Abstract
Background
Systolic left ventricular function strongly influences the blood pressure waveform. Therefore, pressure-derived parameters might potentially be used as non-invasive, diagnostic markers of left ventricular impairment. The aim of this study was to investigate the performance of pressure-based parameters in combination with electrocardiography (ECG) for the detection of left ventricular systolic dysfunction defined as severely reduced ejection fraction (EF).
Methods and results
Two populations, each comprising patients with reduced EF and pressure-matched controls, were included for the main analysis (51/102 patients) and model testing (44/88 patients). Central pressure was derived from radial readings and used to compute blood flow. Subsequently, pulse wave analysis and wave intensity analysis were performed and the ratio of the two peaks of forward intensity (SDR) was calculated as a novel index of ventricular function. SDR was significantly decreased in the reduced EF group (2.5 vs. 4.4, P<0.001), as was central pulse pressure, augmentation index and ejection duration (ED), while the QRS-duration was prolonged. SDR and ED were independent predictors of ventricular impairment and when combined with QRS in a simple decision tree, a reduced EF could be detected with a sensitivity of 92% and a specificity of 80%. The independent power of ED, SDR and QRS to predict reduced EF was furthermore confirmed in the test population.
Conclusion
The detection or indication of reduced ejection fraction from pressure-derived parameters seems feasible. These parameters could help to improve the quality of cardiovascular risk stratification or might be used in screening strategies in the general population.
Introduction
Heart failure represents a serious global health problem that is becoming more and more frequent in the aging population: prognoses in the US predict prevalence to rise by 46% from 2012 to 2030 [1]. Asymptomatic systolic or diastolic dysfunction is common in the population, and is associated with a high risk for hospitalization and mortality [2]. In patients with asymptomatic left ventricular systolic dysfunction, pharmacological treatment may delay or even halt the progression of the disease [2] and the early detection of a potentially impaired systolic function, ideally at a primary care level, may therefore be of great value.
In clinical practice, ventricular function is commonly assessed by echocardiography. Also, electrocardiography (ECG) is recommended as first line investigation for suspected heart failure [2,3]. However, parameters describing pulsatile hemodynamics might be used for diagnosis as well, since an impairment of systolic ventricular function strongly influences the resulting pressure and flow waveforms.
For the noninvasive assessment of aortic pressure, different devices have been validated that provide central pressure from peripheral readings by the means of generalized transfer functions [4,5]. Aortic blood flow can be measured noninvasively using Doppler ultrasound or alternatively estimated from the pressure waveform to simplify the procedure [6].
In this context, an approach based on pressure and flow known as wave intensity analysis (WIA) seems especially promising [7,8]. In WIA, blood pressure and flow velocity measured at the same arterial site are considered and a separation into forward and backward travelling waves can be achieved. Characteristics of wave intensity have been shown to be related to left ventricular ejection and relaxation dynamics [7,8].
Of particular relevance for heart failure, the shape of pressure and flow in the systemic arteries results not only from the mechanical properties of the arteries but from a complex interaction of the ejecting heart and the vasculature. In patients with systolic dysfunction, indices of wave reflections are generally lower than in controls [9–12], indicating a pseudo-healthy status, and the relation of common risk indicators like pulse pressure to outcome was found to be reversed [13], or u-shaped [14]. However, it has not yet been evaluated if these differences can be used as diagnostic markers.
Therefore, the aim of this study was to investigate the performance of measures of pulsatile hemodynamics, derived by pulse wave analysis (PWA) and WIA from non-invasive pressure readings, alone and in combination with ECG-measurements for the discrimination between normal and impaired left ventricular systolic function.
Methods
Study population
51 patients with reduced ejection fraction (EF<45%) and 102 controls with normal EF (EF>50%, including 13 patients with heart failure with preserved ejection fraction, HFpEF) matched for gender, age, body mass index and brachial blood pressure were collected at the university teaching hospital Wels-Grieskirchen, Austria. These 153 patients formed the main study population used for primary analysis and the development of classification models. In a second step, a test data set comprising 44 patients with reduced EF and 88 matched controls (including 15 patients with HFpEF) was assembled in the same manner. Patient recruitment for both populations lasted from January 2005 to December 2012. Exclusion criteria for all subjects were arrhythmias (mainly atrial fibrillation), unstable clinical conditions and valvular heart disease exceeding mild severity. In order to have two distinct groups in this pilot study, patients with intermediate EF (45%-50%) were not included. The study was performed within the framework of ongoing studies investigating the role of pulsatile hemodynamics in cardiology [9,15], which were approved by the ethics committee of Upper Austria and written informed consent was obtained from all participants.
Measurements
Radial pressure waveforms were recorded by applanation tonometry (Millar SPT 301, Millar, Inc., Houston, Texas), calibrated with oscillometric brachial pressure (Omron M5-I, Omron Healthcare, Kyoto, Japan), and processed with the SphygmoCor system (AtCor Medical Pty. Ltd, West Ryde, Australia) to obtain aortic pressure waveforms by applying a generalized transfer function [5]. The estimate of ejection duration (ED) provided by the SphygmoCor system was indexed to heart rate (HR) to obtain the gender-specific ejection time index LVETI [16]. Echocardiographic examination was carried out immediately before or after pressure measurement using a Philips iE33 Ultrasound machine (Philips Medical Systems, Andover, Massachusetts). Left ventricular systolic function was evaluated according to published recommendations [17] and blood flow in the left ventricular outflow tract was acquired by Doppler ultrasound in the apical five chamber view. The duration of cardiac depolarization (QRS-interval) was automatically derived from 12-lead ECGs with the MAC 5500 (GE Healthcare, Little Chalfont, UK) and its inbuilt analysis software and manually checked for reliability.
