Abstract
Understanding the interactions between drought and acute ozone (O3) stress in terms of signaling molecules and cell death would improve the predictions of plant responses to climate change. The aim was to investigate whether drought stress influences the responses of plants to acute episodes of O3 exposure. In this study, the behavior of 84 Mediterranean evergreen Quercus ilex plants was evaluated in terms of cross-talk responses among signaling molecules. Half of the sample was subjected to drought (20% of the effective daily evapotranspiration, for 15 days) and was later exposed to an acute O3 exposure (200 nL L-1 for 5 h). First, our results indicate that in well-water conditions, O3 induced a signaling pathway specific to O3-sensitive behavior. Second, different trends and consequently different roles of phytohormones and signaling molecules (ethylene, ET; abscisic acid, ABA; salycilic acid, SA and jasmonic acid, JA) were observed in relation to water stress and O3. A spatial and functional correlation between these signaling molecules was observed in modulating O3-induced responses in well-watered plants. In contrast, in drought-stressed plants, these compounds were not involved either in O3-induced signaling mechanisms or in leaf senescence (a response observed in water-stressed plants before the O3-exposure). Third, these differences were ascribable to the fact that in drought conditions, most defense processes induced by O3 were compromised and/or altered. Our results highlight how Q. ilex plants suffering from water deprivation respond differently to an acute O3 episode compared to well-watered plants, and suggest new effect to be considered in plant responses to environmental changes. This poses the serious question as to whether or not multiple high-magnitude O3 events (as predicted) can change these cross-talk responses, thus opening it up possible further investigations.
Keywords: climate change, holm oak, mediterranean plant species, phytohormones, hypersensitive response, stress combination
Introduction
Mediterranean plants are threatened by several abiotic stress factors [e.g., warming, drought, tropospheric ozone (O3), UV radiation, salinity] due to environmental changes characterized by new types of stress conditions and stress combinations, which are expected to be more extreme in the Mediterranean than in other areas worldwide (Matesanz and Valladares, 2014; Gray and Brady, 2016; Guidi et al., 2017). Today, drought is the major factor limiting plant performance, and revealing the mechanisms that enable plants to survive or acclimatize to such conditions is crucial (Claeys and Inzé, 2013). On the other hand, O3 is by far the most phytotoxic air pollutant with deleterious effects on growth and productivity (Vainonen and Kangasjärvi, 2015; Cotrozzi et al., 2016a; Yang et al., 2016). Both drought and O3 are co-occurring, increasing stressors in future climate change scenarios (Bates et al., 2008). Given that high-level O3 episodes and drought often occur together in Mediterranean areas, especially during the summer, their interaction needs to be understood (Iyer et al., 2013; Cotrozzi et al., 2016b). O3 enters the leaf through open stomata, then drought-triggered stomatal closure limits O3 uptake, thereby limiting foliar damage (Panek et al., 2002). However, other studies have highlighted that these factors have a synergistic effect, with increased O3 sensitivity observed in droughted plants (Alonso et al., 2014; Pollastrini et al., 2014). The response of plants to a combination of stresses is species-specific and depends on the intensity and duration of each stress factor (Ramegowda and Senthil-Kumar, 2015).
Plant exposure to acute O3 (high O3 concentration within a short period) commonly occurs during hot, dry Mediterranean summers (Matesanz and Valladares, 2014), which often results in a programmed cell death (PCD) response. This is a physiological process that selectively targets and eliminates unwanted cells in response to a variety of biotic and abiotic stimuli (Apel and Hirt, 2004). PCD resembles the hypersensitive response (HR) observed in several plant-pathogen interactions, which often precedes the acquisition of a systemic resistance by plants (Kangasjärvi et al., 1994; Rao et al., 2000; Pellegrini et al., 2013; Vainonen and Kangasjärvi, 2015; Pellegrini et al., 2016). O3 entering the leaves first induces a biphasic oxidative burst with a massive, rapid and transient increase in apoplastic reactive oxygen species (ROS), which is the main event leading to PCD activation (Langebartels et al., 2002) Similarly, an oxidative burst was usually observed in plants under drought (Smirnoff, 1993; Miller et al., 2010; Noctor et al., 2014) and the drought-triggered ROS production can elicit acclimatory events (Smirnoff, 1993). However, HR-like response has never been observed following drought stress. Therefore, the role of ROS in cell signaling and in regulating gene expression is a key aspect (Baxter et al., 2014), in particular in plant, subjected to abiotic stresses, including O3 and drought (Wilkinson and Davies, 2010).
The signaling pathways activated by O3 are integrated into a complex regulatory system involving ROS, plant hormones [e.g., ethylene (ET) and abscisic acid (ABA)], signaling molecules [e.g., salicylic acid (SA) and jasmonic acid (JA)], and secondary messengers (e.g., Ca2+). Signaling and cell death in O3-exposed plants have been reviewed by several authors (e.g., Rao et al., 2000; Rao and Davis, 2001; Kangasjärvi et al., 2005; Tamaoki, 2008; Vainonen and Kangasjärvi, 2015; Carmody et al., 2016; Pellegrini et al., 2016). Different plants use many hydraulic and chemical signals to tune their sensing of water deficit (Wilkinson and Davies, 2010). Thus, the interactions between drought and acute O3 stress in terms of signaling molecules and cell death need to be studied in depth in order to improve predictions of plant acclimation/adaptation strategies to climate change (Carmody et al., 2016). Signaling in acute O3 exposure has mainly been studied in the test plant Arabidopsis thaliana, however, few works have evaluated these mechanisms in tree species [e.g., on hybrid poplar, by Kock et al. (2000)].
To the best of our knowledge, no studies have assessed signaling molecules and cell death in Mediterranean tree species exposed to O3. O3 can also be used as a non-invasive tool to mimic signaling pathways triggered by active apoplastic ROS formation induced by pathogens (Vainonen and Kangasjärvi, 2015), also enabling conclusions to be drawn on drought-biotic stress interactions. The responses of Mediterranean species to the interaction of drought and O3 have yet to be extensively investigated as shown by the scarce information available in the literature (Kurz et al., 1998; Vitale et al., 2008; Calderòn Guerrero et al., 2013; Alonso et al., 2014; Cotrozzi et al., 2016b), especially in relation to acute exposure to the pollutant.
Holm oak (Quercus ilex L.) is probably the most widely studied Mediterranean evergreen tree species which has been defined as ‘drought-avoidant’ and ‘water saver’ with regard to its ecophysiological behavior (Bussotti et al., 2002), although adverse impacts of drought have also been reported in this species (e.g., Gimeno et al., 2008; Cotrozzi et al., 2016b). This species has also been referred to as the most tolerant to O3 stress among several other Quercus species (Calatayud et al., 2011). In a previous study carried out by this research group (Cotrozzi et al., 2016b), Q. ilex subjected to drought (30% of the effective daily evapotranspiration) and/or chronic O3 (80 nL L-1, 5 h d-1, for 77 consecutive days) showed that the major determinant was the water deficit; however, oxidative stress (revealed by a significant build-up of MDA by-products) occurred only when drought was applied with O3 (Cotrozzi et al., 2016b).
In the present study, we evaluated the behavior of Q. ilex saplings, subjected or not to drought, and later exposed to acute O3 exposure by characterizing different components of O3 stress signaling. Our aim was to answer the following questions: (i) can acute O3 exposure initiate an HR? (ii) What role do phytohormones and signaling molecules play in the perception and transduction of drought and/or O3 stress? (iii) Do drought conditions compromise/alter the signaling responses to acute O3 exposure?
Materials and Methods
Plant Material and Experimental Design
Three-year old Q. ilex saplings grown under field conditions were potted in 6.5-L pots with growing medium containing a mixture of standard soil Einhetserde Topfsubstrat ED 63 (Sinntal-Altengronau, Germany) and sand (3.5:1, in volume), according to Cotrozzi et al. (2016b). Two weeks before the beginning of the O3 treatment, 42 plants (WS) received 20% of the effective daily evapotranspiration (calculated by the average 24-h weight loss of five well-watered plants), whereas another 42 plants (WW) were kept at field water capacity. The two groups of plants were then subdivided into four sets (WW-O3, WS-O3, WW+O3, WS+O3; 21 plants per set) and transferred into four controlled fumigation facilities (temperature 23 ± 1°C, relative humidity 85 ± 5% and photon flux density of 530 μmol photons m-2 s-1 at plant height provided by incandescent lamps with L/D 14:10 photoperiod; lights were switched on from 7:00 to 21:00 to simulate environmental light conditions).
WW-O3 and WS-O3 plants were randomly distributed into two chambers, whereas WW+O3 and WS+O3 plants were randomly distributed in the other two chambers. After one week of acclimation, WW+O3 and WS+O3 plants were exposed to an acute O3 stress (200 nL L-1, 5 h day-1, in the form of a square wave between the 2nd and the 7th h of the light period). On the other hand, WW-O3, WS-O3 plants were maintained under charcoal-filtered air, in which the O3 concentration was less than 5 nL L-1. During the O3-exposure, environmental factors were maintained as reported above.
The O3 exposure was performed according to Lorenzini et al. (1994) with minor modifications to avoid pseudo-replications. At the end of the drought exposure, plant water status was evaluated. Photosynthetic parameters were measured at 0, 5, 24 and 48 h from the beginning of the O3 exposure (FBE, From the Beginning of Exposure). Five fully expanded mature leaves per plant per treatment were taken at 0, 1, 2, 5, 8, and 24 h FBE, stored at –20°C and subsequently used for chemical analyses, with the exception of ET determination, which was performed immediately. At the same measuring times, staining, and microscopic assays were also performed on fresh material.
Water Status of Plants
Pre-dawn leaf water potential (PDΨW) was measured on three plants per treatment (one fully expanded mature leaf per plant) with a pressure chamber (PMS model 600, PMS Instrument Company, Albany, OR, USA). On the very same plants, relative water content (RWC) was calculated (one fully expanded mature leaf per plant) as: RWC (%) = (FW-DW)/(TW-DW) × 100, where FW is the fresh weight, TW is the turgid weight after rehydrating samples for 24 h, and DW is the dry weight after oven-drying samples at 85°C for 24 h.
