Abstract
Background: Regardless of its volume, hemoptysis is a concerning symptom. Mild hemoptysis and its significance in patients with solid malignancies has not been studied.
Methods: We conducted a retrospective chart review of patients with solid malignancies who presented for evaluation of mild hemoptysis. In this population, we studied the impact of bronchoscopic findings and endobronchial therapies on overall survival and bleeding recurrence. Patients were categorized into four groups on the basis of the presence or absence of active bleeding and endobronchial disease at the time of initial bronchoscopy: active bleeding with endobronchial lesion (AB/EBL), active bleeding without endobronchial lesion (AB/no-EBL), absence of active bleeding but with endobronchial lesion (no-AB/EBL), and absence of active bleeding and endobronchial lesion (no-AB/no-EBL).
Measurements and Main Results: Ninety-five of the 112 patients with solid malignancies and mild hemoptysis underwent bronchoscopies. There was a significantly lower median survival time for patients with bronchoscopic findings of active bleeding and endobronchial lesion compared with patients with no active bleeding and/or no endobronchial lesion (3.48 mo; 95% confidence interval [CI], 2.14–6.05). On a multivariate analysis, factors independently associated with improved survival were higher hemoglobin values (hazard ratio [HR], 0.78; 95% CI, 0.67–0.91) and cessation of hemoptysis without recurrence at 48 hours (HR, 0.43; 95% CI, 0.22–0.84). Variables independently associated with worse survival were disease stage (HR, 10.8; 95% CI, 2.53–46.08) and AB/EBL (HR, 3.20; 95% CI, 1.74–5.89).
Conclusions: In patients with solid malignancies presenting with mild hemoptysis, bronchoscopic findings of AB/EBL are associated with decreased survival. Hemoptysis control without recurrence at 48 hours after endobronchial intervention may improve survival.
Keywords: hemoptysis, cancer, bronchoscopy
Hemoptysis is a distressing symptom for patients with or without a known history of cancer. It disrupts patients’ quality of life and provokes significant anxiety. For these reasons, evaluation and treatment of mild hemoptysis is important (1).
Morbidity and mortality in patients with hemoptysis depend not only on the volume of expectorated blood but also on the rate of bleeding, the ability of the patient to clear blood from the airways, and the extent and severity of underlying lung disease. A large body of literature on hemoptysis exists for the general population without known history of cancer (1–11). However, most studies have focused on massive hemoptysis because of its high mortality rate (2). Mild hemoptysis may be considered less meaningful because < 20% is due to an underlying malignancy and classically ascribed to inflammatory disease, such as acute or chronic bronchitis (1, 3, 4, 8, 12). Mild or minor hemoptysis is generally defined as blood-tinged, blood-streaked sputum; small blood clots within the sputum; or hemoptysis that is < 20 ml in 24 hours (3, 4).
We conducted this study to better understand the association of bronchoscopic findings and overall survival of patients with solid malignancies presenting with mild hemoptysis. We also wanted to evaluate the impact of invasive treatments on bleeding recurrence and outcomes in this population.
Methods
The University of Texas MD Anderson Cancer Center Institutional Review Board approved this study (protocol DR08–0273).
We conducted a retrospective chart review of inpatients and outpatients with solid malignancies referred to the departments of Pulmonary Medicine and Thoracic and Cardiovascular Surgery at The University of Texas MD Anderson Cancer Center for evaluation of and treatment for hemoptysis from November 2003 to July 2007.
Subjects and Data Collection
We defined mild hemoptysis as blood-tinged, blood-streaked sputum or small blood clots within the sputum. All patients with solid malignancies and mild hemoptysis were included. Patients with hematologic malignancies were excluded. Patients in whom hemoptysis exceeded the above definition were also excluded.
A diagnosis of acute bronchitis was made if patients presented with cough with or without phlegm production lasting for up to 3 weeks with no infiltrate on chest X-ray.
The diagnosis of pneumonia was made on patients who were given a new antibiotic prescription and had at least two of the following findings: new infiltrate on radiographic imaging; increase in cough; and increase in purulent sputum, fever, white blood cell count, or neutropenia.
For the patients who met the inclusion criteria, data on demographics, tumor histology, comorbid conditions, smoking history, Eastern Cooperative Oncology Group (ECOG) performance status, and treatment with antiplatelet or anticoagulant therapy were extracted from the patient records. Data on the stage of the disease were collected. We categorized the patients into early versus advanced disease based on their stage using the TNM system (early stage TNM stage I and II and advanced TNM stage III and IV). The results of laboratory studies, including renal function, hemoglobin values, platelet counts, and coagulation profile, were collected. Bronchoscopy date, time from onset of hemoptysis to bronchoscopy, and findings at bronchoscopy were also noted.
The treatment modalities for hemoptysis and results of the intervention were collected. We also recorded whether hemoptysis had stopped and not recurred 48 hours after bronchoscopy.
Overall survival (OS) was calculated as the time (in months) from the date of bronchoscopy to the date of death or censoring. Survival was censored at the date of last follow-up if death had not been reported.
Statistical Analysis
Summary statistics were used to describe the clinical and demographic characteristics of the study population. Wilcoxon rank-sum tests or t tests were used to assess differences between patients with and without active bleeding for continuous variables.
