Summary
1. The environmental filtering hypothesis predicts that the abiotic environment selects species with similar trait values within communities. Testing this hypothesis along multiple – and interacting – gradients of climate and soil variables constitutes a great opportunity to better understand and predict the responses of plant communities to ongoing environmental changes.
2. Based on two key plant traits, maximum plant height and specific leaf area (SLA), we assessed the filtering effects of climate (mean annual temperature and precipitation, precipitation seasonality), soil characteristics (soil pH, sand content and total phosphorus) and all potential interactions on the functional structure and diversity of 124 dryland communities spread over the globe. The functional structure and diversity of dryland communities were quantified using the mean, variance, skewness and kurtosis of plant trait distributions.
3. The models accurately explained the observed variations in functional trait diversity across the 124 communities studied. All models included interactions among factors, i.e. climate – climate (9% of explanatory power), climate – soil (24% of explanatory power) and soil – soil interactions (5% of explanatory power). Precipitation seasonality was the main driver of maximum plant height, and interacted with mean annual temperature and precipitation. Soil pH mediated the filtering effects of climate and sand content on SLA. Our results also revealed that communities characterized by a low variance can also exhibit low kurtosis values, indicating that functionally contrasting species can co-occur even in communities with narrow ranges of trait values.
4. Synthesis We identified the particular set of conditions under which the environmental filtering hypothesis operates in drylands worldwide. Our findings also indicate that species with functionally contrasting strategies can still co-occur locally, even under prevailing environmental filtering. Interactions between sources of environmental stress should be therefore included in global trait-based studies, as this will help to further anticipate where the effects of environmental filtering will impact plant trait diversity under climate change.
Keywords: climate, community assembly, determinants of plant community diversity and structure, functional biogeography, functional diversity, plant height, pH, precipitation seasonality, specific leaf area, trait distribution
Introduction
Environmental filtering is one of the most pervasive concept in ecology, being central in many studies of plant community assembly, biogeography (e.g. Swenson et al. 2012; de Bello et al. 2013), and trait-based modelling (see Laughlin & Laughlin 2013 for a review). The environmental filtering hypothesis predicts that the abiotic environment selects species with similar trait values within communities (Keddy 1992; Weiher et al. 1998; Grime 2006). The effect of environmental filtering on plant communities has been traditionally assessed along local or regional environmental gradients (e.g. Fonseca et al. 2000; Gross et al. 2008; de Bello et al. 2013; Butterfield & Munson 2016). However, the effect of environmental filtering, sensu stricto, is difficult to isolate from that of local biotic interactions along these gradients (Maire et al. 2012; Gross et al. 2013; Kraft et al. 2015). In a recent paper, Kraft et al. (2015) called for testing the environmental filtering hypothesis explicitly along marked abiotic gradients. This can be typically achieved using large scale (e.g. continental and global) observational surveys focusing on functional trait diversity (e.g. Coyle et al. 2014; Lamanna et al. 2014; Simova et al. 2015). Although they are still sparse, these studies may inform us on the importance of environmental filtering for shaping in the diversity of plant forms and functions globally.
Multiple sources of abiotic stresses are likely to interact and may determine the outcome of environmental filtering on functional trait diversity at the global scale (e.g. Reich et al. 2006; Simpson & Laughlin 2016). For instance, ongoing climate change involves simultaneous shifts in both temperature and precipitation regimes (IPCC 2013). Large-scale climate gradients such as temperature and precipitation regimes are expected to interact (climate – climate interactions), and impact on plant communities and associated ecosystem processes in complex ways (see Peñuelas et al. 2013 for a review). In addition, large-scale climate gradients are prone to interact with local soil conditions (i.e. climate – soil interactions: Ordonez et al. 2009; Fridley et al. 2011; Liancourt et al. 2013). Pervasive climate – soil interactions may explain the large variation in diversity of foliar traits observed between co-occurring species for a given temperature and precipitation level (Wright et al. 2004; Freschet et al. 2011). Yet, the effect of climate–climate or climate-soil interactions on plant functional trait diversity has been barely quantified (Simpson & Laughlin 2016). Testing the environmental filtering hypothesis along multiple gradients of climate and soil variables, and their interactions, constitutes a great opportunity to better understand and predict the response of plant trait diversity under climate change (Violle et al. 2014; Enquist et al. 2015).
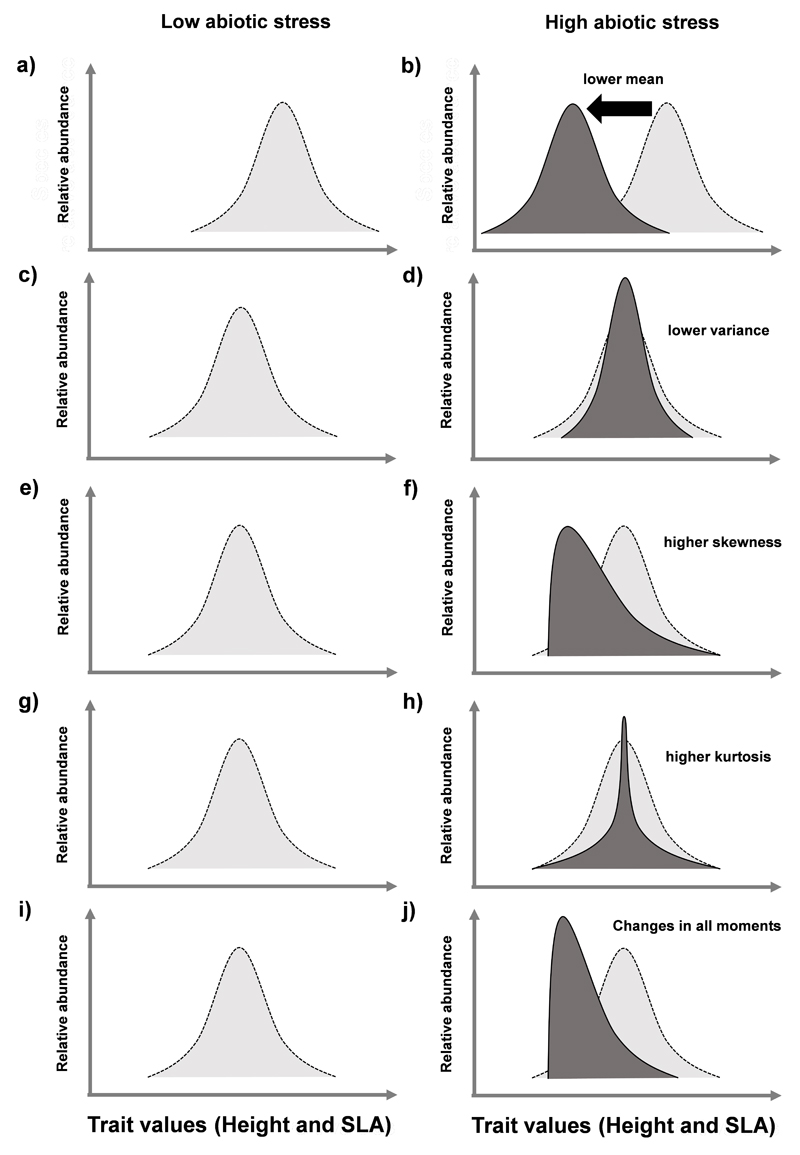
The environmental filtering hypothesis predicts a shift in the trait values of plant species that confers higher stress tolerance with increased environmental stress (e.g. Grime 2006, see Fig. 1a and b for detailed hypothesis). A second prediction is a reduction in the range of trait values observed within communities, because lower stress tolerant species may be filtered out of the community (Cornwell & Ackerly 2009, Fig. 1c and d). These two predictions implicitly assume that a single, most favorable, functional strategy characterized by a narrow set of suitable trait values, allows plant species to establish and persist under a given level of abiotic stress (Enquist et al. 2015). However, the predictions of the environmental filtering hypothesis contrast with the high functional trait diversity that can be observed within plant communities (Wright et al. 2004), even in stressful environments (Chesson et al. 2004; Freschet et al. 2011; Gross et al. 2013).
