Abstract
The incidence of visceral leishmaniasis (VL) in Albania is higher than in other countries of southern Europe, however the role of local sand fly species in the transmission of Leishmania infantum was not addressed conclusively. In 2006, a country-wide collection of sand flies performed in 14 sites selected based on recent occurrence of VL cases showed that Phlebotomus neglectus was by far the most prevalent species (95.6%). Furthermore, 15% of pools made from 422 P. neglectus females tested positive for Leishmania sp. genomic DNA. In the same year, Culicoides trapping was performed for bluetongue disease surveillance in 91 sites of southern Albania, targeting livestock farms regardless recent occurrence of VL in the surveyed areas. In 35 sites where sand flies were collected along with midges, Phlebotomus perfiliewi was the most prevalent among the Phlebotomus species identified, however search for leishmanial DNA in females of this species was unsuccessful. In 2011, sand flies were trapped in 4 sites of north Albania characterized by high VL incidence, and females were dissected to search for Leishmania infections. Both P. neglectus and P. tobbi were collected at high densities. Two positive specimens were detected from a sample of 64 P. neglectus trapped in one site (3.1%). Parasites were successfully cultured from one specimen and characterized as belonging to Leishmania infantum zymodeme MON-1, the only zymodeme so far identified as the agent of human and canine leishmaniasis in the country. Altogether our studies indicate that P. neglectus is the main leishmaniasis vector in Albania.
Introduction
Among the Mediterranean countries endemic for visceral leishmaniasis (VL), Albania is recorded as one of the most affected [1]. Unlike the other South West European countries, the disease occurs predominantly in children [2] although cases of HIV/Leishmania co-infection are recently increasing in adults. VL cases occur in urban settlements on the Adriatic coastal plain and the adjoining river valleys. About 80% of patients notified to the Institute of Public Health from 1995 through 2014 were from peripheral districts of Shkoder and Lezhë in the north, Tirana in the centre and Lushnjë, Fier, Berat and Vlorë in the south. The remaining cases were from rural areas in coastal or lake territories. It is likely that a proportion of VL cases occurred in remote rural areas are left undiagnosed or unreported. Although very underreported, cutaneous leishmaniasis (CL) is seen with higher rates from Lezhë, Elbasan, Krujë, Tiranë and Berat districts (data recorded by the Institute of Public Health, Tirana).
Since late 1980s, a number of parasite isolates from human (VL and CL) and canine leishmaniasis cases were obtained and identified biochemically or molecularly as Leishmania infantum. Some of them, typed by Multilocus Enzyme Electrophoresis (MLEE), were found to belong to zymodeme Montpellier (MON)-1, the commonest zymodeme agent of VL in the Mediterranean region (Table 1).
Table 1. Albanian Leishmania isolates identified from 1988 through 2012 by MultiLocus Enzyme Electrophoresis (MLEE) or PCR-restriction fragments length polymorphism of ribosomal internal transcribed spacer-1 (ITS-1 PCR-RFLP).
| Strain/Sample Code | Year | Host | Clinical form | Place, District | Typing method | Leishmania identification |
|---|---|---|---|---|---|---|
| MCAN/AL/1988/ISS429 | 1988 | Dog | Canine leishmaniasis | Tirane | MLEE | L. infantum MON-1 |
| MCAN/AL/1998/C78L574-ISS1761 | 1998 | Dog | Canine leishmaniasis | Tirane | MLEE | L. infantum MON-1 |
| MHOM/AL/2006/ISS2840-ZR | 2006 | Human | Visceral leishmaniasis | Patos, Fier | MLEE | L. infantum MON-1 |
| MCAN/AL/2006/ISS2841 | 2006 | Dog | Canine leishmaniasis | Tirane | MLEE | L. infantum MON-1 |
| MHOM/AL/2007/ISS2849-FT | 2007 | Human | Visceral leishmaniasis | Lushnje | ITS-1 PCR-RFLP | L. infantum |
| MHOM/AL/2007/ISS2850-KH | 2007 | Human | Visceral leishmaniasis | Devoll | ITS-1 PCR-RFLP | L. infantum |
| MHOM/AL/2010/ISS2996-ED | 2010 | Human | Visceral leishmaniasis | Burrel, Mat | ITS-1 PCR-RFLP | L. infantum |
| RK2012-CL | 2012 | Human | Cutaneous leishmaniasis | Shijak, Durres | ITS-1 PCR-RFLP | L. infantum |
Robust knowledge of the domestic/peridomestic phlebotomine sand fly fauna was acquired through several entomological surveys performed all over Albania during the 1958–1989 period (reviewed by Adhami and Murati [3]). Results from these investigations showed the endemic presence of three members of the Phlebotomus (Larroussius) subgenus, namely Ph. neglectus, Ph. perfiliewi and Ph. tobbi, all species incriminated as L. infantum vectors elsewhere in the Mediterranean [4–6]. Some years later (2002), a study on the sand fly fauna in two areas of central (Kruje district) and northern Albania (Lezhë district) confirmed the presence of the above Larroussius species, among which P. neglectus was found predominant (75.6%) [7]. In this study, natural Leishmania infections where searched by molecular techniques in a small number of P. neglectus females, which were found negative. Ultimately, the role of Larroussius phlebotomine members in the transmission of L. infantum in Albania was not substantially addressed so far. In this paper, we report the results of three entomological surveys carried out in several leishmaniasis endemic areas and provide evidence for the definitive incrimination of Ph. neglectus as the main VL vector in Albania.