We used Pearson’s χ2 test or Fisher’s exact test to assess differences between patients with and without active bleeding for categorical variables.
Univariate Cox proportional hazards regression models were used to determine the association between each potential prognostic factor and OS. The Kaplan-Meier product limit method was used to estimate the median OS. Full and reduced multivariate Cox proportional hazards regression models were used to assess the prognostic factors and OS. Variables considered clinically relevant and those with P value of ≤ 0.10 in the univariate analysis were included in the multivariate model. A backward elimination strategy was used to choose the most parsimonious model. A P value of < 0.05 was considered statistically significant.
Statistical analysis was performed using STATA/SE version 12.1 statistical software (Stata Corp., College Station, TX).
Results
Patient Characteristics
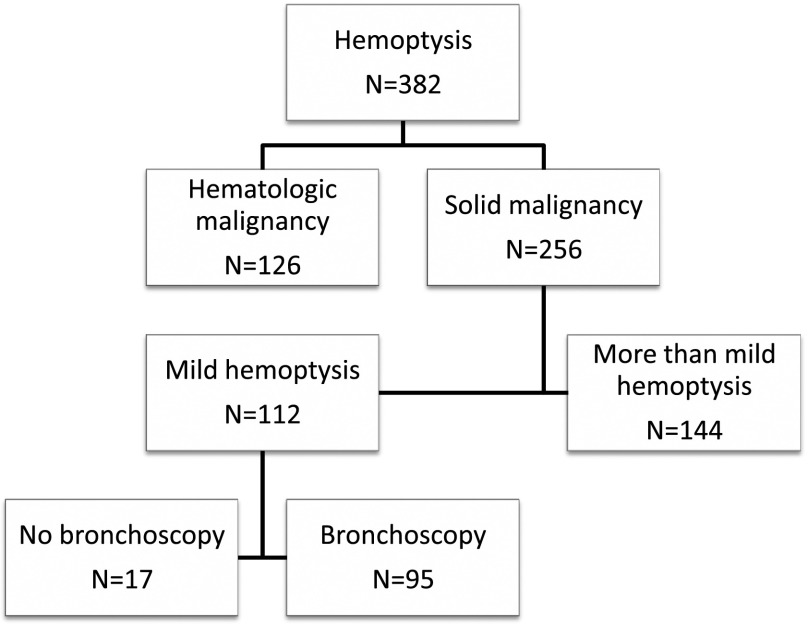
A total of 382 patients with hemoptysis were evaluated from November 2003 to July 2007; 126 had hematologic malignancy upon referral, and 256 had a solid malignancy. Of those with a solid malignancy, 112 (44%) had mild hemoptysis, and 95 (85%) underwent bronchoscopy (Figure 1).
Figure 1.
Number of patients evaluated and number of patients who underwent bronchoscopy.
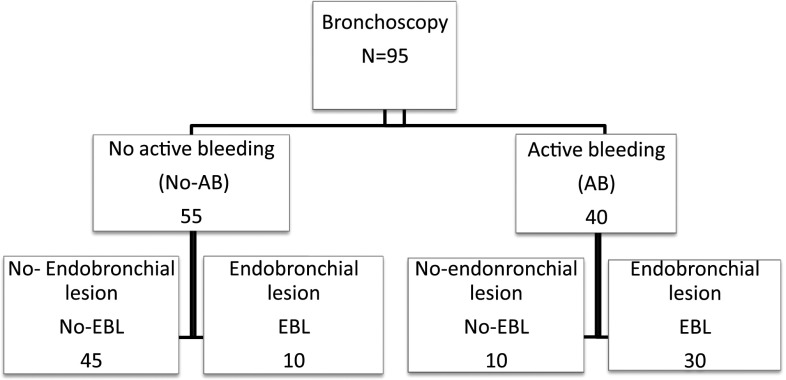
The 95 patients who underwent bronchoscopy were categorized into two main groups based on the finding of active bleeding (AB) or absence of active bleeding (no-AB) at the time of initial bronchoscopic evaluation. These two groups were further divided into the following subgroups: active bleeding with endobronchial lesion (AB/EBL), active bleeding without endobronchial lesion (AB/no-EBL), absence of active bleeding but with endobronchial lesion (no-AB/EBL), and absence of active bleeding and endobronchial lesion (no-AB/no-EBL) (Figure 2).
Figure 2.
Number of patients with active bleeding (AB)/no active bleeding (no-AB) and endobronchial lesion (EBL)/no endobronchial lesion (no-EBL).
Table 1 illustrates a comparison of the baseline characteristics of the patients with and without active bleeding. The most common malignancy was lung cancer, comprising 48% of patients. In the no-AB group, there were 26 patients with non–small lung cancer staged at the time of bronchoscopy as stage I (no. 4), stage III (no. 11), and stage IV (no. 11). In the AB group, there were 20 patients with non–small lung cancer staged at the time of bronchoscopy as stage I (no. 2), stage III (no. 7), and stage IV (no. 11). There was one patient with small cell lung cancer in the no-AB group, and there were five patients in the AB group.
Table 1.