Fig. 1.
Schematic representation of shifts in trait distributions for maximum plant height and specific leaf area (SLA), following the environmental filtering concept. We represent the shifts in mean (a,b), variance (c,d), skewness (d,e), kurtosis (f,g), and all moments together (h,i) of the trait distributions under low and high abiotic stress.
Dryland ecosystems typically reflect the discrepancy between predictions and in situ observations. According to the environmental filtering hypothesis, dryland species should exhibit a stress-tolerant strategy, (sensu Grime 1974), e.g., having thick evergreen leaves [low specific leaf area (SLA)] and short stature (Wright et al. 2001; Moles et al. 2009). However, stress-tolerant species can often coexist in arid regions with stress-avoidant species with thin and summer-deciduous leaves (Noy-Meier 1973; Grime 1977; Chesson et al. 2004), and this coexistence increases trait diversity within dryland plant communities (Gross et al. 2013). Understanding the discrepancy between predictions of the environmental filtering hypothesis and the high functional diversity observed in global drylands is crucial. Maintaining a high functional trait diversity can enhance their resistance to aridity (Valencia et al. 2015), which is forecasted to increase in drylands worldwide by the end of this century (Huang et al. 2016).
We aimed to test the effect of multiple climate and soil drivers on functional trait diversity using a unique data set of 124 arid, semi-arid, and dry-subhumid plant communities spread over all continents, except Antarctica (Appendix S1). The studied environmental drivers included (i) large-scale climate gradients of mean annual temperature (MAT), mean annual precipitation (MAP) and precipitation seasonality (PS); (ii) three soil variables representing the physico-chemical properties of the bedrock, and influencing soil fertility (Maire et al. 2015): soil pH, sand content and total phosphorus (TP); (iii) all potential interactions between the environmental drivers, i.e. climate – climate, climate – soil and soil – soil interactions. Functional trait diversity was quantified as the abundance-weighted distributions within communities of specific leaf area (SLA) and maximum plant height (trait distributions hereafter). These two traits capture the global spectrum of plant form and function in terrestrial ecosystems (Diaz et al. 2016), and are key determinants of functional diversity and ecosystem functioning in semi-arid plant communities (Gross et al. 2013; Le Bagousse-Pinguet et al. 2015; Valencia et al. 2015). We considered the mean (location), the variance (dispersion), and the skewness and kurtosis (shape) of trait distributions, which are all central to understanding how species assemble within communities, and how plant communities respond to environmental change (Enquist et al. 2015).
Following the environmental filtering hypothesis, dryland communities should converge toward shorter statured and conservative plant strategies with increased abiotic stress. This convergence will decrease both their SLA and maximum plant height (lower mean: Fig. 1a and b) and the range of trait values observed (smaller variance, c and d). It will also lead to asymmetric distributions with “optimal” trait values for the shortest and most conservative species occurring within communities (positive skewness, e and f), and decrease the evenness of distributions (high kurtosis, g and h) altogether (i and j).
Material and Methods
Study Area
Based on data availability, we used a subset of 124 sites from the global dryland network presented in Maestre et al. (2012a). The 124 study sites are located in 13 countries (Argentina, Australia, Chile, China, Ecuador, Israel, Kenya, Mexico, Morocco, Spain, Tunisia, USA and Venezuela; Appendix S1). Our dataset included representative sites from the major vegetation types found in drylands (excluding hyper arid areas, which usually have little or no perennial vegetation), and differed widely in climate conditions: mean annual temperature and precipitation ranged from -1.8°C to 27.8°C, and from 79 mm to 1177 mm, respectively.
Climate Variables
The climate features of the 124 studied sites included mean annual temperature (MAT), mean annual precipitation (MAP) and precipitation seasonality (PS: coefficient of variation of 12 monthly rainfall totals), all major determinants of ecosystem structure and functioning in drylands worldwide (see Maestre et al. 2012b for a review). We selected these large-scale climate gradients because: i) they are important drivers of trait variation both at regional and global scales (e.g., Wright et al. 2004; Swenson et al. 2012; Moles et al. 2014); ii) they are key variables for explaining global variation in dryland ecosystem functioning (Maestre et al. 2012a); and (iii), MAT, MAP and PS describe largely independent features of site climate in the studied dataset (bivariate correlations, r < 0.3 in all cases, Appendix S2). Standardized climate data for all study sites were obtained from Worldclim (www.worldclim.org), a high resolution (30 arc seconds or ~ 1km at equator) global database (Hijmans et al. 2005). We did not include irradiance in our models despite being an important abiotic factor in drylands (Noy-Meier 1973) and a main driver of specific leaf area (Poorter et al 2009). We did so because irradiance presented a low coefficient of variation in our dataset (11% in comparison with other climate variables with coefficient of variation above 50%), and was highly correlated with MAT (r = 0.84). Temperature seasonality (standard deviation of monthly temperatures * 100) was also not considered due to its correlation with MAT in the studied dataset (r = 0.59).
Soil Variables
We aimed to select only soil variables that are largely independent from any biological activities (plants, microbes) to effectively assess the true abiotic filtering effect of soil variables on functional trait diversity. We considered the physico-chemical properties of the bedrock using the soil sand content, soil pH and total phosphorus (TP), measured for each site in bare soil (i.e. avoiding vegetation patches). The physico-chemical properties widely differed among the 124 sites: soil sand content, soil pH and TP ranged from 28% to 95%, from 5.15 to 9.28, and from 0.05 to 1.45 mg P. g-1 soil, respectively. These three physico-chemical properties are considered as primordial master soil variables (Maire et al. 2015), play key roles in the availability of water and nutrients in drylands, and are major drivers of the composition and diversity of dryland microbial communities (Delgado-Baquerizo et al. 2016). Soil fertility is expected to be higher in less sandy soils (sand content strongly covaries with soil organic matter and silt content but not with clay content, data not shown), in soils with pH between 7.5 and 8.5 (soil enzymatic activities of N, P and C cycles peak between this range, Delgado-Baquerizo et al. 2015), and with high phosphorus content (Jenny 1941). Soil water retention is then expected to be highest in less sandy soils. These variables were measured in five soil samples per site as described in Maestre et al. (2012a), and were averaged for further statistical analyses. Sand, clay and silt contents were measured in soil samples (0-7.5 cm depth) in open areas devoid of vascular vegetation. Soil pH was measured with a pH meter, in a 1: 2.5 mass: volume soil and water suspension. Total phosphorus was measured using a SKALAR San++ Analyzer (Skalar, Breda, The Netherlands) after digestion with sulphuric acid. Clay and silt contents were not used in our analyses due to their correlation with sand content (r = -0.52 and -0.55, respectively).
Other Variables
Changes in the functional trait diversity of plant communities observed along environmental gradients may be partly driven by changes in the local species pools (species richness), historical context and topography. We considered species richness, the latitude and longitude of our study sites, as well as topography (slope angle; it ranged between 0.2° to 27.8° in our dataset) in our analyses to control for all these potential confounding effects. We used the sinus and cosinus of the longitude to avoid any bias due to intrinsic circularity of longitude in the statistical models (i.e., Longitude (sin) and Longitude (cos) hereafter, respectively).