Blood flow model and wave intensity analysis
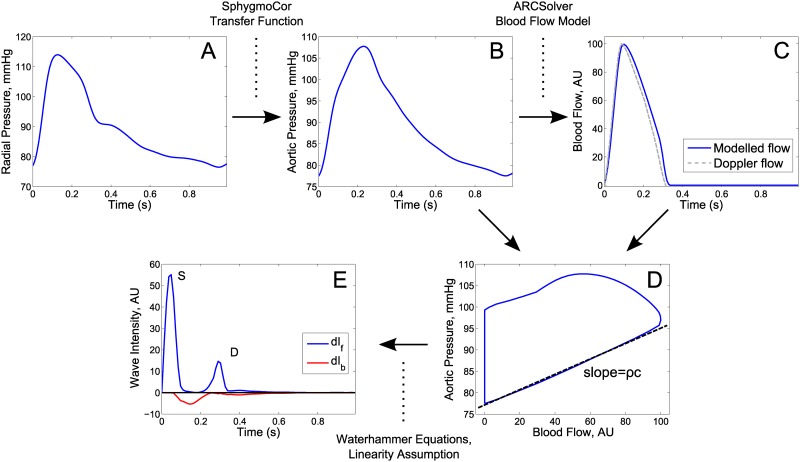
All computational steps are exemplarily depicted in Fig 1. Aortic pressure P was used to obtain aortic blood flow Q with the ARCSolver algorithms (AIT Austrian Institute of Technology GmbH, Vienna, Austria), which are based on a Windkessel model to describe the dynamic relation between pressure and flow plus a minimal work criterion [6]. Q scaled between a minimum of 0 arbitrary units to a maximum of 100 arbitrary units was used as an estimate of the shape of flow velocity U. For WIA, the changes dP and dU per time step were computed and separated into forward (subscript f) and backward (subscript b) travelling components using the Waterhammer equations dPf,b = ±ρc dUf,b and a linearity assumption: dP = dPf+dPb, dU = dUf+dUb [7]. ρ thereby denotes the blood density (1050 kg/m3) and c the wave speed which was estimated with the PU-loop method [18]. The forward and backward wave intensities are then given by dIf,b = dPf,b*dUf,b. dIf generally shows two peaks, denoted as S and D, which are supposed to hold information on systolic and late-systolic/early-diastolic ventricular function [8,19]. Since wave intensity is based on differences of pressure and flow, the S-peak is related to the maximum derivative of left ventricular pressure, while the D-peak is related to the time constant of the pressure decay. The S to D ratio (SDR) is intended as a novel, relative index of systolic function, combining early- and late-systolic pressure and flow dynamics. SDR is independent of the absolute scaling of flow velocity, as this cancels out due to the quotient of WIA parameters. All computations were carried out using Matlab R2011b (The Mathworks, Inc, Natick, Massachusetts).
Fig 1. Computational steps applied for wave intensity analysis.
From tonometrically measured radial pressure (A), aortic pressure (B) is obtained by a generalized transfer function (SphygmoCor). Aortic blood flow (C) is modelled from aortic pressure (ARCSolver) (or measured by Doppler ultrasound for model validation only). Pulse wave velocity c times blood density ρ is estimated from the slope of the approximately linear part of the PU-loop during early systole (D) to derive forward and backward wave intensity (E).
Evaluation of the diagnostic value
The diagnostic value of the various parameters was analyzed in four stages. First, all parameters were evaluated separately in univariable analyses and their performance was compared. In the second stage, combinations of pressure-derived parameters were analyzed and statistical models based on logistic regression analysis were used to identify the strongest independent predictors of a reduced EF. In the third stage, stepwise classification schemes based on the results of the two previous stages were formulated and investigated. These schemes represent easily applicable decision trees that, in contrast to the results of classic statistical models, could be directly transferred to clinical practice. Finally, the proposed scheme was tested in the second data set using the thresholds obtained in stage three.
Statistics
For the statistical evaluation, Medcalc Statistical Software version 15.6.1 (MedCalc Software bvba, Ostend, Belgium) and Matlab R2011b were used. Continuous data are presented as mean (standard deviation) or median [inter-quartile range], according to the results of a Kolmogorov-Smirnov test for normality, categorical data as total number (percentage). Between-group differences of continuous data were quantified with an unpaired t-test (equal variances) or Welch test (unequal variances) if normally or log-normally distributed and with the Mann-Whitney-U test elsewise. For proportions, the chi squared test was used. The performance of the different parameters as binary classifiers of a reduced EF was analyzed using logistic regression, receiver-operator characteristics (ROC) and the area under the ROC curve (AUC). AUCs were compared using the method of DeLong as implemented in MedCalc. The added predictive value of single variables in the stepwise classification schemes was evaluated by the net reclassification improvement as described in [20]. Statistical significance was assumed at a 5% level.
Results
Presented results refer to the main (development) population only, unless explicitly indicated as pertaining to the test population.
The main study population consisted predominantly of male patients (93%) and mean age was 59.9 years (range 27 to 87) for patients with reduced EF and 59.7 years (range 33 to 80) for controls. Anthropometric measures (height, weight, body mass index) as well as the prevalence of coronary artery disease, hypertension and diabetes were comparable between the groups, but antihypertensive medication was more common in reduced EF patients. See Table 1 for a detailed summary of the baseline characteristics for both the development and the test population.
Table 1. Baseline characteristics of both patient groups in the main and the test population.
| Main population | Test population | |||
|---|---|---|---|---|
| Severely reduced EF | Normal EF | Severely reduced EF | Normal EF | |
| Patients | 51 | 102 | 44 | 88 |
| Gender (male/female) | 47/4 | 96/6 | 39/5 | 78/10 |
| Age, years | 59.9 (12.3 SD) | 59.8 (10.8 SD) | 60.5 (10.7 SD) | 60.1 (10.4 SD) |
| Height, cm | 174 (7.15 SD) | 175 (7.8 SD) | 173 (7.08 SD) | 173 (8.76 SD) |
| Weight, kg | 86.9 (16.4 SD) | 86.9 (13.8 SD) | 85.7 (16.3 SD) | 86.1 (18.3 SD) |
| Body mass index, kg/m^2 | 28.7 (4.49 SD) | 28.6 (4.27 SD) | 28.6 (5.35 SD) | 28.7 (5.5 SD) |
| Body surface area, m^2 | 2.01 (0.206 SD) | 2.02 (0.172 SD) | 2.00 (0.188 SD) | 2.00 (0.228 SD) |
| Ejection fraction, % | 27.8 (8.10 SD) * | 69.7 (7.56 SD) * | 28.3 (7.31 SD) * | 68.2 (9.07 SD) * |
| Hypertension | 30 (59%) | 66 (65%) | 25 (57%) | 61 (69%) |
| Diabetes | 14 (27%) | 17 (17%) | 10 (23%) | 13 (15%) |
| Coronary artery disease | 27 (53%) | 45 (44%) | 17 (39%) | 41 (47%) |
| ACE/ARB, n(%) | 43 (84%) * | 35 (34%) * | 39 (89%) * | 47 (53%) * |
| Beta blocker, n (%) | 42 (82%) * | 51 (50%) * | 34 (77%) * | 38 (43%) * |
| Calcium channel blocker, n (%) | 5 (10%) | 9 (9%) | 5 (11%) | 12 (14%) |
| Diuretic, n (%) | 30 (59%) * | 24 (24%) * | 30 (68%) * | 18 (20%) * |
| NO donator, n (%) | 5 (10%) | 11 (11%) | 7 (16%) | 11 (13%) |
| Acetylsalicylic acid, n (%) | 35 (69%) | 83 (81%) | 35 (80%) | 68 (77%) |
| Statin, n (%) | 17 (33%) | 42 (41%) | 14 (32%) | 40 (45%) |
EF, ejection fraction; ACE, angiotensin converting enzyme inhibitors; ARB, angiotensin receptor blocker. Hypertension was defined as office BP > 140/90 or preexisting treatment for hypertension. Values are given as mean (standard deviation) or number (percentage).
