Abstract
Cardiovascular disease (CVD) is the main cause of death among adult men and women in the USA and impacts millions around the globe. Traditional risk factors for CVD include obesity, diabetes, hypertension and dyslipidemia. The modern-day epidemic of obesity not only increased the rate of CVD but also ushered in an additional CVD risk factor to be added to the list; that is obstructive sleep apnea (OSA). In this review, we discuss the growing epidemic of obesity and OSA, highlighting the common pathogenic hypotheses linking these risk factors to CVD. We will also highlight the therapeutic rationale of OSA as a way to reduce CVD risk.
Keywords: Obesity, Cardiovascular disease, Sleep disorders
Introduction
Obesity is a major public health problem in the United States; with more than 2 out of 3 adults considered to be either overweight or obese [1]. The rise in obesity has caused increase in the incidence of obesity-related diseases, such as diabetes mellitus type 2 (DM2), hypertension (HTN), chronic kidney disease (CKD) and cardiovascular diseases (CVD). This epidemic has in turn placed a significant amount of burden on healthcare expenditure in the management of these chronic diseases. Diabetes alone costs more than $245 billion every year in America, with one in three Medicare dollars being spent on care for people with diabetes [2]. Costs for other obesity-related diseases are astronomical as well, with the figures only expected to rise in the future. It is imperative to understand the cause of obesity and its pathophysiology with other chronic conditions that are becoming increasingly expensive.
Obesity is generally understood as a condition of energy imbalance, where there is a surplus of energy consumption compared to its expenditure. Increased caloric intake with modern American diet that is high in simple carbohydrates and saturated fatty acids, combined with sedentary lifestyle are thought to be the main culprits in the obesity pandemic in America.
Obstructive sleep apnea (OSA) has been studied closely as one of the major associated illness in obese patients. Not only is obesity one of the main causes of OSA, affecting 70% of the patients with OSA, it also shares some of the underlying pathophysiologic mechanisms with obesity-related diseases, such as DM2, HTN and CVD [3,4]. Furthermore, OSA increases risk of end-organ damage in critically ill patients. In a propensity matched study, OSA patients were at statistically significant risk of Acute Kidney Injury compared to non-OSA patients [5]. Similarly, CPAP treatment has shown to improve glucose control in type 2 diabetics with blood sugars not controlled with medications. This effect is achieved by increasing insulin secretion and reduced counter-regulatory hormone production [6]. Therefore, development and treatment of OSA carries wide ranging systemic implications and prognosis for patients.
CVD is the number one causes of death in the United States with 1 in every 4 death are attributed to CVD [7]. Modifiable risk factors, such as being overweight and obese account for more than 70% of the risk for CVD and mortality caused by CVD. [8] Pathophysiology and prevention of CVD is also very complex as there are multiple risk factors besides obesity that could increase the risk. These risk factors include but are not limited to, smoking, alcohol and drug abuse along with HTN, DM2 and/or hyperlipidemia, which are all modifiable [9].
It has been shown that low socioeconomic status is positively correlated with obesity and its complications, such as DM2 [10]. It must be noted that obesity, associated with OSA and CVD, is also disproportionately high in black population, as 63% of men and 77% of women in this ethnic group is either overweight or obese [11]. Per the Multi-ethnic study of atherosclerosis (MESA), black population demonstrated the highest levels of sleep disturbance, shorter sleep duration, worse sleep quality and daytime sleepiness compared to Caucasians, Hispanics and Asians [12]. Higher poverty rate is also associated with higher apnea-hypopnea index (AHI) with relatively lower continuous positive airway pressure (CPAP) therapy acceptance rate [13,14].
A similar pattern is observed in CVD and its many risk factors, as the prevalence is disproportionately higher in black population compared to other ethnic groups. [15] People of low income (<$35,000 per year) are at increased risk of developing CVD, compared to individuals with higher income (HR 1.48, 95% CI 1.21–1.81) [16]. With recent studies showing evidence of correlation between socioeconomic status and risk of developing OSA and CVD, there seems to be more than just biological pathophysiology in the development of these diseases.
There are many proposed hypotheses as to why these discrepancies may exist, including poor access to health care services, non-adherence to treatment recommendations, inadequate training and environmental and genetic variations [12,15]. Although the existence of association in these conditions are widely acknowledged, the understanding of the roles in how these diseases influence each other is not well investigated. Summary of prevalence and risk factors of Obesity, OSA and CVD are illustrated in Table 1.
Table 1.
Summary of prevalence and risk factors of obesity, obstructive sleep apnea, and cardiovascular disease.
| Obesity | OSA | CVD | |
|---|---|---|---|
| Prevalence | 35.70% | 3% to 7% (male) | 28.90% |
| (In U.S.) | 68.8% (either overweight or obese) | 2% to 5% (female) | |
| Higher amongst Blacks | |||
| (48.1%; 63% males, 77% females either overweight or obese) | Higher amongst Blacks | Higher amongst Blacks (46% males, 48% females) | |
| (OR=1.78, compared with Whites) | |||
| Sedentary lifestyle | Obesity (70%) | Obesity (>70%) | |
| Risk factors | Increased caloric intake | DM2 | DM2 |
| Diet high in simple carbohydrates and saturated fatty acids | HTN | HTN | |
| CVD | Smoking | ||
| Poor diet | |||
| Physical inactivity |
In this review article, we aim to better understand the relationship between OSA and CVD, by analyzing their underlying pathophysiology from molecular level to non-medical factors that have not been investigated from previous studies, which can significantly influence the clinical outcome. This information will be helpful in guiding future treatment strategies, especially in communities that have high representation of vulnerable ethnic groups. Figure 1 summarizes the interrelationship between Obesity, OSA, and CVD.