Trait Distributions
Trait distributions were quantified for each of the 124 sites, by using two independent datasets: (i) a detailed dataset containing the cover of each perennial plant species measured in 80 quadrats of 2.25 m2 within each site, where the sum of the cover for each species is used as a proxy of species abundance at site (Maestre et al. 2012a); and (ii) data for SLA and maximum plant height, retrieved from the TRY database (Kattge et al. 2011). The 124 sites were selected because trait data were available for: (1) all the perennial species that together accounted for a cumulative relative abundance >80%, and (2) the four most dominant species to avoid any breaks in the trait distributions. We used averaged values when multiple trait data were available for a given species in the TRY database. Trait data were available for 316 and 526 species out of 622 species, for SLA and maximum plant height respectively. Specific Leaf Area is a key trait indexing leaf-level carbon gain strategies (Wright et al. 2004). Plant height reflects a trade-off for biophysical constraints in determining water fluxes within the plant (Diaz et al. 2016), and is related to its competitive ability (e.g. Schamp et al. 2008). Specific leaf area and height load heavily along two important independent axes of plant ecological strategies (Diaz et al. 2016). Maximum plant height and SLA were log-transformed before analysis to amplify the probability of detecting functional community patterns (Majekova et al. 2016).
We calculated the mean, variance, skewness and kurtosis (all weighted by the relative abundance of species) of the 124 trait distributions for SLA and maximum plant height separately:
| (Eqn 1) |
| (Eqn 2) |
| (Eqn 3) |
| (Eqn 4) |
where pi and Ti are the relative abundance and the trait value of the species i respectively, n is the total number of species in a community with available trait values. For each community, the sum of relative abundance equal to 100%, i.e.
The skewness and the kurtosis are unitless, and inform on the shape of the trait distribution. The skewness represents the asymmetry of the distributions. Highly negative or positive values of skewness occur when trait distributions are strongly right-or left-skewed, with a few species that have extreme trait values compared to the bulk of the distribution. Skewed distributions typically result from phenomena such as environmental changes or asymmetric competition (Schamp et al. 2008; Enquist et al. 2015). Kurtosis represents the relative peakiness of the trait distribution and the heaviness of its tails. Low kurtosis reflects the evenness in abundance of trait values occurring within the community, i.e., a high functional diversity (Enquist et al. 2015).
Statistical Analyses
We built four competing models using multiple linear regressions to assess the effect of climate, soil variables and their interactions on each moment of the trait distributions for SLA and plant height separately. We included in the first model species richness, geography, topography and climate variables as predictors (model “CLIMATE”). The second model included species richness, geography, topography, and soil variables as predictors (model “SOIL”). The third model included all predictors of these models (model “CLIMATE + SOIL”). Finally, the fourth model includes all predictors of the model “CLIMATE + SOIL” plus all possible two-way interactions between MAT, MAP, PS, sand content, pH and TP (model “CLIMATE + SOIL + INTERACTIONS”). The variance inflation factors among the predictors used were far below 10 in all cases, hence multicollinearity was low (Appendix S2). Note that we also considered quadratic terms for all predictors since functional structure and trait diversity do not necessarily change linearly along strong gradients (e.g. Gross et al. 2013; Le Bagousse-Pinguet et al. 2015; Valencia et al. 2015).
We used a model selection procedure, based on minimizing the corrected Akaike information criterion (AICc), to select the best predictors of trait distributions. In a first step, we performed model simplification using a backward regression procedure. We subsequently removed non-significant quadratic and interaction terms that did not impact model predictive ability (r2), and further kept all models with lower AICc (ΔAICc < 10). Then, a model selection procedure based on AICc selection (ΔAICc < 2) was applied on the resulting full models to select the best predictors most supported by the data. This procedure was performed using the function dredge in the R package MuMIn (Barton 2013). Species richness, geography and topography were always maintained during the model selection procedure. Model averaging was performed based on AICc thresholds (ΔAICc < 2; Burhnam & Anderson 2002) when multiple models were selected. Model residuals were inspected for constant variance and normality. All predictors were standardized before analyses using the Z-score to interpret parameter estimates on a comparable scale. Response variables were log-transformed when necessary before analysis to meet the assumptions of the tests used.
We evaluated the relative effect of each predictor on the four moments of the trait distributions. We used an analogue of the variance decomposition analysis based on Z-scores. Since predictors were all Z-scored prior analyses, the relative effect of each predictor can be simply calculated as the ratio between its parameter estimate and the sum of all parameter estimates, and expressed in %. Then, the obtained relative effects of predictors are grouped into five identifiable variance fractions: i) climate – climate interactions, ii) climate, iii) climate – soil interactions, iv) soil, v) soil – soil interactions, vi) species richness, vii) geography (latitude, longitude (sin), longitude (cos), slope), and viii) unexplained variance.
We also used the parameter estimates of interacting predictors to illustrate how climate – climate, climate – soil and soil – soil interactions impact the moments of the trait distributions. We fixed one of the two interacting predictors at either low or high value, and examined the effect of the other predictor on the four moments of trait distributions, while the parameter estimates of all other predictors were fixed to their mean value (i.e. 0 since all predictors were Z-scored). All statistical analyses were performed using the R statistical software 2.15.1 (R Core Team 2012).
Results
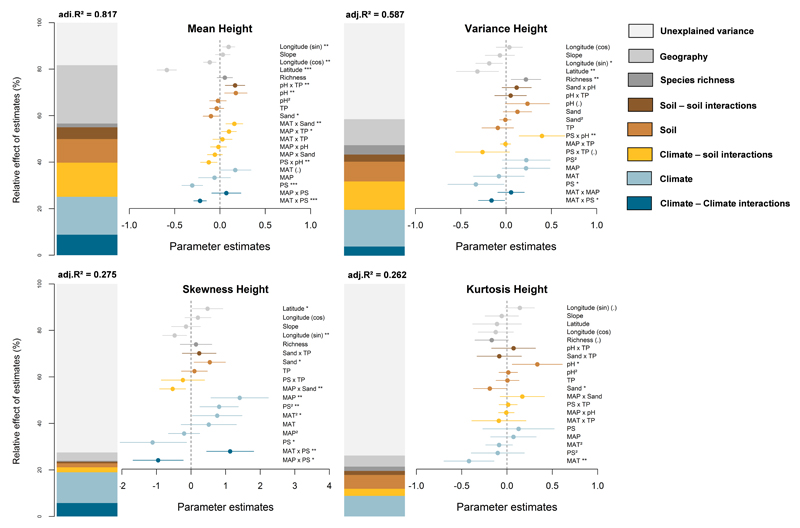
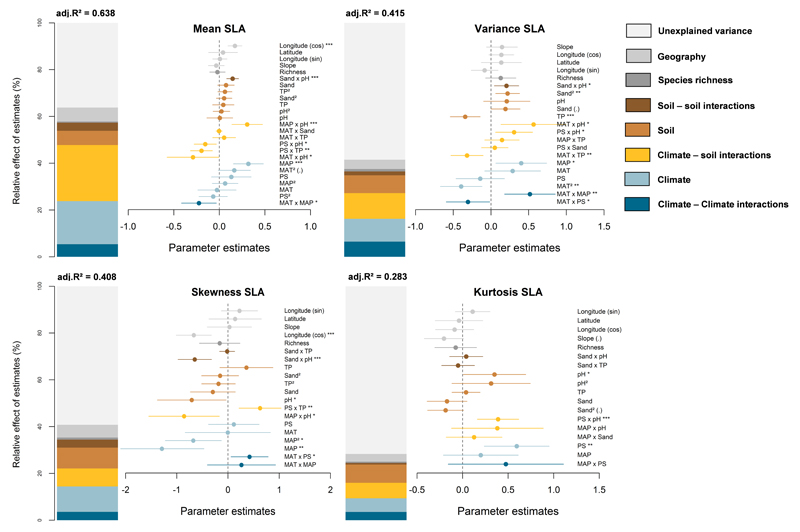
The predictive power of our models was high, but gradually decreased when explaining higher moments of trait distributions for maximum plant height (Fig. 2) and specific leaf area (Fig. 3). For plant height, the predictive power of the models was higher for the mean (Fig. 2: adjusted r2 = 0.817) and variance (0.587), compared to skewness (0.275) and kurtosis (0.262). For specific leaf area, the predictive power of the models on trait distributions was the highest for the mean (Fig. 3: adjusted r2 = 0.638), and also reached more than 40% for the variance (0.415) and skewness (0.408).