* indicates a significant difference in group-wise comparison (severely reduced vs. normal ejection fraction); P-values were <0.001 for all detected differences.
Table 2 depicts the results of PWA, WIA and ECG for both patient groups in the main population. HR and QRS-duration were increased, whereas ED and ED indexed to HR (LVETI) were reduced in the low EF group compared to controls. Although brachial pressures were well matched, estimated central pulse pressure (PP) showed statistically significant differences between the groups and augmentation index was lower for reduced EF. Also, the S to D ratio derived by WIA was significantly lower in the reduced EF group (SDR: 2.5 [1.0, 3.6] vs. 4.4 [3.4, 5.9], P<0.001). This reduction was comparable to the one obtained using Doppler flow measurements (SDR Doppler: 2.5 [1.7,3.3] vs. 5.1 [4.0,6.6], P<0.001) and the difference between SDR and SDR Doppler was non-significant across the study population.
Table 2. Pulse wave analysis, wave intensity analysis and ECG parameters.
| Severely reduced EF | Normal EF | P-value | |
|---|---|---|---|
| Pressure-derived parameters | |||
| Heart rate, bpm | 74.8 (13.4 SD) | 64.3 (10.3 SD) | <0.001 |
| Ejection duration, ms | 268 (27.6 SD) | 309 (26.0 SD) | <0.001 |
| LVETI, ms | 394 (19.8 SD) | 417 (18.5 SD) | <0.001 |
| brachial SBP, mmHg | 123 (20.8 SD) | 127 (13.3 SD) | 0.30 |
| central SBP, mmHg | 111 (18.7 SD) | 117 (12.2 SD) | 0.06 |
| brachial DBP. mmHg | 78.0 (13.8 SD) | 79.0 (9.13 SD) | 0.63 |
| central DBP, mmHg | 78.7 (14.1 SD) | 79.8 (9.18 SD) | 0.62 |
| brachial PP, mmHg | 45.5 (14.1 SD) | 47.8 (11.7 SD) | 0.28 |
| central PP, mmHg | 32.3 (12.2 SD) | 36.7 (9.89 SD) | 0.02 |
| AIx, - | 0.165 (0.086 SD) | 0.247 (0.108 SD) | <0.001 |
| SDR, - | 2.5 [1.98,3.61] | 4.41 [3.4,5.93] | <0.001 |
| Doppler Ultrasound | |||
| Doppler SDR, - | 2.49 [1.71,3.31] | 5.05 [4.03,6.56] | <0.001 |
| ECG | |||
| QRS-interval, ms | 110 [97,133] | 92 [86,100] | <0.001 |
Results are presented as mean (standard deviation) and median [inter-quartile range] for normally and non-normally distributed data respectively. P-values present the results of group-wise comparisons (severely reduced vs. normal ejection fraction). LVETI, left ventricular ejection time index; SBP, systolic blood pressure; DBP, diastolic blood pressure; PP, pulse pressure; AIx, augmentation index; SDR, ratio of the first (S) to second (D) peak of forward wave intensity.
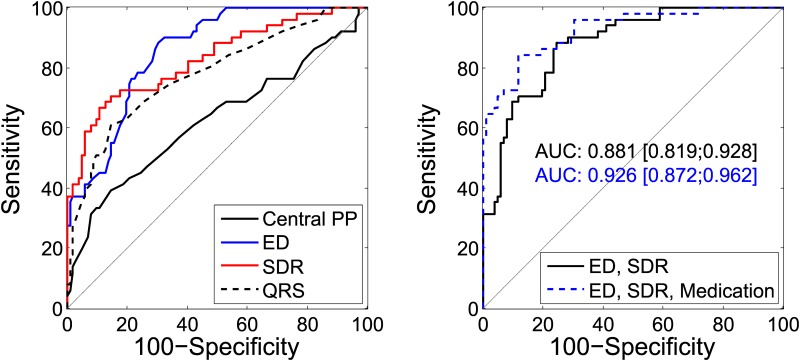
The performance of the different parameters as binary classifiers of a systolic dysfunction are quantitatively presented in Table 3 and graphically depicted in Fig 2. Blood pressure levels showed the worst performance, whereas ED, LVETI and SDR all reached an AUC of above 0.8. The AUC obtained with SDR was smaller than its counterpart obtained with the flow velocity acquired by Doppler ultrasound (0.83 to 0.88). However, a pairwise comparison showed no evidence of a statistical difference (difference AUC: 0.05, 95% confidence interval of [-0.32,0.14], P = 0.22). Using Doppler flow, the S and D peak values could also be evaluated individually. Here the AUCs were significantly lower than for the SDR (difference SDR to S: 0.12, 95% confidence interval of [0.05,0.19], P<0.001; difference SDR to D: 0.28, 95% confidence interval of [0.18,0.39], P<0.001).