Figure 1.
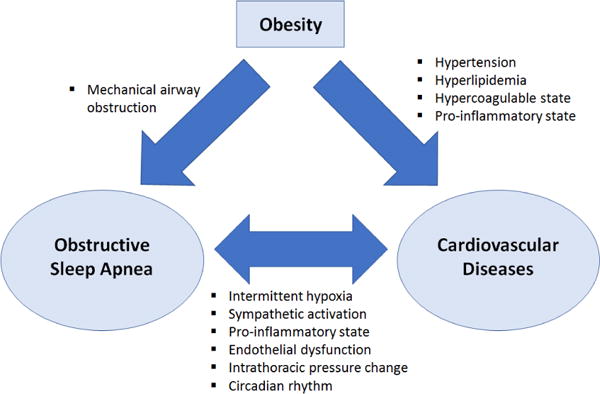
Interrelation between obesity, obstructive sleep apnea, and cardiovascular disease.
Pathophysiologic Mechanisms
Intermittent hypoxia
While in sleep, OSA causes intermittent periods of hypoxia followed by reoxygenation. Recurrent episodes of hypoxia stimulate the carotid chemoreceptors and results in secondary rise in blood pressure from sympathetic activation [17,18]. These episodes of intermittent hypoxia can range anywhere from 5 to more than 100 events per hour [18]. Although acute hypoxia is capable of activating responses that can lead to acute nocturnal cardiac event [19], the chronicity in recurrent sympathetic activation and its consequent vasoconstriction over many years can cause unique profile of biological consequences in OSA patients [20]. For this reason, intermittent hypoxia is thought to be a major culprit in patients with OSA that can lead to CVD. It is also interesting to note that intermittent hypoxia can increase the risk of CVD at the molecular level. Intermittent hypoxia has shown to increase the levels of angiopoietin-like 4 (Angptl4), a potent inhibitor of lipoprotein lipase. This change decreases the body’s clearance of lipoprotein and increases fasting serum levels of triglycerides and very low density lipoprotein cholesterol [21]. In addition, hypoxia-inducible factor-1 (HIF-1), a transcription factor that modulates the body’s response to ischemic injuries has been shown to be up regulated in hypoxia, further demonstrating the evidence of molecular genetic association between hypoxia and ischemic CVD, secondary to impaired lipoprotein turnover (Figure 1) [22].
Sympathetic activation
It is known that sympathetic activations are caused by nocturnal intermittent hypoxia in OSA patients. However, it is interesting to note that, the sympathetic activations in these patients persist during daytime wakefulness in normoxic conditions [20,23]. This persistent sympathetic drive promotes systemic hypertension and increased cardiac sympathetic tone [24]. It also augments subsequent response to sympathetic stimuli [25]. In addition, sympathetic influence on renin-angiotensin system is another critical factor in the pathogenesis of systemic hypertension in OSA patients [19]. This change in autonomic regulation of blood pressure due to impaired baroreflex and renin-angiotensin system in these patients puts them at a higher risk of developing systemic HTN, persistent tachycardia and ultimately CVD.
Pro-inflammatory state
Chronic hypoxic stress can activate systemic inflammatory pathways, as OSA patients have increased level of plasma cytokines, serum amyloid-A and C-reactive protein (CRP) [20,26]. Obesity has also been shown to increase the level of pro-inflammatory cytokines and may cause hypercoagulable state in the bloodstream along with OSA [27]. Adipocyte hypertrophy results in altered expression of adipokines. Adipokines play crucial role in vascular function by influencing glucose and lipid metabolism. In fact, dysregulation of adipocytes leads to altered levels in pro- and anti-inflammatory adipokine expression. While altered adipokine expression has been demonstrated to be a predicative marker and associate with CVD in experimental in vitro and in vivo models, this impact of is less clear in humans [28]. Along with the buildup and the rupture of atherosclerotic plaques, these phenomena of pro-inflammatory and hypercoagulable state can be fatal in the development of CVDs, such as acute myocardial infarction (MI).
Endothelial dysfunction
OSA patients suffer from endothelial dysfunction due to decreased availability of nitric oxide (NO) and cell apoptosis, secondary to increased oxidative stress and systemic inflammation [20]. It is also thought that chronic hypoxic stress causes release of endothelin-1, a potent vasoconstrictor, in human endothelium [29]. Impaired NO production compounded with increase in endothelin-1 release in the vasculature can predispose OSA patients into systemic HTN and consequent CVD. Accumulation of reactive oxygen species also likely contributes to the pathogenesis of endothelial dysfunction in OSA patients by directly forming vascular lesions and CAD [19].
