Abstract
Rationale: Cytological analysis of pleural effusions (PEs) has a sensitivity of approximately 60%. We hypothesized that the CELLSEARCH technology (Janssen Research and Development, Huntingdon Valley, PA) currently used to detect circulating tumor cells could be adapted for the identification of tumor cells in PEs.
Methods: This was a single-center, prospective, observational study. Pleural fluid from subjects with undiagnosed PEs were analyzed by CELLSEARCH technology, which uses an epithelial cell adhesion molecule antibody–based capture system/cytokeratin antibodies to identify tumor cells. Subjects were prospectively monitored by periodic chart review to determine the etiology of the PE.
Measurements and Main Results: One hundred thirty-two subjects were analyzed. A malignant etiology was established in 81 subjects. The median number of “positive” pleural epithelial cells (PECs) detected per milliliter of pleural fluid was 6 in the benign group. The number of PECs was 52 in the malignant nonepithelial group (NS) and 526 in the malignant epithelial group (P < 0.001). Unlike blood, there was a baseline number of “positive” cells in benign pleural fluids; however, any cutoff greater than 852 positive cells/ml had 100% specificity. The area under the receiver operating characteristic curve was 0.86. Nine percent of our cancer cases had high numbers of PECs (>280/ml) but a negative or nondefinitive cancer diagnosis by cytology.
Conclusions: The pleural CELLSEARCH assay may serve as a valuable addition to traditional cytology and provide useful information regarding the diagnosis of malignant effusions. Major advantages include that it is well standardized, relatively inexpensive, has a rapid turnaround, and is easily available. Our data support the conduct of additional studies of this approach to assist in the diagnosis of malignant PEs.
Keywords: pleural effusion, malignant effusion, circulating tumor cell, CELLSEARCH, pleural fluid cytology
Approximately 1.3 million pleural effusions are diagnosed each year in the United States with congestive heart failure, infection, and malignancy being the leading causes (1). If a diagnosis cannot be made on the basis of clinical data alone, sampling of the pleural effusion by thoracentesis is mandatory (1, 2).
Effusions are common in the setting of cancer and can be due to metastatic disease or other benign causes such as postobstructive pneumonia, lymphatic obstruction, or atelectasis. In this context, it is important to diagnose a malignant pleural effusions (MPEs), as this denotes an advanced stage of disease, poor prognosis, and in many instances, defines treatment options (3). An MPM can occur as a complication of virtually any type of tumor; however, they are most commonly associated (∼85%) with epithelial cancers from the lung, breast, genitourinary tract, and gastrointestinal tract (1, 2). Less common causes include lymphomas and malignant mesothelioma.
Pleural fluid cytology is the least invasive means of obtaining a definitive diagnosis of a MPE; however, it carries a sensitivity of only 60% (4–7). Diagnostic yield depends on sample composition, preparation, amount of fluid obtained, experience of the pathologist, and tumor type (2). A number of challenges have been identified (8), including (1) difficulties have been encountered in the distinction between mesothelial, neoplastic, and reactive cells; (2) some tumors display relatively low degrees of nuclear atypia and do not form easily recognized tissue fragments; and (3) some samples have large amounts of blood and inflammatory cells with only rare interspersed tumor cells. One solution has been the use of immunohistochemistry (IHC) (2). For example, specimens can be stained with an epithelial cell marker such as claudin-4 (C4) that was found to be present in malignant cells of epithelial origin but not in mesothelial cells (9). It should be noted, however, that a panel of IHC stains can be relatively expensive, takes additional time, and is not readily available at all laboratories and is thus not routinely performed.
A nondiagnostic cytologic analysis in the setting of a high clinical suspicion for malignancy often leads to more invasive procedures. Pleural biopsy (usually via thoracoscopy) is the most accurate way to exclude malignant pleural involvement. However, this test adds significant patient morbidity and increases health care costs (10–12). An additional approach that could easily identify malignant cells within pleural fluid would thus be extremely useful.
The purpose of this study was to evaluate advances in technology that allow the automated detection in blood of rare epithelial cancer cells called circulating tumor cells (CTCs). Several methods exist for the isolation and characterization of CTCs (13). The most robust and well standardized is the CELLSEARCH technology developed by Janssen Research and Development (Huntingdon Valley, PA). It uses a sensitive immunomagnetic enrichment method to capture epithelial cells in peripheral blood through ferrofluids coupled with an antibody to epithelial cell adhesion molecule (EpCAM or CD326). EpCAM is highly expressed on most cancers of epithelial origin. The detection of just 5 CTCs/7.5 ml of blood in patients with breast and prostate cancer and 3 CTCs/7.5 ml of blood in patients with colorectal cancer was found to be an independent marker of poor survival (14–17). CELLSEARCH is the only U.S. Food and Drug Administration–approved test for the detection of CTCs in whole blood for these tumors.
We hypothesized that the ability of the CELLSEARCH technology to detect these rare tumor cells in blood (sometimes just 1 in 10 million cells) presented an opportunity to identify tumor cells in other body fluids, such as pleural effusions. Accordingly, we determined whether the enumeration of pleural fluid epithelial cells, using the CELLSEARCH technology, could be used for the diagnosis of malignant pleural effusions. In addition, we asked whether the addition of an anti–claudin-4 antibody, as an additional epithelial cell marker, improved the diagnostic accuracy of this test.
