery chemosurgery in 24 patients.
Enucleations
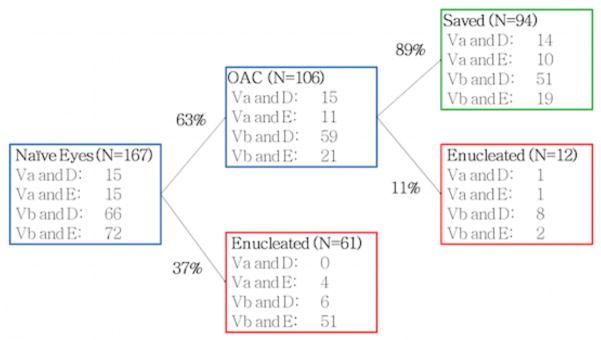
We have seen 167 naïve advanced eyes in our clinic during the specified time frame (Figure 1). The naïve eyes received either OAC (n=106; 63%) or were enucleated (n=61; 37%), these enucleations were done primarily (n=59; 97%) or in the course of non OAC treatment (n=2; 3%). The vast majority (n=51; 83%) of these enucleated eyes were classified as Vb/E (Both RE “Vb” and ICRb COG “E”). Eleven percent of the 106 advanced naïve eyes primarily treated with OAC were subsequently enucleated.
Figure 1. Enucleations in naïve advanced eyes.
Reese Ellsworth Group “Va” or “Vb” and ICRb COG Group “D” or “E” eyes are tabulated for all naïve eyes since May 2006. Naïve advanced eyes were enucleated or treated with OAC. For the enucleated eyes 59/61 were primarily enucleated and 2/61 eyes did receive treatment other than OAC but were enucleated ultimately (both Vb/E). For the 106 eyes that received OAC treatment, 94 eyes were saved until the last follow-up and 12 eyes were enucleated.
Ocular Survival
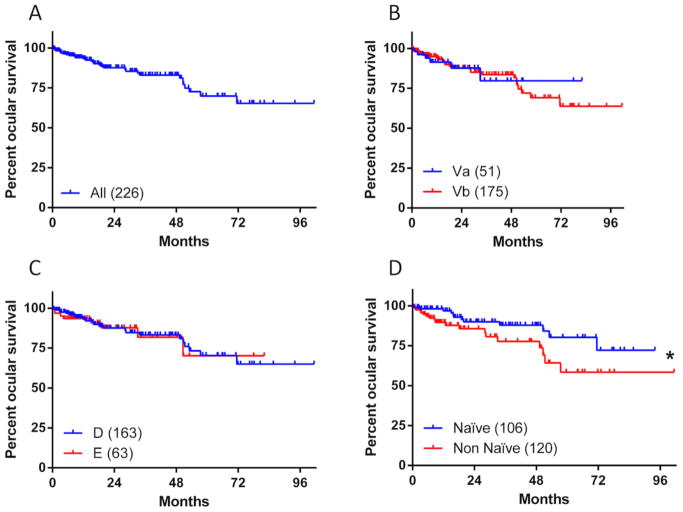
The Kaplan-Meier estimate of the 5-year (60 months) ocular survival for the OAC treated eyes was 70.2% (95% Confidence Interval [CI], 57.3%–79.8%) (Figure 2A). Using the Reese-Ellsworth classification there were 51 Group Va and 175 Group Vb OAC treated eyes in our series. Ocular survival curves were not significantly different (p=0.974), with five-year survival of respectively 79.7% in “Va” (95% CI: 54.7%–91.9%) versus 69.3% in “Vb” (95% CI, 55.2%–79.8%) (Figure 2B). When the eyes were classified by ICRb classification there was no difference in five-year ocular survival between the 163 group “D” versus the 63 group “E” eyes (p=0.827) respectively 70.4% (95% CI, 56.2%–80.8%) and 70.1% (95% CI, 38.7%–87.6%) (Figure 2C).
Figure 2. Ocular survival in advanced eyes treated with OAC.
Ocular Survival Kaplan-Meier curves for 226 eyes which have been treated with OAC (A). Patients’ eyes were divided in groups according to Reese-Ellsworth (B), ICRb COG (C), and Naivety (D). The number of eyes in each group are depicted in the legend, *depicts a significant change; Mantel-Cox test p-value=0.041.
Naïve vs Non-Naïve Eyes
Ocular survival was different when naïve eyes were compared to non-naïve eyes. When naïve eyes (n=106) were compared to non-naïve (n=120) eyes the 5-year ocular survival was significantly better in the naïve cases (p=0.041), respectively 80.2% (95% CI, 64.2–89.6%) vs 58.4% (95% CI, 37.6%–74.4%) (Figure 2D).