Materials and methods
Periods of sand fly collection and description of sites
In total, 132 sites were investigated for sand flies during 2001–2014 (Fig 1), of which 109 across three studies (Fig 2). No specific permissions were required for these locations/activities. Landowners gave permission to conduct the studies on their properties. The field studies did not involve endangered or protected species.
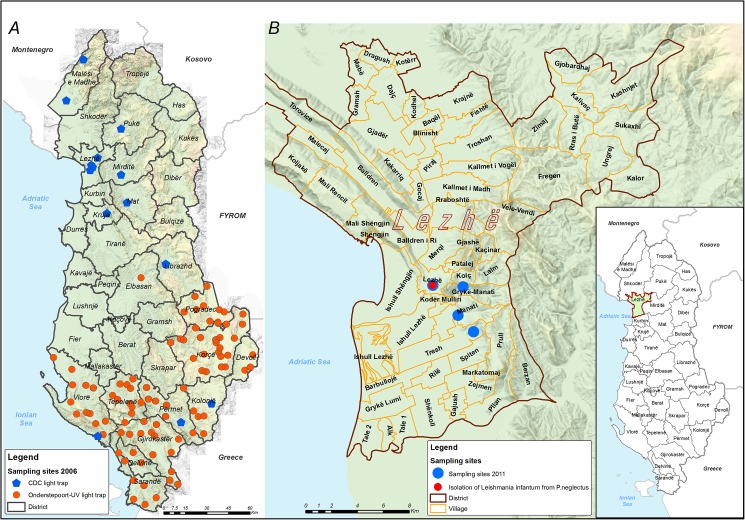
Fig 1. Spatial distribution of sand fly sampling sites and human leishmaniasis cases in the study area for the period 2001–2014.
Fig 2.
Sand fly collection sites (A and B). Map showing: (A) 2006 sampling sites targeting foci of recent VL transmission and Culicoides midges monitoring in the frame of a bluetongue-disease surveillance program (see text for the sampling frame design); (B) 2011 sampling sites targeting foci of recent VL transmission.
Study 1. Country-wide collections targeting VL endemic sites
Between June and September 2006 sand flies were collected in 10/36 districts, representative of northern (4), central (3) and southern territories (3), with the aim of providing further information on sand fly species distribution and prevalence, and to search for Leishmania infections in putative vectors by means of molecular methods. The survey included a total of 14 sites. Target areas consisted of small villages or peri-urban quarters of towns where VL cases were recently reported in resident population. Sites consisted of cow barns and chicken pens near houses. A census of potential sand fly hosts revealed communities made of humans (4–15), dogs (1–4), cats (1–6), cows (1–4), sheep (0–40), pigs (0–10), chickens (5–15) and rabbits (0–8). Altitude of sites varied between the districts, ranging 5–499 m above sea level (a.s.l.) for most of them (8 districts), and 806–1000 m for 2 mountainous districts.
Study 2. Sites monitored in three southern districts
Between May and October 2006 sand flies were collected by taking advantage of a systematic Culicoides midges trapping in the frame a bluetongue disease surveillance program. A total of 91 sites in 9 counties were investigated, located in three southernmost districts of Vlorë, Gjirokastër and Korçë. Target sites were farms with presence of pigs, equines or ruminant livestock. A sampling frame based on 88 km2 sections was applied due to the cross-sectional nature of the study and the wide range of habitats present over a small geographical territory. Using Arcview9 GIS software (ESRI, 2005), the sampling frame was superimposed on a map layer of the study area. A code was given to each section in order to identify that specific area and the closest village to the middle of the section was used as the sampling point. Altitudes of sites varied between the three districs, ranging from 2–702 m a.s.l. in Vlorë, 149–977 m a.s.l. in Gjirokastër and 517–1322 m a.s.l. in Korçë. Farms chosen as trapping sites satisfied specific criteria including the presence of at least 5 livestock units (e.g. cattle, goats, sheep, pigs, horses or donkeys), location at least 2.5 km from the sea coast, and no insecticides used during the past 6 months.
Study 3. Sites with recent high VL incidence
A survey designed for Leishmania isolation and characterization from dissected sand fly specimens, was performed in September 2011 in rural and peri-urban sites of 4 villages of Lezhë district. The choice of representative sites for sand fly collections was based on the following: i) high cumulative incidence of VL in the population; ii) previous knowledge (from Study 1) of the endemic presence of potential L. infantum vector species, and iii) logistic considerations, such as a convenient distance from the equipped laboratory necessary for microscopy dissections and cultures to be performed in aseptic conditions.
Sand fly collection and identification
Catches were performed using two methods, standard CDC miniature light traps (Hausherr’s Machine Works, Toms River, NJ, USA) worldwide used for sand flies collection, and Onderstepoort-type blacklight traps with 8 W UV-light bulbs and downdraught suction motors which have Culicoides as main target species. At best of our knowledge, no studies are available on the comparative effectiveness between these two types of traps for the collection of sand flies. In Studies 1 and 3, two-three CDC traps per site were set in protected habitats such as inside or close to animal pens, in courtyards adjacent to houses or under the eaves of buildings. Traps were suspended at approximately 1.5 meters above the ground and were operated from one hour before sunset until one hour after sunrise. The light traps were retrieved each morning and the collected insects taken alive to laboratory for processing.
In Study 2, catches were carried out using 10 Onderstepoort-type traps for Culicoides monitoring. In each collecting site, one trap was used for one night only. The traps were positioned outdoor within 25 m from the housed livestock, 1.5–2 m above ground, and were operated overnight. The resultant insect catch was poured through a fine gauze square and then transferred to a plastic jar containing 70% alcohol for transportation.