Table 3. Univariable logistic regression models for EF-status (0: Normal, 1: Reduced).
| Parameter | OR | 95% CI OR | AUC | SE | 95% CI AUC | Percent correctly classified | Criterion |
|---|---|---|---|---|---|---|---|
| Heart rate, per bpm | 1.077 | [1.043,1.112] | 0.736 | 0.0437 | [0.659,0.804] | 71.24% | ≥70 |
| Ejection duration, per ms | 0.948 | [0.931,0.965] | 0.855 | 0.0295 | [0.789,0.906] | 75.82% | ≤297 |
| LVETI, per ms | 0.940 | [0.919,0.961] | 0.814 | 0.0342 | [0.744,0.873] | 71.90% | ≤414 |
| brachial PP, per mmHg | 0.985 | [0.958,1.013] | 0.559 | 0.0524 | [0.476,0.639] | 69.93% | ≤33 |
| central PP, per mmHg | 0.961 | [0.929,0.994] | 0.624 | 0.0513 | [0.543,0.701] | 69.93% | ≤26 |
| AIx, per 0.01 | 0.923 | [0.889,0.959] | 0.744 | 0.0407 | [0.667,0.811] | 66.01% | ≤0.24 |
| SDR, per 1 | 0.385 | [0.268,0.551] | 0.829 | 0.0371 | [0.760,0.885] | 81.70% | ≤2.88 |
| QRS-interval, per ms | 1.060 | [1.036,1.085] | 0.778 | 0.0412 | [0.703,0.841] | 77.12% | ≥104 |
| S peak Doppler, per 1 | 0.970 | [0.957, 0.984] | 0.760 | 0.0428 | [0.684, 0.825] | 77.78% | ≤46.1 |
| D peak Doppler, per 1 | 1.025 | [0.992, 1.058] | 0.594 | 0.0488 | [0.512, 0.673] | 55,56% | ≥16.5 |
| SDR Doppler, per 1 | 0.341 | [0.239, 0.487] | 0.879 | 0.0315 | [0,816, 0.926] | 81,05% | ≤3.87 |
Percent correctly classified and the corresponding criterion refers to the point with maximum Youden Index J = Sensitivity+Specificity-1. OR, odds ratio; CI, confidence interval; AUC, area under receiver operating characteristics curve; SE, standard error; abbreviations as in Table 2, additionally S (or D) peak Doppler: S (or D) peak value of wave intensity calculated with pressure and Doppler flow waves.
Fig 2. ROC curve analysis.
Left: Comparison of the ROC curves obtained with central pulse pressure (PP), ejection duration (ED), S to D ratio (SDR) and QRS-duration. Right: ROC curves obtained with a combination of ED and SDR, and ED and SDR when adjusted for medication. Area under the curve (AUC) and 95% confidence interval are given in the same colors.
Stepwise logistic regression analysis was applied to all pressure-derived parameters to identify the best predictors. The final model consisted of ED and SDR and reached a combined AUC of 0.88 [0.82, 0.93], while HR, LVETI, augmentation index as well as central and brachial PP were not included. With this model, the Youden index was maximal at a sensitivity of 76% and a specificity of 88% resulting in 80% correctly classified subjects. Adjustment for medication as a confounding factor increased the AUC further to 0.93 [0.87, 0.96] and the percentage of correctly classified subjects to 87% with a sensitivity of 88% and a specificity of 84%, compare Fig 2 and Table 4.
Table 4. Stepwise multivariable logistic regression models for EF-status (0: Normal, 1: Reduced) using pressure-derived parameters with and without adjustment for medication.
| Parameter | 95% CI | |
|---|---|---|
| Model including pressure-derived parameters | ||
| ED, OR per ms | 0.957 | [0.939, 0.975] |
| SDR, OR per 1 | 0.533 | [0.364, 0.780] |
| Not included | HR, LVETI, central PP, brachial PP, AIx | |
| AUC | 0.881 | [0.819, 0.928] |
| Model including pressure-derived parameters and medication | ||
| ED, OR per ms | 0.956 | [0.935, 0.977] |
| SDR, OR per 1 | 0.608 | [0.404, 0.915] |
| Beta blocker, yes = 1 | 4.190 | [1.306, 13.440] |
| ACE/ARB, yes = 1 | 6.133 | [1.861, 20.215] |
| ASA, yes = 1 | 0.246 | [0.069,0.881] |
| Not included | HR, LVETI, central PP, brachial PP, AIx, CCB, diuretic, NO-donator, statin | |
| AUC | 0.926 | [0.872, 0.962] |
A variable was included if P<0.05 and removed if P>0.1. OR, odds ratio; AUC, area under the ROC curve; ED, ejection duration; SDR, S to D ratio; HR, heart rate; LVETI, left ventricular ejection time index; AIx, augmentation index; ACE, angiotensin-converting enzyme inhibitors, ARB: angiotensin II receptor blocker; ASA, acetylsalicylic acid; CCB, calcium channel blocker;
Table 5 shows the results of a stepwise classification scheme using first SDR, then ED and finally QRS to determine or rule-out a reduced EF. The corresponding criteria were chosen such that the false positive rate (100-Specificity) and false negative rate (100-Sensitivity) were ≤5%. In the first step, patients with SDR≤2.5 were classified as “YES”, having reduced EF, those with SDR>5.6 as “NO”, having normal EF, leaving 91 patients unclassified. These were further analyzed using ED (“YES” for ED≤257 ms, “NO” for ED>310 ms), resulting in a total net reclassification improvement of 43%, which was mainly due to an improved classification of patients with normal EF. The remaining 45 subjects were finally classified with QRS (“YES” for QRS≥126 ms, “NO” for QRS<84 ms), significantly improving the classification further by 23%. This improvement was again stronger for the normal EF group. Overall, 132 out of 153 could be classified, thereof 119 correctly. More specifically, 73% of the patients with reduced EF were correctly assigned to “YES”, and 80% of the controls to “NO”, while 20% of the reduced EF and 11% of the normal EF group remained unclassified. Since the order of the variables may influence the results, a second scheme is given in Table 5 for comparison, which is again based on SDR, ED and QRS, yet starting with QRS. Also in this case, each step significantly improved the overall classification.