Intrathoracic pressure change
Repetitive inspiratory effort against closed upper airway generates intrathoracic pressure change that subsequently causes an increase in transmural gradients across the atria, ventricles and aorta [20,30]. Severe OSA can compromise left and right ventricular function, diastolic filling, and increase the afterload. These all impose risk of developing CVD, arrhythmias, and heart failure (HF) [20,31,32].
Circadian rhythm
Disruption in circadian rhythm and sleep homeostat has also shown its association with CVD. In OSA patients, insufficient duration, quality and timing of sleep, in addition to intermittent hypoxia can promote the development of CVD [33]. It is also interesting to note that people of African descent, who have a higher prevalence of CVD than others also have more disrupted sleep pattern, including period length and phase shifting, compared to the people from European heritage [33]. Figure 2 Sumarizes the pathophysiologic mechanisms linking OSA to CVD.
Figure 2.
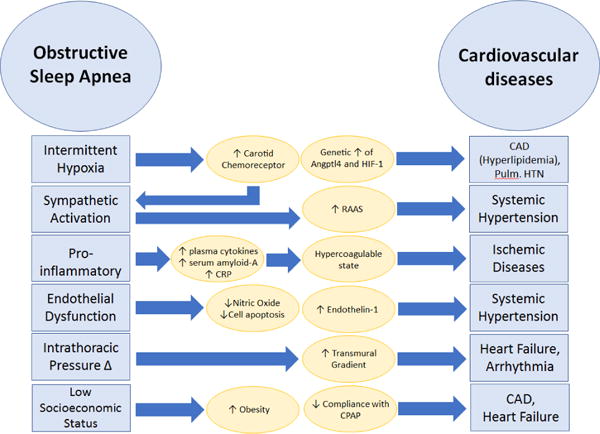
Pathophysiology of Obstructive sleep apnea leading to cardiovascular disease.
Clinical Association between CVD and OSA
Ischemic cardiovascular alterations
Coronary artery disease (CAD)
Chronic intermittent hypoxia induced atherosclerosis with endothelial dysfunction are the thought to be the main causes of CAD in OSA patients. Many clinical studies have shown significant evidence to support the association between the two diseases. Severe OSA patients with AHI>30 showed significantly higher risk of cardiovascular events, including acute coronary syndrome (ACS), myocardial infarction (MI) and stroke [34]. AHI was also found to have strong correlation with atherosclerotic volume when measured by imaging through intravascular ultrasound. [20,35] An interesting finding to note is that MI’s occurring in patients with OSA have a different timing pattern compared to others, as one study has shown that almost half of OSA patients had their MI’s during sleeping hours (22:00 to 06:00) whereas in the general population, the most likely time for the onset of MI is between 06:00 and 11:00 [20,36]. This is likely explained by the fact that nocturnal hypoxia caused by OSA is predisposing this group of patients to nocturnal MI. Treating severe OSA patient has also shown significant reduction in the risk of CAD as patients who received successful CPAP therapy had lower incidence of fatal and nonfatal cardiovascular events [34,37].
Cerebrovascular accidents (CVA)
Ischemic strokes or cerebrovascular accidents were found to be higher in patients with OSA from multiple cross-sectional and prospective cohort studies [38–40]. It is important to note that this higher risk of CVA in OSA patients are independent of other CVD risk factors whose incidence may have also been influenced by the presence of OSA, such as HTN, HF, Atrial Fibrillation(AF) and DM2 [19]. Association was also found between OSA and CVA, as higher AHI was correlated with higher risk of CVA (HR 2.85, 95% CI 1.10–7.39) and greater extent of metabolic impairment of cerebral white matter [40,41]. Proposed mechanisms of pathogenesis include altered cerebral autoregulation, endothelial dysfunction and pro-thrombotic/inflammatory state that place these patients at a higher risk of reduced cerebral blood flow, leading to ischemic stroke [20]. Potential therapeutic benefit from the treatment of OSA is unclear at the moment as limitations in maintaining chronic CPAP treatment with CVA patients have been identified [38]. Nevertheless, it may be beneficial to offer CPAP treatment initially to CVA patients as it would reduce the influence and recurrence of other cardiovascular risk factors as outlined previously (Figure 2).
Nonischemic cardiovascular alterations
Systemic hypertension
From the Wisconsin cohort study, there was a dose-dependent relationship between the OSA status of an individual and the risk of developing HTN, as AHI of 15 events/hour or more showed 3-fold increase in the incidence of HTN [42]. Evidence of positive linear relationship between the severity of OSA and the incidence of CVD was also shown in the Sleep Heart Health Study, a large multi-center cross-sectional study [43]. As mentioned previously, the combination of sympathetic activation and renin-angiotensin system are thought to be possible mechanisms of OSA leading to HTN. One particularly important variant to consider is resistant HTN, defined as BP>140/90 mmHg despite being on combination of 3 or more anti-hypertensive medications titrated to their maximal doses OSA was found more frequently in the resistant HTN population, compared to controlled HTN (71% vs. 38%) [19,44]. This relationship was further strengthened by the evidence of decrease in daytime BP with the treatment of OSA in patients with resistant HTN [20,45].