Fig. 2.
Effects of multiple sources of environmental stress and their interactions on the trait distributions for maximum plant height. Results are presented for the mean, variance, skewness and kurtosis of trait distributions. We show the averaged parameter estimates (standardized regression coefficients) of model predictors, the associated 95% confidence intervals and the relative importance of each factor, expressed as the percentage of explained variance. The adj.r2 of the averaged models and the p-value of each predictor are given as: (.), p < 0.1; *, p < 0.05; ** p > 0.01; *** p < 0.001.
MAT: mean annual temperature; MAP: mean annual precipitation; PS: precipitation seasonality; TP: total phosphorus.
Fig. 3.
Effects of multiple sources of environmental stress and their interactions on the trait distributions for specific leaf area (SLA). Rest of legend as in Fig. 2.
MAT: mean annual temperature; MAP: mean annual precipitation; PS: precipitation seasonality; TP: total phosphorus.
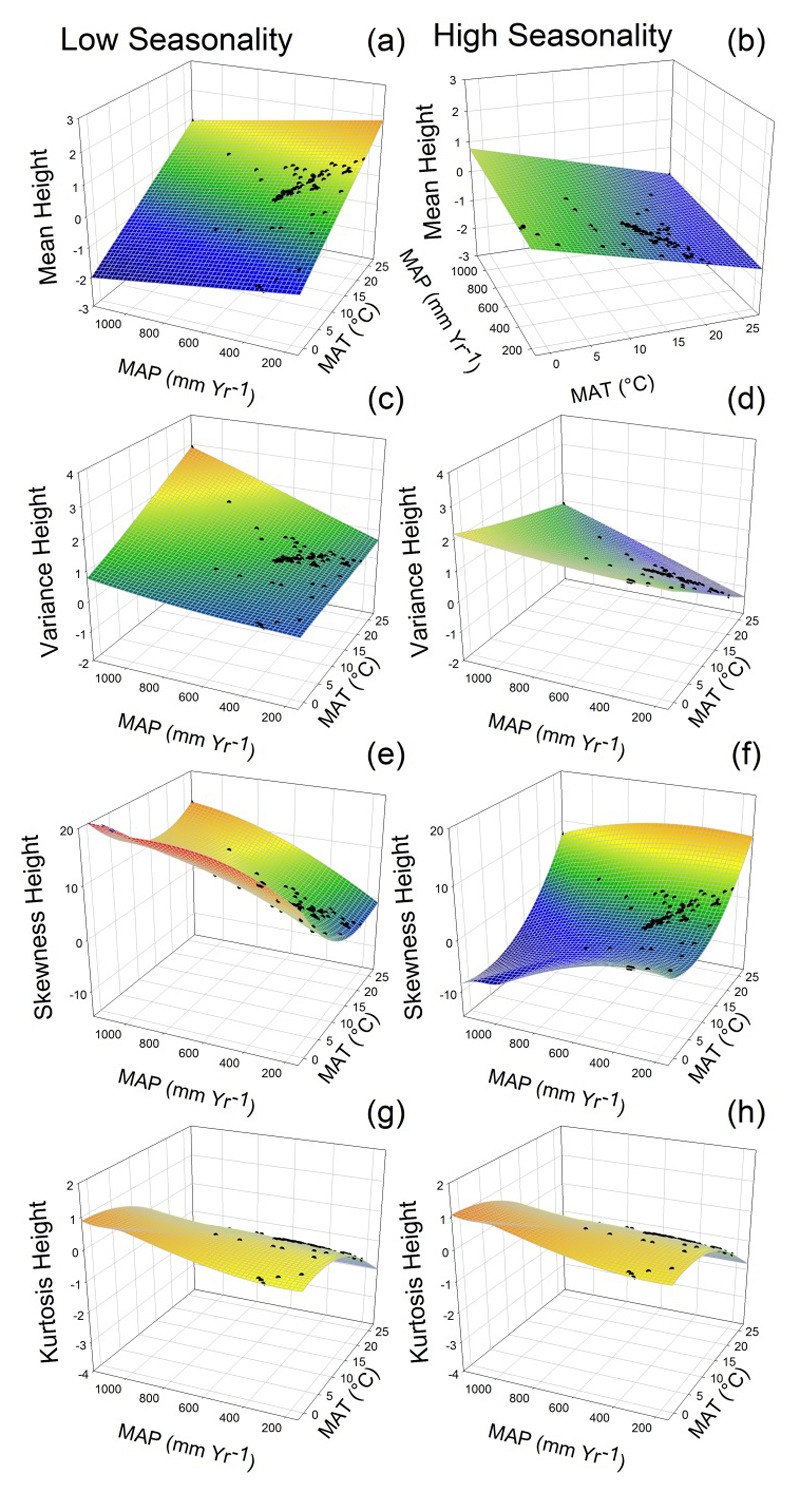
Models including climate – climate, climate – soil and soil – soil interactions explained more variance than the additive models for the four moments of both plant height and specific leaf area (Appendices S3, S4 and S5). These results highlight the importance of considering interactions between multiple sources of abiotic stress when assessing functional trait diversity at global scale. Climate – climate interactions explained up to 9% of the model variance for maximum plant height (Fig. 2), and up to 7% for SLA (Fig. 3). For instance, increasing precipitation seasonality significantly interacted with mean annual temperature and precipitation (Fig. 2). Under low seasonality, higher aridity (i.e. an increase in MAT together with a decrease in MAP) increased mean plant height (Fig. 4a), weakly impacted the variance (c), and decreased the skewness (e) and kurtosis (g). Under low seasonality, these results indicated a weak effect of increased aridity on functional trait diversity. In contrast, we observed a strong filtering effect of aridity under high precipitation seasonality (Fig. 4, right panels). Under high seasonality, aridity decreased the mean (Fig. 4b) and variance for plant height (d), and increased the skewness (f). Note that kurtosis of plant height also strongly decreased in the harshest conditions (Fig. 4h: low MAP and high MAT), suggesting for the local co-occurrence of functionally contrasting strategies.
Fig. 4.
Predicted trait distributions (black dots) from the interactions between mean annual temperature (MAT) and precipitation seasonality, and between mean annual precipitation (MAP) and precipitation seasonality for maximum plant height in a 3D plot. We represented the effects of interactions using the standardized parameter estimates of MAT and MAP (Fig. 2). Predictions were calculated for low and high precipitation seasonality (CV seasonality = 12 and CV seasonality = 124, respectively). All other standardized parameter estimates were fixed at their mean value. The colours of the predicted planes change from blue (low values of the moments) to red (high values).
