Abstract
Objective
Identify hospital-level care variations and association with length of stay (LOS) and hospital revisit in children with tracheostomies hospitalized for bacterial respiratory tract infections (bRTI).
Method
Multicenter retrospective cohort study using the Pediatric Health Information System database between 2007–2014 of patients with tracheostomies aged ≤18 years with a primary diagnosis of bRTI (e.g. tracheitis) or a primary diagnosis of bRTI symptom (e.g. cough) and secondary diagnosis of bRTI. Primary outcomes were LOS and 30-day all-cause revisit rates. Secondary outcomes included hospital-level diagnostic testing and anti-Pseudomonas antibiotic use. We used mixed effects negative binomial (for LOS) and logistic (for revisit) to explore the relationship between hospital-level diagnostic test utilization and the outcomes.
Results
Data representing 4137 unique patients with a median age of three years old (IQR 1–9 years) were included. Median LOS was 4 days (IQR: 3–8 days) and 30-day revisit rate was 24.9%. Use of diagnostic testing and empiric anti-Pseudomonas antibiotics varied significantly among hospitals (all p-values <0.001). After adjusting for patient and hospital characteristics, compared to low test utilization hospitals, there were no differences in 30-day all cause revisit rates in moderate (aOR=1.19; 95% CI: 0.93–1.52) or high (aOR=1.07; 95% CI: 0.82–1.39) utilization hospitals. LOS in hospitals with moderate (% difference= −0.8%; 95% CI: −14.4–14.9%) or high (% difference=13.9%; 95% CI: −0.7–30.6%) test utilization hospitals was not significantly longer.
Conclusions
Given that care variations were not associated with outcomes, future research should focus on standardizing diagnosis and treatment of bRTI and readmission prevention in this population.
In the United States, children with tracheostomies account for over $2.6 billion in estimated hospital charges each year1, 2. Of the 4000 pediatric tracheostomies placed each year3, 4, in-hospital post-tracheotomy mortality rates are 7–10%4, 5 and six-month all-cause hospital readmission rates are as high as 60%.6 Because tracheostomies bypass the respiratory tract’s normal immunologic protection, children with tracheostomies have increased risk for bacterial respiratory tract infections (bRTI) requiring repeated hospitalizations and antibiotic courses. A retrospective study identified bacterial pneumonia as the most common reason for which pediatric patients with tracheostomies were admitted to the hospital, accounting for over 2000 admissions and $100 million in 20097. In addition to bacterial pneumonia, children with tracheostomies are at risk for aspiration pneumonia and bacterial tracheitis. Although individual hospitals may have clinical pathways for inpatient care, unlike pediatric community-acquired pneumonia8 or adult ventilator-associated pneumonia9, there are no national guidelines for diagnosis or treatment of suspected bRTI in tracheostomized children10–13. Further, the contribution of chronic bacterial colonization and viral co-infection may make differentiation between the heterogeneous respiratory infections challenging.
While studies of children hospitalized for community-acquired bacterial pneumonia have shown wide variations in diagnostic test use and outcomes14, 15, care variation and outcomes in children with tracheostomies admitted with bRTI are unknown. The objective of the current study is to identify hospital-level diagnostic and treatment variations in the care of children with tracheostomies hospitalized at children’s hospitals for bRTI and to assess associations between hospital level-care variations, length of stay (LOS), and 30-day all-cause revisit rate.
Materials and Methods
Study Design/Data Source
This is a multicenter retrospective cohort study utilizing administrative data from the Pediatric Health Information System (PHIS) database between 2007 and 2014. PHIS contains inpatient, emergency department, ambulatory surgery and observation unit resource utilization data from 48 not-for-profit, tertiary care pediatric hospitals in the United States. De-identified data are subjected to a number of reliability and validity checks before inclusion in the database16. For the period studied, 45 hospitals contributed data and were evaluated for inclusion. The study was reviewed by the Children’s Hospital Los Angeles Institutional Review Board and was granted an exemption per 45 CFR 46.101[b][4].
Patient selection
We included any patient aged 0–18 years with an International Classification of Diseases-9 (ICD-9) code consistent with tracheostomy status (V44.0, V55.0, 519.00, 519.01, 519.02, and 519.09)4, 7, 17 who had the following: (1) an ICD-9 code consistent with a primary discharge diagnosis of bRTI [those previously validated for bacterial community-acquired pneumonia14, 18, as well as aspiration pneumonia (507.0, 507.8) and acute tracheitis (464.1x)] or (2) a primary discharge diagnosis of a symptom of a bRTI (e.g. tachypnea) with a secondary diagnosis of a bRTI.
