Abstract
Background
Deimination (also known as citrullination), the conversion of arginine in a protein to citrulline, is catalyzed by a family of enzymes called peptidyl-arginine deiminases (PADs). Three PADs are expressed in the epidermis, one of their targets being filaggrin. Filaggrin plays a central role in atopic dermatitis and is a key protein for the epidermal barrier. It aggregates keratins and is cross-linked to cornified envelopes. Following its deimination, it is totally degraded to release free amino acids, contributing to the natural moisturizing factor (NMF). The mechanisms controlling this multistep catabolism in human are unknown.
Objective
To test whether external humidity plays a role, and investigate the molecular mechanisms involved.
Methods
Specimens of reconstructed human epidermis (RHEs) produced in humid or dry conditions (>95% or 30–50% relative humidity) were compared.
Results
RHEs produced in the dry condition presented structural changes, including a thicker stratum corneum and a larger amount of keratohyalin granules. The transepidermal water loss and the stratum corneum pH were decreased whereas the quantity of NMF was greater. This highly suggested that filaggrin proteolysis was up-regulated. The expression/activity of the proteases involved in filaggrin breakdown did not increase while PAD1 expression and the deimination rate of proteins, including filaggrin, were drastically enhanced. Partial inhibition of PADs with Cl-amidine reversed the effect of dryness on filaggrin breakdown.
Conclusion
These results demonstrate the importance of external humidity in the control of human filaggrin metabolism, and suggest that deimination plays a major role in this regulation.
Keywords: atopic dermatitis, citrullination, skin, posttranslational modification, peptidyl-arginine deiminase, proteolysis
1. Introduction
Atopic dermatitis (AD; OMIM #603165) is a chronic inflammatory skin disease affecting up to 20% of children and around 3% of adults in industrialized countries. The pathophysiology of AD involves genetic and environmental factors. Most of the susceptibility genes encode proteins involved in the immune response and epidermal barrier formation [1–3]. Loss-of-function mutations in the gene FLG encoding filaggrin are the strongest known risk factor for AD [4–6]. FLG mutations appear to define a subgroup of AD patients with more severe clinical signs, such as reduced skin hydration, increased transepidermal water loss (TEWL), reduced levels of components of the natural moisturizing factor (NMF) and higher skin surface pH [7–10]. Moreover, filaggrin down-regulation has been reported in adult patients regardless of FLG mutations status, its expression being influenced by inflammatory cytokines [11–13]. Therefore, filaggrin deficiency appears to have a central place in AD pathogenesis.
Human filaggrin is an essential epidermal protein synthesized as a large precursor (400 kDa) named profilaggrin and stored in keratohyalin granules. Profilaggrin consists of a large central repetitive domain comprising 10–12 filaggrin repeats, flanked by two single N- and C-terminal domains. During the stratum granulosum to stratum corneum transition, profilaggrin is proteolytically processed to release basic filaggrin monomers (~37 kDa). These monomers associate with keratin filaments and facilitate their aggregation leading to the corneocyte matrix formation [14]. In addition, some monomers are cross-linked to the cornified envelope by transglutaminases [15]. In the lower stratum corneum, filaggrin is deiminated by peptidyl-arginine deiminases 1 and/or 3 (PAD1 and PAD3). This post-translational modification (transformation of arginyl residues into citrullyl) induces a decrease in the number of filaggrin positive charge leading to filaggrin acidification, and thus promotes its detachment from the keratin filaments. Subsequently, filaggrin is fully proteolyzed into free amino acids [16–18].
Filaggrin degradation is accomplished by bleomycin hydrolase, caspase 14, calpain 1 [19–22] and probably other proteases. Some of the amino acids generated are further modified. In particular, glutamine spontaneously transforms into pyrrolidone-5-carboxylic acid (PCA), a highly hygroscopic molecule, and histidine is modified by histidase to form trans-urocanic acid (UCA), a major UVB-absorbing chromophore [23–25]. The amino acids and their derivatives produced from filaggrin degradation participate in the formation of the NMF, and contribute to skin photoprotection and to stratum corneum hydration and acidification (reviewed in [26]). The low pH of the stratum corneum in turn prevents skin colonization by pathogenic microorganisms, is important for the activity of enzymes involved in lipid synthesis and controls desquamation [27, 28].
Despite the importance of filaggrin in both epidermal homeostasis and AD pathogenesis, the molecular mechanisms controlling its degradation are still not well understood. In rodents, the proteolysis of filaggrin appears to be modulated by environmental humidity [29]. To know whether the humidity controls filaggrin breakdown in humans and to investigate the mechanisms involved, specimens of tridimensional reconstructed human epidermis (RHEs) were produced under either >95% relative humidity (RH) (humid condition) or 30–50% RH (dry condition). We compared the stratum corneum morphology and functional properties. Then we focused on filaggrin and on the expression/activity of the enzymes involved in its metabolism.
2. Materials and methods
2.1. RHE production in humid and dry conditions
Primary normal human keratinocytes were isolated after abdominal dermolipectomy of four healthy female (34–48 years old) subjects who had given their informed consent. Four keratinocyte banks were thus constituted. FLG was sequenced using next-generation deep sequencing, and no loss-of-function mutations were observed. RHEs were produced as previously described [30,31], except that after exposure to the air–liquid interface they were cultivated in either humid (>95% RH) or dry (30–50% RH) conditions at 37°C and 5% CO2. Low humidity was induced by removal of the water pan from the incubator. Humidity rate was continuously monitored using the 176H1 data logger (Testo, Lenzkirch, Germany). To avoid water condensation, RHEs produced in the dry condition were cultivated without culture plate covers which were replaced by gas-permeable films (Breathe-Easy; Diversified Biotech, Dedham, MA). When RHEs were maintained in the dry condition, the culture medium was renewed each day to avoid changes in osmolarity since a higher medium evaporation occurred. The medium was renewed every 2 days in the humid condition. RHEs were harvested 10 days after air-liquid interface exposure.
2.2. Transmission electron microscopy analysis
RHEs, produced with keratinocytes from 3 different donors, were processed as previously described [32] and observed with an HT7700 electron microscope (Hitachi, Tokyo, Japan). Quantifications were performed using 2 RHEs/condition/donor. The numbers of corneocyte layers and of granular and living keratinocyte layers were quantified on 3 independent areas for each epidermis (2500x and 300x magnification pictures, respectively). The density of keratohyalin granules (area of granules/total area of cytoplasm) and their mean size were measured for each epidermis on 6 pictures (2500x) of the stratum granulosum just behind the stratum corneum, using ImageJ software (National Institutes of Health, Bethesda, MD). Briefly, for all pictures, positive areas were picked using the “wand tool” with an 8-connected mode and a fixed tolerance.
2.3. Transepidermal water loss measurement
Transepidermal water loss (TEWL) of RHEs produced with keratinocytes from 3 different donors (3 RHEs/condition/donor) was measured using the tewameter TM300 (Courage & Khazaka, Cologne, Germany). For further information see Supplementary Methods.
2.4. Protein extractions
Different protein extracts were produced: (i) total proteins were extracted in Laemmli buffer 5X [175 mM Tris-HCl pH 6.8, 7.5% SDS, 25% glycerol, 12.5% β-mercaptoethanol, and 0.4% bromophenol blue] (2 pooled RHEs/condition/donor; 4 different donors), (ii) detergent-soluble proteins were extracted in the presence of Nonidet-P40 (NP-40) [40 mM Tris–HCl pH 7.5, 0.5% Nonidet-P40, 10 mM EDTA, 0.25 mM PMSF, 1/100 (v/v) mammalian protease inhibitor cocktail] to get the so-called TE-NP40 extracts (3 pooled RHEs/condition/donor; 3 donors), and (iii) deiminated filaggrin-enriched extracts (3 pooled RHEs/condition/donor; 3 donors) were prepared as previously described [33]. Briefly, TE-NP40 extracts were precipitated with absolute ethanol. After centrifugation, pellets were dried at 80°C for 20 min and resuspended in water. After homogenization, all extracts were cleared by centrifugation for 15 min at 15,000 rpm.
