Nonalcoholic fatty liver disease (NAFLD) is characterized by the presence of hepatic steatosis in individuals who consume little or no alcohol and can lead to nonalcoholic steatohepatitis. Patients with nonalcoholic steatohepatitis, and in particular those with advanced fibrosis, are at significant risk of cirrhosis, hepatic decompensation, and hepatocellular carcinoma.1,2 Recently, magnetic resonance elastography (MRE), a magnetic resonance–based imaging technique, has been shown to accurately diagnose fibrosis in patients with NAFLD.3–5
Weight loss remains the mainstay of treatment for NAFLD and nonalcoholic steatohepatitis; however, there are limited data regarding the effect of weight loss on liver fibrosis. In addition, whether MRE-estimated liver stiffness decreases with weight loss has not been previously assessed. In this study, we aim to determine the quantitative effect of weight loss on MRE-estimated liver stiffness in patients with NAFLD.
Methods
This is a secondary analysis of the placebo arms of 2 randomized controlled trials assessing the effect of ezetimibe and sitagliptin on biochemical and imaging outcomes in NAFLD.6,7 All patients enrolled in this study were diagnosed with NAFLD based on liver biopsy and/or a magnetic resonance imaging fat-fraction of at least 5%. Patients underwent clinical evaluation, biochemical testing, and MRE at baseline and after 24 weeks. Two cohorts for this secondary analysis were derived according to those who had at least a 5% decrease in body mass index (BMI) (weight-loss group) and those who did not have at least a 5% decrease in BMI (no-weight-loss group).
Two-dimensional MRE was performed at baseline and after 24 weeks using a previously described technique.3–5 An acoustic passive driver was secured to the patient’s body wall and connected to an acoustic driver that generated vibrations in the liver. A two-dimensional echo MRE pulse sequence is performed while the vibrations are being transmitted and generates shear waves within the liver. The waves are processed to create quantitative cross-sectional maps (elastograms) that depict tissue stiffness (measured in kilopascals). The elastograms are then analyzed by an image analyst and 4 regions of interest are created in parts of the liver where corresponding wave images show clear wave propagation, while avoiding liver edges, large blood vessels, and artifact. The regions of interest stiffness values at each of the 4 locations are averaged to calculate the mean two-dimensional MRE stiffness.
Results
Thirty-nine patients with NAFLD were included in this study. Between the start of the study (Week 0) and the completion (Week 24), 5 patients had at least a 5% decreased in BMI and 34 patients had less than a 5% decreased in BMI or a weight gain. Overall, patients who had a 5% or greater decreased in BMI lost an average of 5.72 kg (± 1.09), whereas patients who either gained weight or had less than a 5% decrease in BMI had an average loss of 0.07 kg (± 1.85). Baseline age, BMI, and alanine aminotransferase (ALT) values at Week 0 were similar for these 2 groups.
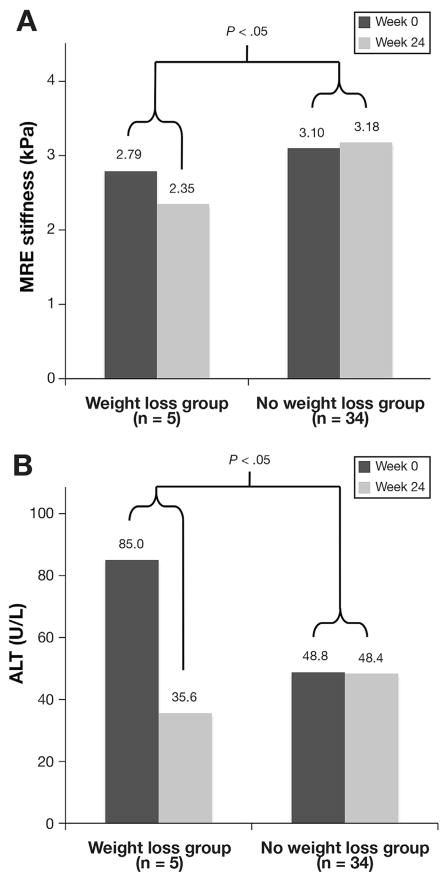
Patients with a 5% or greater decrease in BMI had a mean decreased in MRE-estimated liver stiffness from 2.79 kPa (± 0.74) to 2.35 kPa (± 0.39) (Figure 1), a decrease of 15.8%. Patients with a 5% or less weight loss or weight gain had a mean increased in MRE-estimated liver stiffness from 3.10 kPa (± 1.08) to 3.18 kPa (± 1.32), an increase of 2.5%. The difference in MRE-estimated liver stiffness between these groups was statistically significant (P < .05). There was also a decrease in mean ALT in the weight-loss group from 85.0 (± 111.8) to 35.6 (± 14.1). The difference in ALT change over the study period between both groups was statistically significant (P = .01).