In both types of catches, phlebotomines were sorted under a binocular microscope, counted and stored in alcohol prior to their identification following standard taxonomic keys [8,9].
Examination of sand fly specimens for Leishmania infection
Detection of Leishmania genomic DNA
Nested (n)-PCR was used to detect Leishmania genomic DNA in groups of females pooled by species, site and date of collection. The specimens were homogenized in 1.5 ml sterile tubes using a plastic pestle. 40 μL lysis buffer (100 mM TRIS-HCI, 100 mM NaCI, 25 mM EDTA, 0.5% SDS, pH 8) was added and the homogenate was digested overnight at 37°C by 2 μg/μL proteinase K (Promega). Genomic DNA was extracted by phenol-chloroform and precipitated with 100% ethanol and then centrifuged for 30 min at 13,000g. The DNA pellet was resuspended in 50 μL of sterile water and stored at—20°C until use. Small-subunit ribosomal DNA was amplified by n-PCR technique by sequential amplification of gene fragments using kinetoplastid specific primers R221 and R332, and Leishmania sp. primers R223 and R333 [10]. The cycling conditions were denaturation at 94°C for 30 s, annealing at 60°C for 30 s (65°C for 30 s for the second PCR) and extension at 72°C. Two negative controls (DNA from uninfected flies and no DNA) and two positive controls (L. infantum DNA and Phlebotomus plus L. infantum DNA) were used in all experiments. Finally, the amplification products were analysed through a 1.5% agarose gel and visualized under UV light. Positive samples were anticipated to yield a predicted n-PCR product of 358 bp.
Isolation of Leishmania parasites
Before dissection the flies were anaesthetized for 5 min in a deep freezer and stored in sterile phosphate-buffered saline containing gentamicin (250 g/ml), 5-fluorocytosine (500 g/ml) and commercial baby shampoo (1 drop/30 ml). The specimens were identified by the morphology of pharyngeal armature and spermathecae according to Léger et al [9]. When flies were found to harbor promastigotes, some drops of Evans' Modified Tobie's Medium (EMTM) liquid phase were added to the dissected material and then the entire gut was aspirated and inoculated into screw-top vials containing EMTM solid phase [11].
Characterization of Leishmania isolates
Leishmania typing was performed by both molecular and biochemical methods. PCR-RFLP analysis used primers LITSR and L.5.8S amplifying the internal transcribed spacer-1 (ITS-1) sequence separating the genes coding for SSU rRNA and 5.8S rRNA [12,13]. Leishmania DNA from the WHO reference strain for L. infantum (MHOM/TN/1980/IPT-1) and from two Albanian L. infantum strains of human and canine origin (MHOM/AL/2006/ISS2840 and MCAN/AL/2006/ISS2841, respectively), were used as reference. Ten μl of PCR products were digested overnight in a total volume of 20 μl, with 10U of HaeIII restriction enzyme, as recommended by the manufacturer (Promega). PCR-RFLP products were subjected to electrophoresis by 4% MethaPhor gel (EuroClone) or by Qiaxcel capillary electrophoresis (Qiagen GmbH, Hilden, Germany).
MLEE was employed for the analysis of 15 enzymatic systems following the Montpellier (MON) methodology and zymodeme nomenclature [14]: phosphoglucomutase (PGM; E.C.2.7.5.1); glucose-phosphate isomerase (GPI; E.C.5.3.1.9); glutamate-oxaloacetate transaminases (GOT1, GOT2; E.C.2.6.1.1.); malic enzyme (ME; E.C.1.1.1.40); phosphogluconate dehydrogenase (6PGD; E.C.1.1.1.44); glucose-6-phosphate dehydrogenase (G6PD; E.C.1.1.1.49); malate dehydrogenase (MDH; E.C.1.1.1.37); nucleoside phosphorylases 1 and 2 (NP1, NP2; E.C.2.4.2.1, E.C.4.2.1.*); mannose-phosphate isomerase (MPI; E.C.5.3.1.8); isocitrate dehydrogenase (ICD; E.C.1.1.1.42); diaphorase NADH (DIA; E.C.1.6.2.2); glutamate-dehydrogenase (GLUD; E.C.1.4.1.3); fumarate hydratase (FH; E.C.4.2.1.2) [14]. L.infantum MON-1 (MHOM/TN/80/IPT-1), L.major MON-4 (MHOM/SU/73/5-ASKH) and L.tropica MON-60 (MHOM/SU/74/K27) were used as reference.
Results
Sand fly monitoring
Sand fly species identified and relative abundance
A total of 1,666 sand fly specimens (28.5% males) was collected in the 3 studies (Table 2).
Table 2. Sand fly species diversity and cumulative relative abundance recorded during three entomological studies carried out in Albania in 2006 and 2011.
| Study year | Specimens(M%) | Species (%) | |||||
|---|---|---|---|---|---|---|---|
| P. neglectus | P. tobbi | P. perfiliewi | P. papatasi | P. similis | S. minuta | ||
| 2006a | 549 (28.3) | 525 | 5 | 1 | 0 | 0 | 18 |
| 2006b | 730 (43.7) | 100 | 52 | 213 | 0 | 14 | 351 |
| 2011c | 387 (NR) | 216 | 145 | 0 | 5 | 0 | 21 |
| Total | 1666 (28.5) | 841 (50.5) | 202 (12.2) | 214 (12.8) | 5 (0.3) | 14 (0.8) | 390 (23.4) |
a VL-targeted country-wide survey (Study 1)
b Bluetongue-disease surveillance in south Albania, (Study 2)
c Study aimed at Leishmania identification from dissected sand flies in sites of elevated VL transmission (Study 3); M: male; NR: species unrecorded in males.
