Abstract
Liver fibrosis results from chronic liver injury of different etiologies. It is characterized by dysregulation of physiological remodeling, activation of myofibroblasts, and formation of a fibrous scar. Myofibroblasts develop contractile functions and secrete the extracellular matrix proteins that form this fibrous scar. Myofibroblasts are not present in the normal liver but activate and proliferate in response to injury and inflammation. This review summarizes the understanding and controversies on the contribution of cell populations to the myofibroblasts in liver fibrosis.
Myofibroblasts are absent from normal tissues. During normal tissue repair, transient activation of myofibroblasts contributes to restoration of integrity of the tissue by forming a mechanical scar that is usually dissolved when the tissue is repaired. At this stage myofibroblasts are cleared by apoptosis or by inactivation.(1,2) In contrast, persistent myofibroblast activation causes accumulation and contraction of collagenous extracellular matrix (ECM), a condition called “fibrosis.” Myofibroblasts are defined by de novo development of stress fibers and contractual forces and the secretion of ECM proteins forming a fibrous scar. The most widely used marker of myo-fibroblasts in research and clinical diagnostics is the de novo expression of alpha–smooth muscle actin. Other useful markers of myofibroblasts are F-actin, vinculin, and extra domain A–containing fibronectin. The activation process describes newly acquired cell contraction, migration, proliferation, cytokine production, ECM secretion, and ECM degradation.(2) Myofibroblasts are the source of the fiber scar in the kidney, lung, and liver. Myofibroblasts are a heterogeneous population of cells. The origin of myofibroblasts depends on the tissue that is injured and the type of injury of a specific tissue. The proposed sources of myofibroblasts in liver fibrosis are epithelial cells, mesenchymal stromal cells (MSCs), fibrocytes, mesothelial cells, hepatic stellate cells (HSCs), and portal fibroblasts (PFs). Although advanced genetic techniques have provided strong evidence that HSCs are the predominant source of myofibroblasts in many types of experimental liver injury, some controversies remain. These include the contribution of recruited fibrocytes to the myofibroblast population, the contribution of PFs/myofibroblasts to early cholestatic liver injury, and a possible role for mesothelial cells in capsular or intra-hepatic liver fibrosis (see Fig. 1).
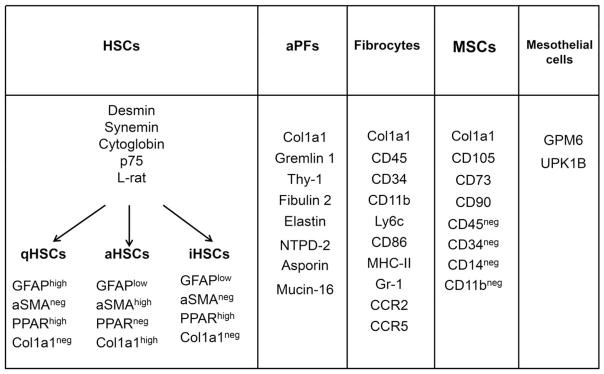
FIG. 1.
Specific markers of activated HSCs, quiescent HSCs, and inactivated HSCs, activated PFs, fibrocytes, and mesothelial cells (explanations are in the text). Abbreviations: aHSC, activated HSC; aSMA, alpha–smooth muscle actin; CCR, chemokine (C-C motif) receptor; CD, cluster of differentiation; Col1a1, collagen type 1a1; GFAP, glial fibrillary acidic protein; iHSC, inactivated HSC; MHC-II, major histocompatibility complex II; PPAR, peroxisome proliferator–activated receptor; qHSC, quiescent HSC.
In general, three methods have been used to trace the origin of myofibroblasts in the liver. Probably the best-validated method is the use of inducible genetic tracing studies in which the purported precursor cell is inducibly marked, such as using a cell-specific inducible cyclization recombination (Cre; CreERT2) crossed with a floxed reporter gene (such as green fluorescent protein [GFP]) and then inducing a prototypical liver injury in a mouse to induce fibrosis. If the resulting myofibroblast cells, which are identified by the markers discussed above, also express the reporter gene expressed in the precursor cell, then the logical conclusion is that the myofibroblasts originated from the precursor cell population. However, this fate-tracing approach is only available for a limited number of cell types, such as for cholangiocytes. Tamoxifen, the most widely used agent to induce CRE expression, may give false-positive results by modifying liver cell physiology. An alternative approach has been to identify myofibroblasts using established myofibroblast markers or genetic reporter genes (such as type 1 collagen-GFP or alpha–smooth muscle actin–GFP)(3) and then to analyze the heterogeneous myofibroblast population to identify markers consistent with their precursor origin. Using immunohistochemistry of fibrotic liver and fluorescence-activated cell sorting of liver cell preparations, many markers have been identified for cell populations that may potentially become myofibroblasts (Fig. 1). The limitation of this approach is that genetic markers may be acquired or lost during activation, creating a false impression of the cell of origin. The third approach used to assess whether the bone marrow (BM) is the source for myofibroblasts is the most robust and conceptually the simplest. With this approach mice undergo lethal irradiation, followed by BM transplant with genetically marked cells (such as type 1 collagen–GFP). The BM chimeric mouse then is subjected to liver injury (e.g., CCl4 gavage, bile duct ligation [BDL]) so that any BM-derived myofibroblasts are readily identified.