2.5. Quantification of UCA and PCA
At day 10, RHEs were homogenized in PBS-Triton X100 0.1%, and UCA and PCA were analyzed by HPLC on a C18 reverse-phase column (3 RHEs/condition/donor; 3 different donors). Their amount was expressed in μg/mg of proteins.
2.6. pH measurement
As recommended by the manufacturer’s instructions, 2 μL of ultra-pure water was topically applied to RHEs (3 RHEs/condition/donor; 3 different donors) and surface pH was measured using the HI-99181a skin pH meter (HANNA instruments, Woonsocket, RI).
2.7. In situ transglutaminase activity assay
In situ transglutaminase assay was performed as described previously [34, 35]. For further information see Supplementary Methods. For each RHE, the area of fluorescence was measured on 3 images with ImageJ software using a fixed threshold and mean ± standard deviation was calculated.
2.8. PAD inhibition
To inhibit PAD activity, Cl-amidine [36] was added into the culture medium to reach a concentration of 800 μM, at day 9 of the culture period. Each RHE specimen was harvested at day 10.
2.9. Statistical analysis
All data are presented as mean ± standard deviation. Statistical differences were determined with bilateral paired Student’s t-tests for paired samples. For unpaired samples, we used unpaired Student’s t-tests if the equality of sample variances was verified by the Fisher’s test with an α-risk of 0.05, and Welch’s t-tests if not. Differences were considered significant when the p-value (p) was less than 0.05.
3. Results
3.1. Low environmental humidity induces stratum corneum thickening and improves barrier function in RHEs
Primary keratinocytes from normal human skin were used to produce RHEs as described in details in the methods’ section. As a prerequisite, DNA was extracted from keratinocytes, and FLG gene was sequenced. No loss-of-function mutations were detected (data not shown). RHEs were produced in either humid (> 95% RH) or dry (30–50% RH) conditions for 10 days after air-liquid interface exposure. We first verified that the dry condition did not alter cell viability (Supplementary Fig. S1a). The morphological consequences of humidity lowering were then analyzed by hematoxylin-eosin staining and transmission electron microscopy. RHEs produced in the dry condition (dry RHEs) showed a thicker stratum corneum than RHEs cultivated in a humid environment (humid RHEs) (Fig. 1a and b), with a 1.8 fold increase in the number of corneocyte layers (Supplementary Fig. S2a). They presented a 1.5 fold thicker stratum granulosum while the thickness of total living keratinocyte layers was not modified. The density and size of keratohyalin granules were increased by factors of 1.6 and 1.8, respectively (Supplementary Fig. S2b and c).
Fig. 1. Thicker stratum corneum and reduced transepidermal water loss in dry RHEs.
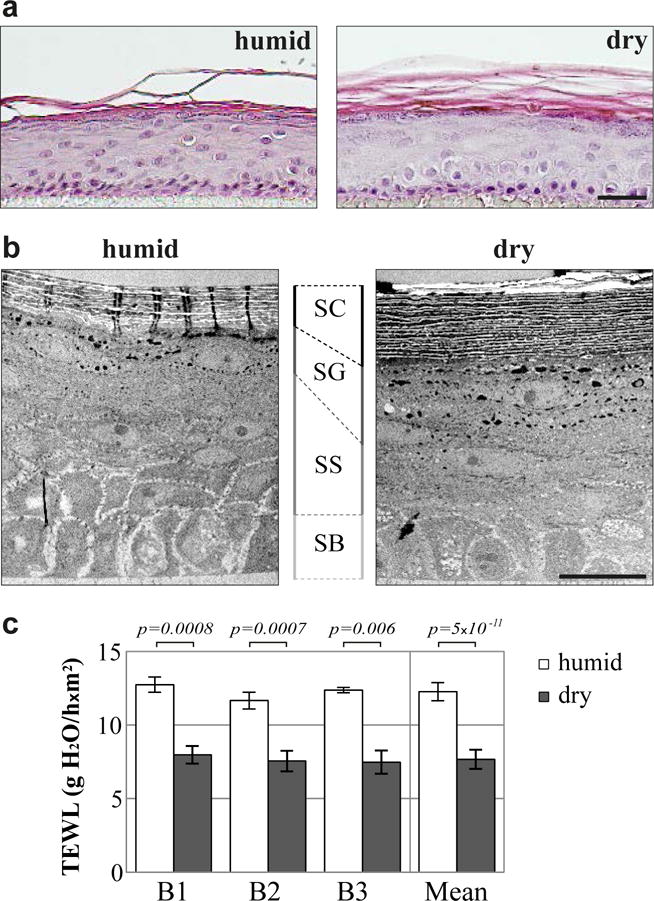
(a and b) Hematoxylin-eosin staining (a) and transmission electron micrographs (b) of humid and dry RHEs produced with keratinocytes from 3 different donors, only one being illustrated. (c) Transepidermal water loss (TEWL) measured for humid and dry RHEs produced with keratinocytes from 3 different donors (B1–B3). Scale bars = 20 μm. SC: stratum corneum, SG: stratum granulosum, SS: stratum spinosum, SB: stratum basale.
To investigate the effect of the dry environment on the stratum corneum permeability, two complementary tests were performed: a lucifer yellow assay to evaluate the outside-in permeability and the TEWL measurement to assess the inside-out barrier. No differences in the penetration of the fluorescent dye were observed between RHEs produced in humid and dry conditions, a negligible quantity being detected in the medium whatever the culture conditions (Supplementary Fig. S1b). However, dry RHEs showed a decreased TEWL (7.7 g H2O/hxm2 vs 12.3 for humid RHEs; Fig. 1c).
3.2. Low environmental humidity modulates keratinocyte differentiation in RHEs
To compare keratinocyte proliferation in RHEs, Ki67 labelling was performed. The number of Ki67 positive nuclei detected in the basal layer was similar in both culture conditions (Supplementary Fig. S1c). Moreover keratin 14 expression, analyzed by RT-qPCR (Supplementary Fig. S3a) and western blotting (Fig. 2a), was not modified. Thus, keratinocyte proliferation was not changed by environmental humidity variations.
Fig. 2. Modulation of the expression of differentiation markers in dry RHEs.