Methods
This was a single-center, prospective, observational study conducted at the Hospital of the University of Pennsylvania (Philadelphia, PA) between January 2010 and April 2012. The goal of the study was to determine the performance characteristics of pleural fluid epithelial cell (PEC) enumeration, using the CELLSEARCH technology as a test for the diagnosis of MPEs. All study participants were enrolled under a waiver of informed consent. The study was performed according to the principles of the Declaration of Helsinki and was approved by the institutional review board. The first and last authors vouch for the accuracy and completeness of the reported data.
Study Population and Sample Collection
Subjects diagnosed with a pleural effusion, who underwent a diagnostic thoracentesis as part of their routine clinical care, and had a sample sent for cytologic analysis were enrolled in the study. Patients and treating physicians were blinded to the results of the study.
A 7.5-cm3 aliquot of unprocessed pleural fluid was collected in a CellSave preservative tube (Veridex, Huntingdon Valley, PA) within 24 hours of collection and sent to Janssen Research and Development for PEC enumeration within 96 hours. We validated that the 96 hours of storage time did not affect the accuracy of the test, using control samples. All samples were kept at room temperature (refrigeration for up to 1 hr was allowed).
CELLSEARCH Technology
Enumeration of CTCs in blood by the CELLSEARCH technology has been described elsewhere (18), and we adapted the technology to enumerate PECs (equivalent to CTCs in blood). In brief, 3.0-ml pleural fluid samples from the CellSave tubes were incubated with ferrofluid particles coated with EpCAM antibodies. After repeated immunomagnetic separation, enriched cells were labeled with a mixture of anti-cytokeratin (CK) antibodies to identify epithelial cells, CD45 antibodies to identify and exclude white blood cells, and 4′,6-diamidino-2-phenylindole (DAPI) to identify nucleated cells. In a subset of patients, cells were also stained for immunofluorescence with claudin-4 antibody (see below, Claudin-4 Evaluation). Using a CellTrack analyzer II, a semiautomated fluorescence microscope system (Veridex), nucleated (DAPI+) EpCAM+/CK+/CD45– cells were enumerated and reported as PECs per milliliter of pleural fluid. In the subset of patients in whom claudin-4 was tested, cells were stained, enumerated, and reported as PEC-C4 cells per milliliter of pleural fluid if they stained for this antibody.
Determination of Pleural Fluid Etiology
The etiology of a subject’s pleural effusion was determined by chart review in a blinded fashion (i.e., the reviewer did not know the PEC results), using the following prospectively defined criteria. A definite benign PE was defined as a transudative effusion (using Light’s criteria [1]) or had a negative pathologic evaluation of an open pleural biopsy or had negative cytology on two separate occasions and no history of malignancy with the patient being cancer free for 9 months. A probable benign PE had at least one negative cytologic evaluation and the patient had no evidence of malignancy and a strong alternative etiology that was suspected. A definite malignant PE had positive cytology or a positive pleural biopsy. A probable malignant PE was exudative by Light’s criteria (1) and the patient had clinical or radiographic evidence of metastatic disease and had no alternative cause for the effusion.
Claudin-4 Evaluation
During a planned interim analysis of the first 40 samples, we observed that there was a baseline number of PECs in the effusions of subjects with definitely benign etiologies. We thus elected to additionally stain the cells with an Alexa Fluor 488–labeled anti–claudin-4 antibody (catalog no. 329488; Life Technologies, Carlsbad, CA) to possibly increase the specificity of the test. All analyses were performed separately for PECs and PECs positive for claudin-4 (PEC-C4) (see CELLSEARCH Technology above).
Statistical Analysis
The PEC and PEC-C4 counts were analyzed as continuous variables. We compared distributions of cell counts between disease groups by analysis of variance (ANOVA) of the log counts with a Bonferroni correction for multiplicity. We assessed the diagnostic performance of the PEC and PEC-C4 enumeration, using receiver operating characteristic (ROC) curves, computing diagnostic specificity, sensitivity, positive predictive value (PPV), negative predictive value (NPV), and area under the ROC curve (AUC). Optimal cutoff points were selected by minimizing the distance between the ROC curve and upper left corner of the unit graph, providing maximal accuracy. All statistical analyses were conducted in R 2.13.1 (R Development Core Team, Vienna, Austria).