They included 5 Phlebotomus species belonging to three subgenera (Larroussius, Phlebotomus and Paraphlebotomus) and one species of Sergentomyia. Ph. neglectus was the most abundant species (50.5% among all phlebotomines; 65.9% among Phlebotomus species) followed by other Larroussius species, Ph. perfiliewi (12.8%/16.8%) and Ph. tobbi (12.2%/15.8%). A few specimens of Ph. similis and Ph. papatasi were also collected, whereas S. minuta represented 23.4% of all phlebotomine specimens.
Spatial distribution
Tables 3 and 4 show the relative abundance by county and district of the sand fly species recorded in Studies 1 and 2, respectively.
Table 3. Prevalence and sand fly species identified by county and district in the 2006 country-wide study targeting VL endemic sites (Study 1).
| Prefecture | District | Specimens (M%) | Species (%) | |||
|---|---|---|---|---|---|---|
| P. neglectus | P. tobbi | P. perfiliewi | S. minuta | |||
| Vlorë | Vlorë | 26 (7.7) | 7 | 0 | 1 | 18 |
| Total (%) | 26 (7.7) | 7 (26.9) | 0 (0.0) | 1 (3.8) | 18 (69.3) | |
| Durrës | Krujë | 31 (0.0) | 31 | 0 | 0 | 0 |
| Total (%) | 31 (0.0) | 31 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Gjirokastër | Përmet | 22 (36.4) | 17 | 5 | 0 | 0 |
| Total (%) | 22 (36.4) | 17 (77.3) | 5 (22.7) | 0 (0.0) | 0 (0.0) | |
| Korçë | Kolonjë | 40 (7.5) | 40 | 0 | 0 | 0 |
| Total (%) | 40 (7.5) | (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Lezhë | Lezhë | 130 (19.2) | 130 | 0 | 0 | 0 |
| Mirditë | 1 (100.0) | 1 | 0 | 0 | 0 | |
| Total (%) | 131 (19.8) | 131 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Elbasan | Librazhd | 2 (100.0) | 2 | 0 | 0 | 0 |
| Total (%) | 2 (100.0) | 2 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Shkodër | Malësi e Madhe | 220 (37.7) | 220 | 0 | 0 | 0 |
| Pukë | 65 (52.3) | 65 | 0 | 0 | 0 | |
| Total (%) | 285 (41.4) | 285 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Dibër | Mat | 12 (0.0) | 12 | 0 | 0 | 0 |
| Total (%) | 12 (0.0) | 12 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Overtotal | 549 (28.3) | 525 (95.6) | 5 (0.9) | 1 (0.2) | 18 (3.3) | |
M = male
Table 4. Prevalence and sand fly species by county and district collected in the 2006 Culicoides-monitoring study in southernmost counties of Albania (Study 2).
| Prefecture | District | Specimens (M%) | Species (%) | ||||
|---|---|---|---|---|---|---|---|
| P. neglectus | P. tobbi | P. perfiliewi | P. similis | S. minuta | |||
| Vlorë | Delvinë | 23 (0.0) | 0 | 0 | 0 | 0 | 23 |
| Sarandë | 258 (34.5) | 24 | 6 | 56 | 0 | 172 | |
| Vlorë | 144 (82.1) | 4 | 15 | 116 | 0 | 9 | |
| Total (%) | 425 (47.1) | 28 (6.6) | 21 (4.9) | 172 (40.5) | 0 | 204 (48.0) | |
| Gjirokastër | Gjirokastër | 187 (31.5) | 46 | 21 | 14 | 14 | 92 |
| Përmet | 61 (27.9) | 7 | 5 | 12 | 0 | 37 | |
| Tepelenë | 27 (88.9) | 13 | 3 | 9 | 0 | 2 | |
| Total (%) | 275 (40.4) | 66 (24.0) | 29 (10.5) | 35 (12.8) | 14 (5.1) | 131 (47.6) | |
| Korçë | Devoll | 2 (0.0) | 0 | 0 | 1 | 0 | 1 |
| Korçë | 26 (30.7) | 6 | 2 | 4 | 0 | 14 | |
| Pogradec | 2 (0.0) | 0 | 0 | 1 | 0 | 1 | |
| Total (%) | 30 (26.7) | 6 (20.0) | 2 (6.7) | 6 (20.0) | 0 (0.0) | 16 (53.3) | |
| Overtotal | 730 (43.7) | 100 (13.7) | 52 (7.1) | 213 (29.2) | 14 (1.9) | 351 (48.1) | |
M = male
In Study 1, Ph. neglectus was found to be the prevalent species in all districts monitored (95.6% among all phlebotomines; 98.9% among Larroussius species). It was collected into and around sites close to recent VL cases, especially near chicken coops and animal pens. The other Larroussius species identified were Ph. tobbi (0.9%) and Ph. perfiliewi (0.2%). A few specimens of S. minuta (18) were also recorded.
In Study 2, only 35/91 sites monitored (38.5%) were positive for sand flies: 15/20 (75.0%) in Vlorë, 23/30 (76.7%) in Gjirokastër and 7/41 (17.1%) in Korçë. The majority of sand fly specimens (425) were from Vlorë country (58.2%), particularly from Sarandë and Vlorë districts. In this study the prevalent species was found to be Ph. perfiliewi (29.9% among all phlebotomines; 56.2% among Larroussius species), whereas Ph. neglectus and Ph. tobbi accounted for 13.7% and 7.1% of specimens, respectively. Ph. similis (1.9%) was only recorded in three close sites of Dropull, Gjirokastër district. The highest elevation of a positive site was at 1,185 m a.s.l., where one Ph. neglectus and one S. minuta were caught. The latter species represented 48.1% of all specimens collected in this study.
