Abstract
Background
Immunosuppression is common even in the early stage of severe sepsis. Interleukin-10 (IL-10) secretion and lymphocyte exhaustion are the main features of sepsis-induced immunosuppression. However, the relationship between IL-10 and the lymphocyte is still unclear. We investigated if IL-10/lymphocyte ratio (IL10LCR) were associated with mortality in severe septic patients.
Methods
Adult patients with severe sepsis admitted to ICU of the First Affiliated Hospital of Guangzhou Medical University were identified from October 2012 to August 2013. Within 24 hours of ICU admission, peripheral whole blood was collected for the measurement of IL-10 using commercial multiplex bead-based assay kits and determination of lymphocyte count from laboratory data. The primary outcome was 28-day mortality.
Results
A total of 63 severe sepsis patients were identified. There were 20 (32%) patients died within 28 days. IL10LCR in non-survival patients was significantly higher than survival patients (median (IQR) 36.78 (12.34–79.63) ng/ml2 versus 11.01(5.41–27.50) ng/ml2, P = 0.002). Correlation analysis showed that IL10LCR was significantly correlated with APACHE II score (Spearman’s rho = 0.424, P<0.001). The receiver operating characteristic (ROC) curves showed the area under the curve was 0.749 for IL10LCR level to predict 28-day mortality with sensitivity and specificity at 70.0% and 74.4%, respectively. At an optimal cutoff of 23.39ng/ml2, Kaplan-Meier curve showed survival in patients with IL10LCR level above 23.39ng/ml2 was significantly lower than in patients with IL10LCR level less than 23.39ng/ml2 (P = 0.001 by log-rank test).
Conclusion
IL10LCR level is significantly associated with the severity and outcome of severe septic patients. It may serve as a biomarker for sepsis-induced immunosuppression.
Introduction
Sepsis is the one of the leading causes of mortality in intensive care units (ICUs). The mortality of sepsis is around 25%-30% and the current treatments of sepsis are very limited[1,2]. Early studies suggested that uncontrolled “cytokine storm” contribute to major mortality of severe sepsis. However, the anti-inflammatory therapies have failed, showing no improvement in clinical outcomes[3]. Nowadays, it is believed that sepsis initiates a complex immunologic response to upsetting the balance between pro-inflammatory and anti-inflammatory processes, leading to immunosuppressive phase and resulting in uncontrolled primary infection or secondary hospital-acquired infections, which contributes to mortality increasing[4].
The mainly features of sepsis-induced immunosuppression was overproduction of anti-inflammatory cytokines and apoptosis-related immune cells[5]. Interleukin-10 (IL-10) is one of the most important anti-inflammatory cytokines, whose concentrations in the blood rang from 12pg/ml to 2400pg/ml in septic patients[6]. Previous researches reported that higher plasma IL-10 concentration contributed to higher mortality of sepsis[7,8]. Additionally, lymphocyte exhaustion, including CD4 and CD8 T cells, B cells, is common in septic patients[9,10]. Drewry et.al showed that persistent lymphopenia on the fourth day after diagnosis of sepsis can predict mortality[11]. Therefore, higher IL-10 and lymphopenia are important in sepsis-induced immunosuppression.
However, the relationship between IL-10/lymphocyte ratio(IL10LCR) and the mortality of severe sepsis patients remains unknown. Here, we investigated whether IL10LCR is associated with the outcome of severe sepsis.
Methods
Study design
We conducted a single-center, retrospective cohort study. Patients were recruited from the intensive care unit (ICU) of the First Affiliated Hospital of Guangzhou Medical University from October 2012 to August 2013. Written informed consent was obtained from their relatives. The study was approved by the Ethic Review Committee of the First Affiliated Hospital of Guangzhou Medical University.
Study setting and population
All the patients admitted to the hospital with severe sepsis were included. Sepsis was defined as the presence of documented or clinically diagnosed infection with at least two of the following criteria: a) core temperature above 38°C or below 36°C; b) heart rate of more than 90 beats per minute; c) respiratory rate more than 20 breaths per minute or partial pressure of carbon dioxide below 32 mmHg; and d) leukocytosis (white blood cell count >12×109/L) or leukopenia (white blood cell count <4×109/L) or more than 10% bands in peripheral blood. Severe sepsis was defined as septic patients with at least one organ dysfunction[12].
