Abstract
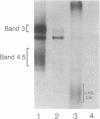
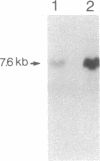
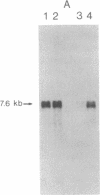
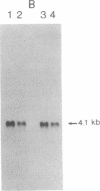
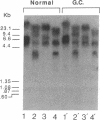
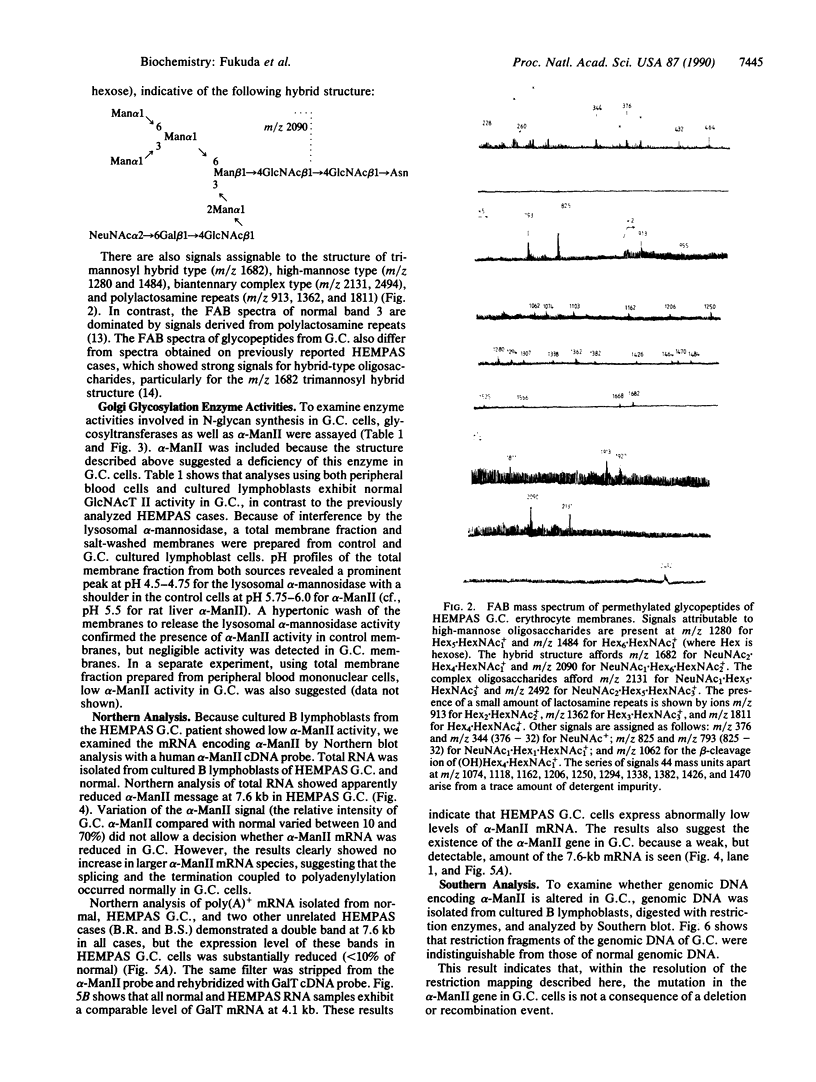
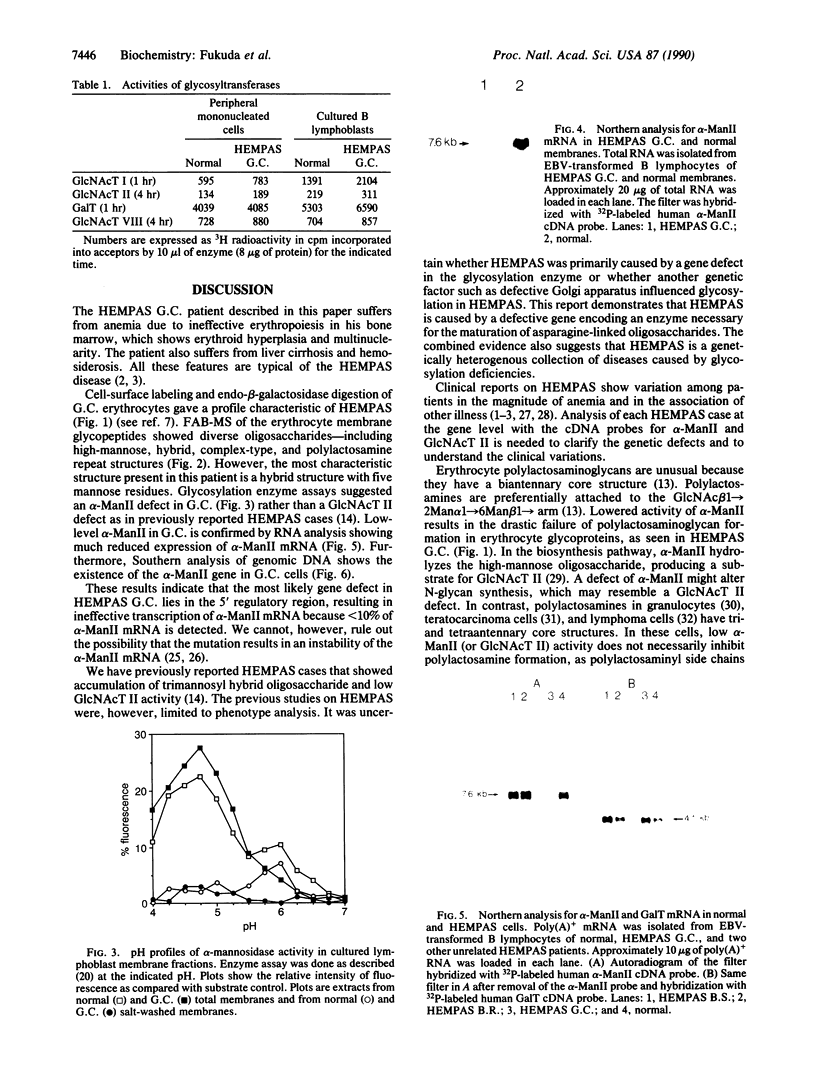
Congenital dyserythropoietic anemia type II, or hereditary erythroblastic multinuclearity with a positive acidified-serum-lysis test (HEMPAS), is a genetic anemia in humans inherited by an autosomally recessive mode. The enzyme defect in most HEMPAS patients has previously been proposed as a lowered activity of N-acetylglucosaminyltransferase II, resulting in a lack of polylactosamine on proteins and leading to the accumulation of polylactosaminyl lipids. A recent HEMPAS case, G.C., has now been analyzed by cell-surface labeling, fast-atom-bombardment mass spectrometry of glycopeptides, and activity assay of glycosylation enzymes. Significantly decreased glycosylation of polylactosaminoglycan proteins and incompletely processed asparagine-linked oligosaccharides were detected in the erythrocyte membranes of G.C. In contrast to the earlier studied HEMPAS cases, G.C. cells are normal in N-acetylglucosaminyltransferase II activity but are low in alpha-mannosidase II (alpha-ManII) activity. Northern (RNA) analysis of poly(A)+ mRNA from normal, G.C., and other unrelated HEMPAS cells all showed double bands at the 7.6-kilobase position, detected by an alpha-ManII cDNA probe, but expression of these bands in G.C. cells was substantially reduced (less than 10% of normal). In Southern analysis of G.C. and normal genomic DNA, the restriction fragment patterns detected by the alpha-ManII cDNA probe were indistinguishable. These results suggest that G.C. cells contain a mutation in alpha-ManII-encoding gene that results in inefficient expression of alpha-ManII mRNA, either through reduced transcription or message instability. This report demonstrates that HEMPAS is caused by a defective gene encoding an enzyme necessary for the synthesis of asparagine-linked oligosaccharides.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alloisio N., Jaccoud P., Dorleac E., Morle L., Philippe N., Margueritte G., Bryon P. A., Delaunay J. Alterations of globin chain synthesis and of red cell membrane proteins in congenital dyserythropoietic anemia I and II. Pediatr Res. 1982 Dec;16(12):1016–1021. doi: 10.1203/00006450-198212000-00010. [DOI] [PubMed] [Google Scholar]
- Anselstetter V., Horstmann H. J., Heimpel H. Congenital dyserythropoietic anaemia, types I and II: aberrant pattern of erythrocyte membrane proteins in CDA II, as revealed by two-dimensional polyacrylamide gel electrophoresis. Br J Haematol. 1977 Feb;35(2):209–215. doi: 10.1111/j.1365-2141.1977.tb00577.x. [DOI] [PubMed] [Google Scholar]