Gas Exchange and Chlorophyll a Fluorescence Measurements
Gas exchange and chlorophyll a fluorescence measurements were determined between 10:00 and 13:00 (solar time) on one fully expanded mature leaf per plant, on three plants per treatment. CO2 assimilation rate (A), stomatal conductance to water vapor (gs) and intercellular CO2 concentration (Ci) in light-saturated conditions and ambient CO2 concentration were measured using an Infrared Gas Analyzer (LI-COR Inc., Lincoln, NE, United States) as reported by Cotrozzi et al. (2016b). Modulated chlorophyll a fluorescence of photosystem II (PSII) was measured with a PAM-2000 fluorometer (Walz, Effeltrich, Germany) on the same leaves used for the gas exchange after 40 min of dark adaptation using a dark leaf clip provided by the producer. The maximal PSII photochemical efficiency [Fv/Fm = (Fm–F0)/Fm] and the photochemical efficiency in light conditions [ΦPSII = (Fm’–Fs)/Fm’)] were calculated (Genty et al., 1989). Maximal fluorescence, Fm, when all PSII reaction centers were closed, was determined by applying a saturating light pulse (0.8 s) at 8,000 μmol m-2 s-1 in dark-adapted leaves. Fluorescence was induced with actinic light (about 480 μmol m-2 s-1), superimposed with 800 ms saturating pulses (10,000 μmol m-2 s-1) at 20 s intervals to determine maximal fluorescence in the light-adapted state (F’m). Minimal fluorescence in the light-adapted state (F’0) was determined immediately after turning off the actinic source in the presence of a far-red (>710 nm) background for 10 s to ensure maximal oxidation of PSII electron acceptors. The saturation pulse method was used to analyze the quenching components, as described by Schreiber et al. (1986).
Staining and Microscopic Assays
For the detection of dead cells, Evan’s blue staining was used according to Tonelli et al. (2015). Leaf material was boiled for 1 min in a mixture of phenol, lactic acid, glycerol and distilled water (1:1:1:1, in vol.) containing 20 mg mL-1 Evan’s blue and, after clarification with an aqueous chloral hydrate solution, examined under a light microscope (DM 4000 B, Leica, Wetzlar, Germany). To detect H2O2 accumulation, fresh leaf samples were stained with 3,30-diaminobenzidine (DAB) following Tonelli et al. (2015). Leaf parts were incubated for at least 8 h in a DAB solution (1 mg mL-1) in HCl adjusted to pH 5.6. The samples were then soaked in 70% (v/v) boiling ethanol and clarified overnight in a solution of 2.5 g L-1 aqueous chloral hydrate solution. The cellular H2O2 accumulation was visualized under a light microscope as a reddish-brown precipitation.
ROS determination
H2O2 production was measured using the Amplex Red Hydrogen Peroxide/Peroxidase Assay Kit (Molecular Probes, Invitrogen, Carlsbad, CA, United States), according to Pellegrini et al. (2013). Spectrofluorimetric determinations were performed with a fluorescence/absorbance microplate reader (Victor3 1420 Multilabel Counter Perkin Elmer, Waltham, MA, United States) at 530 and 590 nm (excitation and emission resorufin fluorescence, respectively). concentration was measured according to Tonelli et al. (2015), after extraction with a Tris-HCl buffer (50 mM, pH 7.5), with a spectrophotometer (6505 UV-Vis, Jenway, United Kingdom) at 470 nm, and using a buffer solution as a blank.
Phytohormone and Signaling Molecule Bioassays
Two minutes after excision, ET production was measured by enclosing six intact leaves (cut a few millimeters below the petiole by a scalpel) in air-tight glass containers (80 mL). Gas samples (2 mL) were taken from the headspace of containers after 1 h incubation at room temperature. Separations were performed with a gas chromatograph (HP5890, Hewlett-Packard, Ramsey, MN, United States) equipped with a stainless steel column (150 × 0.4 cm i.d. packed with Hysep T) and a flame ionization detector. Analytical conditions were as follows: injector and transfer line temperature at 70 and 350°C, respectively, and carrier gas nitrogen at 30 mL min-1 (Pellegrini et al., 2013). SA was determined according to Vitti et al. (2016) with some minor modifications. High performance liquid chromatography (HPLC) separations were performed with a liquid chromatograph (Dionex, Sunnyvale, CA, United States) equipped with a reverse-phase Dionex column (Acclaim 120, C18 5 μm particle size, 4.6 mm i.d. × 150 mm length) and RF 2000 Fluorescence Detector. Analytical conditions were as follows: excitation and emission at 305 and 407 nm, respectively, mobile phase containing 0.2 M sodium acetate buffer, pH 5.5 (90%) and methanol (10%), and the flow-rate at 0.8 mL min-1. JA was determined according to Pellegrini et al. (2013). HPLC separations were performed with the Dionex column described above and a UVD 170U UV/VIS detector. Analytical conditions were as follows: absorbance at 210 nm, mobile phase containing 0.2% (v/v) acidified water, and the flow-rate at 1 mL min-1. ABA was measured after extraction in distilled water (water:tissue ratio, 10:1) overnight at 4°C. The indirect ELISA determinations, based on the use of DBPA1 monoclonal antibody, raised against S(+)-ABA, as described by Trivellini et al. (2011), were performed at 415 nm with a microplate reader (MDL 680, Perkin-Elmer, Waltham, MA, United States).
Proline Content
Proline content was measured as reported in Cotrozzi et al. (2016b), after extraction with sulfosalicylic acid (3%, v/v). Spectrophotometric determinations were performed at 520 nm, using toluene as a blank.
Statistical Analysis
Three repeated experiments were set up following a randomized design and the experimental plot consisted of one plant per container. Ecophysiological and biochemical measurements were carried out on three replicates for each treatment. The normality of data was preliminary tested by the Shapiro–Wilk W test. The effects of drought exposure vs. well-watering were analyzed by the Student’s t-test. The effects of O3 on ecophysiological parameters were tested using one-way repeated measures ANOVA with treatment (WW+O3, WS+O3) as the variability factor. The effects of O3 on biochemical parameters were evaluated by two-way ANOVA with treatment and time as variability factors. For both ecophysiological and biochemical analyses, Fisher’s LSD was used as the post-test, with a significance level of P ≤ 0.05. Since data obtained by control plants maintained in filtered air (WW-O3 and WS-O3) did not show significant differences during the time course (data not shown), a comparison among means was carried out using as WW+O3 and WS+O3 plants controls before beginning the fumigation. Analyses were performed by NCSS 2000 Statistical Analysis System Software (Kaysville, UT, United States).
Results
Effects of Drought Stress
After 15 days of drought, plants did not show visible foliar injury. Physiological responses are reported in Table 1. In WS plants, PDΨW decreased significantly to -4.0 MPa at the end of water deprivation compared to WW controls (–0.5 MPa). However, no changes in RWC were recorded in WS leaves. The net carbon gain was reduced by about 73% in response to drought, which was a larger effect compared with the reduction of gs (–50%). Values of Ci increased in WS leaves (+7%). Chlorophyll fluorescence measurements revealed a reduction in ΦPSII (–39%) and qP (–18%) in WS compared to WW leaves, but no changes in the Fv/Fm ratio. An increase of qNP (+30%) was found after drought stress.
Table 1.
Water status and ecophysiological parameters in Quercus ilex plants well-watered (WW) or water stressed (20% of the effective evapotranspiration daily for 15 days, WS).
| WW | WS | P | ||
|---|---|---|---|---|
| PDΨW | (–MPa) | 0.5 ± 0.06 | 4.0 ± 0.70 | ∗∗ |
| RWC | (%) | 86 ± 7.4 | 82 ± 1.7 | ns |
| A | (μmol CO2 m-2 s-1) | 7.4 ± 0.23 | 2.0 ± 0.13 | ∗∗∗ |
| gs | (mol H2O m-2 s-1) | 0.16 ± 0.001 | 0.08 ± 0.008 | ∗∗∗ |
| Ci | (μmol CO2 mol-1) | 284 ± 2.1 | 304 ± 8.1 | ∗ |
| Fv/Fm | 0.83 ± 0.003 | 0.84 ± 0.004 | ns | |
| ΦPSII | 0.36 ± 0.008 | 0.22 ± 0.045 | ∗∗ | |
| qP | 0.60 ± 0.008 | 0.49 ± 0.034 | ∗∗ | |
| qNP | 0.64 ± 0.027 | 0.83 ± 0.043 | ∗∗ |
Data are shown as mean ± standard deviation. Asterisks show the significance of Student’s t-test: ∗∗∗P ≤ 0.001, ∗∗P ≤ 0.01, ∗P ≤ 0.05, ns P > 0.05. ΦPSII, photochemical efficiency in light conditions; A, CO2 assimilation rate; Ci, intercellular CO2 concentration; Fv/Fm, potential PSII photochemical efficiency; gs, stomatal conductance to water vapor; PDΨW, pre-dawn leaf water potential; qP, photochemical quenching coefficient; qNP, non-photochemical quenching coefficient; RWC, relative water content.
The biochemical responses at the end of drought exposure are summarized in Table 2. In comparison to the controls, H2O2 levels did not change in WS leaves, while accumulation of was 1.6-fold higher under drought. A strong increase in Pro content (+39%) was observed in WS leaves. The endogenous concentration of ABA and SA measured in WS leaves decreased significantly at the end of the experimental period (–33% and –39%, respectively). However, the JA and ET amounts accumulated by WS leaves increased significantly (about 7-fold and +66%, respectively).
Table 2.
