Abstract
Hyaluronan, CD44 and the Receptor for Hyaluronan-Mediated Motility (RHAMM, gene name HMMR) regulate stem cell differentiation including mesenchymal progenitor differentiation. Here, we show that CD44 expression is required for subcutaneous adipogenesis, whereas RHAMM expression suppresses this process. We designed RHAMM function blocking peptides to promote subcutaneous adipogenesis as a clinical and tissue engineering tool. Adipogenic RHAMM peptides were identified by screening for their ability to promote adipogenesis in culture assays using rat bone marrow mesenchymal stem cells, mouse pre-adipocyte cell lines and primary human subcutaneous pre-adipocytes. Oil red O uptake into fat droplets and adiponectin production were used as biomarkers of adipogenesis. Positive peptides were formulated in either collagen I or hyaluronan (Orthovisc) gels then assessed for their adipogenic potential in vivo following injection into dorsal rat skin and mammary fat pads. Fat content was quantified and characterized using micro CT imaging, morphometry, histology, RT-PCR and ELISA analyses of adipogenic gene expression. Injection of screened peptides increased dorsal back subcutaneous fat pad area (208.3 ± 10.4 mm2 versus control 84.11 ± 4.2 mm2; p < 0.05) and mammary fat pad size (45 ± 11 mg above control background, p=0.002) in female rats. This effect lasted >5 weeks as detected by micro CT imaging and perilipin 1 mRNA expression. RHAMM expression suppresses while blocking peptides promote expression of PPARγ, C/EBP and their target genes. Blocking RHAMM function by peptide injection or topical application is a novel and minimally invasive method for potentially promoting subcutaneous adipogenesis in lipodystrophic diseases and a complementary tool to subcutaneous fat augmentation techniques.
Keywords: RHAMM, adipogenesis, hyaluronan, peptides, mesenchymal progenitor cells, pre-adipocytes, adipocyte differentiation, fat augmentation
Introduction
In contrast to visceral fat, subcutaneous fat tissue functions as an active endocrine organ that secretes key factors (e.g., adipokines) regulating systemic metabolism and cognition[1-3]. Subcutaneous fat depots are depleted in congenital disorders, following certain medical treatments (e.g. highly active anti-retroviral therapy), trauma and during aging. The loss of subcutaneous adipose depots coupled with increased organ fat can result in profound insulin resistance, the development of a metabolic syndrome (which increases the risk of heart disease, stroke and diabetes), and a high incidence of pancreatitis, fatty liver, and neurologic co-morbidities. Current adipokine replacement therapies manage aspects of lipodystrophy morbidities but they do not reverse them. Modification of adipogenic signaling pathways using PPARγ agonists such as rosiglitazone have shown efficacy in restoring subcutaneous fat depots in a small number of clinical trials but drug side effects, including increased incidence of heart attacks, will likely prevent their widespread adoption[4]. Currently, autologous fat grafting remains the gold standard technique used to replenish subcutaneous fat for these conditions [5-8]. Fat grafting is also utilized in breast augmentation and reconstruction as a tool to restore overall skin health by temporizing the fibrotic responses to radiation[8-10] and to secondarily address any remaining contour irregularities[11-13]. Although there are many benefits to autologous fat grafting, unpredictable retention patterns, the requirement of a donor site involving liposuction surgery, loss of volume over time and the formation of micro-calcifications remain as drawbacks[14]. Autologous fat graft survival is also heavily influenced by the harvesting and implantation methods. Since adipose stem cell (ASC)–enriched autologous fat grafts potentiate the viability of mature adipocyte grafts[15-17], an ability to reliably stimulate indigenous and graft-associated populations of these progenitor cells would likely circumvent current clinical drawbacks to autologous fat transplantation and provide a tool that in many cases could circumvent the need for the transplantation process.
Adipose stem cells include subpopulations of mesenchymal stem cells (MSC), resident adipose-derived stem cells and pre-adipocytes[18-20]. In order to contribute to the formation of subcutaneous fat pads, subpopulations of these cells must undergo clonal expansion that has been linked to the display of PDGFR∂ and CD44. Additionally, several soluble growth factors and cytokines[21-25] in the extracellular matrix (ECM)[26, 27] have a role in directing the fate of these cells. Native hyaluronan (HA) and osteopontin, which are ligands for CD44, support the proliferation and differentiation of these adipose derived stem cells into adipocytes[28-32]. While native, high molecular weight HA supports survival, proliferation and differentiation of pre-adipocytes, fragmentation of HA suppresses adipocyte maturation. The HA receptors that regulate these divergent effects of HA have not yet been clearly defined. CD44 expression is not required for terminal differentiation of expanded pre-adipocytes into mature adipocytes in response to hypoxia although its role in adipogenesis may be context dependent. HA fragments bind to extracellular forms of RHAMM (gene name, HMMR)[33, 34], which has extra and intracellular functions linked to adipogenesis. Thus, genetic deletion and RNA interference of RHAMM expression promotes pre-adipocyte differentiation[29, 35]. Extracellular RHAMM:HA fragment interactions activate key adipogenic regulators such as MAP kinases (e.g. ERK1) [36, 37] while intracellular RHAMM partners with and regulates ERK1 activity and subcellular distribution[38]. Intracellular RHAMM also partners with ANKRYN26, a protein that suppresses adipogenesis[36, 39]. We reasoned that blocking RHAMM signaling functions in skin would promote subcutaneous adipogenesis for use as a tool in tissue engineering, for treating lipodystrophies and for managing other metabolic diseases.
Here, we use micro CT imaging to show that RHAMM loss enhances adiposity in female mice. We also report the design of synthetic RHAMM-based function blocking peptides that stimulate adipogenesis of mesenchymal progenitors and pre-adipocytes in culture and in vivo. We further show that these function blocking peptides release a RHAMM-mediated inhibition of the master adipogenic transcription factors PPARγ and C/EBP.
Experimental procedures
RHAMM-/- and wildtype mice
RHAMM-/- mice were generated as previously described[40]. All mice were fed regular chow and were bred and housed according to University of Western Ontario animal use committee protocol #2009-060.
Preparation of RHAMM antibodies
Polyclonal RHAMM anti-peptide antibodies were prepared against human synthetic peptides: KRFNDPSGCAPSPGAYD (PAb1), ELMKLRNKRETKMR (PAb2), KLKDENSQLKSEVSK (PAb 3) and CYRAPMECQESW (PAb4) (ProSci), chosen by analysis of hydrophilicity (Kyte-Doolittle), antigenic index (Jamesson-Wolf) and surface probability plots (Emini) for rat RHAMM (accession number NP_037096).
Synthesis of RHAMM peptides
RHAMM peptides 644KLKDENSQLKSEVSK (denoted NPI-0102), KSEVSK (denoted NPI-0104), FTEAESNMNDLV (denoted NPI-0109), KRFNDPSGCAPSPGAYD, ELMKLRNKRETKMR and CYRAPMECQESW were synthesized and purified to >95% purity (ProSci and kind gift of Dr. L. Luyt, Western University and Novare Pharmaceuticals Inc.).
Culture of Rat Mesenchymal Stem Cells (RMSC), 3T3L1 pre-adipocytes and 10T1/2 mesenchymal progenitors
RMSCs (Millipore Corporation) were cultured as described by manufacturer. Human pre-adipocytes were purchased from Lonza Bioresearch and were cultured as recommended by the manufacturer. NIH-3T3L1 and 10T1/2 cells were maintained in low glucose (1 g/L) DMEM + 10% FCS (Gibco) in 100 mm dishes and sub-cultured every 3 days. For adipogenesis assays, cells were subcultured into 96-well plates (Costar®) at a density of 1.7×104 cells/cm2. 10T1/2 parental (empty vector transfected) and RHAMM-transfected (stable) 10T1/2 cells were prepared as described previously[41].
Oil Red O assays
Adipogenesis assays for rat mesenchymal stem cells and NIH-3T3-L1 cells were performed as described by manufacturer (Merck Millipore, mesenchymal stem cell adipogenesis kit, SCR020 and adipogenesis assay ECM950) except that 10 μg/ml insulin was added to the initiating medium for both cell types. Low glucose (1g/L) DMEM was used for both cell types Following initiation stimulus, either RHAMM-based peptides or insulin, which served as the positive control, were added to the “primed” cells. After 2-3 weeks, monolayers were fixed in 3% paraformaldehyde solution and processed for oil red O staining as described by the manufacturer.
Immunohistochemistry
Adiponectin
Skin was processed for paraffin embedded histology slides. Anti-adiponectin monoclonal antibody (Abcam, ab22554) or control, non-immune IgG was incubated with de-paraffin-processed tissues sections (1 μg/ml) overnight at 4°C then incubated with Streptavidin-goat anti-mouse secondary IgG (Abcam) at 2 μg/ml for 1 hr at room temperature followed by HRP-biotin. Colour was developed with 3,3-diaminobenzidine (DAB) and counterstained with hematoxylin.
Picro Sirius Red staining
Paraffin processed tissue sections of skin were stained using Picro Sirius red (Abcam) according to the manufacturer's instructions.
Adiponectin ELISA
NIH-3T3L1 cells were subcultured into 96-well plates (Costar®), stimulated as above for adipogenesis and adiponectin was quantified using ELISA (KMP0041; Life Technologies, Frederick, MD).
Subcutaneous injection of peptides
Animal work performed at Lawrence Berkeley National Laboratories conformed to animal use protocol 0531. Animal work performed at London Health Sciences Centre conformed to animal use protocol 2009-060. Peptides dissolved in PBS (2 mg/ml) were sterilized by filtration through 0.22 μm filter and mixed 1:1 with collagen gel, (Becton Dickinson) then injected subcutaneously in anesthetized (isoflurane inhalation) retired breeder female rats (>7 months old Sprague Dawley, Charles River). Animals received a single injection of 100 μg peptide+vehicle (in 100μl solution) or vehicle alone (used as a negative control) subcutaneously in the dorsal skin or 4th mammary fat pad. Rats were euthanized at day 7-180 after the injection. This peptide concentration has anti-fibrotic and anti-inflammatory activity in rodent models[42, 43]. Animals were euthanized in a CO2 chamber followed by bilateral thoracotomy procedure to ensure death.