Heart failure (HF)
HF is also commonly associated with OSA. Sleep-disordered breathing (SDB), which encompasses OSA with central sleep apnea (CSA) are often found with HF patients up to 50% to 70% [19,46]. HF patient’s exhibit mixed sleep apnea, characterized by initial CSA event followed by an obstructive component [47]. The initial CSA causes sympathetic activation, followed by elevated blood pressure and heart rate, which in turn causes left ventricular remodeling. When this remodeling process is combined with fluid retention from renin-angiotensin system activation, these phenomena can together drive patients into HF [48]. It is important to note that OSA can cause HF via same mechanisms responsible for sympathetic activation and HTN, namely intermittent hypoxia leading to alteration of carotid chemoreceptors. Respiration against occluded nasopharynx can also cause increased intrathoracic pressure and left ventricular transmural gradient, which can lead to an increase in cardiac afterload and decrease in cardiac output [19]. Symptomatic HF with peripheral edema can cause rostral fluid shift at night with supine position, which when occurs simultaneously with decreased pharyngeal muscle tone from Cheyne-Stokes respiration can cause airway collapse with subsequent worsening of OSA [47]. Treating primary symptoms of HF, including diuretics, angiotensin-converting enzyme inhibitors and implantable device therapy has shown improvement in SDB [19,48]. CPAP therapy has also improved left ventricular ejection fraction (LVEF) and quality of life in HF patients [49]. OSA and HF are closely intertwined in their pathogenesis and patients with both diseases can benefit from treatment of HF and/or OSA.
Cardiac arrhythmia
Prevalence of SDB in patients with AF is 40% to 50% [50]. AF is the most common sustained arrhythmia associated with OSA. The pathogenic mechanism of AF in OSA patients largely deals with the stretching of the atria caused by intrathoracic pressure variation as mentioned previously. Additionally, intermittent hypoxia caused by obstructive respiratory episodes leads to hemodynamic fluctuation, modulated by sympathetic activation. The imbalance between the sympathetic action from apneic episodes and natural predominance of parasympathetic system during sleep results in sympatho-vagal imbalance, a mechanism widely thought to be responsible for initiation and maintenance of AF in humans [50,51]. Long term OSA is associated with extensive atrial remodeling that disturbs local conduction pathway and causes longer sinus node recovery period [50]. A recent meta-analysis has also shown the effectiveness of CPAP therapy in patients with AF, as patients treated with CPAP had 42% decreased risk of AF compared to ones who were not treated [52]. Recurrence of AF after radiofrequency ablation was also 25% higher amongst patients with OSA [53]. CPAP therapy was associated with lower rate of recurrent AF compared to untreated patients (HR 0.41, 95% CI, 0.22–0.76) [54]. Overall, the data supports a strong relationship between OSA and AF in their pathophysiology and reflects a potential benefit of CPAP therapy in improving cardiovascular outcomes in patients with AF.
Pulmonary hypertension
Several studies have shown association between OSA and pulmonary HTN, where prevalence of pulmonary HTN in OSA is estimated to be up to 20% [19]. Several risk factors for pulmonary HTN can be commonly found in OSA patients, such as high BMI, obesity-hypoventilation syndrome, left heart disease and nocturnal as well as daytime hypoxemia. Clinical significance in the coexistence of these two conditions was outlined from an observational study with 83 patients, as it was found that those with pulmonary HTN at the time of diagnosis of OSA had poorer outcomes at 1, 4, 8-year survival rate than those who did not [55]. Primary mechanisms of pulmonary HTN in OSA patients are thought to be hypoxic vasoconstriction in pulmonary vasculature and vascular remodeling after chronic stress [20]. CPAP treatment can effectively lower the pulmonary artery pressure and vascular reactivity to hypoxia, leading to treatment benefit of OSA in patients with Pulmonary HTN [56,57].
Treatment of OSA as a therapeutic guide to CVDs
CPAP is the treatment of choice in OSA patients that has been shown to be efficacious in the reduction of CVD. Of the cardiovascular comorbidities discussed, systemic HTN has shown the strongest evidence for benefit [19]. It is recommended that OSA patients with HTN, especially for those with moderate to severe symptoms, and those with resistant HTN be treated with CPAP [19].
Much of the existing literature supporting the use of CPAP as an augment therapy are single-center or smaller randomized trials. A larger, recent randomized controlled trial with 2,717 patients, where the treatment group received CPAP and the control group received usual care, showed no significant effect of CPAP therapy on the composite cardiovascular endpoint (HR 1.10, 95% CI 0.91–1.32), despite lowering the AHI. [58] Based on current literature, it seems unclear if there is a definitive treatment benefit from CPAP beyond symptomatic relief.
Other therapies besides CPAP may also be beneficial to OSA patients in reducing cardiovascular risks, as simple as lifestyle modifications such as weight loss, avoidance of alcohol and sedative medications before bedtime. These lifestyle modifications can all reduce symptoms in patients with mild OSA [19, 59].