We excluded any patient hospitalized over 30 days because this represented a population outlier prior to applying any exclusion criteria (median=7 days, IQR=4–14 days). We excluded any patient transferred from an outside hospital given our interest in test utilization and LOS upon hospital admission. We also excluded any patient who did not receive antibiotics on hospital day 0 or 1 due to the likelihood that these patients did not have a bRTI as their primary reason for admission. One hospital was excluded for not reporting test utilization data. To decrease potential bias that might be introduced by recurrent bRTI admissions represented in the PHIS database, we selected one unique hospitalization per patient at random for inclusion (median number of admissions=1; IQR: 1–2). The final sample included 8369 encounters from 44 hospitals, and 4137 unique patients for possible inclusion.
Outcomes
Primary outcomes were LOS and 30-day all-cause revisit rates. We defined LOS as total number of hospital days at midnight. We defined a 30-day revisit as any inpatient, observation unit or emergency room visit within 30 days of discharge from the index admission. Secondary outcomes included hospital-level diagnostic testing, identified by Clinical Transaction Classification (CTC) codes in the PHIS database, such as laboratory tests [complete blood count (CBC), respiratory culture, blood culture, electrolytes, blood gas, viral testing, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR)] and chest radiographs. In examining respiratory cultures, many sites did not code specifically for respiratory cultures. We attempted to resolve this by contacting several participating hospitals about their practices, but given variability in respiratory culture coding across participating hospitals, we categorized codes for respiratory, unspecified, other source, or subcultures all as respiratory cultures. In addition to diagnostic testing, we also analyzed the use of antibiotics targeting Pseudomonas aeruginosa (P. aeruginosa; Supplemental Table 1) on hospital day 0 or 1, receipt of mechanical ventilation and ICU admission at any time during the hospitalization. Of the 44 hospitals providing data, for each specific item, between 41 and 44 hospitals each submitted data.
Covariates
Covariates of interest included age at admission, gender, race (categorized as white, black or other), Hispanic ethnicity, and insurance (categorized as public, private or other). We used Feudtner et al.’s complex chronic condition (CCC; version 1)19 to describe significant medical comorbidities in the patient population. We defined gastrostomy tube status through the presence of an ICD-9- procedure code (43.1x, and 97.02) or diagnosis code (v44.1, v55.1, and 536.4x)20. We defined ventilator dependency by the presence of an ICD-9 code of V46.1X at discharge. To compare disease severity across different All Patient Refined Diagnostic Related Groups (APR-DRGs), we used the National Association of Children’s Hospitals and Related Institutions Pediatric LOS weight; this is a normalized value of a patient’s expected LOS, given their principal APR-DRG and severity of illness. Similar to the case mix index, this allows us to compare hospital-level patient severity across multiple different APR-DRGs.
Statistical methodology
Descriptive statistics for demographic variables included means and standard deviations for normally distributed continuous data, medians and interquartile ranges (IQR) for non-normally distributed continuous data, and proportions for categorical data. To assess hospital-level care variations in diagnostic testing, antibiotic use, mechanical ventilation and ICU use, observed proportions of use were computed by hospital. Hospital-level proportions were adjusted using mixed effects logistic regression; patient-level use as the dependent variable was adjusted for patient-level covariates (age, gender, race, Hispanic ethnicity, type of insurance) and hospital-level covariates (average daily census, geographic region, average patient severity). Hospital-level random intercepts were specified. Model-predicted adjusted proportions were estimated for each hospital, representing the average use of each item for hospitals with the same patient- and hospital-level characteristics.
For each hospital, a measure of overall use of diagnostic testing (CBC, respiratory culture, blood culture, electrolytes, blood gas, viral testing, CRP, ESR and chest radiograph) relative to other hospitals was computed in the following manner: for each test, relative hospital-level use (calculated as the proportion of patients in that hospital’s cohort receiving each test) was evaluated using mixed effects logistic regression, with hospital-level random intercepts estimating the deviation of each hospital from the average use over hospitals. Hospital-level test statistics were computed as the estimated random intercept divided by standard error; the statistics were ranked over all hospitals, with ranks representing relative use (from lowest to highest) of the diagnostic test. Overall utilization for each hospital was then calculated as the sum of ranks over all diagnostic tests. To standardize this for hospitals not reporting all nine elements, we calculated the average rank for those reported and multiplied by nine.
To assess the associations of overall hospital-level diagnostic test utilization with patient outcomes (LOS and 30-day all-cause revisit), categories of low, moderate, and high resource utilization were defined by tertiles of the summed rank measure. Primary patient-level outcomes evaluated were hospital LOS (using mixed effects negative binomial regression) and 30-day all-cause revisit (using mixed effects logistic regression). Hospital-level random intercepts were specified. The associations of tertiles of hospital-level utilization with these outcomes were estimated and tested in unadjusted models and in models adjusted for patient age, gender, race, Hispanic ethnicity, type of insurance, hospital region, and average daily census. The 30-day all-cause revisit analysis additionally adjusted for hospital average pediatric disease severity. Using the low utilization tertile as the reference group, associations were summarized as adjusted odds ratios (aORs) with 95% confidence intervals for 30-day revisit, and percentage difference in means for LOS. The association between hospital-level use of antibiotics for P. aeruginosa and these outcomes was evaluated with these same patient outcomes in similar mixed effects logistic regression models. All analyses were conducted using STATA statistical software, version 13 (Stata Statistical Software: Release 13. College Station, TX: StataCorp LP).