PCR analysis
Mammary gland RNA was isolated using TRIZOL following the manufacturer's instructions. Real time PCR was performed on a Stratagene Mx3000P cycler using Sso Advanced Universal SYBR green supermix (Biorad). Amplification profile: 10 min 95 °C, 40 cycles of 30 sec 95 °C, 1 min 60 °C, 1 min 72 °C. Primer sequence: Gapdh forward: CTCATG ACCACAGTCCATGC, Gapdh reverse: TTCAGCTCTGGGATGACCTT, Adiponectin forward: TAAGGGTGACCCAGGAGATG, Adiponectin reverse: GGAACATTGGGGACAGTGAC, PPARγ forward: CCCTGGCAAAGCATTTGTAT, PPARγ reverse: ACTGGCACCCTTGAAAAATG, Perilipin forward: TGCAAFCATTCTGACAAGG, reverse: GGAGCCTTCTGCATCTTTTG. ΔCT and fold expression change was calculated using Gapdh as housekeeping gene.
Microarray analysis
mRNA transcriptome analyses were performed as previously described[43].
Gross Pathology
Dissected dorsal back injection sites were photographed using Nikon D7000 DSLR and photographs were analyzed for fat pad area using Image J freehand selections option to trace around the fat pad and the Measure function to quantify the outlined area. Fat pad areas were converted to mm2.
Whole animal micro-computed tomography
Micro-computed tomography (micro-CT) imaging of both mice and rats was performed on Locus Ultra scanner (GE Healthcare, London, ON, Canada). The euthanized animals were imaged with an x-ray tube voltage of 80 kV and a tube current of 55 mA. Each whole-body mouse was scanned in one segment since the mouse fitted into the scanner's axial field-of-view (FOV) of 9 cm and an in-plane FOV of 14 cm in diameter. The whole-body rat was scanned in three segments and stitched together. In each segment, 900 projections of data were acquired at an angular increment of 0.4 degrees around the animal in exposure time of 16 s. Data were then reconstructed into a 3D volume image with a voxel size of 154 μm.
Quantification of total visceral and subcutaneous adipose tissue
Adipose tissue was estimated using image values of -380 HU to -30 HU. These values correspond to adipose tissue and were obtained from the histogram plot of the image values of the whole-body mouse or rat. The volume of the adipose tissue was calculated as the product of the number of voxels between the two thresholds and the voxel size. By multiplying the volume by the adipose tissue density (0. 9 g/cm3), the mass of the adipose tissue was obtained. Volume and mass of subcutaneous adipose tissue (SubQ) was estimated from the region outside the abdominal cavity. These analyses were carried out using a custom software program[44].
Micro-CT analyses of rat mammary fat pads
Nipples were used to identify the injected rectangular region of interest (ROI) located about 1 mm beneath the abdominal skin surface. The ROI size was 10 mm × 5 mm × 3 mm, and was measured using the ROI tool in MicroView, a 3D analysis and visualization software (GE Health Care, London, ON, Canada). The ROI was placed 10 mm from nipple 4 toward nipple 5, the mouse tail; 5 mm sideward, centered on the nipple; and 3 mm deep, inside the abdomen. The image was re-oriented so that the ROI length was parallel to the rat's abdominal surface. This ROI size and location were expected to capture the adipose tissue formed by the injected peptide.
Ingenuity pathway analysis
Ingenuity pathway analysis (IPA) was used to comprehensively identify and analyze networks that are common to the adipogenic genes regulated by RHAMM expression in mesenchymal progenitor (10T1/2) cells. The Core Analysis function identified one network (score 18) as significant[45], which was used to generate hypotheses.
Statistical Analyses
Statistical significance between means were assessed using 2-tailed 2 sample Student “T”-tests. Values were considered to be significantly different at p < 0.05.
Results
CD44 and RHAMM expression alter adipose tissue mass in female mice
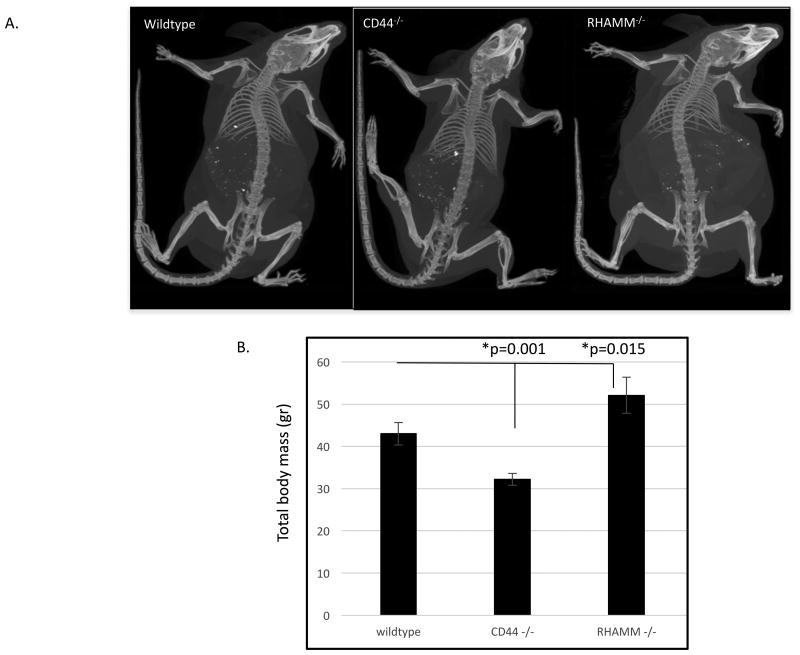
Since two HA receptors, CD44 and RHAMM have been implicated in mesenchymal differentiation of progenitor cells[46-49], we used animal models that lack expression of these two proteins to initially probe aberrant mesenchymal differentiation using Micro CT imaging. HA-receptor mediated changes in body mass were obvious in female (Figure 1) but not male (data not shown) mice. Female mice lacking CD44 expression weighed significantly less than wildtype counterparts, whereas the body mass of RHAMM-/- mice was significantly greater than wildtype (Figure 1A,B).
Figure 1. RHAMM and CD44 loss in female mice alters body mass.
A. Micro-CT images of 8-month female Wildtype, CD44-/- and RHAMM-/-. B. Quantification of total body mass. CD44-/- mice have significantly smaller body mass than wildtype counterparts while RHAMM-/- mice exhibit a significantly increased body mass. Values are the mean and S.E.M. n=3 mice/genotype. Asterisks indicate the difference between means is statistically significant and p values indicate the level of statistical significance using a two-tailed Student's T test.
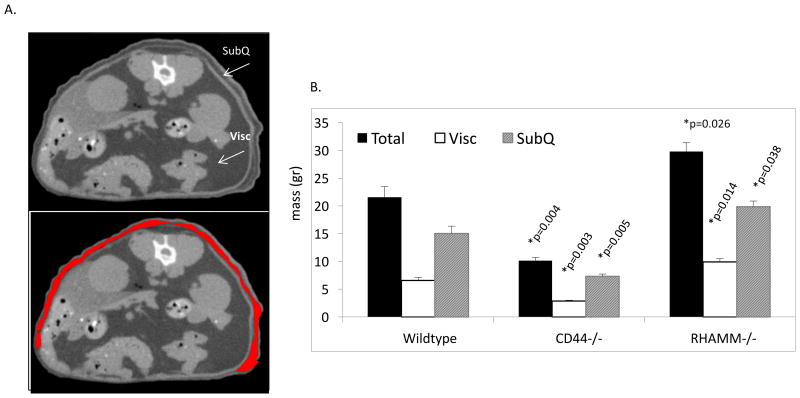
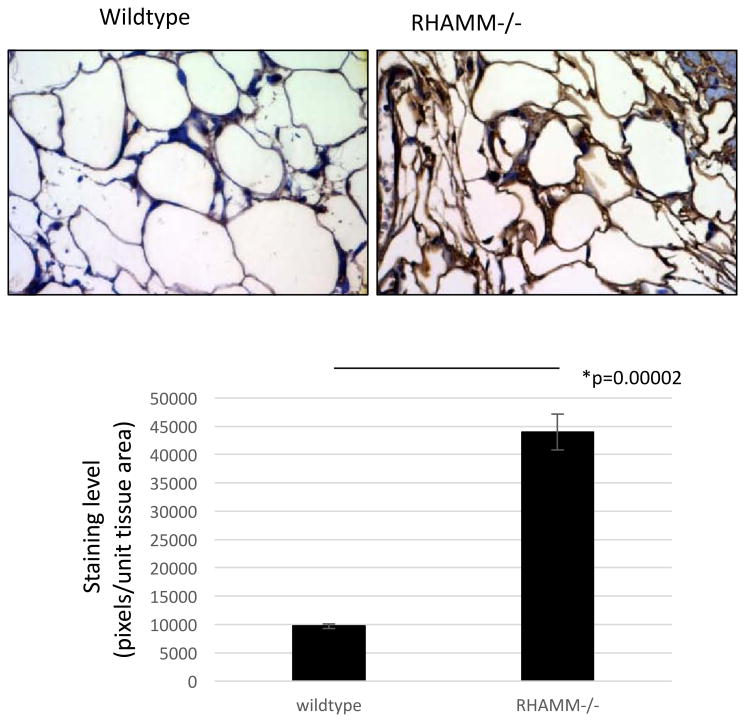
Body mass is contributed to by adiposity, lean muscle mass and skeletal density. No significant change in lean muscle mass or skeletal tissue density was observed amongst genotypes (data not shown). However, the significant differences in total adipose tissue mass (Figure 2A,B) accounted for the differential effect of CD44 loss and RHAMM loss on body mass. Thus, CD44-/- mice have significantly less adipose tissue than wildtype while RHAMM-/- mice have significantly more. The loss of adipose tissue in CD44-/- mice is consistent with a previous report linking CD44 display to the expansion of pre-adipocytes[50]. Our results conclusively show that CD44 expression contributes to adipose tissue deposition. Further analyses showed that CD44 loss reduced both visceral (Visc) and subcutaneous (SubQ) fat depots while RHAMM loss increased both of these fat depots (Figure 2B). The increase in subcutaneous adipose tissue influenced by RHAMM loss and quantified by micro CT was further verified by staining skin sections of wildtype and RHAMM-/- mice with the subcutaneous adipocyte marker gene, adiponectin (Figure 3). Results showed that adiponectin staining was strongly enhanced in RHAMM-/- vs. wildtype subcutaneous adipose depots. Adiponectin, which is a circulating adipokine specifically expressed by subcutaneous fat tissue, performs critical anti-fibrotic and metabolic functions. Therefore, enhancing its expression by blocking RHAMM function has potential therapeutic utility in metabolic diseases. Moreover, localized function blocking of RHAMM has tissue engineering potential for restoring skin health following burns, rescuing morbidity associated with lipodystrophy and tissue reconstruction. Thus, we developed RHAMM antibodies and peptides, which were screened for their adipogenic activity (Figure 4) in order to identify reagents that phenocopy the effects of RHAMM loss on adipogenesis.