- Ashwell G., Morell A. G. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):99–128. doi: 10.1002/9780470122860.ch3. [DOI] [PubMed] [Google Scholar]
- Baines A. J., Banga J. P., Gratzer W. B., Linch D. C., Huehns E. R. Red cell membrane protein anomalies in congenital dyserythropoietic anaemia, type II (HEMP AS). Br J Haematol. 1982 Apr;50(4):563–574. doi: 10.1111/j.1365-2141.1982.tb01956.x. [DOI] [PubMed] [Google Scholar]
- Boogaerts M. A., Verwilghen R. L. Variants of congenital dyserythropoietic anaemia: an update. Haematologia (Budap) 1982;15(2):211–219. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crookston J. H., Crookston M. C., Burnie K. L., Francombe W. H., Dacie J. V., Davis J. A., Lewis S. M. Hereditary erythroblastic multinuclearity associated with a positive acidified-serum test: a type of congenital dyserythropoietic anaemia. Br J Haematol. 1969 Jul;17(1):11–26. doi: 10.1111/j.1365-2141.1969.tb05660.x. [DOI] [PubMed] [Google Scholar]
- Cummings R. D., Kornfeld S. The distribution of repeating [Gal beta 1,4GlcNAc beta 1,3] sequences in asparagine-linked oligosaccharides of the mouse lymphoma cell lines BW5147 and PHAR 2.1. J Biol Chem. 1984 May 25;259(10):6253–6260. [PubMed] [Google Scholar]
- Fukuda M. N., Dell A., Oates J. E., Fukuda M. Embryonal lactosaminoglycan. The structure of branched lactosaminoglycans with novel disialosyl (sialyl alpha 2----9 sialyl) terminals isolated from PA1 human embryonal carcinoma cells. J Biol Chem. 1985 Jun 10;260(11):6623–6631. [PubMed] [Google Scholar]
- Fukuda M. N., Dell A., Scartezzini P. Primary defect of congenital dyserythropoietic anemia type II. Failure in glycosylation of erythrocyte lactosaminoglycan proteins caused by lowered N-acetylglucosaminyltransferase II. J Biol Chem. 1987 May 25;262(15):7195–7206. [PubMed] [Google Scholar]
- Fukuda M. N., Fukuda M., Hakomori S. Cell surface modification by endo-beta-galactosidase. Change of blood group activities and release of oligosaccharides from glycoproteins and glycosphingolipids of human erythrocytes. J Biol Chem. 1979 Jun 25;254(12):5458–5465. [PubMed] [Google Scholar]
- Fukuda M. N., Klier G., Yu J., Scartezzini P. Anomalous clustering of underglycosylated band 3 in erythrocytes and their precursor cells in congenital dyserythropoietic anemia type II. Blood. 1986 Aug;68(2):521–529. [PubMed] [Google Scholar]
- Fukuda M. N., Masri K. A., Dell A., Thonar E. J., Klier G., Lowenthal R. M. Defective glycosylation of erythrocyte membrane glycoconjugates in a variant of congenital dyserythropoietic anemia type II: association of low level of membrane-bound form of galactosyltransferase. Blood. 1989 Apr;73(5):1331–1339. [PubMed] [Google Scholar]