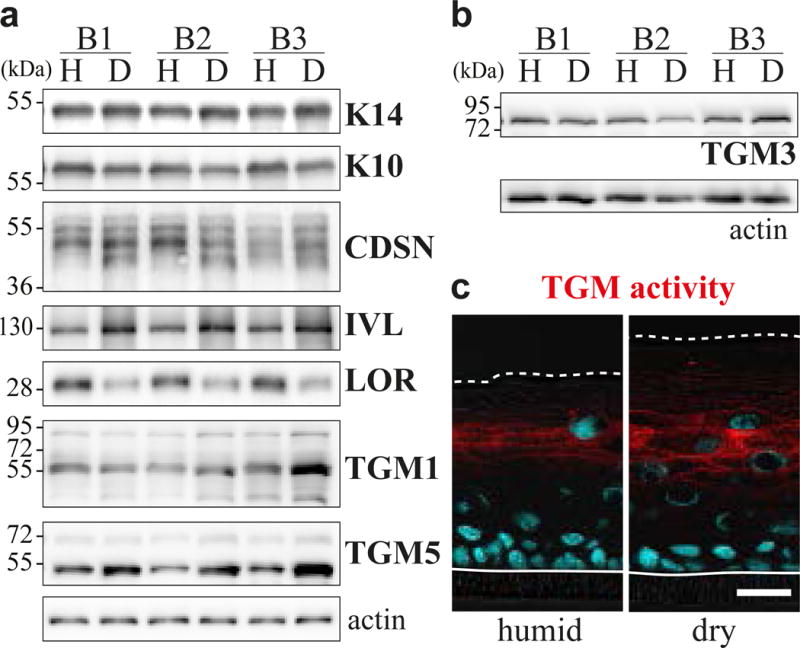
(a and b) Expression of actin (loading control), keratin14 (K14) and various differentiation markers analyzed by western blotting of either total (a) or TE-NP40 extracted (b) proteins of humid (H) and dry (D) RHEs produced with keratinocytes from 3 different donors (B1-B3). (K10, keratin 10; CDSN, corneodesmosin; IVL, involucrin; LOR, loricrin) (c) In situ transglutaminase (TGM) activity assay performed on cryosections of humid and dry RHEs produced with keratinocytes from 3 different donors, only one being illustrated. Scale bar = 20 μm.
The expression of various differentiation markers was then analyzed. At the mRNA level (Supplementary Fig. S3a), no significant differences were observed in the expression of keratin 10, loricrin or transglutaminases, except a ~2 fold increase of transglutaminase 5. A significant increase was also noted for corneodesmosin and involucrin. At the protein level (Fig. 2a and b), detections of keratin 10 and transglutaminases 1 and 3 were not modified, but changes were observed for the others. In dry RHEs, higher amounts of involucrin (1.60 ± 0.12 arbitrary units vs 1.00 in humid RHEs; n = 3; p = 0.013), of transglutaminase 5 active form at 53 kDa (2.16 ± 0.42; n = 4; p = 0.011) and of fragments (≤ 40 kDa) of corneodesmosin (1.67 ± 0.22; n = 4; p = 0.009) were detected, whereas the detection of loricrin was significantly decreased (0.45 ± 0.10; n = 3; p = 0.012). Transglutaminase activity was compared by an in situ enzymatic assay (Fig. 2c). The fluorescent signal resulting from the crosslink of Alexa-Fluor-555–cadaverine at the cell periphery in the stratum granulosum was of similar intensity whatever the condition but more cell layers were labelled in dry RHEs, the area of detection increasing 1.80 ± 0.53 fold (p = 0.001).
3.3. Low environmental humidity stimulates filaggrin breakdown and increases the production of UCA and PCA
To explore the impact of environmental dryness on filaggrin metabolism, its expression was studied by RT-qPCR, and by western blotting and indirect immunofluorescence performed with AHF3 monoclonal antibody directed to both profilaggrin and filaggrin monomers. At the mRNA level, FLG expression was increased in the dry condition (1.58 ± 0.38 fold; n = 6; p = 0.02) (Supplementary Fig. S3b). Profilaggrin immunodetection tended to increase in dry RHEs (1.43 ± 0.30 fold; n = 4; p = 0.06) whereas that of filaggrin monomers significantly decreased (0.64 ± 0.06 fold; n = 4; p = 0.006) (Fig. 3a). Furthermore, labelling patterns obtained by indirect immunofluorescence were different (Fig. 3b and Supplementary Fig. S4). In humid RHEs, a granular labelling of the granular keratinocytes – probably corresponding to profilaggrin stored in the keratohyalin granules – and a diffuse intense staining of the lower stratum corneum – probably corresponding to filaggrin monomers – were observed. In dry RHEs, the stratum granulosum staining clearly increased while the stratum corneum labelling decreased drastically.
Fig. 3. Increased filaggrin breakdown in dry RHEs.
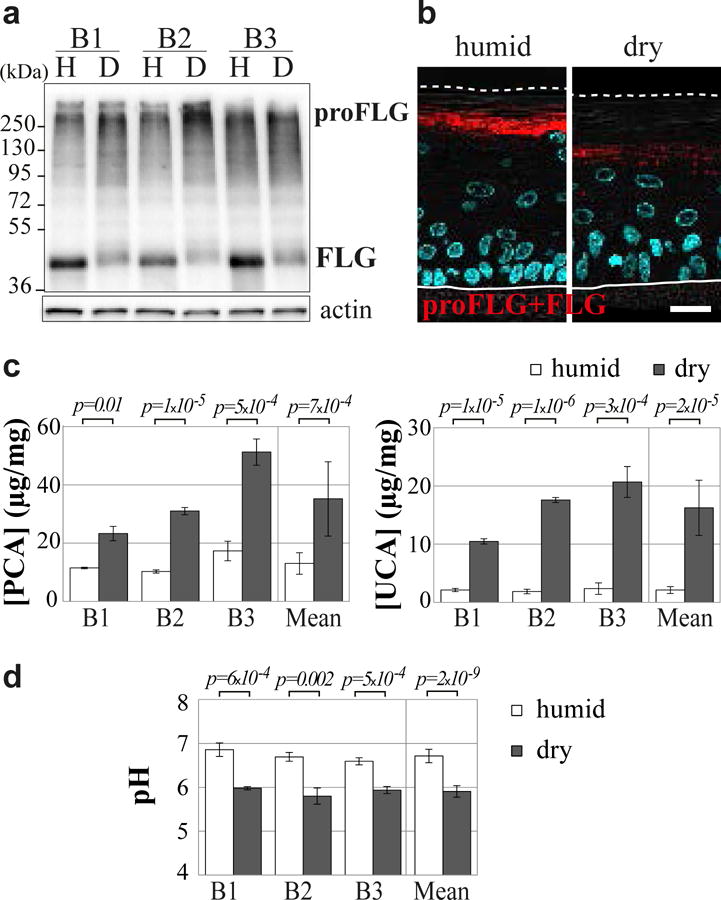
(a and b) Humid (H) and dry (D) RHEs were produced with keratinocytes from 3 different donors (B1-B3) and analyzed. Immunodetection of profilaggrin (proFLG) and filaggrin (FLG) in total protein extracts (a) and on fixed-tissue sections (b). Differential interference contrast images are superimposed; nuclei are stained in blue by DAPI. SC: stratum corneum, SG: stratum granulosum. Scale bar = 20 μm. (c) Amounts of pyrrolidone carboxylic acid (PCA) and urocanic acid (UCA) in RHE lysates are expressed in μg/mg of proteins. (d) Surface pH.
The decreased immunodetection of filaggrin monomers and the reduced immunolabelling of the stratum corneum in the dry condition could be due either to a lower affinity of AHF3 to the deiminated filaggrin, as previously observed [17], or to an enhanced degradation of filaggrin, or both. To test if filaggrin degradation was increased, PCA and UCA contents were analyzed (Fig. 3c). The concentrations of both amino acid derivatives were significantly enhanced in the dry condition (2.71 and 7.62 fold, respectively). Furthermore, the concentration of serine, a very abundant (25.3%) amino acid in filaggrin, was also shown to increase in the dry condition (36.23 ± 4.70 versus 10.29 ± 0.91μg/mg; 3.52 ± 0.55 fold; n = 9; p = 3.7×10−7). This showed that filaggrin breakdown was enhanced. In link with this observation, NMF being known to partly acidify the stratum corneum, the pH of RHE surface was measured. The dry condition induced a statistically significant lowering of pH from 6.7 to 5.9 (Fig. 3d).
