Abstract
Orally administered vaccine bacteria usually persist for a period of time in the intestinal tracts of immunized individuals, and are excreted in feces to the environment resulting in a potential biosafety issue. The releasing risk can be minimized by immediate elimination of the persistent vaccine bacteria once adequate protective immune responses have been elicited by the vaccine bacteria. In a previous study, inducible expression of truncated yncE gene (yncE*) was found lethal to host bacteria. This feature has an application potential in biosafety control. Here, we assessed the efficacy of YncE* in eliminating an attenuated strain of Salmonella enterica serovar Typhimurium in a mouse model. To this end, a pBAD-derived plasmid containing yncE* under the control of the Ara promoter was transformed into a ΔphoPQ mutant of S. Typhimurium. Our data show that the induced expression of yncE* in the presence of arabinose eliminated the vaccine bacteria both in vitro and in vivo. BALB/c mice with or without streptomycin-pretreatment were used to assess the efficacy of YncE* in vivo. Oral administration of 500 μl of 20% arabinose at 24 h postvaccination removed the vaccine bacteria from the guts of the tested mice without streptomycin-pretreatment. For streptomycin-pretreated mice, which were colonized with higher levels of Salmonella, an additional gavage of arabinose was required to completely eliminate the vaccine bacteria in the guts of the tested mice. The orally administered arabinose did not affect the persistence of bacteria that had penetrated the intestinal mucosa of the immunized mice. Furthermore, there was no significant difference in the protection rate between the routine immunization and the immunization with the arabinose treatment. The results indicate that the yncE* element improves the biosafety of the bacterial vaccine, and can be taken in consideration in future design of live bacterial vaccines.
Introduction
Live bacterial vaccines possess multiple advantages including simple manufacture, high acceptance, low rates of side-effects, and a convenient vaccination process which triggers systemic immune and mucosal immune responses [1]. Additionally, the attenuated vaccines can be used as vectors for vaccines against other infections or antitumor agents [2, 3]. Nevertheless, although the excellent safety of the licensed live vaccines had been demonstrated in a number of clinical investigations, there are health and biosafety concerns regarding the release of genetically modified bacteria into the environment [4, 5]. For example, orally administered vaccine bacteria usually persist in the guts of immunized individuals after vaccination, and display fecal shedding for a period of time, from several days to several weeks [6–10].
To minimize the release risk, the fecal shedding is ideally terminated as soon as possible once the protective immunization has been established in the immunized individual. In an attempt to shorten the shedding duration, Ghany et al. identified several mutations in Salmonella enterica serovar Typhimurium, including hdA, misL, and ratB, that reduced shedding in mice without alteration in immunogenicity [11]. Another strategy is to built a conditional lethal system in recombinant bacteria [12]. Many suicidal genetic elements have been employed in these systems, including gef and relF of Escherichia coli [12–14], the E gene of bacteriophage ΦX174 [15], the hok gene of the plasmid R1 [16], colicin E3-encoding gene [17], etc. These conditional lethal genetic elements harbored in the vaccine bacteria can be triggered by environmental circumstances, and specifically kill the host bacteria without interfering with the normal flora.
In a previous study, we found that the inducible expression of truncated yncE gene (yncE*) impaired bacterial membrane structure, leading to lysis of host bacteria [18]. The yncE gene is conserved in Enterobacteriaceae with unknown physiological function [19]. The YncE protein is characterized by a typical seven-bladed β-propeller structure [19, 20], and had been identified as a prominent antigen expressed uniquely in patients who carried S. Typhi in their biliary tracts, suggesting YncE might contribute to bacterial survival in the biliary tract [21]. YncE is also a conserved immunogenic antigen of E. coli able to protect against acute systemic infection in mice [22]. In the present study, we explored whether or not yncE* can be used to clear bacterial cells of an attenuated S. Typhimurium strain administered in a mouse model. Moreover, the impact of the elimination treatment on the efficacy of the vaccine bacteria was also assessed.
Materials and methods
Ethics statement
The protocol of animal experiment was reviewed and approved by the laboratory animal welfare and ethics committee of Third Military Medical University. The animal experiments were performed according to the Guideline on the Humane Treatment of Laboratory Animals of the Ministry of Science and Technology of China.