Results
For the 4137 discharges included, the median age on admission was 3 years (IQR: 1–9 years). Patients were primarily male (57.4%; n=2375), Caucasian (53.5%; n=2214), and on public insurance (71.7%; n=2965). The population had a high level of technology dependence, with 75% (n=3103) having gastrostomy tubes and 32.4% (n=1342) having ventilator dependence. Nearly 52 % (n=2134) received mechanical ventilation at some point during their hospitalization and 37% (n=1530) of patients were admitted to an intensive care unit (ICU; Table 1). Median LOS for the cohort was 4 days (IQR: 3–8 days). In-hospital mortality for this cohort was 0.7% (n=28). Of the 4137 discharges, 24.9% (n=1030) had a hospital revisit within 30 days, with 68.6% (n=707) to inpatient status, 6% (n=62) to observation status, and 25.3% (n=261) to the emergency room. There was no association between hospital-level LOS and revisit rates (Spearman r=−0.22; p=0.15). Primary discharge diagnoses were bacterial pneumonia (43.5%, n=1799), tracheitis (43.3%; n=1792), aspiration pneumonia (8.7%; n=360) and other (4.5%; n=186; Supplemental Table 2). Patients with a primary diagnosis in the other category had higher proportions of cardiovascular or hematological CCCs, higher rates of mechanical ventilation while hospitalized and higher ICU utilization (Supplemental Table 2).
Table 1.
Demographic characteristics of pediatric patients with tracheostomies admitted with bacterial respiratory tract infections in the study population (n=4137 unique patients)
| Variable | Total Sample |
|---|---|
| N (%) | 4137 (100%) |
| Age (median, IQR) | 3 (1,9) |
| Gender | |
| Male | 2375 (57.4%) |
| Female | 1762 (42.6%) |
| Racea | |
| White | 2214 (53.5%) |
| Black | 906 (21.9%) |
| Other/Missing | 1042 (25.2%) |
| Hispanic | 917 (22.2%) |
| Insurance | |
| Public | 2965 (71.7%) |
| Private | 1027 (24.8%) |
| Self-pay, other, missing | 145 (3.5%) |
| Complex Chronic Conditions | |
| Total Number (median, IQR) | 3 (2,4) |
| Cardiovascular | 738 (17.8%) |
| Gastrointestinal | 3367 (81.4%) |
| Hematological | 147 (3.6%) |
| Oncological | 99 (2.4%) |
| Metabolic | 272 (6.6%) |
| Neuromuscular | 2024 (48.9%) |
| Congenital | 1324 (32.0%) |
| Renal | 300 (7.3%) |
| Respiratory | 4137 (100%) |
| Other Comorbidities | |
| Gastrostomy Tube | 3103 (75.0%) |
| Ventilator Dependency | 1342 (32.4%) |
| Received Mechanical Ventilation during Hospitalization | 2134 (51.6%) |
| Admitted to ICU | 1530 (37.0%) |
| Discharge Disposition | |
| Home | 3827 (92.5%) |
| Expired | 28 (0.7%) |
| Transfer to another, other, missing | 282 (6.8%) |
Race numbers add up to more than number of patients, as some reported multiple race categories
Unadjusted and adjusted diagnostic test, antibiotic, ICU and mechanical ventilation utilization is presented in Table 2. The most frequently used diagnostic tests were chest radiographs, CBCs, and respiratory cultures, while the least used resources were ESRs, CRPs, and viral testing. Utilization varied significantly among hospitals in both the unadjusted and adjusted analyses (all p-values for hospital-specific differences <0.001), with respiratory cultures, blood cultures, blood gas and viral testing showing the widest variations. Respiratory cultures were obtained in all patients admitted at some hospitals, but in fewer than 20% of patients admitted at other hospitals.
Table 2.