In Study 3, Ph. neglectus was the most prevalent species (55.8%) followed by Ph. tobbi (37.5%). The other two species identified, not associated to VL transmission, were Ph. papatasi (1.3%) and S. minuta (5.4%).
Detection of Leishmania infections in sand flies
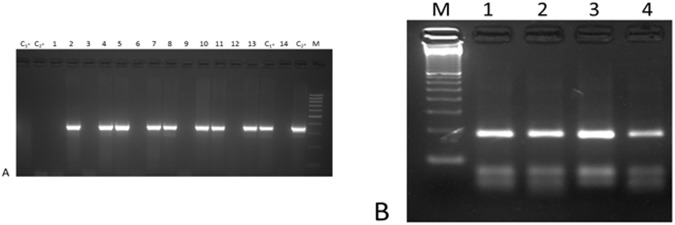
In Study 1, n-PCR was used to detect genomic Leishmania DNA in 425 Phlebotomus (Larroussius) females after species identification. The average number of specimens per pool was 8 (range: 3–12). In total, 8/54 pools (14.8%) were found positive for leishmanial DNA, of which 7/47 pools made from 422 Ph. neglectus, and one pool made from Ph. tobbi, consisting of the only 3 specimens collected in Permet district (Fig 3A).
Fig 3. Molecular detection and characterization of Leishmania from wild-caught sand flies in Albania.
(A) Nested-PCR targeting a Leishmania sp. small-subunit ribosomal DNA sequence. Lane C1-: uninfected reared sand fly DNA; lane C2-: PCR Master Mix with no DNA; lanes 2, 4, 5, 7, 8, 10, 11: positive P. neglectus pools; lane 13: positive P. tobbi pool; lanes 1, 3, 6, 9, 12, 14: negative P. perfiliewi pools; lane C1+: L. infantum promastigotes DNA; C2+: L. infantum promastigotes DNA mixed with uninfected reared sand fly DNA; lane M: 100 base pair ladder (Promega). (B) ITS-1 n-PCR-RFLP for Leishmania species characterization. Lane M: 100 base pair ladder (Promega); lane 1: Leishmania isolate from Albanian P. neglectus (IMJN/AL/2011/MJN2-ISS3056); lane 2: L. infantum (human isolate from Albania, MHOM/AL/2006/ISS2840); lane 3: L. infantum (dog isolate from Albania, MCAN/AL/2006/ISS2841); lane 4: WHO reference strain for L. infantum (MHOM/TN/1980/IPT-1).
Regarding the spatial distribution of Ph. neglectus-positive pools, 2 were from Kolonje, 1 from Kruje, and 4 from Lezhë district, of which 3 from the Lezhë suburban VL focus of Koder-Marlekay. None of the 7 Ph. perfiliewi pools collected from Vlorë and Sarandë districts (a total of 44 specimens) was found positive for leishmanial DNA.
In Study 3, two of 387 sand fly females dissected were found to harbour promastigotes (0.9%). Table 5 reports the number of females examined and the species identified for each site.
Table 5. Leishmania infections detected in wild-caught sand flies in sites of Lezhë district, September 2011 (Study 3).
| Site | Altitudes (m a.s.l.) |
VL cases recorded ** | No. of flies dissected | Species (no. of Leishmania positive; percentage) | |||
|---|---|---|---|---|---|---|---|
| P. neglectus | P. tobbi | P. papatasi | S. minuta | ||||
| Kodër Marlekaj* | 43 | 2 | 69 | 64 (2; 3.1%) | 5 (0) | 0 | 0 |
| Tresh | 96 | 2 | 308 | 147 (0) | 138 (0) | 5 (0) | 18 (0) |
| Grykë Manati | 31 | 1 | 7 | 2 (0) | 2 (0) | 0 | 3 (0) |
| Manati | 26 | 1 | 3 | 3 (0) | 0 | 0 | 0 |
| Total | 6 | 387 | 216 (2; 0.9%) | 145 (0) | 5 (0) | 21(0) | |
(*) This site is a peri-urban settlement of Lezhë town.
(**) In the previous two years
The two positive specimens were from a sample of 64 Ph. neglectus collected in Koder-Marlekaj (3.1%). Interestingly, a similar n-PCR positive rate (3.5%) was estimated in a pool of this species collected in this site in 2006 (see above). One specimen had a scanty infection with few parasites attached to the wall of the stomodeal valve. The other fly harboured a massive mature infection, with a abundant metacyclic promastigotes. (S1 Video). Parasites have been successfully cultured only from this specimen. The strain (IMJN/AL/2011/MJN2-ISS3056) was identified by as belonging to L. infantum. ITS-1 n-PCR-RFLP showed the specific pattern of this species (184-72-55 bp bands) (Fig 3B), whereas MLEE identified the strain as belonging to zymodeme MON-1.