Strains, plasmids and medium
S. Typhimurium CMCC50115 was obtained from the National Center for Medical Culture Collections (Beijing, China), and was used as a wild-type strain. E. coli S17-1/λpir was a gift from Dr. Victor de Lorenzo of the Centro Nacional de Biotecnologia CSIC, Spain. A suicide vector pYG4 described previously was used for construction of deletion mutant [23]. Recombinant plasmid pBAD24-yncE* harboring the truncated yncE gene (the 5’-terminal 408 bp) under the control of the Ara promoter was constructed previously [18]. Unless otherwise noted, bacteria were grown in an animal product free medium composed of 1% vegetable peptone, 0.5% yeast extract and 1% NaCl. When required, kanamycin and ampicillin were used at 50 μg/ml and 100 μg/ml, respectively.
Construction of phoPQ-deletion mutant of S. Typhimurium
Deletion of the phoPQ locus in S. Typhimurium was performed via homologous recombination as described previously [23, 24]. Briefly, the upstream and downstream fragments of phoPQ were PCR-amplified from the genomic DNA of S. Typhimurium with primers P1 (cccgggcccccttacaccacccagattga) and P2 (cccgttataaatttggagtgtgaaggttattgcgtgctcttctcccttgtgttaac), as well as primers P3 (ccccacgcaataaccttcacactccaaatttataacacatttctgtgcgttcttcc) and P4 (cccgagctcccggatcgctgtagtatgta), and spliced by overlap PCR with primers P1 and P4. After restriction enzyme digestion by ApaI and SacI, the fused fragment was inserted into pYG4 pretreated with the same restriction enzymes, resulting in pYG4-ΔphoPQ. The recombinant pYG4-ΔphoPQ was transformed into competent cells of S. Typhimurium by electroporation, and screened for chromosomal integration of the plasmid with kanamycin selection. After an overnight-incubation in antibiotic-free medium, plasmid-excised strains were reversely selected on agar supplemented with 5% sucrose. The ΔphoPQ strain was then screened and identified by PCR with primers P5 (gaagaggtcatcaaactcac) and P6 (catcgtaaatcagcgtcatg), which amplify an internal fragment of phoPQ.
Determination of 50% lethal dose (LD50)
Fresh cultures of the wild-type strain and the ΔphoPQ mutant of S. Typhimurium were washed and resuspended in phosphate-buffered saline (PBS) to get ten-fold dilutions (2×103, 2×104, 2×105, 2×106, and 2×107 CFU/ml for the wild-type strain; 2×106, 2×107, 2×108, 2×109, and 2×1010 CFU/ml for the mutant). Female BALB/c mice (6–8 weeks, 5 mice per group) were administered orally with 0.5 ml of bacterial dilutions using a 1.5-in. curved gavage needle with a 2.25-mm ball. After inoculation, mice were weighed and monitored daily for 14 days. Any mouse that lost more than 20% of body weight or that showed signs of extreme morbidity (e.g., shallow breathing and hunched posture) was euthanized by CO2 overdose, and scored as a death.
Assessment of the lethal effect of the induced expression of yncE* on the vaccine bacteria in vitro
Bacteria of the ΔphoPQ strain harboring pBAD24-yncE* or the empty pBAD24 vector were serially diluted in PBS. Two microliters of bacterial dilution were inoculated on agar containing 0.1% arabinose. After an overnight-incubation, bacterial growth was observed.
Evaluation of the lethal effect of the induced YncE* on the vaccine bacteria in the guts of mice
Fresh cultures of the ΔphoPQ mutant harboring pBAD24-yncE* were harvested and resuspended in PBS. Female BALB/c mice aged 6–8 weeks (three groups, 10 or 11 mice per group) were orally inoculated with 1×109 CFU of the ΔphoPQ mutant harboring pBAD24-yncE* suspended in 0.5 ml PBS, or 0.5 ml PBS alone (as control). At 24 h postvaccination, one group of the immunized mice were orally administered with 0.5 ml of 20% arabinose to induce expression of yncE* in the vaccine bacteria. To quantitate the shedding vaccine bacteria, bacteria were recovered from fecal samples and quantitated for up to 30 days after vaccination (fecal samples were collected everyday in the first week, every 3 days in the remaining weeks). To do that, three to five fecal pellets were collected from each immunized mouse and suspended in PBS at 100 mg/ml. A 100 μl of suspension was plated onto LB agar supplemented with ampicillin to numerate Salmonella. The limit of detection is 10 CFU/g feces.
On the 30th day postvaccination, all mice were orally challenged with 1×108 CFU of the wild-type strain. Mice were weighed and monitored daily for 30 days as described in the assessment of LD50.