3.4. In dry RHEs, peptidyl-arginine deiminase expression and activity were increased but those of proteases involved in filaggrin breakdown were not
In order to further understand the molecular mechanisms involved in the increase of filaggrin breakdown under dry vs humid conditions, the expression of three proteases known to target filaggrin monomers was compared. RT-qPCR revealed no differences in their mRNA amounts (Supplementary Fig. S3c). Western blotting showed an unexpected decreased expression of bleomycin hydrolase in dry RHEs (0.50 ± 0.07 versus 1.00) whereas calpain 1 and caspase 14 (both the zymogen (~28 kDa) and active (~17 kDa) forms) expression was similar (Fig. 4a). An in vitro assay performed on RHE extracts showed that calpain activity was not different between dry and humid conditions (Fig. 4b). We also assessed the expression of other proteases proposed to be implicated in the control of filaggrin proteolysis, namely kallikrein 5, skin aspartic protease, matriptase and elastase 2 [37–40]. At the mRNA level, no significant variation of their expression was measured. However, the amounts of skin aspartic protease, matriptase and elastase 2 detected by western blotting were divided by two in dry RHEs whereas that of kallikrein 5 was not modified (Supplementary Fig. S5). Finally, when analyzing the expression of histidase (responsible for UCA production), we detected three different variants of the enzyme but no differences in their expression (Supplementary Fig. S6).
Fig. 4. Increased filaggrin deimination and peptidyl-arginine deiminase expression in the dry condition.
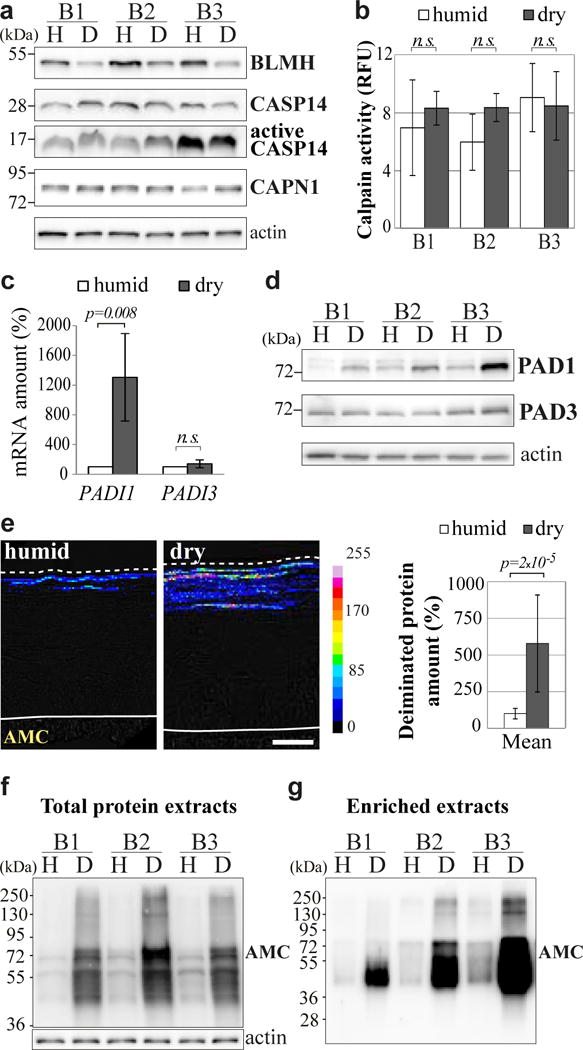
(a) Expression of bleomycin hydrolase (BLMH), procaspase-14 (CASP14), active caspase-14 and calpain-1 (CAPN1) analyzed by western blotting of total proteins of humid (H) and dry (D) RHEs produced with keratinocytes from 3 different donors (B1-B3). (b) Calpain activity measured in lysates of humid and dry RHEs. Results are expressed as relative fluorescence unit (RFU). n.s. = non-significant. (c) Expression of PADI genes analyzed by RT-qPCR (4 different donors). (d) Expression of actin, PAD1 and PAD3 analyzed by western blotting of TE-NP40 extract of the RHEs. (e) Immunodetection of deiminated proteins with AMC antibody on sections of RHEs produced with keratinocytes from 3 different donors. A representative result is shown on the left. Quantification of the signal intensities is shown on the right. Scale bar = 20 μm. (f and g) Equal amounts of total proteins (f) and equal volumes of deiminated filaggrin-enriched extracts (g) were immunodetected with AMC. Note that deiminated filaggrin migrates in the form of a smear between 40 to 72 kDa (fully deiminated), as previously described [43].
Since no protease overexpression seemed to explain the increased filaggrin degradation in the dry condition, we looked for the expression and activity of PADs, and for filaggrin deimination. PADI4 and PADI6 transcripts were not detected in RHEs by RT-qPCR, as was to be expected since they are not expressed in human epidermis. Unlike human epidermis, RHEs showed no PADI2 expression under the experimental conditions used [41–43]. PADI1 mRNA level was strongly increased (13.06 fold) in dry RHEs whereas PADI3 level was not changed (Fig. 4c). The increased expression of PAD1 was also significant at the protein level (3.89 ± 1.38 fold; n = 4; p = 0.025; Fig. 4d). The protein deimination rate was analyzed using the anti-modified citrulline antibody (AMC), as previously described [43, 44], and was shown to be increased in dry RHEs, both by indirect immunofluorescence (5.46 fold; Fig. 4e) and by western blotting (7.68 ± 3.95 fold; n = 4; p = 0.043; Fig. 4f). To study the deimination of filaggrin more specifically, extracts enriched in the neutral to acidic low-salt soluble forms of the protein (essentially the deiminated forms), were prepared from dry and humid RHEs, as previously described for human epidermis [33]. Protein staining confirmed the high degree of filaggrin purity (data not shown). Western blotting with AMC antibody of an equal volume of each extract indicated that filaggrin deimination was increased by a factor of 13.4 ± 4.9 in the dry condition (n = 3; p = 0.048; Fig. 4g).
To sum up, in the dry condition filaggrin deimination and degradation were enhanced, PAD1 expression and PAD activity were increased whereas the proteases known to target filaggrin were not. Altogether, these results suggest that filaggrin deimination rate may control its terminal breakdown.
3.5. Deimination is involved in the regulation of filaggrin proteolysis
To test the above hypothesis and try to reverse the effect of dryness on filaggrin deimination and degradation, dry RHEs were treated with Cl-amidine, a specific pan PAD inhibitor [36,45], for 24 hours before their harvest at day 10. Cl-amidine treatment induced a slight decrease of global deimination assessed by western blotting of total proteins with AMC (data not shown). This is not surprising because stable deiminated proteins such as keratins had probably accumulated in the stratum corneum for several days, deimination being an irreversible post-translational modification. However, the effect on filaggrin was evident as shown by western blotting of the deiminated filaggrin-enriched extracts (0.07 ± 0.04 vs 1.00 arbitrary units; n = 3; p = 0.0006; Fig. 5a). In turn, the amount of filaggrin monomers in the total protein extracts tended to increase (1.25 ± 0.44 fold; n = 3; p = 0.4) and to return to the level observed in humid RHEs (Fig. 5b), while the amount of filaggrin monomers in TE-NP40 extracts (corresponding to low ionic strength-soluble forms) was down-regulated from 100 to 20 ± 7% (n = 3; p = 0.0006; Fig. 5c). This shows that partial inhibition of PAD activity induced accumulation of filaggrin monomers and reduced their solubility. However, Cl-amidine treatment did not change the expression of caspase 14, calpain 1 and bleomycin hydrolase, as tested by western blotting (data not shown).
Fig. 5. Partial inhibition of deimination by Cl-amidine reverses the effect of dryness on filaggrin metabolism.