Biochemical parameters in Quercus ilex plants WW or water stressed (20% of the effective evapotranspiration daily for 15 days, WS).
| WW | WS | P | ||
|---|---|---|---|---|
| H2O2 | (μmol g-1 DW) | 0.18 ± 0.011 | 0.17 ± 0.004 | ns |
| (nmol min-1 g-1 DW) | 24.0 ± 0.20 | 38.7 ± 1.47 | ∗∗∗ | |
| ET | (pl g-1 FW h-1) | 136 ± 15.0 | 226 ± 10.8 | ∗∗ |
| SA | (nmol g-1 DW) | 7.1 ± 1.04 | 4.3 ± 0.08 | ∗∗ |
| JA | (μmol g-1 DW) | 3.5 ± 0.08 | 24.0 ± 0.35 | ∗∗∗ |
| ABA | (nmol g-1 DW) | 4.2 ± 0.03 | 2.8 ± 0.28 | ∗∗∗ |
| Pro | (mmol g-1 DW) | 0.23 ± 0.001 | 0.32 ± 0.010 | ∗∗∗ |
Data are shown as mean ± standard deviation. Asterisks show the significance of Student t-test: ∗∗∗P ≤ 0.001, ∗∗P ≤ 0.01, ns P > 0.05. ABA, abscisic acid; DW, dry weight; ET, ethylene; FW, fresh weight; H2O2, hydrogen peroxide; JA, jasmonic acid; O2-, superoxide anion; Pro, proline; SA, salicylic acid.
Influence of Drought Stress on the Response to Acute O3 Exposure
Macroscopic and Microscopic Ozone-Induced Symptoms
At the end of the O3 treatment (alone and in combination with drought), leaves appeared macroscopically symptomless. However, O3-injuries were already detectable at the microscopic level after 5 h FBE, as confirmed by the appearance of dead cells observed in WW+O3 and WS+O3 (Figures 1A–D). Histological staining showed local accumulation of H2O2 evidenced by reddish-brown areas in O3-treated material (Figures 1G,H) (regardless of drought stress; Figures 1E,F).
FIGURE 1.
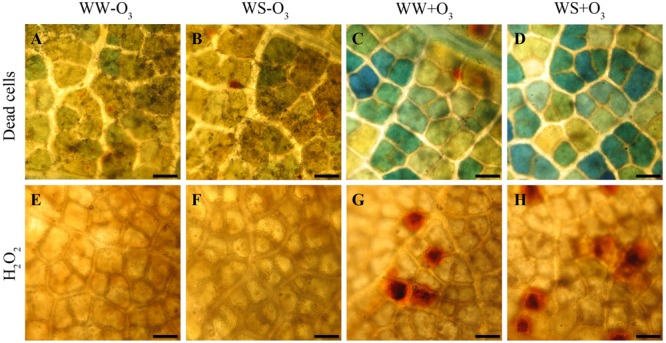
Localization of dead cells visualized with Evans blue staining (A–D) and of hydrogen peroxide (H2O2) visualized the 3,3′-diaminobenzidine (DAB) uptake method (E-H) in Quercus ilex leaves (i) well-watered (WW) and exposed to charcoal filtered air (WW-O3); (ii) water stressed (20% of effective evapotranspiration daily for 15 days) and exposed to charcoal filtered air (WS-O3); (iii) well-watered and exposed to acute ozone (200 nL L-1 for 5 h) (WW+O3); (iv) water stressed and O3 fumigated (WS+O3). The assays were performed 96 h FBE. Bars 50 μm.
Ozone-Induced Physiological Responses
The photosynthetic rate in light saturation conditions decreased strongly following O3 exposure in both WW+O3 and WS+O3 plants, and especially under drought (–23 and –41% in WW+O3 and WS+O3 plants, respectively) (Figure 2A). However, A values continued to decrease only in WW+O3 leaves, also after the end of fumigation, reaching values of about 4 μmol CO2 m-2s-1 at 48 h FBE with a reduction of about 50% compared to the values determined before O3 exposure (Figure 2A). WS+O3 plants showed low levels of A already before the beginning of O3 exposure (–73% in comparison with WW plants) and these values did not decrease further after the fumigation (Figure 2A). Ozone also induced a strong decrease in gs in WW+O3 and even more in WS+O3 leaves at the end of the exposure (-13 and -38% in WW+O3 and WS+O3 leaves, respectively) and the effect of O3 on gs remained at 24 and 48 h FBE (Figure 2B). However, in WS+O3 plants, gs values were 50% lower than those recorded in WW+O3 plants. Finally, Ci values increased significantly following O3 exposure in both WW+O3 and WS+O3 leaves (+8%) although the values recorded in WS+O3 leaves were significantly higher compared to those found in WW+O3. In both WW+O3 and WS+O3 leaves, the Ci values reached at the end of the exposure were maintained up to the end of the experimental period, although a slight decrease was observed for WW+O3 leaves at 24 h FBE (Figure 2C).
FIGURE 2.
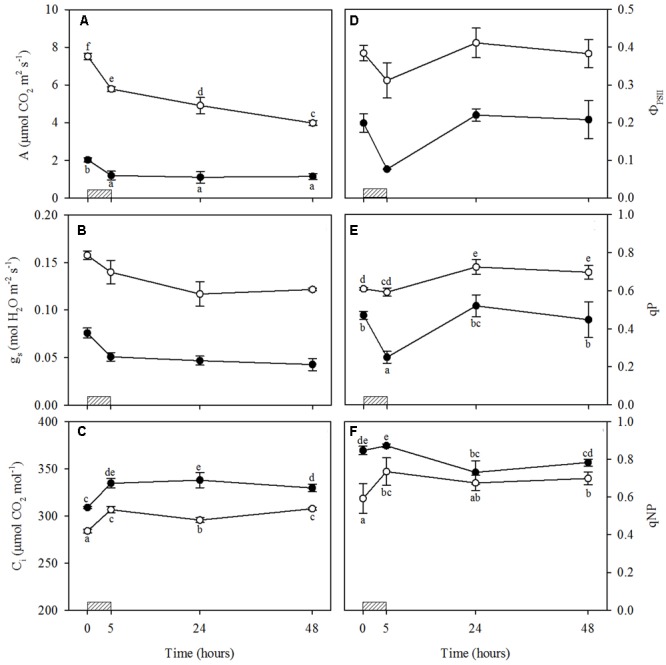
Time course of leaf gas exchange and chlorophyll fluorescence parameters in Quercus ilex plants well-watered (open circle) or water stressed (20% of the effective evapotranspiration daily for 15 days, closed circle) and exposed to acute ozone (200 nL L-1 for 5 h). Data are shown as mean ± standard deviation. The measurements were carried out 0, 5, 24 and 48 h from the beginning of exposure. According to the one-way repeated measures ANOVA with treatment as variability factor, different letters indicate significant differences (P = 0.05). The absence of letters in B and D indicates not significant interaction between variability factors (see Supplementary Table S1). (A) CO2 assimilation rate (A); (B) stomatal conductance to water vapor (gs); (C) intercellular CO2 concentration (Ci); (D) photochemical efficiency in light conditions (ΦPSII); (E) photochemical quenching coefficient (qP); (F) non-photochemical quenching coefficient (qNP). The thick bottom line indicates the time (5 h) of ozone exposure.
Actual ΦPSII decreased at the end of exposure in both WW+O3 and WS+O3 leaves (-19 and -62%, respectively). However, ΦPSII recovered completely 48 h FBE in both WW+O3 and WS+O3 leaves (Figure 2D). In WW+O3 plants, qP values were higher than those observed before the beginning of exposure, both at 24 and 48 h FBE (+19 and +14%, respectively) (Figure 2E). Conversely, in WS+O3 plants, qP values decreased at the end of exposure (-47%), but recovered completely from 24 h onward (Figure 2E). Values of qNP increased in WW+O3 leaves at the end of the fumigation (+24%), and similar values were maintained until 48 h FBE (Figure 2F). Conversely, in WS+O3 plants, qNP decreased at 24 and 48 h FBE (–14 and –8%, respectively, in comparison with the pre-treatment values) (Figure 2F). These mechanisms were sufficient to protect PSII from photoinhibition given that the decrease in Fv/Fm observed at the end of exposure in both WW+O3 and WS+O3 leaves was completely recovered 48 h FBE (data not shown).
Ozone-Induced ROS Accumulation
A biphasic time course of H2O2 production in response to O3 was observed irrespective of drought stress (Figures 3A and Supplementary Table S2). In both WW+O3 and WS+O3 plants, H2O2 content increased at 2 h FBE (+33 and +43% as compared to time 0, respectively), showed a significant decline at 5 h FBE, and increased again at 8 h FBE. This second peak was higher in WW+O3 than in WS+O3 plants (+62% vs. +38%, compared to their respective time 0). In addition, only in WW+O3 leaves was the second peak higher than the first, and H2O2 levels at 24 h FBE remained higher than those at time 0 (+24%).
FIGURE 3.
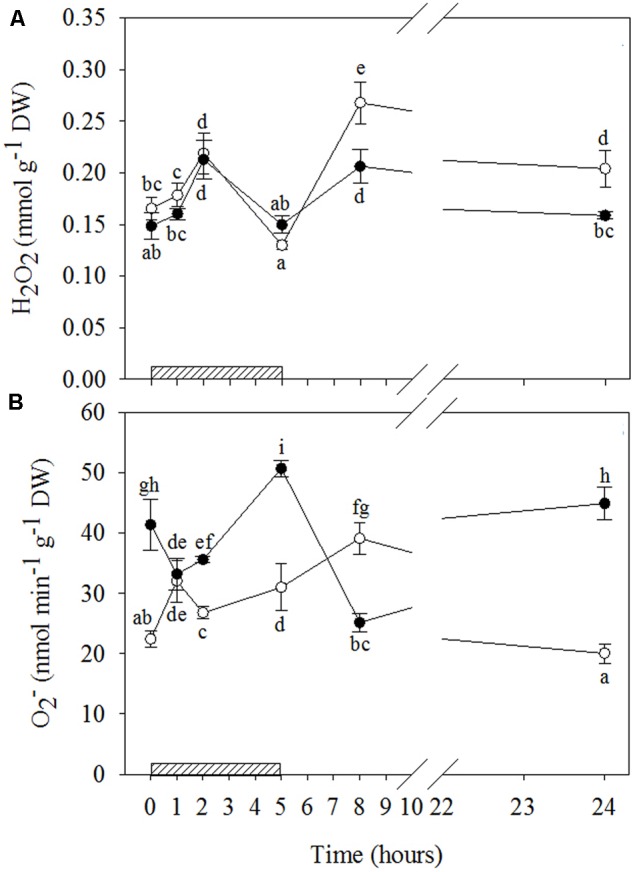
Time course of reactive oxygen species (ROS) in Quercus ilex plants well-watered (open circle) or water stressed (20% of the effective evapotranspiration daily for 15 days, closed circle) and exposed to acute ozone (200 nL L-1 for 5 h). Data are shown as mean ± standard deviation. The measurements were carried out at 0, 1, 2, 5, 8, and 24 h from the beginning of exposure. According to the two-way ANOVA with treatment and time as variability factors, different letters indicate significant differences (P = 0.05). DW, dry weight; H2O2, hydrogen peroxide (A); , superoxide anion (B). The thick bottom line indicates the time (5 h) of ozone exposure.