Evaluation of the lethal effect of the induced YncE* on the vaccine bacteria in the guts of streptomycin-pretreated mice
BALB/c mice were treated with orally administration of streptomycin (20 mg per mouse) dissolved in sterile water. At 20 h after the streptomycin-treatment, water and food were withdrawn for 4 h before the mice were orally inoculated with 1×108 CFU of the ΔphoPQ mutant harboring pBAD24-yncE* (two groups of six mice each) or the empty pBAD24 vector (one group of six mice) suspended in 0.5 ml of PBS.
At 24 h and 48 h postvaccination, the immunized mice were orally given 0.5 ml of 20% arabinose or PBS. Fecal samples were collected and suspended in PBS at 100 mg/ml. After serially diluted, a 100 μl of suspension was plated onto Salmonella-Shigella agar (SS agar) with ampicillin to quantitate Salmonella. For the samples that produced no colony on SS agar with ampicillin, a 100 μl of suspension was inoculated on SS agar without ampicillin to examine the possible existence of Salmonella, which escaped from YncE*-killing by losing plasmid or mutation. On the 30th day postvaccination, the tested mice were euthanized by CO2 overdose, and their livers, spleens and mesenteric lymph nodes were removed by sterile surgery. The homogenized suspensions of the tissues were plated onto LB agar to enumerate persistent bacteria.
Flagellin purification
Fresh cultures of S. Typhimurium were pelleted, washed and resuspended in PBS. The suspension was shaken violently for 45 min. Bacterial cells were pelleted by centrifugation at 7000 g for 10 min. The supernatant was then centrifuged at 100000 g for 3 h to harvest flagellin. The flagellin protein was dissolved in water, and incubated at 4°C overnight. After a centrifugation at 7000 g, the supernatant was collected. Protein concentration was determined with the BCA assay. The flagellin solution was then preserved at -80°C for future use.
Determination of IgG levels of anti-flagellin and anti-lipopolysaccharide (anti-LPS) in the serum samples of the immunized mice
Female BALB/c mice (three groups, four mice per group) were immunized as described above. On the 30th day postvaccination, blood samples were collected. Determination of IgG levels of anti-flagellin and anti-LPS in the serum samples was performed with ELISA as described elsewhere [24]. The LPS of S. Typhimurium was purchased from Sigma-Aldrich.
Statistical analysis
LD50 was determined by probit analysis. Group means were compared by Kruskal-Wallis test with Dunn’s post-hoc test. Fisher’s exact test was used to analyze survival rates. Values of P < 0.05 were regarded as statistically significant.
Results
Construction of the ΔphoPQ mutant of S. Typhimurium
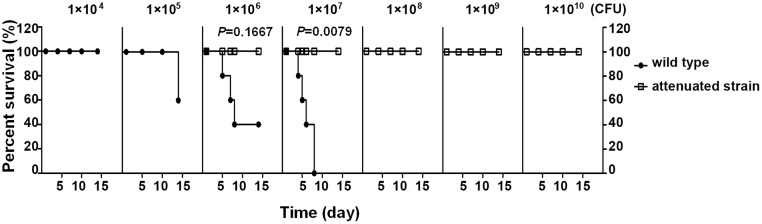
To create an attenuated strain, the phoPQ locus was deleted from S. Typhimurium CMCC50115 via homologous recombination (S1 Fig). The phoPQ locus encodes a two-component regulatory system controlling expression of a large number of genes (>300), many of which are associated with bacterial virulence [25–31]. Therefore, the PhoPQ regulatory system plays a critical role in pathogenicity. Deficiency of phoPQ causes reduced bacterial virulence [29, 32, 33]. The phoPQ locus has been extensively used in attenuating bacteria [34]. As shown in Fig 1, LD50 of the ΔphoPQ mutant (>2×1010 CFU) was markedly increased compared to that of the wild-type strain (7.58×105 CFU), suggesting that the virulence of the ΔphoPQ mutant was largely reduced. The mutant was then used in the subsequent experiment.
Fig 1. Survival curves of mice challenged with Salmonella enterica serovar Typhimurium wild-type or ΔphoPQ.
Female BALB/c mice aged 6–8 weeks (5 mice per group) were orally challenged with bacteria at indicated doses. Mice were monitored daily for 14 days as described in the section of Materials and methods.