Bi-level positive airway pressure (BiPAP) is also an option of treatment for patients who cannot tolerate CPAP treatment and for those with OSA associated with chronic obstructive pulmonary disease (COPD) [20]. At the moment, there is no definitive evidence of benefit from the use of BiPAP in lowering CVD risk.
Positional therapy with avoidance of supine position during sleep and mandibular advancement devices have also shown to reduce the AHI and symptoms of OSA, such as snoring. Currently these therapeutic modalities have not shown consistent therapeutic benefit in reducing CVD directly. (Table 2) [19].
Table 2.
Association between OSA and its impact on known CVDs with available data.
| CVD | Association with OSA | Literature |
|---|---|---|
| Systemic Hypertension | Positive, dose-dependent relationship with AHI | Wisconsin cohort (2000) |
| Sympathetic activation | Sleep Heart Health (2000) | |
| RAAS activation | ||
| More frequent in resistant hypertension | ||
| Ischemic Heart Disease | Higher risk of myocardial infarction (AHI>30) | Lancet (2005) |
| Coronary Artery Disease (CAD) | Positive relationship between atherosclerotic volume and AHI | American College of Cardiology (2008) |
| Increased nocturnal MIs | International Journal of Cardiology (2009) | |
| Nocturnal hypoxia | ||
| Heart Failure | High degree of association (50–70%) | European Journal of Heart Failure (2007) |
| Initial central apnea causing sympathetic stimulation | Nature Reviews: Cardiology (2016) | |
| HTN, tachycardia causing cardiac remodeling | ||
| RAAS activation | ||
| Arrhythmias | Moderate degree of association (40–50%) | American College of Cardiology (2014) |
| Intrathoracic pressure change causing stretching of atria | Sleep Medicine (2016) | |
| Atrial remodeling disturbing conduction pathways | ||
| Sympatho-vagal imbalance from apneic episodes | ||
| Strokes (CVA) | Risk independent of other CVD risk factors | Lancet (2004) |
| Dose-dependent relationship with AHI | American Thoracic Society (2010) | |
| Altered cerebral autoregulation | ||
| Endothelial dysfunction | ||
| Pro-thrombic/inflammatory state | ||
| Pulmonary Hypertension | Evidence of association (20%) | American Journal of Cardiology (2009) |
| Obesity-hypoventilation syndrome |
Conclusion
Obesity in the United States has reached its epidemic state and the prevalence of its related diseases, such as OSA and CVD are also rising. Table 1 summarizes the pathophysiologic and clinical implications of OSA. With the trend expected to rise in the future, it is important to understand the association between these two obesity-related diseases, in efforts to reduce the burden on the rising healthcare expenditure that is increasing.
It is also important to understand that many of the patients with such conditions are minorities with considerable portion that remain undiagnosed. Current hypotheses suggest low socioeconomic status and lack of education on proper nutrition may have contributed to predisposition of this population into more risk factors.
In addition to environmental factors, variations in molecular genetics of different ethnicities are likely associated with differences in risk of progression to OSA and CVD As the number one cause of death in the United States, it is essential to recognize the pathophysiology of CVD and factors potentially contributing to its onset. In this article, we recognize OSA as an emerging risk factor for CVD and pathophysiologic relationship between OSA and CVD; these are summarized in Figure 3.
Figure 3.
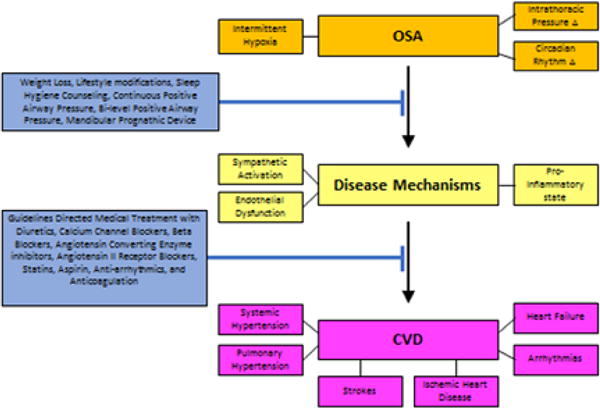
Progression of Obstructive Sleep Apnea to Cardiovascular Disease with potential treatments to inhibit or slow the progression from Obstructive Sleep Apnea to Cardiovascular Disease.
Figure 3 also provides possible interventions available currently to possibly halt the progression from OSA to CVD. It is not yet clear if the treatment of OSA would reduce the risk of CVD, due to conflicting results in current literature. Larger, multi-center trials are needed. Despite the lack of well-defined evidence based guidelines, clinicians should always keep prevention of obesity as one of their priorities, especially if practicing in an at-risk population (Figure 3).
Acknowledgments
This work is sponsored in part by the Brooklyn Health Disparities Center NIH grant #P20 MD006875.