The time patterns of induced by O3 were notably different in relation to water stress (Figures 3B and Supplementary Table S2). In WW+O3 conditions, content also exhibited a clear biphasic time course. It peaked already at 1 h FBE (+47%, compared to the beginning of O3-exposure), and again at 8 h FBE (+75%), although it remained higher than time 0 at 2 and 5 h FBE (+20 and +38%, respectively). At 24 h FBE, the content decreased at the same levels as time 0. Conversely, in WS+O3 plants (where levels were already higher in WS+O3 than WW+O3 plants), content decreased during the first two hours of O3 treatment (–20 and –14%, after 1 and 2 h FBE, respectively). It then peaked at 5 h FBE (+22%), decreased again at 8 h FBE (reaching the lowest values of the whole treatment), and finally increased again reaching the levels shown at time 0.
Ozone-Induced Signaling Molecule Stimulation
The results of signaling molecules indicate that O3 only significantly stimulated ET emission in WW+O3 leaves (Figures 4A and Supplementary Table S2). In these plants, starting from 1 h of treatment onwards, ET emission values remained higher than those shown at the beginning of O3-exposure throughout the treatment period, and reached the maximum 8 h FBE (+49, +52, +79, +128, and +76% after 1, 2, 5, 8, and 24 h FBE, respectively). Conversely, a clear biphasic time course of SA production was observed in response to O3, irrespective of drought stress. However, the average concentration throughout the treatment and the changes induced by O3 were higher and more pronounced in WW+O3 than in WS+O3 plants, respectively (Figures 4B and Supplementary Table S2). In WW plants, total SA levels increased already at 1 h FBE, reaching their maximum values (three-fold higher than before the O3-treatment). They then progressively decreased to constitutive levels at 5 h FBE, and to even lower values at 8 h FBE, but increased again at the end of the experiment (+73% compared to time 0). In WS+O3 conditions, SA concentrations peaked at 2 and 8 h FBE (+79 and +80%, respectively), whereas SA levels were similar before the beginning of O3 treatment than at the other analysis times.
FIGURE 4.
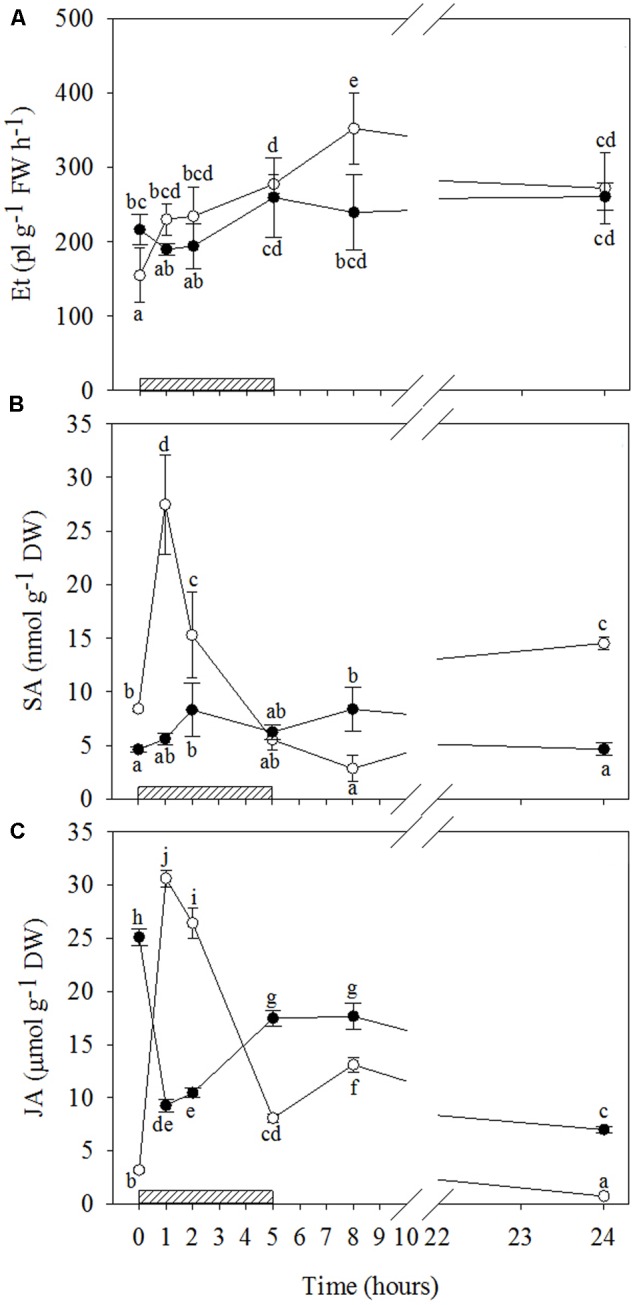
Time course of phytohormones in Quercus ilex plants well-watered (open circle) or water stressed (20% of the effective evapotranspiration daily for 15 days, closed circle) and exposed to acute ozone (200 nL L-1 for 5 h). Data are shown as mean ± standard deviation. The measurements were carried out 0, 1, 2, 5, 8, and 24 h from the beginning of exposure. According to the two-way ANOVA with treatment and time as variability factors, different letters indicate significant differences (P = 0.05). DW, dry weight; ET, ethylene (A); FW, fresh weight; JA, jasmonic acid (C); SA, salycilic acid (B). The thick bottom line indicates the time (5 h) of ozone exposure.
The time patterns of JA induced by O3 were also notably different in relation to drought (Figures 4C and Supplementary Table S2). A biphasic time course of JA production was shown by WW+O3 plants. Similarly to SA (and ABA, as reported below), a first marked peak in JA levels (tenfold higher than controls) was shown by WW+O3 plants at 1 h FBE. Then, JA progressively decreased until 5 h FBE (remaining at higher levels than those recorded at time 0), peaked again 8 h FBE (four times higher than time 0), and, finally, reached lower values than before the beginning of O3-treatment at 24 h FBE. Conversely, in WS+O3 plants (where JA levels, similarly to , were already higher in WS+O3 than WW+O3 plants) a marked decrease in JA concentrations was observed starting from 1 h onwards (–63%, in comparison to controls). Throughout the period of O3-treatment, the values of this phytohormone remained lower than those shown before the exposure, although a recovery was shown at 5 and 8 h FBE.
Ozone-Induced ABA and Osmolyte Accumulation
O3 significantly stimulated ABA production only in WW+O3 leaves (Figures 5A and Supplementary Table S2), where a clear biphasic response to the pollutant was observed. In comparison to the levels shown before the O3 treatment, ABA in WW+O3 leaves already increased at 1 h FBE (overall three times), showed no differences at 2 and 5 h FBE, slightly increased again at 8 h FBE (+44%) and, finally, reached lower values at 24 h FBE. In WW+O3 conditions, O3 induced a slight increase in Pro only at the first two hours of exposure (+46 and +33%, respectively at 1 and 2 h FBE), whereas during the post-fumigation period, Pro values remained lower than those at time 0 (Figures 5B and Supplementary Table S2). Conversely, in WS+O3 plants, Pro content peaked after 1 h FBE (+96%, in comparison to controls), then declined at 2 h FBE (at the same concentrations shown before the beginning of O3-exposure), increased again at 5 h FBE (+69%) and reaching a maximum at 8 h FBE, with the maximum values (sixfold higher than at time 0). Finally, Pro concentration of WS+O3 leaves decreased at 24 h FBE, although they remained higher than before the O3-exposure (more than two fold).
FIGURE 5.
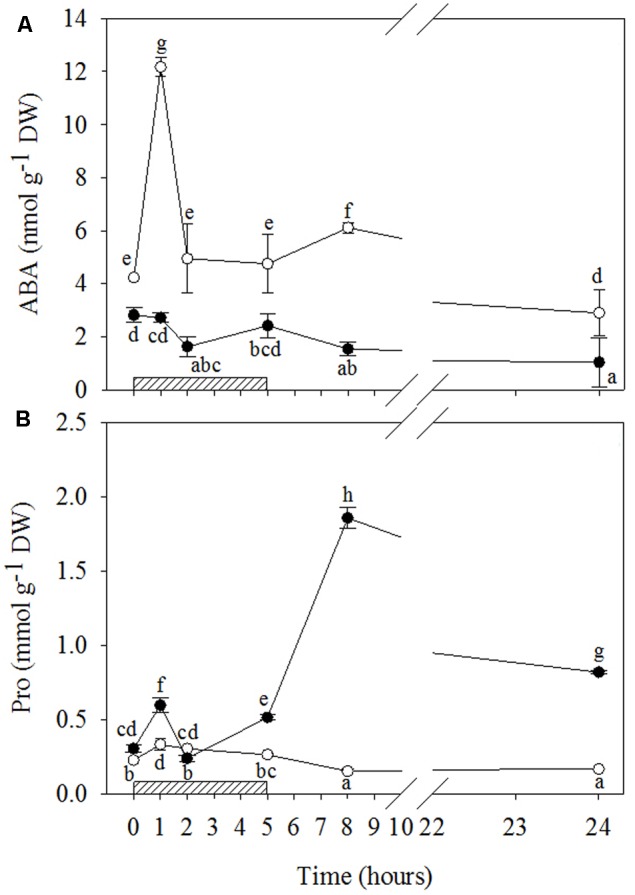
Time course of abscisic acid (ABA; A) and proline (Pro; B) in Quercus ilex plants well-watered (open circle) or water stressed (20% of the effective evapotranspiration daily for 15 days, closed circle) and exposed to acute ozone (200 nL L-1 for 5 h). Data are shown as mean ± standard deviation. The measurements were carried out at 0, 1, 2, 5, 8, and 24 h from the beginning of exposure. According to the two-way ANOVA with treatment and time as variability factors, different letters indicate significant differences (P = 0.05). DW, dry weight. The thick bottom-line indicates the time (5 h) of ozone exposure.