Results
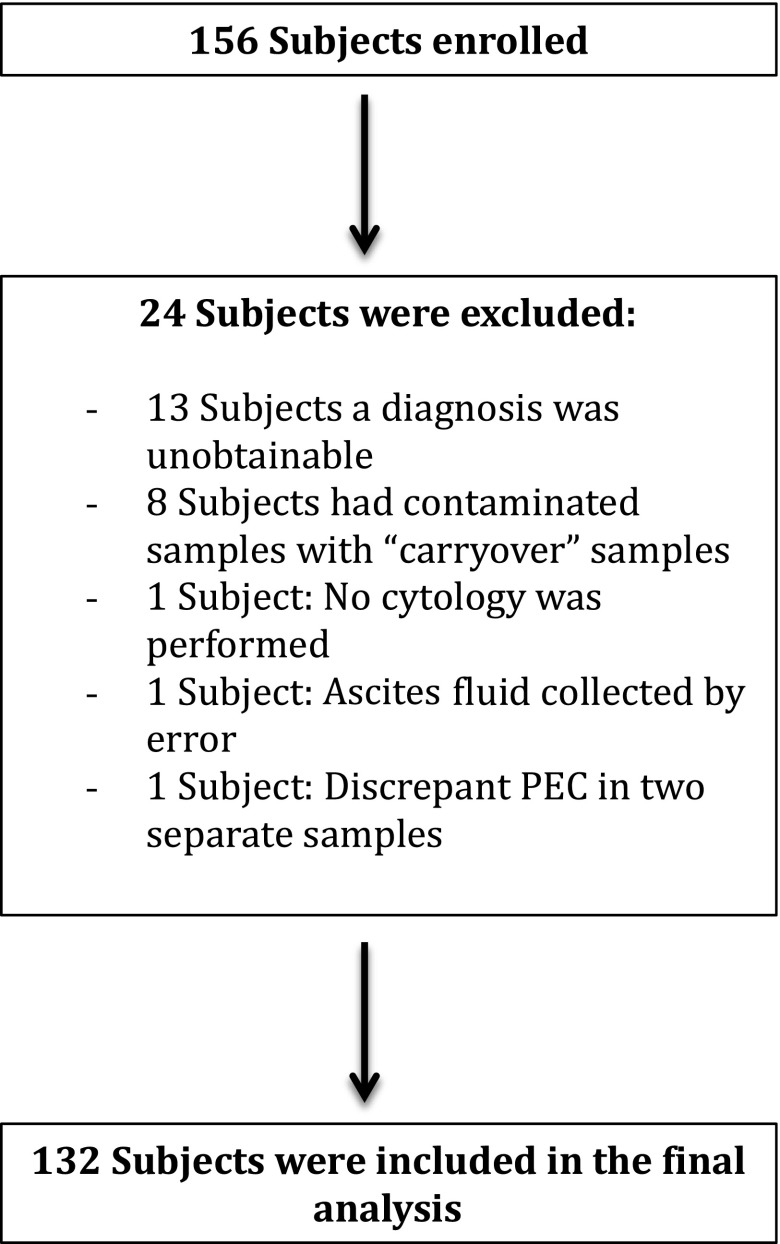
One hundred and fifty-six consecutive subjects were enrolled, and 132 subjects were included in the final analysis (Figure 1). All, except for five samples, were obtained by standard needle-based thoracentesis. The characteristics of the study population are summarized in Table 1. A malignant etiology was established in 81 subjects and most (75.3%) were pathologically confirmed (cytology or thoracoscopy). Cytology was positive in 55 subjects and had an overall sensitivity of 67.9%. In the MPE group, the diagnosis was judged as definitive in 69 patients and probable in 12. There were 51 benign effusions. In the majority of benign diseases, the diagnosis was established clinically (88.2% of cases) (Table 2). In this group, the diagnosis was judged as definitive in 36 and probable in 15. Eleven subjects in the benign effusion group had a transudative effusion.
Figure 1.
Flow diagram for subject enrollment. PEC = pleural epithelial cells.
Table 1.
Characteristics of study population
| Characteristic | n (%) |
|---|---|
| Total number of subjects | 156 |
| Subjects included in analysis | 132 |
| Sex | |
| Male | 73 (55%) |
| Female | 59 (45%) |
| Age (median/range) | 66 (21–90) |
| Etiology | |
| Benign | 51 (38.6%) |
| Malignant | 81 (61.4%) |
| Malignant nonepithelial | 14 (17.3%) |
| Malignant epithelial | 67 (82.7%) |
| Method of diagnosis | |
| Cytology | 55 (41.7%) |
| Clinical basis | 66 (50.0%) |
| Thoracoscopy | 11 (8.3%) |
Table 2.
Diagnosis of pleural effusions
| Malignant (%) | Benign (%) | |
|---|---|---|
| Total | 81 | 51 |
| Certainty of diagnosis | ||
| Definitive (n = 105) | 69 (85%) | 36 (70%) |
| Probable (n = 27) | 12 (15%) | 15 (30%) |
| Method of diagnosis | ||
| Pathologically confirmed | 61 (75.3%) | 6 (11.8%) |
| Cytology | 55 | NA |
| Thoracoscopy | 6 | 6 |
| Clinical basis | 20 (24.7%) | 45 (88.2%) |
Definition of abbreviation: NA = not applicable.