Unilateral vs Bilateral Eyes
Ocular survival curves were not significantly different (p=0.825), with five-year survival of respectively 67.0% in bilateral eyes (95% CI: 48.2%–80.3%) versus 73.5% in unilateral eyes (95% CI, 54.6%–85.5%) (Supplemental Figure 1).
Electroretinography Data
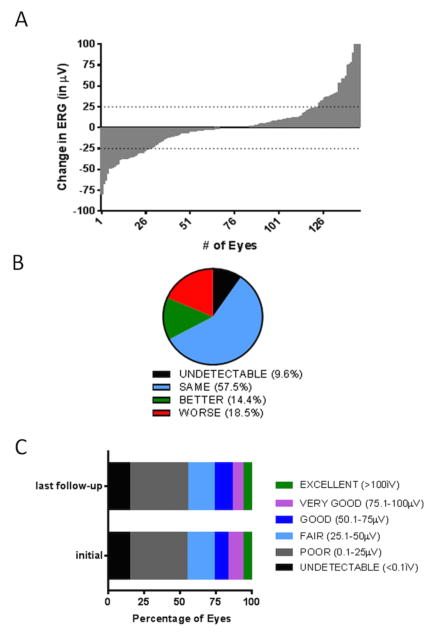
ERG amplitudes were recorded at two time points in 189 eyes; before the start of OAC treatment and at the last follow-up. Included in this group were the 43 eyes that also received intravitreal injections of chemotherapy; since this treatment is known to affect the retinography negatively these 43 eyes were analysed seperately.27 Changes in ERG amplitude of each individual eye at the last follow-up versus the initial measurement are visualized in a waterfall plot (Figure 3A).
Figure 3. Electroretinography response in patients treated with OAC.
The change in ERG amplitude between the initial measurement and the last follow-up has been plotted in a waterfall plot (A). Eyes with an ERG change of more than 25 μV or −25 μV are respectively doing ‘better’ or ‘worse’. Furthermore, eyes are categorized ‘undetectable’ when ERG values <0.1 μV are measured at the initial visit and at the last follow-up and eyes were categorized ‘same’ when the ERG change is less than 25 μV (B). Eyes have been divided in 6 groups (see legend) according to ERG amplitude (C).
For the 146 eyes that had not received additional intravitreal treatments ERG responses were ‘undetectable’ in 9.6%, ‘same’ in 57.5 %, ‘better’ in 14.4% or ‘worse’ in 18.5% (Figure 3B). The distribution of ERG amplitudes in the 146 eyes were very similar before and after OAC treatment (Figure 3C).
For eyes which also received intravitreal treatment (n=43), ERG changes were ‘undetectable’ in 9.3%, ‘same’ in 55.8 % or ‘worse’ (34.9%) (data not shown). As expected, the intravitreally treated eyes had a significantly higher fraction of ‘worse’ eyes compared to non-intravitreally treated eyes (Chi-square, p=0.023).
Retinoblastoma patient survival
Three patients (1.5%) developed metastatic retinoblastoma (all unilateral, also described in,28) upon this diagnosis these patients received multiagent systemic chemotherapy. Each of these patients is disease-free with follow-up of 3, 4 and 5 years after metastasis diagnosis, (total follow-up for these patients is respectively 4,7,8 years). CNS involvement was seen for one of these patients with metastatic disease (the patient with 7 years total follow-up).
Second Cancers
Four patients (2.0%, all bilateral) have developed a second, nonocular cancer and have been managed with systemic chemotherapy and surgery. One patient (0.5%) died 1 year after diagnosis of the second cancer, a pineoblastoma. In our study period, the other 3 patients with second cancer remain alive (2 with pineoblastoma and 1 with osteosarcoma) respectively 4 months, 1 year and 6 months after diagnosis. The total follow-up in these 3 patients is respectively 3, 2 and 5 years.
DISCUSSION
The decision of OAC versus enucleation is made on a case-to-case basis. In general, we advise that eyes with buphthalmos, rubeotic glaucoma or anterior chamber involment be enucleated. However, some parents are absolutely not willing to consent to an enucleation procedure and consequently these eyes will be treated with OAC.
In our current study actual vision is not reported since we were dealing with preverbal patients. Instead, ERG amplitude measurements were taken before treatment and at follow-up appointments. Forty-six percent of the advanced eyes treated with OAC show ‘fair’ to ‘excellent’ ERG amplitudes at last follow-up. Future research will aim to determine actual vision according to “Snellen” acuity testing in this population.