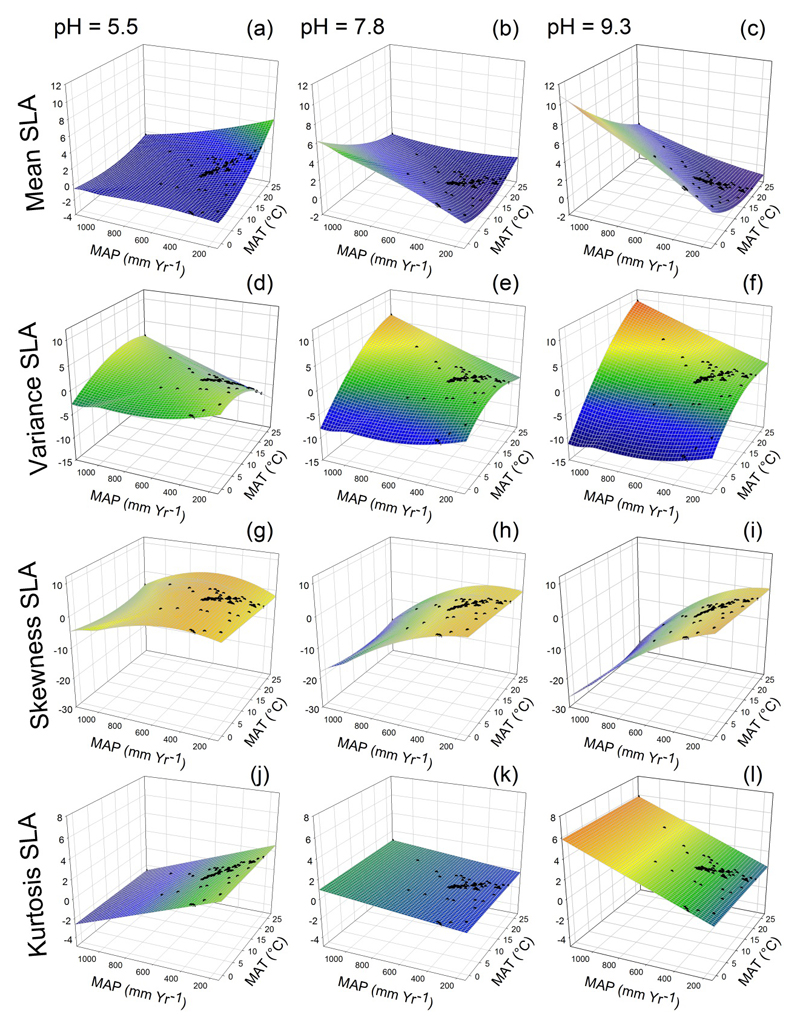
Climate – soil interactions explained up to 15% for maximum plant height, and up to 24% for SLA. The effects of climate on trait distributions were significantly modulated by soil pH, and notably for SLA (Fig. 3). In acidic conditions, the mean SLA increased, and the skewness decreased with lower MAP and higher MAT (Fig. 5a and g). The variance of SLA decreased in the most arid sites while its kurtosis increased (Fig. 5d and j), indicating a decline in functional trait diversity. Communities developing under basic soil conditions were dominated by more stress-tolerant species exhibiting low SLA values with increasing aridity (Fig. 5c: low mean SLA; 5i: high skewness). A higher SLA variance and a lower kurtosis were also observed in most arid sites (Fig. 5f and l). These results indicate an increase in functional trait diversity with environmental stress.
Fig. 5.
Predicted trait distributions (black dots) from the interactions between mean annual temperature (MAT) and pH, and between mean annual precipitation (MAP) and pH for specific leaf area (SLA) in a 3D plot. We represented the effects of interactions using the standardized parameter estimates of MAT and MAP (Fig. 3). Predictions were calculated for acidic, slightly basic and basic conditions (pH = 5.5, pH = 7.8, pH = 9.3, respectively). All other standardized parameter estimates were fixed at their mean value. The colours of the predicted planes change from blue (low values of the moments) to red (high values).
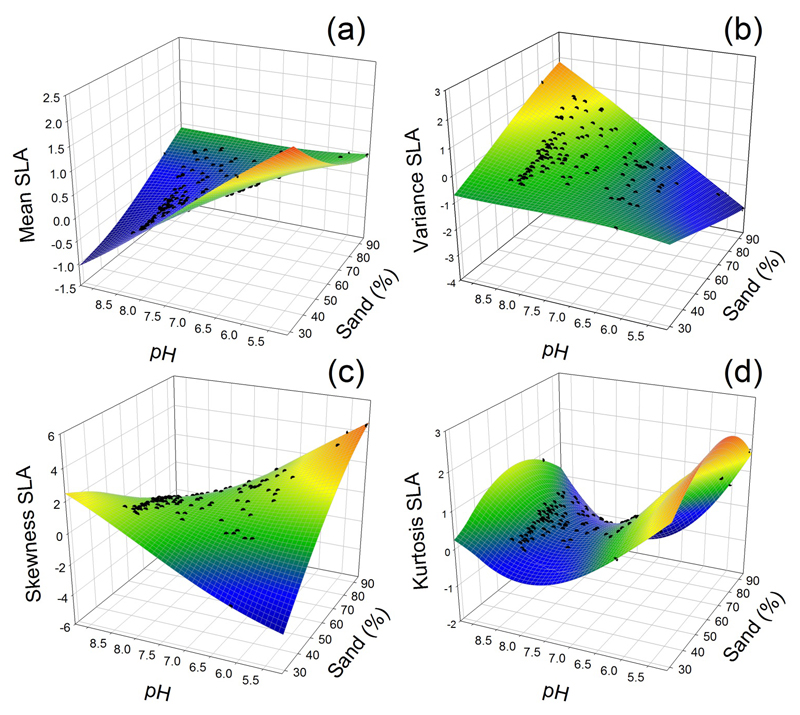
Finally, soil – soil interactions explained a smaller, but significant fraction of the variation in functional trait diversity observed (up to 5%, Fig. 3), mostly due to the interaction between sand content and soil pH for SLA (Fig. 6). Both the lowest and the highest mean SLA occurred at low sand content (Fig. 6a). The lowest mean SLA occurred under basic soil conditions (soil pH ~ 8), whereas the highest mean SLA was observed under acidic conditions (pH ~ 5.5). Also, the variance of SLA strongly increased with soil pH in sandy soils (high sand content), but it was not sensitive to soil pH at low sand content (Fig. 6b). Finally, we also observed lowest values in the kurtosis of SLA for pH ~ 7 (Fig. 6d), indicating that trait diversity was the highest under neural conditions.
Fig. 6.
Predicted trait distributions (black dots) from the interactions between pH and sand content for specific leaf area (SLA) in a 3D plot. We represented the effects of interactions using the standardized parameter estimates of pH and sand content (Fig. 3). All other standardized parameter estimates were fixed at their mean value. The colours of the predicted planes change from blue (low values of the moments) to red (high values).
Discussion
Interactions between multiple abiotic stress sources are key for predicting functional trait diversity at a global scale. By considering the interactions among abiotic drivers, and by controlling for the local species pool, we identified the particular sets of environmental conditions under which the environmental filtering hypothesis operates in drylands worldwide. Shifts in functional trait diversity along abiotic drivers were trait-specific, with a major role of climate-climate interactions in driving the abundance distributions of maximum plant height. Climate – soil and soil – soil interactions had a predominant effect on SLA.
Functional Trait Diversity Responses to Climate and Soil Conditions in Drylands
Precipitation seasonality was a major driver of functional trait diversity for maximum plant height in the drylands studied, and strongly modulated the effects of MAT and MAP on this diversity (Fig. 4). Under high precipitation seasonality, increased MAT and lower MAP not only filtered plant communities toward the dominance of shorter species (Fig. 4b: lower mean), but also narrowed the range of trait values (Fig. 4d: lower variance). Therefore, intense drought periods in the most arid part of the studied gradient filtered plant communities toward a narrow set of suitable trait values allowing them to cope with the strong abiotic constraint, supporting for the environmental filtering hypothesis (Keddy 1992; Weiher et al. 1998; Grime 2006). The observed reduction in plant height in the harshest conditions of our climate gradients (i.e., high precipitation seasonality and temperature, and low annual precipitation) supports the hypotheses of height limitation due to hydraulic constraints (e.g. Koch et al. 2004). Although a loss of hydraulic conductivity following embolisms can also be common for shorter plant species, tall plants show low recovery capacity after the loss of hydraulic functions (Koch et al. 2004).
Soil pH was an important driver explaining functional trait diversity for SLA, but its effect was modulated by the climate drivers and the sand content (Figs. 3, 5, 6). A negative correlation between soil pH and mean SLA has been documented at the global scale (Maire et al. 2015), but we found that this is true only under low sand content conditions in drylands (Fig. 6a). When SLA decreases, leaf nitrogen content (per area) can increase, favoring leaf photosynthesis for a given water use (Maire et al. 2015). Our results would accord with the theory and observations that predict the dominance of species with high leaf nitrogen strategy to increase water use efficiency (Wright et al. 2003). This leaf nitrogen strategy is viable only when plant nitrogen uptake is less expensive (in terms of energy cost) than water uptake and transport from soil to leaves (Prentice et al 2014). In arid ecosystems, this may occur under high soil fertility conditions, i.e., under intermediate/high soil pH, low sandy soils (Fig. 6a), and warm temperatures favoring soil organic matter decomposition (Fig. 5b and c).