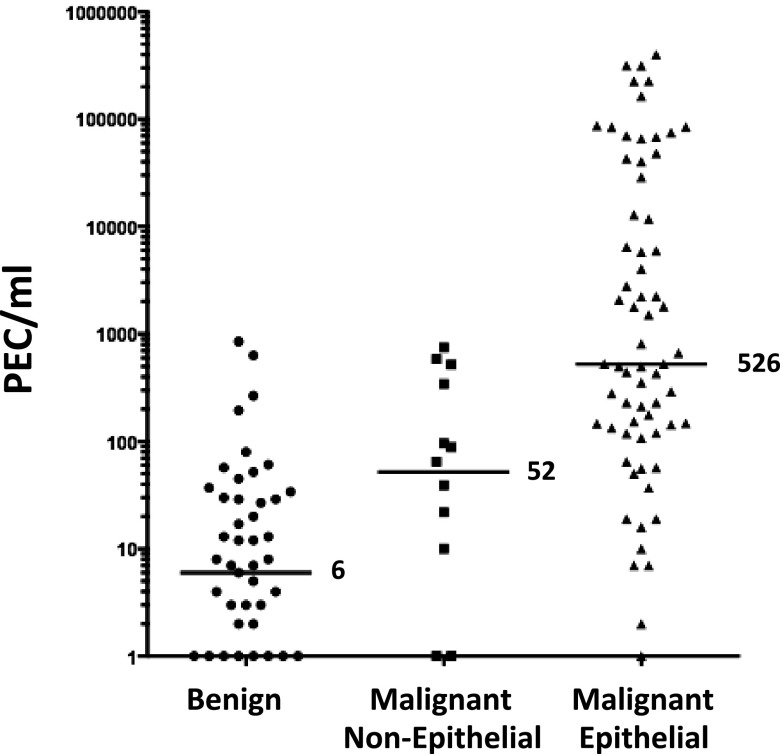
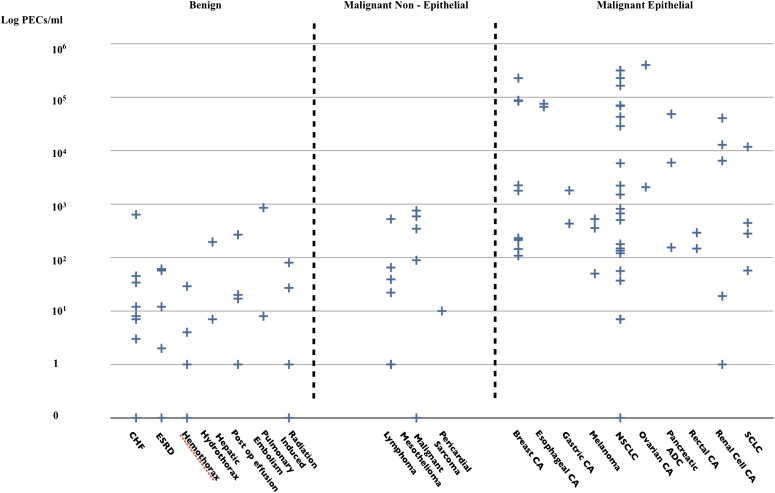
The primary outcome of our study was the number of PECs per milliliter of pleural fluid. As the CELLSEARCH technology is designed to detect only EpCAM-expressing (epithelial) cells, we subclassified malignant diagnoses as epithelial and nonepithelial (Figure 2). The median number of PECs in the benign effusions was 6/ml of pleural fluid (range, 0–852). The median number of PECs in subjects with malignant nonepithelial cancers was 52/ml (range, 0–755). This difference was not statistically significant (P = 0.3). The median number of PECs in the malignant epithelial effusion category was 526/ml (range, 0–400,000), which was statistically significantly higher (P < 0.001 by ANOVA on the log counts) than in the benign and malignant nonepithelial categories. The distribution of PEC levels in specific representative diseases is shown in Figure 3.
Figure 2.
Distribution of pleural epithelial cells (PECs) per milliliter according to diagnosis type.
Figure 3.
Distribution of pleural epithelial cells (PECs) per milliliter according to disease. ADC = adenocarcinoma; CA = cancer; CHF = congestive heart failure; ESRD = end-stage kidney disease; NSCLC = non–small cell lung cancer; SCLC = small cell lung cancer.
To determine whether the certainty of the diagnosis (definitive vs. probable) would alter the results of our analysis, we compared the number of PECs among those with definitive and probable diagnoses according to disease type (benign, malignant nonepithelial, and malignant epithelial) (see Figure E1 in the online supplement). There were relatively few “probable” diagnoses and the median values of PECs were similar between each definite and probable diagnosis. We therefore grouped subjects with definitive and probable diagnoses for subsequent analyses.