Discussion
In this study, the behavior of the Mediterranean evergreen Q. ilex subjected or not to drought, and later exposed to an acute O3 exposure, was evaluated in terms of cross-talk responses among signaling molecules. The aim was to confirm or disentangle the hypothesis according to which drought stress influences the responses of plants to acute episodes of O3 exposure.
Physiological and Biochemical Responses to Drought
Although drought induced a strong decrease in PDΨW (reaching lower values than those reported in a previous study; Cotrozzi et al. (2016b), attributable to different growing seasons between experiments), the RWC of WS leaves did not significantly change in comparison to the WW leaves. This indicates that a good level of leaf hydration was also maintained under drought conditions, RWC being a reliable indicator of leaf water content (Rosales-Serna et al., 2004). This result is in accordance with the accumulation of Pro, a metabolite that is considered an important compatible solute which (i) facilitates water absorption by increasing the cell osmotic potential (Ashraf and Foolad, 2007), and (ii) reduces cell damage (Filippou et al., 2014). The important role of Pro in response to water stress has already been reported in this species where Pro played a key role in the high plasticity of Q. ilex under a long period of moderate water stress (Cotrozzi et al., 2016b).
In WS plants, the strong decline in CO2 photo-assimilation was attributable to coordinated and concomitant stomatal and mesophyll limitations, which is in line the results obtained by several authors (e.g., Centritto et al., 2009; Flexas et al., 2013). The drop in A levels was higher than that in gs, thus leading to lower values of intrinsic water use efficiency (data not shown), as already reported in this species when subjected to water stress (Cotrozzi et al., 2016b).
These outcomes indicate that CO2 assimilation was strongly influenced not only by stomatal behavior but also by mesophyll limitations, as demonstrated by the increase in Ci. Drought compromised the PSII photochemical efficiency in light adapted leaves with decreases in ΦPSII and qP levels, although this inhibition did not determine PSII photoinhibition, as confirmed by unchanged values in the Fv/Fm ratio. In WS conditions, the lower CO2 assimilation rate induced, in turn, a reduced consumption of ATP and NADPH synthesized into the chloroplasts and, consequently, led to an excess of excitation energy in the thylakoid membrane, which was only partially dissipated, via non-photochemical mechanisms (increase in qNP). The remaining excess of reducing power in WS plants altered the ROS levels (although H2O2 did not change, a strong increase of was observed). This led to a modification in phytohormones and other signaling molecule cross-talk in terms of (i) promoting oxidative stress and (ii) modulating leaf senescence (Munné-Bosch and Alegre, 2004; Miller et al., 2010; Baxter et al., 2014), which is a defense commonly activated in response to both a plethora of abiotic and biotic stresses (Wingler and Roitsch, 2008).
Among phytohormones, the important roles of ABA and ET in plant responses to drought is well known (Munné-Bosch and Alegre, 2004) as ABA represents the most important regulator of stomata functioning (Wilkinson and Davies, 2002), whereas ET is a shoot growth inhibitor and a promoter of ripening, senescence and abscission (Abeles et al., 1992). However, ET can inhibit ABA-induced stomatal closure (Tanaka et al., 2005). Wilkinson and Davies (2009, 2010) reported that under stress-oxidative conditions, an ET-antagonism of the stomatal response to ABA occurs. This interaction was confirmed by our data (ABA decreased, while ET increased), suggesting that drought-induced ET biosynthesis could be considered a marker of leaf senescence (Dangl et al., 2000; Dolferus, 2014).
In addition, SA and JA have been shown to play a central role in leaf senescence (Abreu and Munné-Bosch, 2008), although they are well known for triggering defense reactions against biotrophic and necrotrophic pathogens such as induced resistance (Barna et al., 2012; Shigenaga and Argueso, 2016). In particular, SA and JA interact at physiological levels in many growth and developmental processes, and they play a role in controlling gene expression during leaf senescence (Abreu and Munné-Bosch, 2008). However, as only the JA levels increased in WS leaves, it is reasonable that only JA participated in senescence-associated signaling and degradative processes of membranes. The significantly higher levels of JA shown by WS compared to WW plants indicate that lipid peroxidation producing substrates for octadecanoid pathways was exacerbated in limited water conditions. In particular, JA could be a promoter of leaf senescence in response to drought, thus leading to stomatal closure and accumulation of osmo-compatible solutes (in our case, only Pro), in line with Dar et al. (2015).
Influence of Drought Stress on the Physiological and Biochemical Responses to O3 Exposure
The physiological responses observed in O3-stressed plants were similar to those shown at the end of water deprivation, and in accordance with a previous study by our research group on Q. ilex exposed to O3 (Cotrozzi et al., 2016b). At the end of the fumigation, the O3-induced stomatal closure found in both WW+O3 and WS+O3 leaves led to significant reductions in CO2 assimilation. The more pronounced decrease in A in WW+O3 compared to WS+O3 leaves, as well as the lack of a further decrease in A observed in WS+O3 plants throughout the recovery phase, was probably attributable to the very low CO2 assimilation rate shown by water stressed plants before the beginning of the fumigation. The increase in Ci level in plants exposed to O3 indicates that the pollutant gas influenced not only stomatal conductance but, as with after water stress, also the mesophyll activity. In fact, an impairment of PSII activity was recorded. Although Fv/Fm and ΦPSII values decreased significantly after O3 exposure in both WW+O3 and WS+O3 plants, the reduction was more pronounced in WS+O3 plants. This behavior was linked to different quenching responses to the leaf-water status of plants. In WW+O3 plants, where qP did not decrease, a mechanism aimed at dissipating the excess excitation energy was activated (qNP increased). By contrast, in WS+O3 leaves, the O3-induced decrease in qP was ascribable to the fact that qNP values were already high (probably at their maximum in relation to the capability of plants to activate this mechanism) after drought, and the leaves were not able to enhance this type of dissipation mechanism. The complete recovery of PSII photochemical activity during the recovery time after drought and O3 exposure indicates that the decrease in PSII activity was sufficient to prevent the photosynthetic apparatus from undergoing irreversible damage.
Unlike the ecophysiological measurements, microscopic analyses highlighted significant differences between plants exposed (WW+O3, WS+O3) or not (WW and WS) to the gaseous pollutant. Although visible symptoms were not shown by any of the plants irrespectively of the applied treatment, DAB staining and Evan’s blue incorporation observed in WW+O3 and WS+O3 leaves 5 h FBE indicated that H2O2 deposition and cell death had already occurred at the end of exposure. This confirms that O3 resembles the HR occurring in incompatible plant-pathogen interactions (Iriti and Faoro, 2008; Vainonen and Kangasjärvi, 2015). An integrated perspective has been proposed to explain how phytohormones and signaling molecules might be involved in molecular events (namely lesion initiation, propagation, and containment) leading to O3-induced HR-mimicking foliar symptoms (Overmeyer et al., 2003, 2005; Kangasjärvi et al., 2005). ROS, phytohormones and other signaling molecules have a pivotal role in both HR-mimicking responses induced by acute O3 and in promoting leaf senescence under drought (as shown by WS plants). The trends of these molecules were monitored in both well-watered and drought-stressed plants during and after O3 exposure, in order to test the hypotheses of this work.
Given that O3 induces an endogenous, active and self-propagating ROS generation in the apoplast and a subsequent cellular oxidative burst, some authors have proposed that short-term O3 exposure mimics pathogen infection (Rao et al., 2000; Kangasjärvi et al., 2005; Carmody et al., 2016). The two O3-induced H2O2 peaks observed in our saplings, irrespectively of the water conditions, are analogous to the biphasic response usually observed during the establishment of the HR of plants against pathogens. The first H2O2 peak usually reflects elicitation by pathogen-associated molecular patterns, and the second reflects the interaction between a pathogen-encoded virulence gene product with a plant resistance gene (Mur et al., 2009). In our study, the first peak observed during the fumigation was attributable to O3-decomposition, whereas the second peak, in the recovery period, could be entirely ascribable to the plant metabolism, in line with Mahalingam et al. (2006), Di Baccio et al. (2012), and Pellegrini et al. (2013) in herbaceous species.
Although the similarity in H2O2 profiles over time between WW+O3 and WS+O3 conditions, the divergence in the magnitude of their relative peaks (the second peak of the WW+O3 plants was much greater than their first peak and greater than the second peak of the WS+O3 plants, where the two peaks were not significantly different from each other) suggests that drought partially inhibited the response to O3-stress. As H2O2 is one of the most important products of oxidative stress (Gill and Tuteja, 2010), it is reasonable to speculate that the biphasic trend of H2O2 observed in Q. ilex, irrespectively of drought stress, might reflect the biphasic oxidative burst in response to O3, in line with several authors (e.g., Wohlgemuth et al., 2002; Di Baccio et al., 2012).
However, this hypothesis is strengthened by only in WW+O3 plants, where a biphasic time course of levels was shown concurrently with H2O2. Kangasjärvi et al. (2005) reported similar temporal changes in ROS for O3-sensitive genotypes of several species (e.g., tobacco, tomato, birch), whereas only a modest increase in the first hours of exposure was observed in O3-tolerant genotypes. Conversely, the different patterns of the WS+O3 plants suggest a possible dual function of this radical depending on water stress. In fact, the significant decrease in observed in WS+O3 plants during the first hours of exposure suggests that under drought+O3 superoxide anion may act as a precursor of H2O2 and even more toxic radical derivatives. However, the accumulation of had already been induced by drought before the beginning of O3-exposure. On the other hand, the marked increase in content at the end of the exposure demonstrates that this radical may also be directly involved in the O3-oxidative burst.