Induced expression of yncE* inhibits the growth of the ΔphoPQ mutant in vitro
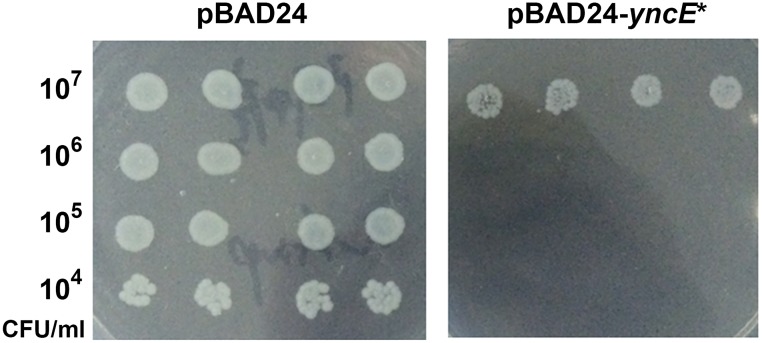
Prior to in vivo experiments, we assessed the lethal effect of the induced YncE* on the growth of the ΔphoPQ mutant in vitro. The data show that colony formation of the ΔphoPQ mutant harboring pBAD24-yncE* in the presence of 0.1% arabinose was remarkably inhibited compared to the mutant harboring the empty pBAD24 (Fig 2).
Fig 2. Growth inhibition of Salmonella enterica serovar Typhimurium by induced expression of yncE*.
Fresh cultures of S. Typhimurium harboring pBAD24- yncE* or empty pBAD24 plasmid were serially diluted. Two microliters of dilutions were inoculated and grown on LB agar supplemented with 0.1% of arabinose.
Induced expression of YncE* eliminates the vaccine bacteria in the intestinal tracts of mice
Because there are no ampicillin-resistant bacteria in the intestinal tract of the mice used in our animal experiments (confirmed by our preliminary experiments), a simple culture assay with agar supplemented with 100 μg/ml ampicillin was used to quantitate the ΔphoPQ mutant bearing the ampicillin-resistant pBAD-derived plasmid. After an oral administration of the ΔphoPQ mutant harboring pBAD24-yncE*, fecal samples were collected, and subjected to cultivation and colony counting. A number of live vaccine bacteria were recovered from the fecal samples of the immunized mice without arabinose treatment after inoculation (Fig 3). The amount of the shedding bacteria was slowly decreased. However, even on the 30th day postvaccination, the vaccine bacteria were still detectable in the feces of the immunized mice without arabinose treatment (Fig 3). The results demonstrate that the administrated vaccine bacteria colonized the guts of the immunized mice, and were excreted in the feces for a period of time.
Fig 3. Elimination of the vaccine bacteria in the guts of mice by induced expression of yncE*.
Female BABL/c mice (10 or 11 mice per group) were orally immunized with the ΔphoPQ mutant of Salmonella enterica serovar Typhimurium harboring pBAD24-yncE* (1×109 CFU/mouse). One group of the immunized mice were orally administered with 0.5 ml of 20% arabinose at 24 h postvaccination. The shedding vaccine bacteria in the fecal samples were quantitated by plate cultivation with ampicillin-containing LB agar. The detection limit is 10 CFU/g feces.
In contrast to the persistent presence of the vaccine bacteria in the guts of the immunized mice without arabinose treatment, the vaccine bacteria were completely eliminated in the guts of the immunized mice with a single oral arabinose treatment, and never recovered from the feces samples (Fig 3). The results show that the induced expression of YncE* eliminated the live vaccine bacteria in the guts of the mice.
Oral treatment with streptomycin can disrupt the indigenous flora of mice, and improves intestinal colonization by exogenous bacteria [35, 36]. Thus, we used streptomycin-pretreated BALB/c mice to further analyze the efficacy of the arabinose-inducible eradication. As expected, higher levels of the vaccine bacteria colonized in the guts of the tested mice with streptomycin treatment (Fig 4A). However, one dose of arabinose was insufficient to remove the vaccine bacteria in the guts. An additional oral arabinose treatment was required for complete clearance of the vaccine bacteria (Fig 4A). For the samples that produced no colony on SS agar with ampicillin, SS agar without ampicillin was used to confirm the results. From the fecal samples that produced no colony on ampicillin-containing SS agar, no vaccine bacteria were recovered on SS agar either. However, the results can not rule out the escape possibility of the vaccine bacteria from the YncE*-killing, which has been observed in vitro. The few escape bacteria were probably eliminated by the multiple hostile factors in the gut, such as low pH, bile, the normal flora, etc.