Discussion
Both VL and CL caused by L. infantum are emerging diseases in Albania. In 2003, Velo et al [15] reported an increasing trend of VL cases, from 144 to 209, occurred between 1997 and 2001. More recently, Petrela et al [2] have confirmed this trend in a retrospective analysis of data recorded from 1995 to 2009 at the national pediatric reference hospital of Tirana. This analysis showed that VL is largely a paediatric disease, with an incidence rate of 25/100,000 in the age group 0–6 years for that hospital area which is much higher than in other endemic countries of southern Europe [1]. A most recent gap analysis from 2005–2013 shows a decreasing trend of VL from 2.5/100000 to 0.8/100000. Dogs are the only confirmed reservoir of zoonotic VL in Albania. As a result of uncontrolled increase in number of stray and owned dogs, canine leishmaniasis is being increasingly observed in several Albanian districts although systematic monitoring of the disease in dogs has not been performed yet. Serosurveys performed on some 400 dogs from 7 districts disclosed an IFAT rate of 15.8% [16].
Overall, our studies indicate that Ph. neglectus is the dominant Larroussius species associated to foci of VL in Albania. This species, also referred as to Ph. major neglectus [17], is a sand fly member of the Ph. major complex [18], which has long been suspected to be involved in the transmission of VL in eastern Mediterranean countries [19,20]. In 1988 Léger et al [6] firstly reported from the island of Corfu (Greece) the natural infection of this species with L. infantum zymodeme MON-1. One year later, Garifallou et al. [20] reported on the natural infection by L. donovani sensu lato (most probably L. infantum) of a Ph. neglectus specimen from the island of Zakinthos (Greece). Ivović et al [21] found promastigotes in dissected females of this species collected in the Bar area of Montenegro, but parasites were not identified. Taking into account morphological characters that distinguish Ph. neglectus from other members of the Ph. major group [22], the geographical distribution of this species appears to be restricted to the eastern Mediterranean countries of Europe and western border of Middle East. Besides Albania, this species was recorded in Bulgaria [23], Cyprus [24], Croatia [25], Greece [26], Hungary [27], Kosovo [28], Italy [29], Malta [30], Montenegro [21], Romania [31], Serbia [32], Slovenia [29], and Turkey [33].
The biology of Ph. neglectus in Albania needs to be investigated further; however some data on seasonal dynamics and host preferences have been recorded from 6 collecting sites of Kruje and Lezhe districts in 2002. First adults were collected by CDC traps on June 11th and the last ones on October 16th. The highest number of specimens was recorded at the end of July. Interestingly, blood-meal analysis from about fifty blood-fed specimens collected, showed that among 5 Phlebotomus species examined, Ph. neglectus was the only species found with human (6%) and canine blood (17%) [7].
Despite a pool made from 3 specimens of Ph. tobbi collected in the southernmost part of the country tested Leishmania positive by a molecular method in Study 1 in 2006, we failed to demonstrate microscopically the occurrence of natural infections in 145 Ph. tobbi specimens examined in north Albania in Study 3. In general, Ph. tobbi was found low prevalent in several Albanian sites associated to VL transmission as compared with Ph. neglectus (see Table 2). However this species may play the role of a secondary vector in Albania, considering that Ph. tobbi has been incriminated as L. infantum vector in Cyprus [6], in western and eastern parts of Turkey [34,35], and it is suspected to have such a role in neighbouring countries such as Croatia and Greece [36].
Ph. perfiliewi was found abundant only in catches performed using UV light-Onderstepoort-type traps placed near farms housing large livestock, and in sites selected for purposes unrelated to VL transmission. Conversely, the relative prevalence of this species was almost nul in sites targeted because of recent occurrence of VL cases. Furthermore, no Leishmania-PCR positive specimens were recorded in 7 pools from this species. We cannot exclude that the use of a trap developed for Culicoides collection may have provided a biased species prevalence of the sand fly fauna.
The Leishmania typing methods employed by us either require a sufficient amount of intact DNA (for PCR-RFLP), or parasite isolation and mass cultivation (for MLEE). DNA material left from the eight nPCR-Leishmania positive pools proved insufficient, or possibly degraded, for the PCR-RFLP amplification protocol. Furthermore, one of the two infected P. neglectus specimens had a scanty infection of promastigotes which did not grow in culture and were lost for molecular typing.
Altogether our studies reasonably indicate that Ph. neglectus is the main vector of human leishmaniasis in Albania, as three main vector incrimination criteria (recently reviewed in [36]) were satisfied: i) Ph. neglectus is associated with biotopes of human VL transmission; ii) it exhibits anthropophily and also feeds on the canine reservoir host; iii) this species was found to harbour infectious stages of the Leishmania species and zymodeme causing human and canine disease in Albania.
Supporting information
(WMV)
Acknowledgments
The authors are grateful to Eng. Migel Ali, GIS expert at the Company Geo Consulting Albania, who helped us to prepare the maps specifically for this manuscript; and we thank the families in Albania, which allowed us to carry out the field work in their properties. Moreover we thank Erion Muhaxhiri, Viola Jani and Gjergji Sino for technical assistance.
Data Availability
All relevant data are within the paper and its Supporting Information file.
Funding Statement
This work has been funded by the Institute of Public Health, Tirana and partially funded by EU grant FP7-261504 EDENext and is catalogued by the EDENext Steering Committee as EDENext471 (http://www.edenext.eu). The contents of this publication are the responsibility of the authors and do not necessarily reflect the views of the European Commission. MM was supported by a grant of the World Health Organization, Office in Albania, Consulting Research Service 2011/170992-0.
