Abstract
Despite conflicting evidence from clinical trials, rituximab continues to be used off-label in the treatment of systemic lupus erythematosus (SLE). A new study has now investigated the use of this drug for SLE in Europe, including indications for use and patient characteristics.
The ongoing search for effective therapies for systemic lupus erythematosus (SLE) is illustrated by a recent study by Ryden-Aulin et al.1 investigating the off-label use of rituximab for SLE in Europe. Effective, safe and affordable treatments are urgently needed for SLE. The clinical needs include medicines for induction and maintenance of disease remission, alternative treatments to corticosteroids, and medicines to attenuate cumulative damage and improve overall outcomes. These goals are obviously a tall order in any disease and have proven particularly challenging for SLE2. As discussed in multiple publications, the reasons for the limitations faced in the treatment of SLE are numerous, including disease heterogeneity, study size and design, and the beneficial effects of background therapies that might blunt the ability of short-term studies to demonstrate added value of investigational drugs.
Given that B cells are assumed to have a central pathogenic role in SLE, therapies targeting these cells have been of major interest over the past decade3. In addition to a strong immunological foundation, this interest has been predicated on a favourable safety profile and the documented benefit of rituximab in rheumatoid arthritis (RA) and, more recently, in antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis4,5. Although the high hopes for B cell therapies have been somewhat supported by the approval of belimumab for the treatment of SLE, they have also been thwarted by the failure of two seminal trials of rituximab in non-renal lupus and in lupus nephritis (the EXPLORER and LUNAR trials, respectively). Nevertheless, clinicians continue to be interested in the use of rituximab owing to some promising exploratory end points in LUNAR, including potential benefit in African American patients, as well as clinical experience and observational studies6–9. The off-label use of rituximab is enabled by its approval for RA and ANCA-associated vasculitis by the FDA and the European Medicines Agency, and supported by its selection as one option for the treatment of refractory lupus nephritis in both the EULAR and ACR guidelines10.
Despite anecdotal evidence for the use of rituximab in clinical practice, the actual frequency of use, clinical indications and other variables affecting its utilization remain understudied. The new publication by Ryden- Aulin et al.1 represents an important step in addressing this deficiency. An international group of rheumatologists and autoimmune disease specialists used the International Registry for Biologics In SLE (IRBIS), a retrospective and prospective registry launched in 2010, to survey patients with SLE from across European countries. In addition to identifying patients treated with rituximab at their sites, the participating centres provided additional information, both on patients treated with rituximab and control patients treated with conventional immunosuppressive agents, including demographics and clinical variables. A nationwide estimate of SLE prevalence and rituximab use in SLE was calculated from case report forms. When case report forms were not available, rituximab use was estimated through published reports and personal communication. When these sources were not available either, national estimates were made by assumed similarity with neighbouring countries. The study included patients treated from 2010–2013. In all, 29 centres from 12 countries (with almost half of the centres being in Spain) participated, in some cases drawing information from established national registries. 19 additional countries were included on the basis of geographical proximity.
“Effective, safe and affordable treatments are urgently needed for SLE”
The estimated prevalence of SLE for contributing countries (1–13 per 10,000 population) and the one extrapolated for all of Europe (0.9–13.2 per 10,000 population) was within the expected range for predominantly white populations. The calculated frequency of rituximab use in SLE for reporting countries was 0.6–1.6% of the estimated number of patients per country and it was, not surprisingly given the methodology used, essentially the same for all of Europe (0.5–1.5%). Unfortunately, the information is presented as a calculated aggregate for the individual countries without a breakdown for individual centres. This lack of breakdown limits the ability of the study to understand patterns and determinants of rituximab utilization and the influences of personal experience and preference, sense of efficacy, safety concerns, and financial and regulatory limitations. The variability found between individual reporting countries further highlights the need for more detailed data. For example, 4–20% of patients were estimated to have received rituximab in Sweden, perhaps owing to the good reimbursement policies in this country, and 0–11% in Spain, but only 1–2% in Belgium, France and Germany (FIG. 1). Moreover, Spain and Sweden had a wide range of utilization, which is difficult to interpret with the given data.
Figure 1.
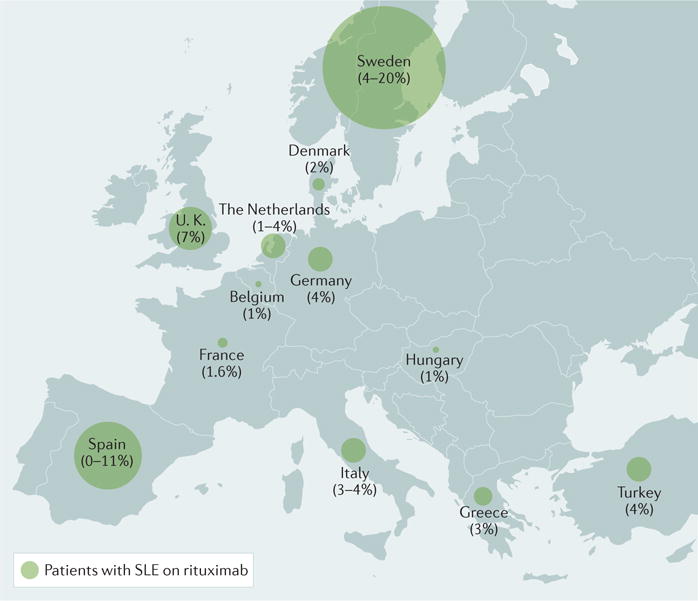
Percentage of patients with systemic lupus erythematosus receiving rituximab in 12 European countries1.