Reactive oxygen species should not be considered as exclusively deleterious and harmful. They can (i) play a key role in intracellular communication which triggers the acclimation ability, and (ii) indirectly orchestrate PCD (Mittler et al., 2011; Xia et al., 2015; Carmody et al., 2016). The amplification of ROS signals and the complete induction of defense genes seem to require signal molecules (Overmeyer et al., 2003). The differences observed in the present study in O3-induced ROS extent dynamics in relation to water stress suggest a rather complex network of events in signal transduction, involving other molecules (e.g., phytohormones) and processes. Metabolites such as ET, ABA, SA and JA may interact at the physiological level in many growth and developmental processes, with a key role in controlling gene expression during leaf senescence. Most of the genes regulated by these metabolites are defense-related (Fossdal et al., 2007), participating therefore in responses to O3 (Xu et al., 2015).
Under both biotic and abiotic stresses, SA is required for the induction of PCD, controlling and potentiating the oxidative burst together with ET, whereas JA is involved in limiting the spread of lesions (van Loon et al., 2006; Shigenaga and Argueso, 2016). There are three phases that highlight the influence of ABA on stress conditions (Rejeb I.B. et al., 2014). First, ABA induces stomatal closure, which leads to a reduction in water loss (in this phase, SA, JA and ET may not yet be activated and ABA can antagonize their induction). In the second step, there is a post-infection reaction- an intact ABA signaling pathway is required to increase callose accumulation in affected plants, and the presence of ABA can induce or repress additional callose accumulation depending on the environmental conditions. The third phase begins when pathogen-associated molecular patterns stimulate the accumulation of specific SA, JA, and ET hormones in order to regulate the defense reaction.
In our study, the patterns of phytohormones during and after O3 treatment were completely different in WW+O3 and WS+O3 plants, showing how drought stress has a pivotal role in O3 responses, and how these signal molecules may be altered in relation to water stress. The ET and ABA accumulations observed throughout the entire period of O3 exposure occurred only in well-watered conditions. On the other hand, when plants had been previously subjected to water stress, their unchanged values suggest that ET and ABA were not involved in either signaling-responses to O3, or senescence strategies (as shown for WS plants).
It is worth noting that (i) the maximal ET emission in WW+O3 plants coincided with the second peak of H2O2 and ; (ii) the first peak of ABA (during O3 treatment) preceded that of H2O2, suggesting that ABA could act as a stress messenger by inducing H2O2 (Jiang and Zhang, 2002) and consequently stomatal closure (as confirmed by the decrease in gs values observed at the end of exposure), and (iii) the weaker second ABA peak (in the recovery phase) was concomitant with the maximum H2O2 and levels and the maximal ET emission. These outcomes confirm a spatial and functional correlation between ROS and the accumulation of these phytohormones.
The SA induction observed, irrespectively of whether the plants had been subjected to drought or not, suggests that this metabolite is also an important modulator of O3-induced responses (Pasqualini et al., 2002; Horváth et al., 2007). However, the differences between WW+O3 and WS+O3 plants show that the functioning of SA is dependent on water stress. In WW+O3 conditions, the strong increase in SA during the first hours of the treatment and at the end of the O3 fumigation, confirms the central role of this metabolite in lesion initiation and progression in response to O3 (Tamaoki, 2008). In addition, the greater SA concentration corresponded with the maximal ABA stimulation and the first increase in ET, thus demonstrating the synergistic action of these hormones in the regulation of defense reactions (Roychoudhury et al., 2013; Wang et al., 2013). By contrast, the biphasic time course of SA (similar to that of H2O2) shown by WS+O3 plants (although slight) recalls the biphasic induction that develops during biotrophic pathogen infection (Mur et al., 2009). Here, the similarity in magnitude of the two SA peaks suggests that the first accumulation of this metabolite (concomitant with the first peak of H2O2) did not actuate the second increase in H2O2 and hence did not affect the level of plant defense.
The highest concentration of JA observed in WW+O3 plants during the first hour of fumigation coincided with the initial increase in ET and the maximum accumulation of SA and ABA, thus also demonstrating a spatial and functional correlation between these compounds (Thaler et al., 2012). The significant O3-induced decrease in JA levels observed in WS+O3 plants during and after the exposure suggests that JA did not promote leaf senescence in O3-treated leaves in spite of the high concentrations of this metabolite observed in WS+O3 plants, not excluding the involvement of JA in senescence-associated signaling (Abreu and Munné-Bosch, 2008). In fact, the JA level in WS+O3 plants increased again after the end of fumigation, reaching higher values than those found in the WW+O3 counterpart during the recovery. JA is known to rapidly inhibit the expression of genes involved in photosynthesis by inducing chlorophyll loss and cellular changes that cause less photochemical damage (Santino et al., 2013).
Proline plays several roles in plant responses to abiotic and biotic stresses, and under stress its metabolism is affected by multiple and complex regulatory pathways which can profoundly influence cell death and survival in plants (Zhang and Becker, 2015). The slight increase in Pro observed in WS plants compared to WW likely indicates its role as an osmoprotectant. By the same token the O3-induced increase in Pro observed in WW+O3 plants only during the first hours of treatment and coinciding with the maximal ABA, SA and JA stimulation and the first increase in ET, H2O2 and , suggests a potential cross-talk among signaling molecules in regulating Pro metabolism, as previously reported by Rejeb K.B et al. (2014). The role of Pro as an ROS scavenger has also been reported (Szabados and Savouré, 2009; Banu et al., 2010; Zhang and Becker, 2015). In WW+O3 plants the lack of accumulation in Pro at 8 h FBE was concurrent with a strong increase in H2O2, whereas in WS+O3 the huge increase in Pro at 8 h FBE suppressed the increase in H2O2 (which remained at the same levels shown at 1 h FBE), thus suggesting an H2O2-scavenging role in these water conditions. This mechanism was also confirmed at 24 h FBE. Several studies have attributed an antioxidant feature to Pro, suggesting the capability of Pro in and H2O2 quenching (e.g., Szabados and Savouré, 2009; Wang et al., 2009).
Author Contributions
The work presented here was carried out in collaboration among all authors. GL and RM defined the research theme and obtained funding. LC, DR, EP, MT, AT, and ML designed methods, carried out laboratory experiments and analyzed the data. LG, CN, and PV co-designed experiments, discussed analyses, interpreted the results, and wrote the paper. All authors have contributed to, seen and approved the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study was supported by MIUR, Rome, project PRIN 2010–2011 “Planning the green city in the global change era: urban tree functions and suitability for predicted future climates (TreeCity)”. The authors are grateful to Mr Andrea Bianchi and Ms Romina Papini for technical support.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2017.01020/full#supplementary-material
References
- Abeles F. B., Morgan P. W., Saltveit M. E., Jr. (1992). Ethylene in Plant Biology. San Diego, CA: Academic Press. [Google Scholar]
- Abreu M. E., Munné-Bosch S. (2008). Salicylic acid may be involved in the regulation of drought-induced leaf senescence in perennials: a case study in field-grown Salvia officinalis L. plants. Environ. Exp. Bot. 64 105–112. 10.1016/j.envexpbot.2007.12.016 [DOI] [Google Scholar]
- Alonso R., Elvira S., Gonzalez-Fernandez I., Calvete H., Garcia-Gomez H., Bermejo V. (2014). Drought stress does not protect Quercus ilex L. from ozone effects: results from a comparative study of two subspecies differing in ozone sensitivity. Plant Biol. 16 375–384. 10.1111/plb.12073 [DOI] [PubMed] [Google Scholar]
- Apel K., Hirt H. (2004). Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55 373–399. 10.1146/annurev.arplant.55.031903.141701 [DOI] [PubMed] [Google Scholar]
- Ashraf M., Foolad M. R. (2007). Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 59 206–216. 10.1016/j.envexpbot.2005.12.006 [DOI] [Google Scholar]
- Banu N. A., Hoque A., Watanabe-Sugimoto M., Islam M. M., Uraji M., Matsuoka K., et al. (2010). Proline and glycinebetaine ameliorated NaCl stress via scavenging of hydrogen peroxide and methylglyoxal but not superoxide or nitric oxide in tobacco cultured cells. Biosci. Biotechnol. Biochem. 74 2043–2049. 10.1271/bbb.100334 [DOI] [PubMed] [Google Scholar]