Summary statistics of clinical and demographic characteristics
|
Active
Bleeding |
P Value | ||
|---|---|---|---|
| Yes | No | ||
| Age, yr | 40 | 55 | |
| Median (range) | 61.6 (23.9–82.1) | 61.1 (20.3–86.9) | |
| Gender, n (%) | |||
| Male | 24 (60.0) | 23 (41.8) | |
| Female | 16 (40.0) | 32 (58.2) | |
| Ethnicity, n (%) | |||
| White | 29 (72.5) | 42 (76.4) | |
| Black | 6 (15.0) | 7 (12.7) | |
| Hispanic | 3 (7.5) | 5 (9.1) | |
| Asian | 2 (5.0) | 1 (1.8) | |
| Basal malignancy, n (%) | |||
| Lung | 20 (50.0) | 26 (47.3) | |
| Metastatic cancer | 20 (50.0) | 29 (52.7) | |
| Smoking, n (%) | |||
| Positive | 7 (17.5) | 15 (27.3) | |
| Negative | 33 (82.5) | 40 (72.7) | |
| Heart disease, n (%) | |||
| No | 25 (62.5) | 27 (49.1) | |
| Yes | 15 (37.5) | 28 (50.9) | |
| Lung disease, n (%) | |||
| No | 19 (47.5) | 23 (41.8) | |
| Yes | 21 (52.5) | 32 (58.2) | |
| Kidney disease, n (%) | |||
| No | 38 (95.0) | 52 (94.5) | |
| Yes | 2 (5.0) | 3 (5.5) | |
| Liver disease, n (%) | |||
| No | 39 (97.5) | 54 (98.2) | |
| Yes | 1 (2.5) | 1 (1.8) | |
| Radiation therapy to chest, n (%) | |||
| No | 27 (67.5) | 37 (67.3) | |
| Yes | 13 (32.5) | 18 (32.7) | |
| Antiplatelet therapy, n (%) | |||
| No | 32 (80.0) | 45 (81.8) | |
| Yes | 8 (20.0) | 8 (14.5) | |
| Anticoagulant therapy, n (%) | |||
| No | 38 (95.0) | 52 (94.5) | |
| Yes | 2 (5.0) | 2 (3.6) | |
| Hemoglobin, g/dl | 0.124* | ||
| n | 38 | 48 | |
| Mean (SD) | 11.5 (1.8) | 12.1 (2.1) | |
| PTT | 0.466* | ||
| n | 37 | 49 | |
| Mean (SD) | 28.4 (3.4) | 29.0 (4.0) | |
| INR | 0.103† | ||
| n | 37 | 50 | |
| Median (range) | 1.1 (0.8–1.5) | 1.1 (0.8–3.3) | 0.103† |
| Platelets | |||
| n | 38 | 50 | |
| Mean (SD) | 320.1 (136.9) | 277.2 (107.3) | |
| Median | 288.5 | 273.5 | |
| Creatinine, mg/dl | 0.060† | ||
| n | 38 | 52 | |
| Median (range) | 0.9 (0.5–1.8) | 1.0 (0.5–2.3) | |
| Time from hemoptysis onset to consultation, d | 0.265† | ||
| n | 40 | 54 | |
| Median (range) | 14.0 (1.0–180.0) | 14.0 (1.0–150.0) | |
| Time from consultation to bronchoscopy, d | 0.073† | ||
| n | 40 | 55 | |
| Median (range) | 1.0 (0.5–7.0) | 1.0 (0.5–14.0) | |
| ECOG PS, n (%) | 0.197‡ | ||
| PS 0 | 3 (7.5) | 7 (12.7) | |
| PS 1 | 17 (42.5) | 32 (58.2) | |
| PS 2 | 13 (32.5) | 12 (21.8) | |
| PS 3 | 7 (17.5) | 4 (7.3) | |
| Resolved in 48 h, n (%) | 0.068‡ | ||
| No | 12 (30.0) | 8 (14.5) | |
| Yes | 28 (70.0) | 47 (85.5) | |
| Blood transfusion, n (%) | 0.229 | ||
| No | 35 (89.7) | 53 (96.4) | |
| Yes | 4 (10.3) | 2 (3.6) | |
| Endobronchial lesion, n (%) | <0.001§ | ||
| No | 10 (25.0) | 45 (81.8) | |
| Yes | 30 (75.0) | 10 (18.2) | |
| Stage, n (%) | 0.010‡ | ||
| Early | 3 (7.5) | 16 (29.1) | |
| Advanced | 37 (92.5) | 39 (70.9) | |
Definition of abbreviations: ECOG = Eastern Cooperative Oncology Group; INR = international normalized ratio; PS = performance status; PTT = partial thromboplastin time.
t Test.
Wilcoxon rank-sum test.
Fisher’s exact test.
Pearson chi-square test.
In the no-AB group, there were 10 patients with localized non–lung cancer solid malignancies and 18 with advanced non–lung cancer solid malignancies. In the AB group, there was one patient with a localized non–lung cancer solid malignancy, and there were 14 with advanced non–lung cancer solid malignancies. Non–lung cancer solid malignancies included laryngeal carcinoma, thyroid carcinoma, breast carcinoma, renal carcinoma, melanoma, mesothelioma, prostate carcinoma, colorectal carcinoma, sarcoma, cervical carcinoma, parotid carcinoma, pancreas, adenocarcinoma of unknown origin, chordoma, malignant ciliary epithelioma, thymoma, carcinoma of the mouth, hepatocellular carcinoma, nasopharyngeal carcinoma, and bladder carcinoma.