Epithelial–Mesenchymal Transition
Epithelial–mesenchymal transition is a process in which parenchymal cells (epithelial cells) become myofibroblasts. Studies that supported this concept were based on an overlap of cells that expressed markers for epithelial cells and myofibroblasts (for example, expressing both cytokeratin 19 and type 1 collagen) and of transforming growth factor β–induced conversion of parenchymal cells to fibroblast-like cells in culture.(4–6) However, lineage-tracing experiments that permanently mark cholangiocytes (K19), hepatocytes (albumin), or epithelial precursor cells (alpha-fetoprotein) have clearly demonstrated that myofibroblasts induced in liver fibrosis injury models are not derived from epithelial cells. However, epithelial cells such as cholangiocytes and hepatocytes change their gene expression profiles during liver injury and express some mesenchymal markers. This is most clearly demonstrated in the role of de novo snail expression in hepatocytes during CCl4-induced liver fibrosis.(7) Because parenchymal cells from fibrotic liver do not express all myofibroblast markers or secrete ECM proteins, perhaps the concept of partial epithelial–mesenchymal transition should be ascribed to this change in gene expression.
BM-Derived Cells
There are two potential BM sources of myofibroblasts, mesenchymal stem cells and fibrocytes. MSCs (also called “mesenchymal stem cells”) are found in many tissues, including adipose tissue and BM. As alleged mesenchymal stem cells, MSCs have been proposed as a source of myofibroblasts in injured tissues to which they are recruited. More recent studies using MSCs have demonstrated that they are actually short-lived in the recruited tissue, are antifibrotic when added to injured tissue, and may actually provide a protective microenvironment, perhaps through immunosuppression.(8) Current clinical trials are assessing the role of MSCs as therapy for liver fibrosis.
Fibrocytes are defined as CD34+ type 1 collagen+ cells. They are myeloid cells derived in the BM and then recruited to different sites of injury. In the injured tissue they may differentiate into macrophages or myofibroblasts. Studies in liver report a range of respective fibrocyte contributions to myofibroblasts of between 3% and 50%.(9,10) Nonetheless, most studies do not reveal a significant contribution of fibrocytes to the myofibroblast population in fibrotic liver disease. Importantly, many studies performed with the genetic lineage-tracing approach account for nearly all of the myofibroblasts coming from sources other than the BM, so their quantitative contribution to myofibroblasts should be small.(11,12)
Mesothelial Cells
Cell fate mapping experiments using mesothelin, Msx2 (msh-like 2), or Wilms tumor 1 homolog as markers have provided strong evidence that epithelial mesothelial cells are the origin of both PFs and HSCs during embryonic development.(13,14) Whether mesothelial cells, the epithelial cells lining the peritoneum and other body cavities, can be a source of myofibroblasts in liver fibrosis is unclear. A recent study used fluorescent-activated cell sorting to purify three different populations of cells. Mesothelial cells, identified by the mesothelial cell marker GPM6, did not express type 1 collagen mRNA. Therefore, these cells designated as originating from mesothelial cells are not the source of myofibroblasts in the BDL experimental model.(15) However, mesothelial cells may contribute to fibrosis of the liver capsule,(10) a common finding in cirrhosis, and the peritoneum. Perhaps some of the confusion about the relative contribution of HSCs, PFs, and mesothelial cells may result from the fact that the three cell types probably all originate from the same cell during development and may express common markers.