Variations in hospital-level diagnostic testing, ICU/ventilator use and anti-Pseudomonas antibiotic use
| Unadjusted distribution across hospitals (% patients receiving) |
Adjusted distribution across hospitals (% patients receiving)a |
|||
|---|---|---|---|---|
|
| ||||
| Resource (number of hospitals) | Median Percentage (IQR) | Range | Median Percentage (IQR) | Range |
| Chest radiograph (n=44) | 83.8 (76.3, 90.2) | 60.0, 97.9 | 84.9 (81.0, 88.3) | 76.4, 94.1 |
|
| ||||
| Laboratory Tests | ||||
| Complete blood count (n=44) | 82.4 (76.3, 88.4) | 17.0, 96.6 | 83.3 (78.3, 88.8) | 74.9, 98.8 |
| Respiratory culture (n=44) | 81.2 (76.8, 86.5) | 8.3, 96.8 | 78.6 (72.6, 82.8) | 64.3, 92.7 |
| Blood culture (n=43) | 66.7 (50.5, 72.2) | 0, 87.2 | 63.3 (57.4, 69.8) | 44.1, 84.8 |
| Serum electrolytes (n=42) | 62.6 (41.1, 75.4) | 0, 93.1 | 55.3 (47.8, 61.7) | 31.2, 77.4 |
| Blood gas (n=44) | 50.3 (38.3, 62.8) | 9.4, 84.7 | 52.1 (49.1, 57.5) | 38.6, 71.4 |
| Viral testing (n=41) | 23.7 (12.6, 35.9) | 0, 88.2 | 21.0 (14.2, 29.9) | 4.3, 66.1 |
| C-reactive protein (n=44) | 14.3 (5.2, 36.2) | 0, 78.9 | 17.1 (12.3, 21.9) | 6.9, 33.0 |
| ESR (n=44) | 3.0 (1.3, 5.5) | 0, 13.0 | 3.1 (2.7, 3.6) | 2.0, 6.1 |
|
| ||||
| Antibiotics targeting Pseudomonas on hospital day 0 or 1 (n=44) | 67.3 (55.2, 82.0) | 31.2, 90.4 | 68.9 (66.1, 71.9) | 55.9, 79.2 |
|
| ||||
| Patients receiving mechanical ventilation during hospitalization (n=44) | 50.4 (43.2, 59.8) | 20.7, 82.3 | 49.4 (45.7, 53.6) | 42.0, 69.5 |
|
| ||||
| Patients with ICU admission during hospitalization (n=44) | 37.6 (20.5, 52.8) | 2.0, 91.5 | 36.1 (32.3, 40.1) | 18.4, 58.0 |
Adjusted for patient age, gender, race, Hispanic ethnicity, type of insurance (public, private, other), hospital region (Midwest, Northeast, South, West), average daily census, and patient severity.
After dividing included hospitals into tertiles (low, moderate, high) based upon diagnostic test use, we calculated the mean patient severity for hospitals included in each group. Compared to the lowest tertile, there was no significant difference in patient severity at moderate (adjusted % difference= −6.9%; 95% CI: −17.1% to 4.5%) and high tertile hospitals (adjusted % difference= 2.4%; 95% CI: −8.9% to 15.3%).
Hospital-level median LOS varied from 3–7 days in the low tertile of diagnostic test use, 2–6.5 days in the moderate tertile and 3.5–6 days in the high tertile (Table 3). When compared to the lowest tertile, average LOS for patients in the middle tertile was not significantly different in adjusted analysis (percentage difference= −0.8%; 95% CI: −14.4% to 14.9%). Compared to the lowest tertile, average LOS for patients in the highest tertile was significantly longer in unadjusted analysis (% difference 16.4%; 95% CI: 2.3% to 32.4%; p=0.02) but not in adjusted analysis (% difference 13.9%; 95% CI: −0.7 to 30.6%; p=0.06). Therefore, there was no association between hospital-level diagnostic test utilization and LOS.
Table 3.
Associations between Degree of Hospital-Level Diagnostic Test Use, LOS and Return Visit Outcomesa
| Hospital-Level Resource Utilization (Tertile)
|
||||||
|---|---|---|---|---|---|---|
| Unadjusted Analysis | Adjusted Analysisb | |||||
|
|
||||||
| Low | Moderate | High | Low | Moderate | High | |
| LOS | ||||||
| Range of Hospital-Level Medians | 3–7 days | 2–6.5 days | 3.5–6 days | N/A | N/A | N/A |
| % difference (95% CI)c | REF | 0.2% (−13.8%, 16.4%) | 16.4% (2.3%, 32.4%) | REF | −0.8% (−14.4%, 14.9%) | 13.9% (−0.7%, 30.6%) |
| p-value | — | 0.98 | 0.02 | — | 0.91 | 0.06 |
|
| ||||||
| 30-Day Revisit Rate | ||||||
| All-Cause Revisit Rate | 6.2–38.5% | 17.7–33.3% | 10.3–36.9% | N/A | N/A | N/A |
| OR (95% CI) | REF | 1.10 (0.83,1.47) | 0.97 (0.72,1.31) | REF | 1.19 (0.93,1.52) | 1.07 (0.82,1.39) |
| p-value | — | 0.49 | 0.85 | — | 0.16 | 0.63 |
| Inpatient/Observation Readmissions Only | 0–35.9% | 12.8–25.9% | 10.3–31.0% | |||
| OR (95% CI) | REF | 1.01 (0.72, 1.42) | 0.88 (0.62, 1.25) | REF | 1.08 (0.80, 1.45) | 0.93 (0.68, 1.28) |
| p-value | — | 0.97 | 0.48 | — | 0.63 | 0.66 |
| ED revisits only | 0–12.2% | 0–15.7% | 0–13.2% | |||
| OR (95% CI) | REF | 1.40 (0.77, 2.55) | 1.35 (0.76, 2.39) | REF | 1.46 (0.76, 2.78) | 1.50 (0.84, 2.68) |
| p-value | — | 0.27 | 0.30 | — | 0.25 | 0.17 |
30-day revisit outcomes analyzed by mixed effects logistic regression, with random intercept for hospital. LOS outcome analyzed by mixed effects negative binomial regression, with random intercept for hospital.