No differences in age, sex, ethnicity, comorbid conditions, smoking history, or antiplatelet/anticoagulant therapy were found between the AB and the no-AB groups. Also, no differences were observed between the groups on values of renal function, platelet counts, and coagulation profiles. The ECOG performance status of most patients was between 1 and 2.
Bronchoscopic evidence of endobronchial lesion was found more often in the AB group than in the no-AB group (75 vs. 25%; P < 0.001). Additionally, patients in the AB group were more likely to have advanced stage disease.
Bronchitis and pneumonia as a cause of mild hemoptysis was found in 8 out of 95 patients.
In patients who had evidence of metastatic disease to the chest on radiographic imaging, we presumed the bleeding was from distal metastatic lung parenchymal lesions once all other causes of bleeding were ruled out.
Treatment Modalities
In the AB/EBL group (n = 30), bleeding was managed bronchoscopically in 26 (87%) patients. Argon plasma coagulation (APC) was used in 25 patients, and neodymium-doped yttrium aluminum perovskite lasers were used in the remaining patient. Endoscopic treatment resulted in immediate cessation of hemoptysis without recurrence during the following 48 hours in 23 (88%) of these 26 patients. All three patients who had recurrence of bleeding at 48 hours were successfully treated a second time (two patients with APC and one with arterial embolization).
In the AB/no-EBL group (n = 10), one patient was effectively treated with bronchial artery embolization, and this patient had no evidence of recurrence at 48 hours after treatment. Nine patients did not undergo intervention for mild hemoptysis. In five (55%) of these patients, hemoptysis resolved with medical treatment at 48 hours after presentation.
In the no-AB/EBL group (n = 10), one patient underwent APC treatment to the endobronchial lesion that had stigmata of prior bleed and had no recurrence of bleeding at 48 hours after therapy. Of nine patients who did not undergo therapeutic intervention, three (33%) had a recurrence of bleeding by 48 hours after presentation.
In the no-AB/no-EBL group (n = 45), none of the patients underwent endobronchial intervention, and five (11%) patients had recurrence of bleeding at 48 hours after presentation.
The presumed bleeding source in most patients without EBL was distal airway metastatic disease; only eight patients were treated as bronchitis or pneumonia as a cause of mild hemoptysis.
Overall Survival
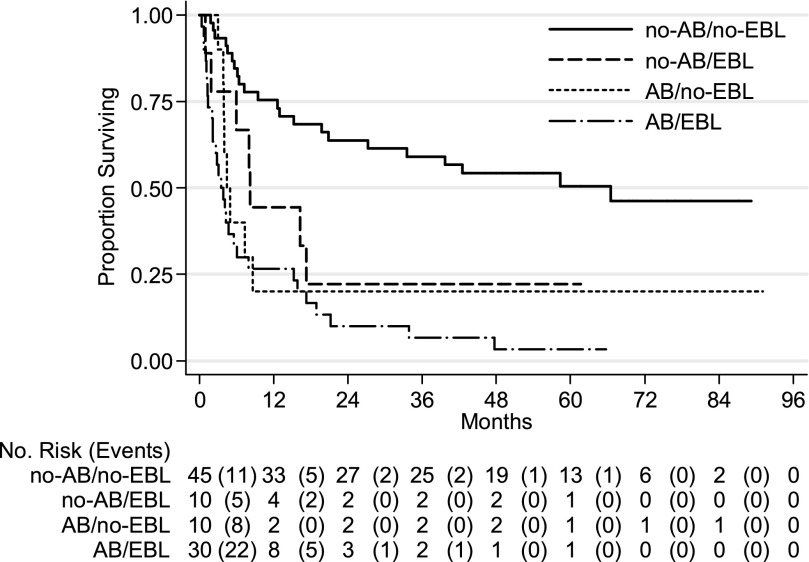
The median survival times for patients in the subgroups were as follows: AB/EBL, 3.48 months (95% CI, 2.14–6.05); AB/no-EBL, 4.40 months (95% CI, 2.99–8.54); no-AB/EBL, 8.15 months (95% CI, 0.95–NE); and no-AB/no-EBL, 66.50 months (95% CI, 20.80–NE) (Table 2).
Table 2.