HSCs
Genetic cell fate mapping using platelet-derived growth factor receptor α,(16) glial fibrillar acidic protein,(17) and lecithin retinol acyl-transferase (LRAT)(11) have all provided strong evidence that the predominant cell of origin of myofibroblasts in most types of experimental liver disease are the HSCs. While quiescent HSCs are present in the uninjured liver as the vitamin A lipid droplet–containing cells in the space of Disse, activated HSCs maintain most of their quiescent markers and acquire a myofibroblast phenotype. However, because none of these quiescent markers are inducible, a pulse-chase experiment cannot be performed and they do not fulfill the classic experimental design for cell fate mapping. LratCre may not provide an inducible fate-tracing approach and might possibly label additional cells in the course of fibrogenesis. All LratCre-labeled cells in the liver contained vitamin A following cholestatic liver injury, whereas LratCre-negative PF-like cells did not contain vitamin A. This suggests that LratCre labels vitamin A+ HSCs.(11) However, an independent second method also demonstrated that HSCs are the predominant cells that become myofibroblasts throughout the response to toxic liver injury. PFs are a major source of myofibroblasts during early cholestatic injury, but HSCs become the predominant source as the injury progresses(12) (see below).
PFs
PFs are believed to be a local quiescent fibroblast population. Because there are no known single markers to discriminate fibroblasts from other mesenchymal cells, it is difficult to identify or purify quiescent PFs. Activated PFs were first described in clinical and experimental cholestatic liver disease by histology, immunohistochemistry, electron microscopy, and immunoelectron microscopy(18–20) that clearly distinguished two cell populations. Further descriptions identified markers that were positive on PFs but not on HSCs, including elastin, Thy1, and Ntpdase2(21) (see Fig. 1). The experimental studies emphasized the contribution of PFs to fibrosis during the early onset of the cholestatic injury, which is consistent with the later studies using genetically marked cells.(12) However, there are no established methods to use inducible cell-specific markers to do pulse labeling to establish a genetic lineage tracing for PFs. Although PFs are reported to be rare in normal liver, they have been purified in rat liver.(21) A marker, Ntpdase2, has been reported for quiescent activated fibroblasts but not for myofibroblasts. In cholestatic injury (BDL), two studies have generated similar results using the same type 1 collagen–GFP reporter mouse.(12,15) In each case, two distinct populations of type 1 collagen mRNA–positive cells consistent with myofibroblasts were identified as HSCs and PFs. The experimental error in quantifying the contribution of HSCs and PFs could mask a minor contribution of other cell types such as fibrocytes to cholestatic liver injury. Differences in the proposed contribution of PFs to the myofibroblast population in BDL may reflect differences in fibrotic time course in different mouse strains, different microbiota, and other differences in mouse house environment as well as the above-discussed limitations of the different types of methodologies for assessing this quantification.
Conclusions
Fibrogenic hepatic myofibroblasts arise mostly from liver-resident HSCs, activated PFs, and mesothelial cells. Activated HSCs are the predominant contributor to myofibroblasts during toxic liver injury and late cholestatic injury, while activated PFs contribute to early cholestatic injury. BM-derived fibrocytes make a quantitatively small contribution to liver fibrosis; they migrate to the fibrosing liver in response to both cholestatic and toxic liver injury. Identifying the origin of myofibroblasts in different fibrotic diseases will lead to a greater understanding of the pathophysiology of fibrosis, provide new insights into the regression of fibrosis, and lead to more targeted therapy of fibrosis.
Acknowledgments
Supported by the National Institutes of Health (AA022614, DK099205, DK101737).
Abbreviations
- BDL
bile duct ligation
- BM
bone marrow
- Cre
cyclization recombination
- ECM
extracellular matrix
- GFP
green fluorescent protein
- HSC
hepatic stellate cell
- LRAT
lecithin retinol acyl-transferase
- MSC
mesenchymal stromal cell
- PF
portal fibroblast
Footnotes
Potential conflict of interest: Nothing to report.