Table 5. Approach to diagnose or exclude a reduced ejection fraction by a stepwise classification scheme.
| Criteria | Categories | ||||||
|---|---|---|---|---|---|---|---|
| YES | NO | YES (rEF/nEF) | Inconclusive (rEF/nEF) | NO (rEF/nEF) | NRI total/rEF/nEF | P-value total/rEF/nEF | |
| Model 1 | |||||||
| SDR | ≤2.5 | >5.6 | 31 (26/5) | 91 (23/68) | 31 (2/29) | ||
| ED | ≤257 | >310 | 41 (33/8) | 45 (15/30) | 67 (3/64) | 0.43/0.12/0.31 | <0.001/0.03/<0.001 |
| QRS | ≥138 | <98 | 46 (37/9) | 21 (10/11) | 86 (4/82) | 0.23/0.06/0.17 | <0.001/0.18/<0.001 |
| Model 2 | |||||||
| QRS | ≥124 | <84 | 23 (18/5) | 111 (31/80) | 19 (2/17) | ||
| SDR | ≤2.5 | >5.6 | 42 (33/9) | 67 (15/52) | 44 (3/41) | 0.47/0.27/0.20 | <0.001/<0.001/<0.001 |
| ED | ≤254 | >299 | 50 (39/11) | 24 (8/16) | 79 (4/75) | 0.41/0.10/0.31 | <0.001/0.06/<0.001 |
For this approach, subjects were classified as having severely reduced EF (YES) or not (NO) according to the criteria given in the first two columns, starting with the parameter in the first row. If none of the criteria applied, results were inconclusive and the parameter in the next row was used for further classification. Criteria were optimized with regards to the false positive and false negative rates (max 5%). NRI indicates the improvement from one step to the next and is given for the total population as well as for the reduced and normal EF group separately. NRI, net reclassification improvement; rEF, reduced ejection fraction; nEF, normal ejection fraction; ED, ejection duration; SDR, S to D ratio.
Baseline characteristics of the test population were very similar to the main, development population, see Table 1, and pressure-derived parameters as well as QRS-duration were in the same range, compare Table 6. However, differences in HR were less pronounced between the groups and even though central PP was again lower in the reduced EF group, this reduction did not reach statistical significance.
Table 6. Pulse wave analysis, wave intensity analysis and ECG parameters in the test population.
| Severely reduced EF | Normal EF | P-value | |
|---|---|---|---|
| Pressure-derived parameters | |||
| Heart rate, bpm | 72.1 (12.3 SD) | 66.4 (12.3 SD) | 0.01 |
| Ejection duration, ms | 266 (27.0 SD) | 305 (27.8 SD) | <0.001 |
| LVETI, ms | 388 (24.1 SD) | 417 (18.6 SD) | <0.001 |
| brachial SBP, mmHg | 124 (19.3 SD) | 125 (15.7 SD) | 0.75 |
| central SBP, mmHg | 111 (18.1 SD) | 114 (15.3 SD) | 0.37 |
| brachial DBP. mmHg | 78.3 (12.8 SD) | 77.9 (11.9 SD) | 0.86 |
| central DBP, mmHg | 78.9 (12.9 SD) | 78.8 (12.1 SD) | 0.99 |
| brachial PP, mmHg | 45.4 (12.1 SD) | 46.8 (9.79 SD) | 0.48 |
| central PP, mmHg | 32.4 (10.5 SD) | 35.1 (8.57 SD) | 0.11 |
| AIx, - | 0.190 (0.121 SD) | 0.221 (0.105 SD) | 0.13 |
| SDR, - | 2.61 [1.88,3.28] | 4.75 [3.45,6.37] | <0.001 |
| ECG | |||
| QRS-interval, ms | 120 [98,146] | 91 [86,102] | <0.001 |
Results are presented as mean (standard deviation) and median [inter-quartile range] for normally and non-normally distributed data respectively. P-values present the results of group-wise comparisons (severely reduced vs. normal ejection fraction). LVETI, left ventricular ejection time index; SBP, systolic blood pressure; DBP, diastolic blood pressure; PP, pulse pressure; AIx, augmentation index; SDR, S to D ratio.
Results obtained with the classification scheme from Table 5 (model 1) using prescribed cutoff-values are finally presented in Table 7. Again, each step resulted in a significant improvement and 70% of the patients with reduced EF and 76% of the normal EF group were classified correctly, while 11% and 13% of the two groups remained unclassified.
Table 7. Approach to diagnose or exclude a reduced ejection fraction by a stepwise classification scheme with fixed criteria in the test population.
| Criteria | Categories | ||||||
|---|---|---|---|---|---|---|---|
| YES | NO | YES (rEF/nEF) | Inconclusive (rEF/nEF) | NO (rEF/nEF) | NRI total/rEF/nEF | P-value total/rEF/nEF | |
| SDR | ≤2.5 | >5.6 | 27 (20/7) | 72 (22/50) | 33 (2/31) | ||
| ED | ≤257 | >310 | 37 (28/9) | 38 (11/27) | 57 (5/52) | 0.33/0.11/0.22 | <0.001/0.13/<0.001 |
| QRS | ≥138 | <98 | 41 (31/10) | 16 (5/11) | 75 (8/67) | 0.16/0.00/0.16 | 0.03/1.00/<0.001 |
Discussion
In this study, a new marker of impaired systolic function, the ratio of the first to second peak of forward wave intensity (SDR) derived from pressure waveforms alone, was introduced and investigated as a discriminator between subjects with reduced EF and controls. Furthermore, the combination of SDR with other pressure-derived measures and QRS-duration from ECG was examined.
The influence of left ventricular impairment on the shape of the central pressure wave is reflected in the reduction of central PP and augmentation index found in this study as well as in previous works [9–11]. Also, ED and HR are important determinants of the pulse waveform and were both altered in the reduced EF group, whereby the differences in LVETI imply that ED was reduced beyond the effect of HR elevation. These changes in HR and ED have been reported before [9–11,21] and might be explained by the inability of the impaired ventricle to overcome late systolic load (shorter ED), leading to an increase in heart rate to compensate the resulting lower stroke volume [22]. Of note, HR was higher despite the higher prevalence of beta blocker use in the reduced EF group. PP amplification, the ratio of peripheral to central PP, assessed with the SphygmoCor system has been found to increase linearly with HR [23]. This is in line with our finding that PP amplification tends to be higher in patients with reduced EF compared to controls resulting in a larger difference in central than in brachial PP between the groups. More precisely, due to the matching criteria, peripheral PP did not differ significantly between groups for both populations, while central PP differed with a borderline significance (p = 0.02 for main population, p = 0.11 for test population). Also the augmentation index has been reported to be influenced by temporal effects [24,25] as well as cardiac properties [26].
While augmentation index and PP are both indirect measures of and strongly influenced by wave reflections, forward wave intensity is comprised of forward travelling waves only. These forward waves are generated by the left ventricle and result in two dominant peaks in the wave intensity dIf. The first peak S is related to left ventricular contractility [8], whereas the second peak D is associated with the beginning of relaxation [7]. Their ratio could thus be seen as an index that combines the characteristics of the left ventricle during the entire mechanical systole. The lower values of SDR observed in the reduced EF group are in line with these theoretical considerations as well as with results from Sugawara et al. [8] and Curtis et al. [19], who both found a reduction in the first but not the second peak of wave intensity in subjects with systolic heart failure compared to controls. Results from the present study using Doppler flow also indicate that the main effect is a reduction of the S-peak, but the ratio of S and D has a significantly higher AUC and thus an even higher discriminative power than the S-peak alone.