This study confirms that the majority of eyes with advanced retinoblastoma can be managed with primary OAC and mostly avoid the need for enucleation or external beam irradiation (used only in 1% of the OAC treated eyes) or systemic chemotherapy (used only in 2% of the OAC treated eyes). Sixty-three percent of our naïve advanced eyes were treated primarily with OAC and 37% were primarily enucleated. Additional local therapies are often given to OAC treated eyes and these therapies likely contribute to the ocular survival of OAC treated eyes. Most frequently, transpupillary chemotherapy (54%) and cryotherapy (38%) were given but also intravitreal chemotherapy (19%), plaque brachytherapy (8%) periocular chemotherapy (6%) were used.
Eyes that have failed prior systemic chemotherapy (or external beam irradiation) have few options other than enucleation. If recurrent disease is limited focal techniques such as laser, cryotherapy or plaques often allow the eye to be saved, but for advanced eyes (with vitreous and or subretinal seeding) the options are few. When radiation is added to chemotherapy the incidence of ocular complications increases and so does the incidence of (usually) fatal second cancers.29 This present series demonstrates that even in cases that have failed prior systemic chemotherapy and are advanced the majority can be salvaged with OAC.
The introduction of OAC has improved ocular survival rates for eyes with advanced retinoblastoma (naive or not) and our 9-year experience shows that we have been able to save the majority of eyes with advanced retinoblastoma.
Previously, we have shown that unilateral advanced eyes treated with OAC survival had better ocular survival in the period of 2010–2014 compared to 2006–2009. We have speculated that this is the result of a combination of factors: experience, introduction of multi-agent chemotherapy in OAC and the introduction of intravitreal injections in 2012 in our clinic.28
Shields and colleagues have also shown successful OAC treatment in a smaller group of advanced eyes, they have treated 32 advanced eyes with OAC. After a mean follow-up of 19 months they have shown that in naïve patients the ocular survival is respectively 94% in “D” eyes and 36% in “E” eyes.22 In addition, Tuncer and colleagues recently reported a 62.9% 2-year ocular survival in 24 “D” eyes that were primarily treated with OAC in Turkey.30 In comparison, we found an overall 80.2% 5-year ocular survival in naïve advanced eyes but we have not observed an ocular survival difference between “D” versus “E” eyes.
This study reports a metastatic rate of 1.5%, which is low compared to the 5% described in literature. Around 90% of metastatic cases will present in the first year after retinoblastoma diagnosis.31 Since our median follow-up is 19 months we have likely captured almost all metastatic cases in our study population. Furthermore, it is notable that despite the fact that all eyes in this series were advanced no patient has died of metastatic disease. Moreover, a recent report in which combined data from four of the world’s largest OAC centers on three continents also shows that metastasis or metastatic deaths have not increased by OAC (634 patients from which 202 overlap with our cohort).20
Four cases of second cancer were diagnosed in our series, three of which were in bilateral patients (3/130; 2.3%) diagnosed with pineoblastomas. A recent systemic review performed a meta-analysis for pineoblastoma incidence in literature and they found a 2.9% incidence in bilateral patients that were diagnosed after 1995,32 which is comparable to the 2.3% pineoblastoma rate that we have found in our bilateral OAC-treated patients. Moreover, another meta-analysis has shown that about half of the patients with pineoblastoma survive,33 which is comparable to this study in which 2/3 patients have survived pineoblastoma.
Altogether, in this series the incidence of metastasis and pineoblastoma were respectively low and comparable to the rates reported in literature, so were the metastatic and pineoblastoma death rates.31–33
Acknowledgments
FUNDING
This work was supported by the Fund for Ophthalmic Knowledge, Inc. (no grant number; philantropic fund); and Perry’s Promise Fund (no grant number; philantropic fund) and funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Color versions of one or more figures in the article can be found online at http://www.tandfonline.com/iopg.
Supplemental data for this article can be accessed on the publisher’s website at http://dx.doi.org/10.1080/13816810.2016.1244695.