Figure 2. The RHAMM and CD44 mediated change in body mass in female mice results from alterations in adipose tissue.
A. Micro CT image shows a typical optical section through the midsection of a mouse that was used to re-construct a 3-D image of subcutaneous fat (SubQ, extra-abdominal, arrow, lower image, SubQ quantified is outlined in red) and visceral fat (Visc, arrow) from which the values shown in the histogram were derived (as described in Experimental procedures). B. Micro CT analysis of total adipose tissue shows a significant increase in RHAMM-/- vs. wildtype mice and decrease in CD44-/- mice compared to wildtype counterparts. RHAMM-/- mice exhibit significantly increased subcutaneous (p=0.038) adipose tissue compared to wildtype mice while CD44-/- mice exhibit reduced SubQ and Visc. Asterisks indicate the difference between Wildtype and CD44-/- or between Wildtype and RHAMM-/- means are statistically significant and p values indicate the level of statistical significance using a two-tailed Student's T test.
Figure 3. The adipokine adiponectin is increased in subcutaneous fat of RHAMM-/- vs. wildtype mice.
Paraffin processed histology sections of wildtype and RHAMM-/- skin were stained for adiponectin (brown stain) and counterstained with hematoxylin (blue stain). The adiponectin-positive brown stain was quantified using image analysis as described in Methods and results show that adiponectin protein levels are increased in RHAMM-/- subcutaneous adipose tissue. Values are the mean and S.E.M n=5 tissue sections. Asterisk indicates statistical significance between means, and p value indicates the level of statistical significance using a two-tailed Student's T test.
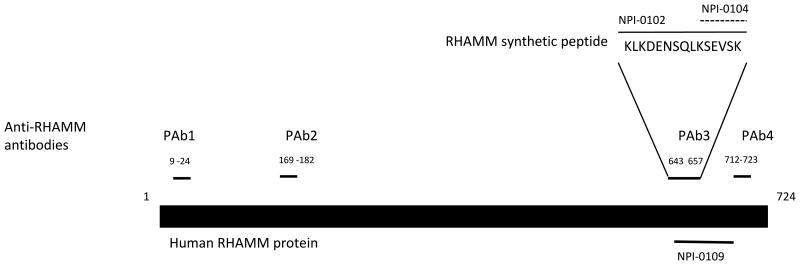
Figure 4. Diagram of strategy for identifying RHAMM function blocking peptides that promote adipogenesis.
Polyclonal antibodies to RHAMM sequence were prepared as shown in the diagram. These were tested for their ability to promote adipogenesis (See Figure 5). Since PAb3 was the only antibody that promoted adipogenesis, synthetic peptides of varying lengths were prepared from the sequence used to generate PAb3 (NPI-0102, NPI0104). These were then tested for their effect on adipogenesis. The tubulin derived peptide, NPI-0109, which binds to the HA binding region of RHAMM and behaves as an HA fragment function blocking peptide mimic, was also tested for its ability to promote adipogenesis.
RHAMM function blocking reagents promote adipogenesis of mesenchymal progenitor cells in culture
Polyclonal antibodies were raised against 4 RHAMM-based peptide sequences predicted to be displayed on the protein surface (see methods) including regions known to be required for RHAMM signaling (Figure 4). Antibodies and the RHAMM peptides used to produce these antibodies were screened for their ability to promote adipogenesis of mesenchymal stem cells and pre-adipocytes (e.g. Figure 5A-C). Of the 4 antibodies tested only PAb3 promoted adipogenesis in these assays. PAb3 detects sequence within the HA binding region of RHAMM, which also contains a leucine zipper, necessary for protein interactions required for RHAMM signaling[51, 52]. This peptide sequence binds weakly to HA (data not shown). This RHAMM sequence (NPI-0102) and a truncation of this sequence (NPI-0104) used for producing PAb3 were therefore assessed for their ability to interrupt RHAMM regulated adipogenic signaling in the above culture assays (Figure 5). Both peptides strongly promoted adipogenesis of rat mesenchymal cells (NPI-0104 shown) and human subcutaneous pre-adipocytes (NPI-0102 shown) in these assays (Figure 5).
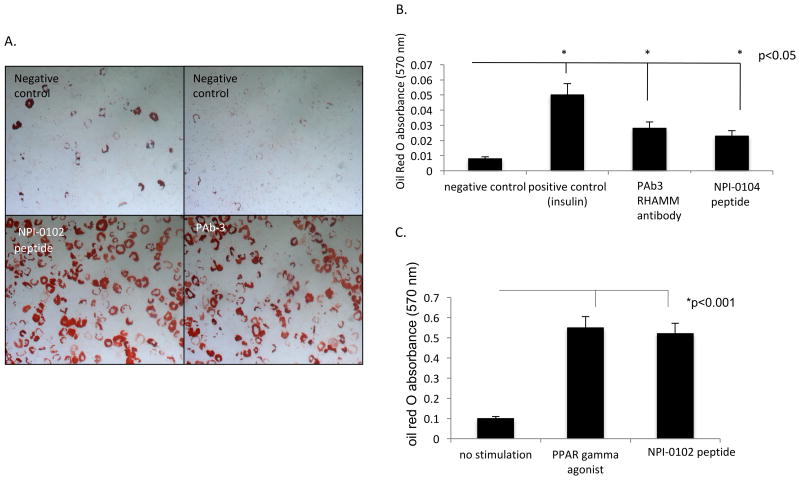
Figure 5. The RHAMM antibody and function blocking peptide promote adipogenesis of mesenchymal progenitor cells.
A. Bright field micrographs of rat bone marrow mesenchymal stem cells primed with glucocorticoids and then exposed to either medium alone (negative control), insulin (positive control, not shown), RHAMM antibody (PAb3) or a RHAMM peptide (NPI-0102 shown). Differentiation was detected by the presence of fat droplets, which were visualized by staining with the lipophilic dye Oil Red O. Both PAb-3 and RHAMM peptide promoted fat droplet accumulation. B. Oil Red O was extracted and quantified by measuring absorbance at 520 nm. Insulin, PAb-3 and RHAMM peptide (NPI-0104 shown) significantly stimulated (marked by asterisks) Oil Red O uptake above negative controls. C. RHAMM peptide (NPI-0102 shown) also stimulates adipocyte differentiation in human primary pre-adipocytes isolated from subcutaneous adipose depots as detected by oil red O uptake in lipid droplets. Adipogenesis stimulated by NPI-0102 is similar to the effect of a PPARγ agonist included as a positive control for these assays. Values are the means of n=5 replicates. P values indicate the level of statistical significance using a two-tailed Student's T test.
We have previously reported the isolation of tubulin derived peptides that bind with nM affinity to RHAMM and that mimic HA fragments that are ligands for extracellular RHAMM [53]. We therefore next compared the adipogenic dose response of mouse pre-adipocytes (3T3-L1) to one of these peptides (NPI-0109) with the response to NPI-201 and NPI-104 using promotion of adiponectin protein expression as a marker for adipogenesis (Figure 6). All peptides were bioactive in the low nM concentration range and activity tended to drop at high μM concentration (Figure 6). Since mesenchymal progenitor cells, adipose-derived stem cells and pre-adipocytes are present in the subcutaneous fat layers of skin and mammary fat pads [15] , we next assessed if these peptides promote adipogenesis in these tissues in vivo.
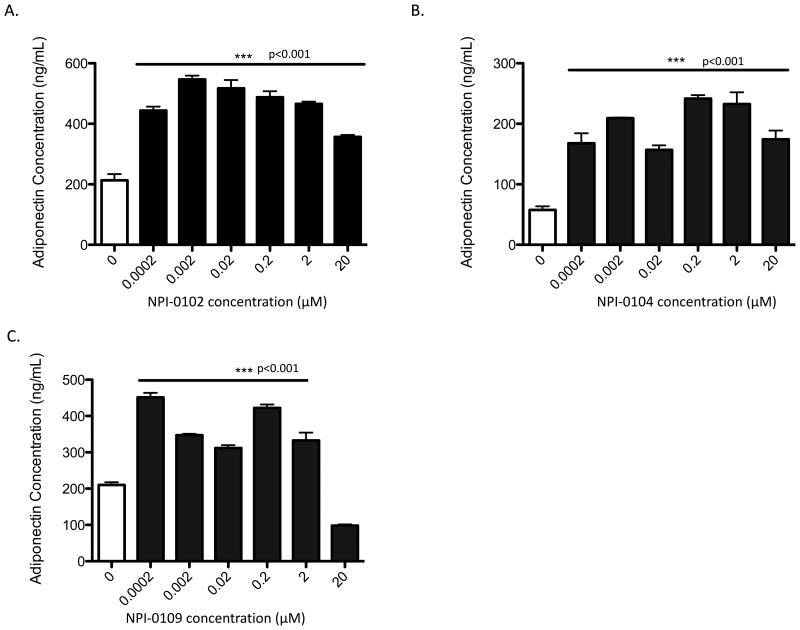
Figure 6. RHAMM function blocking peptides stimulate adiponectin expression in mouse pre-adipocytes.