Fig 4. Elimination of the vaccine bacteria in the guts of streptomycin-pretreated mice by induced expression of yncE*.
(A) Fecal shedding of the immunized mice. Female BABL/c mice (6 mice per group) were orally pretreated with streptomycin at 24 h prior to immunization with 1×108 CFU of the ΔphoPQ mutant harboring pBAD24-yncE* or pBAD24. At 24 h and 48 h postvaccination, the immunized mice were orally administered with 0.5 ml of 20% arabinose or PBS. The shedding vaccine bacteria were recovered on Salmonella-Shigella agar with ampicillin and quantitated. (B) Persistent bacteria in the internal organs. On the 30th day postvaccination, the tested mice were euthanized, and their livers, spleens and mesenteric lymph nodes were removed by sterile surgery. The homogenized suspensions of the tissues were plated onto LB agar to enumerate persistent bacteria.
In contrast to the vaccine bacteria in the guts of mice, the vaccine bacteria resident in internal organs were not significantly impacted by the gavage of arabinose. Amounts of persistent Salmonella in the mesenteric lymph nodes, livers and spleens of the immunized mice with arabinose treatment are comparable to those of the mice without arabinose treatment, or the mice immunized with the vaccine bacteria harboring the empty pBAD24 vector (Fig 4B). The data indicate that orally administered arabinose can not reach the bacteria that have penetrated the intestinal mucosa of the tested mice.
The protective efficacy of vaccination was not significantly altered by the elimination treatment
To explore whether or not the elimination treatment impairs the protective efficacy of the live vaccine, we measured the levels of protective antibodies, the anti-flagellin IgG and the anti-LPS IgG, in the serum samples of the immunized mice. The results show that the IgG levels of anti-flagellin and anti-LPS in the serum samples of the arabinose-treated mice are comparable to those in the serum samples of the mice without arabinose treatment; the mice in both immunized groups, with or without arabinose treatment, produced increased levels of anti-flagellin and anti-LPS IgGs relative to the mice with the mock vaccination (Fig 5, P<0.05 or P<0.01). The data indicate that the elimination of the vaccine bacteria in the guts of the immunized mice at 24 h postvaccination did not significantly impair the strength of humoral immune responses elicited by flagellin or LPS, two important protective antigens for Salmonella, probably due to the persistent systemic vaccine bacteria.
Fig 5. The levels of IgG antibodies in the immunized mice with arabinose treatment are comparable to those in the immunized mice without arabinose treatment.
BALB/c mice were orally immunized with the ΔphoPQ mutant harboring pBAD24-yncE* at a dose of 1×109 CFU/mouse, or PBS as control. One group of the immunized mice were administered with 0.5 ml of 20% arabinose at 24 h postvaccination. On the 30th day postvaccination, serum samples were harvested. The levels of anti-flagella and anti-LPS IgGs were determined with ELISA.
To assess the level of immunoprotection, on the 30th day postvaccination, the immunized mice were orally challenged with the wild-type strain at a dose of 1×108 CFU/mouse. Consistent with the detection results of the antibody levels, the vaccination with the elimination treatment showed a significant protection against the wild-type challenge compared with the mock immunization (P<0.01), at a level comparable to the normal vaccination without the arabinose treatment (Fig 6).
Fig 6. Immunoprotection of orally administered Salmonella enterica serovar Typhimurium ΔphoPQ with or without a subsequent arabinose-induced bacterial elimination.
BALB/c mice were orally immunized with the ΔphoPQ mutant harboring pBAD24-yncE* at a dose of 1×109 CFU/mouse, or PBS as control. One group of the immunized mice were administered with 0.5 ml of 20% arabinose at 24 h postvaccination. On the 30th day postvaccination, the tested mice were challenged with the wild-type strain at a dose of 1×108 CFU per mouse. Mice were monitored daily for 30 days as described in the section of Materials and methods.
Discussion
With the rise of synthetic biology and the extensive application of recombinant DNA technology in the past decades, the concerns about the biosafety of genetically modified microbes are growing [37, 38]. One of these concerns is that the microorganisms may escape their intended habitats and cause unpredictable biohazard to the environment [5].
In order to minimize environment release, which can not be completely avoided, to an acceptable level, a large number of studies have focused on the construction of engineered microbes with elaborate designs that allow them to be removed in a non-designated environment [37, 38]. Generally, three strategies are involved in these designs: addiction to an exogenously supplied ligand; self-killing outside of a designated environment; and self-destroying encoded DNA circuitry outside of a designated environment [37]. The use of self-killing switches involves certain toxic genes, whose expression has robust lethal effect on host bacteria. The encoding products of these toxic genes include toxin-antitoxin systems, restriction enzymes, bacteriocins, bacteriophage lytic systems, etc [38].