LOS analyses adjusted for patient age, gender race, Hispanic ethnicity, type of insurance (public, private, other), hospital region (Midwest, Northeast, South, West), and average daily census. 30-day re-visit analysis additionally adjusted for hospital average pediatric LOS weight.
LOS interpreted as percent mean difference in outcome (from low resource utilization). OR = odds ratio, CI = confidence interval.
30-day all-cause revisit rates varied from 6.2–38.5% in the low tertile of diagnostic test use, 17.7–33.3% in the moderate tertile, and 10.3–36.9% in the tertile (Table 3). On adjusted analyses, when compared to the lowest tertile, 30-day all-cause revisit rates for patients hospitalized at moderate (aOR= 1.19; 95% CI: 0.93–1.52) or high (aOR=1.07; 95% CI: 0.82–1.39) utilization hospitals were not significantly different. Our subgroup analysis examining emergency room visits and hospital readmissions separately found no difference between both outcomes and degree of diagnostic test use (Table 3). Thus, there was no association between revisit rate and diagnostic test utilization.
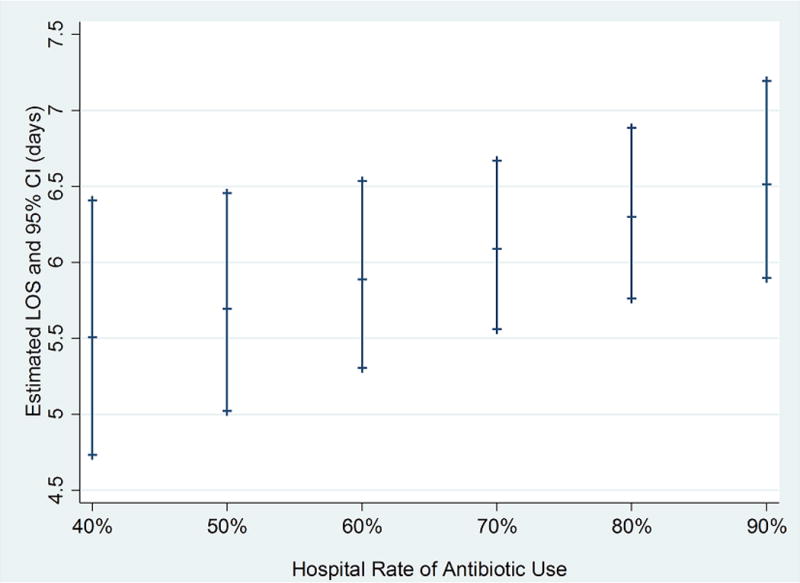
In this cohort, hospital-level median rates of empiric antibiotics targeting P. aeruginosa were 67.3% (IQR: 55.2–82.0%, Table 2). There were no association between patient severity and hospital use of antibiotics targeting P. aeruginosa on adjusted analyses (% difference per 10% increase in antibiotic usage=1.6%; 95% CI: −1.4% to 4.7%). Increased hospital-level use of antibiotics targeting P. aeruginosa was associated with significantly longer LOS (adjusted % difference in average LOS per 10% increase in antibiotic usage=3.4%; 95% CI: 0% to 7.0%; p=0.05; Figure 1). There was no relationship between hospital-level use of anti-Pseudomonas antibiotics and 30-day revisit rate (aOR=0.96 per 10% increase in antibiotic use; 95% CI: 0.91–1.01).
Figure 1.
Discussion
Children with tracheostomies hospitalized for treatment of bRTIs have high 30-day all-cause revisit rates with wide care variations on initial hospital presentation. On admission, most patients received chest radiographs and certain labs (CBC, blood cultures, and respiratory cultures), whereas fewer hospitals routinely used markers of inflammation (ESR, CRP). These care variations upon admission did not appreciably change upon controlling for patient demographics, illness severity and hospital factors. After adjusting for patient demographics, patient severity, hospital region and average daily census, we found no statistically significant associations between overall hospital-level admission diagnostic test utilization and 30-day all-cause revisit rates or LOS. Decreasing variation in diagnostic testing may standardize clinical care without increasing LOS or readmission rates.