Dose response of NPI-0102 (A), NPI-0104 (B) and NPI-0109 (C) on 3T3-L1 pre-adipocytes show that a wide concentration range of peptide promotes adiponectin expression. Higher doses of all peptides (20μg/ml) reduced expression although this effect was most prominent for NPI-0109. Values are the mean of n=6 replicates, p values indicate the level of statistical significance using a two-tailed Student's T test.
RHAMM peptides promote subcutaneous adipogenesis in dorsal skin and mammary fat pads in vivo
RHAMM peptides were mixed in either a collagen I vehicle that solidifies at 37°C or a viscous high molecular weight HA gel (Orthovisc) to favor peptide retention at the dorsal skin and the 4th mammary fat pad injection sites. The choice of vehicle did not alter the outcome of these experiments (data not shown). Macroscopic fat pads in dorsal skin were observed adhering to the lower dermis within 7 days of a single injection of peptide/collagen gel (Figure 7). Similarly, an enlarged mammary fat pad was visible 7 days after peptide injection in the orthovisc vehicle (Figure 8). Measurement of the areas of these fat pads verified that they were significantly enlarged relative to vehicle only controls (Figures 7, 8). Micro-CT images of the mammary tissue confirmed these results for mammary site injections and demonstrate neoadipogenesis is well integrated into the fat pad (Figure 9).
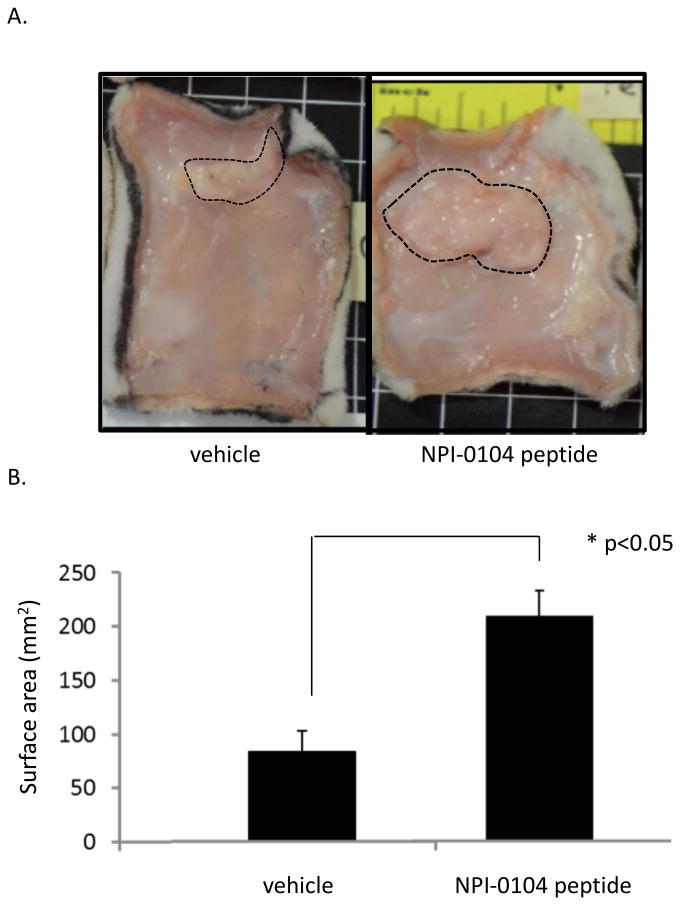
Figure 7. RHAMM function blocking peptides promote formation of subcutaneous fat pads in the dorsal skin of female rats.
A. The NPI-0104 peptide was mixed with a rat tail collagen I gel as described in Methods and injected subcutaneously into the right dorsal skin. Collagen gel only injected into the alternate side of the same rat served as a negative control. Fat pads were photographed 7 days later. B. The areas of fat pads such as shown in the images were quantified using image analysis. The NPI-0104 peptide significantly increases the fat pad area when compared to the vehicle control. Values are the mean and S.E.M. of n=5 rats. The asterisk indicates significant differences between means (p<0.05), p value indicates the level of statistical significance using a two-tailed Student's T test
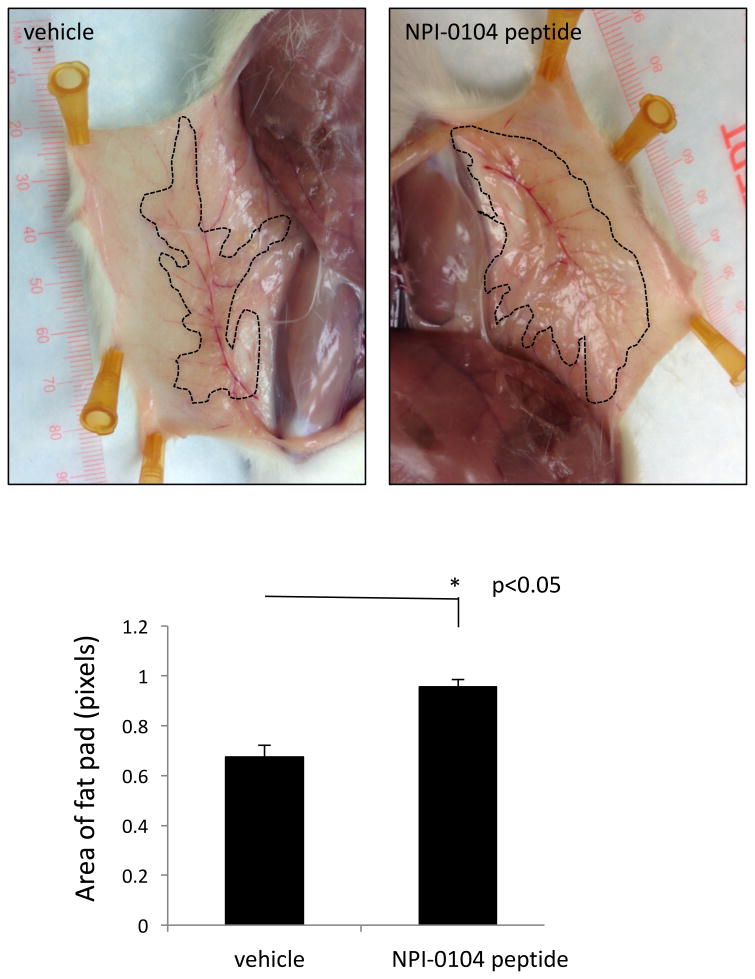
Figure 8. NPI-0104 RHAMM function blocking peptide increases the area of mammary fat pads.
The NPI-0104 peptide was injected under the right 4th nipple while the vehicle only control was injected under the nipple of the left 4th fat pad. Fat pads were photographed 7 days after injections. The areas of the treated and control fat pads were calculated from the photographs using image analysis. Results show that the NPI-0104 RHAMM function blocking peptide significantly increases mammary fat pad area (asterisks, p<0.05). Values are the means and S.E.M. of n=5 rats for each group. P value indicates the level of statistical significance using a two-tailed Student's T test.
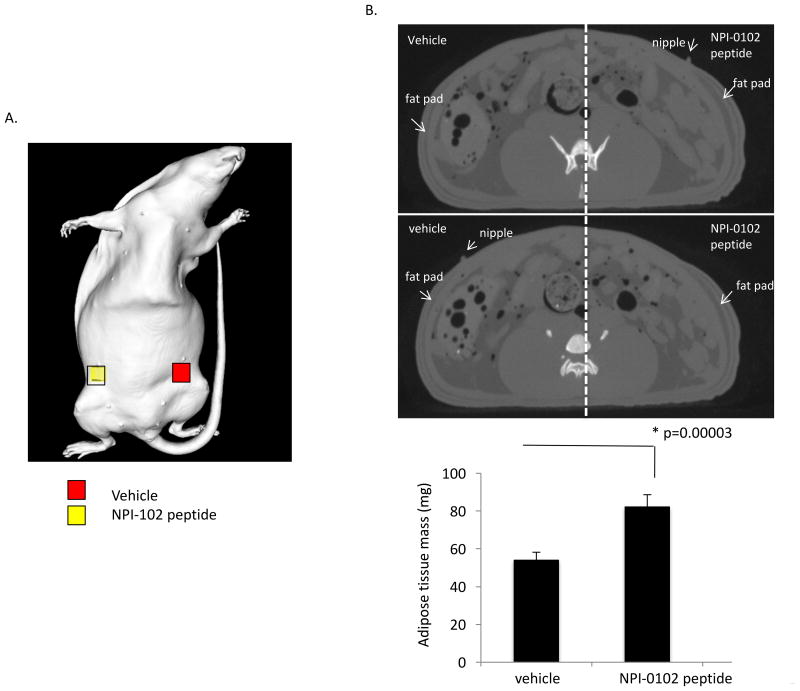
Figure 9. RHAMM function blocking peptide NPI-0102 injection increases mammary gland fat pad mass.
A. Micro-CT images were taken at the level of the 4th nipple on the 7th day after injection of either the NPI-0102 RHAMM function blocking peptide (right side of images) or vehicle alone (left side of images). B. Micro CT Images show the increase in subcutaneous fat in the area of the mammary fat pads resulting from RHAMM function blocking peptide injection. Left images are vehicle only and right images are peptide injected. All Images were taken 7 days after peptide injection. Following 3D reconstruction of the ROI shown in (A), the mass of the fat pads was calculated. Peptide treatment significantly increased fat pad mass. The values are the Means and S.E.M. n=5 rats, p value indicates the level of statistical significance using a two-tailed Student's T test.