Exclusion criteria were as follows: a) age<18 year, b) hematological or immunological disease, c) treatment with radiation therapy or chemotherapy for an underlying malignancy within 6 months prior to hospital admission, d) continuous administration corticosteroids (at least 0.5 mg/kg per day of prednisolone or an equivalent drug) at least a month during the 3 months, d) no plasma sample was collected within 24 hours.
Plasma interleukin-10 measurements
Plasma samples were collected from each patient at the time of enrollment. Whole blood was collected in sodium citrate tubes and centrifuge at 1,000 ×g for 10 minutes. Plasma was removed and frozen at -80°C until use.
The plasma level of Interleukin-10 was measured using commercial multiplex bead-based assay kits (Bio-Plex Cytokines Assay, Bio-Rad Inc., USA) according to the manufacturer’s instructions[13].
Data collection
Lymphocyte counts were recorded from laboratory data for the first day admitted to ICU. We also collected the following data: age, sex, underlying disease or comorbidities, the source of infection, the white blood cell count, neutrophil count, Acute Physiology and Chronic Health Evaluation II (APACHE II) score, the length of mechanical ventilation, the length of ICU and hospital stays. The primary outcome for the study was the 28-day mortality in ICU. All the data was recorded at the time of plasma samples collection.
Statistical analysis
Quantitative data were expressed as mean and standard deviation (SD) for normal distribution, while median and interquartile range (IQR) for abnormal distribution. Qualitative data were presented as numbers(%). For continuous variables, two-group comparisons were performed using unpaired t-tests or Mann-Whitney tests according to test of normality. Spearman correlation coefficient was used to test the correlations between IL10LCR levels and other clinical variables. Receiver operating characteristic (ROC) curve was used to determine the sensitivity and specificity of prediction of IL10LC for clinical outcomes. Kaplan-Meier curves were performed by using IL10LCR groups as strata and compared by log-rank test. To identify risk factors for 28-day mortality, a simple and multiple Cox proportional hazards regression model was performed. All analyses and figures were performed by using SPSS 18.0 (SPSS Inc., Chicago, IL) or Prism 5.0 (Graphpad Software, La Jolla, CA). A P<0.050 (two-sided) was considered statistically significant.
Results
Study population
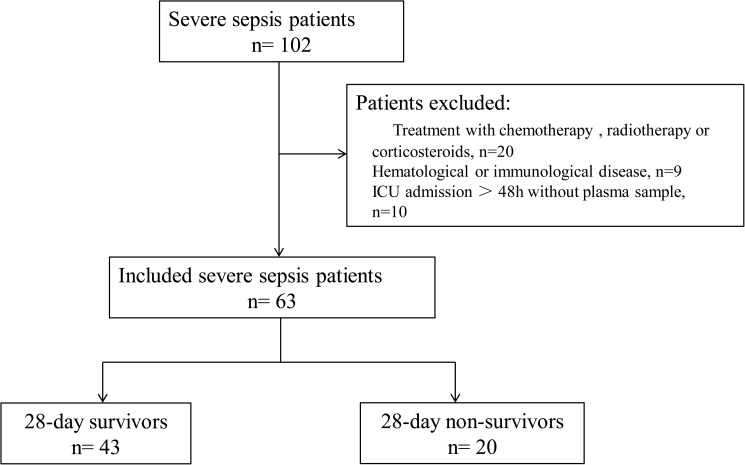
There were 102 patients who met the criteria. However, 29 patients were excluded because of hematological or immunological disease, use of chemotherapy, radiation therapy or use of glucocorticoids. Ten patients were also excluded because of no plasma samples within 24 hours ICU admission. Finally, a total of 63 severe sepsis patients were included. Among them, 58(92%) patients had lung infection while 54(86%) patients had definitely presented of a microbiologically documented. There were 20(32%) patients died within 28-day follow-up (Fig 1).
Fig 1. Flowchart of included and excluded patients.
Comparison of clinical parameters and IL10LCR between the survivor and non-survivors
Patients were classified as survivors or non-survivors according to their 28-day clinical outcome of sepsis. No significant difference was observed between survivors or non-survivors in terms of age and sex.