In the present study, we evaluated the elimination effect of YncE*, a genetic element with bactericidal capability that we determined previously in vitro, on the orally administered Salmonella in the guts of immunized mice. The results show that the expression of YncE*, induced by oral addition of arabinose, was able to reduce the vaccine bacteria in the guts of mice to an undetectable level, though multiple treatments were required sometimes. However, the oral arabinose treatment did not affect the systemic vaccine bacteria, probably due to low level of arabinose that entered into circulation by the oral administration. Thus, the eliminating approach is limited to reducing the intestinal luminal vaccine burden, but cannot replace other attenuations that limit systemic bacterial survival and pathogenicity.
For an attenuated vaccine, an optimal balance must be kept between safety and immunogenicity. Excessive attenuation leads to the weakening of immunogenicity. In the case of our experiment, removing the vaccine bacteria too soon may cause reduced immune responses, and consequently decreased efficacy of the vaccine. Since the orally inoculated bacteria of S. Typhimurium can develop infection loci in the Peyer’s patches of the small intestine within 3 h [39], we performed the elimination treatment at 24 h postvaccination, a time point that we proposed is sufficient for the vaccine bacteria to penetrate intestinal mucosa and elicit immune responses. Our data show that removal of bacteria from the guts of the immunized mice at this time point did not significantly impair the IgG levels of anti-LPS and anti-flagellin. The immunization by the vaccine bacteria with arabinose treatment effectively protected mice from the wild-type challenge.
Conclusion
In summary, our data show that the inducible expression of yncE* can effectively reduce the fecal shedding of live vaccine bacteria of S. Typhimurium in the mice model. The elimination treatment at 24 h postvaccination does not significantly impact the immunogenicity and efficacy of the vaccine bacteria. This lethal element, yncE*, has an application potential for the biosafety control of oral live bacterial vaccine.
Supporting information
M: molecular marker; lanes 1 and 3: PCR products of phoPQ-deletion mutant with primers P1 and P4, as well as P5 and P6; lanes 2 and 4: PCR products of the wild-type strain with primers P1 and P4, as well as P5 and P6.
(TIF)
(DOCX)
Acknowledgments
This study was supported by the national natural science foundation of China (31500116) and a scientific and technological research project of Chongqing (cstc2012gg-yyjs10057).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by the national natural science foundation of China (31500116) to KX and a scientific and technological research project of Chongqing (cstc2012gg-yyjs10057) to YC. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Aziz MA, Midha S, Waheed SM, Bhatnagar R. Oral vaccines: new needs, new possibilities. BioEssays. 2007;29(6):591–604. doi: 10.1002/bies.20580 [DOI] [PubMed] [Google Scholar]
- 2.Yuki Y, Kiyono H. Mucosal vaccines: novel advances in technology and delivery. Expert Rev Vaccines. 2009;8(8):1083–97. doi: 10.1586/erv.09.61 [DOI] [PubMed] [Google Scholar]
- 3.Lin IY, Van TT, Smooker PM. Live-Attenuated Bacterial Vectors: Tools for Vaccine and Therapeutic Agent Delivery. Vaccines (Basel). 2015;3(4):940–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kotton CN, Hohmann EL. Enteric pathogens as vaccine vectors for foreign antigen delivery. Infect Immun. 2004;72(10):5535–47. doi: 10.1128/IAI.72.10.5535-5547.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davison J. Towards safer vectors for the field release of recombinant bacteria. Environ Biosafety Res. 2002;1(1):9–18. [DOI] [PubMed] [Google Scholar]
- 6.Viret JF, Dietrich G, Favre D. Biosafety aspects of the recombinant live oral Vibrio cholerae vaccine strain CVD 103-HgR. Vaccine. 2004;22(19):2457–69. doi: 10.1016/j.vaccine.2003.12.033 [DOI] [PubMed] [Google Scholar]