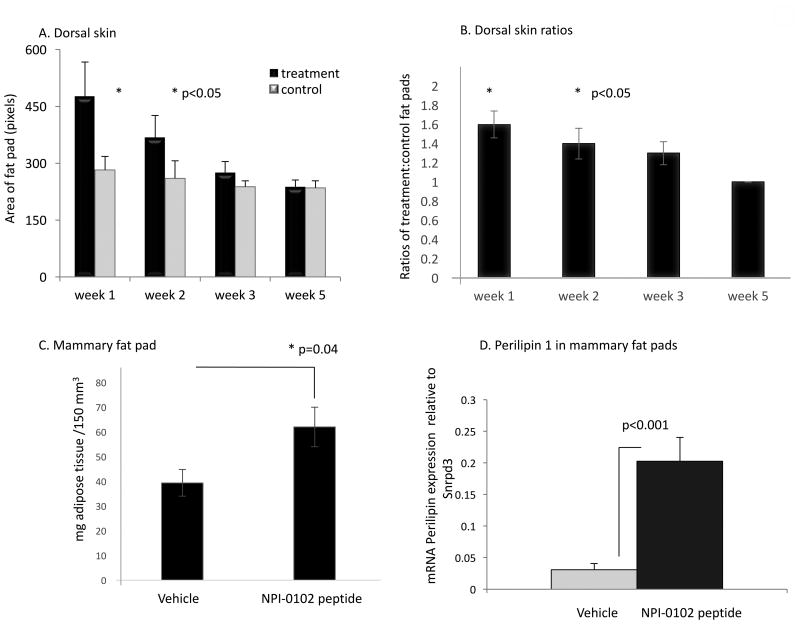
We next assessed the longevity of the fat pads induced in vivo. For dorsal skin, animals were injected with peptide on Day 0 then groups were euthanized at Day 7, 21, and 35 days post-injection for morphometry measurements. For analysis of fat retention in mammary glands, peptides were injected at D0 and imaged with micro CT at day 35 (Week 5). Peptide-induced fat pad surface area in dorsal skin tissues was significantly larger than vehicle controls at day 21 (Figure 10). In mammary glands, the peptide-induced increase in fat pad volume was still significantly higher than control injection sites at Day 35 (Figure 10B). This was verified by demonstrating significantly higher levels of the lipid marker protein, perilipin 1 in the peptide vs. control vehicle injection site (Figure 10C).
Figure 10. RHAMM peptide induced adipose tissue is retained for at least 5 weeks after a single injection.
A. The NPI-0104 RHAMM function blocking peptide was injected subcutaneously into dorsal back skin and fat pad dimensions were measured at weekly intervals. Results show that fat pad retention was significantly above vehicle only controls for at least 2 weeks following a single injection of peptide (p<0.05). Values are the Mean and S.E.M. n=6 replicates/group. B. Values in A. are presented as the fold increase relative to vehicle controls. C. The NPI-0102 RHAMM function blocking peptide was injected into the right mammary fat pad and fat pad volume was quantified by micro CT. Mammary fat pads injected with peptide were significantly larger than those injected with vehicle only at 5 weeks. Values are the mean and S.E.M. n=7 animals/group. D. m-RNA was isolated from mammary fat pads in (B) and QPCR performed for perilipin 1 as described in methods. Results show that expression of perilipin 1 is strongly increased in peptide-treated mammary fat pads verifying that the tissue volume increase detected by micro CT in B results from increased adipogenesis. Values are the mean and S.E.M. n=4 replicates. P values indicate the level of statistical significance using a two-tailed Student's T test.
RHAMM peptides promote PPARγ expression and expression of target genes
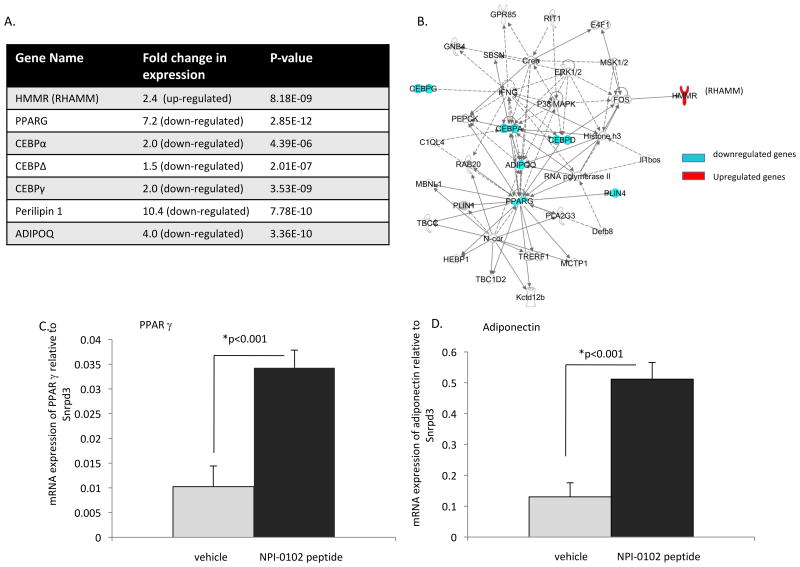
To begin to identify the mechanism(s) by which RHAMM and RHAMM peptide function blocking peptides regulate adipogenesis, RHAMM was overexpressed in mesenchymal progenitor cells and the consequences of overexpression on the adipocyte transcriptome was assessed. RHAMM transfected and parental cells total mRNA expression was compared using mRNA microarrays. Results show that overexpression of RHAMM downregulates mRNA expression of a subset of key adipogenic genes in particular the master adipogenic transcription factors PPARγ and C/EBP family of adipogenic transcription factors (Figure 11A). Expression of targets of these transcription factors including adiponectin and perilipin 1 were also downregulated. These mRNA microarray results were verified by Q-PCR (not shown) and altered gene expression profiles were analyzed for their network interactions and functions using Ingenuity Pathway Analysis (IPA). A single network links most of the genes listed in Figure 11A including RHAMM (gene name HMMR). The signaling functions of this network include cellular development, cellular growth and proliferation, and connective tissue development and function. IPA analysis shows that the top canonical pathway of this signaling network is adipogenesis, confirming the expression analysis. Based upon these results, we hypothesized that RHAMM function blocking peptides would release RHAMM suppression of PPARγ signaling. We therefore quantified the effects of NPI-0102 on the expression of PPARγ and adiponectin by comparing peptide-treated vs. vehicle control-treated mammary fat pads. Results show that this RHAMM function blocking peptide significantly increased the expression of these genes (Figure 11C, D).
Figure 11. Expression of a RHAMM cDNA in mesenchymal progenitor cells suppresses while NPI peptides promote expression of key adipogenic genes.
A. mRNA transcriptome analyses of 10T1/2 mesenchymal progenitor cells stably overexpressing RHAMM show that master adipogenic transcription factors and target genes are suppressed by RHAMM expression, particularly PPARγ and adiponectin (ADIPOQ). B. A network of these RHAMM suppressed adipogenic genes was constructed using IPA software that identifies individual causal relationships curated from the literature. A connective tissue development and function network incorporated most of these genes including RHAMM (HMMR) MAP kinases ERK1,2 and p38, which regulate adipogenesis. C. NPI-0102 promotes mRNA expression of PPARγ and its target gene adiponectin. Values are the mean and S.E.M. n=4 replicates. P values indicate the level of statistical significance using a two-tailed Student's T test.
Discussion
We show that CD44 expression is required for the formation of a normal adipose tissue mass while RHAMM expression appears to counter this since loss of its expression promotes adipogenesis. The adipogenic suppressing effect of RHAMM is associated with reduced expression of adipogenic transcription factors PPARγ and C/EBP and the expression of a subset of their target genes. We identify RHAMM peptide mimics and RHAMM binding peptides that phenocopy RHAMM loss. We propose that these peptides will be useful in restoration of subcutaneous adipose tissue following full thickness burns, lipodystrophy resulting from genetic disease or medical treatment (e.g. HIV) as well as for reconstructive and cosmetic indications. A good safety profile is predicted given the restricted expression of RHAMM in homeostatic tissues (31) and by the lack of apparent toxicity in the injected tissues used in the present study, as detected by histology and evidence for induction of adipocyte differentiation rather than cell death. Consistent with this prediction, RHAMM vaccines have shown acceptable safety profiles in leukemia patients[54, 55].
Use of fat in volume restoration for both reconstructive and cosmetic indications evolved significantly during the 20th century. Its biocompatibility, lack of immunogenicity, natural feel, and relative permanency make it an ideal soft tissue filler, and ancillary uses as a reconstructive adjunct continue to grow[56, 57]. Fat grafting has improved from the initial methods described by Neuber in 1893 through efforts to preserve progenitor cells and development of minimally-invasive volume restoration techniques. Fat grafting using the widely accepted Coleman technique now provides solutions to difficult clinical problems. For example, fat grafting provides cosmetic rescue of localized pathological facial fat atrophy of HIV lipodystrophy patients[58], and in patients with Parry-Romberg syndrome[59, 60]. Fat grafting also results in improved skin quality of radiated, burned, or badly scarred tissues by reducing radiation-induced tissue damage such as inflammation, hyperpigmentation, and ulceration. Properties of immunomodulation, anti-fibrosis, skin regeneration, and neovascularization have been newly linked to fat grafting, and these are attributed to the transfer of mesenchymal stem cells and growth factors, along with the fat graft [61-63]. Successful restoration of subcutaneous fat stores also has the potential to impact metabolic deficiencies associated with lipoatrophy since adipocytes are now understood to perform critical endocrine functions that regulate systemic metabolism and cognition by producing adipokines such as leptin and adiponectin. Therefore, developing technologies that can promote adipogenesis are critical.
Minor deficiencies in current fat-grafting include procedural complications and donor site morbidity (infection, bruising and seroma/hematoma) and in the longer term, scarring, and small contour deformities[64, 65]. More serious but rare complications include intravascular embolization that can lead to significant vascular occlusion including stroke[66] and pneumothorax infection, which has been described as a complication of fat grafting breasts[67]. However, major obstacles to wider cosmetic and medical use of fat transplantation include commonly observed fat necrosis and calcifications at the recipient site, and patient variation in graft “take” and lifespan, as well as limitations to the expanse of tissue that can be injected in a cosmetically acceptable manner. Our peptide method has the potential to address these limitations since it would not involve the complications associated with a requirement for survival of transplanted mesenchymal stem cells. Future work will determine if this method could also be used in an adjuvant setting to increase fat graft success and longevity and if sufficient subcutaneous fat depots could be generated to correct metabolic syndrome resulting from lipoatrophy.