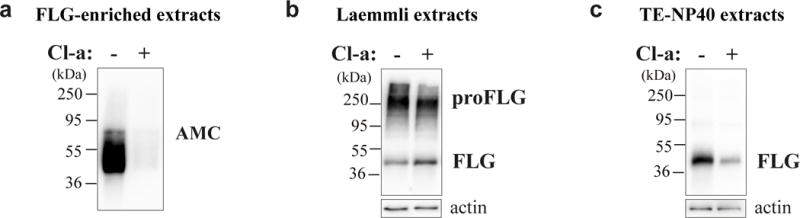
(a–c) Dry RHEs produced with keratinocytes from 3 different donors were treated for 24 hours (+) with Cl-amidine (Cl-a) or were untreated (−), the results being illustrated for only one donor. (a) Equal volumes of deiminated enriched-filaggrin extracts were immunodetected with AMC antibody to detect the deiminated protein. (b) Total protein extracts were immunodetected with AHF3 antibody to detect both profilaggrin (proFLG) and filaggrin (FLG). (c) TE-NP40 extracts were immunoblotted with AHF3 to detect the low ionic strength-soluble form of FLG.
4. Discussion
We showed that lowering the RH in the incubator to 30–50% during the production of RHEs profoundly altered the properties of the epidermis at the structural, functional and biochemical levels (Fig. 6). In particular, the stratum corneum of RHEs at low humidity level was thicker, with an increased number of corneocyte layers, and its TEWL decreased. In parallel, the amount of PCA, a highly hygroscopic NMF component, was higher. This suggests that the epidermis adapted to its environment so as to retain water in the cornified layer. This is in agreement with Sun et al.‘s recent report of normalization of lipid lamellar bilayer formation in the stratum corneum of RHEs grown at 50% RH, associated with an increased expression of glucosylceramide synthase [46]. Hyperkeratosis, decreased TEWL and increased secretion of lamellar bodies have also been reported in hairless mice maintained in a very dry environment (RH < 10%) as compared to humid one (> 80%) [47]. In addition, the detected amounts of loricrin and involucrin, two main components of cornified envelopes, were inversely modified, whereas their encoding mRNA levels did not change. We also observed an increased number of cells with transglutaminase activity, and overexpression of transglutaminase 5, one of the enzymes necessary for envelope formation. This suggests that the difference of loricrin and involucrin solubility was due to differences in their cross-linking rather than their synthesis. Therefore the composition of the envelopes may vary. In agreement, Sun et al. reported modification in the thickness of these structures in RHEs grown at 50% RH [46].
Fig. 6. Schematic representation of the effect of the dry condition on RHEs and filaggrin metabolism.
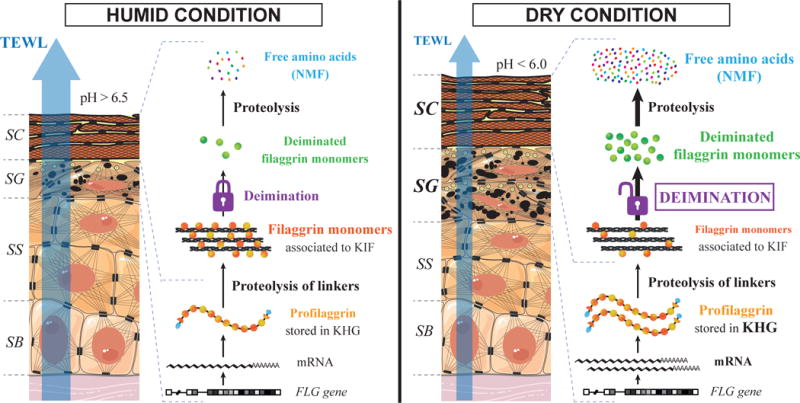
For details, see the text. SC: stratum corneum, SG: stratum granulosum, SS: stratum spinosum, SB: stratum basale, FLG: filaggrin, KHG: keratohyalin granules, KIF: keratin intermediate filaments, NMF: natural moisturizing factor, TEWL: transepidermal water loss.
We demonstrated that the multi-step proteolysis of human filaggrin was increased in the low humidity condition, which was likely to produce more hygroscopic molecules. This indicates that filaggrin degradation is dependent on the environmental water content. Similar observations have been made in rodents. For example, during late foetal development of rats, filaggrin accumulates through the entire thickness of the stratum corneum and, immediately after birth, normal filaggrin proteolysis occurs in the outer stratum corneum. If the newborn rats are maintained in 100% humidity, mimicking the in utero milieu, the activation of filaggrin degradation is blocked. Similarly, application of occlusive patches to adult rats to maintain 100% humidity at the skin surface prevents the normal proteolysis of filaggrin [29].
In our conditions, deimination appeared as a major regulator of filaggrin breakdown (Fig. 6). Expression and activity of proteases known to be involved in the process was not found to be modified whereas the expression of PAD1, known to act on filaggrin monomers, and PAD activity were highly enhanced in dry RHEs. In agreement, the deimination of filaggrin is up-regulated two hours after birth of mice [48], when filaggrin degradation would probably occur. An increase of deimination thus seems to be sufficient to drive filaggrin degradation. This is consistent with the fact that deimination of rat filaggrin has previously been shown to induce its detachment from keratin filaments [49], and that human filaggrin is more efficiently proteolyzed in vitro by calpain 1 when it is deiminated [20, 50]. Moreover, partial inhibition of PADs for 24 hours with the PAD inhibitor Cl-amidine (this work) tends to reverse the effect of culture humidity lowering on filaggrin metabolism (association with intermediate filaments and degradation), suggesting that deimination is necessary for filaggrin degradation to occur.
In line with these observations, it has been shown that genes encoding filaggrin, PAD1 and PAD3 are not present in the genome of fishes and amphibian [51, 52], animals living in extremely humid environment.
We previously described deimination as a multilock controlled process [43]. Here we show that, in normal keratinocytes, environmental dryness unlocks this process. The mechanisms involved remain to be deciphered, but the data reported by Sun et al. give a clue. Calcium concentration is probably involved as dry RHEs expressed larger amounts of osmolyte transporters and displayed a higher calcium concentration and a marked calcium increase at the granular layer level, with a calcium gradient similar to native epidermis [46]. On the other hand, PADs are known to be calcium-dependent, and in native epidermis the peak of calcium at the granular/lower cornified layers is suspected to activate the enzymes (deiminated proteins are only detected in the stratum corneum).
Do our data have any other relevance to native healthy human epidermis? In extremely preterm neonates, the lack of an effective skin barrier leads to high TEWL with a high risk of dehydration. A progressive decrease of ambient humidity (from 75% to 50% RH) after the first post-natal week has been shown to accelerate barrier development in the premature infants [53]. Workers exposed to low RH have a lower TEWL (reviewed in [54]). In both cases, an adaptive mechanism similar to the one described in this study is probably involved. Whether filaggrin degradation is affected is not known but can be suspected.