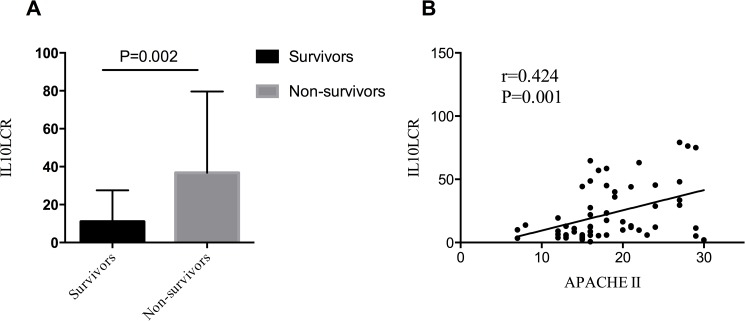
As it is shown in Table 1, APACHE II score in non-survivor was higher than the survivor group (median (IQR) 24 (18–31) versus 16 (14–20), P = 0.000). Moreover, IL10LCR was also significantly higher than the survivors (median (IQR) 36.78 (12.34–79.63) versus 11.01 (5.41–27.50), P = 0.002)(Fig 2A).
Table 1. Comparison of baseline characteristics and clinical data between survivors and non-survivors with severe sepsis.
| Survivors | Non-survivors | P value | |
|---|---|---|---|
| n = 43 | n = 20 | ||
| Age (years), mean±SD | 62±16 | 57±13 | 0.268 |
| Male, n(%) | 24(56%) | 12(60%) | 0.791 |
| APACHE II | 16(14–20) | 24(18–31) | 0.000 |
| Source of infection, n(%) | |||
| Lung | 38(88%) | 20(100%) | |
| Abdomen | 2(5%) | 0 | |
| Urinary tract | 2(5%) | 0 | |
| Blood | 1(2%) | 0 | |
| Underlying diseases or conditions, n(%) | |||
| Hypertension | 22(51%) | 5(25%) | |
| Diabetes mellitus | 7(16%) | 4(2%) | |
| Chronic renal failure | 4(9%) | 1(5%) | |
| Connective tissue disease | 1(2%) | 1(5%) | |
| Cerebrovascular attack | 2(5%) | 1(5%) | |
| Malignancy | 4(9%) | 1(5%) | |
| Temperature (°C), media(IQR) | 37.9±1.2 | 38.0±1.0 | 0.846 |
| Hate rate (bmp), media(IQR) | 123±26 | 107±33 | 0.050 |
| Respiration (bmp), media(IQR) | 30(26–36) | 27(34–43) | 0.201 |
| White blood count (×109/L), media(IQR) | 12.42(9.00–15.91) | 19.30(12.39–28.23) | 0.015 |
| Lymphocyte (×109/L), media(IQR) | 0.9(0.5–1.3) | 0.6(0.4–0.8) | 0.051 |
| IL-10 (pg/ml), media(IQR) | 9.02(6.73–13.38) | 21.07(8.40–53.50) | 0.006 |
| IL10LCR (ng/ml2), media(IQR) | 11.01(5.41–27.50) | 36.78(12.34–79.63) | 0.002 |
SD: standard deviation; APACHE II: Acute Physiology and Chronic Health Evaluation; IQR: 25%-75% interquartile range; bmp: beats per minute; P value less than 0.05 was considered statistically significant.
Fig 2. IL10LRC in severity of sepsis.
(A) IL10LCR between survivors and non-survivors (median (IQR) 36.78 (12.34–79.63) versus 11.01 (5.41–27.50), P = 0.002). (B) IL10LCR was positively correlated with APACHE II score (Spearman’s rho = 0.424, P = 0.0009).
Correlations between levels of IL10LRC and APACHE II
Spearman correlation coefficient was performed to test the correlations between IL10LCR levels and APACHE II. We use the ROUT method for identifying outliers by Prism 5.0. It is showed that the levels of plasma IL10LCR were positively correlated with APACHE II score (Spearman correlation 0.424, P = 0.001)(Fig 2B).