In order to form substantial adipose tissue, mesenchymal progenitor cell subpopulations must undergo clonal expansion prior to differentiating into mature adipocytes[36, 37, 68]. ERK1,2 kinases are required for expansion and loss of ERK1 expression results in lean mice[36]. However, this MAP kinase plays complex roles in adipogenesis since sustained activity blocks adipocyte maturation and promotes insulin resistance [36, 37, 69, 70]. RHAMM expression has previously been reported to be high in dormant stem cells but is down-regulated during embryonic stem cell differentiation suggesting that lowering RHAMM levels, which affect ERK1,2 activity, may be an important step in initiating differentiation of a variety of stem cells [33, 71, 72]. Our results show that RHAMM function loss is a necessary and rather specific step in directing the differentiation of mesenchymal progenitors into mature adipocytes. The molecular mechanisms by which RHAMM, which is a multifunctional protein with both extracellular and intracellular functions that affect mesenchymal differentiation[51, 73], controls adipocyte maturation require further study. However, clues are provided by previous reports. For example, extracellular RHAMM binds to HA fragments in the size range that has recently been reported to suppress adipogenesis[74, 75] and associates with transmembrane receptors such as CD44 and PDGFR[73, 76, 77] to regulate activation of ERK1,2 signaling pathways controlling mesenchymal differentiation [73, 77]. Furthermore, intracellular RHAMM protein isoforms complex with, sustain activity and regulate subcellular targeting of the MEK1/ERK1,2 complexes that affect adipogenesis [38]. Intracellular RHAMM also partners with anti-adipogenic proteins such as the ERK1,2 target protein ANKRYD26[39, 78], which results in suppression of the activation and expression of master adipogenic transcription factors such as PPARγ[79].
Our results showing that CD44 loss results in adipose tissue depletion is consistent with its proposed role in expansion of pre-adipocyte subsets[50]. However, previous studies have shown that CD44-/- mice fed a high fat diet[80], CD44 deficient NOD mice [81]or NOD mice treated with CD44 function blocking antibodies[82] have reduced susceptibility to hepatic steatosis, adipose tissue-associated inflammation and insulin resistance. Interestingly, these mice exhibited increased adipose tissue mass unlike our results showing loss of adipose tissue when CD44 expression is deficient. This difference between these previous results and our present study may be due to gender differences, mouse background and high fat vs. normal diet. Our observation that adiposity of female but not male mice are affected by RHAMM and CD44 expression raise the interesting possibility that responses to high fat diets that are regulated by CD44 and, possibly, RHAMM may be affected by estrogen and testosterone.
Gender specific differences in both adipocyte differentiation and tissue depoting have previously been reported[83, 84]. For example, major adipogenic genes are sex steroid regulated (e.g. PLIN1, ATGL and MEDA-4[85, 86]) and the effect of genetic deletion of SERT[87], GPER[88] and CD24[89] on adipogenesis in mice is gender dependent. The mechanisms for this difference appears to be complex including differential sensitivity to activation of the adipogenic transcription factor PPARG[90] and to differences in tissue microenvironments. For example, the balance between TIMP4 and MMP3 expression, which differs in males and females[91], is critical for remodeling the microenvironment to permit adipocyte proliferation. Understanding the mechanisms by which RHAMM blocks adipogenesis in females but not males warrants further investigation.
To date, the consequences of adipose tissue increase resulting from RHAMM loss on susceptibility to adipose tissue inflammation and insulin resistance have not to our knowledge been reported. We predict that adipose tissue inflammation is reduced as a result of blunted signaling through both HA fragments[43, 92] and ERK1,2, both of which promote inflammation and obesity [93-96]. This effect combined with the RHAMM-loss dependent increase in adiponectin protein expression, which is a circulating anti-inflammatory, anti-fibrotic adipokine [97-100] predicts that the increased adipogenesis that occurs with RHAMM loss will not be associated with increased inflammation/insulin resistance normally observed in visceral fat depots.
Conclusions
Collectively, our results show that RHAMM function blocking peptides act on progenitor cells known to be present within skin and mammary gland subcutaneous fat depots to significantly expand adipose tissue mass at these sites. Administration of RHAMM function blocking peptides could circumvent the challenges associated with poor adipocyte survival encountered in current surgical strategies, which employ autologous fat transplantation. Further research is required to determine if this is a clinically sound alternative or supplemental approach to fat augmentation and treatment of adipogenesis associated metabolic diseases.
Insight, Innovation and Integration statement.
Hyaluronan, CD44 and the Receptor for Hyaluronan-Mediated Motility (RHAMM) have previously been reported to regulate mesenchymal progenitor cell differentiation. Here we more specifically show that both of these HA receptors play key roles in adipocyte differentiation. CD44 expression is required for adipogenesis in vivo. In contrast, RHAMM expression suppresses adipogenesis by inhibiting differentiation of pre-adipocytes into mature adipocytes. We further identify reagents that block RHAMM signaling and show that these promote adipogenesis in culture and in vivo. These reagents have translational potential spanning from restorative tissue engineering to treatment of lipodystrophic disorders.
Acknowledgments
We thank Arya Nikjoo, Partow Imani, Shahrzad Afghani, Anne Marie Jeng and Jenny Ma for their technical assistance. This work was supported by Canadian Institutes of Health Research to ET, Chairman's Fund Professor in Cancer Research to JBM, and Novare Pharmaceuticals, Boston MA, to MJB and ET. MJB laboratory was supported by funds from the National Cancer Institute (R01 CA064786) and by a grant from the Breast Cancer Research Foundation. MV was supported by National Cancer Institute of the National Institute of Health Ruth L. Kirschstein National Research Service award (F32 CA132491). SBB was supported by Novare Pharmaceuticals Inc. ET and JBM laboratories were supported by NIH CA119092. ET laboratory was also supported by Novare Pharmaceuticals Inc.
Footnotes
Financial disclosure: EAT, SBB and MJB are named inventors on a patent application describing the RHAMM function blocking peptides that were the subject of and described in this research. This patent application and other intellectual property has been licensed to Novare Pharmaceuticals (www.novarepharma.com, formerly known as ProGDerm), of which EAT and MJB have shares, or an equity interest. Novare sponsored in whole or in part this research conducted at LBNL and LHSC. EAT and SBB were paid consultants of Novare during the time of this research.
Author contribution: Experimental design: Bahrami SB, Tolg C, Peart T, Symonette C, Veiseh M, Umoh JU, Holdworth DW, McCarthy JB, Luyt LG, Bissell MJ, Yazdani A, Turley EA
Data acquisition: Bahrami SB, Tolg C, Peart T, Symonette C, Umoh JU
Data analysis and interpretation: Bahrami SB, Tolg C, Peart T, Symonette C, Veiseh M, Umoh JU, Holdworth DW, McCarthy JB, Luyt LG, Bissell MJ, Yazdani A, Turley EA
Manuscript preparation: Bahrami SB, Tolg C, Peart T, Symonette C, Veiseh M, Umoh JU, Holdworth DW, McCarthy JB, Luyt LG, Bissell MJ, Yazdani A, Turley EA
References
- 1.Booth BW, Yang CC, Burg KJ. Assessment of a Chitosan/Hyaluronan Injectable Composite for Fat Reconstruction. J Biomater Sci Polym Ed. 2012;23(18):2303–20. doi: 10.1163/156856211X615274. [DOI] [PubMed] [Google Scholar]
- 2.Kiliaan AJ, Arnoldussen IA, Gustafson DR. Adipokines: a link between obesity and dementia? Lancet Neurol. 2014;13(9):913–23. doi: 10.1016/S1474-4422(14)70085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Booth A, et al. Adipose tissue, obesity and adipokines: role in cancer promotion. Horm Mol Biol Clin Investig. 2015;21(1):57–74. doi: 10.1515/hmbci-2014-0037. [DOI] [PubMed] [Google Scholar]
- 4.Magkos F, Mantzoros CS. Body fat redistribution and metabolic abnormalities in HIV-infected patients on highly active antiretroviral therapy: novel insights into pathophysiology and emerging opportunities for treatment. Metabolism. 2011;60(6):749–53. doi: 10.1016/j.metabol.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Isik S, Sahin I. Contour restoration of the forehead by lipofilling: our experience. Aesthetic Plast Surg. 2012;36(4):761–6. doi: 10.1007/s00266-011-9800-2. [DOI] [PubMed] [Google Scholar]
- 6.DeFatta RJ, Williams EF., 3rd Fat transfer in conjunction with facial rejuvenation procedures. Facial Plast Surg Clin North Am. 2008;16(4):383–90. doi: 10.1016/j.fsc.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Pu LL, Yoshimura K, Coleman SR. Future Perspectives of Fat Grafting. Clin Plast Surg. 2015;42(3):389–94. ix–x. doi: 10.1016/j.cps.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Hsu VM, et al. Fat grafting's past, present, and future: why adipose tissue is emerging as a critical link to the advancement of regenerative medicine. Aesthet Surg J. 2012;32(7):892–9. doi: 10.1177/1090820X12455658. [DOI] [PubMed] [Google Scholar]
- 9.Rigotti G, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg. 2007;119(5):1409–22. doi: 10.1097/01.prs.0000256047.47909.71. discussion 1423-4. [DOI] [PubMed] [Google Scholar]
- 10.Salgarello M, Visconti G, Barone-Adesi L. Fat grafting and breast reconstruction with implant: another option for irradiated breast cancer patients. Plast Reconstr Surg. 2012;129(2):317–29. doi: 10.1097/PRS.0b013e31822b6619. [DOI] [PubMed] [Google Scholar]