Univariate COX proportional survival analysis
| No. of Points | No. of Events | Median Survival | 95% CI for Median | HR | 95% CI for HR | P Value | |
|---|---|---|---|---|---|---|---|
| Age, yr | |||||||
| Unit change | 95 | 66 | 12.62 | 6.05–19.75 | 1.00 | 0.99–1.02 | 0.618 |
| Hemoglobin, g/dl | |||||||
| Unit change | 95 | 66 | 12.62 | 6.05–19.75 | 0.78 | 0.68–0.88 | <0.001 |
| PTT | |||||||
| Unit change | 95 | 66 | 12.62 | 6.05–19.75 | 0.96 | 0.89–1.03 | 0.244 |
| INR | |||||||
| Unit change | 95 | 66 | 12.62 | 6.05–19.75 | 1.15 | 0.64–2.06 | 0.646 |
| Platelets | |||||||
| <50,000 | 2 | 2 | 2.99 | 2.99–NE | 1.00 | 0.08–1.34 | 0.118 |
| ≥50,000 | 86 | 59 | 12.62 | 6.05–18.86 | 0.32 | ||
| Creatinine, mg/dl | |||||||
| Unit change | 95 | 66 | 12.62 | 6.05–19.75 | 0.70 | 0.32–1.54 | 0.373 |
| Time for hemoptysis onset to consultation, d | |||||||
| Unit change | 95 | 66 | 12.62 | 6.05–19.75 | 0.99 | 0.99–1.00 | 0.189 |
| Time to bronchoscopy, d | |||||||
| Unit change | 95 | 66 | 12.62 | 6.05–19.75 | 0.91 | 0.82–1.00 | 0.057 |
| Gender | |||||||
| Male | 47 | 36 | 6.14 | 3.78–16.23 | 1.00 | ||
| Female | 48 | 30 | 17.28 | 7.20–66.50 | 0.61 | 0.37–0.99 | 0.044 |
| Ethnicity | |||||||
| White | 71 | 47 | 15.84 | 5.59–33.87 | 1.00 | ||
| Black | 13 | 10 | 6.34 | 3.48–9.40 | 1.42 | 0.71–2.81 | 0.321 |
| Hispanic | 8 | 7 | 6.14 | 0.69–20.80 | 1.58 | 0.71–3.51 | 0.265 |
| Asian | 3 | 2 | 17.28 | 17.28–NE | 1.08 | 0.26–4.46 | 0.913 |
| Basal malignancy | |||||||
| Lung cancers | 46 | 33 | 7.33 | 4.93–16.23 | 1.00 | ||
| Other cancers | 49 | 33 | 17.25 | 5.29–47.70 | 0.80 | 0.49–1.30 | 0.362 |
| Smoking | |||||||
| Positive | 22 | 15 | 17.28 | 4.24–NE | 1.00 | ||
| Negative | 73 | 51 | 7.89 | 5.55–18.86 | 1.27 | 0.71–2.25 | 0.423 |
| Heart | |||||||
| No | 52 | 38 | 12.88 | 5.98–20.80 | 1.00 | ||
| Yes | 43 | 28 | 8.54 | 5.29–42.48 | 0.89 | 0.55–1.45 | 0.641 |
| Lung | |||||||
| No | 42 | 33 | 7.20 | 4.24–17.25 | 1.00 | ||
| Yes | 53 | 33 | 20.80 | 6.34–58.38 | 0.63 | 0.39–1.03 | 0.064 |
| Kidney | |||||||
| No | 90 | 62 | 12.62 | 6.05–18.86 | 1.00 | ||
| Yes | 5 | 4 | 19.75 | 2.99–NE | 1.07 | 0.39–2.93 | 0.901 |
| Liver | |||||||
| No | 93 | 64 | 12.62 | 6.05–19.75 | 1.00 | ||
| Yes | 2 | 2 | 6.14 | 6.14–NE | 1.36 | 0.33–5.59 | 0.668 |
| Antiplatelet therapy | |||||||
| No | 77 | 56 | 8.54 | 5.59–17.25 | 1.00 | ||
| Yes | 16 | 10 | 18.86 | 3.48–NE | 0.73 | 0.37–1.44 | 0.368 |
| Anticoagulant therapy | |||||||
| No | 90 | 62 | 12.88 | 6.14–19.75 | 1.00 | ||
| Yes | 4 | 4 | 1.87 | 1.02–NE | 2.62 | 0.95–7.24 | 0.063 |
| ECOG PS | |||||||
| PS 0 | 10 | 4 | NR | 7.98–NE | 1.00 | ||
| PS 1 | 49 | 32 | 20.80 | 6.34–58.38 | 2.29 | 0.81–6.47 | 0.119 |
| PS 2 | 25 | 21 | 5.98 | 3.81–9.40 | 4.35 | 1.48–12.76 | 0.007 |
| PS 3 | 11 | 9 | 2.99 | 1.12–12.62 | 6.51 | 1.98–21.41 | 0.002 |
| Active bleeding | |||||||
| No-AB | 55 | 29 | 42.48 | 16.23-NE | 1.00 | ||
| AB | 40 | 37 | 4.01 | 2.99-5.55 | 3.65 | 2.22–6.02 | <0.001 |
| Blood transfusion | |||||||
| No | 88 | 60 | 15.24 | 7.20-21.19 | 1.00 | ||
| Yes | 6 | 5 | 2.99 | 0.66-NE | 2.27 | 0.91–5.69 | 0.080 |
| Resolved in 48 h | |||||||
| No | 20 | 17 | 4.40 | 2.99-7.89 | 1.00 | ||
| Yes | 75 | 49 | 16.23 | 7.98-39.75 | 0.50 | 0.28–0.87 | 0.014 |
| Active bleeding and endobronchial lesion | |||||||
| No-AB/no-EBL | 45 | 22 | 66.50 | 20.80-NE | 1.00 | ||
| No-AB/EBL | 10 | 7 | 8.15 | 0.95-NE | 2.40 | 1.02–5.66 | 0.045 |
| AB/no-EBL | 10 | 8 | 4.40 | 2.99-8.54 | 2.96 | 1.31–6.71 | 0.009 |
| AB/EBL | 30 | 29 | 3.48 | 2.14-6.05 | 4.93 | 2.78–8.74 | <0.001 |
| Endobronchial lesion | |||||||
| No-EBL | 55 | 30 | 39.75 | 12.62-NE | 1.00 | ||
| EBL | 40 | 36 | 4.21 | 2.14-7.98 | 3.30 | 2.01–5.44 | <0.001 |
| Stage | |||||||
| Early | 19 | 4 | NR | 66.50-NE | 1.00 | ||
| Advanced | 76 | 62 | 6.14 | 4.40-12.62 | 8.81 | 3.17–24.46 | <0.001 |
Definition of abbreviations: AB = active bleeding; CI = confidence interval; EBL = endobronchial lesion; ECOG = Eastern Cooperative Oncology Group; INR = international normalized ratio; NE = not estimable; NR = not reached; PS = performance status; PTT = partial thromboplastin time.