References
- 1.Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. (2012) Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLoS One 7 (5): e35671 doi: 10.1371/journal.pone.0035671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petrela R, Kuneshka L, Foto E, Zavalani F, Gradoni L (2010) Pediatric visceral leishmaniasis in Albania: A retrospective analysis of 1,210 consecutive hospitalized patients (1995–2009). PLoS Negl Trop Dis 4: e814 doi: 10.1371/journal.pntd.0000814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adhami J, Murati N (2000) Phlebotomine sandflies (Diptera: Psychodidae) of domestic places in Albania. Revista Mjeksore 1: 60–75 [in Albanian]. [Google Scholar]
- 4.Léger N, Gramiccia M, Gradoni L, Madulo-Leblond G, Pesson B, Ferté H, et al. (1988) Isolation and typing of Leishmania infantum from Phlebotomus neglectus on the island of Corfu, Greece. Trans R Soc Trop Med Hyg 82: 419–420. [DOI] [PubMed] [Google Scholar]
- 5.Maroli M, Gramiccia M, Gradoni L (1987) Natural infection of sandfly Phlebotomus perfiliewi with Leishmania infantum in a cutaneous leishmaniasis focus of the Abruzzi region, Italy. Trans Roy Soc Trop Med Hyg 81: 596–598. [DOI] [PubMed] [Google Scholar]
- 6.Léger N, Depaquit J, Ferté H, Rioux JA, Gantier JC, Michaelides A, et al. (2000) [Phlebotomine sandflies (Diptera-Psychodidae) of the isle of Cyprus. II-Isolation and typing of Leishmania (Leishmania infantum Nicolle, 1908 (zymodeme MON 1) from Phlebotomus (Larroussius) tobbi Adler and Theodor, 1930]. Parasite 7: 143–146 [Article in French]. doi: 10.1051/parasite/2000072143 [DOI] [PubMed] [Google Scholar]
- 7.Velo E, Paparisto A, Bongiorno G, Di Muccio T, Khoury C, Bino S, et al. (2005) Entomological study on phlebotomine sandflies in central and northern Albania. Parasite 12: 45–49. doi: 10.1051/parasite/2005121045 [DOI] [PubMed] [Google Scholar]
- 8.Theodor O (1958) Psychodidae-Phlebotomine. In: Lindner, E. (Ed.), Die Fliegen der Palaearktischen Region, 9c. E. Schweizerbart’sche Verlagsbuchhandlung, Stuttgart, pp 1–55.
- 9.Léger N, Pesson B, Madulo-Leblond G, Abonnenc E (1983) [Differentiation of females of the subgenus Larroussius Nitzulescu 1931 (Diptera-Phlebotomidae) of the Mediterranean region]. Ann Parasitol Hum Comp 58: 611–623 [Article in French]. [PubMed] [Google Scholar]
- 10.Van Eys GJ, Schoone GJ, Kroon NC, Ebeling SB (1992) Sequence analysis of small subunit ribosomal RNA genes and its use for detection and identification of Leishmania parasites. Mol Biochem Parasitol 51: 133–142. [DOI] [PubMed] [Google Scholar]
- 11.Maroli M, Gramiccia M, Gradoni L, Troiani M, Ascione R (1994) Natural infection of Phlebotomus perniciosus with an enzymatic variant of Leishmania infantum in the Campania region of Italy. Acta Trop 57: 333–335. [DOI] [PubMed] [Google Scholar]
- 12.Schönian G, Nasereddin A, Dinse N, Schweynoch C, Schallig HD, Presber W, et al. (2003) PCR diagnosis and characterization of Leishmania in local and imported clinical samples. Diagn Microbiol Infect Dis 47: 349–358. [DOI] [PubMed] [Google Scholar]
- 13.Alcover MM, Gramiccia M, Di Muccio T, Ballart C, Castillejo, Picado A, et al. (2012) Application of molecular techniques in the study of natural infection of Leishmania infantum vectors and utility of sandfly blood meal digestion for epidemiological surveys of leishmaniasis. Parasitol Res 111: 515–523. doi: 10.1007/s00436-012-2863-4 [DOI] [PubMed] [Google Scholar]
- 14.Gramiccia M (2003) The identification and variability of the parasites causing leishmaniasis in HIV-positive patients in Italy. Ann Trop Med Parasitol 97 (Suppl 1): 65–73. [DOI] [PubMed] [Google Scholar]
- 15.Velo E, Bino S, Kuli-Lito G, Pano K, Gradoni L, Maroli M (2003) Recrudescence of visceral leishmaniasis in Albania: retrospective analysis of cases during 1997 to 2001 and results of an entomological survey carried out during 2001 in some districts. Trans R Soc Trop Med Hyg 97: 288–290. [DOI] [PubMed] [Google Scholar]
- 16.Cani E, Myrseli T, Petrela R, Minarolli P, Pano K (2001) Visceral leishmaniasis—a zoonosis with high potential risk in Albania. Revista Veterinaria 5: 81–92. [in Albanian]. [Google Scholar]
- 17.Léger N, Pesson B (1987) [Taxonomy and geographic distribution of Phlebotomus (Adlerius) chinensis s. l. and P. (Larroussius) major s. l. (Psychodidae-Diptera). Status of species present in Greece]. Bull Soc Pathol Exot Filiales 80: 252–60 [Article in French]. [PubMed] [Google Scholar]
- 18.Adler S, Theodor O (1935) Investigations on Mediterranean Kala-Azar. IX Feeding Experiments with Phebotomus perniciosus and other species on animals infected with Leishmania infantum. Proc R Soc 116: 516–542. [Google Scholar]