References
- 1.Hinz B, Phan SH, Thannickal VJ, Prunotto M, Desmouliere A, Varga J, et al. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol. 2012;180:1340–1355. doi: 10.1016/j.ajpath.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hinz B. Myofibroblasts. Exp Eye Res. 2016;142:56–70. doi: 10.1016/j.exer.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Magness ST, Bataller R, Yang L, Brenner DA. A dual reporter gene transgenic mouse demonstrates heterogeneity in hepatic fibrogenic cell populations. Hepatology. 2004;40:1151–1159. doi: 10.1002/hep.20427. [DOI] [PubMed] [Google Scholar]
- 4.Scholten D, Osterreicher CH, Scholten A, Iwaisako K, Gu G, Brenner DA, et al. Genetic labeling does not detect epithelial-to-mesenchymal transition of cholangiocytes in liver fibrosis in mice. Gastroenterology. 2010;139:987–998. doi: 10.1053/j.gastro.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taura K, Miura K, Iwaisako K, Osterreicher CH, Kodama Y, Penz-Osterreicher M, et al. Hepatocytes do not undergo epithelial-mesenchymal transition in liver fibrosis in mice. Hepatology. 2010;51:1027–1036. doi: 10.1002/hep.23368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chu AS, Diaz R, Hui JJ, Yanger K, Zong Y, Alpini G, et al. Lineage tracing demonstrates no evidence of cholangiocyte epithelial-to-mesenchymal transition in murine models of hepatic fibrosis. Hepatology. 2011;53:1685–1695. doi: 10.1002/hep.24206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rowe RG, Lin Y, Shimizu-Hirota R, Hanada S, Neilson EG, Greenson JK, et al. Hepatocyte-derived Snail1 propagates liver fibrosis progression. Mol Cell Biol. 2011;31:2392–2403. doi: 10.1128/MCB.01218-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prockop DJ. Inflammation, fibrosis, and modulation of the process by mesenchymal stem/stromal cells. Matrix Biol. 2016;51:7–13. doi: 10.1016/j.matbio.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kisseleva T, Uchinami H, Feirt N, Quintana-Bustamante O, Segovia JC, Schwabe RF, et al. Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. J Hepatol. 2006;45:429–438. doi: 10.1016/j.jhep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Lua I, Li Y, Pappoe LS, Asahina K. Myofibroblastic conversion and regeneration of mesothelial cells in peritoneal and liver fibrosis. Am J Pathol. 2015;185:3258–3273. doi: 10.1016/j.ajpath.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mederacke I, Hsu CC, Troeger JS, Huebener P, Mu X, Dapito DH, et al. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun. 2013;4:2823. doi: 10.1038/ncomms3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iwaisako K, Jiang C, Zhang M, Cong M, Moore-Morris TJ, Park TJ, et al. Origin of myofibroblasts in the fibrotic liver in mice. Proc Natl Acad Sci USA. 2014;111:E3297–E3305. doi: 10.1073/pnas.1400062111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rinkevich Y, Mori T, Sahoo D, Xu PX, Bermingham JR, Jr, Weissman IL. Identification and prospective isolation of a mesothelial precursor lineage giving rise to smooth muscle cells and fibroblasts for mammalian internal organs, and their vasculature. Nat Cell Biol. 2012;14:1251–1260. doi: 10.1038/ncb2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asahina K, Zhou B, Pu WT, Tsukamoto H. Septum transversum-derived mesothelium gives rise to hepatic stellate cells and perivascular mesenchymal cells in developing mouse liver. Hepatology. 2011;53:983–995. doi: 10.1002/hep.24119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lua I, Li Y, Zagory JA, Wang KS, French SW, Sevigny J, et al. Characterization of hepatic stellate cells, portal fibroblasts, and mesothelial cells in normal and fibrotic livers. J Hepatol. 2016 doi: 10.1016/j.jhep.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henderson NC, Arnold TD, Katamura Y, Giacomini MM, Rodriguez JD, McCarty JH, et al. Targeting of alphav integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat Med. 2013;19:1617–1624. doi: 10.1038/nm.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puche JE, Lee YA, Jiao J, Aloman C, Fiel MI, Munoz U, et al. A novel murine model to deplete hepatic stellate cells uncovers their role in amplifying liver damage in mice. Hepatology. 2013;57:339–350. doi: 10.1002/hep.26053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang L, Tanaka Y, Marumo F, Sato C. Phenotypic change in portal fibroblasts in biliary fibrosis. Liver. 1994;14:76–82. doi: 10.1111/j.1600-0676.1994.tb00051.x. [DOI] [PubMed] [Google Scholar]
- 19.Herbst H, Frey A, Heinrichs O, Milani S, Bechstein WO, Neuhaus P, et al. Heterogeneity of liver cells expressing procollagen types I and IV in vivo. Histochem Cell Biol. 1997;107:399–409. doi: 10.1007/s004180050126. [DOI] [PubMed] [Google Scholar]
- 20.Cassiman D, Libbrecht L, Desmet V, Denef C, Roskams T. Hepatic stellate cell/myofibroblast subpopulations in fibrotic human and rat livers. J Hepatol. 2002;36:200–209. doi: 10.1016/s0168-8278(01)00260-4. [DOI] [PubMed] [Google Scholar]
- 21.Fausther M, Goree JR, Lavoie EG, Graham AL, Sevigny J, Dranoff JA. Establishment and characterization of rat portal myofibroblast cell lines. PLoS One. 2015;10:e0121161. doi: 10.1371/journal.pone.0121161. [DOI] [PMC free article] [PubMed] [Google Scholar]