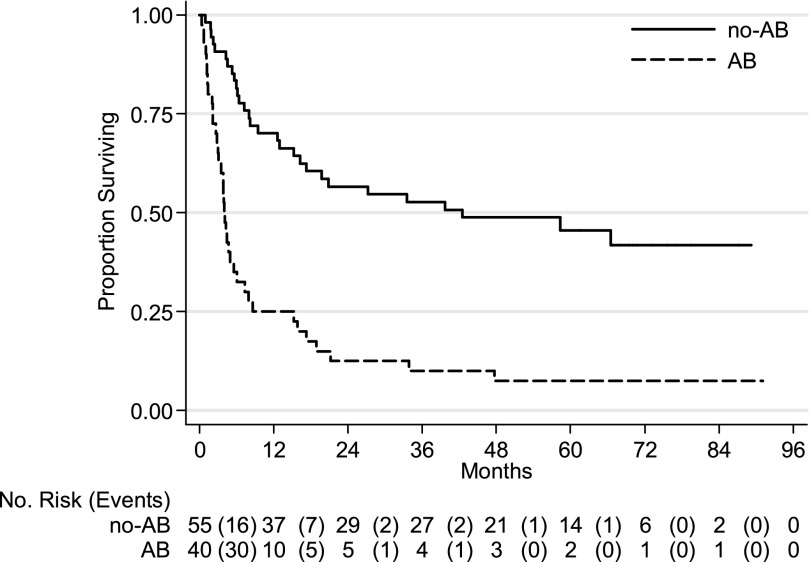
Hazard ratios in the multivariate Cox survival analysis were significantly higher for the AB/EBL group compared with the no-AB/no-EBL group; the HRs of the no-AB/EBL and AB/no-EBL groups were not statistically different when compared with the no-AB/no-EBL group. As expected in the multivariate analysis, advanced stage disease was associated with worse survival (HR, 10.80; 95% CI, 2.53–46.08). Factors independently associated with improved survival in the multivariate analysis were higher hemoglobin levels (HR, 0.78; 95% CI. 0.67–0.91) and cessation of hemoptysis without recurrence at 48 hours after bronchoscopy (HR, 0.43; 95% CI, 0.22–0.84) (Table 3). Kaplan-Meier survival estimation curves for patients in the AB and no-AB groups are shown in Figure 3; curves for AB/EBL, AB/no-EBL, no-AB/EBL, and no-AB/no-EBL are shown in Figure 4.
Table 3.
Multivariate COX proportional survival analysis
| Full Model* |
Reduced Model† |
|||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age | ||||
| Unit change | 1.01 (0.99–1.03) | 0.379 | ||
| Hemoglobin, g/dl | ||||
| Unit change | 0.83 (0.71–0.98) | 0.025 | 0.78 (0.67–0.91) | 0.001 |
| Time from consultation to bronchoscopy, d | ||||
| Unit change | 1.00 (0.99–1.01) | 0.689 | ||
| Gender | ||||
| Male | ||||
| Female | 0.66 (0.37–1.17) | 0.152 | ||
| Heart | ||||
| No | ||||
| Yes | 0.89 (0.47–1.70) | 0.724 | ||
| Anticoagulant therapy | ||||
| No | ||||
| Yes | 3.07 (0.97–9.69) | 0.056 | ||
| ECOG PS | ||||
| PS 0 | ||||
| PS 1 | 2.00 (0.54–7.36) | 0.298 | ||
| PS 2 | 3.61 (0.88–14.86) | 0.075 | ||
| PS 3 | 6.28 (1.28–30.78) | 0.023 | ||
| Stage | ||||
| Early | ||||
| Advanced | 9.53 (2.09–43.41) | 0.004 | 10.80 (2.53 –46.08) | 0.001 |
| Active bleeding and endobrochial lesion | ||||
| no-AB/no-EBL | ||||
| no-AB/EBL | 1.58 (0.57–4.40) | 0.383 | 1.29 (0.53 –3.16) | 0.572 |
| AB/no- EBL | 3.24 (1.16–9.04) | 0.025 | 2.40 (0.98 –5.85) | 0.055 |
| AB/EBL | 2.80 (1.37–5.72) | 0.005 | 3.20 (1.74 –5.89) | <0.001 |
| Resolved in 48 h | ||||
| No | ||||
| Yes | 0.66 (0.29–1.46) | 0.302 | 0.43 (0.22–0.84) | 0.014 |
| Blood transfusion | 0.84 (0.28–2.54) | 0.759 | ||
| No | ||||
| Yes | ||||
P < 0.10 in the univariate analysis.