Our results are consistent with previous studies showing wide hospital-level care variation in respiratory diseases (e.g., community-acquired pneumonia14, 21, bronchiolitis22, 23) and other pediatric diseases (e.g., tonsillectomy24, abscess incision and drainage25). The exact reasons for the wide differences in diagnostic testing and other resource use in this population are unclear. As noted, there are no national guidelines for respiratory infections in pediatric patients with tracheostomies. Further, there are few clinical studies examining tracheostomy-associated respiratory infections, limiting the ability to create evidence-based care guidelines. Finally, the care variations may be due to patient differences not accounted for in our current study. Our results are consistent with previous pediatric studies that show no correlation between LOS and hospital readmission in community-acquired pneumonia and other common diseases14, 26–28. This suggests that there may be potential to decrease LOS without adversely affecting hospital revisit rates and represents an area for future research. The overall high 30-day revisit rate suggests that there is room for improvement of post-discharge care. There are potential cost savings by decreasing LOS and readmission rates, if the decreased LOS does not lead to higher readmission rates for treatment failures or complications from the index admission. Prospective studies are needed to evaluate the contribution of specific care practices on patient outcomes.
We found high rates of empiric use of antibiotics targeting P. aeruginosa (adjusted hospital median=68.9%), but due to lack of clinical test results from the PHIS database, conclusions are limited. While receipt of anti-Pseudomonas antibiotics is an imperfect measure for a history of Pseudomonas-positive cultures, this suggests either a present or past history of a positive P. aeruginosa culture that lead to coverage of this organism during subsequent admissions. The use of broad-spectrum antibiotics may lead to increased development of resistant organisms and suggests an increased role for antimicrobial stewardship programs. Indeed, variations in hospital-level use of empiric anti-Pseudomonas antibiotics may be due to the presence of antimicrobial stewardship programs that affect overall use of these antibiotics. We also found a significant association between the use of antibiotics targeting P. aeruginosa and longer LOS. The longer LOS may be due to limited enteral alternatives, antibiotic resistance patterns, difficulty obtaining home intravenous antibiotic therapy, hesitance to transition from the intravenous to enteral route, or unmeasured confounders. Future studies should correlate culture results and susceptibilities with appropriate antibiotic utilization.
The current study has several limitations. First, this is an observational, retrospective design that relies on accurate coding and translation of data from patients admitted at PHIS member children’s hospitals. While we used bacterial pneumonia ICD-9 codes validated in previous work18, in children with complex chronic conditions, they identified correctly 71.8% of provider-confirmed community-acquired pneumonia cases, with a high specificity of 91.4%. Given our inability to confirm diagnoses via chart review, by maximizing specificity, we limited inclusion of patients who may not meet our inclusion criteria. Second, the database only provides administrative billing data and, does not include results of tests. There may be some patients treated with antibiotics for positive respiratory cultures that represent chronic colonization and not acute infection. However, we included only patients who received antibiotic therapy and who had a diagnostic code of a bacterial respiratory infection. Third, the data do not include patients hospitalized at community hospitals and may not be representative of all children with tracheostomies admitted with bRTIs. We were not able to capture patient readmission to non-PHIS hospitals; thus, this analysis may underestimate the all-cause revisit rates at 30 days. However, given the medical complexity of the patient population studied, we believe the number of patients readmitted to non-PHIS hospitals is low. Fourth, although we excluded any lateral (inpatient-to-inpatient) hospital transfers, we may have included patients with tests and medications given by a non-hospital referral source (e.g. outpatient clinic, skilled nursing facilities). This, however, would lead to underestimation of resource utilization. Finally, some resource utilization may be influenced by hospital-level care practices rather than patient acuity (e.g. at some hospitals, most or all patients with tracheostomies are admitted to the ICU); we were not able to account for all potential differences between hospitals given the inherent limitations of the dataset.
Despite these limitations, this is the first study to demonstrate high hospital-level 30-day all-cause revisit rates and variable LOS and test utilization in patients with tracheostomies admitted for bRTIs. Because this population accounts for disproportionately high utilization of health care dollars and resources, development of evidence-based best practices for the prevention and treatment of respiratory tract infections is crucial to decreasing expenditures and hospital admissions for this vulnerable population. Given the heterogeneity of this patient population, future research should identify patient-level risk factors for prolonged LOS and readmission in children with tracheostomies admitted with bacterial respiratory infections, controlling for hospital-level factors that may influence test utilization and patient-level outcomes. This information can guide development of evidence-based guidelines for diagnosis and management of children with tracheostomies admitted with bRTIs.