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- 1.Abramson DH. Retinoblastoma: Saving Life with Vision. Annu Rev Med. 2014;65(1):171–184. doi: 10.1146/annurev-med-061312-123455. [DOI] [PubMed] [Google Scholar]
- 2.Abramson DH, Beaverson KL, Chang ST, Dunkel IJ, McCormick B. Outcome Following Initial External Beam Radiotherapy in Patients WithReese-Ellsworth Group Vb Retinoblastoma. Arch Ophthalmol. 2004;122(9):1316–1323. doi: 10.1001/archopht.122.9.1316. [DOI] [PubMed] [Google Scholar]
- 3.Abramson DH, Schefler AC. Update on Retinoblastoma. Retina. 2004;24(6):828–848. doi: 10.1097/00006982-200412000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Chan H, Gallie B, Munier F, Beckpopovic M. Chemotherapy for Retinoblastoma. Ophthalmology Clinics of North America. 2005;18(1):55–63. doi: 10.1016/j.ohc.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Chung SE, Sa HS, Koo HH, Yoo KH, Sung KW, Ham D-I. Clinical manifestations and treatment of retinoblastoma in Korea. British Journal of Ophthalmology. 2008;92(9):1180–1184. doi: 10.1136/bjo.2008.140046. [DOI] [PubMed] [Google Scholar]
- 6.Gündüz K, Köse K, Kurt RA, et al. Retinoblastoma in Turkey: Results From a Tertiary Care Center in Ankara. J Pediatr Ophthalmol Strabismus. 2013;50(5):296–303. doi: 10.3928/01913913-20130730-02. [DOI] [PubMed] [Google Scholar]
- 7.Lim FPM, Soh SY, Iyer JV, Tan AM, Swati H, Quah BL. Clinical Profile, Management, and Outcome of Retinoblastoma in Singapore. J Pediatr Ophthalmol Strabismus. 2012;50(2):106–112. doi: 10.3928/01913913-20121211-01. [DOI] [PubMed] [Google Scholar]
- 8.Mallipatna AC, Sutherland JE, Gallie BL, Chan H, Héon E. Management and outcome of unilateral retinoblastoma. Journal of American Association for Pediatric Ophthalmology and Strabismus. 2009;13(6):546–550. doi: 10.1016/j.jaapos.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Reese AB, Hyman GA, Merriam GR, Forrest AW, Kligerman MM. Treatment of retinoblastoma by radiation and triethylenemelamine. AMA Arch Ophthalmol. 1954;53(4):505–513. doi: 10.1001/archopht.1955.00930010507007. [DOI] [PubMed] [Google Scholar]
- 10.Yamane T, Kaneko A, Mohri M. The technique of ophthalmic arterial infusion therapy for patients with intraocular retinoblastoma. Int J Clin Oncol. 2004;9(2):69–73. doi: 10.1007/s10147-004-0392-6. [DOI] [PubMed] [Google Scholar]
- 11.Abramson DH, Dunkel IJ, Brodie SE, Kim JW, Gobin YP. A Phase I/II Study of Direct Intraarterial (Ophthalmic Artery) Chemotherapy with Melphalan for Intraocular Retinoblastoma. Ophthalmology. 2008;115(8):1398–1404. e1. doi: 10.1016/j.ophtha.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 12.Abramson DH. Chemosurgery for Retinoblastoma. Arch Ophthalmol. 2011;129(11):1492–1494. doi: 10.1001/archophthalmol.2011.354. [DOI] [PubMed] [Google Scholar]
- 13.Chantada G, Schaiquevich P. Management of Retinoblastoma in Children: Current Status. Pediatr Drugs. 2015;17(3):185–198. doi: 10.1007/s40272-015-0121-9. [DOI] [PubMed] [Google Scholar]
- 14.Shields CL, Kaliki S, Shah SU, Bianciotto CG, Jabbour P, Shields JA. Effect of intraarterial chemotherapy on retinoblastoma-induced retinal detachment. Retina (Philadelphia, Pa) 2012;32(4):799–804. doi: 10.1097/IAE.0b013e31823d8e1e. [DOI] [PubMed] [Google Scholar]
- 15.Abramson DH, Marr BP, Brodie SE, Dunkel I, Palioura S, Gobin YP. Ophthalmic artery chemosurgery for less advanced intraocular retinoblastoma: five year review. In: Abramson DH, Marr BP, Brodie SE, Dunkel I, Palioura S, Gobin YP, editors. PLoS ONE. 4. Vol. 7. 2012. p. e34120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muen WJ, Kingston JE, Robertson F, Brew S, Sagoo MS, Reddy MA. Efficacy and Complications of Super-selective Intra-ophthalmic Artery Melphalan for the Treatment of Refractory Retinoblastoma. Ophthalmology. 2012;119(3):611–616. doi: 10.1016/j.ophtha.2011.08.045. [DOI] [PubMed] [Google Scholar]