Demographics and disease characteristics were established through the analysis of 103 patients who were treated with rituximab (>90% of whom were white individuals), but unfortunately no information is provided as to how those particular patients were selected for analysis. Compared with patients on conventional immunosuppressive drugs, patients treated with rituximab were slightly older and had much longer and significantly more active disease (measured by the SLE Disease Activity Index (SLEDAI) and Systemic Lupus International Collaborative Clinics (SLICC) damage index). The authors reasonably conclude that rituximab is used for more severe disease and also suggest that its primary indication is to control disease rather than to spare corticosteroids. Along these lines, as might be expected, the main disease manifestation for which rituximab was used was lupus nephritis, whereas cutaneous and musculoskeletal disease did not lead to rituximab treatment. Regrettably, no information is provided regarding the type of lupus nephritis or whether the disease had been refractory to other treatments. Thrombocytopaenia and haemolytic anaemia were the secondary indication for rituximab use, probably owing to the documented benefit and prevalent use of rituximab in idiopathic autoimmune haemolytic anaemia and thrombocytopaenia.
In summary, this study represents a welcome effort to understand rituximab use in SLE. The results might inform future use, particularly, as pointed out by the investigators, if affordable biosimilars overcome financial constraints. However, the information provided has some important limitations, some of which are acknowledged by the authors. For example, the study excluded other subspecialists including nephrologists, dermatologists, neurologists and haematologists. Therefore, the study is likely to underestimate the use of rituximab in patients in whom the corresponding manifestations could be prominent enough to prompt direct referral by primary care physicians to other subspecialists. In fact, this possibility is even more likely given the long disease duration in patients treated with rituximab, as many acute presentations in specific organ systems might be referred to other subspecialists before a diagnosis of SLE is established. It would have also been helpful to assess what the ‘intention-to-treat’ preferences of rheumatologists would have been if cost, approval and other barriers were removed from the decisionmaking process and instead if one could consider only the perceived clinical need and potential benefit. Along these lines, at face value, the data presented suggest that either the frequency of refractory SLE in this study was rather low or, instead, rituximab was not considered a viable option for most patients with refractory disease whether due to lack of reimbursement, cost-benefit ratio analysis, physicians’ perception of clinical efficacy and potential adverse effects, infusion logistics or patients’ preference. A discussion of these issues would have been desirable and hopefully will represent future inquiries through this important registry.
The actual benefit and clinical indications of rituximab in SLE continue to be debated, and studies are underway with new B cell depleting antibodies or in the absence of large doses of corticosteroids. Undoubtedly, positive studies will foster the approval of this modality and provide support and clarity for wider clinical application. In the meantime, both clinical experience, observational studies and some guidelines support the use of this approach for the treatment of severe SLE as well as disease with sustained activity that remains unabated by conventional immune-suppression. In addition, B cell depletion, which can potentially induce prolonged effects, should be an important consideration for active disease in patients with unacceptable adverse effects from other therapies as well as in patients with poor adherence to treatments requiring daily administration.
Acknowledgments
The work of the author is supported by a grant from NIH National Institute of Allergy and Infectious Diseases (NIAID) - U19 A1110483.
Footnotes
Competing interests statement
The author declares no competing interests.
References
- 1.Rydén-Aulin M, et al. Off-label use of rituximab for systemic lupus erythematosus in Europe. Lupus Sci Med. 2016;3:e000163. doi: 10.1136/lupus-2016-000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahieu MA, Strand V, Simon LS, Lipsky PE, Ramsey-Goldman R. A critical review of clinical trials in systemic lupus erythematosus. Lupus. 2016;25:1122–1140. doi: 10.1177/0961203316652492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanz I, Lee FE. B cells as therapeutic targets in SLE. Nat Rev Rheumatol. 2010;6:326–337. doi: 10.1038/nrrheum.2010.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edwards JCW, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350:2572–2581. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 5.Stone JH, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363:221–232. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rovin BH, et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum. 2012;64:1215–1226. doi: 10.1002/art.34359. [DOI] [PubMed] [Google Scholar]
- 7.Lightstone L. The landscape after LUNAR: rituximab’s crater-filled path. Arthritis Rheum. 2012;64:962–965. doi: 10.1002/art.34362. [DOI] [PubMed] [Google Scholar]
- 8.Lu T, et al. A retrospective seven-year analysis of the use of B cell depletion therapy in systemic lupus erythematosus at University College London Hospital: the first fifty patients. Arthritis Rheum. 2009;61:482–487. doi: 10.1002/art.24341. [DOI] [PubMed] [Google Scholar]
- 9.Condon MB, et al. Prospective observational singlecentre cohort study to evaluate the effectiveness of treating lupus nephritis with rituximab and mycophenolate mofetil but no oral steroids. Ann Rheum Dis. 2013;72:1280–1286. doi: 10.1136/annrheumdis-2012-202844. [DOI] [PubMed] [Google Scholar]
- 10.Wilhelmus S, et al. Lupus nephritis management guidelines compared. Nephrol Dial Transplant. 2015;31:904–913. doi: 10.1093/ndt/gfv102. [DOI] [PubMed] [Google Scholar]