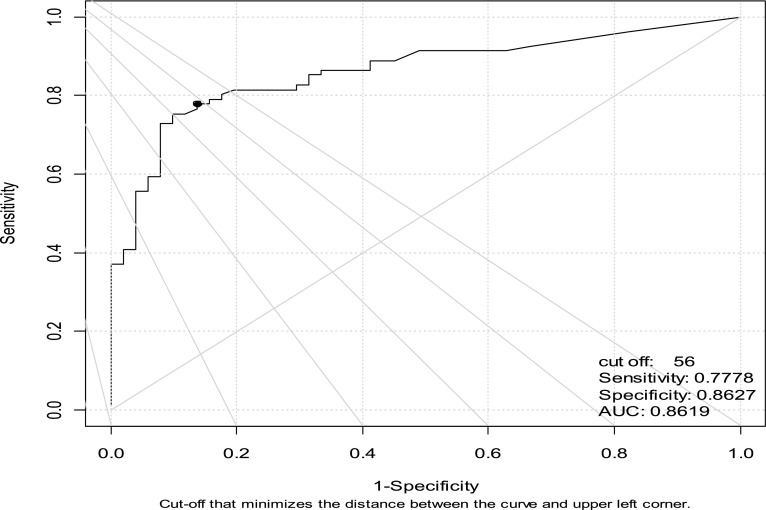
The performance of PEC enumeration for the diagnosis of all types of MPEs was assessed using ROC curves (Figure 4). The area under the ROC curve (95% confidence interval) was 0.86 (0.82–0.92), indicating that this test exhibits excellent discriminatory ability. The optimal cutoff point using the minimal distance between the ROC curve and the upper left corner is a count of 56 PECs/ml, providing a sensitivity of 0.78 and a specificity of 0.86. However, a clinician may weigh the importance of sensitivity and specificity differently, such as choosing a cutoff in the range from 852 to 1,508 that yields specificity of 100% with maximal sensitivity of 37%. Some examples of different cutoff values with their attendant sensitivity, specificity, PPV, and NPV are shown in Table E1.
Figure 4.
Receiver operating characteristic (ROC) curve for pleural epithelial cells (PECs) does discriminate between benign and malignant pleural effusions. AUC = area under the curve.
Given the relatively high number of epithelial cells in benign effusions, we also stained samples from a subgroup of patients for expression of claudin-4. In 81 subjects, we defined PEC–claudin-4 (PEC-C4) cells as DAPI+/EpCAM+/CK+/claudin-4+/CD45– cells in an attempt to improve the discriminatory properties of the test. Compared with the EpCAM+/CK+ cells (PECs), the number of PEC–claudin-4 cells was lower across all groups (Figure E2). Although the maximal number of PEC-C4 cells in benign effusions was reduced to less than 50/ml, the area under the ROC curve was only 0.78 (worse than the AUC of 0.86 we saw when using PECs alone), and the optimal point using the minimal distance between the ROC curve and the upper left corner was a count of 2 PEC-C4/ml. This cutoff provided a sensitivity and specificity of 0.67 and 0.86, respectively (Figure E3). Thus, although the addition of claudin-4 staining lowered our background, it did not improve the discriminatory properties of the test.
Examples of Subjects with High PEC Levels but Negative Cytology Examinations
One potentially important use for this technology would be the identification of patients with possible malignant effusions and for whom cytology had been negative. In our study, we identified seven subjects with PECs/ml greater than 280 (which has a specificity and PPV of >95%) but who had a negative cytology with a presumptive diagnosis of malignancy. The details of these cases are shown in Table 3.
Table 3.
Cases with high pleural epithelial cell levels but negative cytology examinations
| Subject | PECs (cells/ml) | Diagnosis | How Dx Made/Explanation |
|---|---|---|---|
| 021 | 431 | Gastric cancer | Patient had two negative cytology examinations. Eventually diagnosed with widely metastatic gastric cancer with pleural metastases |
| 048 | 12,827 | Renal cell carcinoma | Patient with metastatic RCC and PET-positive lesion involving the pleura |
| 090 | 588 | Malignant mesothelioma | Biopsy-proven malignant mesothelioma by VATS |
| 092 | 668 | Lung adenocarcinoma | Patient with known metastatic NSCLC with recurrent R effusion. Cytology neg × 1. Patient had PET CT on 9/27 showing focal FDG avid uptake in the L pleural margin. Primary oncologist diagnosed patient with osseous and pleural metastases |
| 114 | 2,218 | Lung squamous cell carcinoma | Patient with known metastatic NSCLC and evidence of pleural nodularity on CT indicating pleural metastasis |
| 121 | 5,804 | Lung cancer; not otherwise specified | Cytology at PENN showed rare atypical cells in a background of blood and scant cellularity, precluding a definitive diagnosis. However, an outside hospital cytology was positive for malignancy |
| 123 | 343 | Malignant mesothelioma | Diagnosis made by VATS |
Definition of abbreviations: CT = computed tomography; Dx = diagnosis; FDG = fluorodeoxyglucose; L = left; NSCLC = non–small cell lung cancer; PENN = University of Pennsylvania; PET = positron emission tomography; R = right; RCC = renal cell carcinoma; VATS = video-assisted thoracoscopic surgery.
Discussion
Cytologic examination remains the best test to diagnose malignant pleural effusions because of its wide availability and high specificity, and the relatively easy accessibility of pleural fluid for analysis. Unfortunately, the sensitivity of cytology ranges widely, averaging about 60% (4–7). In addition to the issues discussed previously, a major factor contributing to this limited sensitivity is recognition of the range of nuclear atypia that may be displayed by reactive mesothelial cells. It is well recognized by cytopathologists that mesothelial cells can display a number of atypical nuclear changes that may mimic those seen in malignancy. As a result, the diagnostic threshold for the diagnosis of malignancy is elevated when reactive mesothelial cells are noted in the background, resulting in a significantly lowered sensitivity for these specimens.