- Fukuda M. N., Papayannopoulou T., Gordon-Smith E. C., Rochant H., Testa U. Defect in glycosylation of erythrocyte membrane proteins in congenital dyserythropoietic anaemia type II (HEMPAS). Br J Haematol. 1984 Jan;56(1):55–68. doi: 10.1111/j.1365-2141.1984.tb01271.x. [DOI] [PubMed] [Google Scholar]
- Fukuda M., Dell A., Fukuda M. N. Structure of fetal lactosaminoglycan. The carbohydrate moiety of Band 3 isolated from human umbilical cord erythrocytes. J Biol Chem. 1984 Apr 25;259(8):4782–4791. [PubMed] [Google Scholar]
- Fukuda M., Fukuda M. N., Papayannopoulou T., Hakomori S. Membrane differentiation in human erythroid cells: unique profiles of cell surface glycoproteins expressed in erythroblasts in vitro from three ontogenic stages. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3474–3478. doi: 10.1073/pnas.77.6.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow R. W., Lowenthal R. M. Erythrocyte membrane proteins in an unusual case of congenital dyserythropoietic anaemia type II (CDA II). Br J Haematol. 1982 Jan;50(1):35–41. doi: 10.1111/j.1365-2141.1982.tb01888.x. [DOI] [PubMed] [Google Scholar]
- Heimpel H., Wendt F. Congenital dyserythropoietic anemia with karyorrhexis and multinuclearity of erythroblasts. Helv Med Acta. 1968 Mar;34(2):103–115. [PubMed] [Google Scholar]
- Hughes R. C., Feeney J. Ricin-resistant mutants of baby-hamster-kidney cells deficient in alpha-mannosidase-II-catalyzed processing of asparagine-linked oligosaccharides. Eur J Biochem. 1986 Jul 15;158(2):227–237. doi: 10.1111/j.1432-1033.1986.tb09742.x. [DOI] [PubMed] [Google Scholar]
- Kadowaki T., Kadowaki H., Taylor S. I. A nonsense mutation causing decreased levels of insulin receptor mRNA: detection by a simplified technique for direct sequencing of genomic DNA amplified by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Jan;87(2):658–662. doi: 10.1073/pnas.87.2.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Lowenthal R. M., Marsden K. A., Dewar C. L., Thompson G. R. Congenital dyserythropoietic anaemia (CDA) with severe gout, rare Kell phenotype and erythrocyte, granulocyte and platelet membrane reduplication: a new variant of CDA type II. Br J Haematol. 1980 Feb;44(2):211–220. doi: 10.1111/j.1365-2141.1980.tb01203.x. [DOI] [PubMed] [Google Scholar]
- Masri K. A., Appert H. E., Fukuda M. N. Identification of the full-length coding sequence for human galactosyltransferase (beta-N-acetylglucosaminide: beta 1,4-galactosyltransferase). Biochem Biophys Res Commun. 1988 Dec 15;157(2):657–663. doi: 10.1016/s0006-291x(88)80300-0. [DOI] [PubMed] [Google Scholar]
- Mawby W. J., Tanner M. J., Anstee D. J., Clamp J. R. Incomplete glycosylation of erythrocyte membrane proteins in congenital dyserythropoietic anaemia type II (CDA II). Br J Haematol. 1983 Oct;55(2):357–368. doi: 10.1111/j.1365-2141.1983.tb01257.x. [DOI] [PubMed] [Google Scholar]