Levels of IL10LRC and severe sepsis outcome
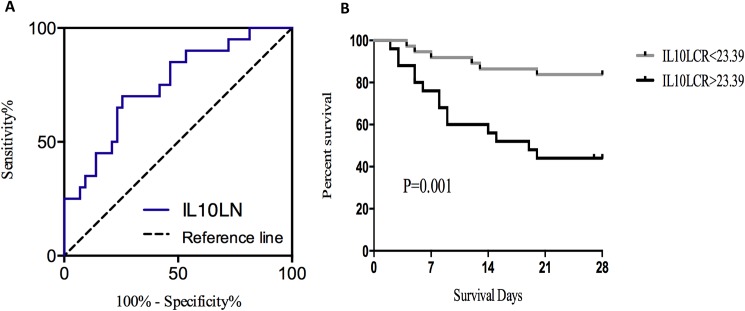
We found that the area under the ROC curve of 0.749 (95%CI 62.1%-87.7%, P = 0.002) (Fig 3A). At an optimal cutoff value of 23.39 ng/ml2, the sensitivity and specificity of IL10LCR for predicting 28-day mortality were 70.0% and 74.4%, respectively.
Fig 3. Levels of IL10LRC and severe sepsis outcome.
(A) The receiver operating characteristic (ROC) curves showed the area under the curve was 0.749 (95%CI 62.1%-87.7%, P = 0.002) for IL10LRC level to predict 28-day mortality with sensitivity and specificity at70.0% and 74.4%, respectively. (B) Patients stratified according to ROC-determined cutoff point of 23.39ng/ml2, Kaplan-Meier curve showed survival in patients with IL10LCR level above 23.39ng/ml2 was significantly lower than in patients less than 23.39ng/ml2 (P = 0.001 by log-rank test).
Patients were categorized into strata according to IL10LCR level above and below 23.39 ng/ml2 as described. Notably, the Kaplan-Meier survival curve showed that the patients with IL10LCR above 23.39 ng/ml2 were at greater risks of death than others (P = 0.001 by log-rank test) (Fig 3B). This indicated that higher levels of IL10LCR were significantly associated with higher mortality of server septic patients.
For further risk assessment, we performed simple and múltiple Cox regression to analyze hazard ratios (Table 2). Among all collected parameters, APACHE II score, IL10LCR, IL-10 were associated with mortality in simple Cox regression. The hazard ratio and 95% confidence interval (95%CI) for APACHE II score, IL10LCR, IL-10 were 1.211(1.060–1.187), 1.005(1.002–1.008), 1.014(1.007–1.021), respectively. However, only APACHE II score remained significant after adjusting for age and gender in a multiple Cox regression, with a hazard ratio of 1.101 (95%CI, 1.026–1.181).
Table 2. Cox proportional hazards models for mortality prediction.
| Variable | Simple Cox model | Multiple Cox model | ||
|---|---|---|---|---|
| HR(95%CI) | P value | HR(95%CI) | P value | |
| Age | 0.984(0.958–1.011) | 0.253 | NA | NA |
| Sex | 1.129(0.461–2.763) | 0.791 | NA | NA |
| IL10LNC | 1.005(1.002–1.008) | 0.000 | 0.997(0.988–1.006) | 0.500 |
| IL-10 | 1.014(1.007–1.021) | 0.000 | 1.012(0.991–1.034) | 0.269 |
| Lymphocyte | 0.381(0.132–1.100) | 0.075 | NA | NA |
| APACHE II | 1.211(1.060–1.187) | 0.000 | 1.101(1.026–1.181) | 0.008 |
HR: hazard ratio; NA: not application; P value less than 0.05 was considered statistically significant.
Discussion
In the present study, we found that the ratio of IL-10 to lymphocyte in non-survivors,at ICU admission was significant higher than the survivors in severe sepsis. IL10LCR may become a good indicator to predict mortality in severe septic patients.
Sepsis is complex immune response and is accompanied with considerable derangements of both the innate and adaptive immune systems[14]. Invasive infection triggers a pro-inflammatory and anti-inflammatory response, the magnitude of which depends on multiple factors, including pathogen site of infection, virulence, host genetics, and comorbidities[15]. Finally, most septic patients died of immunoparalysis. Recently, some post-mortem studies have shown that the patients who died of sepsis were in the stage of immunoparalysis, mainly displayed in massively depleted immune effector cells and opportunistic infections[16,17]. Therefore, restoring host immunity is a potential way to cure sepsis patients[18]. Hence, it is of great importance to find a good indicator to detect immune status and serve as a predictor of death. The increase of anti-inflammatory cytokines (eg. IL-10) and apoptosis of lymphocyte were the main features during immunosuppression in sepsis[11,19]. Therefore, we combined with IL-10 and lymphocyte to reflect the status of sepsis-induced immunosuppression. Fortunately, we found that IL10LCR was significantly higher than the survivors of severe sepsis (median (IQR) 36.78 (12.34–79.63) versus 11.01 (5.41–27.50), P = 0.002). Furthermore, IL10LCR was positively correlated with APACHE II and was related to sepsis mortality.