Accordingly, additional tools to make this diagnosis are needed and a number of approaches have been used. Although IHC can increase sensitivity, no universal marker of malignancy exists, necessitating a broad panel of markers; this can be time-consuming and expensive. Newer experimental approaches have also been explored with varying levels of success, such as fluorescence in situ hybridization (FISH) to detect common chromosomal aberrations (19) and PCR-based tests to detect pleural fluid cell–free DNA integrity (20). Neither test is standardized nor quantitative, they are relatively time-consuming, and they would be expensive if applied to all effusions.
We hypothesized that the commercially accessible CELLSEARCH system could be used to identify “pleural epithelial cells” (PECs) as a marker for the diagnosis of MPE. Despite intrinsic methodologic characteristics that limit the diagnoses of nonepithelial malignancies (see below), using ROC analysis, the CELLSEARCH system had an AUC of 0.86 for all effusions, an extremely good performance.
As is true of virtually all diagnostic tests, there is some overlap between diagnostic groups, potentially limiting this as a “yes–no” type of test. However, because the test is quantitative, the clinician could use the pleural CELLSEARCH data in a variety of ways, depending on the situation. There would be some cutoff value of high PEC numbers that would make the diagnosis of MPE virtually certain and possibly obviate the need for further tests (100% specificity for malignancy). In our study, this value was 852 cells/ml, but because of the discreteness of the data, any cutoff between 853 and 1,508 gave an identical estimated sensitivity and specificity for our data set. A final cutoff number would eventually be established in independent replication and would likely be slightly different because of interstudy variability. Lower numbers of PECs could be used to determine pretest probabilities and assist in decisions about the value of additional staining approaches or further invasive diagnostic procedures. On the other hand, if lymphoma or melanoma was clinically suspected a priori, pleural CELLSEARCH would not be a useful test.
We can also imagine a number of potential clinical niches for pleural CELLSEARCH for pleural effusion diagnosis. The most important use would be in conjunction with cytology where in a number of cases a definitive conclusion can be difficult, leading to a diagnosis of “atypical” or “highly atypical” cells. The finding of a high number of PECs by pleural CELLSEARCH could provide strong reassurance in making a malignant diagnosis when less than definite criteria are present or could provide a signal to move forward with immunohistochemical stains.
As examples, we identified seven cancer cases (approximately 9% of our malignant diagnoses) with high numbers of PECs (>280/ml) but a negative or nondefinitive cancer diagnosis by cytology. In two cases, a diagnosis of mesothelioma was made, a condition for which cytology has traditionally been difficult (21). There was one case of renal cell carcinoma, a disease in which the nuclear atypia is often subtle, making cytological diagnosis challenging. Gastric carcinoma (seen in one case) often presents with small cells that can resemble reactive mesothelial cells. In one lung cancer case, only rare atypical cells among a large inflammatory background were seen on cytology. The reason for the false negative diagnoses in the other cases was not obvious. Interestingly, a number of the cases described in Table 3 were re-reviewed with the aid of IHC stains and revealed highly suspicious or positive findings.
Given the capability of pleural CELLSEARCH to display malignant cells in a format that allows easy immunostaining or FISH analysis, a second use of our approach could be efficient tumor cell analysis. For example, lung cancer specimens could be rapidly evaluated for epidermal growth factor receptor mutations or EML4–ALK (echinoderm microtubule-associated protein like-4–anaplastic lymphoma kinase) translocations. The CELLSEARCH system has been used for immunohistochemistry and FISH to analyze circulating tumor cells.
There are a number of limitations to our study. First, we studied only 132 patients, all at one institution. Although this serves as a proof-of-principle trial, validation in a larger, multicenter study is required. Second, we did not obtain a definitive pathologic diagnosis in all cases, a nearly impossible achievement, especially for many benign effusions from which biopsies are never taken. It is thus possible that a small number of effusions may have been misclassified. To address this issue, we established clear prospective criteria based on the literature and clinical consensus and used these definitions to define “definite” and “probable” categories in a fashion blinded to the PEC results. Using these criteria, we had enough information to classify 132 of 156 patients. To test for bias introduced by using definite versus probable diagnoses, we compared the PECs in each group and found no significant differences (Figure E1), and therefore felt justified in combining the data.
Because the capture antibody used recognizes the epithelium-specific molecule EpCAM, this technology has an inherent limitation in sensitivity, in that it is limited to the detection of epithelial malignancies. By definition, EpCAM will be negative on all lymphomas and leukemias and this limitation is seen in our data: we saw low levels of EpCAM-positive cells (<100 PECs/ml) in four of five of our lymphoma cases. Fortunately, EpCAM positivity rates are greater than 85–90% in cancers deriving from lung, breast, colon, esophagus, stomach, pancreas, endometrium, ovary, and prostate (22).