- 11.Biazus JV, et al. Immediate Reconstruction with Autologous fat Transfer Following Breast-Conserving Surgery. Breast J. 2015;21(3):268–75. doi: 10.1111/tbj.12397. [DOI] [PubMed] [Google Scholar]
- 12.Missana MC, et al. Autologous fat transfer in reconstructive breast surgery: indications, technique and results. Eur J Surg Oncol. 2007;33(6):685–90. doi: 10.1016/j.ejso.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Kato H, et al. Degeneration, regeneration, and cicatrization after fat grafting: dynamic total tissue remodeling during the first 3 months. Plast Reconstr Surg. 2014;133(3):303e–313e. doi: 10.1097/PRS.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 14.Yoshimura K, Coleman SR. Complications of Fat Grafting: How They Occur and How to Find, Avoid, and Treat Them. Clin Plast Surg. 2015;42(3):383–8. ix. doi: 10.1016/j.cps.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Yoshimura K, et al. Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg. 2008;32(1):48–55. doi: 10.1007/s00266-007-9019-4. discussion 56-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu S, et al. Fate of adipose-derived stromal vascular fraction cells after co-implantation with fat grafts: evidence of cell survival and differentiation in ischemic adipose tissue. Plast Reconstr Surg. 2013;132(2):363–73. doi: 10.1097/PRS.0b013e31829588b3. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto D, et al. Cell-assisted lipotransfer: supportive use of human adipose-derived cells for soft tissue augmentation with lipoinjection. Tissue Eng. 2006;12(12):3375–82. doi: 10.1089/ten.2006.12.3375. [DOI] [PubMed] [Google Scholar]
- 18.Stoltz JF, et al. Stem Cells and Regenerative Medicine: Myth or Reality of the 21th Century. Stem Cells Int. 2015;2015:734731. doi: 10.1155/2015/734731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forcales SV. Potential of adipose-derived stem cells in muscular regenerative therapies. Front Aging Neurosci. 2015;7:123. doi: 10.3389/fnagi.2015.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Francesco F, et al. Human Adipose Stem Cells: From Bench to Bedside. Tissue Eng Part B Rev. 2015;21(6):572–84. doi: 10.1089/ten.TEB.2014.0608. [DOI] [PubMed] [Google Scholar]
- 21.Scott MA, et al. Current methods of adipogenic differentiation of mesenchymal stem cells. Stem Cells Dev. 2011;20(10):1793–804. doi: 10.1089/scd.2011.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsuji W, Rubin JP, Marra KG. Adipose-derived stem cells: Implications in tissue regeneration. World J Stem Cells. 2014;6(3):312–21. doi: 10.4252/wjsc.v6.i3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang P, Skinner KA, Hicks DG. Molecular classification of breast carcinomas by immunohistochemical analysis: are we ready? Diagn Mol Pathol. 2009;18(3):125–32. doi: 10.1097/PDM.0b013e31818d107b. [DOI] [PubMed] [Google Scholar]
- 24.Margoni A, Fotis L, Papavassiliou AG. The transforming growth factor-beta/bone morphogenetic protein signalling pathway in adipogenesis. Int J Biochem Cell Biol. 2012;44(3):475–9. doi: 10.1016/j.biocel.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 25.Kim WS, Park HS, Sung JH. The pivotal role of PDGF and its receptor isoforms in adipose-derived stem cells. Histol Histopathol. 2015;30(7):793–9. doi: 10.14670/HH-11-598. [DOI] [PubMed] [Google Scholar]
- 26.Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol. 2011;12(11):722–34. doi: 10.1038/nrm3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mariman EC, Wang P. Adipocyte extracellular matrix composition, dynamics and role in obesity. Cell Mol Life Sci. 2010;67(8):1277–92. doi: 10.1007/s00018-010-0263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cantu DA, Kao WJ. Combinatorial biomatrix/cell-based therapies for restoration of host tissue architecture and function. Adv Healthc Mater. 2013;2(12):1544–63. doi: 10.1002/adhm.201300063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jha AK, et al. Controlling the adhesion and differentiation of mesenchymal stem cells using hyaluronic acid-based, doubly crosslinked networks. Biomaterials. 2011;32(10):2466–78. doi: 10.1016/j.biomaterials.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marquez-Curtis LA, et al. The ins and outs of hematopoietic stem cells: studies to improve transplantation outcomes. Stem Cell Rev. 2011;7(3):590–607. doi: 10.1007/s12015-010-9212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ji E, et al. Inhibition of adipogenesis in 3T3-L1 cells and suppression of abdominal fat accumulation in high-fat diet-feeding C57BL/6J mice after downregulation of hyaluronic acid. Int J Obes (Lond) 2014;38(8):1035–43. doi: 10.1038/ijo.2013.202. [DOI] [PubMed] [Google Scholar]
- 32.Wang A, et al. Hyperglycemia diverts dividing osteoblastic precursor cells to an adipogenic pathway and induces synthesis of a hyaluronan matrix that is adhesive for monocytes. J Biol Chem. 2014;289(16):11410–20. doi: 10.1074/jbc.M113.541458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Solis MA, et al. Hyaluronan regulates cell behavior: a potential niche matrix for stem cells. Biochem Res Int. 2012;2012:346972. doi: 10.1155/2012/346972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tolg C, et al. Hyaluronan and RHAMM in wound repair and the “cancerization” of stromal tissues. Biomed Res Int. 2014;2014:103923. doi: 10.1155/2014/103923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams K, et al. CD44 integrates signaling in normal stem cell, cancer stem cell and (pre)metastatic niches. Exp Biol Med (Maywood) 2013;238(3):324–38. doi: 10.1177/1535370213480714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bost F, et al. The role of MAPKs in adipocyte differentiation and obesity. Biochimie. 2005;87(1):51–6. doi: 10.1016/j.biochi.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 37.Scioli MG, et al. The biomolecular basis of adipogenic differentiation of adipose-derived stem cells. Int J Mol Sci. 2014;15(4):6517–26. doi: 10.3390/ijms15046517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tolg C, et al. RHAMM promotes interphase microtubule instability and mitotic spindle integrity through MEK1/ERK1/2 activity. J Biol Chem. 2010;285(34):26461–74. doi: 10.1074/jbc.M110.121491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu XF, et al. ANKRD26 and its interacting partners TRIO, GPS2, HMMR and DIPA regulate adipogenesis in 3T3-L1 cells. PLoS One. 2012;7(5):e38130. doi: 10.1371/journal.pone.0038130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tolg C, et al. Genetic deletion of receptor for hyaluronan-mediated motility (Rhamm) attenuates the formation of aggressive fibromatosis (desmoid tumor) Oncogene. 2003;22(44):6873–82. doi: 10.1038/sj.onc.1206811. [DOI] [PubMed] [Google Scholar]
- 41.Zhang S, et al. The hyaluronan receptor RHAMM regulates extracellular-regulated kinase. J Biol Chem. 1998;273(18):11342–8. doi: 10.1074/jbc.273.18.11342. [DOI] [PubMed] [Google Scholar]
- 42.Savani RC, et al. A role for hyaluronan in macrophage accumulation and collagen deposition after bleomycin-induced lung injury. Am J Respir Cell Mol Biol. 2000;23(4):475–84. doi: 10.1165/ajrcmb.23.4.3944. [DOI] [PubMed] [Google Scholar]
- 43.Tolg C, et al. A RHAMM mimetic peptide blocks hyaluronan signaling and reduces inflammation and fibrogenesis in excisional skin wounds. Am J Pathol. 2012;181(4):1250–70. doi: 10.1016/j.ajpath.2012.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Granton PV, et al. Rapid in vivo whole body composition of rats using cone beam muCT. J Appl Physiol. 2010;109(4):1162–9. doi: 10.1152/japplphysiol.00016.2010. [DOI] [PubMed] [Google Scholar]
- 45.Muurling T, Stankovic KM. Metabolomic and network analysis of pharmacotherapies for sensorineural hearing loss. Otol Neurotol. 2014;35(1):1–6. doi: 10.1097/MAO.0000000000000254. [DOI] [PubMed] [Google Scholar]
- 46.Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells - current trends and future prospective. Biosci Rep. 2015;35(2) doi: 10.1042/BSR20150025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Astachov L, et al. Hyaluronan and mesenchymal stem cells: from germ layer to cartilage and bone. Front Biosci (Landmark Ed) 2011;16:261–76. doi: 10.2741/3687. [DOI] [PubMed] [Google Scholar]
- 48.Nikitovic D, et al. The roles of hyaluronan/RHAMM/CD44 and their respective interactions along the insidious pathways of fibrosarcoma progression. Biomed Res Int. 2013;2013:929531. doi: 10.1155/2013/929531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prosdocimi M, Bevilacqua C. Exogenous hyaluronic acid and wound healing: an updated vision. Panminerva Med. 2012;54(2):129–35. [PubMed] [Google Scholar]
- 50.Lee YH, Petkova AP, Granneman JG. Identification of an adipogenic niche for adipose tissue remodeling and restoration. Cell Metab. 2013;18(3):355–67. doi: 10.1016/j.cmet.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maxwell CA, McCarthy J, Turley E. Cell-surface and mitotic-spindle RHAMM: moonlighting or dual oncogenic functions? J Cell Sci. 2008;121(Pt 7):925–32. doi: 10.1242/jcs.022038. [DOI] [PubMed] [Google Scholar]
- 52.Jiang J, et al. Multifunctional proteins bridge mitosis with motility and cancer with inflammation and arthritis. ScientificWorldJournal. 2010;10:1244–57. doi: 10.1100/tsw.2010.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Esguerra KV, et al. Identification, design and synthesis of tubulin-derived peptides as novel hyaluronan mimetic ligands for the receptor for hyaluronan-mediated motility (RHAMM/HMMR) Integr Biol (Camb) 2015;7(12):1547–60. doi: 10.1039/c5ib00222b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brayer JB, Pinilla-Ibarz J. Developing strategies in the immunotherapy of leukemias. Cancer Control. 2013;20(1):49–59. doi: 10.1177/107327481302000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lokeshwar VB, Mirza S, Jordan A. Targeting hyaluronic acid family for cancer chemoprevention and therapy. Adv Cancer Res. 2014;123:35–65. doi: 10.1016/B978-0-12-800092-2.00002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coleman SR. Structural fat grafts: the ideal filler? Clin Plast Surg. 2001;28(1):111–9. [PubMed] [Google Scholar]