- 7.Angelakopoulos H, Hohmann EL. Pilot study of phoP/phoQ-deleted Salmonella enterica serovar typhimurium expressing Helicobacter pylori urease in adult volunteers. Infect Immun. 2000;68(4):2135–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hindle Z, Chatfield SN, Phillimore J, Bentley M, Johnson J, Cosgrove CA, et al. Characterization of Salmonella enterica derivatives harboring defined aroC and Salmonella pathogenicity island 2 type III secretion system (ssaV) mutations by immunization of healthy volunteers. Infect Immun. 2002;70(7):3457–67. doi: 10.1128/IAI.70.7.3457-3467.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kotton CN, Lankowski AJ, Scott N, Sisul D, Chen LM, Raschke K, et al. Safety and immunogenicity of attenuated Salmonella enterica serovar Typhimurium delivering an HIV-1 Gag antigen via the Salmonella Type III secretion system. Vaccine. 2006;24(37–39):6216–24. doi: 10.1016/j.vaccine.2006.05.094 [DOI] [PubMed] [Google Scholar]
- 10.Tacket CO, Losonsky G, Taylor DN, Baron LS, Kopecko D, Cryz S, et al. Lack of immune response to the Vi component of a Vi-positive variant of the Salmonella typhi live oral vaccine strain Ty21a in human studies. J Infect Dis. 1991;163(4):901–4. [DOI] [PubMed] [Google Scholar]
- 11.Abd El Ghany M, Jansen A, Clare S, Hall L, Pickard D, Kingsley RA, et al. Candidate live, attenuated Salmonella enterica serotype Typhimurium vaccines with reduced fecal shedding are immunogenic and effective oral vaccines. Infect Immun. 2007;75(4):1835–42. doi: 10.1128/IAI.01655-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molin S, Boe L, Jensen LB, Kristensen CS, Givskov M, Ramos JL, et al. Suicidal genetic elements and their use in biological containment of bacteria. Annu Rev Microbiol. 1993;47:139–66. doi: 10.1146/annurev.mi.47.100193.001035 [DOI] [PubMed] [Google Scholar]
- 13.Jensen RB, Gerdes K. Programmed cell death in bacteria: proteic plasmid stabilization systems. Mol Microbiol. 1995;17(2):205–10. [DOI] [PubMed] [Google Scholar]
- 14.Knudsen S, Saadbye P, Hansen LH, Collier A, Jacobsen BL, Schlundt J, et al. Development and testing of improved suicide functions for biological containment of bacteria. Appl Environ Microbiol. 1995;61(3):985–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ronchel MC, Molina L, Witte A, Lutbiz W, Molin S, Ramos JL, et al. Characterization of cell lysis in Pseudomonas putida induced upon expression of heterologous killing genes. Appl Environ Microbiol. 1998;64(12):4904–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bej AK, Molin S, Perlin M, Atlas RM. Maintenance and killing efficiency of conditional lethal constructs in Pseudomonas putida. J Ind Microbiol. 1992;10(2):79–85. [DOI] [PubMed] [Google Scholar]
- 17.Diaz E, Munthali M, de Lorenzo V, Timmis KN. Universal barrier to lateral spread of specific genes among microorganisms. Mol Microbiol. 1994;13(5):855–61. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Xiong K, Zou L, Chen Z, Wang Y, Hu X, et al. Lytic Action of the Truncated yncE Gene in Escherichia coli. Curr microbiol. 2015. [DOI] [PubMed] [Google Scholar]
- 19.Baba-Dikwa A, Thompson D, Spencer NJ, Andrews SC, Watson KA. Overproduction, purification and preliminary X-ray diffraction analysis of YncE, an iron-regulated Sec-dependent periplasmic protein from Escherichia coli. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2008;64(Pt 10):966–9. doi: 10.1107/S1744309108029515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kagawa W, Sagawa T, Niki H, Kurumizaka H. Structural basis for the DNA-binding activity of the bacterial beta-propeller protein YncE. Acta Crystallogr D Biol Crystallogr. 2011;67(Pt 12):1045–53. doi: 10.1107/S0907444911045033 [DOI] [PubMed] [Google Scholar]