Supplementary Material
Acknowledgments
Acknowledgements: We thank Sharis Mardirosian, MPH and Eugene Nguyen, BA for assistance with data management and Joyce Koh, MD, Vivian Lee, MD and Margaret Trost, MD and other members of the Children’s Hospital Los Angeles PHIS Research Group for providing input on study design and data interpretation. We thank Tamara D. Simon, MD, MSPH for providing feedback.
Funding source: Dr. Russell is a KL2 Scholar awarded under the KL2 Mentoring Research Career Development Award through Southern California Clinical and Translational Science Institute at University of Southern California, Keck School of Medicine. As part of his career development, Dr. Russell was supported by the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), through Grant Award Number KL2TR000131. The content is solely the responsibility of the author(s) and does not necessarily represent the official view of the NIH.
Abbreviations
- aOR
adjusted odds ratio
- APR-DRGs
All Patient Refined Diagnostic Related Groups
- bRTI
bacterial respiratory tract infection(s)
- CCC
complex chronic condition
- CRP
C-reactive protein
- ESR
erythrocyte sedimentation rate
- ICD
International Classification of Diseases
- ICU
intensive care unit
- IQR
interquartile range
- LOS
length of stay
- PHIS
Pediatric Health Information System
Footnotes
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Conflicts of Interest: The authors have no conflicts of interest relevant to this article to disclose.
Contributors’ Statement:
Dr. Russell conceptualized and designed the study, drafted the analytic plan and the initial manuscript, and approved the final manuscript as submitted.
Dr. Mack conducted the statistical analyses, revised the manuscript and approved the final manuscript as submitted.
Drs. Schrager and Wu reviewed and critically revised the manuscript and approved the final manuscript as submitted.
References
- 1.Zhu H, Das P, Roberson DW, Jang J, Skinner ML, Paine M, et al. Hospitalizations in children with preexisting tracheostomy: a national perspective. Laryngoscope. 2015;125(2):462–468. doi: 10.1002/lary.24797. [DOI] [PubMed] [Google Scholar]
- 2.AHRQ. Agency for Healthcare Research and Quality. HCUP KID Database. 2012 Accessed March 1, 2016. [Google Scholar]
- 3.Lewis CW, Carron JD, Perkins JA, Sie KC, Feudtner C. Tracheotomy in pediatric patients: a national perspective. Arch Otolaryngol Head Neck Surg. 2003;129(5):523–529. doi: 10.1001/archotol.129.5.523. [DOI] [PubMed] [Google Scholar]
- 4.Berry JG, Graham RJ, Roberson DW, Rhein L, Graham DA, Zhou J, et al. Patient characteristics associated with in-hospital mortality in children following tracheotomy. Arch Dis Child. 2010;95(9):703–710. doi: 10.1136/adc.2009.180836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu C, Heffernan C, Saluja S, Yuan J, Paine M, Oyemwense N, et al. Indications, Hospital Course, and Complexity of Patients Undergoing Tracheostomy at a Tertiary Care Pediatric Hospital. Otolaryngol Head Neck Surg. 2014;151(2):232–239. doi: 10.1177/0194599814531731. [DOI] [PubMed] [Google Scholar]
- 6.Graf JM, Montagnino BA, Hueckel R, McPherson ML. Pediatric tracheostomies: a recent experience from one academic center. Pediatr Crit Care Med. 2008;9(1):96–100. doi: 10.1097/01.PCC.0000298641.84257.53. [DOI] [PubMed] [Google Scholar]
- 7.Zhu H, Das P, Roberson DW, Jang J, Skinner ML, Paine M, et al. Hospitalizations in children with preexisting tracheostomy: A national perspective. Laryngoscope. 2014 doi: 10.1002/lary.24797. [DOI] [PubMed] [Google Scholar]
- 8.Bradley JS, Byington CL, Shah SS, Alverson B, Carter ER, Harrison C, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25–76. doi: 10.1093/cid/cir531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brook I. Treatment of aspiration or tracheostomy-associated pneumonia in neurologically impaired children: effect of antimicrobials effective against anaerobic bacteria. Int J Pediatr Otorhinolaryngol. 1996;35(2):171–177. doi: 10.1016/0165-5876(96)01332-8. [DOI] [PubMed] [Google Scholar]
- 11.Cline JM, Woods CR, Ervin SE, Rubin BK, Kirse DJ. Surveillance tracheal aspirate cultures do not reliably predict bacteria cultured at the time of an acute respiratory infection in children with tracheostomy tubes. Chest. 2012;141(3):625–631. doi: 10.1378/chest.10-2539. [DOI] [PubMed] [Google Scholar]
- 12.Graf J, Stein F. Tracheitis in pediatric patients. Semin Pediatr Infect Dis. 2006;17(1):11–13. doi: 10.1053/j.spid.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Rusakow LS, Guarin M, Wegner CB, Rice TB, Mischler EH. Suspected respiratory tract infection in the tracheostomized child: the pediatric pulmonologist’s approach. Chest. 1998;113(6):1549–1554. doi: 10.1378/chest.113.6.1549. [DOI] [PubMed] [Google Scholar]
- 14.Brogan TV, Hall M, Williams DJ, Neuman MI, Grijalva CG, Farris RW, et al. Variability in processes of care and outcomes among children hospitalized with community-acquired pneumonia. Pediatr Infect Dis J. 2012;31(10):1036–1041. doi: 10.1097/INF.0b013e31825f2b10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leyenaar JK, Lagu T, Shieh MS, Pekow PS, Lindenauer PK. Variation in Resource Utilization for the Management of Uncomplicated Community-Acquired Pneumonia across Community and Children’s Hospitals. J Pediatr. 2014 doi: 10.1016/j.jpeds.2014.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pennington A, Dobies CG. PHIS Description when Referenced as Data Source. http://bit.ly/1tEESzM. Published 2014. Accessed August 1, 2014.