- 57.Jatana KR, Smith SP., Jr The scientific basis for lipotransfer: is it the ideal filler? Facial Plast Surg Clin North Am. 2008;16(4):443–8. vi–vii. doi: 10.1016/j.fsc.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 58.Shuck J, et al. Autologous fat grafting and injectable dermal fillers for human immunodeficiency virus-associated facial lipodystrophy: a comparison of safety, efficacy, and long-term treatment outcomes. Plast Reconstr Surg. 2013;131(3):499–506. doi: 10.1097/PRS.0b013e31827c6df5. [DOI] [PubMed] [Google Scholar]
- 59.Hunstad JP, Shifrin DA, Kortesis BG. Successful treatment of Parry-Romberg syndrome with autologous fat grafting: 14-year follow-up and review. Ann Plast Surg. 2011;67(4):423–5. doi: 10.1097/SAP.0b013e31820b3aa8. [DOI] [PubMed] [Google Scholar]
- 60.Rodby KA, et al. Evaluating Autologous Lipofilling for Parry-Romberg Syndrome-Associated Defects: A Systematic Literature Review and Case Report. Cleft Palate Craniofac J. 2016;53(3):339–50. doi: 10.1597/14-232. [DOI] [PubMed] [Google Scholar]
- 61.Banyard DA, et al. Implications for human adipose-derived stem cells in plastic surgery. J Cell Mol Med. 2015;19(1):21–30. doi: 10.1111/jcmm.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Trojahn Kolle SF, et al. Importance of mesenchymal stem cells in autologous fat grafting: a systematic review of existing studies. J Plast Surg Hand Surg. 2012;46(2):59–68. doi: 10.3109/2000656X.2012.668326. [DOI] [PubMed] [Google Scholar]
- 63.Wetterau M, et al. Autologous fat grafting and facial reconstruction. J Craniofac Surg. 2012;23(1):315–8. doi: 10.1097/SCS.0b013e318241e1de. [DOI] [PubMed] [Google Scholar]
- 64.Gutowski KA, Force AFGT. Current applications and safety of autologous fat grafts: a report of the ASPS fat graft task force. Plast Reconstr Surg. 2009;124(1):272–80. doi: 10.1097/PRS.0b013e3181a09506. [DOI] [PubMed] [Google Scholar]
- 65.Kim YH, et al. Analysis of postoperative complications for superficial liposuction: a review of 2398 cases. Plast Reconstr Surg. 2011;127(2):863–71. doi: 10.1097/PRS.0b013e318200afbf. [DOI] [PubMed] [Google Scholar]
- 66.Yu NZ, et al. A systemic review of autologous fat grafting survival rate and related severe complications. Chin Med J (Engl) 2015;128(9):1245–51. doi: 10.4103/0366-6999.156142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Delay E, et al. Fat injection to the breast: technique, results, and indications based on 880 procedures over 10 years. Aesthet Surg J. 2009;29(5):360–76. doi: 10.1016/j.asj.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 68.Farmer SR. Regulation of PPARgamma activity during adipogenesis. Int J Obes (Lond) 2005;29(Suppl 1):S13–6. doi: 10.1038/sj.ijo.0802907. [DOI] [PubMed] [Google Scholar]
- 69.Sul HS. Minireview: Pref-1: role in adipogenesis and mesenchymal cell fate. Mol Endocrinol. 2009;23(11):1717–25. doi: 10.1210/me.2009-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jager J, et al. Deficiency in the extracellular signal-regulated kinase 1 (ERK1) protects leptin-deficient mice from insulin resistance without affecting obesity. Diabetologia. 2011;54(1):180–9. doi: 10.1007/s00125-010-1944-0. [DOI] [PubMed] [Google Scholar]
- 71.Choudhary M, et al. Putative role of hyaluronan and its related genes, HAS2 and RHAMM, in human early preimplantation embryogenesis and embryonic stem cell characterization. Stem Cells. 2007;25(12):3045–57. doi: 10.1634/stemcells.2007-0296. [DOI] [PubMed] [Google Scholar]
- 72.Jiang J, Mohan P, Maxwell CA. The cytoskeletal protein RHAMM and ERK1/2 activity maintain the pluripotency of murine embryonic stem cells. PLoS One. 2013;8(9):e73548. doi: 10.1371/journal.pone.0073548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Turley EA, Wood DK, McCarthy JB. Carcinoma Cell Hyaluronan as a “Portable” Cancerized Prometastatic Microenvironment. Cancer Res. 2016;76(9):2507–12. doi: 10.1158/0008-5472.CAN-15-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Park BG, et al. Enzymatic fragments of hyaluronan inhibit adipocyte differentiation in 3T3-L1 pre-adipocytes. Biochem Biophys Res Commun. 2015;467(4):623–8. doi: 10.1016/j.bbrc.2015.10.104. [DOI] [PubMed] [Google Scholar]
- 75.Park BG, et al. Anti-obesity potential of enzymatic fragments of hyaluronan on high-fat diet-induced obesity in C57BL/6 mice. Biochem Biophys Res Commun. 2016;473(1):290–5. doi: 10.1016/j.bbrc.2016.03.098. [DOI] [PubMed] [Google Scholar]
- 76.Naor D. Editorial: Interaction Between Hyaluronic Acid and Its Receptors (CD44, RHAMM) Regulates the Activity of Inflammation and Cancer. Front Immunol. 2016;7:39. doi: 10.3389/fimmu.2016.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nikitovic D, et al. Hyaluronan/Hyaladherins - a Promising Axis for Targeted Drug Delivery in Cancer. Curr Drug Deliv. 2016;13(4):500–11. doi: 10.2174/1567201813666151109103013. [DOI] [PubMed] [Google Scholar]
- 78.Fei Z, et al. Ankrd26 gene disruption enhances adipogenesis of mouse embryonic fibroblasts. J Biol Chem. 2011;286(31):27761–8. doi: 10.1074/jbc.M111.248435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang S, Dougherty EJ, Danner RL. PPARgamma signaling and emerging opportunities for improved therapeutics. Pharmacol Res. 2016;111:76–85. doi: 10.1016/j.phrs.2016.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kang HS, et al. CD44 plays a critical role in regulating diet-induced adipose inflammation, hepatic steatosis, and insulin resistance. PLoS One. 2013;8(3):e58417. doi: 10.1371/journal.pone.0058417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Assayag-Asherie N, et al. Can CD44 Be a Mediator of Cell Destruction? The Challenge of Type 1 Diabetes. PLoS One. 2015;10(12):e0143589. doi: 10.1371/journal.pone.0143589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kodama K, et al. Anti-CD44 antibody treatment lowers hyperglycemia and improves insulin resistance, adipose inflammation, and hepatic steatosis in diet-induced obese mice. Diabetes. 2015;64(3):867–75. doi: 10.2337/db14-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jeffery E, et al. The Adipose Tissue Microenvironment Regulates Depot-Specific Adipogenesis in Obesity. Cell Metab. 2016;24(1):142–50. doi: 10.1016/j.cmet.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhou P, et al. High fat diet rescues disturbances to metabolic homeostasis and survival in the Id2 null mouse in a sex-specific manner. Biochem Biophys Res Commun. 2014;451(3):374–81. doi: 10.1016/j.bbrc.2014.07.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wend K, et al. ERalpha regulates lipid metabolism in bone through ATGL and perilipin. J Cell Biochem. 2013;114(6):1306–14. doi: 10.1002/jcb.24470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang H, Chen X, Sairam MR. Novel genes of visceral adiposity: identification of mouse and human mesenteric estrogen-dependent adipose (MEDA)-4 gene and its adipogenic function. Endocrinology. 2012;153(6):2665–76. doi: 10.1210/en.2011-2008. [DOI] [PubMed] [Google Scholar]
- 87.Homberg JR, la Fleur SE, Cuppen E. Serotonin transporter deficiency increases abdominal fat in female, but not male rats. Obesity (Silver Spring) 2010;18(1):137–45. doi: 10.1038/oby.2009.139. [DOI] [PubMed] [Google Scholar]
- 88.Sharma G, et al. GPER deficiency in male mice results in insulin resistance, dyslipidemia, and a proinflammatory state. Endocrinology. 2013;154(11):4136–45. doi: 10.1210/en.2013-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fairbridge NA, et al. Loss of CD24 in Mice Leads to Metabolic Dysfunctions and a Reduction in White Adipocyte Tissue. PLoS One. 2015;10(11):e0141966. doi: 10.1371/journal.pone.0141966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bragdon B, et al. Intrinsic Sex-Linked Variations in Osteogenic and Adipogenic Differentiation Potential of Bone Marrow Multipotent Stromal Cells. J Cell Physiol. 2015;230(2):296–307. doi: 10.1002/jcp.24705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wu Y, et al. High-fat diet-induced obesity regulates MMP3 to modulate depot- and sex-dependent adipose expansion in C57BL/6J mice. Am J Physiol Endocrinol Metab. 2017;312(1):E58–E71. doi: 10.1152/ajpendo.00128.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tolg C, Telmer P, Turley E. Specific sizes of hyaluronan oligosaccharides stimulate fibroblast migration and excisional wound repair. PLoS One. 2014;9(2):e88479. doi: 10.1371/journal.pone.0088479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maytin EV. Hyaluronan: More than just a wrinkle filler. Glycobiology. 2016;26(6):553–9. doi: 10.1093/glycob/cww033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lauer ME, et al. The Rise and Fall of Hyaluronan in Respiratory Diseases. Int J Cell Biol. 2015;2015:712507. doi: 10.1155/2015/712507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hull RL, et al. Hyaluronan: A Mediator of Islet Dysfunction and Destruction in Diabetes? J Histochem Cytochem. 2015;63(8):592–603. doi: 10.1369/0022155415576542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schwertfeger KL, et al. Hyaluronan, Inflammation, and Breast Cancer Progression. Front Immunol. 2015;6:236. doi: 10.3389/fimmu.2015.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Park PH, Sanz-Garcia C, Nagy LE. Adiponectin as an anti-fibrotic and anti-inflammatory adipokine in the liver. Curr Pathobiol Rep. 2015;3(4):243–252. doi: 10.1007/s40139-015-0094-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Freitas Lima LC, et al. Adipokines, diabetes and atherosclerosis: an inflammatory association. Front Physiol. 2015;6:304. doi: 10.3389/fphys.2015.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu D, Luo S, Li Z. Multifaceted roles of adiponectin in rheumatoid arthritis. Int Immunopharmacol. 2015;28(2):1084–90. doi: 10.1016/j.intimp.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 100.Matsuda M, Shimomura I. Roles of adiponectin and oxidative stress in obesity-associated metabolic and cardiovascular diseases. Rev Endocr Metab Disord. 2014;15(1):1–10. doi: 10.1007/s11154-013-9271-7. [DOI] [PubMed] [Google Scholar]