A similar approach was taken by Ntsinjana and coworkers to analyze ventricular function in children with HFpEF [27]. Equivalently to SDR, they calculated the ratio of forward compression wave and forward expansion wave from wave intensity derived with area and velocity obtained from cardiovascular magnetic resonance phase-contrast data. A significant reduction was found for patients with HFpEF compared to controls. Although patients had a normal ejection fraction, the authors speculated that this indicates a load-independent systolic dysfunction. This finding should be interpreted in context with another recent work by Gu and coworkers in patients with diastolic dysfunction [28]. The authors linked systolic to diastolic function via an impaired shortening deactivation and a reduced so-called early ejection fraction, despite preserved total ejection fraction. This early ejection fraction was determined during early systole, thus it is potentially related to the S-peak in WIA. Clearly, further studies are needed to investigate these relations and the role of SDR in heart failure beside patients with HFrEF, who have been in focus in this current study.
As a ratio, SDR is virtually independent of the sampling frequency, making its value comparable between different investigators using different instruments. Furthermore, the computation of SDR is not affected by the absolute scaling of blood flow, which enables the use of a (volumetric) blood flow model for the estimation of flow velocity. The model used here has been investigated previously in patients with and without heart failure with reduced EF [6,24,29]. In both cases, modelled flow showed a good agreement with Doppler flow measurements and the qualitative and quantitative behavior of the derived PWA parameters was comparable. Also in the present work, differences in SDR as well as in the derived AUC between modelled and measured flow were non-significant. The use of a flow model for the computation of SDR greatly facilitates the data acquisition process and represents an important criterion to enable its implementation in day-to-day clinical practice.
In ROC curve analysis, ED and SDR reached the largest AUC from all pressure-derived parameters considered in this study. Furthermore, the results of multivariable logistic regression analysis indicated no independent additive association of either PP, augmentation index, HR or LVETI to EF-status beyond ED and SDR. This might be explained by the intrinsic link between HR and ED on the one hand and the impact of HR and ED on both PP and augmentation index on the other hand [23,25]. In contrast, SDR seems to reflect aspects of ventricular function that are not covered by ED. This is further underlined by the different shapes of their ROC curves, while using SDR and ED together combines the advantages of both, as presented in Fig 2. Overall, the AUC of 0.88 obtained with the combination of ED and SDR implies a satisfactory discrimination between patients with normal and reduced EF, which is further improved if medication is adjusted for. Sensitivity and specificity lie within the range of 76%–92%. Despite high values for the AUC, the sensitivity might not be sufficiently high for a rigorous clinical test, but since the maximum Youden-index is used here as cutoff-criteria, application-specific cutoff-values can enhance the sensitivity if needed.
Systolic heart failure is reflected in various abnormalities in the ECG signal, including temporal and morphological characteristics [3], and a normal ECG makes left ventricular systolic dysfunction (LVSD) very unlikely [3,30]. Assessment of the QRS-duration is explicitly recommended as part of the diagnostic investigation in suspected heart failure by the European Society of Cardiology [3] and it has been investigated before as an identifier of LVSD [31,32]. The observed prolongation in patients with reduced EF is in line with previous findings [31,32] and the specificity of 85% obtained in the present work for QRS≥104 ms coincides with the 84% obtained by Murkofsky et al. [31] for QRS≥100 ms. Sensitivity was even higher (61% compared to 44%).
Based on a community-based study performed in Germany, Fischer et al. concluded that echocardiographic screening for LVSD cannot be recommended in the unselected, middle-aged population because asymptomatic LVSD is rare and most patients with LVSD present with cardiovascular comorbidities [33]. However, even in patients with suspected heart failure, left ventricular function is often not assessed by primary care physicians, leading to possible misdiagnoses [34]. Therefore, non-invasive methods for either preselection or for indicating further examination could help to improve the early diagnosis and treatment decision in a primary care setting. This idea has been investigated before with regards to ECG, yet using ECG alone was found to be of limited clinical use [30]. The decision tree given in Table 7 (model 1) represents a theoretical pre-echocardiographic screening test based on a combination of pressure-derived parameters and ECG. A similar approach has been presented by Weber et al. for the diagnosis of heart failure with preserved EF [35]. The results obtained with the second model, i.e. with a changed order of the variables, confirmed the independency between the measures: improvement was still significant for each parameter included and the cut-off values remained mostly unchanged. The association of the chosen parameters to an impairment of ventricular systolic function was further corroborated by the classification results achieved in the test population, indicating that the observed relations are not restricted to the initial population used for model development.
Recently, operator-independent methods based on oscillometric pressure cuffs have been introduced for the assessment of central hemodynamics [4]. Thus, all parameters used in this work could potentially be obtained automatically using a pressure cuff and an ECG, making them easily assessable also at a primary care level.
Limitations
The study population was collected in a 1:2 fashion regarding the proportion of patients with reduced EF to controls, which does not reflect the real, low prevalence of approximately 1% for systolic heart failure and 2% for LVSD in general population [3,33]. However, the prevalence of systolic heart failure increases with age, reaching more than 5% in age groups above 70 [3]. The control groups included a number of patients with HFpEF, but due to the limited amount of patients with HFpEF in the study fulfilling the matching criteria, two control groups solely based on HFpEF patients could not be set up. This would allow distinguishing whether controls with and without HFpEF will be identified correctly in a comparable amount. The fact that predominantly male subjects (91%) were included in this study has to be stated as another limitation. However, also previous studies in systolic heart failure show an unbalanced gender distribution [10,11] (between 80% and 97% male), which might be caused by the unequal prevalence of LVSD with regards to gender, which was found to be about 2.5 times higher in men than in women [36]. The applicability of the proposed scheme in the general population has to be investigated in future studies and bigger data sets are necessary to derive optimal threshold values. In the current work, the false negative rate was rather high (~18%) in the test population, but higher sensitivity could be achieved by adopting the cutoff values. Moreover, sensitivity and specificity were rated equally important in this study, but could be chosen differently for clinical application.