- 17.Gobin YP, Dunkel IJ, Marr BP, Francis JH, Brodie SE, Abramson DH. Combined, sequential intravenous and intra-arterial chemotherapy (bridge chemotherapy) for young infants with retinoblastoma. In: Gobin YP, Dunkel IJ, Marr BP, Francis JH, Brodie SE, Abramson DH, editors. PLoS ONE. 9. Vol. 7. 2012. p. e44322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jabbour P, Chalouhi N, Tjoumakaris S, et al. Pearls and pitfalls of intraarterial chemotherapy for retinoblastoma. J Neurosurg Pediatr. 2012;10(3):175–181. doi: 10.3171/2012.5.PEDS1277. [DOI] [PubMed] [Google Scholar]
- 19.Yousef YA, Soliman SE, Astudillo PPP, et al. Intra-arterial Chemotherapy for Retinoblastoma: A Systematic Review. JAMA Ophthalmol. 2016;134(5):584–591. doi: 10.1001/jamaophthalmol.2016.0244. [DOI] [PubMed] [Google Scholar]
- 20.Abramson DH, Shields CL, Munier FL, Chantada GL. Treatment of Retinoblastoma in 2015. JAMA Ophthalmol. 2015 Sep;:1. doi: 10.1001/jamaophthalmol.2015.3108. [DOI] [PubMed] [Google Scholar]
- 21.Lin P, O’Brien JM. Frontiers in the Management of Retinoblastoma. American Journal of Ophthalmology. 2009;148(2):192–198. doi: 10.1016/j.ajo.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Shields CL, Manjandavida FP, Lally SE, et al. Intra-arterial Chemotherapy for Retinoblastoma in 70 Eyes. Ophthalmology. 2014;121(7):1453–1460. doi: 10.1016/j.ophtha.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 23.Abramson DH, Marr BP, Francis JH, et al. Simultaneous Bilateral Ophthalmic Artery Chemosurgery for Bilateral Retinoblastoma (Tandem Therapy) In: Vavvas DG, editor. PLoS ONE. 6. Vol. 11. 2016. p. e0156806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Francis JH, Abramson DH, Gaillard M-C, Marr BP, Beck-Popovic M, Munier FL. The classification of vitreous seeds in retinoblastoma and response to intravitreal melphalan. Ophthalmology. 2015;122(6):1173–1179. doi: 10.1016/j.ophtha.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 25.Francis JH, Abramson DH, Gobin YP, et al. Electroretinogram Monitoring of Dose-Dependent Toxicity after Ophthalmic Artery Chemosurgery in Retinoblastoma Eyes: Six Year Review. In: Cinti C, editor. PLoS ONE. 1. Vol. 9. 2014. p. e84247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Francis JH, Abramson DH, Gobin YP, et al. Efficacy and Toxicity of Second-Course Ophthalmic Artery Chemosurgery for Retinoblastoma. Ophthalmology. 2015;122(5):1016–1022. doi: 10.1016/j.ophtha.2014.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Francis JH, Schaiquevich P, Buitrago E, et al. Local and Systemic Toxicity of Intravitreal Melphalan for Vitreous Seeding in Retinoblastoma. Ophthalmology. 2014;121(9):1810–1817. doi: 10.1016/j.ophtha.2014.03.028. [DOI] [PubMed] [Google Scholar]
- 28.Abramson DH, Fabius AWM, Issa R, et al. Advanced Unilateral Retinoblastoma: The Impact of Ophthalmic Artery Chemosurgery on Enucleation Rate and Patient Survival at MSKCC. In: Vavvas D, editor. PLoS ONE. 12. Vol. 10. 2015. p. e0145436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong JR, Morton LM, Tucker MA, et al. Risk of subsequent malignant neoplasms in long-term hereditary retinoblastoma survivors after chemotherapy and radiotherapy. J Clin Oncol. 2014;32(29):3284–3290. doi: 10.1200/JCO.2013.54.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tuncer S, Sencer S, Kebudi R, Tanyıldız B, Cebeci Z, Aydın K. Superselective intra-arterial chemotherapy in the primary management of advanced intra-ocular retinoblastoma: first 4-year experience from a single institution in Turkey. Acta Ophthalmol. 2016 May; doi: 10.1111/aos.13077. [DOI] [PubMed] [Google Scholar]
- 31.Ramasubramanian A, Shields CL. Retinoblastoma. JP Medical Ltd; 2012. [Google Scholar]
- 32.de Jong MC, Kors WA, de Graaf P, Castelijns JA, Moll AC, Kivelä T. The Incidence of Trilateral Retinoblastoma: A Systematic Review and Meta-Analysis. American Journal of Ophthalmology. 2015;160(6):1116–1126. e5. doi: 10.1016/j.ajo.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 33.de Jong MC, Kors WA, de Graaf P, Castelijns JA, Kivelä T, Moll AC. Trilateral retinoblastoma: a systematic review and meta-analysis. Lancet Oncol. 2014;15(10):1157–1167. doi: 10.1016/S1470-2045(14)70336-5. [DOI] [PubMed] [Google Scholar]