We also observed an increase in SLA variance with soil pH (Fig. 3). Over evolutionary time scale, soil pH has also been recognized as creating an environmental backdrop under which species diversity is shaped (Laliberté et al. 2014). As such, we expect the size of the calcicolous trait pool to be larger in drylands, where the regional soil pH, which can be different from the local soil pH, is on average alkaline (Hengl et al. 2014). This may favor the highest functional diversity observed in our alkaline sites, especially under warm climate conditions where a larger set of species may benefit from higher soil fertility and faster growing conditions (Fig. 6b). On the other hand, when climate is cold, soil organic matter decomposition slows down and soil fertility decreases, while residual negative impact of high soil pH (e.g. salinity) may increase the environmental stress and act as a strong filter (decreasing SLA variance).
The Environmental Filtering Hypothesis in Global Drylands
It is imperative to consider pervasive interactions between environmental drivers in order to identify the circumstances under which environmental filtering will impact functional trait diversity (Simpson & Laughlin 2016). Previous large-scale studies reported that higher abiotic stress does not necessarily filter plant communities toward a narrower range of trait values (Coyle et al. 2014; Simova et al. 2015). Our study reveals the environmental conditions under which functional trait diversity may decrease in global dryland in response to abiotic filtering processes: e.g., under the combining effect of high precipitation seasonality, high MAT and low MAP (Fig 4: right panels), or under high MAT and low MAP in acidic conditions (Fig. 5: left panels).
Importantly, our study also shows that abiotic stress should not necessarily imply a reduction in functional trait diversity. For instance, higher MAT and lower MAP did not affect the functional trait diversity of height in the studied drylands when precipitation seasonality was low (Fig. 4c). We even observed an increase in functional trait diversity (variance) for SLA with higher MAT and lower MAP in basic soil conditions (Fig. 5e and f). Our results support the view that multiple sets of trait values can allow functionally contrasting species to cross the filtering effect imposed by an abiotic stress, where they can equally perform in term of abundance in a given community (e.g. Gross et al. 2013). In dry and hot conditions, high trait variance can reflect the co-occurrence of stress-avoidant vs. stress tolerant species within communities for a given level of stress (Poorter et al. 2009; Gross et al. 2013), the occurrence of positive interactions (e.g. Butterfield & Briggs 2011; Butterfield & Munson 2016), or spatial / temporal storage effects (Chesson 2000; Chesson et al. 2004).
Our approach focusing on the four moments of trait distributions also reveals the existence of additional mechanisms that can promote the local co-occurrence of functionally contrasting species within communities. We showed that variance and kurtosis varied independently along environmental stress gradients. For instance, we observed an increased evenness in the abundance of trait values for maximum plant height under high MAT and low MAP (i.e. low kurtosis value, Fig. 4g and h), while variance slightly or strongly decreased under low and high precipitation seasonality, respectively (Fig. 4c and d). Also, we observed that kurtosis was minimized for neutral pH, a signal that was not observed with the variance (Fig. 6b and d). Our results indicated that functionally contrasting species can still co-occur even under prevailing environmental filtering, i.e., even when the abiotic environment selects for narrower ranges of trait values within communities (see also Cornwell & Ackerly 2009 and Gross et al. 2013 for similar evidences along local environmental gradients).
Finally, it is worth noting that we observed an overall decrease in the predictive power of our statistical models using the higher moments for maximum plant height (Fig. 2) and specific leaf area (Fig. 3). The predictive power of our models was very high for the mean of trait distributions, intermediate for the variance, and low for the shape parameters (skewness and kurtosis). These results may arise from a higher sensitivity of the skewness and the kurtosis to sampling effort. When considering frequency distribution, skewness and kurtosis might be very sensitive to the local species richness, making their estimation potentially difficult in species-poor communities. However, we focused on abundance-weighted skewness and kurtosis using an extensive field survey. This should circumvent such a methodological limitation because: (i) the shape of the distribution is driven by the abundance of traits within the community; (ii) the sampling effort for species relative abundance is standardized across communities; and (iii) skewness and kurtosis were largely independent from local species richness in our dataset (Figs. 2 and 3). Instead, the observed decrease in the predictive power of our models when using the higher moments likely reflects a decrease in the abiotic determinism of the moments of trait distributions. The mean and the variance of trait distributions reflect the functional type and diversity of plant communities (Mouillot et al. 2011); in turn, this reflects the effect of the abiotic environment in sorting species with a given set of traits values. By contrast, the shape parameters reflect the intrinsic structure of plant communities and how the abundance and trait diversity are assembled and distributed locally (see Gross et al. 2009 for an experimental test on how biotic interactions can shape trait abundance distribution). Skewness and kurtosis are then likely encompassing not only abiotic factors, but also the biotic processes involved in shaping plant diversity (Schamp et al. 2008; Gross et al. 2009; Butterfield & Munson 2016). Hence, this work provides strong evidence that these parameters are crucial for improving our predictions of the effects of climate change on plant communities and associated ecosystem functions (Enquist et al. 2015).
Conclusions
Our study, which is based on the four moments of trait distributions and that considers interactions between multiple abiotic stress drivers, plays an important role in depicting the complex effects of environmental filtering on plant functional trait diversity in global drylands. This approach would certainly gain predictive power by integrating intraspecific trait variability that can strongly impact plant community assembly (e.g. Le Bagousse-Pinguet et al. 2014, 2015; Siefert et al. 2015), and particularly by considering complex shapes of individual-level trait distributions (Laughlin et al. 2015). We show that interactions between climate and soil variables highlight the importance of environmental filtering and are fundamental in the understanding of trait diversity patterns. Identifying the combinations of environmental factors leading to lower functional diversity is of primary importance to better understand and predict how global environmental change will impact plant communities in drylands.
Supporting Information
Additional supporting information may be found in the online version of this article
Acknowledgements
We specially acknowledge all the member of the EPES-BIOCOM network for their contribution to the global dryland database used. We are grateful to Drs. I. J. Wright, M. Avolio, and A.T. Austin, as well as two anonymous reviewers for valuable comments on earlier versions, to Dr. A. Siefert for providing plant trait data, and to Dr. A. MacRae-Cerar for editing the english. This research was supported by the European Research Council under the European Community's Seventh Framework Programme (FP7/2007-2013)/ERC Grant agreement 242658 (BIOCOM). Y.L.B.P. is supported by the project Postdoc USB (reg.no. CZ.1.07/2.3.00/30.0006) realized through EU Education for Competitiveness Operational Programme. This project is funded by European Social Fund and Czech State Budget. Y.L.B.P is also supported by a Marie Sklodowska-Curie Actions Individual Fellowship (MSCA-IF) within the European Program Horizon 2020 (DRYFUN Project 656035). N.G. has received the support of the EU in the framework of the Marie-Curie FP7 COFUND People Programme, through the award of an AgreenSkills + fellowship (under grant agreement n° 609398). F.T.M. acknowledges support from the Salvador de Madariaga program of the Spanish Ministry of Education, Culture and Sports (PRX14/00225) and the Research Exchange Program of the Hawkesbury Institute for the Environment during the writing of this article. V.M. is supported by the grant NSERC-2016-05716. F.d.B. is funded by the Czech Science Foundation, grant GA16-15012S. C.R.F. is supported by a fellowship from the Conselho Nacional de Desenvolvimento Científico e Tecnológico - Brazil (PQ 305304/2013-5). P.L. received support from the European Union’s Seventh Framework Programme for research, technological development and demonstration under grant agreement no GA-2010-267243 – PLANT FELLOWS.