Conclusion
The detection or indication of reduced ejection fraction from parameters derived from pulse wave readings seems feasible. These parameters could help to improve the quality of cardiovascular risk stratification and might potentially be incorporated into cheap, noninvasive screening strategies in the general population.
Data Availability
All patients who participated in this study gave written informed consent. However, this consent did not include a provision stating that individual raw data can be made freely accessible to the public. Therefore, in accordance with the Austrian data protection act (Datenschutzgesetz 2000 - DSG 2000, Federal Law Gazette I No. 165/1999) the underlying data cannot be made accessible to the public. Researchers meeting the criteria for access to confidential data may access the data upon request, involving the documentation of data access. The principal investigator of the Wels cohort Thomas Weber can be contacted at "thomas.weber3@liwest.at". Alternatively, the AIT Cardiovascular Diagnostics research database can be contacted at "data.cv-diagnostics@ait.ac.at".
Funding Statement
This work was supported by the program „Talente“ by the Austrian Ministry for Transport, Innovation and Technology BMVIT (FEMCOR Project, FFG No. 849808). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. , on behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics—2016 Update. Circulation 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350 [DOI] [PubMed] [Google Scholar]
- 2.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure. Circulation 2013;128(16):e240–e327. doi: 10.1161/CIR.0b013e31829e8776 [DOI] [PubMed] [Google Scholar]
- 3.Ponikowski P, Voors AA, Anker SD, Bueno Hé, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128 [DOI] [PubMed] [Google Scholar]
- 4.Weber T, Wassertheurer S, Rammer M, Maurer E, Hametner B, Mayer CC, et al. Validation of a Brachial Cuff-Based Method for Estimating Central Systolic Blood Pressure. Hypertension 2011;58(5):825–32. doi: 10.1161/HYPERTENSIONAHA.111.176313 [DOI] [PubMed] [Google Scholar]
- 5.Karamanoglu M, O'Rourke M, Avolio A, Kelly R. An analysis of the relationship between central aortic and peripheral upper limb pressure waves in man. Eur Heart J 1993;14(2):160–7. [DOI] [PubMed] [Google Scholar]
- 6.Hametner B, Wassertheurer S, Kropf J, Mayer C, Holzinger A, Eber B, et al. Wave reflection quantification based on pressure waveforms alone-Methods, comparison, and clinical covariates. Comput Meth Programs Biomed 2013;109(3):250–9. [DOI] [PubMed] [Google Scholar]
- 7.Parker K, Jones C. Forward and backward running waves in the arteries: analysis using the method of characteristics. J Biomech Eng 1990;112(3):322–6. [DOI] [PubMed] [Google Scholar]
- 8.Sugawara M, Niki K, Ohte N, Okada T, Harada A. Clinical usefulness of wave intensity analysis. Med Biol Eng Comput 2009;47(2):197–206. doi: 10.1007/s11517-008-0388-x [DOI] [PubMed] [Google Scholar]
- 9.Weber T, Auer J, Lamm G, O'Rourke MF, Eber B. Arterial Stiffness, Central Blood Pressures, and Wave Reflections in Cardiomyopathy-Implications for Risk Stratification. J Card Fail 2007;13(5):353–9. doi: 10.1016/j.cardfail.2007.02.008 [DOI] [PubMed] [Google Scholar]
- 10.Denardo SJ, Nandyala R, Freeman GL, Pierce GL, Nichols WW. Pulse Wave Analysis of the Aortic Pressure Waveform in Severe Left Ventricular Systolic Dysfunction. Circ Heart Fail 2010;3(1):149–56. doi: 10.1161/CIRCHEARTFAILURE.109.862383 [DOI] [PubMed] [Google Scholar]
- 11.Tartiere J-M, Logeart D, Safar M, Cohen-Solal A. Interaction between pulse wave velocity, augmentation index, pulse pressure and left ventricular function in chronic heart failure. J Hum Hypertens 2006;20(3):213–9. doi: 10.1038/sj.jhh.1001965 [DOI] [PubMed] [Google Scholar]
- 12.Parragh S, Hametner B, Bachler M, Kellermair J, Eber B, Wassertheurer S, et al. Determinants and covariates of central pressures and wave reflections in systolic heart failure. Int J Cardiol 2015;190:308–14. doi: 10.1016/j.ijcard.2015.04.183 [DOI] [PubMed] [Google Scholar]
- 13.Regnault V, Lagrange J, Pizard A, Safar ME, Fay R, Pitt B, et al. Opposite Predictive Value of Pulse Pressure and Aortic Pulse Wave Velocity on Heart Failure With Reduced Left Ventricular Ejection Fraction: Insights From an Eplerenone Post–Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) Substudy. Hypertension 2014;63(1):105–11. doi: 10.1161/HYPERTENSIONAHA.113.02046 [DOI] [PubMed] [Google Scholar]
- 14.Laskey WK, Wu J, Schulte PJ, Hernandez AF, Yancy CW, Heidenreich PA, et al. Association of Arterial Pulse Pressure With Long-Term Clinical Outcomes in Patients With Heart Failure. J Am Coll Cardiol HF 2016;4:42–9. [DOI] [PubMed] [Google Scholar]
- 15.Weber T, Wassertheurer S, Hametner B, Parragh S, Eber B. Noninvasive methods to assess pulse wave velocity: comparison with the invasive gold standard and relationship with organ damage. J Hypertens 2015;33:1023–31. doi: 10.1097/HJH.0000000000000518 [DOI] [PubMed] [Google Scholar]
- 16.Haiden A, Eber B, Weber T. U-Shaped Relationship of Left Ventricular Ejection Time Index and All-Cause Mortality. Am J Hypertens 2014;27(5):702–9. doi: 10.1093/ajh/hpt185 [DOI] [PubMed] [Google Scholar]
- 17.Lang R, Bierig M, Devereux R, Flachskampf F, Foster E, Pellikka P, et al. Recommendations for Chamber Quantification: A Report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, Developed in Conjunction with the European Association of Echocardiography, a Branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005 [DOI] [PubMed] [Google Scholar]
- 18.Khir A, O'Brien A, Gibbs J, Parker K. Determination of wave speed and wave separation in the arteries. J Biomech 2001;34(9):1145–55. [DOI] [PubMed] [Google Scholar]