- Barna B., Fodor J., Harrach B. D., Pogány M., Király Z. (2012). The Janus face of reactive oxygen species in resistance and susceptibility of plants to necrotrophic and biotrophic pathogens. Plant Physiol. Biochem. 59 37–43. 10.1016/j.plaphy.2012.01.014 [DOI] [PubMed] [Google Scholar]
- Bates B. C., Kundzewicz Z. W., Wu S., Palutikof J. P. (2008). Climate Change and Water. Technical Paper of the Intergovernmental Panel on Climate Change. Geneva: IPCC Secretariat. [Google Scholar]
- Baxter A., Mittler R., Suzuki N. (2014). ROS as key players in plant stress signalling. J. Exp. Bot. 65 1229–1240. 10.1093/jxb/ert375 [DOI] [PubMed] [Google Scholar]
- Bussotti F., Bettini D., Grossoni P., Mansuino S., Nibbi R., Soda C., et al. (2002). Structural and functional traits of Quercus ilex in response to water availability. Environ. Exp. Bot. 47 11–23. 10.1016/S0098-8472(01)00111-3 [DOI] [Google Scholar]
- Calatayud V., Cerveró J., Calvo E., García-Brejio F. J., Reig-Armiñana J., Sanz M. J. (2011). Responses of evergreen and deciduous Quercus species to enhanced ozone levels. Environ. Pollut. 159 55–63. 10.1016/j.envpol.2010.09.024 [DOI] [PubMed] [Google Scholar]
- Calderòn Guerrero C. C., Günthardt-Goerg M. S., Vollenweider P. (2013). Foliar symptoms triggered by ozone stress in irrigated holm oaks from the city of Madrid, Spain. PLoS ONE 8:e69171 10.1371/journal.pone.0069171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmody M., Waszczak C., Idänheimo N., Saarinen T., Kangasjärvi J. (2016). ROS signaling in a destabilized world: a molecular understanding of climate change. J. Plant Physiol. 203 69–83. 10.1016/j.jplph.2016.06.008 [DOI] [PubMed] [Google Scholar]
- Centritto M., Lauteri M., Monteverdi M. C., Serraj R. (2009). Leaf gas exchange, carbon isotope discrimination, and grain yield in contrasting rice genotypes subjected to water deficits during the reproductive stage. J. Exp. Bot. 60 2325–2339. 10.1093/jxb/erp123 [DOI] [PubMed] [Google Scholar]
- Claeys H., Inzé D. (2013). The agony of choice: how plants balance growth and survival under water-limiting conditions. Plant Physiol. 162 1768–1779. 10.1104/pp.113.220921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotrozzi L., Campanella A., Pellegrini E., Lorenzini G., Nali C., Paoletti E. (2016a). Phenylpropanoids are key players in the antioxidant defense to ozone of European ash, Fraxinus excelsior. Environ. Sci. Pollut. Res. 10.1007/s11356-016-8194-8 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Cotrozzi L., Remorini D., Pellegrini E., Landi M., Massai R., Nali C., et al. (2016b). Variations in physiological and biochemical traits of oak seedlings grown under drought and ozone stress. Physiol. Plant. 157 69–84. 10.1111/ppl.12402 [DOI] [PubMed] [Google Scholar]
- Dangl J., Dietrich R., Thomas H. (2000). “Senescence and programmed cell death,” in Biochemistry and Molecular Biology of Plants eds Buchanan B., Gruissemand W., Jones R. (Rochville, MD: American Society of Plant Physiologists; ) 1044–1100. [Google Scholar]
- Dar T. A., Uddin M., Khan M. M. A., Hakeem K. R., Jaleel H. (2015). Jasmonates counter plant stress: a review. Environ. Exp. Bot. 115 49–57. 10.1016/j.envexpbot.2015.02.010 [DOI] [Google Scholar]
- Di Baccio D., Ederli L., Marabottini R., Badiani M., Francini A., Nali C., et al. (2012). Similar foliar lesions but opposite hormonal patterns in a tomato mutant impaired in ethylene perception and its near isogenic wild type challenged with ozone. Environ. Exp. Bot. 75 286–297. 10.1016/j.envexpbot.2011.08.001 [DOI] [Google Scholar]
- Dolferus R. (2014). To grow or not to grow: a stressful decision for plants. Plant Sci. 229 247–261. 10.1016/j.plantsci.2014.10.002 [DOI] [PubMed] [Google Scholar]
- Filippou P., Bouchagier P., Skotti E., Fotopoulos V. (2014). Proline and reactive oxygen/nitrogen species biosynthesis is involved in the tolerant response of the invasive plant species Ailanthus altissima to drought and salinity. Environ. Exp. Bot. 97 1–10. 10.1016/j.envexpbot.2013.09.010 [DOI] [Google Scholar]
- Flexas J., Niinemets Ü, Gallé A., Barbour M. M., Centritto M., Diaz-Espejo A., et al. (2013). Diffusional conductances to CO2 as a target for increasing photosynthesis and photosynthetic water-use efficiency. Photosynth. Res. 117 45–59. 10.1007/s11120-013-9844-z [DOI] [PubMed] [Google Scholar]
- Fossdal C. G., Nagy N. E., Johnsen Ø, Dalen L. S. (2007). Local and systemic stress responses in Norway spruce: similarities in gene expression between a compatible pathogen interaction and drought stress. Physiol. Mol. Plant Pathol. 70 161–173. 10.1016/j.pmpp.2007.09.002 [DOI] [Google Scholar]
- Genty B., Briantais J. M., Baker N. R. (1989). The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 990 87–92. 10.1016/S0304-4165(89)80016-9 [DOI] [Google Scholar]
- Gill S. S., Tuteja N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 48 909–930. 10.1016/j.plaphy.2010.08.016 [DOI] [PubMed] [Google Scholar]
- Gimeno T. E., Pías B., Lemos-Filho P., Valladares F. (2008). Plasticity and stress tolerance override local adaptation in the responses of Mediterranean holm oak seedlings to drought and cold. Tree Physiol. 29 87–98. 10.1093/treephys/tpn007 [DOI] [PubMed] [Google Scholar]
- Gray S. B., Brady S. M. (2016). Plant developmental responses to climate change. Dev. Biol. 419 64–77. 10.1016/j.ydbio.2016.07.023 [DOI] [PubMed] [Google Scholar]
- Guidi L., Remorini D., Cotrozzi L., Giordani T., Lorenzini G., Massai R., et al. (2017). The harsh life of an urban tree: the effect of a single pulse of ozone in salt-stressed Quercus ilex saplings. Tree Physiol. 37 246–260. 10.1093/treephys/tpw103 [DOI] [PubMed] [Google Scholar]
- Horváth E., Szalai G., Janda T. (2007). Induction of abiotic stress tolerance by salicylic acid signaling. J. Plant Growth Regul. 26 290–300. 10.1007/s00344-007-9017-4 [DOI] [Google Scholar]
- Iriti M., Faoro F. (2008). Chemical diversity and defence metabolism: how plants cope with pathogens and ozone pollution. Int. J. Mol. Sci. 10 3371–3399. 10.3390/ijms10083371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer N. J., Tang Y., Mahalingam R. (2013). Physiological, biochemical and molecular responses to a combination of drought and ozone in Medicago truncatula. Plant Cell Environ. 36 706–720. 10.1111/pce.12008 [DOI] [PubMed] [Google Scholar]
- Jiang M., Zhang J. (2002). Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves. J. Exp. Bot. 53 2401–2410. 10.1093/jxb/erf090 [DOI] [PubMed] [Google Scholar]
- Kangasjärvi J., Jaspers P., Kollist H. (2005). Signalling and cell death in ozone-exposed plants. Plant Cell Environ. 28 1021–1036. 10.1111/j.1365-3040.2005.01325.x [DOI] [Google Scholar]
- Kangasjärvi J., Talvinen J., Utriainen M., Karjalainen R. (1994). Plant defence systems induced by ozone. Plant Cell Environ. 17 783–794. 10.1111/j.1365-3040.1994.tb00173.x [DOI] [Google Scholar]
- Kock J. R., Creelman R. A., Eshita S. M., Seskar M., Mullet J. E., Davis K. R. (2000). Ozone sensitivity in hybrid poplar correlates with insensitivity to both salycilic acid and jasmonic acid. The role of programmed cell death in lesion formation. Plant Physiol. 123 487–496. 10.1104/pp.123.2.487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz C., Schmieden U., Strobel P., Wild A. (1998). The combined effect of CO2, ozone and drought on the radical scavenging system of young oak trees (Quercus petraea) – a phytothron study. Chemosphere 36 783–788. 10.1016/S0045-6535(97)10124-2 [DOI] [Google Scholar]
- Langebartels C., Schraudner M., Heller W., Ernst D., Sandermann H. (2002). “Oxidative stress and defense reactions in plants exposed to air pollutants and UV-B radiation,” in Oxidative Stress in Plants eds Inzé D., Van Montagu M. (London: Taylor & Francis; ) 105–135. [Google Scholar]
- Lorenzini G., Medeghini Bonatti P., Nali C., Baroni Fornasiero R. (1994). The protective effect of rust infection against ozone, sulphur dioxide and paraquat toxicity symptoms in broad bean. Physiol. Mol. Plant Pathol. 45 263–279. 10.1016/S0885-5765(05)80058-X [DOI] [Google Scholar]
- Mahalingam R., Jambunathan N., Gunjan S. K., Faustin E., Weng H., Ayoubi P. (2006). Analysis of oxidative signalling induced by ozone in Arabidopsis thaliana. Plant Cell Environ. 29 1357–1371. 10.1111/j.1365-3040.2006.01516.x [DOI] [PubMed] [Google Scholar]
- Matesanz S., Valladares F. (2014). Ecological and evolutionary responses of Mediterranean plants to global change. Environ. Exp. Bot. 103 53–67. 10.1016/j.envexpbot.2013.09.004 [DOI] [Google Scholar]
- Miller G., Suzuki N., Ciftci-Yilmaz S., Mittler R. (2010). Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 33 453–467. 10.1111/j.1365-3040.2009.02041.x [DOI] [PubMed] [Google Scholar]
- Mittler R., Vanderauwera S., Suzuki N., Miller G., Tognetti V. B., Vandepele K., et al. (2011). ROS signaling: the new wave? Trends Plant Sci. 16 1360–1385. 10.1016/j.tplants.2011.03.007 [DOI] [PubMed] [Google Scholar]
- Munné-Bosch S., Alegre L. (2004). Die and let live: leaf senescence contributes to plant survival under drought stress. Funct. Plant Biol. 31 203–213. 10.1071/FP03236 [DOI] [PubMed] [Google Scholar]