Do our data have any relevance for the understanding of AD? Climatic variations impact the disease, as recently discussed [54], but the mechanisms involved are incompletely understood. For example, a negative correlation between indoor RH and disease severity has been reported [55]; children with AD and FLG mutations have a higher severity score and their lesions are more often located in air-exposed skin areas [56]; FLG mutations are a weaker risk factor for AD in subtropical climates [57], and children with AD experienced a reduction in the disease severity after one month spent in such a humid climate [58]. In a large-scale study of the influences of US climate on childhood eczema prevalence, a lower prevalence has been found in areas with higher outdoor RH [59]. All these observations are consistent with our results showing that more filaggrin and more filaggrin proteolysis is required in a dryer environment. In fact, we can suspect that the epidermis of AD patients, when the patients change from a humid to a dryer environment, cannot adapt because it is unable to increase filaggrin synthesis/degradation, whatever the reason (FLG loss-of-function mutations, effects of Th2-type inflammatory cytokines, others). Therefore the epidermal barrier is more altered, and the disease worsens. On the opposite, when the patients move from a dry to a more humid environment, their epidermis needs less filaggrin to be effective; therefore their symptoms regress. Altogether, these data convince us that eczema predisposed children should be encouraged to increase their indoor humidity. Our data also suggest that PAD expression/activity could be of importance in AD, although no genetic variations in PADI genes have been associated with the disease susceptibility until now. PADs could therefore constitute therapeutic skin targets.
Supplementary Material
Acknowledgments
We are indebted to Dr Pascal Descargues (Genoskin, Toulouse, France) for the human skin samples. We thank Carole Pons for her excellent technical assistance, Dr Marie Reynier for her helpful contribution to qPCR primer design (UDEAR), and Dr Gabrielle Le Provost for her advice on the Lucifer Yellow protocol. We acknowledge Pr. Yves Poumay and all members of his team (University of Namur, Belgium) for warmly hosting Laura Cau in his lab during one week and for their useful advice on RHE production. We thank all members of the electron microscopy facility of Toulouse University, Florence Capilla of the histopathology facility, Sophie Allart, Astrid Canivet and Danièle Daviaud from the cellular imaging facility (CPTP, INSERM U1043, Toulouse Rio Imagerie, Toulouse), and Dr Alain Moga (Synelvia, Toulouse, France) for the NMF dosage. This work was supported by grants from CNRS, Toulouse University, INSERM and the French Society for Dermatology (SFD). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. Laura Cau was supported by the French Ministry of Research and Technology, and gratefully acknowledges the SILAB–Jean PAUFIQUE Corporate Foundation for its support.
Abbreviations
- AD
atopic dermatitis
- NMF
natural moisturizing factor
- PAD
peptidyl-arginine deiminase
- PCA
pyrrolidone-5-carboxylic acid
- RH
relative humidity
- RHE
reconstructed human epidermis
- TEWL
transepidermal water loss
- UCA
urocanic acid
References
- 1.Bieber T. Atopic dermatitis 2.0: from the clinical phenotype to the molecular taxonomy and stratified medicine. Allergy. 2012;67:1475–1482. doi: 10.1111/all.12049. [DOI] [PubMed] [Google Scholar]
- 2.Kezic S, Novak N, Jakasa I, Jungersted JM, Simon M, Brandner JM, Middelkamp-Hup MA, Weidinger S. Skin barrier in atopic dermatitis. Front Biosci Landmark Ed. 2014;19:542–556. doi: 10.2741/4225. [DOI] [PubMed] [Google Scholar]
- 3.Al-Shobaili HA, Ahmed AA, Alnomair N, Alobead ZA, Rasheed Z. Molecular Genetic of Atopic dermatitis: An Update. Int J Health Sci. 2016;10 [PMC free article] [PubMed] [Google Scholar]
- 4.Palmer CNA, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, Goudie DR, Sandilands A, Campbell LE, Smith FJD, O’Regan GM, Watson RM, Cecil JE, Bale SJ, Compton JG, DiGiovanna JJ, Fleckman P, Lewis-Jones S, Arseculeratne G, Sergeant A, Munro CS, El Houate B, McElreavey K, Halkjaer LB, Bisgaard H, Mukhopadhyay S, McLean WHI. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38:441–446. doi: 10.1038/ng1767. [DOI] [PubMed] [Google Scholar]
- 5.Baurecht H, Irvine AD, Novak N, Illig T, Bühler B, Ring J, Wagenpfeil S, Weidinger S. Toward a major risk factor for atopic eczema: Meta-analysis of filaggrin polymorphism data. J Allergy Clin Immunol. 2007;120:1406–1412. doi: 10.1016/j.jaci.2007.08.067. [DOI] [PubMed] [Google Scholar]
- 6.Rodríguez E, Baurecht H, Herberich E, Wagenpfeil S, Brown SJ, Cordell HJ, Irvine AD, Weidinger S. Meta-analysis of filaggrin polymorphisms in eczema and asthma: Robust risk factors in atopic disease. J Allergy Clin Immunol. 2009;123:1361–1370.e7. doi: 10.1016/j.jaci.2009.03.036. [DOI] [PubMed] [Google Scholar]
- 7.Kezic S, Kemperman PMJH, Koster ES, de Jongh CM, Thio HB, Campbell LE, Irvine AD, McLean IWH, Puppels GJ, Caspers PJ. Loss-of-Function Mutations in the Filaggrin Gene Lead to Reduced Level of Natural Moisturizing Factor in the Stratum Corneum. J Invest Dermatol. 2008;128:2117–2119. doi: 10.1038/jid.2008.29. [DOI] [PubMed] [Google Scholar]
- 8.Jungersted JM, Scheer H, Mempel M, Baurecht H, Cifuentes L, Høgh JK, Hellgren LI, Jemec GBE, Agner T, Weidinger S. Stratum corneum lipids, skin barrier function and filaggrin mutations in patients with atopic eczema. Allergy. 2010;65:911–918. doi: 10.1111/j.1398-9995.2010.02326.x. [DOI] [PubMed] [Google Scholar]
- 9.Thyssen JP, Kezic S. Causes of epidermal filaggrin reduction and their role in the pathogenesis of atopic dermatitis. J Allergy Clin Immunol. 2014;134:792–799. doi: 10.1016/j.jaci.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 10.Mlitz V, Latreille J, Gardinier S, Jdid R, Drouault Y, Hufnagl P, Eckhart L, Guinot C, Tschachler E. Impact of filaggrin mutations on Raman spectra and biophysical properties of the stratum corneum in mild to moderate atopic dermatitis. J Eur Acad Dermatol Venereol. 2011;26:983–990. doi: 10.1111/j.1468-3083.2011.04198.x. [DOI] [PubMed] [Google Scholar]
- 11.Howell MD, Kim BE, Gao P, Grant AV, Boguniewicz M, DeBenedetto A, Schneider L, Beck LA, Barnes KC, Leung DYM. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol. 2009;124:R7–R12. doi: 10.1016/j.jaci.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Kezic S, O’Regan GM, Yau N, Sandilands A, Chen H, Campbell LE, Kroboth K, Watson R, Rowland M, Irwin McLean WH, Irvine AD. Levels of filaggrin degradation products are influenced by both filaggrin genotype and atopic dermatitis severity. Allergy. 2011;66:934–940. doi: 10.1111/j.1398-9995.2010.02540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pellerin L, Henry J, Hsu C-Y, Balica S, Jean-Decoster C, Méchin M-C, Hansmann B, Rodriguez E, Weindinger S, Schmitt A-M, Serre G, Paul C, Simon M. Defects of filaggrin-like proteins in both lesional and nonlesional atopic skin. J Allergy Clin Immunol. 2013;131:1094–1102. doi: 10.1016/j.jaci.2012.12.1566. [DOI] [PubMed] [Google Scholar]