IL-10 is a molecule with immune-regulatory properties. Secretion of IL-10 in sepsis could limit and ultimately terminate inflammatory responses[20], which called as anti-inflammation cytokine. Besides, high levels of IL-10 could induce a state of functional immunoparalysis, leading to an incontrollable infection[21]. Previous studies demonstrated that overproduction of IL-10 was a predictor of severity and fatal outcome[7,8]. Our study also showed that IL-10 in non-survivors were higher than survivors(Table 1). We also obtained the area under the ROC curve of IL-10 was 0.715 (Date not shown in article), but when combining with lymphocyte, the ROC curve for predicting death increased to 0.749 (95%CI 62.1%-87.7%, P = 0.002).
We found lymphocytes in non-survivors were less than in survivors (P = 0.051). Previous studies have demonstrated that the level of lymphocyte, mainly B and T cells, decreased in septic non-survivors compared to survivors[10,11,22–25]. Furthermore, Wyllie et.al highlighted that lymphocytopenia was a useful diagnostic and prognostic marker of bacteremia in adult medical emergency admissions. Lymphocyte count was significantly associated with bacteremia in multivariate analysis[26]. Moreover, previous studies showed that sepsis patients with persistent lymphopenia had a greatly increased risk of nosocomial infections compared to patients whose lymphocyte level recovered to normal[11,27]. In the sepsis model of mice, Hotchkiss and his collogues showed that the prevention of lymphocyte apoptosis resulted in a marked improvement in survival and a therapy of caspase inhibitors which could prevent lymphocyte from apoptosis and improving survival[28,29].
The level of IL-10 and lymphocyte had a close interaction. On one hand, the lymphocytopenia in sepsis resulted from both depletion and apoptosis[30], IL-10 may play a role in the apoptosis of T-lymphocytes[31–33]. On the other hand, T cell and B cell could express and secret IL-10[34]. Therefore, to better understanding the change of physiopathology in sepsis, it is good way to combining with IL-10 and lymphocyte.
Limitation
Our sample amount is relatively small and is a single center study. It may influence the analysis of multivariate Cox regression model. Besides, as a retrospective study, blood sample was limited to examine other known biomarkers of sepsis-induced immunosuppression, such as monocyte HLA-DR expression, PD-1 on T cells, TNF levels. For this reason, we couldn’t definitely evaluate their relationship with L10LCR and the status of immunosuppression. In the end, a large prospective study should be going on to evaluate whether IL10LCR can serve as a biomarker for sepsis-induced immunosuppression.
Conclusions
The current study demonstrated that IL10LCR was significantly correlated with APACHE II score and associated with worse outcomes in severe septic patients. It is an indicator to reflect the severity of critical illness and may serve as a biomarker for sepsis-induced immunosuppression.
Supporting information
(XLSX)
(SAV)
Acknowledgments
The authors would like to thank all the medical doctors and nurses of the ICU of the First Affiliated Hospital of Guangzhou Medical University for their careful and generous collaboration while doing this work. This study was supported by National Natural Science Foundation of China (Grant No. 81400060 to YH and 81490534 to YL); Natural Science Foundation of Guangdong Province, China (Grant No. 2015A030313480 to YL and 2015A030313456 to X.Q.L); Guangzhou Healthcare Collaborative Innovation Major Project (Grant No. 201400000002 to YL) and Science and Technology Program of Guangzhou, China (Grant No. 2014Y-00065 to YL). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- ICU
intensive care units
- IL-10
Interleukin-10
- IL10LCR
Interleukin-10 –lymphocyte count ratio
- APACHE II
Acute Physiology and Chronic Health Evaluation II