P < 0.05 in the univariate analysis.
Figure 3.
Kaplan-Meier survival estimation curves for patients in the active bleeding (AB) and no active bleeding (no-AB) groups (P < 0.001).
Figure 4.
Kaplan-Meier survival estimation curves for patients in the groups with active bleeding with endobronchial lesion (AB/EBL), active bleeding with no endobronchial lesion (AB/no-EBL), no active bleeding with endobronchial lesion (no-AB/EBL), and no active bleeding and no endobronchial lesion (no-AB/no-EBL) (P < 0.001).
Discussion
This study suggests that the presence of active bleeding and endobronchial lesion during the initial bronchoscopic evaluation of patients with mild hemoptysis and advanced stage diseases is independently associated with decreased survival. Higher hemoglobin levels at the time of bronchoscopy and bleeding control at 48 hours were significantly associated with improved survival. No other statistically significant associations between outcome and other prognostic factors were found.
Our study shows that patients with lung cancer and non–lung cancer solid malignancies presenting with mild hemoptysis are very likely to have active bleeding and endobronchial disease that is identifiable at bronchoscopy and amenable to bronchoscopic therapies. Etiologies of hemoptysis reported in various series depend on the year of the publication and the health care environment and geographic location of patients. Ours is the first study addressing the significance of mild hemoptysis in patients with a known history of a solid malignancy.
Hirshberg and colleagues reviewed 208 patients without history of malignancy who presented with hemoptysis at a university hospital in Jerusalem, Israel, between 1980 and 1995 (3). Of these, 80 patients presented with mild hemoptysis, defined similarly as in our study. The most common cause of mild hemoptysis reported in this publication was bronchitis, followed in decreasing frequency by pneumonia, lung cancer, and bronchiectasis. These authors reported “positive results” after bronchoscopy in 54% of patients with mild hemoptysis. However, neither the definition of “positive results” nor the bronchoscopic findings was described. Also, although 18% of these patients with mild hemoptysis were diagnosed with lung cancer, the presence or absence of endobronchial disease was not reported.
Johnston and colleagues retrospectively reviewed 148 patients with hemoptysis who underwent diagnostic bronchoscopy (1). Seventy-two patients had mild hemoptysis, defined as < 20 ml per 24 hours. Similar to the study by Hirshberg and colleagues (3), the most common cause of mild hemoptysis in this publication was bronchitis (47%).
In our study, 46% of the patients had endobronchial disease visible by bronchoscopy, and 42% had active bleeding at the time of bronchoscopy. Only 8 of these 95 patients in our cohort had bronchitis or pneumonia as a cause of mild hemoptysis, which is a much lower frequency than described for the general population. In patients who had evidence of metastatic disease to the chest without other clear cause for bleeding, we presumed that the bleeding was from distal metastatic lesions. Our findings are different from those reported in the literature because our studied population included only patients with a known history of a solid malignancy.
Knowing the site of bleeding is critical for managing hemoptysis, and bronchoscopy is essential to accomplishing this goal. Not only the bronchoscopic intervention itself can be therapeutic, but we believe the bronchoscopic findings can help plan additional measures in case the bleeding increases or assist in guiding bronchial artery embolization procedures if necessary. In the current study, bronchoscopic treatments for bleeding control were implemented when active bleeding and endobronchial lesions were identified. Of these, APC was most commonly used. Similar to a previous report from our group, APC was successful for achieving hemostasis in most cases (13).
Hemoptysis is a common symptom in patients with intrathoracic tumors and one that is responsible for significant alteration in quality of life (17). Given the overall poor outcome of bronchopulmonary malignancies, symptom palliation is of utmost importance.
The observed decreased survival in the AB/EBL group could be a reflection of advanced disease rather than the presence of active bleeding and endobronchial lesion during bronchoscopy. However, after adjusting for different factors, including disease stage, AB/EBL remained as an independent risk factor for worse survival. We hypothesize that central airway involvement by metastatic disease has a worse prognosis than parenchymal lung metastasis. Smaller, centrally located tumors can result in major complications, such as respiratory insufficiency from airway obstruction, postobstructive pneumonia, and asphyxiation from bleeding. In contrast, distal parenchymal metastatic disease often remains without symptoms or complications until tumor burden is large. Bleeding control without recurrence at 48 hours after bronchoscopy had a favorable effect on survival in our study, and endobronchial treatment was also effective to alleviate hemoptysis. One of the strengths of our study is that it consists of data averaged over a long period (mature cohort) instead of single-year data. This is useful for reducing measurement error and capturing a long-term effect. Also, our database accurately recorded all the predictive variables considered important for this multivariate analysis. Although the 48-hour cut off for bleeding cessation is not meant to predict recurrence of hemoptysis, it is clinically useful to define prognosis shortly after bronchoscopy.