- 19.Adler S, Theodor O (1957) Transmission of disease agents by phlebotomine sandflies. Ann Rev Entomol 2: 203–226. [Google Scholar]
- 20.Garifallou A, Hadziandoniou M, Schnur LF, Yuval B, Warburg A, Jacobson RL, et al. (1989). Epidemiology of human and canine leishmaniasis of the island of Zakinthos. In: DT Hart (Editor), Leishmaniasis. NATO ASI Series, Plenum Press: 1011–1015
- 21.Ivović V, Depaquit J, Léger N, Urano A, Papadopoulos B. (2004) Sandflies (Diptera: Psychodidae) in the Bar area of Montenegro (Yugoslavia). 2. Presence of promastigotes in Phlebotomus neglectus and first record of P. kandelakii. Ann Trop Med Parasitol 98: 425–427. doi: 10.1179/000349804225003352 [DOI] [PubMed] [Google Scholar]
- 22.Killick-Kendrick R, Tang Y, Killick-Kendrick M, Sang DK, Sirdar MK, Ke L., et al. (1991) The identification of female sandflies of the subgenus Larroussius by the morphology of the spermathecal ducts. Parassitologia 33: S335–S347. [PubMed] [Google Scholar]
- 23.Tsachev I, Papadogiannakis El, Kontos V, Ivanon A, Chakarova B, Stojankev K, et al. (2007) Seroepidemiology of Leishmania infantum exposure among healthy dogs in Bulgaria. Turk J Vet Anim Sci 31: 73–74. [Google Scholar]
- 24.Rastgeldi S, Ozbel Y, Ozensoy Toz S, Ertabaklar H, Göcmen B. (2005) Phlebotominae sand flies (Diptera: Psychodidae) of the Northern part of Cyprus Island. Proc 5th Int Symp Phlebotomine Sandflies April 17–21, 2005. Arch Inst Pasteur Tunis 82: 121. [Google Scholar]
- 25.Bosnić S, Gradoni L, Khoury C, Maroli M (2006) A review of leishmaniasis in Dalmatia (Croatia) and results from recent surveys on phlebotomine sandflies in three southern counties. Acta Trop 99: 42–49. doi: 10.1016/j.actatropica.2006.06.009 [DOI] [PubMed] [Google Scholar]
- 26.Chaniotis B, Gozalo Garcia G, Tselentis Y (1994) Leishmaniasis in greater Athens, Greece. Entomological studies. Ann Trop Med Parasitol 88: 659–663. [DOI] [PubMed] [Google Scholar]
- 27.Farkas R, Tanczos B, Bongiorno G, Maroli M, Dereure J, Ready PD. (2011) First surveys to investigate the presence of canine leishmaniasis and its phlebotomine vectors in Hungary. Vector Borne Zoonotic Dis 11: 823–834. doi: 10.1089/vbz.2010.0186 [DOI] [PubMed] [Google Scholar]
- 28.Lewis DJ (1982). Taxonomic review of the genus Phlebotomus (Diptera: Psychodidae). B Brit Museum (Natural History) (Entomology) 45: 121–209. [Google Scholar]
- 29.Maroli M, Khoury C, Bianchi R, Ferroglio E, Natale A (2002) Recent findings of Phlebotomus neglectus Tonnoir, 1921 in Italy and its western limit of distribution. Parassitologia, 44: 103–109. [PubMed] [Google Scholar]
- 30.Léger N, Marchai R, Madulo-Leblond G, Pesson B, Kristensen A, Ferté H, et al. (1991) Les phlébotomes impliqués dans la transmission des leishmanioses dans l’ıle de Gozo (Malte). Ann Parasitol Hum Comp 66: 33–41. [Google Scholar]
- 31.Dancesco P (2008) Les espèces de phlébotomes (Diptera: Psychodidae) de Roumanie, certains aspects de leur écologie et nouvelles stations de capture. Travaux du Muséum National d’Histoire Naturelle “Grigore Antipa” 51: 185–199. [Google Scholar]
- 32.Zivkovic V (1982) Faunistic and ecological investigations of sandflies (Diptera, Phlebotomidae) in Serbia. Acta Vet 32: 295–306. [Google Scholar]
- 33.Ozbel Y, Balcioğlu IC, Olgen MK, Simsek FM, Töz SÖ, Ertabaklar H, et al. (2011) Spatial distribution of phlebotomine sand flies in the Aydin Mountains and surroundings: the main focus of cutaneous leishmaniasis in western Turkey. J Vector Ecol 36: S99–S105. doi: 10.1111/j.1948-7134.2011.00118.x [DOI] [PubMed] [Google Scholar]
- 34.Svobodová M, Alten B, Zídková L, Dvorák V, Hlavacková J, Myšková J, et al. (2009) Cutaneous leishmaniasis caused by Leishmania infantum transmitted by Phlebotomus tobbi. Int J Parasitol 39: 251–256. doi: 10.1016/j.ijpara.2008.06.016 [DOI] [PubMed] [Google Scholar]
- 35.Ozbel Y, Karakuş M, Arserim SK, Kalkan ŞO, Töz S. (2016) Molecular detection and identification of Leishmania spp. in naturally infected Phlebotomus tobbi and Sergentomyia dentata in a focus of human and canine leishmaniasis in western Turkey. Acta Trop 155: 89–94. doi: 10.1016/j.actatropica.2015.12.018 [DOI] [PubMed] [Google Scholar]
- 36.Maroli M, Feliciangeli MD, Bichaud L, Charrel RN, Gradoni L (2013) Phlebotomine sandflies and the spreading of leishmaniases and other diseases of public health concern. Med Vet Entomol 27: 123–147. doi: 10.1111/j.1365-2915.2012.01034.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(WMV)
Data Availability Statement
All relevant data are within the paper and its Supporting Information file.