Footnotes
Author Contribution
Y.L.B.P., N.G., V.M., F.T.M. and P.L. developed the conceptual and methodological foundation of this study. Y.L.B.P. and N.G. conducted statistical analyses. F.T.M designed the field study and coordinated field data acquisition. Y.L.B.P., N.G., C.R.T., J.K., E.V and F.T.M. provided plant trait data. Y.L.B.P., N.G. and P.L. wrote the first draft, and all authors substantially contributed to the subsequent drafts.
Data Accessibility
All data associated with this manuscript are available from figshare: https://figshare.com/s/25987d7f8d8fda8206cc (Le Bagousse-Pinguet et al. 2017), as well as in Appendix S6.
References
- Barton K. MuMIn: Multi-model inference. R package version 1.9.0 ed 2013. [Google Scholar]
- Burnham KP, Anderson DR. Model selection and multi-model inference: a practical information-theoretic approach. Second editon. Springer-Verlag; New Yourk, USA: 2002. [Google Scholar]
- Butterfield BJ, Briggs JM. Regeneration niche differentiates functional strategies of desert woody plant species. Oecologia. 2011;165:477–478. doi: 10.1007/s00442-010-1741-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield BJ, Munson SM. Temperature is better than precipitation as a predictor of plant community assembly across a dryland region. Journal of Vegetation Science. 2016 doi: 10.1111/jvs.12440. [DOI] [Google Scholar]
- Chesson P. Mechanisms of maintenance of species diversity. Annual Review of Ecology and Systematics. 2000;31:343–366. [Google Scholar]
- Chesson P, Gebauer RLE, Schwinning S, Huntly N, Wiegand K, Ernest MSK, et al. Resource pulses, species interactions, and diversity maintenance in arid and semi-arid environments. Oecologia. 2004;141:236–253. doi: 10.1007/s00442-004-1551-1. [DOI] [PubMed] [Google Scholar]
- Coyle JR, Halliday FW, Lopez BE, Palmquist KA, Wilfahrt PA, Hurlbert AH. Using trait and phylogenetic diversity to evaluate the generality of the stress-dominance hypothesis in eastern North American tree communities. Ecography. 2014;37:814–826. [Google Scholar]
- Cornwell WK, Ackerly DD. Community assembly and shifts in plant trait distributions across an environmental gradient in coastal California. Ecological Monographs. 2009;79:109–126. [Google Scholar]
- Delgado-Baquerizo M, Garcia-Palacios P, Milla R, Gallardo A, Maestre FT. Soil characteristics determine soil carbon and nitrogen availability during leaf litter decomposition regardless of litter quality. Soil Biology and Biochemistry. 2015;81:134–142. [Google Scholar]
- Delgado-Baquerizo M, Maestre FT, Reich PB, Jeffries TC, Gaitan JJ, Encinar D, et al. Microbial diversity drives multifunctionality in terrestrial ecosystems. Nature Communications. 2016;7:10541. doi: 10.1038/ncomms10541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bello F, Lavorel S, Lavergne S, Albert CH, Boulangeat I, Mazel F, et al. Hierarchical effects of environmental filters on the functional structure of plant communities: a case study in the French Alps. Ecography. 2013;36:393–402. [Google Scholar]
- Diaz S, Kattge J, Cornelissen JHC, Wright IJ, Lavorel S, Dray S, et al. The global spectrum of plant form and function. Nature. 2016;529:167–171. doi: 10.1038/nature16489. [DOI] [PubMed] [Google Scholar]
- Enquist BJ, Norrberg J, Bonser SP, Violle C, Webb CT, Henderson A, et al. Scaling from traits to ecosystems: developing a General Trait Theory via integrating Trait-based and Metabolic Scaling theories. Advances in Ecological Research. 2015 in press. [Google Scholar]
- Fonseca CR, Overton JM, Collins B, Westoby M. Shifts in trait-combinations along rainfall and phosphorus gradients. Journal of Ecology. 2000;88:964–977. [Google Scholar]
- Freschet GT, Dias ATC, Ackerly DD, Aerts R, Van Bodegom PM, Cornwell WK, et al. Global to community scale differences in the prevalence of convergent over divergent leaf trait distributions in plant assemblages. Global Ecology and Biogeography. 2011;5:755–765. [Google Scholar]
- Fridley JD, Grime JP, Askew AP, Moser B, Stevens CJ. Soil heterogeneity buffers community response to climate change in species-rich grassland. Global Change Biogeography. 2011;17:2002–2011. [Google Scholar]
- Grime JP. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. American Naturalist. 1977;111:1169–1194. [Google Scholar]
- Grime JP. Trait convergence and trait divergence in herbaceous plant communities: mechanisms and consequences. Journal of Vegetation Science. 2006;17:255–260. [Google Scholar]
- Gross N, Robson TM, Lavorel S, Albert CH, Le Bagousse-Pinguet Y, Guillemin R. Plant response traits mediate the effects of subalpine grasslands on soil moisture. New Phytologist. 2008;180:652–662. doi: 10.1111/j.1469-8137.2008.02577.x. [DOI] [PubMed] [Google Scholar]
- Gross N, Kunstler G, Liancourt P, De Bello F, Suding KN, Lavorel S. Linking individual response to biotic interactions with community structure: a trait-based framework. Functional Ecology. 2009;23:1167–1178. [Google Scholar]
- Gross N, Börger L, Soriano-Morales SI, Le Bagousse-Pinguet Y, Quero J-L, Garcia-Gomez M, Valencia-Gomez E, Maestre FT. Uncovering multiscale effects of aridity and biotic interactions on the functional structure of Mediterranean shrublands. Journal of Ecology. 2013;101:637–649. [Google Scholar]
- Hengl T, Mendes De Jesus J, Macmillan RA, Batjes NH, Heuvelink GBM, Ribeiro EC, et al. SoilGrids1 km - global soil information based on automated mapping. PLoS ONE. 2014;9(8):e105992. doi: 10.1371/journal.pone.0105992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology. 2005;25:1965–1978. [Google Scholar]
- IPCC. Climate change 2013: the physical science basis. In: Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM, editors. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press; Cambridge, UK: 2013. [Google Scholar]
- Jenny H. Factors of soil formation: a system of quantitative pedology. edn. McGraw-Hill; New York: 1941. [Google Scholar]
- Kattge J, Díaz S, Lavorel S, Prentice IC, Leadley P, Bönisch G, Garnier E, Westoby M, Reich PB, Wright IJ, et al. TRY–a global database of plant traits. Global Change Biology. 2011;17:2905–2935. [Google Scholar]
- Keddy PA. Assembly and response rules: two goals for predictive community ecology. Journal of Vegetation Science. 1992;3:157–164. [Google Scholar]
- Koch GW, Sillett SC, Jennings GM, Davis SD. The limits to tree height. Nature. 2004;428:851–854. doi: 10.1038/nature02417. [DOI] [PubMed] [Google Scholar]
- Kraft NJB, Adler PB, Godoy O, James EC, Fuller S, Levine JM. Community assembly, coexistence and the environmental filtering metaphor. Functional Ecology. 2015;29:592–599. [Google Scholar]
- Huang J, Yu H, Guan X, Wang G, Guo R. Accelerated dryland expansion under climate change. Nature Climate Change. 2016;6:166–171. [Google Scholar]
- Laliberté E, Zemumik G, Turner BL. Environmental filtering explains variation in plant diversity along resource gradients. Science. 2014;345:1602–1605. doi: 10.1126/science.1256330. [DOI] [PubMed] [Google Scholar]
- Lamanna C, Blonder B, Violle C, Kraft NJB, Sandel B, Simova I, et al. Functional trait space and the latitudinal diversity gradient. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:13745–13750. doi: 10.1073/pnas.1317722111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin DC, Laughlin DE. Advances in modelling trait-based plant community assembly. Trends in Plant Science. 2013;18:584–593. doi: 10.1016/j.tplants.2013.04.012. [DOI] [PubMed] [Google Scholar]