- 19.Curtis SL, Zambanini A, Mayet J, Thom SAM, Foale R, Parker KH, et al. Reduced systolic wave generation and increased peripheral wave reflection in chronic heart failure. Am J Physiol Heart Circ Physiol 2007;293(1):H557–62. doi: 10.1152/ajpheart.01095.2006 [DOI] [PubMed] [Google Scholar]
- 20.Pencina MJ, D' Agostino RB, D' Agostino RB Sr, Vasan RS. Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat Med 2008;27(2):157–72. doi: 10.1002/sim.2929 [DOI] [PubMed] [Google Scholar]
- 21.Weissler AM, Harris WS, Schoenfeld CD. Systolic time intervals in heart failure in man. Circulation 1968;37(2):149–59. [DOI] [PubMed] [Google Scholar]
- 22.Westerhof N, O'Rourke MF. Haemodynamic basis for the development of left ventricular failure in systolic hypertension and for its logical therapy. J Hypertens 1995;13(9):943–52. [DOI] [PubMed] [Google Scholar]
- 23.Wilkinson IB, Mohammad NH, Tyrrell S, Hall IR, Webb DJ, Paul VE, et al. Heart rate dependency of pulse pressure amplification and arterial stiffness. Am J Hypertens 2002;15(1):24–30. [DOI] [PubMed] [Google Scholar]
- 24.Parragh S, Hametner B, Bachler M, Weber T, Eber B, Wassertheurer S. Non-invasive wave reflection quantification in patients with reduced ejection fraction. Physiol Meas 2015;36(2):179–90. doi: 10.1088/0967-3334/36/2/179 [DOI] [PubMed] [Google Scholar]
- 25.Sharman JE, Davies JE, Jenkins C, Marwick TH. Augmentation index, left ventricular contractility, and wave reflection. Hypertension 2009; 54(5):1099–105. doi: 10.1161/HYPERTENSIONAHA.109.133066 [DOI] [PubMed] [Google Scholar]
- 26.Fok H, Guilcher A, Li Y, Brett S, Shah A, Clapp B, et al. Augmentation Pressure Is Influenced by Ventricular Contractility/Relaxation Dynamics: Novel Mechanism of Reduction of Pulse Pressure by Nitrates. Hypertension 2014;63(5):1050–5. doi: 10.1161/HYPERTENSIONAHA.113.02955 [DOI] [PubMed] [Google Scholar]
- 27.Ntsinjana HN, Chung R, Ciliberti P, Muthurangu V, Schievano S, Marek J, et al. Utility of cardiovascular Magnetic resonance-Derived Wave intensity analysis as a Marker of Ventricular Function in children with heart Failure and normal ejection Fraction. Front. Pediatr. 2017;5:65 doi: 10.3389/fped.2017.00065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gu H, Li Y, Fok H, Simpson J, Kentish JC, Shah AM, et al. Reduced First-Phase Ejection Fraction and Sustained Myocardial Wall Stress in Hypertensive Patients With Diastolic Dysfunction. A Manifestation of Impaired Shortening Deactivation That Links Systolic to Diastolic Dysfunction and Preserves Systolic Ejection Fraction. Hypertension. 2017;69:633–640. doi: 10.1161/HYPERTENSIONAHA.116.08545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hametner B, Parragh S, Mayer C, Weber T, Van Bortel L, De Buyzere M, et al. Assessment of Model Based (Input) Impedance, Pulse Wave Velocity, and Wave Reflection in the Asklepios Cohort. PLoS ONE 2015;10(10):e0141656 doi: 10.1371/journal.pone.0141656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khan NK, Goode KM, Cleland JG, Rigby AS, Freemantle N, Eastaugh J, et al. , for the EuroHeart Failure Survey Investigators. Prevalence of ECG abnormalities in an international survey of patients with suspected or confirmed heart failure at death or discharge. Eur J Heart Fail 2007;9:491–501. doi: 10.1016/j.ejheart.2006.11.003 [DOI] [PubMed] [Google Scholar]
- 31.Murkofsky RL, Dangas G, Diamond JA, Mehta D, Schaffer A, Ambrose JA. A prolonged QRS duration on surface electrocardiogram is a specific indicator of left ventricular dysfunction. J Am Coll Cardiol 1998;32(2):476–82. [DOI] [PubMed] [Google Scholar]
- 32.Cincin A, Ozben B, Erdogan O. Diagnostic utility of specific electrocardiographical parameters in predicting left ventricular function. Exp Clin Cardiol 2012;17(4):210–4. [PMC free article] [PubMed] [Google Scholar]
- 33.Fischer M, Baessler A, Holmer SR, Muscholl M, Bröckel U, Luchner A, et al. Epidemiologie der linksventrikulären systolischen Dysfunktion in der Allgemeinbevölkerung Deutschlands. Z Kardiol 2003;92(4):294–302. doi: 10.1007/s00392-003-0899-2 [DOI] [PubMed] [Google Scholar]
- 34.Hobbs FDR, Jones MI, Allan TF, Wilson S, Tobias R. European survey of primary care physician perceptions on heart failure diagnosis and management (Euro-HF). Eur Heart J 2000;21(22):1877–87. doi: 10.1053/euhj.2000.2170 [DOI] [PubMed] [Google Scholar]
- 35.Weber T, Wassertheurer S, O'Rourke MF, Haiden A, Zweiker R, Rammer M, et al. Pulsatile Hemodynamics in Patients With Exertional Dyspnea: Potentially of Value in the Diagnostic Evaluation of Suspected Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol 2013;61(18):1874–83. doi: 10.1016/j.jacc.2013.02.013 [DOI] [PubMed] [Google Scholar]
- 36.Mosterd A, Hoes A, de Bruyne M, Deckers J, Linker D, Hofman A, et al. Prevalence of heart failure and left ventricular dysfunction in the general population; The Rotterdam Study. Eur Heart J 1999;20(6):447–55. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All patients who participated in this study gave written informed consent. However, this consent did not include a provision stating that individual raw data can be made freely accessible to the public. Therefore, in accordance with the Austrian data protection act (Datenschutzgesetz 2000 - DSG 2000, Federal Law Gazette I No. 165/1999) the underlying data cannot be made accessible to the public. Researchers meeting the criteria for access to confidential data may access the data upon request, involving the documentation of data access. The principal investigator of the Wels cohort Thomas Weber can be contacted at "thomas.weber3@liwest.at". Alternatively, the AIT Cardiovascular Diagnostics research database can be contacted at "data.cv-diagnostics@ait.ac.at".