The primary limitation to specificity was an unexpectedly high rate of background positivity. When compared with blood, where background levels are extremely low, we were somewhat surprised to see some benign effusions having hundreds of PECs per milliliter (Figure 3). The exact nature of these cells is uncertain. One possibility is that they are respiratory epithelial cells shed into the pleural space. However, because it has been reported that EpCAM (Ber-EP4) staining is positive in 5–10% of reactive mesothelial cells (23), we believe a more likely explanation for our high background is that we are detecting reactive mesothelial cells that have undergone a partial mesenchymal-to-epithelial transition and have started to express low levels of EpCAM. Reactive mesothelial cells are commonly seen in benign effusions, even those associated with congestive heart failure. CELLSEARCH was designed to capture cells with even low amounts of cell surface EpCAM and after capture does not differentiate between cells with high and low EpCAM expression levels. Although this poses no issues in blood (where normal blood cells do not express any EpCAM), it likely accounts for the relatively high background level of “EpCAM-positive” cells (100–1,000/ml) in some benign effusions where mesothelial cells with low levels of EPCAM expression were captured. In future studies, it may be possible to reduce this background level by lowering the efficiency of the capturing EpCAM antibody system so that cells with only higher levels of EpCAM are captured and enumerated.
As another approach to increase the specificity, we added an additional antibody, anti–claudin-4, to the panel. The claudins form key functional constituents of epithelial tight junctions. Claudin-4 is generally overexpressed in malignant cells while being negative in normal mesothelium (9, 24, 25). Although adding claudin-4 positivity to our definition of a PEC did lower the overall number of PECs detected in our benign effusions, this shift did not lead to increased performance characteristics (Figure E3).
We are planning to study other ways to lower the background staining by using antibodies that can more specifically identify reactive mesothelial cells. For example, intracellular desmin staining has been seen in more than 85% of reactive mesothelial cells with low expression in mesothelioma or carcinoma (23, 26–28).
In summary, although not ready for clinical implementation at this time, we believe our data support additional studies to validate the use of the pleural CELLSEARCH technology to assist in the diagnosis of malignant pleural effusions. We believe the test could be improved by lower background staining, which should improve the sensitivity and specificity.
Footnotes
Author Contributions: D.E.S.L., J.T., G.Y., B.V., C.R., M.C., and S.A.: study conception and design, hypothesis delineation, data analysis and interpretation, and writing of the manuscript; A.V., K.S.T., and D.F.H.: hypothesis delineation, data analysis and interpretation, and writing of the manuscript; M.U.: data collection and interpretation, database creation, collection and processing of samples, and creation of figures (note that these were later updated with newer data).
This article has an online supplement, which is accessible from this issue’s table of contents online at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Light RW. Clinical practice: pleural effusion. N Engl J Med. 2002;346:1971–1977. doi: 10.1056/NEJMcp010731. [DOI] [PubMed] [Google Scholar]
- 2.Hooper C, Lee YC, Maskell N BTS Pleural Guideline Group. Investigation of a unilateral pleural effusion in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65:ii4–ii17. doi: 10.1136/thx.2010.136978. [DOI] [PubMed] [Google Scholar]
- 3.Lombardi G, Zustovich F, Nicoletto MO, Donach M, Artioli G, Pastorelli D. Diagnosis and treatment of malignant pleural effusion: a systematic literature review and new approaches. Am J Clin Oncol. 2010;33:420–423. doi: 10.1097/COC.0b013e3181aacbbf. [DOI] [PubMed] [Google Scholar]
- 4.Salyer WR, Eggleston JC, Erozan YS. Efficacy of pleural needle biopsy and pleural fluid cytopathology in the diagnosis of malignant neoplasm involving the pleura. Chest. 1975;67:536–539. doi: 10.1378/chest.67.5.536. [DOI] [PubMed] [Google Scholar]
- 5.Nance KV, Shermer RW, Askin FB. Diagnostic efficacy of pleural biopsy as compared with that of pleural fluid examination. Mod Pathol. 1991;4:320–324. [PubMed] [Google Scholar]
- 6.Prakash UB, Reiman HM. Comparison of needle biopsy with cytologic analysis for the evaluation of pleural effusion: analysis of 414 cases. Mayo Clin Proc. 1985;60:158–164. doi: 10.1016/s0025-6196(12)60212-2. [DOI] [PubMed] [Google Scholar]
- 7.Garcia LW, Ducatman BS, Wang HH. The value of multiple fluid specimens in the cytological diagnosis of malignancy. Mod Pathol. 1994;7:665–668. [PubMed] [Google Scholar]