References
- 1.National Center for Health Statistics. Obesity and Overweight. United States: 2015. [Google Scholar]
- 2.American Diabetes Association. The Staggering Costs of Diabetes in America [Google Scholar]
- 3.Fritscher LG, Mottin CC, Canani S, Chatkin JM. Obesity and obstructive sleep apnea-hypopnea syndrome: the impact of bariatric surgery. Obes Surg. 2007;17:95–99. doi: 10.1007/s11695-007-9012-7. [DOI] [PubMed] [Google Scholar]
- 4.Giunta J, Salifu M, McFarlane S. Sleep Disorders and Cardio-Renal Disease: Implications for minority populations. Epidemiology. 2016;6:e120. doi: 10.4172/2161-1165.1000e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dou L, Lan H, Reynolds DJ, Gunderson TM, Kashyap R, et al. Association between obstructive sleep apnea and acute kidney injury in critically Ill patients: A propensity-matched study. Nephron. 2017;135:137–146. doi: 10.1159/000453367. [DOI] [PubMed] [Google Scholar]
- 6.Mokhlesi B, Grimaldi D, Beccuti G, Van Cauter E. Effect of one week of CPAP treatment of obstructive sleep apnoea on 24-hour profiles of glucose, insulin and counter-regulatory hormones in type 2 diabetes. 2015;19:452–456. doi: 10.1111/dom.12823. [DOI] [PubMed] [Google Scholar]
- 7.Sardarinia M, Akbarpour S, Lotfaliany M, Farideh BK, Mohammadreza B, et al. Risk Factors for Incidence of Cardiovascular Diseases and All-Cause Mortality in a Middle Eastern Population over a Decade Follow-up: Tehran Lipid and Glucose Study. PLoS One. 2016;11:e0167623. doi: 10.1371/journal.pone.0167623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McFarlane SI, Sica DA, Sowers JR. Stroke in patients with diabetes and hypertension. J Clin Hypertens. 2005;7:286–292. doi: 10.1111/j.1524-6175.2005.04379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Myers CA, Slack T, Broyles ST, Heymsfield SM, Church TS, et al. Diabetes prevalence is associated with different community factors in the diabetes belt versus the rest of the United States. Obesity (Silver Spring) 2016 doi: 10.1002/oby.21725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Heart Association. African-Americans and Heart Disease, Stroke. 2015 Jul [Google Scholar]
- 11.Chen X, Wang R, Zee P, Lutsey PL, Javaheri S, et al. Racial/Ethnic Differences in Sleep Disturbances: The Multi-Ethnic Study of Atherosclerosis (MESA) Sleep. 2015;38:877–888. doi: 10.5665/sleep.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang R, Dong Y, Weng J, Kontos EZ, Chervin RD, et al. Associations among neighborhood, race and sleep apnea severity in children: a six city analysis. Ann Am Thorac Soc. 2016;14:76–84. doi: 10.1513/AnnalsATS.201609-662OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kendzerska T, Gershon AS, Tomlinson G, Leung RS. The Effect of patient neighborhood income level on the purchase of continuous positive airway pressure treatment among patients with sleep apnea. Ann Am Thorac SocJan. 2016;13:93–100. doi: 10.1513/AnnalsATS.201505-294OC. [DOI] [PubMed] [Google Scholar]
- 14.Jones KM, Carter MM, Schulkin J. Racial and Ethnic Disparities in Cardiovascular Disease: An Assessment of Obstetrician-Gynecologists’ Knowledge, Attitudes, and Practice Patterns. J Racial Ethn Health Disparities. 2015;2:256–266. doi: 10.1007/s40615-015-0088-9. [DOI] [PubMed] [Google Scholar]
- 15.Sumner JA, Khodneva Y, Muntner P, Redmond N, Lewis MW, et al. Effects of concurrent depressive symptoms and perceived stress on cardiovascular risk in low- and high-income participants: findings from the reasons for geographical and racial differences in stroke (REGARDS) study. J Am Heart Assoc. 2016;5:e003930. doi: 10.1161/JAHA.116.003930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fletcher EC, Lesske J, Behm R, Miller CC, Stauss H, et al. Carotid chemoreceptors, systemic blood pressure, and chronic episodic hypoxia mimicking sleep apnea. J Appl Physiol. 1992;72:1978–1984. doi: 10.1152/jappl.1992.72.5.1978. [DOI] [PubMed] [Google Scholar]
- 17.Lesske J, Fletcher EC, Bao G, Unger T. Hypertension caused by chronic intermittent hypoxia–influence of chemoreceptors and sympathetic nervous system. J Hypertens. 1997;15:1593–1603. doi: 10.1097/00004872-199715120-00060. [DOI] [PubMed] [Google Scholar]
- 18.Khayat R, Pleister A. Consequences ofObstructive Sleep Apnea; Cardiovascular Risk of Obstructive Sleep Apnea and Whether Continuous Positive Airway Pressure Reduces that Risk. Sleep Med Clin. 2016;11:273–286. doi: 10.1016/j.jsmc.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Sert Kuniyoshi FH, Pusalavidyasagar S, Singh P, Somers VK. Cardiovascular consequences of obstructive sleep apnoea. Indian J Med Res. 2010;131:196–205. [PubMed] [Google Scholar]