Figure 1.
Change in MRE and ALT with weight loss. Data expressed as mean. (A) Change in MRE with weight loss. (B) Change in ALT with weight loss. Weight-loss group represents patients with >5% decrease in BMI at 24 weeks; no-weight-loss group represents patients with <5% decrease in BMI or gain in BMI at 24 weeks.
Discussion
In this secondary analysis of 2 randomized controlled trials using MRE, we demonstrate that patients with NAFLD with at least a 5% reduction in BMI have a significant decrease in MRE-estimated liver stiffness. This weight loss is also associated with a reduction in ALT.
Previous studies have shown that weight loss is associated with a reduction in histology-determined liver fat and inflammation and magnetic resonance–estimated liver fat.8 Despite this, there are little data on the effect of weight loss on fibrosis in patients with NAFLD. Fibrosis would be a valuable endpoint in both the clinical setting and in future clinical trials studying therapeutics for patients with NAFLD. Our study adds novel information by quantifying the reduction in fibrosis that can be expected with weight loss. By quantifying changes in fibrosis, MRE can be a valuable tool in assessing response to therapeutics in both the clinical and research setting in patients with NAFLD.
Acknowledgments
Funding
Funding provided by Atlantic Philanthropies, Inc; the John A. Hartford Foundation; the Association of Specialty Professors; and the American Gastroenterological Association and grant R01DK106419-01. This research was partially supported by the Clinical & Translational Research Institute at the University of California, San Diego. The study sponsor(s) had no role in the study design, collection, analysis, interpretation of the data, and/or drafting of the manuscript.
Footnotes
Conflicts of interest
The study was conducted at the Clinical and Translational Research Institute, University of California at San Diego. This author discloses the following: Rohit Loomba is supported in part by the American Gastroenterological Association Foundation – Sucampo – ASP Designated Research Award in Geriatric Gastroenterology and by a T. Franklin Williams Scholarship Award. The remaining authors disclose no conflicts.
References
- 1.Bhala N, Angulo P, van der Poorten D, et al. The natural history of nonalcoholic fatty liver disease with advanced fibrosis or cirrhosis: an international collaborative study. Hepatology. 2011;54:1208–1216. doi: 10.1002/hep.24491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh S, Allen AM, Wang Z, et al. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13:643–654. doi: 10.1016/j.cgh.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim D, Kim WR, Talwalkar JA, et al. Advanced fibrosis in nonalcoholic fatty liver disease: noninvasive assessment with MR elastography. Radiology. 2013;268:411–419. doi: 10.1148/radiol.13121193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cui J, Ang B, Haufe W, et al. Comparative diagnostic accuracy of magnetic resonance elastography vs. eight clinical prediction rules for non-invasive diagnosis of advanced fibrosis in biopsy-proven non-alcoholic fatty liver disease: a prospective study. Aliment Pharmacol Ther. 2015;41:1271–1280. doi: 10.1111/apt.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loomba R, Wolfson T, Ang B, et al. Magnetic resonance elastography predicts advanced fibrosis in patients with nonalcoholic fatty liver disease: a prospective study. Hepatology. 2014;60:1920–1928. doi: 10.1002/hep.27362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loomba R, Sirlin CB, Ang B, et al. Ezetimibe for the treatment of nonalcoholic steatohepatitis: assessment by novel magnetic resonance imaging and magnetic resonance elastography in a randomized trial (MOZART trial) Hepatology. 2015;61:1239–1250. doi: 10.1002/hep.27647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui J, Philo L, Nguyen P, et al. Sitagliptin vs. placebo for nonalcoholic fatty liver disease: a randomized controlled trial. J Hepatol. 2016;65:369–376. doi: 10.1016/j.jhep.2016.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrison SA, Fecht W, Brunt EM, et al. Orlistat for overweight subjects with nonalcoholic steatohepatitis: a randomized, prospective trial. Hepatology. 2009;49:80–86. doi: 10.1002/hep.22575. [DOI] [PubMed] [Google Scholar]