- 8.Bedrossian CW. Diagnostic problems in serous effusions. Diagn Cytopathol. 1998;19:131–137. doi: 10.1002/(sici)1097-0339(199808)19:2<131::aid-dc14>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 9.Lonardi S, Manera C, Marucci R, Santoro A, Lorenzi L, Facchetti F. Usefulness of Claudin 4 in the cytological diagnosis of serosal effusions. Diagn Cytopathol. 2011;39:313–317. doi: 10.1002/dc.21380. [DOI] [PubMed] [Google Scholar]
- 10.Davies HE, Nicholson JE, Rahman NM, Wilkinson EM, Davies RJ, Lee YC. Outcome of patients with nonspecific pleuritis/fibrosis on thoracoscopic pleural biopsies. Eur J Cardiothorac Surg. 2010;38:472–477. doi: 10.1016/j.ejcts.2010.01.057. [DOI] [PubMed] [Google Scholar]
- 11.Menzies R, Charbonneau M. Thoracoscopy for the diagnosis of pleural disease. Ann Intern Med. 1991;114:271–276. doi: 10.7326/0003-4819-114-4-271. [DOI] [PubMed] [Google Scholar]
- 12.Venekamp LN, Velkeniers B, Noppen M. Does “idiopathic pleuritis” exist? Natural history of non-specific pleuritis diagnosed after thoracoscopy. Respiration. 2005;72:74–78. doi: 10.1159/000083404. [DOI] [PubMed] [Google Scholar]
- 13.Pantel K, Brakenhoff RH, Brandt B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat Rev Cancer. 2008;8:329–340. doi: 10.1038/nrc2375. [DOI] [PubMed] [Google Scholar]
- 14.Miller MC, Doyle GV, Terstappen LW. Significance of circulating tumor cells detected by the CellSearch system in patients with metastatic breast colorectal and prostate cancer. J Oncol. 2010;2010:617421. doi: 10.1155/2010/617421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 16.Shaffer DR, Leversha MA, Danila DC, Lin O, Gonzalez-Espinoza R, Gu B, Anand A, Smith K, Maslak P, Doyle GV, et al. Circulating tumor cell analysis in patients with progressive castration-resistant prostate cancer. Clin Cancer Res. 2007;13:2023–2029. doi: 10.1158/1078-0432.CCR-06-2701. [DOI] [PubMed] [Google Scholar]
- 17.Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:3213–3221. doi: 10.1200/JCO.2007.15.8923. [DOI] [PubMed] [Google Scholar]
- 18.Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AG, Uhr JW, Terstappen LW. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897–6904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- 19.Han J, Cao S, Zhang K, Zhao G, Xin Y, Dong Q, Yan Y, Cui J. Fluorescence in situ hybridization as adjunct to cytology improves the diagnosis and directs estimation of prognosis of malignant pleural effusions. J Cardiothorac Surg. 2012;7:121. doi: 10.1186/1749-8090-7-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sriram KB, Relan V, Clarke BE, Duhig EE, Windsor MN, Matar KS, Naidoo R, Passmore L, McCaul E, Courtney D, et al. Pleural fluid cell–free DNA integrity index to identify cytologically negative malignant pleural effusions including mesotheliomas. BMC Cancer. 2012;12:428. doi: 10.1186/1471-2407-12-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murugan P, Siddaraju N, Habeebullah S, Basu D. Immunohistochemical distinction between mesothelial and adenocarcinoma cells in serous effusions: a combination panel-based approach with a brief review of the literature. Indian J Pathol Microbiol. 2009;52:175–181. doi: 10.4103/0377-4929.48910. [DOI] [PubMed] [Google Scholar]
- 22.Patriarca C, Macchi RM, Marschner AK, Mellstedt H. Epithelial cell adhesion molecule expression (CD326) in cancer: a short review. Cancer Treat Rev. 2012;38:68–75. doi: 10.1016/j.ctrv.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Davidson B, Nielsen S, Christensen J, Asschenfeldt P, Berner A, Risberg B, Johansen P. The role of desmin and N-cadherin in effusion cytology: a comparative study using established markers of mesothelial and epithelial cells. Am J Surg Pathol. 2001;25:1405–1412. doi: 10.1097/00000478-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Soini Y. Expression of claudins 1, 2, 3, 4, 5 and 7 in various types of tumours. Histopathology. 2005;46:551–560. doi: 10.1111/j.1365-2559.2005.02127.x. [DOI] [PubMed] [Google Scholar]
- 25.Facchetti F, Lonardi S, Gentili F, Bercich L, Falchetti M, Tardanico R, Baronchelli C, Lucini L, Santin A, Murer B. Claudin 4 identifies a wide spectrum of epithelial neoplasms and represents a very useful marker for carcinoma versus mesothelioma diagnosis in pleural and peritoneal biopsies and effusions. Virchows Arch. 2007;451:669–680. doi: 10.1007/s00428-007-0448-x. [DOI] [PubMed] [Google Scholar]
- 26.King J, Thatcher N, Pickering C, Hasleton P. Sensitivity and specificity of immunohistochemical antibodies used to distinguish between benign and malignant pleural disease: a systematic review of published reports. Histopathology. 2006;49:561–568. doi: 10.1111/j.1365-2559.2006.02442.x. [DOI] [PubMed] [Google Scholar]
- 27.Hasteh F, Lin GY, Weidner N, Michael CW. The use of immunohistochemistry to distinguish reactive mesothelial cells from malignant mesothelioma in cytologic effusions. Cancer Cytopathol. 2010;118:90–96. doi: 10.1002/cncy.20071. [DOI] [PubMed] [Google Scholar]
- 28.Su XY, Li GD, Liu WP, Xie B, Jiang YH. Cytological differential diagnosis among adenocarcinoma, epithelial mesothelioma, and reactive mesothelial cells in serous effusions by immunocytochemistry. Diagn Cytopathol. 2011;39:900–908. doi: 10.1002/dc.21489. [DOI] [PubMed] [Google Scholar]