Our study is subject to the potential limitations of the use of observational data to evaluate the effects of outcomes and therapy: it is possible that the observed results are due to unmeasured confounders. Future studies will have to test the potential associations we found on our report. In spite of its retrospective nature, our study is the first to offer information on the survival of patients with solid malignancy presenting with mild hemoptysis. Finally, our study is based on a selected patient population attending a dedicated cancer center, which probably represents a self-selection and different threshold to request and accept advanced interventions when compared with the general population.
In summary, patients with an underlying solid malignancy of any origin who present with mild hemoptysis and have active bleeding and endobronchial lesions during bronchoscopy have decreased survival. Furthermore, these patients are likely to benefit from palliative interventions and should be approached differently than patients with mild hemoptysis without a history of malignancy. Bronchoscopy should be always considered in these patients as part of the diagnostic and prognostic investigations and for possible palliative treatments.
Footnotes
Author Contributions: C.A.J. and R.F.C. conceived the study. R.F.C. and H.B.G. collected the data. C.A.J., R.F.C., and G.M.N.-G performed the statistical analysis. All authors participated in the design of the study and helped to draft the manuscript. All authors read and approved the final manuscript.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Kvale PA, Simoff M, Prakash UB. American College of Chest Physicians. Palliative care. Chest. 2003;123(Suppl):284S–311S. doi: 10.1378/chest.123.1_suppl.284s. [DOI] [PubMed] [Google Scholar]
- 2.Amirana M, Frater R, Tirschwell P, Janis M, Bloomberg A, State D. An aggressive surgical approach to significant hemoptysis in patients with pulmonary tuberculosis. Am Rev Respir Dis. 1968;97:187–192. doi: 10.1164/arrd.1968.97.2.187. [DOI] [PubMed] [Google Scholar]
- 3.Hirshberg B, Biran I, Glazer M, Kramer MR. Hemoptysis: etiology, evaluation, and outcome in a tertiary referral hospital. Chest. 1997;112:440–444. doi: 10.1378/chest.112.2.440. [DOI] [PubMed] [Google Scholar]
- 4.Johnston H, Reisz G. Changing spectrum of hemoptysis. Underlying causes in 148 patients undergoing diagnostic flexible fiberoptic bronchoscopy. Arch Intern Med. 1989;149:1666–1668. doi: 10.1001/archinte.149.7.1666. [DOI] [PubMed] [Google Scholar]
- 5.Tsoumakidou M, Chrysofakis G, Tsiligianni I, Maltezakis G, Siafakas NM, Tzanakis N. A prospective analysis of 184 hemoptysis cases: diagnostic impact of chest X-ray, computed tomography, bronchoscopy. Respiration. 2006;73:808–814. doi: 10.1159/000091189. [DOI] [PubMed] [Google Scholar]
- 6.Unsal E, Köksal D, Cimen F, Taci Hoca N, Sipit T. Analysis of patients with hemoptysis in a reference hospital for chest diseases. Tuberk Toraks. 2006;54:34–42. [PubMed] [Google Scholar]
- 7.Gong H, Jr, Salvatierra C. Clinical efficacy of early and delayed fiberoptic bronchoscopy in patients with hemoptysis. Am Rev Respir Dis. 1981;124:221–225. doi: 10.1164/arrd.1981.124.3.221. [DOI] [PubMed] [Google Scholar]
- 8.Ibrahim WH. Massive haemoptysis: the definition should be revised. Eur Respir J. 2008;32:1131–1132. doi: 10.1183/09031936.00080108. [DOI] [PubMed] [Google Scholar]
- 9.Shigemura N, Wan IY, Yu SC, Wong RH, Hsin MK, Thung HK, Lee TW, Wan S, Underwood MJ, Yim AP. Multidisciplinary management of life-threatening massive hemoptysis: a 10-year experience. Ann Thorac Surg. 2009;87:849–853. doi: 10.1016/j.athoracsur.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Thompson AB, Teschler H, Rennard SI. Pathogenesis, evaluation, and therapy for massive hemoptysis. Clin Chest Med. 1992;13:69–82. [PubMed] [Google Scholar]
- 11.Santiago S, Tobias J, Williams AJ. A reappraisal of the causes of hemoptysis. Arch Intern Med. 1991;151:2449–2451. [PubMed] [Google Scholar]
- 12.Jackson CV, Savage PJ, Quinn DL. Role of fiberoptic bronchoscopy in patients with hemoptysis and a normal chest roentgenogram. Chest. 1985;87:142–144. doi: 10.1378/chest.87.2.142. [DOI] [PubMed] [Google Scholar]
- 13.Morice RC, Ece T, Ece F, Keus L. Endobronchial argon plasma coagulation for treatment of hemoptysis and neoplastic airway obstruction. Chest. 2001;119:781–787. doi: 10.1378/chest.119.3.781. [DOI] [PubMed] [Google Scholar]
- 14.McGuinness G, Beacher JR, Harkin TJ, Garay SM, Rom WN, Naidich DP. Hemoptysis: prospective high-resolution CT/bronchoscopic correlation. Chest. 1994;105:1155–1162. doi: 10.1378/chest.105.4.1155. [DOI] [PubMed] [Google Scholar]