- 20.Drager LF, Yao Q, Hernandez KL, Shin MK, Bevans-Fonti S, Gay J, et al. Chronic intermittent hypoxia induces atherosclerosis via activation of adipose angiopoietin-like 4. Am J Respir Crit Care Med. 2013;188:240–248. doi: 10.1164/rccm.201209-1688OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kasivisvanathan V, Shalhoub J, Lim CS, Shepherd AC, Thapar A, et al. Hypoxia-inducible factor-1 in arterial disease: a putative therapeutic target. Curr Vasc Pharmacol. 2011;9:333–349. doi: 10.2174/157016111795495602. [DOI] [PubMed] [Google Scholar]
- 22.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–1904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oparil S, Zaman MA, Calhoun DA. Pathogenesis of hypertension. Ann Intern Med. 2003;139:761–776. doi: 10.7326/0003-4819-139-9-200311040-00011. [DOI] [PubMed] [Google Scholar]
- 24.Guo GB, Abboud FM. Angiotensin II attenuates baroreflex control of heart rate and sympathetic activity. Am J Physiol. 1984;246:H80–H89. doi: 10.1152/ajpheart.1984.246.1.H80. [DOI] [PubMed] [Google Scholar]
- 25.Hartmann G, Tschop M, Fischer R, Bidlingmaier C, Riepl R, et al. High altitude increases circulating interleukin-6, interleukin-1 receptor antagonist and C-reactive protein. Cytokine. 2000;12:246–252. doi: 10.1006/cyto.1999.0533. [DOI] [PubMed] [Google Scholar]
- 26.Moss JW, Ramji DP. Cytokines: roles in atherosclerosis disease progression and potential therapeutic targets. Future Med Chem. 2016;8:1317–1330. doi: 10.4155/fmc-2016-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruscica M, Baragetti A, Catapano AL, Norata GD. Translating the biology of adipokines in atherosclerosis and cardiovascular diseases: Gaps and open questions. Nutr Metab Cardiovasc Dis. 2016;88:1054–1057. doi: 10.1016/j.numecd.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 28.Buda AJ, Pinsky MR, Ingels NB, Jr, Daughters GT, Stinson EB, et al. Effect of intrathoracic pressure on left ventricular performance. N Engl J Med. 1979;301:453–459. doi: 10.1056/NEJM197908303010901. [DOI] [PubMed] [Google Scholar]
- 29.Romero-Corral A, Somers VK, Pellikka PA, Olson EJ, Bailey KR, et al. Decreased right and left ventricular myocardial performance in obstructive sleep apnea. Chest. 2007;132:1863–1870. doi: 10.1378/chest.07-0966. [DOI] [PubMed] [Google Scholar]
- 30.Otto ME, Belohlavek M, Romero-Corral A, Gami AS, Gilman G, et al. Comparison of cardiac structural and functional changes in obese otherwise healthy adults with versus without obstructive sleep apnea. Am J Cardiol. 2007;99:1298–1302. doi: 10.1016/j.amjcard.2006.12.052. [DOI] [PubMed] [Google Scholar]
- 31.Egan KJ, Knutson KL, Pereira AC, von Schantz M. The role of race and ethnicity in sleep, circadian rhythms and cardiovascular health. Sleep Med Rev. 2016:S1087–S1792. doi: 10.1016/j.smrv.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an obser-vational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 33.Turmel J, Series F, Boulet LP, Poirier P, Tardif JC, et al. Relationship between atherosclerosis and the sleep apnea syndrome: an intravascular ultrasound study. Int J Cardiol. 2009;132:203–209. doi: 10.1016/j.ijcard.2007.11.063. [DOI] [PubMed] [Google Scholar]
- 34.Kuniyoshi FH, Garcia-Touchard A, Gami AS, Romero-Corral A, van der Walt C, et al. Day-night variation of acute myocardial infarction in obstructive sleep apnea. J Am Coll Cardiol. 2008;52:343–346. doi: 10.1016/j.jacc.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doherty LS, Kiely JL, Swan V, McNicholas WT. Long-term effects of nasal continuous positive airway pressure therapy on cardiovascular outcomes in sleep apnea syndrome. Chest. 2005;127:2076–2084. doi: 10.1378/chest.127.6.2076. [DOI] [PubMed] [Google Scholar]
- 36.Bassetti CL, Milanova M, Gugger M. Sleep-disordered breathing and acute ischemic stroke: diagnosis, risk factors, treatment, evolution, and long-term clinical outcome. Stroke. 2006;37:967–972. doi: 10.1161/01.STR.0000208215.49243.c3. [DOI] [PubMed] [Google Scholar]
- 37.Valham F, Mooe T, Rabben T, Stenlund H, Wiklund U, et al. Increased risk of stroke in patients with coronary artery disease and sleep apnea: a 10-year follow-up. Circulation. 2008;118:955–960. doi: 10.1161/CIRCULATIONAHA.108.783290. [DOI] [PubMed] [Google Scholar]
- 38.Redline S, Yenokyan G, Gottlieb DJ, Shahar E, O’Connor GT, et al. Obstruc-tive sleep apnea-hypopnea and incident stroke: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2010;182:269–277. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qureshi WT, bin Nasir U, Alqalyoobi S, O’Neal WT, Mawri S, et al. Meta-Analysis of Continuous Positive Airway Pressure as a Therapy of Atrial Fibrillation in Obstructive Sleep Apnea. Am J Cardiol. 2015;116:e1767–e1773. doi: 10.1016/j.amjcard.2015.08.046. [DOI] [PubMed] [Google Scholar]