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by National Natural Science Foundation of China (Grant No. 81400060 to YH and 81490534 to YL); Natural Science Foundation of Guangdong Province, China (Grant No. 2015A030313480 to YL and 2015A030313456 to XQL); Guangzhou Healthcare Collaborative Innovation Major Project (Grant No. 201400000002 to YL) and Science and Technology Program of Guangzhou, China (Grant No. 2014Y-00065 to YL). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kaukonen K-M, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. Jama. 2014;311(13):1308–1316. doi: 10.1001/jama.2014.2637 [DOI] [PubMed] [Google Scholar]
- 2.Vincent J-L, Marshall JC, Ñamendys-Silva SA, François B, Martin-Loeches I, Lipman J, et al. Assessment of the worldwide burden of critical illness: the Intensive Care Over Nations (ICON) audit. The Lancet Respiratory Medicine. 2014;2(5):380–386. doi: 10.1016/S2213-2600(14)70061-X [DOI] [PubMed] [Google Scholar]
- 3.Cross AS, Opal SM. A new paradigm for the treatment of sepsis: is it time to consider combination therapy? Annals of internal medicine. 2003;138(6):502–505. [DOI] [PubMed] [Google Scholar]
- 4.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nature reviews. Immunology. 2013;13(12):862–874. doi: 10.1038/nri3552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Venet F, Lukaszewicz AC, Payen D, Hotchkiss R, Monneret G. Monitoring the immune response in sepsis: a rational approach to administration of immunoadjuvant therapies. Current opinion in immunology. 2013;25(4):477–483. doi: 10.1016/j.coi.2013.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marchant A, Alegre M-L, Hakim A, Pierard G, Marecaux G, Friedman G, et al. Clinical and biological significance of interleukin-10 plasma levels in patients with septic shock. Journal of clinical immunology. 1995;15(5):266–273. [DOI] [PubMed] [Google Scholar]
- 7.Tamayo E, Fernandez A, Almansa R, Carrasco E, Heredia M, Lajo C, et al. Pro- and anti-inflammatory responses are regulated simultaneously from the first moments of septic shock. European cytokine network. 2011;22(2):82–87. doi: 10.1684/ecn.2011.0281 [DOI] [PubMed] [Google Scholar]
- 8.Gogos CA, Drosou E, Bassaris HP, Skoutelis A. Pro- versus anti-inflammatory cytokine profile in patients with severe sepsis: a marker for prognosis and future therapeutic options. The Journal of infectious diseases. 2000;181(1):176–180. doi: 10.1086/315214 [DOI] [PubMed] [Google Scholar]
- 9.Inoue S, Suzuki-Utsunomiya K, Okada Y, Taira T, Iida Y, Miura N, et al. Reduction of immunocompetent T cells followed by prolonged lymphopenia in severe sepsis in the elderly. Critical care medicine. 2013;41(3):810–819. doi: 10.1097/CCM.0b013e318274645f [DOI] [PubMed] [Google Scholar]
- 10.Monserrat J, de Pablo R, Diaz-Martin D, Rodriguez-Zapata M, de la Hera A, Prieto A, et al. Early alterations of B cells in patients with septic shock. Critical care. 2013;17(3):R105 doi: 10.1186/cc12750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drewry AM, Samra N, Skrupky LP, Fuller BM, Compton SM, Hotchkiss RS. Persistent lymphopenia after diagnosis of sepsis predicts mortality. Shock. 2014;42(5):383–391. doi: 10.1097/SHK.0000000000000234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive care medicine. 2003;29(4):530–538. doi: 10.1007/s00134-003-1662-x [DOI] [PubMed] [Google Scholar]
- 13.Asner S, Waters V, Solomon M, Yau Y, Richardson SE, Grasemann H, et al. Role of respiratory viruses in pulmonary exacerbations in children with cystic fibrosis. Journal of cystic fibrosis: official journal of the European Cystic Fibrosis Society. 2012;11(5):433–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stearns-Kurosawa DJ, Osuchowski MF, Valentine C, Kurosawa S, Remick DG. The pathogenesis of sepsis. Annual review of pathology. 2011;6:19–48. doi: 10.1146/annurev-pathol-011110-130327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiersinga WJ, Leopold SJ, Cranendonk DR, van der Poll T. Host innate immune responses to sepsis. Virulence. 2014;5(1):36–44. doi: 10.4161/viru.25436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306(23):2594–2605. doi: 10.1001/jama.2011.1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torgersen C, Moser P, Luckner G, Mayr V, Jochberger S, Hasibeder WR, et al. Macroscopic postmortem findings in 235 surgical intensive care patients with sepsis. Anesthesia and analgesia. 2009;108(6):1841–1847. doi: 10.1213/ane.0b013e318195e11d [DOI] [PubMed] [Google Scholar]