- 14.Steinert PM, Cantieri JS, Teller DC, Lonsdale-Eccles JD, Dale BA. Characterization of a class of cationic proteins that specifically interact with intermediate filaments. Proc Natl Acad Sci U S A. 1981;78:4097–4101. doi: 10.1073/pnas.78.7.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simon M, Haftek M, Sebbag M, Montézin M, Girbal-Neuhauser E, Schmitt D, Serre G. Evidence that filaggrin is a component of cornified cell envelopes in human plantar epidermis. Biochem J. 1996;317:173–177. doi: 10.1042/bj3170173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Méchin M-C, Sebbag M, Arnaud J, Nachat R, Foulquier C, Adoue V, Coudane F, Duplan H, Schmitt A-M, Chavanas S, Guerrin M, Serre G, Simon M. Update on peptidylarginine deiminases and deimination in skin physiology and severe human diseases. Int J Cosmet Sci. 2007;29:147–168. doi: 10.1111/j.1467-2494.2007.00377.x. [DOI] [PubMed] [Google Scholar]
- 17.Méchin M-C, Enji M, Nachat R, Chavanas S, Charveron M, Ishida-Yamamoto A, Serre G, Takahara H, Simon M. The peptidylarginine deiminases expressed in human epidermis differ in their substrate specificities and subcellular locations. Cell Mol Life Sci. 2005;62:1984–1995. doi: 10.1007/s00018-005-5196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nachat R, Méchin M-C, Takahara H, Chavanas S, Charveron M, Serre G, Simon M. Peptidylarginine Deiminase Isoforms 1–3 Are Expressed in the Epidermis and Involved in the Deimination of K1 and Filaggrin. J Invest Dermatol. 2005;124:384–393. doi: 10.1111/j.0022-202X.2004.23568.x. [DOI] [PubMed] [Google Scholar]
- 19.Resing KA, al-Alawi N, Blomquist C, Fleckman P, Dale BA. Independent regulation of two cytoplasmic processing stages of the intermediate filament-associated protein filaggrin and role of Ca2+ in the second stage. J Biol Chem. 1993;268:25139–25145. [PubMed] [Google Scholar]
- 20.Kamata Y, Taniguchi A, Yamamoto M, Nomura J, Ishihara K, Takahara H, Hibino T, Takeda A. Neutral Cysteine Protease Bleomycin Hydrolase Is Essential for the Breakdown of Deiminated Filaggrin into Amino Acids. J Biol Chem. 2009;284:12829–12836. doi: 10.1074/jbc.M807908200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eckhart L, Tschachler E. Cuts by Caspase-14 Control the Proteolysis of Filaggrin. J Invest Dermatol. 2011;131:2173–2175. doi: 10.1038/jid.2011.282. [DOI] [PubMed] [Google Scholar]
- 22.Hoste E, Kemperman P, Devos M, Denecker G, Kezic S, Yau N, Gilbert B, Lippens S, De Groote P, Roelandt R, Van Damme P, Gevaert K, Presland RB, Takahara H, Puppels G, Caspers P, Vandenabeele P, Declercq W. Caspase-14 Is Required for Filaggrin Degradation to Natural Moisturizing Factors in the Skin. J Invest Dermatol. 2011;131:2233–2241. doi: 10.1038/jid.2011.153. [DOI] [PubMed] [Google Scholar]
- 23.Rawlings AV, Harding CR. Moisturization and skin barrier function. Dermatol Ther. 2004;17:43–48. doi: 10.1111/j.1396-0296.2004.04S1005.x. [DOI] [PubMed] [Google Scholar]
- 24.Barresi C, Stremnitzer C, Mlitz V, Kezic S, Kammeyer A, Ghannadan M, Posa-Markaryan K, Selden C, Tschachler E, Eckhart L. Increased Sensitivity of Histidinemic Mice to UVB Radiation Suggests a Crucial Role of Endogenous Urocanic Acid in Photoprotection. J Invest Dermatol. 2011;131:188–194. doi: 10.1038/jid.2010.231. [DOI] [PubMed] [Google Scholar]
- 25.Gibbs NK, Norval M. Urocanic Acid in the Skin: A Mixed Blessing? J Invest Dermatol. 2011;131:14–17. doi: 10.1038/jid.2010.276. [DOI] [PubMed] [Google Scholar]
- 26.Le Lamer M, Pellerin L, Reynier M, Cau L, Pendaries V, Leprince C, Méchin M-C, Serre G, Paul C, Simon M. Defects of corneocyte structural proteins and epidermal barrier in atopic dermatitis. Biol Chem. 2015;396:1163–1179. doi: 10.1515/hsz-2015-0141. [DOI] [PubMed] [Google Scholar]
- 27.Deraison C, Bonnart C, Lopez F, Besson C, Robinson R, Jayakumar A, Wagberg F, Brattsand M, Hachem JP, Leonardsson G, Hovnanian A. LEKTI Fragments Specifically Inhibit KLK5, KLK7, and KLK14 and Control Desquamation through a pH-dependent Interaction. Mol Biol Cell. 2007;18:3607–3619. doi: 10.1091/mbc.E07-02-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miajlovic H, Fallon PG, Irvine AD, Foster TJ. Effect of filaggrin breakdown products on growth of and protein expression by Staphylococcus aureus. J Allergy Clin Immunol. 2010;126:1184–1190.e3. doi: 10.1016/j.jaci.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scott IR, Harding CR. Filaggrin breakdown to water binding compounds during development of the rat stratum corneum is controlled by the water activity of the environment. Dev Biol. 1986;115:84–92. doi: 10.1016/0012-1606(86)90230-7. [DOI] [PubMed] [Google Scholar]
- 30.Frankart A, Malaisse J, De Vuyst E, Minner F, de Rouvroit CL, Poumay Y. Epidermal morphogenesis during progressive in vitro 3D reconstruction at the air-liquid interface. Exp Dermatol. 2012;21:871–875. doi: 10.1111/exd.12020. [DOI] [PubMed] [Google Scholar]
- 31.Pendaries V, Malaisse J, Pellerin L, Le Lamer M, Nachat R, Kezic S, Schmitt AM, Paul C, Poumay Y, Serre G, Simon M. Knockdown of Filaggrin in a Three-Dimensional Reconstructed Human Epidermis Impairs Keratinocyte Differentiation. J Invest Dermatol. 2014;134:2938–2946. doi: 10.1038/jid.2014.259. [DOI] [PubMed] [Google Scholar]
- 32.Reynier M, Allart S, Gaspard E, Moga A, Goudounèche D, Serre G, Simon M, Leprince C. Rab11a Is Essential for Lamellar Body Biogenesis in the Human Epidermis. J Invest Dermatol. 2016;136:1199–1209. doi: 10.1016/j.jid.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 33.Simon M, Girbal E, Sebbag M, Gomès-Daudrix V, Vincent C, Salama G, Serre G. The cytokeratin filament-aggregating protein filaggrin is the target of the so-called “antikeratin antibodies,” autoantibodies specific for rheumatoid arthritis. J Clin Invest. 1993;92:1387–1393. doi: 10.1172/JCI116713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leclerc EA, Huchenq A, Kezic S, Serre G, Jonca N. Mice deficient for the epidermal dermokine β and γ isoforms display transient cornification defects. J Cell Sci. 2014;127:2862–2872. doi: 10.1242/jcs.144808. [DOI] [PubMed] [Google Scholar]
- 35.Raghunath M, Hennies HC, Velten F, Wiebe V, Steinert PM, Reis A, Traupe H. A novel in situ method for the detection of deficient transglutaminase activity in the skin. Arch Dermatol Res. 1998;290:621–627. doi: 10.1007/s004030050362. [DOI] [PubMed] [Google Scholar]