- Mur L. A. J., Lloyd A. J., Cristesce S. M., Harren F. J. M., Hall M. A., Smith A. R. (2009). Biphasic ethylene production during the hypersensitive response in Arabidopsis. Plant Signal. Behav. 4 610–613. 10.4161/psb.4.7.8904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G., Mhamdi A., Foyer C. H. (2014). The roles of reactive oxygen metabolism in drought: not so cut and dried. Plant Physiol. 164 1636–1648. 10.1104/pp.113.233478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overmeyer K., Brosche M., Kangasjärvi J. (2003). Reactive oxygen species and hormonal control of cell death. Trends Plant Sci. 8 335–342. 10.1016/S1360-1385(03)00135-3 [DOI] [PubMed] [Google Scholar]
- Overmeyer K., Brosché M., Pellinen R., Kuittinen T., Tuominen H., Ahlfors R., et al. (2005). Ozone-induced programmed cell death in the Arabidopsis radical-induced cell death1 mutant. Plant Physiol. 137 1092–1104. 10.1104/pp.104.055681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panek J. A., Kurpius M. R., Goldstein A. H. (2002). An evaluation of ozone exposure metrics for a seasonally drought-stressed ponderosa pine ecosystem. Environ. Pollut. 117 93–100. 10.1016/S0269-7491(01)00155-5 [DOI] [PubMed] [Google Scholar]
- Pasqualini S., Della Torre G., Ferranti F., Ederli L., Piccioni C., Reale L., et al. (2002). Salicylic acid modulates ozone-induced hypersensitive cell death in tobacco plants. Physiol. Plant. 115 204–212. 10.1034/j.1399-3054.2002.1150205.x [DOI] [PubMed] [Google Scholar]
- Pellegrini E., Trivellini A., Campanella A., Francini A., Lorenzini G., Nali C., et al. (2013). Signaling molecules and cell death in Melissa offcinalis plants exposed to ozone. Plant Cell Rep. 32 1965–1980. 10.1007/s00299-013-1508-0 [DOI] [PubMed] [Google Scholar]
- Pellegrini E., Trivellini A., Cotrozzi L., Vernieri P., Nali C. (2016). “Involvement of phytohormones in plant responses to ozone,” in Plants Hormones Under Challenging Environmental Factors eds Ahammed G. J., Yu J.-Q. (Dordrrecht: Springer; ) 215–245. 10.1007/978-94-017-7758-2_9 [DOI] [Google Scholar]
- Pollastrini M., Desotgiu R., Camin F., Ziller L., Gerosa G., Marzuoli R., et al. (2014). Severe drought events increase the sensitivity to ozone on poplar clones. Environ. Exp. Bot. 100 94–104. 10.1016/j.envexpbot.2013.12.016 [DOI] [Google Scholar]
- Ramegowda V., Senthil-Kumar M. (2015). The interactive effects of simultaneous biotic and abiotic stresses on plants: mechanistic understanding from drought and pathogen combination. J. Plant Physiol. 176 47–54. 10.1016/j.jplph.2014.11.008 [DOI] [PubMed] [Google Scholar]
- Rao M. V., Davis K. R. (2001). The physiology of ozone and induced cell death. Planta 213 682–690. 10.1007/s004250100618 [DOI] [PubMed] [Google Scholar]
- Rao M. V., Koch J. R., Davis K. R. (2000). Ozone: a tool for probing programmed cell death in plants. Plant Mol. Biol. 44 345–358. 10.1007/978-94-010-0934-8-8 [DOI] [PubMed] [Google Scholar]
- Rejeb I. B., Pastor V., Mauch-Mani B. (2014). Plant responses to simultaneous biotic and abiotic stress: molecular mechanisms. Plants 3 458–475. 10.3390/plants3040458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rejeb K. B., Abdelly C., Savouré A. (2014). How reactive oxygen species and proline face stress together. Plant Physiol. Biochem. 80 278–284. 10.1016/j.plaphy.2014.04.007 [DOI] [PubMed] [Google Scholar]
- Rosales-Serna R., Kohashi-Shibata J., Acosta-Gallegos J. A., Trejo-Lopez C., Ortiz-Cereceres J., Kelly J. D. (2004). Biomass distribution, maturity acceleration and yield in drought-stressed common bean cultivars. Field Crop Res. 85 203–211. 10.1016/S0378-4290(03)00161-8 [DOI] [Google Scholar]
- Roychoudhury A., Paul S., Basu S. (2013). Cross-talk between abscisic acid-dependent and abscisic acid-independent pathways during abiotic stress. Plant Cell Rep. 32 985–1006. 10.1007/s00299-013-1414-5 [DOI] [PubMed] [Google Scholar]
- Santino A., Taurino M., De Domenico S., Bonsegna S., Poltronieri P., Pastor V., et al. (2013). Jasmonate signaling in plant development and defense response to multiple (a)biotic stresses. Plant Cell Rep. 32 1085–1098. 10.1007/s00299-013-1441-2 [DOI] [PubMed] [Google Scholar]
- Schreiber U., Schliwa U., Bilger W. (1986). Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth. Res. 20 51–62. 10.1007/BF00024185 [DOI] [PubMed] [Google Scholar]
- Shigenaga A. M., Argueso C. T. (2016). No hormone to rule them all: interactions of plant hormones during the responses of plants to pathogens. Semin. Cell Dev. Biol. 56 174–189. 10.1016/j.semcdb.2016.06.005 [DOI] [PubMed] [Google Scholar]
- Smirnoff N. (1993). The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol. 125 27–58. 10.1111/j.1469-8137.1993.tb03863.x [DOI] [PubMed] [Google Scholar]
- Szabados L., Savouré A. (2009). Proline: a multifunctional amino acid. Trends Plant Sci. 15 89–97. 10.1016/j.tplants.2009.11.009 [DOI] [PubMed] [Google Scholar]
- Tamaoki M. (2008). The role of phytohormone signaling in ozone-induced cell death in plants. Plant Signal. Behav. 3 166–174. 10.4161/psb.3.3.5538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y., Sano T., Tamaoki M., Nakajima N., Kondo N., Hasezawa S. (2005). Ethylene inhibits abscisic acid-induced stomatal closure in Arabidopsis. Plant Physiol. 138 2337–2343. 10.1104/pp.105.063503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler J. S., Humphrey P. T., Whiteman N. K. (2012). Evolution of jasmonate and salicylate signal crosstalk. Trends Plant Sci. 17 260–270. 10.1016/j.tplants.2012.02.010 [DOI] [PubMed] [Google Scholar]
- Tonelli M., Pellegrini E., D’Angiolillo F., Petersen M., Nali C., Pistelli L., et al. (2015). Ozone-elicited secondary metabolites in shoot cultures of Melissa officinalis L. Plant Cell Tiss. Organ. Cult. 120 617–629. 10.1007/s11240-014-0628-8 [DOI] [Google Scholar]
- Trivellini A., Ferrante A., Vernieri P., Serra G. (2011). Effects of abscisic acid on ethylene biosynthesis and perception in Hibiscus rosa-sinensis L. flower development. J. Exp. Bot. 15 5437–5452. 10.1093/jxb/err218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainonen J. P., Kangasjärvi J. (2015). Plant signalling in acute ozone exposure. Plant Cell Environ. 38 240–252. 10.1111/pce.12273 [DOI] [PubMed] [Google Scholar]
- van Loon L. C., Geraats B. P. J., Linthorst H. J. M. (2006). Ethylene as a modulator of disease resistance in plants. Trends Plant Sci. 11 184–191. 10.1016/j.tplants.2006.02.005 [DOI] [PubMed] [Google Scholar]
- Vitale M., Salvatori E., Loreto F., Fares S., Manes F. (2008). Physiological responses of Quercus ilex leaves to water stress and acute ozone exposure under controlled conditions. Water Air Soil Pollut. 189 113–125. 10.1007/s11270-007-9560-4 [DOI] [Google Scholar]
- Vitti A., Pellegrini E., Nali C., Lovelli S., Sofo A., Valerio M., et al. (2016). Trichoderma harzianum T-22 induces systemic resistance in tomato infected by Cucumber mosaic virus. Front. Plant Sci. 7:1520 10.3389/fpls.2016.01520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Cui X., Sun Y., Dong C.-H. (2013). Ethylene signaling and regulation in plant growth and stress responses. Plant Cell Rep. 32 1099–1109. 10.1007/s00299-013-1421-6 [DOI] [PubMed] [Google Scholar]
- Wang F., Zeng B., Sun Z., Zhu C. (2009). Relationship between proline and Hg21-induced oxidative stress in a tolerant rice mutant. Arch. Environ. Contam. Toxicol. 56 723–731. 10.1007/s00244-008-9226-2 [DOI] [PubMed] [Google Scholar]
- Wilkinson S., Davies W. J. (2002). ABA-based chemical signaling: the co-ordination of responses to stress in plants. Plant Cell Environ. 25 195–210. 10.1046/j.0016-8025.2001.00824.x [DOI] [PubMed] [Google Scholar]
- Wilkinson S., Davies W. J. (2009). Ozone suppresses soil drying- and abscisic acid (ABA)-induced stomatal closure via an ethylene-dependent mechanism. Plant Cell Environ. 32 949–959. 10.1111/j.1365-3040.2009.01970.x [DOI] [PubMed] [Google Scholar]
- Wilkinson S., Davies W. J. (2010). Drought, ozone, ABA and ethylene: new insights from cell to plant to community. Plant Cell Environ. 33 510–525. 10.1111/j.1365-3040.2009.02052.x [DOI] [PubMed] [Google Scholar]
- Wingler A., Roitsch T. (2008). Metabolic regulation of leaf senescence: interactions of sugar signaling with biotic and abiotic stress responses. Plant Biol. 10 50–62. 10.1111/j.1438-8677.2008.00086.x [DOI] [PubMed] [Google Scholar]
- Wohlgemuth H., Mittelstrass K., Kschieschan S., Bender J., Weigel H.-J., Overmyer K., et al. (2002). Activation of an oxidative burst is a general feature of sensitive plants exposed to the air pollutant ozone. Plant Cell Environ. 25 717–726. 10.1046/j.1365-3040.2002.00859.x [DOI] [Google Scholar]
- Xia X.-J., Zhou Y.-H., Shi K., Zhou J., Foyer C. H., Yu J. Q. (2015). Interplay between reactive oxygen species and hormones in the control of plant development and stress tolerance. J. Exp. Bot. 66 2839–2856. 10.1093/jxb/erv089 [DOI] [PubMed] [Google Scholar]
- Xu E., Vaahtera L., Brosché M. (2015). Roles of defense hormones in the regulation of ozone-induced changes in gene expression and cell death. Mol. Plant 8 1776–1794. 10.1016/j.molp.2015.08.008 [DOI] [PubMed] [Google Scholar]
- Yang N., Wang X., Cotrozzi L., Chen Y., Zheng F. (2016). Ozone effects on photosynthesis of ornamental species suitable for urban green spaces of China. Urban For. Urban Gree. 20 437–447. 10.1016/j.ufug.2016.10.014 [DOI] [Google Scholar]
- Zhang L., Becker D. F. (2015). Connecting proline metabolism and signaling pathways in plant senescence. Front. Plant Sci. 6:552 10.3389/fpls.2015.00552 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