- Moremen K. W. Isolation of a rat liver Golgi mannosidase II clone by mixed oligonucleotide-primed amplification of cDNA. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5276–5280. doi: 10.1073/pnas.86.14.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moremen K. W., Touster O. Biosynthesis and modification of Golgi mannosidase II in HeLa and 3T3 cells. J Biol Chem. 1985 Jun 10;260(11):6654–6662. [PubMed] [Google Scholar]
- Nozawa S., Yajima M., Sakuma T., Udagawa Y., Kiguchi K., Sakayori M., Narisawa S., Iizuka R., Uemura M. Cancer-associated galactosyltransferase as a new tumor marker for ovarian clear cell carcinoma. Cancer Res. 1990 Feb 1;50(3):754–759. [PubMed] [Google Scholar]
- Pierce M., Arango J., Tahir S. H., Hindsgaul O. Activity of UDP-GlcNAc:alpha-mannoside beta(1,6)N-acetylglucosaminyltransferase (GnT V) in cultured cells using a synthetic trisaccharide acceptor. Biochem Biophys Res Commun. 1987 Jul 31;146(2):679–684. doi: 10.1016/0006-291x(87)90582-1. [DOI] [PubMed] [Google Scholar]
- Scartezzini P., Forni G. L., Baldi M., Izzo C., Sansone G. Decreased glycosylation of band 3 and band 4.5 glycoproteins of erythrocyte membrane in congenital dyserythropoietic anaemia type II. Br J Haematol. 1982 Aug;51(4):569–576. doi: 10.1111/j.1365-2141.1982.tb02820.x. [DOI] [PubMed] [Google Scholar]
- Schachter H., Narasimhan S., Gleeson P., Vella G. Control of branching during the biosynthesis of asparagine-linked oligosaccharides. Can J Biochem Cell Biol. 1983 Sep;61(9):1049–1066. doi: 10.1139/o83-134. [DOI] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spooncer E., Fukuda M., Klock J. C., Oates J. E., Dell A. Isolation and characterization of polyfucosylated lactosaminoglycan from human granulocytes. J Biol Chem. 1984 Apr 25;259(8):4792–4801. [PubMed] [Google Scholar]
- Uemura M., Winant R. C., Brandt A. E. Immunoassay of serum galactosyltransferase isoenzyme II in cancer patients and control subjects. Cancer Res. 1988 Sep 15;48(18):5335–5341. [PubMed] [Google Scholar]
- Vainchenker W., Guichard J., Breton-Gorius J. Morphological Abnormalities in cultured erythroid colonies (BFU-E) from the blood of two patients with HEMPAS. Br J Haematol. 1979 Jul;42(3):363–369. doi: 10.1111/j.1365-2141.1979.tb01144.x. [DOI] [PubMed] [Google Scholar]
- Verwilghen R. L., Lewis S. M., Dacie J. V., Crookston J. H., Crookston M. C. Hempas: congenital dyserythropoietic anaemia (type II). Q J Med. 1973 Apr;42(166):257–278. [PubMed] [Google Scholar]
- Zdebska E., Anselstetter V., Pacuszka T., Krauze R., Chełstowska A., Heimpel H., Kościelak J. Glycolipids and glycopeptides of red cell membranes in congenital dyserythropoietic anaemia type II (CDA II). Br J Haematol. 1987 Jul;66(3):385–391. doi: 10.1111/j.1365-2141.1987.tb06928.x. [DOI] [PubMed] [Google Scholar]