- 17.Berry JG, Graham DA, Graham RJ, Zhou J, Putney HL, O’Brien JE, et al. Predictors of clinical outcomes and hospital resource use of children after tracheotomy. Pediatrics. 2009;124(2):563–572. doi: 10.1542/peds.2008-3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams DJ, Shah SS, Myers A, Hall M, Auger K, Queen MA, et al. Identifying pediatric community-acquired pneumonia hospitalizations: Accuracy of administrative billing codes. JAMA Pediatr. 2013;167(9):851–858. doi: 10.1001/jamapediatrics.2013.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feudtner C, Christakis DA, Connell FA. Pediatric deaths attributable to complex chronic conditions: a population-based study of Washington State, 1980–1997. Pediatrics. 2000;106(1 Pt 2):205–209. [PubMed] [Google Scholar]
- 20.Barnhart DC, Hall M, Mahant S, Goldin AB, Berry JG, Faix RG, et al. Effectiveness of fundoplication at the time of gastrostomy in infants with neurological impairment. JAMA Pediatr. 2013;167(10):911–918. doi: 10.1001/jamapediatrics.2013.334. [DOI] [PubMed] [Google Scholar]
- 21.Neuman MI, Graham D, Bachur R. Variation in the use of chest radiography for pneumonia in pediatric emergency departments. Pediatr Emerg Care. 2011;27(7):606–610. doi: 10.1097/PEC.0b013e3182225578. [DOI] [PubMed] [Google Scholar]
- 22.Florin TA, Byczkowski T, Ruddy RM, Zorc JJ, Test M, Shah SS. Variation in the management of infants hospitalized for bronchiolitis persists after the 2006 American Academy of Pediatrics bronchiolitis guidelines. J Pediatr. 2014;165(4):786–792.e781. doi: 10.1016/j.jpeds.2014.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christakis DA, Cowan CA, Garrison MM, Molteni R, Marcuse E, Zerr DM. Variation in inpatient diagnostic testing and management of bronchiolitis. Pediatrics. 2005;115(4):878–884. doi: 10.1542/peds.2004-1299. [DOI] [PubMed] [Google Scholar]
- 24.Goyal SS, Shah R, Roberson DW, Schwartz ML. Variation in post-adenotonsillectomy admission practices in 24 pediatric hospitals. Laryngoscope. 2013;123(10):2560–2566. doi: 10.1002/lary.24172. [DOI] [PubMed] [Google Scholar]
- 25.Uspal NG, Klein EJ, Tieder JS, Oron AP, Simon TD. Variation in the use of procedural sedation for incision and drainage of skin and soft tissue infection in pediatric emergency departments. Hosp Pediatr. 2015;5(4):185–192. doi: 10.1542/hpeds.2014-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knighton AJ, Flood A, Speedie SM, Harmon B, Smith P, Crosby C, et al. Does initial length of stay impact 30-day readmission risk in pediatric asthma patients? J Asthma. 2013;50(8):821–827. doi: 10.3109/02770903.2013.816726. [DOI] [PubMed] [Google Scholar]
- 27.Morse RB, Hall M, Fieldston ES, Goodman DM, Berry JG, Gay JC, et al. Children’s hospitals with shorter lengths of stay do not have higher readmission rates. J Pediatr. 2013;163(4):1034–1038.e1031. doi: 10.1016/j.jpeds.2013.03.083. [DOI] [PubMed] [Google Scholar]
- 28.Berry JG, Toomey SL, Zaslavsky AM, Jha AK, Nakamura MM, Klein DJ, et al. Pediatric readmission prevalence and variability across hospitals. Jama. 2013;309(4):372–380. doi: 10.1001/jama.2012.188351. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