- 36.Luo Y, Arita K, Bhatia M, Knuckley B, Lee YH, Stallcup MR, Sato M, Thompson PR. Inhibitors and Inactivators of Protein Arginine Deiminase 4: Functional and Structural Characterization. Biochemistry (Mosc) 2006;45:11727–11736. doi: 10.1021/bi061180d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.List K, Szabo R, Wertz PW, Segre J, Haudenschild CC, Kim SY, Bugge TH. Loss of proteolytically processed filaggrin caused by epidermal deletion of Matriptase/MT-SP1. J Cell Biol. 2003;163:901–910. doi: 10.1083/jcb.200304161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsui T, Miyamoto K, Kubo A, Kawasaki H, Ebihara T, Hata K, Tanahashi S, Ichinose S, Imoto I, Inazawa J, Kudoh J, Amagai M. SASPase regulates stratum corneum hydration through profilaggrin-to-filaggrin processing. EMBO Mol Med. 2011;3:320–333. doi: 10.1002/emmm.201100140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakabe J, Yamamoto M, Hirakawa S, Motoyama A, Ohta I, Tatsuno K, Ito T, Kabashima K, Hibino T, Tokura Y. Kallikrein-related Peptidase 5 Functions in Proteolytic Processing of Profilaggrin in Cultured Human Keratinocytes. J Biol Chem. 2013;288:17179–17189. doi: 10.1074/jbc.M113.476820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonnart C, Deraison C, Lacroix M, Uchida Y, Besson C, Robin A, Briot A, Gonthier M, Lamant L, Dubus P, Monsarrat B, Hovnanian A. Elastase 2 is expressed in human and mouse epidermis and impairs skin barrier function in Netherton syndrome through filaggrin and lipid misprocessing. J Clin Invest. 2010;120:871–882. doi: 10.1172/JCI41440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ishigami A, Ohsawa T, Asaga H, Akiyama K, Kuramoto M, Maruyama N. Human peptidylarginine deiminase type II: molecular cloning, gene organization, and expression in human skin. Arch Biochem Biophys. 2002;407:25–31. doi: 10.1016/S0003-9861(02)00516-7. [DOI] [PubMed] [Google Scholar]
- 42.Chavanas S, Méchin M-C, Takahara H, Kawada A, Nachat R, Serre G, Simon M. Comparative analysis of the mouse and human peptidylarginine deiminase gene clusters reveals highly conserved non-coding segments and a new human gene, PADI6. Gene. 2004;330:19–27. doi: 10.1016/j.gene.2003.12.038. [DOI] [PubMed] [Google Scholar]
- 43.Méchin M-C, Coudane F, Adoue V, Arnaud J, Duplan H, Charveron M, Schmitt A-M, Takahara H, Serre G, Simon M. Deimination is regulated at multiple levels including auto-deimination of peptidylarginine deiminases. Cell Mol Life Sci. 2010;67:1491–1503. doi: 10.1007/s00018-010-0262-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Senshu T, Akiyama K, Kan S, Asaga H, Ishigami A, Manabe M. Detection of Deiminated Proteins in Rat Skin: Probing with a Monospecific Antibody After Modification of Citrulline Residues. J Invest Dermatol. 1995;105:163–169. doi: 10.1111/1523-1747.ep12317070. [DOI] [PubMed] [Google Scholar]
- 45.Knuckley B, Causey CP, Jones JE, Bhatia M, Dreyton CJ, Osborne TC, Takahara H, Thompson PR. Substrate Specificity and Kinetic Studies of PADs 1, 3, and 4 Identify Potent and Selective Inhibitors of Protein Arginine Deiminase 3. Biochemistry (Mosc) 2010;49:4852–4863. doi: 10.1021/bi100363t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun R, Celli A, Crumrine D, Hupe M, Adame LC, Pennypacker SD, Park K, Uchida Y, Feingold KR, Elias PM, Ilic D, Mauro TM. Lowered Humidity Produces Human Epidermal Equivalents with Enhanced Barrier Properties. Tissue Eng Part C Methods. 2015;21:15–22. doi: 10.1089/ten.tec.2014.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Denda M, Sato J, Masuda Y, Tsuchiya T, Koyama J, Kuramoto M, Elias PM, Feingold KR. Exposure to a Dry Environment Enhances Epidermal Permeability Barrier Function. J Invest Dermatol. 1998;111:858–863. doi: 10.1046/j.1523-1747.1998.00333.x. [DOI] [PubMed] [Google Scholar]
- 48.Akiyama K, Senshu T. Dynamic aspects of protein deimination in developing mouse epidermis. Exp Dermatol. 1999;8:177–186. doi: 10.1111/j.1600-0625.1999.tb00368.x. [DOI] [PubMed] [Google Scholar]
- 49.Harding CR, Scott IR. Histidine-rich proteins (filaggrins): structural and functional heterogeneity during epidermal differentiation. J Mol Biol. 1983;170:651–673. doi: 10.1016/s0022-2836(83)80126-0. [DOI] [PubMed] [Google Scholar]
- 50.Hsu C-Y, Henry J, Raymond A-A, Méchin M-C, Pendaries V, Nassar D, Hansmann B, Balica S, Burlet-Schiltz O, Schmitt A-M, Takahara H, Paul C, Serre G, Simon M. Deimination of Human Filaggrin-2 Promotes Its Proteolysis by Calpain 1. J Biol Chem. 2011;286:23222–23233. doi: 10.1074/jbc.M110.197400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ying S, Dong S, Kawada A, Kojima T, Chavanas S, Méchin M-C, Adoue V, Serre G, Simon M, Takahara H. Transcriptional regulation of peptidylarginine deiminase expression in human keratinocytes. J Dermatol Sci. 2009;53:2–9. doi: 10.1016/j.jdermsci.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 52.Strasser B, Mlitz V, Fischer H, Tschachler E, Eckhart L. Comparative genomics reveals conservation of filaggrin and loss of caspase-14 in dolphins. Exp Dermatol. 2015;24:365–369. doi: 10.1111/exd.12681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ågren J, Sjörs G, Sedin G. Ambient humidity influences the rate of skin barrier maturation in extremely preterm infants. J Pediatr. 2006;148:613–617. doi: 10.1016/j.jpeds.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 54.Engebretsen KA, Johansen JD, Kezic S, Linneberg A, Thyssen JP. The effect of environmental humidity and temperature on skin barrier function and dermatitis. J Eur Acad Dermatol Venereol. 2016;30:223–249. doi: 10.1111/jdv.13301. [DOI] [PubMed] [Google Scholar]
- 55.Weiland S, Husing A, Strachan D, Rzehak P, Pearce N. Climate and the prevalence of symptoms of asthma, allergic rhinitis, and atopic eczema in children. Occup Environ Med. 2004;61:609–615. doi: 10.1136/oem.2002.006809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carson CG, Rasmussen MA, Thyssen JP, Menné T, Bisgaard H. Clinical Presentation of Atopic Dermatitis by Filaggrin Gene Mutation Status during the First 7 Years of Life in a Prospective Cohort Study. Plos One. 2012;7:e48678. doi: 10.1371/journal.pone.0048678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sasaki T, Furusyo N, Shiohama A, Takeuchi S, Nakahara T, Uchi H, Hirota T, Tamari M, Shimizu N, Ebihara T, Amagai M, Furue M, Hayashi J, Kudoh J. Filaggrin loss-of-function mutations are not a predisposing factor for atopic dermatitis in an Ishigaki Island under subtropical climate. J Dermatol Sci. 2014;76:10–15. doi: 10.1016/j.jdermsci.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 58.Byremo G, Rød G, Carlsen KH. Effect of climatic change in children with atopic eczema. Allergy. 2006;61:1403–1410. doi: 10.1111/j.1398-9995.2006.01209.x. [DOI] [PubMed] [Google Scholar]
- 59.Silverberg JI, Hanifin J, Simpson EL. Climatic factors are associated with childhood eczema prevalence in the United States. J Invest Dermatol. 2013;133:1752–1759. doi: 10.1038/jid.2013.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