- 40.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 41.Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. Jama. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 42.Goncalves SC, Martinez D, Gus M, de Abreu-Silva EO, Bertoluci C, et al. Obstructive sleep apnea and resistant hypertension: a case-control study. Chest. 2007;132:1858–1862. doi: 10.1378/chest.07-1170. [DOI] [PubMed] [Google Scholar]
- 43.Logan AG, Tkacova R, Perlikowski SM, Leung RS, Tisler A, et al. Refractory hypertension and sleep apnoea: effect of CPAP on blood pressure and barore ex. Eur Respir J. 2003;21:241–247. doi: 10.1183/09031936.03.00035402. [DOI] [PubMed] [Google Scholar]
- 44.Oldenburg O, Lamp B, Faber L, Teschler H, Horstkotte D, et al. Sleep-disordered breathing in patients with symptomatic heart failure: a contemporary study of prevalence in and characteristics of 700 patients. Eur J Heart Fail. 2007;9:251–257. doi: 10.1016/j.ejheart.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 45.Parati G, Lombardi C, Castagna F, Mattaliano P, Filardi P, et al. Heart failure and sleep disorders. Nature Reviews Cardiology. 2016;13:389–403. doi: 10.1038/nrcardio.2016.71. [DOI] [PubMed] [Google Scholar]
- 46.Stanchina ML, Ellison K, Malhotra A, Anderson M, Kirk M, et al. The impact of cardiac resynchronization therapy on obstructive sleep apnea in heart failure patients: a pilot study. Chest. 2007;132:433–439. doi: 10.1378/chest.06-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Linz D, Linz B, Hohl M, Bohm M. Atrial arrhythmogenesis in obstructive sleep apnea: Therapeutic implications. Sleep Medicine Reviews. 2016;87:e94. doi: 10.1016/j.smrv.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 48.Kaneko Y, Floras JS, Usui K, Plante J, Tkacova R, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med. 2003;348:1233–1241. doi: 10.1056/NEJMoa022479. [DOI] [PubMed] [Google Scholar]
- 49.Linz D, Ukena C, Mahfoud F, Neuberger HR, Bohm M. Atrialautonomic innervation: a target for interventional antiarrhythmic therapy? J Am Coll Cardiol. 2014;63:e24. doi: 10.1016/j.jacc.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 50.Ng CY, Liu T, Shehata M, Stevens S, Chugh SS, et al. Meta-analysis of obstructive sleep apnea as predictor of atrial fibrillation recurrence after catheter ablation. Am J Cardiol. 2011;108:47–51. doi: 10.1016/j.amjcard.2011.02.343. [DOI] [PubMed] [Google Scholar]
- 51.Naruse Y, Tada H, Satoh M, Yanagihara M, Tsuneoka H, et al. Concomitant obstructive sleep apnea increases the recurrence of atrial fibrillation following radiofrequency catheter ablation of atrial fibrillation: clinical impact of continuous positive airway pressure therapy. Heart Rhythm. 2013;10:331–337. doi: 10.1016/j.hrthm.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 52.Yaggi H, Mohsenin V. Obstructive sleep apnoea and stroke. Lancet Neurol. 2004;3:333–342. doi: 10.1016/S1474-4422(04)00766-5. [DOI] [PubMed] [Google Scholar]
- 53.Minai OA, Ricaurte B, Kaw R, Mansour M, McCarthy K, et al. Frequency and impact of pulmonary hypertension in patients with obstructive sleep apnea syndrome. Am J Cardiol. 2009;104:1300–1306. doi: 10.1016/j.amjcard.2009.06.048. [DOI] [PubMed] [Google Scholar]
- 54.Arias MA, Garcia-Rio F, Alonso-Fernandez A, Martínez Isabel, Villamor José. Pulmonary hypertension in obstructive sleep apnoea: effects of continuous positive airway pressure: a randomized, controlled cross-over study. Eur Heart J. 2006;27:1106–1113. doi: 10.1093/eurheartj/ehi807. [DOI] [PubMed] [Google Scholar]
- 55.Sajkov D, Wang T, Saunders NA, Bune AJ, McEvoy RD. Continuous positive airway pressure treatment improves pulmonary hemodynamics in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:152–158. doi: 10.1164/ajrccm.165.2.2010092. 2002. [DOI] [PubMed] [Google Scholar]
- 56.McEnvoy RD, Antic NA, Heeley E, Luo Y, Luo Y, Ou Q, et al. CPAP for Prevention of Cardiovascular Events in Obstructive Sleep Apnea. N Engl J Med. 2016;375:919–931. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- 57.Khayat R, Small R, Rathman L, Krueger Steven, Gocke Becky, et al. Sleep disordered breathing in heart failure: identifying and treating an important but often unrecognized comorbidity in heart failure patients. J Card Fail. 2013;19:431–444. doi: 10.1016/j.cardfail.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