- Laughlin DC, Joshi C, Richardson SJ, Peltzer DA, Mason NWM, Wardle DA. Quantifying multimodal trait distributions improves trait-based predictions of species abundances and functional diversity. Journal of Vegetation Science. 2015;26:46–57. [Google Scholar]
- Le Bagousse-Pinguet Y, de Bello F, Vandewalle M, Leps J, Sykes MT. Species richness of limestone grasslands increases with trait overlap: evidence from within- and between-species functional diversity partitioning. Journal of Ecology. 2014;102:466–474. [Google Scholar]
- Le Bagousse-Pinguet Y, Börger L, Quero J-L, Garcia-Gomez M, Soriano S, Maestre FT, et al. Traits of neighbouring plants and space limitation determine intraspecific trait variability in semi-arid shrublands. Journal of Ecology. 2015;103:1647–1657. [Google Scholar]
- Le Bagousse-Pinguet Y, Gross N, Maestre FT, Maire V, de Bello F, Fonseca C, et al. Testing the environmental filtering concept in global drylands. Figshare. 2017 doi: 10.1111/1365-2745.12735. https://figshare.com/s/25987d7f8d8fda8206cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liancourt P, Spence LA, Song DS, Lkhagva A, Sharkuu A, Boldgiv B, et al. Plant response to climate change varies with topography, interactions with neighbours, and ecotype. Ecology. 2013;94:444–453. doi: 10.1890/12-0780.1. [DOI] [PubMed] [Google Scholar]
- Maestre FT, Quero JL, Gotelli NJ, Escudero A, Ochoa V, Delgado-Baquerizo M, García-Gómez M, Bowker MA, Soliveres S, Escolar C, et al. Plant Species Richness and Ecosystem Multifunctionality in Global Drylands. Science. 2012a;335:214–218. doi: 10.1126/science.1215442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestre FT, Salguero-Gomez R, Quero JL. It is getting hotter in here: determining and projecting the impacts of global environmental change on drylands. Philosophical Transactions of the Royal Society B. 2012b;367:3062–3075. doi: 10.1098/rstb.2011.0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maire V, Gross N, Börger L, Wirth C, Proulx R, da Silveira Pontes L, Soussana J-F, Louault F. Habitat-filtering and niche differentiation jointly determine species relative abundance within grassland communities along fertility and disturbance gradients. New Phytologist. 2012;196:497–509. doi: 10.1111/j.1469-8137.2012.04287.x. [DOI] [PubMed] [Google Scholar]
- Maire V, Wright IJ, Prentice IC, Batjes NH, Bhaskar R, Bodegom PM, et al. Global effects of soil and climate on leaf photosynthetic traits and rates. Global Ecology and Biogeography. 2015;24:706–717. [Google Scholar]
- Majekova M, Paal T, Plowman NS, Bryndova M, Kasari L, Norberg A, et al. Evalutating functional diversity: missing trait data and the importance of species abundance structure and data transformation. PLoS ONE. 2016;11:e0149270. doi: 10.1371/journal.pone.0149270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moles AT, Warton DI, Warman L, Swenson NG, Laffan SW, Zanne AE, et al. Global patterns in plant height. Journal of Ecology. 2009;97:923–932. [Google Scholar]
- Moles AT, Perkins SE, Laffan SW, Flores-Moreno H, Awasthy M, Tindall ML, et al. Which is a better predictor of plant traits: temperature or precipitation? Journal of Vegetation Science. 2014;25:1167–1180. [Google Scholar]
- Mouillot D, Villéger S, Scherer-Lorenzen M, Mason NWH. Functional structure of biological communities predicts ecosystem multifunctionality. PLoS ONE. 2011;6:e17476. doi: 10.1371/journal.pone.0017476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noy Meier I. Desert ecosystems: environment and producers. Annual Review of Ecology and Systematics. 1973;4:25–51. [Google Scholar]
- Ordonez JC, van Bodegom PM, Witte J-PM, Wright IJ, Reich PB, Aerts R. A global study of relationships between leaf traits, climate and soil measures of nutrient fertility. Global Ecology and Biogeography. 2009;18:137–149. [Google Scholar]
- Peñuelas J, Sardans J, Estiarte M, Ogaya R, Carnicer J, Coll M, et al. Evidence of current impact of climate change on life: a walk from genes to the biosphere. Global Change Biology. 2013;19:2303–2338. doi: 10.1111/gcb.12143. [DOI] [PubMed] [Google Scholar]
- Poorter H, Niinemets Ü, Poorter L, Wright IJ, Villar R. Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytologist. 2009;182:565–588. doi: 10.1111/j.1469-8137.2009.02830.x. [DOI] [PubMed] [Google Scholar]
- Prentice IC, Dong N, Gleason SM, Maire V, Wright IJ. Balancing the costs of carbon gain and water transport: testing a new theoretical framework for plant functional ecology. Ecology Letters. 2014;17:82–91. doi: 10.1111/ele.12211. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2012. ISBN 3-900051-07-0, URL http://www.R-project.org/ [Google Scholar]
- Reich PB, Hobbie SE, Lee T, Ellsworth DS, West JB, Tilman D, et al. Nitrogen limitation constrains sustainability of ecosystem response to CO2. Nature. 2006;440:922–925. doi: 10.1038/nature04486. [DOI] [PubMed] [Google Scholar]
- Schamp BS, Chau J, Aarssen LW. Dispersion of traits related to competitive ability in an old-field plant community. Journal of Ecology. 2008;96:204–212. [Google Scholar]
- Siefert A, Violle C, Chalmandrier L, Albert CH, Taudiere A, Fajardo A, et al. A global meta-analysis of the relative extent of intraspecific trait variability in plant communities. Ecology Letters. 2015;18:1406–1419. doi: 10.1111/ele.12508. [DOI] [PubMed] [Google Scholar]
- Simova I, Violle C, Kraft NJB, Storch D, Svenning J-C, Boyle B, et al. Shifts in trait means and variances in North American tree assemblages: species richness patterns are loosely related to the functional space. Ecography. 2015;38:649–658. [Google Scholar]
- Simpson AH, Richardson SJ, Laughlin DC. Soil-climate interactions explain variation in foliar, stem, root and reproductive traits across temperate forests. Global Ecology and Biogeography. 2016;25:964–968. [Google Scholar]
- Swenson NG, Enquist BJ, Pither J, Kerkhoff AJ, Boyle B, Weiser MD, et al. The biogeography and filtering of woody plant functional diversity in North and South America. Global Ecology and Biogeography. 2012;21:798–808. [Google Scholar]
- Valencia E, Maestre FT, Le Bagousse-Pinguet Y, Quéro JL, Tamme R, Börger L, et al. Functional diversity enhances the resistance of ecosystem multifunctionality to aridity in Mediterranean drylands. New Phytologist. 2015;206:660–671. doi: 10.1111/nph.13268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Violle C, Reich PB, Pacala SW, Enquist BJ, Kattge J. The emergence and promise of functional biogeography. Proceedings of the National Academy of Sciences. 2014;111:13690–13696. doi: 10.1073/pnas.1415442111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiher E, Clarke GDP, Keddy PA. Community assembly rules, morphological dispersion, and the coexistence of plant species. Oikos. 1998;81:309–322. [Google Scholar]
- Wright IJ, Reich PB, Westoby M. Strategy-shifts in leaf physiology, structure and nutrient content between species of high and low rainfall, and high and low nutrient habitats. Functional Ecology. 2001;15:423–434. [Google Scholar]
- Wright IJ, Reich PB, Westoby M. Least-cost input mixtures of water and nitrogen for photosynthesis. The American Naturalist. 2003;161:98–111. doi: 10.1086/344920. [DOI] [PubMed] [Google Scholar]
- Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, et al. The worldwide leaf economics spectrum. Nature. 2004;428:821–827. doi: 10.1038/nature02403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.