- 21.Charles RC, Sultana T, Alam MM, Yu Y, Wu-Freeman Y, Bufano MK, et al. Identification of immunogenic Salmonella enterica serotype Typhi antigens expressed in chronic biliary carriers of S. Typhi in Kathmandu, Nepal. PLoS Negl Trop Dis. 2013;7(8):e2335 doi: 10.1371/journal.pntd.0002335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moriel DG, Tan L, Goh KG, Phan MD, Ipe DS, Lo AW, et al. A Novel Protective Vaccine Antigen from the Core Escherichia coli Genome. mSphere. 2016;1(6):e00326–16. doi: 10.1128/mSphere.00326-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiong K, Chen Z, Xiang G, Wang J, Rao X, Hu F, et al. Deletion of yncD gene in Salmonella enterica ssp. enterica serovar Typhi leads to attenuation in mouse model. FEMS Microbiol Lett. 2012;328(1):70–7. doi: 10.1111/j.1574-6968.2011.02481.x [DOI] [PubMed] [Google Scholar]
- 24.Xiong K, Chen Z, Zhu C, Li J, Hu X, Rao X, et al. Safety and immunogenicity of an attenuated Salmonella enterica serovar Paratyphi A vaccine candidate. Int J Med Microbiol. 2015;305(6):563–71. doi: 10.1016/j.ijmm.2015.07.004 [DOI] [PubMed] [Google Scholar]
- 25.Adams P, Fowler R, Kinsella N, Howell G, Farris M, Coote P, et al. Proteomic detection of PhoPQ- and acid-mediated repression of Salmonella motility. Proteomics. 2001;1(4):597–607. doi: 10.1002/1615-9861(200104)1:4<597::AID-PROT597>3.0.CO;2-P [DOI] [PubMed] [Google Scholar]
- 26.Bearson BL, Wilson L, Foster JW. A low pH-inducible, PhoPQ-dependent acid tolerance response protects Salmonella typhimurium against inorganic acid stress. J Bacteriol. 1998;180(9):2409–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Behlau I, Miller SI. A PhoP-repressed gene promotes Salmonella typhimurium invasion of epithelial cells. J Bacteriol. 1993;175(14):4475–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dalebroux ZD, Miller SI. Salmonellae PhoPQ regulation of the outer membrane to resist innate immunity. Curr Opin Microbiol. 2014;17:106–13. doi: 10.1016/j.mib.2013.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fields PI, Groisman EA, Heffron F. A Salmonella locus that controls resistance to microbicidal proteins from phagocytic cells. Science. 1989;243(4894 Pt 1):1059–62. [DOI] [PubMed] [Google Scholar]
- 30.Gunn JS, Miller SI. PhoP-PhoQ activates transcription of pmrAB, encoding a two-component regulatory system involved in Salmonella typhimurium antimicrobial peptide resistance. J Bacteriol. 1996;178(23):6857–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo L, Lim KB, Gunn JS, Bainbridge B, Darveau RP, Hackett M, et al. Regulation of lipid A modifications by Salmonella typhimurium virulence genes phoP-phoQ. Science. 1997;276(5310):250–3. [DOI] [PubMed] [Google Scholar]
- 32.Galan JE, Curtiss R 3rd. Virulence and vaccine potential of phoP mutants of Salmonella typhimurium. Microb Pathog. 1989;6(6):433–43. [DOI] [PubMed] [Google Scholar]
- 33.Miller SI, Kukral AM, Mekalanos JJ. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc Natl Acad Sci U S A. 1989;86(13):5054–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karasova D, Sebkova A, Vrbas V, Havlickova H, Sisak F, Rychlik I. Comparative analysis of Salmonella enterica serovar Enteritidis mutants with a vaccine potential. Vaccine. 2009;27(38):5265–70. doi: 10.1016/j.vaccine.2009.06.060 [DOI] [PubMed] [Google Scholar]
- 35.Que JU, Hentges DJ. Effect of streptomycin administration on colonization resistance to Salmonella typhimurium in mice. Infect Immun. 1985;48(1):169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Waaij D, Berghuis-de Vries JM, Lekkerkerk L-v. Colonization resistance of the digestive tract in conventional and antibiotic-treated mice. The Journal of hygiene. 1971;69(3):405–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simon AJ, Ellington AD. Recent advances in synthetic biosafety. F1000Res. 2016;5:F1000 Faculty Rev-2118. [Google Scholar]
- 38.Wright O, Stan GB, Ellis T. Building-in biosafety for synthetic biology. Microbiology. 2013;159(Pt 7):1221–35. doi: 10.1099/mic.0.066308-0 [DOI] [PubMed] [Google Scholar]
- 39.Hohmann AW, Schmidt G, Rowley D. Intestinal colonization and virulence of Salmonella in mice. Infect Immun. 1978;22(3):763–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
M: molecular marker; lanes 1 and 3: PCR products of phoPQ-deletion mutant with primers P1 and P4, as well as P5 and P6; lanes 2 and 4: PCR products of the wild-type strain with primers P1 and P4, as well as P5 and P6.
(TIF)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.