- 18.Cohen J, Vincent JL, Adhikari NK, Machado FR, Angus DC, Calandra T, et al. Sepsis: a roadmap for future research. The Lancet. Infectious diseases. 2015;15(5):581–614. doi: 10.1016/S1473-3099(15)70112-X [DOI] [PubMed] [Google Scholar]
- 19.Schulte W, Bernhagen J, Bucala R. Cytokines in sepsis: potent immunoregulators and potential therapeutic targets—an updated view. Mediators of inflammation. 2013;2013:165974 doi: 10.1155/2013/165974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annual review of immunology. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683 [DOI] [PubMed] [Google Scholar]
- 21.Abe R, Hirasawa H, Oda S, Sadahiro T, Nakamura M, Watanabe E, et al. Up-regulation of interleukin-10 mRNA expression in peripheral leukocytes predicts poor outcome and diminished human leukocyte antigen-DR expression on monocytes in septic patients. The Journal of surgical research. 2008;147(1):1–8. doi: 10.1016/j.jss.2007.07.009 [DOI] [PubMed] [Google Scholar]
- 22.Monserrat J, de Pablo R, Reyes E, Diaz D, Barcenilla H, Zapata MR, et al. Clinical relevance of the severe abnormalities of the T cell compartment in septic shock patients. Critical care. 2009;13(1):R26 doi: 10.1186/cc7731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hein F, Massin F, Cravoisy-Popovic A, Barraud D, Levy B, Bollaert P-E, et al. The relationship between CD4+ CD25+ CD127-regulatory T cells and inflammatory response and outcome during shock states. Critical care. 2010;14(1):R19 doi: 10.1186/cc8876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheadle WG, Pemberton RM, Robinson D, Livingston DH, Rodriguez JL, Polk HC Jr. Lymphocyte subset responses to trauma and sepsis. The Journal of trauma. 1993;35(6):844–849. [DOI] [PubMed] [Google Scholar]
- 25.Le Tulzo Y, Pangault C, Gacouin A, Guilloux V, Tribut O, Amiot L, et al. Early circulating lymphocyte apoptosis in human septic shock is associated with poor outcome. Shock. 2002;18(6):487–494. [DOI] [PubMed] [Google Scholar]
- 26.Wyllie D, Bowler I, Peto T. Relation between lymphopenia and bacteraemia in UK adults with medical emergencies. Journal of clinical pathology. 2004;57(9):950–955. doi: 10.1136/jcp.2004.017335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rajan G, Sleigh JW. Lymphocyte counts and the development of nosocomial sepsis. Intensive care medicine. 1997;23(11):1187 [DOI] [PubMed] [Google Scholar]
- 28.Hotchkiss RS, Tinsley KW, Swanson PE, Chang KC, Cobb JP, Buchman TG, et al. Prevention of lymphocyte cell death in sepsis improves survival in mice. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(25):14541–14546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hotchkiss RS, Chang KC, Swanson PE, Tinsley KW, Hui JJ, Klender P, et al. Caspase inhibitors improve survival in sepsis: a critical role of the lymphocyte. Nature immunology. 2000;1(6):496–501. doi: 10.1038/82741 [DOI] [PubMed] [Google Scholar]
- 30.Hotchkiss RS, Coopersmith CM, Karl IE. Prevention of lymphocyte apoptosis—a potential treatment of sepsis? Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2005;41 Suppl 7:S465–469. [DOI] [PubMed] [Google Scholar]
- 31.Oberholzer A, Oberholzer C, Moldawer LL. Interleukin-10: a complex role in the pathogenesis of sepsis syndromes and its potential as an anti-inflammatory drug. Critical care medicine. 2002;30(1):S58–S63. [PubMed] [Google Scholar]
- 32.de Waal Malefyt R, Yssel H, de Vries JE. Direct effects of IL-10 on subsets of human CD4+ T cell clones and resting T cells. Specific inhibition of IL-2 production and proliferation. The Journal of Immunology. 1993;150(11):4754–4765. [PubMed] [Google Scholar]
- 33.Taga K, Tosato G. IL-10 inhibits human T cell proliferation and IL-2 production. The Journal of Immunology. 1992;148(4):1143–1148. [PubMed] [Google Scholar]
- 34.Scumpia PO, Moldawer LL. Biology of interleukin-10 and its regulatory roles in sepsis syndromes. Critical care medicine. 2005;33(12 Suppl):S468–471. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(SAV)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.